The impact of coupling assessments on conceptual understanding and connection-making in chemical equilibrium and acid–base chemistry†
Li
Ye
 *a,
Jack F.
Eichler
*a,
Jack F.
Eichler
 *b,
Alex
Gilewski
ac,
Lance E.
Talbert
b,
Emily
Mallory
a,
Mikhail
Litvak
d,
Emily
M. Rigsby
*b,
Alex
Gilewski
ac,
Lance E.
Talbert
b,
Emily
Mallory
a,
Mikhail
Litvak
d,
Emily
M. Rigsby
 b,
Grace
Henbest
e,
Kiana
Mortezaei
b and
Cybill
Guregyan
b
b,
Grace
Henbest
e,
Kiana
Mortezaei
b and
Cybill
Guregyan
b
aDepartment of Chemistry and Biochemistry, California State University, Northridge, 18111 Nordhoff Street, Northridge, California 91330, USA. E-mail: li.ye@csun.edu
bDepartment of Chemistry, University of California, Riverside, 501 Big Springs Road, Riverside, California 92521, USA. E-mail: jack.eichler@ucr.edu
cDepartment of Chemistry, Glendale Community College, 1500 N Verdugo Rd, Glendale, California 91208, USA
dDepartment of Biology, California State University, Northridge, 18111 Nordhoff Street, Northridge, California 91330, USA
eGraduate School of Education, University of California, Riverside, 501 Big Springs Road, Riverside, California 92521, USA
First published on 14th May 2020
Abstract
Science educators have developed a variety of assessment techniques to help students connect their scientific knowledge and bridge conceptual gaps. In chemistry, concept maps and creative exercises are the two notable assessments that have been implemented into multiple chemistry courses and indicated promising effects on students’ conceptual learning and connection-making between chemistry concepts. These two assessment techniques were usually implemented individually in research studies. Herein, we employed a quasi-experimental, mixed-methods approach to explore whether combining concept maps and creative exercises would reveal any synergistic effects for student learning of chemical equilibrium and acid–base chemistry in a college general chemistry course. In this study, student perceptions of the use of the two assessments were examined by open-ended surveys. Interestingly, students perceived creative exercises as an assessment technique while concept maps were viewed as a learning tool for studying or reviewing exams. Additionally, Students believed that concept maps assisted them in answering creative exercises, but not vice versa. The four study groups (control group, concept maps only, creative exercises only, and both concept maps and creative exercises) were compared through concept inventory pre and post-test questions. The results of an ANCOVA indicated that participation in the experimental groups did not significantly impact conceptual learning gains, as measured by the concept inventory post-test scores. However, focus group interviews indicated students from the experimental group that used both concept maps and creative exercises were able to provide more sophisticated scientific explanations for conceptual questions related to the topics of chemical equilibrium and acid–base chemistry. Implications of these research results, best practices for implementation of the two assessments, and future research are discussed.
Introduction
General chemistry introduces students to fundamental concepts that serve as a foundation for other coursework in science, technology, engineering, and mathematics (STEM). As such, students’ understanding of chemistry concepts and the ability to connect related knowledge are critical for their academic success. However, students who successfully complete the general chemistry series may not spontaneously connect chemistry concepts and may still have gaps in their conceptual understanding of certain topics (Cracolice et al., 2008). Science educators have developed a variety of techniques to help students connect the knowledge and bridge conceptual gaps. One such tool is the Concept Map (CM). CMs were pioneered by Joseph Novak in science education in the 1970s, and serve to visually organize connections between different concepts and use linking phrases to explicate their connections (Novak et al., 1984). The use of CMs for improving conceptual understanding and connection-making between concepts has been reported in many science and health-related disciplines, such as nursing (Chan, 2017), medicine (Veronese et al., 2013), engineering (Segalàs et al., 2008), biology (Bramwell-Lalor and Rainford, 2014), and chemistry (Francisco et al., 2002; Lopez et al., 2011; Burrows and Mooring, 2015; Aguiar and Correia, 2016; Turan-Oluk and Ekmekci, 2018; Talbert et al., 2020).In chemistry, CMs have been implemented as learning and assessment tools in several college chemistry courses, including general chemistry, organic chemistry, and analytical chemistry. A subset of studies has incorporated CMs in the general chemistry curriculum. For instance, Francisco and colleagues implemented concept maps in general chemistry as homework, pre-, and post-laboratory exercises, quizzes, and exams (Francisco et al., 2002). The students were given a list of terms and asked to construct individual maps during the above activities. The concept maps gave insight as to why many students were failing to correctly answer the algorithmic questions. That is, a lack of conceptual understanding led to poor algorithmic question performance. In another study, Burrows and Mooring conducted interviews including a concept map exercise of organic chemistry students who had recently completed one year of general chemistry (Burrows and Mooring, 2015). They graded the concept maps and found that students who scored in the highest tercile had a more thorough conceptual understanding of electronegativity in the context of chemical bonding than those who scored in the lowest tercile. A recent quasi-experimental study by Talbert and colleagues (Talbert et al., 2020) implemented concept maps in a large-enrollment general chemistry course. Students receiving the concept maps assessment built upon their maps every week throughout the course. This group of students performed significantly better on a chemical concept post-test than the corresponding control group (who completed weekly journal entries instead of concept maps) as demonstrated through t-tests, though the results were insignificant when covariates related to incoming academic preparation were included in the statistical analysis.
Moreover, concept maps have been used as a diagnostic assessment for students who completed general chemistry. Lopez and colleagues (Lopez et al., 2011) interviewed organic chemistry students four times and assigned them a concept map to complete in each interview in addition to textbook problems. Concept map scores were significant predictors of problem-solving and exam scores, indicating that concept mapping and conceptual organization improved the students’ problem-solving skills and academic performance in organic chemistry. Students in analytical chemistry have shown learning gains after using concept maps. Turan-Oluk and Ekmecki (Turan-Oluk and Ekmekci, 2018) implemented a concept inventory and four concept maps as pre- and post-tests for gravimetric analysis and demonstrated quantitative gains with large effect sizes. Qualitatively, they determined that students viewed the concept maps in a highly positive manner, as evidenced through Likert-type attitude scales and content analysis of open-ended survey responses. While no specific themes were presented, the authors note that students claimed that concept maps greatly helped their conceptual understanding of the material.
