Support for instructional scaffolding with 1H NMR spectral features in organic chemistry textbook problems
Shannon Y. C.
Anderson
 *a,
Whitney S. Y.
Ong
*a,
Whitney S. Y.
Ong
 b and
Jennifer L.
Momsen
b and
Jennifer L.
Momsen
 c
c
aDepartment of Chemistry and Biochemistry, North Dakota State University, P.O. Box 6050, Fargo, ND 58108, USA. E-mail: shannon.y.anderson@ndsu.edu
bDepartment of Chemistry and Biochemistry, The University of Texas at Dallas, 800 West Campbell Road, BSB13, Richardson, TX 75080, USA
cDepartment of Biological Sciences, North Dakota State University, P.O. Box 6050, Fargo, ND 58108, USA
First published on 24th March 2020
Abstract
Nuclear magnetic resonance (NMR) spectroscopy is vital to synthesis and provides rich problem-solving opportunities to organic chemistry students. Using the theories of scaffolding, interleaving, and blocking, our research systematically explores how textbooks introduce and reinforce spectral features when teaching students to solve 1H NMR spectroscopy problems. Specifically, we investigated the 1H NMR spectral features presented in worked examples and practice problems across four undergraduate organic chemistry textbooks. We examined the frequency and ordering of spectral features to explore how the textbooks could support scaffolded instruction. Spectral features like the number of signals and chemical shift were covered by problems more frequently, while integration was covered least. Our findings suggest that textbooks do not provide sufficient practice with all 1H NMR spectral features. We observed no discernible pattern in how textbooks ordered spectral features of 1H NMR in problems, indicating that there is little systematic method to the design of textbook chapters. Implications for textbook authors and editors, instruction, and research are discussed.
Introduction
Nuclear magnetic resonance (NMR) spectroscopy is an indispensable tool for chemists to characterize unknowns and synthesized products. Spectroscopy provides fundamental information on atomic and molecular levels, molecular geometry, chemical bonding, and the mechanisms of chemical reactions (Parker, 1988). Spectral analysis is a vital technique for synthetic chemists in characterizing and analyzing chemical compounds (Thomas, 1991) and is a routine process in learning and practicing organic chemistry. Chemists are proficient at transforming spectral representations into their respective structural representations (Kozma and Russell, 1997).As a result of NMR spectroscopy's importance to chemists and its vital role in synthesis, interpreting these spectra and identifying unknown compounds with spectra serve as a component of organic chemistry instruction. In undergraduate organic chemistry, students learn about both 1H and 13C NMR spectroscopy, along with other spectroscopic and spectrometric methods. While all methods can be used together in the characterization of organic compounds, 1H NMR spectroscopy provides enough information by itself to deduce the whole structure of an unknown organic compound. Instructors often note—anecdotally—that students struggle with 1H NMR spectroscopy, but systematic research exploring these concerns is lacking. Research on student understanding of NMR spectroscopy has focused on the characteristics of graduate-level solvers (Bodner and Domin, 2000; Cartrette and Bodner, 2010; Domin and Bodner, 2012) and on how undergraduate students approach such problems (Topczewski et al., 2017; Connor et al., 2019; Stowe and Cooper, 2019).
While solving 1H NMR spectroscopy problems, undergraduate students have been shown to make incorrect assumptions about spectral features and resort to heuristics to make their interpretations (Connor et al., 2019). While students have been shown to possess adequate procedural knowledge on approaching 1H NMR spectroscopy problems, they have been found to lack reasoning to support their answers (Stowe and Cooper, 2019). Likewise, it has been shown that undergraduate students do not spend their time solving by connecting spectral data with their answers; students lack the checking procedures that experts utilize (Topczewski et al., 2017). Connor and Shultz (2018) examined the pedagogical content knowledge (PCK) in 1H NMR spectroscopy with teaching assistants and found that PCK increased with experience in teaching the subject of NMR. Additionally, they found that TAs struggled to identify what would make similar problems difficult and to provide teaching strategies for those problems, indicating that PCK with NMR may be specific to certain problems and topics. From these studies, we see that undergraduate students need further instruction and opportunities on checking and supporting their answers when solving 1H NMR spectroscopy problems, and extended coverage on each of the spectral features to improve their interpretations of spectral data and prevent heuristic use. These studies have examined students solving spectroscopy problems and provide insight in the area of teaching NMR, but to our knowledge no studies have analyzed how the content of 1H NMR spectroscopy is presented to students.
Textbooks are a reliable resource for learners, created by experts in the field, assigned by professors, and made available to all students. A number of studies have examined undergraduate chemistry textbooks, and have shown that textbooks are important to teaching and learning chemistry (Gkitzia et al., 2011), often guiding the organization of a course's curriculum (Koppal and Caldwell, 2004). Just as different general chemistry textbooks have been shown to present the same concepts differently in the narrative (Pyburn and Pazicni, 2014), in practice problems (Dávila and Talanquer, 2010), and with representations (Nyachwaya and Gillaspie, 2016), we can likewise discern and establish the different ways students are being presented with content on 1H NMR spectroscopy. We therefore focused our research on the potential of textbooks to support instruction on 1H NMR spectroscopy.
Background
Instructional scaffolding can support the learning of spectroscopy
Scaffolded instruction is an idea that emerges from Vygotsky's zone of proximal development (Vygotsky, 1962, 1978). Scaffolding is an interactive process between the instructor and student, whereby the instructor provides supports to the student so they can accomplish a task that might otherwise be too difficult. Well-designed scaffolding adjusts to meet the needs of each student; the support gradually fades to allow the student to complete the task without any instructional scaffolds (van de Pol et al., 2010). While most research on scaffolding does not examine the aspects and benefits of fading (Lin et al., 2012), fading is still regarded as a crucial step of scaffolding. Students with faded support outperform those with either continuous or no support (McNeill et al., 2006). Scaffolding as a whole reduces cognitive load by allowing learners to master the individual steps in the problem-solving process, prior to attempting a problem involving all those steps. In spectroscopy, scaffolding might involve the mastery of one spectral feature before learning and combining multiple spectral features. For example, a student might be presented with how to interpret chemical shift before advancing to using chemical shift and integration together to make an inference on the structure of a compound.While scaffolding holds promise in enabling students to master activities such as problem solving with spectroscopy, the term scaffolding itself has been interpreted and used in different ways. Research has depicted scaffolding as involving an instructor providing structures to support a student's performance (Wood et al., 1976; Rosenshine and Meister, 1992), becoming a participant in a student's learning process (Bruner, 1983; Mercer and Littleton, 2007), helping a learner accomplish a task that they would not have been able to accomplish on their own (Bruner, 1974; Maybin et al., 1992), and systematically devising the sequencing of prompted content, tasks, and support to optimize student learning (Dickson et al., 1993). Most simply stated, scaffolding is a support for learning (as inferred from its metaphor to construction). However, descriptions of scaffolding in practice—and what it looks like in the classroom—are limited. Synthesizing research on scaffolding, van de Pol et al. (2010) proposed a framework to characterize instructional scaffolding. Drawing from research by Tharp and Gallimore (1988) and Wood et al. (1976), van de Pol et al. (2010) describes a scaffolding strategy as involving two dimensions, means and intentions (Table 1). The means of a scaffolding strategy include clear pedagogical moves like providing feedback or demonstrating a particular skill. The second dimension of a scaffolding strategy is the intention, that is the purpose of the scaffolding strategy. The intention can include actions that help the student stay on task or organize their thinking. van de Pol et al. (2010) proposed that scaffolding is comprised of both an intention and means, where the means of scaffolding act to implement the intentions of scaffolding. For example, an instructor may want to aid a student in staying on task toward the current goal (direct maintenance) by the act of asking the student a question (questioning).
| Definition | ||
|---|---|---|
| Intentions | Direct maintenance | Keep the student on target and ensure the student is working toward a particular objective |
| Cognitive structuring | Help the student organize and justify areas of the task | |
| Reduction of the degrees of freedom | Take over parts of the task that the student cannot yet complete in order to simplify the task | |
| Recruitment | Get students interested in the task | |
| Contingency management | Provide incentives to keep students motivated | |
| Frustration control | Minimize or prevent a student's frustration | |
| Means | Feeding back | Providing students with information on their performance |
| Giving hints | Providing clues or suggestions to aid the students in progressing forward | |
| Instructing | Explaining how something must be done and why | |
| Explaining | Providing more detailed information or clarification | |
| Modeling | Demonstrating skills and offering the students actions they can adopt or imitate | |
| Questioning | Asking students questions that require an active answer |
Although the broad use of the term scaffolding has been criticized for being vague and meaning nothing more than support (Pea, 2004; Puntambekar and Hubscher, 2005), the framework of scaffolding as proposed by van de Pol et al. (2010) addressed these criticisms by clearly defining the intentions and means of scaffolding strategies in instruction. We propose that scaffolding is essential to learning 1H NMR spectroscopy because the task is complex, with multiple steps and spectral features dependent on the diverse characteristics of a compound. As shown by van de Pol et al., scaffolding strategies can be infused at different curricular levels, such as the course, unit of instruction, or even the individual item. In fact, Stowe and Cooper (2019) investigated scaffolding at the item level by guiding students’ argumentation to different extents through item prompts. They found that scaffolding at the level of the prompt showed no impact on students’ abilities to construct evidence-based arguments behind their 1H NMR spectroscopy problem solving. We hypothesize that learning 1H NMR spectroscopy could benefit from scaffolding on the level of the unit as a whole, where intentions like reducing the degrees of freedom would provide students with problems requiring the use of the chemical shift, before encountering problems requiring the use of the chemical shift and the splitting.