Another emerging technique for promoting connection-making and conceptual understanding of chemistry concepts in chemistry education literature is the Creative Exercise (CE). In CEs, a student is given a chemistry prompt that is designed based on newly learned concepts in the course and asked to write as many accurate, distinct, and relevant statements as they can, relating their previous chemistry knowledge to the newly learned concepts (Ye and Lewis, 2014). Creative exercises are open-ended activities that are designed to incorporate the gains a student would achieve through an activity, and are easier to objectively assess than traditional concept maps. Rubrics, comprised of a list of possible responses from instructors, can be generated beforehand. Additionally, students are explicitly informed as to the three criteria of accuracy, distinction from previously used concepts, and relevance to the prompt and concepts learned in the course. Student responses that meet the aforementioned criteria are added to the instructor-generated rubrics for grading. Recently, Gilewski and colleagues (Gilewski et al., 2019) have shown that creative exercises implemented in two similar introductory chemistry courses at two institutions increased student academic performance with small and medium effect sizes. CEs were given to the treatment groups one week before exams as a group activity and then individually on exams. Control groups completed close-ended (multiple-choice or short-answer) questions on exams written on the same topics as the CE prompt. Students appeared to make more connections between topics over time and had an overall positive appraisal of the assessment. Four themes emerged from open coding survey responses: conceptual understanding, effective study habits, knowledge integration, and flexibility. These positive themes synergize with the quantitative gains endemic to the treatment groups. Warfa and Odowa (Warfa and Odowa, 2015) implemented CEs three times in a process-oriented guided inquiry learning (POGIL) biochemistry course and demonstrated their utility in supporting foundational concepts in a biochemistry course. Students were able to use CEs to link concepts such as acid/base chemistry and chemical equilibrium to structural biochemistry. The authors noted the enzyme kinetics CE, in particular, was able to illuminate learning gaps regarding free energy diagrams and the connections of chemical equilibrium, kinetics, and energy.
Theoretical framework
CMs and CEs are both based upon a meaningful learning framework. Their impact arises from integrating new knowledge into a learner's existing knowledge network to promote meaningful learning, as first proposed by the psychologist Ausubel (Ausubel, 1968), and further developed by Novak (Novak and Cañas, 2006). Meaningful learning is distinct from rote learning, in which a learner relies on verbatim memorization and makes little effort to connect new knowledge to prior knowledge and understand the meaning of the knowledge. Ausubel suggests that meaningful learning is most likely to occur when learners can integrate new concepts into previously-learned topics. Meaningful learning results in deeper understanding, greater capacity for retention, and the ability to apply the knowledge in novel situations. The common nature of concept maps and creative exercises is that they both require learners to retrieve prior knowledge that is relevant to newly learned knowledge, then select and present the connections between knowledge in response to the assessments. The learning opportunities presented by the two assessments provide scaffolding for learners to gain a more complete knowledge structure and lead to a greater extent of meaningful learning.Scaffolding is a process through which an educator provides support for building students’ experiences and knowledge while they are learning new skills, and this support can be removed once the students master the skills. The idea of scaffolding is closely related to the Zone of Proximal Development theory (ZPD). The ZPD originally pioneered by Vygotsky, describes three cognitive areas: one where students can perform tasks entirely on their own, another in which students cannot perform cognitive tasks even with assistance, and one area in between the two, where students can complete tasks with assistance (Fernández et al., 2001). It is this middle area that is of interest to educators and researchers, where the potential development of students’ capabilities can be determined through learning activities under expert guidance or in collaboration with more capable peers. CMs and CEs fill this role well. These tasks are structured with an appropriate level of difficulty, that is, neither trivial nor frustratingly difficult. With proper guidance, students can make their own connections between concepts, and in doing so, recognize that new concepts can be integrated alongside their prior knowledge and advance their learning to more meaningful learning. While the ZPD is traditionally framed in an asymmetric learning scenario (teacher–student interaction), the paradigm can be shifted to symmetric learning when students collaborate together (Fernández et al., 2001). In fact, the collective ZPD is expanded when a group works together to complete a task and offers a fertile ground for learning to occur. Therefore, collaboration between students with diverse abilities can also fall within the ZPD. When students work as a group to help each other to complete CM and CE tasks, they have opportunities to discuss and learn from each other's answers. This support from peers is likely to enhance connection making and deepen meaningful learning of individuals. In addition to helping students to explicate conceptual connections, CMs have been shown to reduce cognitive load of learners, especially in those with limited verbal capacity (Schroeder et al., 2017). This is achieved through the simplicity of the grammatical structures required to connect concepts together, compared to textual structures, which can be much more complex.
Study purpose
Recent research studies (Gilewski et al., 2019; Talbert et al., 2020) completed by the authors have investigated the efficacy of concept maps and creative exercises in college general and introductory chemistry, respectively, and indicated the positive influence of each tool on enhancing students’ conceptual understanding and the potential to foster meaningful learning. However, no studies were found in the literature to pair or compare these two assessment tools, which share some similar features to promote conceptual understanding and connection-making between concepts. Therefore, we expanded the research design by pairing concept maps with creative exercises in this study. The goal of this quasi-experimental, mixed-methods study was to determine if combining concept maps and creative exercises would reveal any synergistic effects in a general chemistry course. We focused the investigation on the topics of chemical equilibrium and acid–base chemistry, two topics that are common to most general chemistry courses and which require the cumulative use of multiple concepts from the year-long general chemistry curriculum. The study design is set to compare four study groups: a control group; Concept Maps only (CM-only); Creative Exercises only (CE-only); and both Concept Maps and Creative Exercises (CM + CE). Through this partitioning, we are able to examine and compare differences between the two experimental groups (CM-only or CE-only) with a “teaching as usual” control group as well as to explore possible synergistic effects when both concept maps and creative exercises are applied and their interplay on students’ understanding of these two specific chemistry topics. Open-ended surveys were used to explore student perceptions of the use of two assessments. To compare students’ conceptual understanding of chemical equilibrium and acid–base chemistry between groups, concept inventory pre-post test questions and focus group interviews were employed.Research questions
1. (a) What are the similarities and differences between concept maps and creative exercises from students’ perspectives? (b) How do these two assessments influence each other?2. (a) Do students believe the assessments improve their conceptual understanding and connection making in chemical equilibrium and acid–base chemistry? (b) How do coupling concept maps and creative exercises affect the efficacy of learning as compared to using each assessment alone?
Methods
Data collection
The concept map and creative exercise assessments were carried out in a third-quarter general chemistry course. This is the third course in the three-quarter general chemistry sequence offered at a large public research university in the southwestern United States. The topics covered in this course include chemical equilibrium, acid–base chemistry, buffers and titrations, electrochemistry, coordination chemistry, and nuclear chemistry. This course was taught for an “on-sequence” cohort of first-year students in the spring of 2019 (S19), but also included second-year students and upper-division students who may have needed the course as a general college science requirement or a prerequisite for health professional schools. The assessments were administered during the first five weeks of the 10-week quarter, and included only the chemical equilibrium and acid–base chemistry units. Students were invited to participate in the study and all data were collected under the approved Institutional Research Board (IRB) protocol HS-10-135. All participants in this study were given an informed consent and signed to agree to participate in the study groups and interviews.The S19 course consisted of a large-enrollment section, which included two 80 minute lectures and one 50 minute recitation section each week (there were 25–30 students in each recitation section). Approximately one-third of the lecture meetings used flipped classroom modules (Eichler and Peeples, 2016), while the remaining class periods included a mixture of lecture, peer-to-peer discussion, and interactive clicker questions. The recitation sections were taught by Teaching Assistants (TAs) who had prepared in advance to implement the CM and/or CE assessments. One TA (TA-1) taught the CM-only (two recitation sections; total n = 55) and CM + CE (two recitation sections; total n = 59) groups. The second TA (TA-2) taught the CE-only (two recitation sections; total n = 60) and control group (two recitation sections; total n = 59). Students enrolled in the discussion group sections based on their schedule availability, and were not aware of the study during the enrollment period. The TAs were also assigned to the discussion group sections based on schedule availability, and TA-1 was assigned to teach the CM-only and CM + CE sections because this TA had implemented the concept map activities in a previous course. The demographic and academic backgrounds of the students were not known when the TAs were assigned to various study groups/discussion group sections. Students were awarded extra credit for completing the CMs and/or CEs, and the students in the control group sections were awarded equivalent extra credit for attendance. In lieu of completing CMs or CEs in groups, students in the control group sections completed collaborative group problem-solving exercises that included free-response calculation-based questions. Detailed protocols describing how the TAs structured the weekly recitation groups for the different study groups are provided in Appendices 1 and 2 (ESI†). It is noted here that the CMs and CEs were collected on a weekly basis to ensure students were making progress in regard to building up their conceptual frameworks.