Undergraduate organic chemistry students learn about several spectral features (in particular, the number of signals, chemical shift, integration, and splitting), are expected to correctly interpret those features and then use those spectral features together to determine the structure of the compound represented. With intentions (or goals) like direct maintenance, focused cognitive structuring, and reducing the degrees of freedom, an instructor can directly reduce the cognitive demand on a student while interpreting 1H NMR spectra. Furthermore, through means such as instructing, explaining, and modeling, an instructor can show a student how to approach their interpreting of 1H NMR spectra.
Textbooks are reliable instructional tools that can support scaffolded instruction
While textbooks do not accomplish the task of instructional scaffolding alone, they are designed with features to support an instructor when scaffolding content on 1H NMR spectroscopy. Textbook chapters on spectroscopy typically include rich NMR data sets, worked examples, practice problems, figures depicting the instrument and spectra, along with descriptions and applications of the spectral features. As a result, instructors of introductory organic chemistry often rely on textbooks to structure curriculum, reinforce lecture topics, and provide worked examples and practice problems (Chiappetta et al., 1991; Mikk, 2000; Justi and Gilbert, 2002). Students then use textbooks to reinforce or clarify lecture material, explore applications, and evaluate their own problem-solving abilities. Instructors may also rely on worked examples and practice problems to demonstrate critical components and steps of the spectroscopy problem-solving process; learners may use those same worked examples to develop their own problem-solving skills. Textbooks are, therefore, viewed by students and instructors as reliable resources that support, enhance, and reinforce students’ learning of critical spectral features (Knight, 2015). As a consequence, textbooks can have a substantial impact on instructional scaffolding and by extension, student learning.Although textbooks are resources unto themselves, scaffolding is a process between the instructor and student. Therefore, textbooks have the potential to support instructional scaffolding, but how textbooks function as part of the scaffolding process is highly instructor-dependent. For example, an instructor may use a textbook to introduce spectroscopy before going over the information in class. An instructor may also have students refer to the textbook upon introducing the subject in class, where the textbook provides students with clarification and practice on the task. An instructor may also use the textbook problems, both within the chapter and at the end of the chapter, as formative or even summative assessment. Regardless of what an instructor chooses to do, textbooks have the potential to explicitly support scaffolded instruction in the areas of cognitive structuring and reducing the degrees of freedom through content that explains and models, and can fade that support through the order and structure of problems (van de Pol et al., 2010).
Learning to interpret 1H NMR spectra is a complex task, one that benefits from instructional scaffolds. Students must be able to comprehend and interpret each spectral feature, use all the spectral features together, and be able to move forward or backward in that interpretation process (either by predicting what a spectrum should look like based on the compound or by inferring what is represented by a given spectrum). Ideally, textbooks should provide material sufficient for mastering all areas of interpretation. Specifically, textbooks should provide support for cognitive structuring with features to instruct and explain each spectral feature of 1H NMR spectroscopy and then reduce the degrees of freedom by modeling areas of interpretation. Building student understanding of these spectral features one at a time, then moving to two or three spectral features together, before moving to all four spectral features together slowly introduces more degrees of freedom into the problem-solving process. Once all spectral features have been practiced alone and together, practice problems can fade the support further when the arrangement of problems does not foreshadow the approach to take. For example, an organic chemistry textbook could provide examples and questions for the number of signals, chemical shift, integration, and splitting individually and then together, modeling how to use all the spectral features alone and in concert to interpret a full spectrum. Worked examples and practice problems allow textbooks to accomplish this support to instructional scaffolding.
Worked examples and practice problems demonstrate potential support for instructional scaffolding
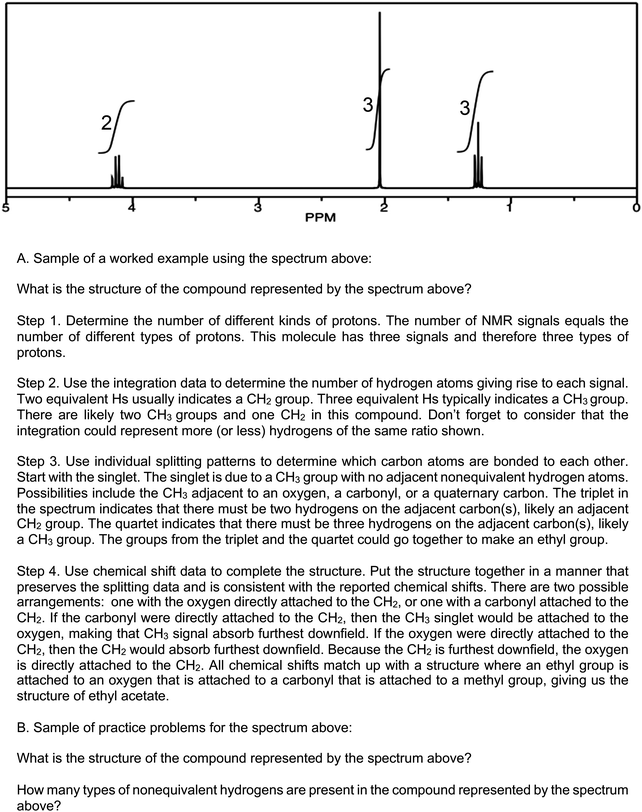
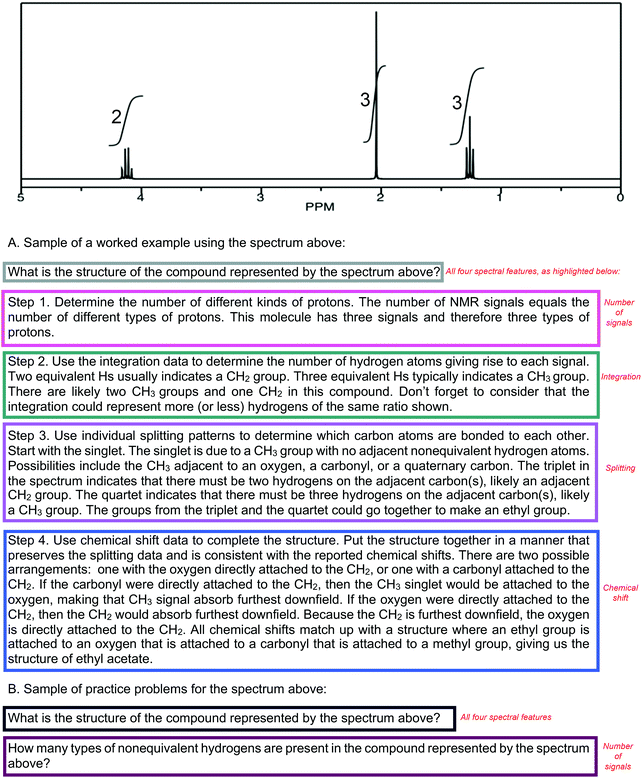
Effective scaffolding supports students’ learning of how and why to do a task (Hmelo-Silver, 2006). Worked examples, with their written explanations (Fig. 1A) are one textbook tool that can convey that information, although the extent to which they actually scaffold learning is unknown. Worked examples consist of a problem statement and the appropriate steps to the solution (Kalyuga et al., 2001). The level of solution detail varies among textbooks, but each worked example must provide a problem statement, answer, and some description of the solution pathway. The description of the solution can vary, where the specific steps are described in detail or briefly. Considering van de Pol's framework, worked examples are particularly useful in supporting learning in scaffolded instruction as they address two intentions of scaffolding: cognitive structuring through organizing and justifying ways to problem solve, and the reduction of degrees of freedom by showing the steps to solve a problem and the solution, allowing the students to focus on those individual details rather than generating a solution path as well as reflecting on the steps they took. Furthermore, worked examples supply two scaffolding means to support learning with explanations on how steps should be done and why, and with the process to solve the problems modeled for the students.Reducing cognitive load, worked examples can help students in identifying how and why steps are taken in a path to solve a problem by allowing them to focus exclusively on the characteristics of that path, rather than both creating a solution path de novo and then evaluating the accuracy or correctness of that path. For the novice solver, evaluating the accuracy or correctness of a path may be cognitively taxing. Evidence from mathematics and the learning sciences demonstrates that worked examples can reduce cognitive load (Cooper and Sweller, 1987; Paas, 1992; Paas and van Merriënboer, 1994; Paas et al., 2016; van Gerven et al., 2002). Moreover, reducing cognitive load functions analogously to the reduction of the degrees of freedom in scaffolding. In 1H NMR spectroscopy, worked examples can reduce cognitive load by allowing the learner to focus on the interpretations that can be made with each individual spectral feature and then use all the spectral features together to come to a solution.
In contrast, practice problems (Fig. 1B) provide a problem statement, but do not provide the answer or goal state. Left as an unknown, students must have a developed problem-solving approach to find the goal state. Thus, practice problems have a very different learning goal, namely to provide students with opportunities to practice, refine, and expand problem-solving approaches. Practice problems serve as an implicit part of the scaffolding process because they do not explicitly indicate which spectral features to use when solving a problem. Practice problems support scaffolded instruction as a diagnostic that addresses areas in need of continued cognitive structuring, and serve as a form of faded support that increases the degrees of freedom. Moreover, the systematic order and combination of problems can implicitly function as a means of scaffolded instruction.
The sequencing of practice problems through interleaving and blocking can impact scaffolding
As practice problems reintroduce degrees of freedom, the sequencing of practice problems with the strategies of interleaving and blocking can reduce or increase the degrees of freedom and thereby add support or fade support in solving. Interleaving deliberately intermixes problems and solving strategies to help learners distinguish between similar concepts and solution pathways. In contrast, blocking drills a single strategy through repetition (Rohrer, 2012). Interleaving may help students develop discrimination skills, thereby supporting their ability to identify an appropriate strategy, rather than having the order of the problems dictate which strategy to use. In contrast, blocking drills a single strategy or concept in a block of questions, with the next question set drilling another strategy. Research in mathematics has found that interleaving promotes students’ problem solving (Rohrer et al., 2014). Although interleaving has been broadly applied to areas like art, language learning, and animal classification, these studies have focused on categorization and identification tasks (Kornell and Bjork, 2008; Vlach et al., 2008; Wahlheim et al., 2011). To our knowledge, there is no research exploring how interleaving might impact students’ use of spectral features when solving spectroscopy problems.The interpretation of spectral features to solve 1H NMR spectroscopy problems may not be directly comparable to the problem-solving approaches in the literature on interleaving and blocking, but the material could benefit from interleaving. In 1H NMR spectroscopy, the strategy or approach does not change between problems: as students interpret spectra, they should attend to all four spectral features each time. However, in each problem, a specific spectral feature or a few spectral features may lead to the structure solution; those spectral features vary with each problem. Additionally, the order in which a solver attends to each spectral feature may differ with the problem and/or the person solving. No matter the order used in solving the problem, the student should still use and interpret all four spectral features. Finally, while there is no evidence that students confuse one spectral feature of 1H NMR spectroscopy with another, interleaving could still be a useful tactic to help students discern how and when to use the spectral features and make the practice problems more diagnostic in nature, so a student knows which spectral feature(s) they do not understand.
Outside the context of categorization and identification tasks, we hypothesize that blocking and interleaving could work together to promote mastery in problem solving. Blocking supports the mastery of a single spectral feature, while interleaving supports mastery of the overall problem-solving process. Blocking can function to aid the student in mastering the interpretations and uses of specific spectral features, and with support from the text, blocking can reduce the degrees of freedom regarding the determination of which spectral features to pay attention to. Students can, therefore, focus on mastering interpretation with one spectral feature at a time. Moreover, interleaving allows for further mastery of problem solving by fading that support, letting students develop and evaluate the solution pathway. While research on interleaving has purported the use of interleaving over blocking, according to van de Pol's framework, the intentions of scaffolding apply to both blocking and interleaving. Thought should go into using both blocking and interleaving to build the problem-solving pathway.
Through the ordering of spectral features within worked examples and practice problems found in organic chemistry textbooks, students are provided with multi-faceted opportunities to develop their knowledge of 1H NMR spectroscopy and problem solving. Systematically examining the worked examples and practice problems for the presence of these spectral features of 1H NMR spectroscopy provides evidence of how textbooks can support the scaffolded instruction of 1H NMR spectroscopy. In this study, we characterized the potential for supporting scaffolded instruction in worked examples and practice problems found in organic chemistry textbooks commonly used in undergraduate instruction. Specifically, we asked:
1. What 1H NMR spectral features do textbook worked examples and practice problems focus on?
2. How do textbook worked examples and practice problems show evidence of interleaving and blocking with 1H NMR spectral features?
Methods
Textbook selection and sampling
We used a convenience sample of eight organic chemistry textbooks commonly used at many institutions; six of the selected textbooks were listed in the top 20 organic chemistry books based on sales on Amazon.com in February 2015 (Table 2). Within each textbook, we identified and analyzed the sections pertaining to 1H NMR spectroscopy. Any problem on 1H NMR spectroscopy within the spectroscopy chapters was analyzed, including worked examples. The number of worked examples and practice problems were counted for each textbook. There was a wide range in the total number of practice problems presented in the textbooks, from 42–154, with a median of 76.5 (Table 3). There was also a wide range in the number of worked examples presented in the textbooks, from 1 to 31 (Table 3), with a median of 7. Further, some textbooks presented many practice problems while presenting few worked examples. Because some textbooks presented a limited number of worked examples, we chose to constrain our examination of the scaffolding of spectral features to the four textbooks that provided the most worked examples (shown in bold in Table 3). Even with their differences, most textbooks have been found to be variations of the same underlying design. Textbook design and structuring emerged over time, guided by personal empiricism (Cooper and Stowe, 2018), where scientists went with gut instinct and personal experience with what had been done before, instead of being driven by empirical evidence. Raker and Holme (2013) in their analysis of organic chemistry curricula through ACS exams, found that little has changed since the 1970s. Cooper et al. (2019) submit that undergraduate organic chemistry textbooks and curricula have not changed in those 50 years, as textbooks use the presentation of synthesis and reactivity of functional groups and the use of electron pushing formalism to show how electrons move in those reactions to guide the compounds formed. With such similarity among undergraduate organic chemistry textbooks, our narrowing down to these four textbooks was further supported by examining the patterns in presenting the spectral features of 1H NMR in each textbook (Table 4). Each of the four selected textbooks reflects a different pattern in the ordering of spectral features.| Title | Authors | Edition | Publisher |
|---|---|---|---|
| Organic Chemistry | W. H. Brown, C. S. Foote, B. L. Iverson and E. Anslyn | 5th edition | Cengage Learning |
| Organic Chemistry | P. Y. Bruice | 4th edition | Prentice Hall |
| Organic Chemistry | F. A. Carey and R. M. Giuliano | 8th edition | McGraw-Hill |
| Organic Chemistry | M. Jones Jr. and S. A. Fleming | 5th edition | W. W. Norton & Company |
| Organic Chemistry | J. E. McMurry | 8th edition | Cengage Learning |
| Organic Chemistry | J. G. Smith | 3rd edition | McGraw-Hill |
| Organic Chemistry | T. W. G. Solomons and C. B. Fryhle | 9th edition | Wiley |
| Organic Chemistry | L. G. Wade Jr. | 5th edition | Prentice Hall |
| Textbook | Practice problems at end of chapter | Practice problem within narrative | Worked examples |
|---|---|---|---|
| Brown et al. | 30 | 12 | 15 |
| Bruice | 57 | 97 | 3 |
| Carey and Giuliano | 34 | 30 | 31 |
| Jones and Fleming | 62 | 11 | 14 |
| McMurry | 50 | 35 | 2 |
| Smith | 82 | 55 | 8 |
| Solomons and Fryhle | 28 | 28 | 1 |
| Wade | 34 | 46 | 6 |
| First feature | Second feature | Third feature | Fourth feature | Textbook(s) following the pattern | |