Implementation of concept maps
To increase the convenience of collecting and assigning extra credit for the CMs, students were required to use a concept mapping program called Cmap. Cmap is a freely available software program that students used to create CMs, and then save and submit their maps as PDF files. TA-1 was familiarized with the use of the Cmap program, and trained to administer and evaluate the concept map activities as previously described (Talbert et al., 2020). TA-1 was able to answer any questions students had with the program and ensured students were able to navigate the Cmap program and properly save their concept maps, and created a video tutorial that the students could view anytime during the term on the online course management system. The CMs were collected by TA-1 electronically (an example concept map created by a student in the study can be found in Appendix 3, ESI†), and if students submitted maps that included most of the relevant concepts with a complete network of connections, they were awarded the designated extra credit. Additionally, students were required to submit a concept map on a weekly basis in order to receive the full extra credit. A total of four concept maps were submitted within the timeframe of the study, and the students were instructed to include all concepts from the equilibrium and acid–base units in their final concept map.Implementation of creative exercises and grading
CE prompts were designed and written based on the current topics (chemical equilibrium and acid–base chemistry) students learned in the course. Three chemistry instructors examined the prompts of the CEs before implementation to ensure the content and level of difficulty of the CEs are appropriate for the students in the course. Author J. F. E worked with TA-1 and TA-2 prior to the start of the quarter to plan out the administration of the CE assignments. This training entailed discussing what highly developed CE responses should look like, how to facilitate collaborative group work among the students during the CE activities, how to provide feedback to students during the CE activities, and how CE responses should be evaluated. Author J. F. E, TA-1, and TA-2 also met on a weekly basis during the quarter to discuss how the implementation of the CE activities was going and decide if any adjustments to the facilitation of the CEs was necessary. Two CEs were implemented in the first four weeks of recitation sections for both the CE-only and CM + CE experimental groups. One CE prompt was used for the chemical equilibrium unit in weeks one and two, and a second CE prompt was used for the acid–base unit in weeks three and four (see Appendix 4, ESI†). Students were prompted to supply six conceptual responses in the first week of each respective CE prompt, then asked to provide ten total responses in the second week. This was done in an effort to help the students build their conceptual models of each broad learning objective. Students in the CE-only and CM + CE experimental groups were then given a CE on the first midterm exam in week five that required the integration of concepts from both the chemical equilibrium and acid–base units (the CE prompt was embedded within the midterm exam; see Appendix 4, ESI†). Students were asked to write at least four statements from the topics of chemical equilibrium and acid–base chemistry to ensure a portion of responses were from current topics learned in the course. The CEs from the weekly recitation sessions and the midterm exam were scanned and uploaded into the Grade Scope system for evaluation. Inter-rater reliability was established by having one of the authors (A. G.) who is not the instructor or teaching assistant of the students to grade 10% of the student responses to the CE on the first midterm independently. The inter-rater agreement of the correctness of the statements of 79% was reached between the teaching assistant and the author A. G.Concept inventory
A 12-item concept inventory was used to assess student gains in conceptual understanding of chemical equilibrium and acid–base chemistry. The five items that tested chemical equilibrium were obtained from an existing chemistry concept inventory published in a library of conceptual questions (JCE Online: Library of Conceptual Questions (CQs): Equilibrium), whereas the seven items that tested acid–base concepts were obtained from an assessment developed by Jensen and Bretz (Jensen, 2013). The concept inventory was input into the course management system and students in all experimental and control groups completed the assessment online in the first recitation meeting in week one (this is termed the “concept inventory pre-test”). The same concept inventory questions were then embedded in the first midterm exam, which all students took in week five of the quarter (this is termed the “concept inventory post-test”). Even though the test items were taken from previously developed concept inventories, the item means for each question were determined and the internal reliability for the combined set of concept inventory items was determined (see Appendix 5, ESI†).Interview and survey
After the first exam, invitation emails were sent separately to the four groups of students (control group and three experimental groups CM-only, CE-only, and CM + CE) to recruit participants for focus group interviews. Focus group interviews were selected because they allow participants with shared experiences to verbalize their feelings or understanding in a more natural conversation (Patton, 2002). The purpose of the focus group interview in this study is to understand students’ experiences of using the two assessments and compare differences in verbal explanations of chemical equilibrium and acid–base chemistry between the groups. There was only one student in the control group who participated in the interview, so that data was excluded from the analysis. The number of students ranged from two to four for the remaining three groups. Participants who volunteered for the focus group were interviewed for about 43 minutes on average and given $25 gift cards as compensation for their time. The focus group interviews consisted of two parts: (1) a series of perception questions to understand the implementation of CM and CE from students’ perspectives; and (2) a series of conceptual questions that explored students’ conceptual understanding of chemical equilibrium and acid–base chemistry. The interview questions are listed in Appendix 6 Table 1 (ESI†). In addition to interview data, open-ended surveys were administered to explore student perceptions on the use of the two assessments at the end of the quarter to all the participants of the three experimental groups (CM-only, CE-only, CM + CE). Survey questions are listed in Appendix 6 Table 2 (ESI†). The study design and timeline of data collection are summarized in Table 1.| Same lecture by author J. F. E. | Week 1 recitation concept inventory pre-test | Week 2 recitation | Week 3 recitation | Week 4 recitation | Week 5 midterm 1 | Week 6 concept inventory post-test |
|---|---|---|---|---|---|---|
| Control by TA1 | Calculation-based questions | Calculation-based questions | Calculation-based questions | Calculation-based questions | Equally valued multiple-choice questions | Focus group interview by author L. Y. + open-ended survey questions |
| CM-only by TA2 | CM | CM | CM | CM | Equally valued multiple-choice questions | |
| CE-only by TA1 | CE1 | CE1 | CE2 | CE2 | CE3 | |
| CM + CE by TA2 | CM + CE1 | CM + CE1 | CM + CE2 | CM + CE2 | CE3 | |
Survey analysis
The percentages of agreement on whether the activity (CE or CM) helped students understand chemistry conceptually and connecting chemistry concepts of the three experimental groups were analyzed and compared using histogram diagrams. Student responses to open-ended survey questions on why the activities helped were also analyzed. The data was used to triangulate the themes found from the focus group interview data.Interview analysis