|---|---|---|---|---|---|
| a McMurry briefly introduced the feature of chemical shift in terms of NMR spectroscopy in general and with 13C NMR spectroscopy before more explaining the topic in depth with respect to 1H NMR spectroscopy. | |||||
| Pattern 1 | #S | CS | INT | SPL | Bruice, Smith, Solomons, McMurrya |
| Pattern 2 | CS | #S | INT | SPL | Carey, Wade |
| Pattern 3 | #S | INT | CS | SPL | Brown |
| Pattern 4 | INT | CS | SPL | Jones | |
Coding and analysis
Within each 1H NMR spectroscopy chapter, two researchers coded all eight textbooks together to categorize the content as narrative, figures, hints, worked examples, and practice problems. Worked examples and practice problems emerged from the analysis as primary resources existent in all the textbooks in supporting the learning of problem solving with 1H NMR spectroscopy, as they offered opportunities to practice problem solving with the spectral features. A worked example was classified as a problem when it contained both an answer and an explanation and/or steps to arrive to the answer. Practice problems were defined as containing a problem statement, without an explanation or steps to arrive at the answer. Practice problems included problems within and at the end of the chapter.To determine which 1H NMR spectral features were the focus of worked examples and practice problems, each problem and worked example was coded for the specific spectral feature(s) indicated by the problem. Those spectral features were the number of signals/proton equivalency (#S), chemical shift (CS), integration (INT), and splitting (SPL). The number of signals in the spectrum indicates the number of equivalent proton groups the compound has and is equal to the number of different types of protons. The chemical shift, or the position of a signal, is determined by shielding and deshielding effects, where shielding shifts an absorption upfield and deshielding shifts an absorption downfield. Integration is the area under a signal and is proportional to the number of absorbing protons. Splitting, the pattern of absorption peaks resulting from spin–spin splitting of nuclei, can be used to determine how many protons reside on the carbon atoms near the absorbing proton. The number and nature of adjacent protons determines the observed splitting pattern. These four spectral features were examined because they encompass the information on 1H NMR spectroscopy most readily and universally available in undergraduate organic chemistry instruction. While some undergraduate courses may cover spectral features like spin–spin coupling and diastereotopic protons, the four spectral features we examined are because they pertain to a baseline of features covered in undergraduate instruction. An a priori coding scheme of the spectral features was established by two researchers, and one researcher independently coded all worked examples and practice problems. The second researcher took a random sample of worked examples and practice problems, comprising 25% of the total sample, and coded them to establish reliability (Cohen's kappa: 0.945).
Once the practice problems and worked examples were coded for the four outlined 1H NMR spectral features (see example in Fig. 2 below), the scaffolding of problems was examined in terms of the ordering and combinations of those spectral features. The ordering of the spectral features for the in-text practice problems and worked examples was compared to the ordering of the spectral features in the narrative. To support our goal of identifying evidence of blocking and interleaving, we used sunburst diagrams to visualize the spatial distributions of spectral features in worked examples and practice problems. We modified a typical sunburst diagram to show the progression of spectral features used in worked examples and practice problems.
Results
We coded 68 worked examples and 316 practice problems across our four selected textbooks (the four textbooks with the most worked examples and different pattern of spectral feature introduction). The 1H NMR spectral feature most frequently identified in either worked examples or practice problems was the number of signals (62% and 69%, respectively); the least frequently identified feature was integration (36% and 55%, respectively).Coverage of spectral features
After coding all textbook problems for the spectral features represented in each, we found that three of the four sampled textbooks included worked examples covering all four spectral features of 1H NMR spectroscopy (Table 5). The number of signals was the most frequent spectral feature included in worked examples in three of the four textbooks (Brown et al., 2008; Carey and Giuliano, 2011; Smith, 2011). The most common spectral feature in the other textbook (Jones and Fleming, 2014) was chemical shift, while the least frequently included spectral feature in the worked examples varied with each book. For Carey and Giuliano, and Smith, integration was the least frequently included spectral feature of the worked examples.| Textbook | Spectral featurea | |||
|---|---|---|---|---|
| Number of signals | Chemical shift | Splitting | Integration | |
| a Note that percentages can add to more than 100 as some worked examples include multiple spectral features of 1H NMR. | ||||
| Brown et al. | 93% (14) | 20% (3) | 33% (5) | 53% (8) |
| Carey and Giuliano | 77% (24) | 16% (5) | 39% (12) | 0% (0) |
| Jones and Fleming | 14% (2) | 93% (13) | 57% (8) | 64% (9) |
| Smith | 63% (5) | 38% (3) | 38% (3) | 25% (2) |
| Average (±St. Dev.) | 62% ± 34% | 42% ± 35% | 42% ± 11% | 36% ± 29% |
All four examined spectral features were featured in the practice problems in all textbooks, both in the in-text and end-of-chapter practice problems (Table 6). The number of signals and chemical shift were the most frequent spectral features among the textbook problems. Among the practice problems, integration was featured the least, comprising of 55% of the problems on average across the textbooks.
| Textbook | Spectral feature | |||
|---|---|---|---|---|
| Number of signals | Chemical shift | Splitting | Integration | |
| Brown et al. | 95% (40) | 76% (32) | 74% (31) | 81% (34) |
| Carey and Giuliano | 70% (28) | 85% (34) | 50% (20) | 55% (22) |
| Jones and Fleming | 40% (29) | 82% (60) | 66% (48) | 42% (31) |
| Smith | 71% (97) | 50% (68) | 60% (82) | 41% (56) |
| Average (±St. Dev.) | 69% ± 23% | 73% ± 16% | 62% ± 10% | 55% ± 19% |
We also explored the number of problems targeting single or multiple spectral features (Table 7). We found that, in general, worked examples were more likely to focus on a single spectral feature at a time, while practice problems focused on all four spectral features together.
| Textbook | One feature | Two features | Three features | All four features |
|---|---|---|---|---|
| Worked examples | 51.3% ± 20.8% | 23.1% ± 11.4% | 19.2% ± 24.1% | 6.5% ± 7.5% |
| Practice problems | 29.2% ± 13.2% | 22.4% ± 9.7% | 8.3% ± 8.4% | 40.2% ± 19.0% |
Ordering of spectral features
We utilized a case-study approach to describe the ordering of spectral features within a textbook, examining each book individually. Modified sunburst diagrams allowed us to visualize the spatial ordering or chronology of the spectral features used in worked examples and practice problems; in addition, these diagrams allowed us to identify instances of blocking and interleaving within the problems.A sunburst diagram is read in a clockwise manner, starting at the topmost notch in the circle. The gray ring indicates the page on which a particular problem was found. Each colored ring conveys the chronology of a different spectral feature within the textbook. For example, pink denotes number of signals, and the light pink signifies the spectral feature was observed in a worked example and dark pink in a practice problem. This convention is used for each spectral feature: blue denotes chemical shift, green denotes integration, and purple denotes splitting. If no color is present in a portion of the ring, the spectral feature for that respective ring was not exercised by the problem in question. Pages without any colored rings indicate a page with no problems.
Sunburst diagrams support our exploration of blocking and interleaving of spectral features within worked examples and practice problems in both within-chapter and end-of-chapter problems. Given our definition of interleaving as the deliberate intermixing of spectral features, we would not expect to see solid rings of color in our sunburst diagrams, but we would see different colors mixed across the problems. With interleaving, we would not see multiple problems of a spectral feature in a row, as that would signal to a student that that is the feature to use. In contrast, if blocking provides a set of problems grouped together that exercise the same spectral feature(s), we would expect to see solid rings of color as evidence of blocking of spectral features.
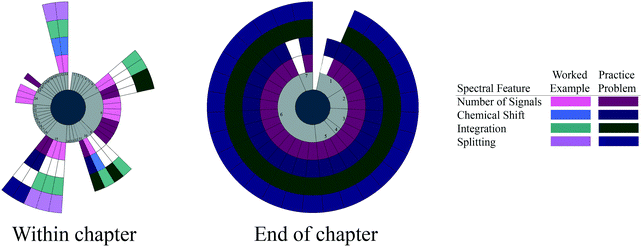 | ||
| Fig. 3 Spectral features targeted by each worked example and practice problem within the chapter and at the end of the chapter for Brown et al. (2008). Pages are conveyed in light gray to show the order of the problems and highlight pages without any problems. | ||
We see little evidence of blocking or interleaving in the Brown et al. within-chapter problems. Rather, we see some evidence of an additive approach, where the problems introduce one spectral feature and add subsequent spectral features in turn.
The end-of-chapter problems in Brown et al. almost exclusively focus on the use of all four spectral features. There is no evidence of interleaving, and the use of all four spectral features in the bulk of problems shows evidence of poor scaffolding.
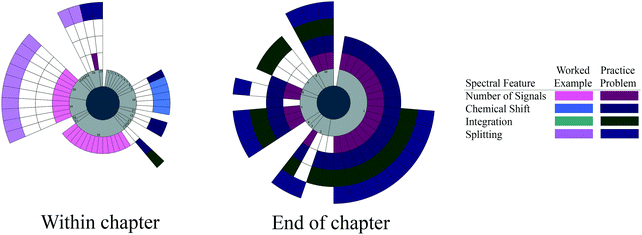 | ||
| Fig. 4 Spectral features targeted by each worked example and practice problem within the chapter and at the end of the chapter for Carey and Giuliano (2011). Pages are conveyed in light gray to show the order of the problems and highlight pages without any problems. | ||
We see evidence of blocking in the within-chapter problems of Carey and Giuliano, with sets of problems devoted to chemical shift, then the number of signals, and later with the number of signals and splitting together. Again, the focus is primarily on one spectral feature at a time and no worked examples or practice problems target all four spectral features together.
The end-of-chapter problems begin with two spectral features, the chemical shift and number of signals, but expand to focus on all four spectral features at a time. Several problems then focus on chemical shift and integration, followed by a single problem on only splitting, and then a set of problems on all four spectral features again. At the end of the chapter narrative, the problems focus on random spectral features and combinations of spectral features before going back to all four spectral features together. We see little evidence of interleaving in the end-of-chapter problems, but there is evidence of blocking, followed by a set of problems with all four spectral features. While the ordering of the spectral features becomes more random toward the end of the problem set, most problems are focused on using three or four spectral features simultaneously.
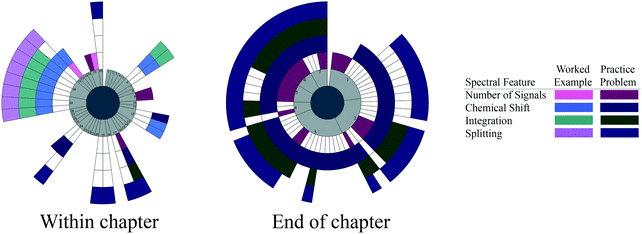 | ||
| Fig. 5 Spectral features targeted by each worked example and practice problem within the chapter and at the end of the chapter for Jones and Fleming (2014). Pages are conveyed in light gray to show the order of the problems and highlight pages without any problems. | ||
With the few within-chapter problems it is difficult to make claims about blocking or interleaving. There is some blocking toward the end of the section. One practice problem in the middle of the chapter targets all four spectral features, while no worked examples target all four spectral features together.
We see evidence of an additive approach to the spectral features in the end-of-chapter practice problems for Jones and Fleming, starting with the number of signals, then moving to chemical shift, and then chemical shift and integration before focusing on all four spectral features together. These problems show evidence of blocking. The ordering of the spectral features gets more unpredictable in the last two-thirds of the problems, focusing several problems on chemical shift then all spectral features together except the number of signals. More random combinations of spectral features are targeted before focusing primarily on all four spectral features together at the end. These random combinations could be possible evidence of interleaving, but with blocking throughout the problem set, the evidence for interleaving is not strong.
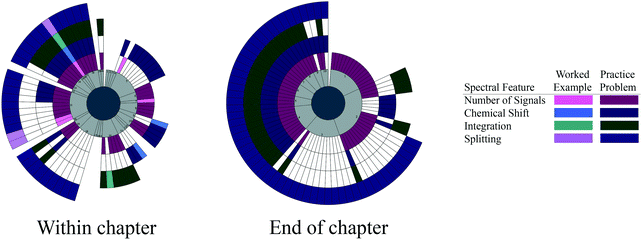 | ||
| Fig. 6 Spectral features targeted by each worked example and practice problem within the chapter and at the end of the chapter for Smith (2011). Pages are conveyed in light gray to show the order of the problems and highlight pages without any problems. | ||
We see evidence of blocking with Smith's within-chapter problems. Overall, the within-chapter problems focus on a single spectral feature at a time throughout much of the chapter; some of the later problems incorporate three or four spectral features, but the spectral features are built up. This additive approach to the spectral features is shown as Smith focuses on the first two spectral features independently and then together. This building is repeated for a fourth spectral feature with a set of problems using only splitting, then using splitting with the three other spectral features. There is no evidence of interleaving.
We see evidence of blocking in the end-of-chapter problems, where a series of problems on the number of signals are given, followed by the number of signals with integration. Chemical shift problems are then provided, followed by problems with the number of signals and splitting. A series of problems on splitting are provided before problems are given on all four spectral features together. Each spectral feature is handled independently before all four spectral features are used together. There is no evidence of interleaving with the Smith end-of-chapter problems.
Across all four sampled textbooks, the ordering and combinations of within-chapter problems varied. Where Brown et al. (2008) utilized an additive approach, beginning with one spectral feature then adding another spectral feature to that previous spectral feature, Carey and Giuliano (2011) focused on each spectral feature independently. Jones and Fleming (2014) moved back and forth from combinations of spectral features, and Smith (2011) was additive with spectral features, but also provided multiple practice problems and worked examples with all four spectral features together. In the end-of-chapter problems it is evident that all four spectral features together are utilized in all the textbooks, but the textbooks’ approaches still differ. Brown et al. (2008) almost exclusively focuses on all four spectral features together, Carey and Giuliano (2011) offer a few problems with all four spectral features in the middle of the problem set and then follows with problems with fewer spectral features and repeating this process. Jones and Fleming (2014) starts out with an additive approach and then gets more random in the combinations of spectral features, while Smith (2011) covers each spectral feature alone before combining multiple features and ending with all four spectral features together. In a full comparison, all four textbooks continue to vary in their approaches. Between all the worked examples and practice problems (within-chapter and end-of-chapter), three of the textbooks showed evidence of blocking (Carey and Giuliano, Jones and Fleming, and Smith). Three textbooks showed some additive approaches with the spectral features (Brown et al., Jones and Fleming, and Smith). Only one textbook showed potential for interleaving (Jones and Fleming).
Discussion
Textbooks serve as reliable resources for students, supplementing and supporting classroom instruction (Knight, 2015). In order to identify how textbooks support learners in developing 1H NMR spectroscopy problem-solving approaches, we analyzed four textbooks to describe how worked examples and practice problems ordered and combined four baseline spectral features. Practice problems in all four textbooks included all four spectral features, with number of signals and chemical shift the most common features observed. The ordering and combinations of within-chapter problems varied across all four of our sampled textbooks, but three textbooks showed evidence of blocking in within- and end-of-chapter problems, with little to no interleaving of the spectral features.Individual spectral features
1H NMR spectra are made up of four distinct features that function to guide problem solving. These spectral features include the number of signals, chemical shift, integration, and splitting, and make up the foundation of cognitive structuring the textbooks can address. We found that worked examples and practice problems in textbooks do not place equal emphasis on each 1H NMR spectral feature, which results in an excess of problems for one feature and a dearth for other features. For example, in Carey and Giuliano's (2011) 31 total worked examples, 24 covered the number of signals, while zero discussed integration. By extension, this may send an implicit signal to students of what is important in the problem-solving process. Understanding how to interpret these spectral features is essential to successful problem solving; Cartrette and Bodner (2010) found that more successful solvers better mined the spectral data. Those successful solvers were guided by their understanding of the features of 1H NMR spectroscopy. We know that understanding and attending to all four spectral features when solving is crucial, but without sufficient assessment, instructors cannot diagnose student difficulty with each spectral feature. If there are many problems on the number of signals and fewer on splitting, a student might assume erroneously that one is more important than the other. In the case of the textbooks we examined, the number of signals was most common across the textbooks, while integration was the least common. This may signal to students to focus on the number of signals while ignoring integration, thus limiting the development of their problem-solving abilities.Without adequate practice with each individual spectral feature, textbooks could be shortchanging the learning process for students. As in many of the textbooks we chose not to include in this study, when there are few worked examples or practice problems on specific spectral features, it can impact the development of fluency in problem solving. Students working with a limited number of problems for a particular spectral feature will not see the diversity in problems and gain the fluency and flexibility necessary in using the spectral features together. As Stowe and Cooper (2019) observed, students do not build evidence-based arguments to support the answers they arrived at. We would argue that textbook coverage of spectral features could build problem solving fluency and by extension, improve students’ argumentation. Furthermore, a lack of worked examples and practice problems could lead students to adhere to heuristics like generalization and rigidity, and students could make assumptions that spectral data should be absolute (Connor et al., 2019). The more problems students have access to, the more ways they can observe spectral features being used, which could help them build fluency in using those spectral features.