Two authors (E. M. & M. L.) each transcribed 50% of the interviews verbatim. The two then cross-checked the entirety of each other's work. Only a few minor typographical or homophonic discrepancies arose, and those were quickly addressed. Subsequently, the qualitative analysis employed by reading the transcriptions multiple times and content analysis (Patton, 2002). The transcription was separated into two different parts for analysis. First, student perspectives on CM and CE were coded and the themes and codes emerging from the data were represented using a concept map. Concept maps have been reported to organize qualitative interview data retrospectively in the literature (i.e. constructed post hoc, not concurrently with or prior to the interview) (Kinchin et al., 2010). Herein, the interviews were coded in aggregate, not individually. At first, content analysis was employed by four of the authors (L. Y., A. G., E. M., and M. L.) to distinguish common themes (i.e. codes) from student responses to the perception questions of interview transcripts. Then, the transcript reviewers employed codes to form concept maps using codes as termini while grouping similar codes under themes and/or subthemes. To increase the efficiency of the coding process, the four coders had a meeting to discuss and agree on some general principles before composing individual concept maps. For example, all of them would use spoke-type maps (one central theme with many offshoots) for organizing themes associated with concept maps or creative exercises alone and then combine into network-type maps (multiple central themes with interconnections) to indicate the themes showing connections between the two assessments. In this manner, the four individually-created maps would have a similar organization to ease the amount of time needed for the next stage. After individually-created maps were generated, the coders then discussed their individual maps while simultaneously generating a cohesive map by including mutually agreed-upon themes and codes; in essence, the new map would incorporate the best components of the individual maps. All four maps were generated as a network connecting two spokes, in which the head of one spoke representing concept maps and the other creative exercises. The network comprised codes that were observed between all three focus groups. This process of consolidation took place over one group meeting, as the individual maps were not exceptionally disparate. The amalgamated map was then used to guide the coding of the transcripts. Two authors (Authors A. G. and M. L.) independently coded 33% of the perception data from the transcript. As their coding was insufficiently consistent, the authors discussed and resolved all discrepancies. They then independently coded another 33% of the data. After this, the two compared their codes and reached an agreement of 72%, which was deemed sufficient. One of the two authors (A. G.) coded the remaining third of the perception data.Second, for students’ verbal explanations of the conceptual questions, an analytical framework for coding student reasoning about acid–base reactions developed by Cooper and colleagues (Cooper et al., 2016) was modified to include both acid–base chemistry and chemical equilibrium topics. According to the nature of participant responses to the conceptual questions during the focus group interview, the responses were coded into four major categories: no response; non-normative; principle/theory descriptive; principle/theory mechanistic. One-fourth of participant responses to conceptual questions in the focus groups were then coded into those four categories independently by authors A. G. and M. L. An inter-coder reliability of 81% was reached, and the discrepancies were resolved after deliberation. One author (A. G.) then coded the remainder of the conceptual question data.
Statistical analysis
To determine if there was a statistically significant difference in concept inventory post-test performance between any of the study groups, an analysis of covariance (ANCOVA) was carried out (author J. F. E). To account for the impact of incoming content knowledge on the variance of the dependent variable, concept inventory pre-test scores were included as a covariate in a model that included study group participation as the fixed factor and concept inventory post-test score as the dependent variable. Bonferroni-corrected pairwise between-groups comparisons were included in the analysis. This provided an opportunity to determine if there were differences in concept inventory post-test scores between any of the specific study groups, while accounting for any potential family-wise error. A second ANCOVA was carried out to determine if there was a statistically significant difference in final CE scores on the first midterm exam between the CE-only and CE + CM groups. This model included group participation as the fixed factor (CE-only vs. CE + CM), final CE score as the dependent variable, and concept inventory pre-test score as the covariate. The Statistical Package for the Social Sciences (SPSS) predictive analytics package was used to run all analyses, and a complete summary of both ANCOVA analyses and associated tests for assumptions are provided in Appendices 7 and 8 (ESI†). The estimated study group sample sizes required to achieve a power of 0.80, an effect size index (f) of 0.25, and a significance level of p = 0.05 was calculated as described by Cohen (Cohen, 1988). The minimum sample size for four study groups (k = 4; u = 3) was determined to be n = 45.Results and discussion
Research question 1
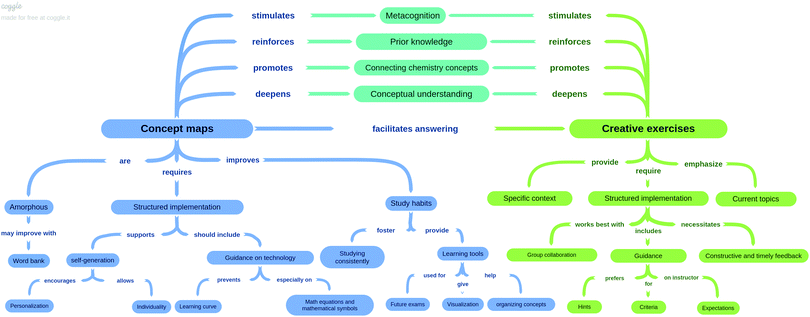
The concordance of the two assessments is shown at the top of the map and represent common themes present in all focus groups. The first theme, metacognition, describes students thinking about their own learning process. For example, a student from one of the groups that used CMs mentioned, “[Concept maps] make you think about what you learned.” Another example quotation for metacognition from the groups used CE, “I believe that it made me think in another way. It made me calculate and write down everything that I know about the question and statement and allowed me to see what are things that I would be able to solve for…”. Metacognition and self-reflection have been shown to be beneficial to learning in many science disciplines, including chemistry, by allowing students to refine ideas about concepts (Rickey and Stacy, 2000; Cook et al., 2013).
Another common theme from the interview data was the reinforcement of prior concepts. This means that students understand that these assessments strengthen their understanding of topics covered in current and previous chemistry courses. A student from the CE group said, “…it kind of forces you to kind of recall basic, basic gen chem concepts to help you come up with statements.” From the CM group perspective, another response was: “…how the [ideas] connected to the acid–base chemistry and also the chemical equilibrium and I also connected some ideas from things I have learned in Chem 1A and Chem 1B.” The setting for the study was the third quarter of the general chemistry series, the first two quarters of which are Chem 1A and Chem 1B. Ideally, these assessment tools would help students not only see the connections between concepts learned in the course, but also make connections to chemistry concepts learned in previous courses.
Two additional common themes between the two assessments were noted: the assessment helped students to connect chemistry concepts and deepen conceptual understanding of the topics of chemical equilibrium and acid–base chemistry. “So the concept map was just – was just essential to me, just make those connections and remember it better than I did in previous classes,” claimed a student. Another mentioned, “[creative exercises] establish more connections to understand Gen Chem better.” With regards to conceptual understanding, a student from one of the groups that used CEs, when asked if he believed he needed to understand concepts rather than just knowing them, said, “These aren't statements that you can just memorize and copy exactly over to something. Because the prompts all differ, so you have to know pretty much everything you're learning. There can't be gaps in your knowledge.” In one of the groups that used CMs, a student said, “I believe that concept maps do help me understand chemistry conceptually because pretty much, concept maps, you can't really lay out – I mean you can lay out equations on there, but you can't really like doing the math so you are forced to like lay out the concepts and actually think about what you are talking about.”
The above themes align with Ausubel's Meaningful Learning framework, as students believe that building upon and making connections with previous concepts are helping them to reinforce their understanding of chemistry and take a more active role in making connections and thinking about their learning process. The themes of deepened conceptual understanding and connection-making resonate with student comments found from previous studies using concept maps (Turan-Oluk and Ekmekci, 2018) or creative exercises in chemistry (Gilewski et al., 2019) and some other science-related disciplines (Veronese et al., 2013; Chan, 2017). Chan found the use of concept maps motivated students in a problem-based nursing class to learn more actively and nurture their creativity. Veronese and colleagues found concept maps were useful to first-year college medical and dental students for systems physiology integration, identification of knowledge deficiencies, and re-examination of learned material.
In addition to similarities between the two assessments, as shown in Fig. 1, many different themes endemic to one assessment arose from the analysis. Representative quotations of the codes unique to CMs and CEs alone can be found in Appendix 9 Tables 1 and 2 (ESI†). The quotations in these two tables are from all three experimental groups. These themes are closely related to the nature of the assessment itself and the implementation of the assessment. Some highlights include students believing that creative exercises were well-suited to group work and collaboration, while concept maps were more personalized. As a result, the CMs might be difficult for others to interpret. Indeed, along those same lines, students identified CEs as an assessment tool while CMs were identified more as a learning tool for self-study for exams. Participants offered suggestions for how to improve the implementation of each assessment. In particular, students wanted thorough and timely feedback for their responses to CEs. To prevent a steep learning curve and to be able to incorporate math equations and mathematical symbols into their conceptual frameworks, students stressed that they want a more comprehensive guidance to new technologies employed and word banks for generating concept maps.
Research question 2
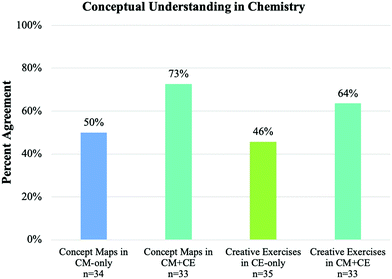
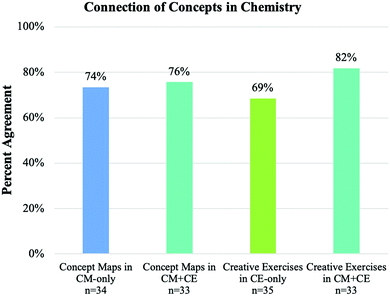
Survey questions comparison. All of the participants were asked to describe whether and why the assessments improved their conceptual understanding and ability to make connections at the end of the quarter. The number of students who responded to the surveys in the three experimental groups (CE-only, CM-only, and CE + CM group) was comparable, ranging from 33 to 35. The summary of affirmative response rates is shown in Fig. 2 and 3. For instance, the students were asked: “Do you think [the exercises] help you understand chemistry conceptually?” Among the students in the CM-only group, 50% found concept maps helped them understand chemistry conceptually, whereas 73% of the students in the CM + CE group found the concept maps to be significantly more helpful. Of those students in the CE-only group, 46% found creative exercises helped them understand chemistry conceptually, while 64% of the students in the CM + CE group found creative exercises were more helpful in improving their conceptual understanding in chemistry. The second question asked was “Do you think [the exercises] help you make connections among chemistry concepts?”. In the CM-only group, 74% of participants found concept maps helped them make connections, while 76% of the participants in the CM + CE group thought concept maps helped them make connections. For the CE-only group, 69% of the students found creative exercise helped them make connections, while 82% of students in the CM + CE group found creative exercises were more helpful for making connections between chemistry concepts.
The above survey results suggest that students believed coupling CMs and CEs improves their conceptual understanding in chemistry and the ability to make connections between chemistry concepts. When students were given either activity individually, no more than half of the students believed the activity helped them understand chemistry conceptually. However, coupling both activities increased the agreement of conceptual understanding by 23% compared to the CM-only group and 18% relative to the CE-only group. Chi-square tests were conducted to examine whether the differences in the percentages of agreement between groups were statistically significant. The results indicated that the proportion of students in CM + CE group believed the assessments helped their conceptual understanding was significantly higher than CM-only group (χ2 = 3.643, p = 0.049) with medium effect size, Cramer's V = 0.233 (Gravetter and Wallnau, 2014). However, the difference between the CM + CE group and CE-only group was not statistically significant (χ2 = 2.119, p = 0.138). In terms of making connections between chemistry concepts, the majority of the students believed each activity helped them make connections. There was a small improvement between pairing both activities compared to the individual activity (2% and 13% respectively), especially when comparing the CM-only group with the CM + CE group. This is likely due to the high agreement already perceived for the CM-only group for making connections. Chi-square tests did not show significant differences between groups for connection making (between CM + CE group and CM-only group, χ2 = 0.044, p = 0.834; between CM + CE group and CE-only group, χ2 = 1.509, p = 0.207). Overall, the evidence from survey data and analysis showed that students believed coupling CMs and CEs was more effective for helping students with conceptual understanding, compared to when the CMs and CEs were implemented individually. Considering these results within the context of the theoretical frameworks described in the introduction (Novak et al., 1984), the coupling of both CMs and CEs offers more opportunities for students to link prior and newly learned knowledge with a deepened understanding of chemistry concepts, therefore more meaningful learning might occur during students' learning process.
Research question 2
Concept inventory post-test score comparison. As described above, the concept inventory included test items previously created for existing instruments. Because the instrument used here included items related to two different content dimensions (chemical equilibrium and acid–base chemistry) the stratified alpha coefficient was calculated as previously described by Widhiarso & Ravand (Widhiarso and Ravand, 2014). This analysis suggests the internal reliability of the concept inventory was moderate (αs = 0.566; see Appendix 5 Table 2, ESI†). An item analysis was also carried out to determine the difficulty of the test items (see Appendix 5 Table 1, ESI;† item difficulty was calculated by determining the percentage of correct answer responses for each question; correct answers were coded 1 and incorrect answers were coded 0). The item difficulty statistics suggest the concept inventory assessed content that closely matched the content covered in the course (i.e., no items had an item difficulty lower than 0.40). The descriptive statistics in Table 2 summarize the concept inventory pre and post-test average scores, average CE scores on the midterm exam, and student demographics.