In our analyses, we found that integration comprised 55% of the practice problems on average across the textbooks, the least of all the spectral features. Students may implicitly rely on those spectral features that are overrepresented, mistakenly believing those are key spectral features for solving problems. By extension, students do not develop a robust problem-solving approach. Without adequate practice on integration, a student could be uncertain in interpreting the number of hydrogens represented by individual signals sets and could therefore fail to see how to connect the structural pieces together. Each 1H NMR spectral feature is important to the solution pathway. With some spectral features overrepresented in worked examples and practice problems, a student may develop an understanding that those lesser assessed spectral features are not as important to the problem-solving process. While it could be argued that some spectral features may be more foundational than others and therefore deem more practice, it is not necessarily the role of a textbook to determine what is needed most by students and a textbook should not signal what could be arguably more foundational—this is a role of the instructor. In their use of textbooks, instructors make decisions on what to use, supplement, and alter from a textbook (Mesa and Griffiths, 2012). Likewise, students search out examples in their use of textbooks (Weinberg et al., 2012; Lee et al., 2013). As sufficient support for scaffolded instruction, textbooks could instead include bountiful examples and problems of all types that an instructor can refer their students to.
Support for instructional scaffolding by combining spectral features
Overall, the worked examples and in-text practice problems we analyzed were geared toward helping students master interpretations with each spectral feature individually, with few of these problems integrating all four spectral features together. Only two textbooks (Brown et al., 2008; Smith, 2011) incorporated worked examples with all four spectral features together, and there were only three in total of these examples. Worked examples that integrate all four spectral features fulfill the ‘expert solver’ role. These problems summarize and synthesize all four spectral features and provide the learner with an evidence-based pathway for problem solving. If the goal of spectroscopy instruction is to equip students with the knowledge and skills necessary to interpret and characterize spectra, it is essential that they learn to incorporate all four spectral features into a solution pathway.Worked examples and practice problems support instructional scaffolding by giving learners many problems and examples involving each individual spectral feature prior to encountering a problem involving all the spectral features. If textbooks are to support instructional scaffolding in the learning process, we would expect to find worked examples and practice problems that examine each spectral feature in isolation as well as in combination to assess students’ ability to interpret and use multiple spectral features. Students could benefit from seeing additional worked examples that combine spectral features to better learn how the four spectral features work in concert to solve a 1H NMR spectroscopy problem. The novices in Topczewski et al. (2017) spent time looking at all resonances in the spectra and did not discern the most critical areas of interest. Thus, organic chemistry students may require more practice using all spectral features together and models on expert approaches to interpreting the features all together. Students need to see how the features work together to be able to discern how to connect resonances with the structures and effectively perform checking procedures like experts and successful solvers (Cartrette and Bodner, 2010). Worked examples that combine multiple spectral features could work well toward the end of the chapter, after the introduction of each spectral feature, and ideally after the students have had opportunities to use and master each spectral feature. Instructors could then point students towards additional models of expert-like solving through worked examples as well as problems to practice their understanding.
As we consider the framework on scaffolding from van de Pol et al. (2010), this additive approach of spectral features within the worked examples and practice problems addresses the intent of scaffolding to reduce the degrees of freedom, allowing students to focus on and master interpretations with one spectral feature before introducing another degree of freedom in the form of another spectral feature. Building a student's fluency using spectral features from one to multiple moves from one degree of freedom to many. Increasing the degrees of freedom functions to fade support in scaffolded instruction. While we pointed out three textbooks that used an additive approach with the spectral features, our textbook analysis does not find any clear evidence of the textbooks helping establish mastery with additive scaffolding, where each spectral feature is first exercised alone and then in combination, in either the worked examples or practice problems. If we were to see evidence of scaffolding by adding in spectral features, we would expect the worked examples and practice problems within the chapter to block a number of problems on one spectral feature, before adding another spectral feature, before moving onto all four spectral features together. The Carey and Giuliano, and Smith textbooks both focus on mastering individual spectral features before moving onto additional spectral features. However, we note that there is not a clear signal for additive scaffolding in these books, as some spectral features are combined with others before covering the new feature on its own. A purposeful additive approach in scaffolding would cover one spectral feature on its own, then another on its own, and then combine the two features. A third spectral feature could then be introduced with practice problems of its own, and then either combined with the other two features or with one of the other features. Additive scaffolding in this sense then offers mastery of a single concept before adding another concept and increasing the degrees of freedom.
Support for instructional scaffolding by ordering spectral features
Beyond the use of individual spectral features and combinations of multiple spectral features in problems, the ordering of spectral features through interleaving and blocking can aid in instructional scaffolding. Blocking spectral features acts to reduce the degrees of freedom when scaffolding instruction. In the textbooks we sampled, we saw little evidence of systematic blocking and interleaving, although we note that end-of-chapter problems tended to block spectral features individually or examined all four spectral features simultaneously. Students may need substantial practice using a single spectral feature to fully understand how the spectral feature is interpreted. Once students have some proficiency with interpreting a spectral feature, they can then begin combining spectral features to develop a problem-solving pathway. Textbook ordering of practice problems can systematically support instructional scaffolding by initially removing degrees of freedom to focus on single spectral features (blocking) and then reintroduce degrees of freedom by interleaving problems, to help learners build fluency.The lack of interleaving found in our sampled textbooks shines a light on an area in need of addressing. If textbooks utilized systematic blocking and interleaving it could be possible to mitigate the difficulties students have shown in problem solving with 1H NMR spectra. Topczewski et al. (2017) showed that students spent time focusing on all resonances in the spectra, struggling to recognize or understand the connections needed to be made. Too much blocking could exacerbate issues such as these, where students could be led to believe that they must focus on and analyze all areas in the spectrum, using more of their working memory capacity in the process. Scaffolding could also aid in helping students see how the spectral data works together. Connor et al. (2019) found that students made assumptions that the N + 1 rule should hold true and that spectral data like typical chemical shift values should be absolute. In these cases, scaffolding and more blocking with each of the spectral features could help in picking out patterns within each of the spectral features alone, before having to use all spectral features together. Stowe and Cooper (2019) observed that students possessed procedural knowledge, but could not make well-supported arguments for the answers they found. In this situation, too much blocking could promote issues in knowing how to use all the information together.
Topczewski et al. (2017) also found in their study that students did not spend time connecting specific resonances with the structures. Here, interleaving could help students gain mastery in finding and connecting the relevant spectral support for a structure. Connor et al. (2019) also found that students used heuristics like rigidity and one-reason decision making, using only some or one of the spectral features to make their decisions. The use of such heuristics could be mitigated through interleaving, so students can understand how to use all spectral features together and see patterns across problems incorporating all spectral features. Since the students in Stowe and Cooper's (2019) study possessed adequate procedural knowledge and were able to arrive at the answers just fine, they needed help in using all the information. Interleaving could help students gain flexibility in using procedural knowledge and help students discern not only how to apply a strategy, but when to use that strategy (Rohrer, 2012). Practice with interleaved problems would help students see how general rules in interpreting spectral features are not absolute and would provide students with opportunities to see the shortcomings of specific heuristics.
Our suggestion to block spectral features before interleaving contrasts with existing research conducted primarily in mathematics education. In math, substantial research supports the efficacy of interleaving in helping students develop robust problem-solving approaches (Mayfield and Chase, 2002; Rohrer and Taylor, 2007; Rohrer et al., 2014). Success in mathematics requires that students learn to choose appropriate strategies when solving problems. However, we believe blocking may be important in solving spectroscopy problems as students need to master interpretations with each spectral feature independently, before combining spectral features to solve a problem. Certainly, further research is warranted that explores the effects of interleaving and blocking on students’ ability to solve spectroscopy problems.