| CE + CM (n = 59) | CE-only (n = 60) | CM-only (n = 55) | Control (n = 59) | |
|---|---|---|---|---|
| Concept inventory pre-test | 6.03 ± 2.48 | 6.21 ± 2.03 | 5.65 ± 1.98 | 6.50 ± 1.73 |
| Concept inventory post-test | 9.25 ± 1.81 | 8.82 ± 2.06 | 8.71 ± 2.05 | 9.02 ± 2.01 |
| CE3 score | 13.2 ± 4.4 | 12.5 ± 3.3 | n/a | n/a |
| Female | 78% | 43% | 44% | 54% |
| Male | 27% | 51% | 54% | 46% |
| Asian | 51% | 54% | 59% | 56% |
| African American | 3% | 3% | 5% | 0% |
| Hispanic/LatinX | 22% | 30% | 23% | 27% |
| White | 14% | 5% | 5% | 8% |
| Multi-racial | 16% | 0% | 3% | 8% |
An ANCOVA was carried out to determine if the concept inventory post-test scores differed significantly between any of the study groups. In particular, it was of interest to determine if the CE + CM study group appeared to positively impact the conceptual learning gains relative to the other study groups. Though the CE + CM study group was observed to have the highest concept inventory post-test scores (see Table 2), the omnibus ANCOVA indicates there was no statistically significant difference in concept inventory post-test scores between any of the groups (i.e., the null hypothesis stating the mean concept inventory post-test scores are equivalent for the study groups could not be rejected; F = 0.911; p = 0.436; see Table 3). It is noted this analysis accounted for the impact incoming content knowledge had on the variance of the dependent variable by including the concept inventory pre-test scores as a covariate in the model. Bonferroni-corrected between-groups pairwise comparisons were also carried out, and indicate there were no statistically significant differences in concept inventory post-test scores between any of the study groups (see Appendix 7, ESI†). It was also desired to determine if the combination of CEs and CMs might lead to greater gains on the final CE assessment scores, therefore a second ANCOVA was carried out in which the final CE scores were compared between the CE + CM and CE-only groups. Because there was a significant correlation between the concept inventory pre-test scores and final CE scores (see Appendix 10, ESI†), the concept inventory pre-test scores were included as a covariate in the model. Though the CE + CM group was observed to have higher CE scores (see Table 2), the ANCOVA indicates this difference in CE scores was not statistically significant (i.e., the null hypothesis stating the mean final CE scores are equivalent between CE and CE + CE groups could not be rejected; F = 1.136; p = 0.289; see Table 3).
| Analysis | Degrees of freedom | Sum of squares | Mean square | F | p | η 2 |
|---|---|---|---|---|---|---|
| Comparison of means on concept inventory post-test between all groups | 3 | 9.711 | 3.237 | 0.911 | 0.436 | 0.012 |
| Comparison of means on final CE scores between CE-only and CE + CM groups | 1 | 14.670 | 14.670 | 1.136 | 0.289 | 0.100 |
The fact none of the experimental groups appeared to significantly improve student performance on the concept inventory post-test assessment is likely explained by two inter-related factors. First, the CM and CE assessments were employed over a four-week period, and this relatively short exposure may have limited the impact of the CM and CE treatments on concept inventory performance. Second, the experimental conditions were embedded within a larger course framework that student groups were given the same lectures on the two topics. This confounding factor may have diminished the impact of the assessments relative to the control group, and relative to one another. If the CM and CE treatments had been administered over a longer timeframe, and/or had been compared to a “teaching as usual” control that was embedded in a different course that had less focus on conceptual learning in the lecture portion of the course, it is possible these treatments would have significantly impacted the concept inventory post-test scores.
Focus group interview comparison
Table 4 lists demographic information, high school GPA, SAT math, and concept inventory post-test scores of all the focus group participants. The analytic framework (see Table 5) for analyzing the focus group interview data includes four categories: 1: No response; 2: Non-normative; 3: Principle/theory Descriptive (what); 4: Principle/theory Mechanistic (what and how). The higher value of the category indicates more sophisticated scientific explanations were provided by the participants. Characterizations and example quotations of the four categories can be found in Table 5. To compare student understanding between study groups on these two topics, the number of coded statements in each category and the number of participants in each focus group are calculated (see Table 6). Because there were different numbers of students per focus group, the number of responses in each code is reported as a proportion of all coded statements given by the respective focus group. The comparison between the four groups in proportions for four categories is presented in Fig. 4.| CM-only (n = 3) | CE-only (n = 4) | CM + CE (n = 2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a All the names are pseudonyms. b n/a means the data is unavailable or the student did not submit certain assessments. | |||||||||
| Namea | Linda | Daisy | James | David | Katie | Rose | Felix | Chris | Jenny |
| Gender | Female | Female | Male | Male | Female | Female | Male | Male | Female |
| Ethnicity | Asian | Black | Asian | Asian | Asian | Multi-racial | Asian | Multi-racial | Hispanic |
| High school GPA | 3.6 | 4.4 | 3.8 | 4.2 | 3.5 | n/ab | 3.6 | 4 | 4.3 |
| Math SAT | 640 | 670 | 670 | 770 | 560 | n/a | 570 | 650 | 610 |
| Concept inventory post-test scores | 6 | 11 | 10 | 7 | 9 | 6 | n/a | 12 | 9 |
| CE scores | n/a | n/a | n/a | 16 | 15 | 11 | 12 | 16 | 20 |
| CM scores | 6 | 8 | 5 | n/a | n/a | n/a | n/a | n/a | 6 |
| Categories and characterizations | Examples of student quotations |
|---|---|
| 1. No response | “I didn’t really get that question.” |
| Students did not provide an answer | “I do not really have anything more to add.” |
| 2. Non-normative | “Basically, one's just like the reactants and the products or something like that, I'm not sure.” |
| Students provide non-normative or unrelated explanations | “I said that the conjugate base, CH3COOH, was able to neutralize the acid by reacting with it.” |
| 3. Principle/theory descriptive (what) | |
| Students provide simplistic description including | “The reaction shifts to the products and that came from Le Chatelier's Principle.” |
| • description of chemical equilibrium as reaction rates are constant for forward and reverse reactions | “I also just put down like, HCl acts as the Brønsted–Lowry acid because it donates the proton and NH3 acts as the base in Brønsted–Lowry because it accepts it.” |
| • identification of the shift of the reaction or mention of Le Chatelier's principle without stating how it changes the chemical equilibrium | |
| • Identification of a strong acid is completely dissociated into its ions while a weak acid is partially dissociated into its ions in water | |
| • identification of acids and bases based on Brønsted–Lowry or Lewis theory | |
| • identification of CH3COOH as weak acid and its conjugate base CH3COONa | |
| 4. Principle/theory mechanistic (what and how) | |
| Students provide more scientific explanation including | “If you have one of Le Chatelier's that the reaction can react some kind of stress placed on the reaction, so if you increase the concentration of the reactants, then the reaction will thus favor the-like it'll favor and proceed towards the products side to counteract the addition of reactants and form more products.” |
| • explanation on chemical equilibrium constant or differences between solid/gaseous/aqueous reactions | “You have HCl and NH3 so according to Brønsted–Lowry acids are proton-proton donors and bases are proton acceptors. So, in this case, HCl is your acid which is the proton donor. So when you draw the reaction, you have your two separate reactions: HCl plus NH3 and that forms – that would form NH4 plus and Cl minus. So what happened was the HCl, the acid, donated its acidic hydrogen to the ammonium to form NH4 plus, and the Cl minus became its – the, the conjugate base.” |
| • application of Le Chatelier's principle and explanation on how it changes the chemical equilibrium | |
| • connections between strong and weak acids with chemical equilibrium or concentration of H+ or pH | |
| • description of the products of the acid–base reaction or the role of proton in Brønsted–Lowry theory or the role of lone pair in Lewis theory | |
| • description of chemical reactions between HCl and CH3COONa or NaOH and CH3COOH | |
| CM-only | CE-only | CM + CE | |
|---|---|---|---|
| No response | 3 (8%) | 3 (9%) | 0 (0%) |
| Non-normative | 9 (25%) | 11 (32%) | 5 (16%) |
| Principle/theory descriptive | 9 (25%) | 12 (35%) | 15 (48%) |
| Principle/theory mechanistic | 15 (42%) | 8 (24%) | 11 (36%) |
| Total | 36 (100%) | 34 (100%) | 31 (100%) |
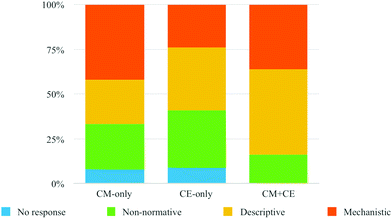 | ||
| Fig. 4 The comparison between the three experimental groups in proportions for the four categories of conceptual understanding. | ||
As indicated in Fig. 4, students in the CM + CE group were able to provide more scientific explanations in the last two categories: Principle/theory Descriptive and Principle/theory Mechanistic, 48% and 36%, respectively. There were no statements coded as No response and relatively low percentage (16%) of statements were coded as Non-normative (i.e., incorrect or irrelevant). Students who had CM-only as an assessment had 8% coded as No response and 25% coded as Non-normative. The rest of the student responses were coded as 25% of Principle/theory Descriptive and 42% Principle/theory Mechanistic category. The CE-only group had similar percentages for No response (9%), and higher percentages for (32%) Non-normative as compared to the CM-only groups. Non-normative and Principle/theory Descriptive were 35% and 24% for this group. When combing the two higher-level categories (Principle/theory Descriptive and Principle/theory Mechanistic) for the three experimental groups, which represented the more scientific and sophisticated explanations of chemical equilibrium and acid–base chemistry concepts, response rates were 59%, 67%, and 84% for CE-only group, CM-only group, and CM + CE group, respectively. Chi-square tests were conducted to examine whether the differences in the proportions of higher-level and lower-level categories (No response and Non-normative) between groups were statistically significant. The results indicated that there was a significant difference between CE-only and CM + CE groups (χ2 = 4.918, p = 0.027) with medium effect size, Cramer's V = 0.275 (Gravetter and Wallnau, 2014). However, the difference between the CM-only and CM + CE groups was not statistically significant (χ2 = 2.604, p = 0.107). These results suggest the students in CM + CE group were able to more frequently describe scientific concepts pertaining to chemical equilibrium or acid–base chemistry in a scientific manner, relative to the CE-only group. In addition, students in the CM-only group were also more likely to provide more scientific descriptions about similar concepts, or what was taking place during the chemical equilibrium or acid–base chemistry, compared to the CE-only group.
Limitations of the study
In terms of the quantitative analysis of the concept inventory post-test scores, the confounding variables associated with the different learning assessments embedded in the main lecture portion of the course could not be controlled. This fact, coupled with the short duration of the study, likely led to the insignificant differences in concept inventory post-test scores among the different study groups. If future studies can extend the length of the assessments across the semester or isolate the CM and CE assessments from other instructional strategies, the impact on conceptual learning might be detected at a statistically significant level. The individual differences between participants of groups might contribute to the variability of the student responses to conceptual questions during the focus group interview. We acknowledge that the findings of the qualitative results might not be used to generalize across populations in other settings. Future studies should consider expanding the sample sizes of groups so as to increase the likelihood of being able to select a good representation of students for interviews from different groups.Conclusions and implications
Concept maps and creative exercises were implemented as assessments to deepen student conceptual understanding and promote connection-making in the chemical equilibrium and acid–base chemistry units, and were implemented in a large-enrollment general chemistry course at a research-oriented public university. Student perspectives on the two assessments were determined through interviews, and it was found common features of the two assessments include enhancing student metacognition, reinforcing prior knowledge, connecting chemistry concepts, and improving conceptual understanding. Interestingly, students perceived creative exercises as an assessment technique and concept maps as a learning tool for studying or reviewing exams. Students believed that concept maps assisted them in answering creative exercises, but not vice versa. The analysis of the open-ended survey responses suggests higher proportions of students in the group that used both concept maps and creative exercises believed the two assessments improved their conceptual understanding and connection-making in chemistry concepts, compared to the groups used each assessment alone. The difference between the CM + CE group and the CM-only or CE-only groups was substantial for conceptual understanding. Conversely, the respective differences were smaller for connection-making, likely due to the fact that each assessment can effectively carry out this role well when implemented individually.Though the CE + CM group appeared to have higher concept inventory post-test scores and higher CE scores relative to the CM-only and CE-only study groups, the ANCOVA results indicate the combined assessment did not significantly impact conceptual learning gains. However, the analysis of survey responses and focus group interviews suggest the promising impact of pairing concept maps and creative exercises on student learning in chemistry. In particular, the focus group interviews suggest the combination of concept maps and creative exercises led to greater gains in conceptual understanding compared to either individual assessment. During the focus group interviews, students who used both concept maps and creative exercises were able to provide more sophisticated scientific explanations for the concepts related to the topics of chemical equilibrium and acid–base chemistry.
Based on the evidence obtained in this study we encourage instructors and educators to adopt concept maps and creative exercises into their curricula and to implement these two assessments together. The benefits of pairing the CMs with CEs include: (1) the combinations of CEs and CMs provides students with multiple tools for understanding and connecting chemistry concepts in addition to traditional assessments; and (2) one assessment is complementary to the other due to the nature of each assessment; CMs are ideal for formative assessment that assists students to transfer textual concepts to visual representations while CEs are suitable for summative assessment because they can be graded in a less subjective manner. To facilitate the implementation of the two assessments for instructors, a set of creatives exercise and example student responses, and detailed teaching notes are provided in the appendices (see Appendices 1 and 2, ESI†). Instructors can use these ready-to-use materials directly or use them as templates to develop their own course materials. Best practices for implementation can be derived from student responses in this study. In particular, instructors should keep in mind students perceive creative exercises as an assessment technique, whereas they view concept maps as a study tool that helps them prepare for the CEs. Students also indicate they would like to generate personalized concept maps with the assistance of word banks and technology tools that incorporate math equations and mathematical symbols, that they prefer to work collaboratively with others for creative exercises, and that prompt and constructive feedback from instructors is essential. Thus, instructors should consider these recommendations when implementing the two assessments. Additionally, since students believe concept mapping helps with answering creative exercises, giving students opportunities to generate concept maps before creative exercises are assigned will likely improve performance, especially if the CEs are incorporated into high-stakes exams.