Conclusions
As instructors and researchers, we know the importance of scaffolding the learning experience. Textbooks can be a tool to support scaffolding, especially when they are designed and written using evidence-based practices like interleaving. In the present research, we identified several practices that could be more fully used by textbooks. For example, textbooks could deliberately structure the narrative, worked examples, and practice problems to focus on each spectral feature independently and then together. Moreover, practice problems could begin by blocking each spectral feature and then move towards interleaving the spectral features.Although we focused on just one textbook chapter, we believe these results underscore a greater need to use the research from the learning and cognitive sciences and discipline-based education research to design textbooks. Much is known about problem solving and the importance of scaffolding and this research can inform textbook design, including the ordering and combination of worked examples and practice problems. A systematic approach to textbook design, including deliberate decisions about the order spectral features are introduced, using blocking and interleaving with practice problems, supports instructional scaffolding and by extension, student learning.
Limitations and future directions
Instructors have discretion in structuring their instruction which may temper or limit the effects of textbooks on student learning. Textbooks, however, influence course design, as instructors use textbooks to help structure courses (Mesa and Griffiths, 2012). As a result, the impact of textbooks on student learning is likely substantial. Textbooks have the potential to support scaffolded instruction, but based on this research, they do not have the worked examples and practice problems designed and ordered to achieve this. Instructors may take the brunt of scaffolding, but more research is needed in this area to figure out if scaffolding is happening in the classroom and if it is, to what extent it promotes learning.Given the lack of worked examples throughout all four textbooks we analyzed, robust conclusions are limited. Examining the chapter narrative could provide further insight into the nuances of how the spectral features of 1H NMR are described and taught. This research focuses on four baseline spectral features, but there are additional spectral features that become pertinent to chemists beyond the undergraduate level.
While we can use principles from the learning sciences to design textbooks, i.e. how we might order problems, further insights from students solving problems would help to refine and systematically shape textbook problems and worked examples. We know from Topczewski et al. (2017) that novice solvers pay attention to all four spectral features, but we do not know how students may struggle in discriminating between the spectral features and if they know when one spectral feature versus another provides them with the information necessary to solve for the structure of the problem. More research is needed that focuses on how students use and integrate the four spectral features of 1H NMR spectroscopy presented here.
Some spectral features involve an understanding of others to be properly applied. For example, splitting and integration involve an understanding of proton equivalence. In our study, we only went as far as the prompts explicitly told students to solve for or reason with. The only problems that had an absence of specific features to solve for were the problems that asked for a whole structure, and those problems were coded for all four spectral features. Textbook analysis therefore contributes to this limitation, as we would not know if students are drawing on interrelated spectral features in that way or not. Stowe and Cooper's (2019) findings showed that as students displayed adequate procedural knowledge, they did not support their reasoning with spectral evidence. Likewise, the findings from Topczewski et al. (2017), where novices did not connect spectral features with the provided structure answer choices, also shows that students are not drawing back to specific spectral features. We would not be sure if students were truly reasoning with the other features or not. Making such determinations turns into an examination of the problem-solving process and is therefore a facet of the individual human that cannot be determined from the textbook analysis itself.
Moreover, one spectral feature in a 1H NMR spectrum can often be more critical than other features in determining the structure of the compound represented. On this level, blocking and interleaving could actually underly problems that would otherwise seem to assess all spectral features, such as the end-of-chapter problems for Brown et al. (2008). It is possible that critical spectral features could be deliberately intermixed within a set of problems that, on the surface, cover all four spectral features. Future research should examine the nature of such critical spectral features and provide an intersection between the difficulties in solving students exhibit, as this involves aspects of the individual problem-solving process and characteristics of novice and expert solvers.
Implications
Implications for instruction
We described different areas in which an instructor might rely on a textbook to support scaffolded instruction. As our study examined the ways in which textbook worked examples and practice problems focused on the spectral features of 1H NMR, we can address how an instructor could use textbooks in the stages of providing students with practice and using problems for different assessments. Findings from this study suggest that textbooks may not provide students with enough practice with interpreting each individual 1H NMR spectral feature. Limited practice could impede the development of problem-solving fluency and may, in fact, support student use of inappropriate heuristics. As we carefully consider the nature of the four 1H NMR spectral features we focused on, the number of signals and chemical shift allow the solver to hypothesize what structural and functional pieces could be present, but splitting and integration allow the solver to connect these structural pieces together. If the practice students get with interpreting the spectral features aligns with our findings from the textbooks, students may be able to find the structural and functional pieces from a 1H NMR spectrum, but may lack adequate practice with putting those pieces together into a whole structure. We encourage instructors to take a closer look into how these spectral features are presented to students and how these spectral features are assessed. If an instructor chooses to incorporate textbook problems in their own assessments, we would encourage them to examine the spectral features focused on by each question to ensure alignment with their unit objectives. Reflective instruction that deliberately introduces and assesses each of the four spectral features could support students as they master the spectroscopy solving process. Likewise, deliberate use of blocking and interleaving with the spectral features could help students develop solving fluency.Implications for research
Using principles from the learning sciences with scaffolding, blocking, and interleaving, our research systematically explores how textbooks introduce and reinforce spectral features when teaching students to solve 1H NMR spectroscopy problems. While textbooks generally introduce students to all spectral features, we find that the approach does not reflect practices of scaffolding, interleaving, or blocking. We recognize that textbooks do not directly reflect classroom practice; thus, we believe there is a need to further study how spectral features of spectroscopy are introduced to students in the natural setting of an organic chemistry classroom. In addition, the ordering of spectral features may impact how students use the spectral features to solve spectroscopy problems. To date, we know of few studies investigating students’ reasoning when solving spectroscopy problems; more work is warranted in this area. To our knowledge, no study has investigated how the spectral features behind 1H NMR spectroscopy are presented to students. This approach with spectral features provides a new way to frame what is seen in student reasoning when solving 1H NMR spectroscopy problems. Studies should explore how students use and reason with each specific spectral feature. Finally, there is a need for research that explores how blocking might help or hinder the 1H NMR spectroscopy solving process. Blocking is generally viewed unfavorably in the learning sciences community, but we posit that blocking may be beneficial when learning to solve complex, multistep spectroscopy problems.Conflicts of interest
There are no conflicts to declare.References
- Bodner G. M. and Domin D. S., (2000), Mental models: the role of representations in problem solving in chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Brown W. H., Foote C. S., Iverson B. L. and Anslyn E. V., (2008), Organic Chemistry, 5th edn, Belmont: Brooks Cole, Cengage Learning.
- Bruice P. Y., (2004), Organic Chemistry, 4th edn, Upper Saddle River: Prentice Hall.
- Bruner J. S., (1974), From communication to language—a psychological perspective, Cognition, 3(3), 255–287.
- Bruner J. S., (1983), Child's Talk - Learning to Use Language, 1st edn, New York: W.W. Norton.
- Carey F. A. and Giuliano R. M., (2011), Organic Chemistry, 8th edn, New York: McGraw-Hill.
- Cartrette D. P. and Bodner G. M., (2010), Non-mathematical problem solving in organic chemistry, J. Res. Sci. Teach., 47(6), 643–660.
- Chiappetta E. L., Fillman D. A. and Sethna G. H., (1991), A method to quantify major themes of scientific literacy in science textbooks, J. Res. Sci. Teach., 28(8), 713–725.
- Cooper M. M. and Stowe R. L., (2018), Chemistry education research—from personal empiricism to evidence, theory, and informed practice, Chem. Rev., 118(12), 6053–6087.
- Cooper M. M., Stowe R. L., Crandell O. M. and Klymkowsky M. W., (2019), Organic chemistry, life, the universe and everything (OCLUE): a transformed organic chemistry curriculum, J. Chem. Educ., 96(9), 1858–1872.
- Connor M. C. and Shultz G. V., (2018), Teaching assistants’ topic-specific pedagogical content knowledge in 1H NMR spectroscopy, Chem. Educ. Res. Pract., 19(3), 653–669.
- Connor M. C., Finkenstaedt-Quinn S. A. and Shultz G. V., (2019), Constraints on organic chemistry students’ reasoning during IR and 1H NMR spectral interpretation, Chem. Educ. Res. Pract., 20(3), 522–541.