Lastly, future directions of research with regards to the two assessments could put more thought on how to measure the differences between study groups using alternative modes for collecting student. For instance, one potential measure could ask students to explain relevant concepts and connections between chemistry concepts and submit the explanations in the form of video or audio files. A rubric similar to our analytic framework in Table 5 for student conceptual understanding in chemical equilibrium and acid–base chemistry can be developed for grading and comparing student explanations between different groups of students. Such a study might increase the sample sizes of participants from each study group because it will not be restricted by location and time, and could allow for a repeated measures study design. Another interesting area of future work might be an investigation of the impact of the TAs on students' concept mapping and connection-making. In our study, participants expressed anecdotally in the interview about how TAs influence their proficiency in completing the assessments. Do students benefit more from working with their peers in groups or TAs? How does each contribute to the potential development of students’ capabilities? A further investigation of these questions might be worthwhile and gain insight into the theory of the Zone of Proximal Development.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank all the students, teaching assistants, and instructors who participated in this research.References
- Aguiar J. G. and Correia P. R. M., (2016), Using concept maps as instructional materials to foster the understanding of the atomic model and matter–energy interaction, Chem. Educ. Res. Pract., 17(4), 756–765.
- Ausubel D. P., (1968), Educational psychology: a cognitive view, Holt, Rinehart and Winston.
- Bramwell-Lalor S. and Rainford M., (2014), The Effects of Using Concept Mapping for Improving Advanced Level Biology Students’ Lower- and Higher-Order Cognitive Skills, Int. J. Sci. Educ., 36(5), 839–864.
- Burrows N. L. and Mooring S. R., (2015), Using concept mapping to uncover students’ knowledge structures of chemical bonding concepts, Chem. Educ. Res. Pract., 16(1), 53–66.
- Chan Z. C. Y., (2017), A qualitative study on using concept maps in problem-based learning, Nurse Educ. Pract., 24, 70–76.
- Cohen J., (1988), Statistical Power Analysis for the Behavioral Sciences, 2nd edn, New York, NY: Lawrence Erlbaum Associates, ch. 9.
- Cook E., Kennedy E. and McGuire S. Y., (2013), Effect of Teaching Metacognitive Learning Strategies on Performance in General Chemistry Courses, J. Chem. Educ., 90(8), 961–967.
- Cooper M. M., Kouyoumdjian H. and Underwood S. M., (2016), Investigating Students’ Reasoning about Acid–Base Reactions, J. Chem. Educ., 93(10), 1703–1712.
- Cracolice M. S., Deming J. C. and Ehlert B., (2008), Concept Learning versus Problem Solving: A Cognitive Difference, J. Chem. Educ., 85(6), 873.
- Eichler J. F. and Peeples J., (2016), Flipped classroom modules for large enrollment general chemistry courses: a low barrier approach to increase active learning and improve student grades, Chem. Educ. Res. Pract., 17(1), 197–208.
- Fernández M., Wegerif R., Mercer N. and Rojas-Drummond S., (2001), Re-conceptualizing “Scaffolding” and the Zone of Proximal Development in the Context of Symmetrical Collaborative Learning, p. 19.
- Francisco J. S., Nakhleh M. B., Nurrenbern S. C. and Miller M. L., (2002), Assessing Student Understanding of General Chemistry with Concept Mapping, J. Chem. Educ., 79(2), 248.
- Gilewski A., Mallory E., Sandoval M., Litvak M. and Ye L., (2019), Does linking help? Effects and student perceptions of a learner-centered assessment implemented in introductory chemistry, Chem. Educ. Res. Pract., 20(2), 399–411.
- Gravetter F. J. and Wallnau L. B., (2014), Statistics for the behavioral sciences, 9th edn, Belmont, CA: Wadsworth.
- JCE Online: Library of Conceptual Questions (CQs): Equilibrium https://www.chemedx.org/JCEDLib/QBank/collection/CQandChP/CQs/LibraryCQ/EquilibriumCQ.html. assessed November 22, 2019.
- Jensen J. D., (2013), Students’ understandings of acid–base reactions investigated through their classification schemes and the acid–base reactions concept inventory, Doctoral dissertation, Miami University, Retrieved from https://etd.ohiolink.edu/.
- Kinchin I. M., Streatfield D. and Hay D. B., (2010), Using Concept Mapping to Enhance the Research Interview, Int. J. Qual. Methods, 9, 52–68.
- Lopez E., Kim J., Nandagopal K., Cardin N., Shavelson R. J. and Penn J. H., (2011), Validating the use of concept-mapping as a diagnostic assessment tool in organic chemistry: implications for teaching, Chem. Educ. Res. Pract., 12(2), 133–141.
- Novak, J. D. and Cañas, A. J., (2006), The Theory Underlying Concept Maps and How to Construct Them.
- Novak J. D. and Gowin D. B., (1984), Learning How to Learn, New York and Cambridge, UK: Cambridge University Press.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, SAGE Publ. Inc.
- Rickey D. and Stacy A. M., (2000), The Role of Metacognition in Learning Chemistry, J. Chem. Educ., 77(7), 915.
- Schroeder N. L., Nesbit J. C., Anguiano C. J. and Adesope O. O., (2017), Studying and Constructing Concept Maps: A Meta-Analysis, Edu. Psychol. Rev., 30(2), 431–455.
- Segalàs J., Ferrer-Balas D. and Mulder K. F., (2008), Conceptual maps: measuring learning processes of engineering students concerning sustainable development, Eur. J. Eng. Educ., 33(3), 297–306.
- Talbert L. E., Bonner J., Mortezaei K., Guregyan C., Henbest G. and Eichler J. F., (2020), Revisiting the use of concept maps in a large enrollment general chemistry course: implementation and assessment, Chem. Educ. Res. Pract., 21(1), 37–50.
- Turan-Oluk N. and Ekmekci G., (2018), The effect of concept maps, as an individual learning tool, on the success of learning the concepts related to gravimetric analysis, Chem. Educ. Res. Pract., 19(3), 819–833.
- Veronese C., Richards J. B., Pernar L., Sullivan A. M. and Schwartzstein R. M., (2013), A randomized pilot study of the use of concept maps to enhance problem-based learning among first-year medical students, Med. Teach., 35(9), e1478–e1484.
- Warfa A.-R. M. and Odowa N., (2015), Creative exercises (CEs) in the biochemistry domain: an analysis of students’ linking of chemical and biochemical concepts, Chem. Educ. Res. Pract., 16(4), 747–757.
- Widhiarso W. and Ravand H., (2014), Estimating reliability coefficient for multidimensional measures: a pedagogical illustration, Rev. Psychol., 21(2), 111–121.
- Ye L. and Lewis S. E., (2014), Looking for links: examining student responses in creative exercises for evidence of linking chemistry concepts, Chem. Educ. Res. Pract., 15(4), 576–586.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0rp00038h |
| This journal is © The Royal Society of Chemistry 2020 |