- Cooper G. A. and Sweller J., (1987), Effects of schema acquisition and rule automation on mathematical problem-solving transfer, J. Educ. Psychol., 79(4), 347.
- Dávila K. and Talanquer V., (2010), Classifying end-of-chapter questions and problems for selected general chemistry textbooks used in the United States, J. Chem. Educ., 87(1), 97–101.
- Dickson S. V., Chard D. J. and Simmons D. C., (1993), An integrated reading/writing curriculum: a focus on scaffolding, LD Forum, 18(4), 12–16.
- Domin D. S. and Bodner G. M., (2012), Using students’ representations constructed during problem solving to infer conceptual understanding, J. Chem. Educ., 89(7), 837–843.
- Gkitzia V., Salta K. and Tzougraki C., (2011), Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12(1), 5–14.
- Hmelo-Silver C. E., (2006), Design principles for scaffolding technology-based inquiry, in O’Donnell A. M., Hmelo-Silver C. M. and Erkens, G. (ed.), Collaborative Learning, Reasoning, and Technology, New York: Routledge, pp. 147–170.
- Jones Jr. M. and Fleming S. A., (2014), Organic Chemistry, 5th edn, New York: W. W. Norton & Company.
- Justi R. S. and Gilbert J. K., (2002), Modelling, teachers’ views on the nature of modelling, and implications for the education of modellers, Int. J. Sci. Educ., 24(4), 369–387.
- Kalyuga S., Chandler P., Tuovinen J. and Sweller J., (2001), When problem solving is superior to studying worked examples, J. Educ. Psychol., 93(3), 579.
- Knight B. A., (2015), Teachers’ use of textbooks in the digital age, Cogent Educ., 2(1), 1–10.
- Koppal M. and Caldwell A., (2004), Meeting the challenge of science literacy: Project 2061 efforts to improve science education, Cell Biol. Educ., 3(1), 28–30.
- Kornell, N. and Bjork R. A., (2008), Learning concepts and categories: is spacing the “enemy of induction”? Psychol. Sci., 19(6), 585–592.
- Kozma, R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Lee C. S., McNeill N. J., Douglas E. P., Koro-Ljungberg M. E. and Therriault D. J., (2013), Indispensable resource? A phenomenological study of textbook use in engineering problem solving, J. Eng. Educ., 102(2), 268–288.
- Lin T., Hsu Y., Lin S., Changlai M., Yang K. and Lai T., (2012), A review of empirical evidence on scaffolding for science education, Int. J. Sci. Math. Educ., 10(2), 437–455.
- Maybin J., Mercer N. and Stierer B., (1992), Scaffolding learning in the classroom, in Norman K. (ed.), Thinking Voices: The Work of the National Oracy Project, London: Hodder and Stoughton, pp. 186–195.
- Mayfield K. H. and Chase P. N., (2002), The effects of cumulative practice on mathematics problem solving, J. Appl. Behav. Anal., 35(2), 105–123.
- McMurry J., (2012), Organic Chemistry, 8th edn, Belmont: Brooks Cole, Cengage Learning.
- McNeill K. L., Lizotte D. J., Krajcik J. and Marx R. W., (2006), Supporting students’ construction of scientific explanations by fading scaffolds in instructional materials, J. Learn. Sci., 15(2), 153–191.
- Mercer N. and Littleton K., (2007), Dialogue and the Development of Children's Thinking: A Sociocultural Approach, New York: Routledge.
- Mesa V. and Griffiths, B., (2012), Textbook mediation of teaching: an example from tertiary mathematics instructors, Educ. Stud. Math., 79(1), 85–107.
- Mikk J., (2000), Textbook: Research and Writing, New York: Peter Lang Publishing, Inc.
- Nyachwaya J. M. and Gillaspie M., (2016), Features of representations in general chemistry textbooks: a peek through the lens of the cognitive load theory, Chem. Educ. Res. Pract., 17(1), 58–71.
- Paas F. G. W. C., (1992), Training strategies for attaining transfer of problem solving skills in statistics: a cognitive-load approach, J. Educ. Psychol., 84(4), 429–434.
- Paas F. G. W. C. and van Merriënboer J. J. G., (1994), Variability of worked examples and transfer of geometrical problem-solving skills: a cognitive-load approach, J. Educ. Psychol., 86(1), 122–133.
- Paas F. G. W. C., van Merriënboer J. J. G. and Adam J. J., (2016), Measurement of cognitive load in instructional research, Percept. Mot. Skills, 79(1), 419–430.
- Parker S. P., (1988), Spectroscopy Source Book, New York: McGraw-Hill.
- Pea R. D., (2004), The social and technological dimensions of scaffolding and related theoretical concepts for learning, education, and human activity, J. Learn. Sci., 13(3), 423–451.
- Puntambekar S. and Hubscher R., (2005), Tools for scaffolding students in a complex learning environment: what have we gained and what have we missed? Educ. Psychol., 40(1), 1–12.
- Pyburn D. T. and Pazicni S., (2014), Applying the multilevel framework of discourse comprehension to evaluate the text characteristics of general chemistry textbooks, J. Chem. Educ., 91(6), 778–783.
- Raker J. R. and Holme T. A., (2013), A historical analysis of the curriculum of organic chemistry using ACS exams as artifacts, J. Chem. Educ., 90(11), 1437–1442.
- Rohrer D., (2012), Interleaving helps students distinguish among similar concepts, Educ. Psychol. Rev., 24(3), 355–367.
- Rohrer D. and Taylor K. M., (2007), The shuffling of mathematics problems improves learning, Instr. Sci., 35(6), 481–498.
- Rohrer D., Dedrick R. F. and Burgess K., (2014), The benefit of interleaved mathematics practice is not limited to superficially similar kinds of problems, Psychon. Bull. Rev., 21(5), 1323–1330.
- Rosenshine B. and Meister C., (1992), The use of scaffolds for teaching higher-level cognitive strategies, Educ. Leadersh., 49(7), 26–33.
- Smith J. G., (2011), Organic Chemistry, 3rd edn, New York: McGraw-Hill.
- Solomons, T. W. G. and Fryhle C. B., (2008), Organic Chemistry, 9th edn, Hoboken: John Wiley.
- Stowe R. L. and Cooper M. M., (2019), Arguing from spectroscopic evidence, J. Chem. Educ., 96(10), 2072–2085.
- Tharp R. G. and Gallimore R., (1988), Rousing Minds to Life: Teaching, Learning, and Schooling in Social Context, New York: Cambridge University Press.
- Thomas N. C., (1991), The early history of spectroscopy, J. Chem. Educ., 68(8), 631.
- Topczewski J. J., Topczewski A. M., Tang H., Kendhammer L. K. and Pienta N. J., (2017), NMR spectra through the eyes of a student: eye tracking applied to NMR items, J. Chem. Educ., 94(1), 29–37.
- van de Pol J., Volman M. and Beishuizen J., (2010), Scaffolding in teacher–student interaction: a decade of research, Educ. Psychol. Rev., 22(3), 271–296.
- van Gerven P. W. M., Paas F. G. W. C., van Merriënboer J. J. G. and Schmidt H. G., (2002), Cognitive load theory and aging: effects of worked examples on training efficiency, Learn. Instr., 12(1), 87–105.
- Vlach H. A., Sandhofer C. M. and Kornell N., (2008), The spacing effect in children's memory and category induction, Cognition, 109(1), 163–167.
- Vygotsky L. S., (1962), Thought and Language, Cambridge: MIT Press.
- Vygotsky L. S., (1978), Mind in Society: The Development of Higher Psychological Processes, Cambridge: Harvard University Press.
- Wade L. G., (2006), Organic Chemistry, 6th edn, Upper Saddle River: Prentice Hall.
- Wahlheim C. N., Dunlosky J. and Jacoby L. L., (2011), Spacing enhances the learning of natural concepts: an investigation of mechanisms, metacognition, and aging, Mem. Cogn., 39(5), 750–763.
- Weinberg A., Wiesner E., Benesh B. and Boester T., (2012), Undergraduate students’ self-reported use of mathematics textbooks, PRIMUS, 22(2), 152–175.
- Wood D., Bruner J. S. and Ross G., (1976), The role of tutoring in problem solving, J. Child Psychol. Psychiatry, 17(2), 89–100.
| This journal is © The Royal Society of Chemistry 2020 |