Implementing collaborative inquiry in a middle school science course†
Müge
Özkanbaş‡
a and
Özgecan
Taştan Kırık
 *b
*b
aİmadettin Levent Ortaokulu, Yüreğir, Adana, Turkey
bÇukurova Üniversitesi Eğitim Fakltesi, Matematik ve Fen Bilimleri Eğitimi Bölümü, Fen Bilgisi Öğretmenliği Anabilim Dalı, Balcalı, Adana, Turkey. E-mail: ozge.deniz@gmail.com
First published on 1st July 2020
Abstract
Process Oriented Guided Inquiry Learning (POGIL®) as a collaborative inquiry method has been widely used in teaching chemistry to high school and college students. This paper presents the impact of POGIL on 65 middle school students’ understanding about nature of matter. It is a quasi-experimental non-equivalent control group design study in which a control group taught by whole class instruction was compared to the experimental group which was taught by POGIL in the context of the “Particulate Nature of Matter (PNM) Unit” including PNM, physical and chemical change and density topics. A Nature of Matter Achievement Test was administered as both pre- and post-test. It was found that a POGIL method improved students’ achievement more than teacher-centered whole-class instruction. This study provides evidence supporting the fact that POGIL is an effective pedagogy to teach nature of matter to middle school students.
Introduction
The National Science Education Standards (National Research Council, 1996) suggest that chemistry concepts such as substances, chemical properties, and chemical reactions are the core content for fifth to eighth graders. However, learning these concepts without associating to the particulate nature of matter (PNM), which is described as the atomic, molecular, and ionic interactions lying behind observed chemical phenomena, can result in learning difficulties (Gabel, 1999). Correspondingly, Johnstone (1993) characterized three levels of chemical representation of matter: submicroscopic (molecular and atomic interactions), macroscopic (chemistry of the touchable, edible and visible) and symbolic (symbols, equations, stoichiometry and mathematical algorithms). He recommended that students studying chemistry must be able to connect their understanding of the symbolic level to the macroscopic and submicroscopic levels to develop conceptual understanding of foundational chemistry concepts (Hernández et al., 2014). Interestingly, Gabel (1993) revealed that when the particle level is emphasized in instruction, the macroscopic (sensory) level or its symbolic representation cannot be isolated, thus students should relate knowledge at two or three levels. On the other hand, the abstract nature of atoms and molecules makes it difficult for middle school graders to fully understand the nature of matter, a foundational domain concept in chemistry. Liu and Lesniak (2005) claimed that “since chemistry is a science of matter and its transformations; appropriate understanding of matter determines students’ understanding of principles and theories of physical and chemical changes” (p. 434). On the other hand, research on students’ learning has indicated that the students hold several alternative conceptions related to PNM (Yezierski and Birk, 2006; Özalp and Kahveci, 2015; Abell and Bretz, 2018), states of matter and phase changes (Lee et al., 1993; Nakhleh and Samarapungavan, 1999; Ayyıldız and Tarhan, 2013), physical and chemical change (Ahtee and Varjola, 1998; Valanides, 2000; Christian and Yezierski, 2012) and density (Gennaro, 1981; Maclin et al., 1997; Dawkins et al., 2008). At this point, it is crucial to use an appropriate strategy in teaching matter to middle grade students.Inquiry-based instructional strategies in chemistry education have been proven to be effective to teach nature of matter by using particulate models especially in secondary school and at university level (Bridle and Yezierski 2012; Vilardo et al., 2017). It is an inductive way of active learning that highlights the engagement of students in the processes of sense making, generating research questions, conducting investigations, discussing ideas, formulating and defending hypotheses and developing evidence-based explanations (Lee, 2011). Guided-inquiry enables students to connect their understandings of the macroscopic and submicroscopic level to their symbolic representations (Vilardo et al., 2017). In guided-inquiry learning environments, students are engaged in specifically designed materials and facilitators guide and scaffold their processes. Nevertheless, there is a relatively small body of research investigating the effect of inquiry-based pedagogy in the context of nature of matter considering submicroscopic representation at middle school level (Marx et al., 2004) although the students in Turkey are actually taught the matter concept at this mentioned (submicroscopic) level at sixth grade. Thus, in this study, one guided-inquiry technique providing students with opportunities to participate in collaborative discourse, Process Oriented Guided Inquiry Learning (POGIL), was implemented to reveal its impact on sixth grade students’ achievement in understanding the nature of matter. Actually, POGIL was originally developed for university level chemistry courses. However, it is a suitable approach for middle school science teaching because of its nature of integrating learning cycle (an inquiry-based model) and cooperative learning, which promotes a number of desired learning outcomes as a result of students’ active participation. For example, Jasperson (2013) used a POGIL approach to teach introductory physics concepts to middle school students. She found that POGIL, as a guided-inquiry method, was effective in comprehending basic physics concepts and that loss of long term memory was slightly less in the POGIL group. Rahayu and Pamelasari (2018), in their experimental study, claimed that the POGIL pedagogy improved seventh grade students’ critical thinking skills. Gülmez Güngörmez (2018) reported that POGIL promoted seventh grade Turkish students’ understanding in the “Force and Energy Unit” and provided a conceptual change. This learning environment was also seen to have developed their scientific reasoning skills. Thus, while the number of studies about the effects of POGIL on science learning performances of middle school students is limited, a consensus that POGIL improves performance at middle school grade seems to be emerging. As POGIL is an effective guided-inquiry learning strategy in secondary school and college level chemistry education, it is necessary to explore middle school learning environments where POGIL is used as a main strategy to teach chemistry. This study attempts to fill this gap by presenting the influence of this approach on middle school students’ content knowledge about the nature of matter including the PNM, physical and chemical change and density topics.
POGIL approach
POGIL is both a philosophy and a student-centred teaching and learning strategy, which has three main components: guided-inquiry, cooperative learning and metacognition. All over the world, the main objective of school science curriculums is to teach through inquiry pedagogy to promote meaningful learning of scientific concepts and develop skills and understanding of nature of science (Anderson, 2007). In “Inquiry and the National Science Education Standards” published by National Research Council (2000), five essential features were attributed to an inquiry-based learning environment, regardless of grade level:1. Scientifically oriented questions that the students address.
2. Evidence gathered by the students.
3. Explanations developed by the students from the evidence they collect to handle the scientifically oriented questions.
4. Evaluation of their explanations.
5. Communication and verification of the explanation they have developed with their peers and teacher.
In a POGIL environment, students work together in learning teams on guided-inquiry activities designed based on the learning cycle paradigm by studying data, models, or examples and also by answering critical thinking questions. Learning cycle is an inquiry-oriented approach involving three phases: exploration, concept invention or formation, and application. In the exploration phase, students are subjected to data through a model to examine or a set of tasks to complete. Critical thinking questions guide them during the exploration of the model to understand the concept deeply. In the second phase, the concept invention or concept formation, the students derive concepts from the data explored previously. In the activities requiring concept invention; the concept is not presented obviously. Efficient guidance facilitates reaching conclusions and making predictions. In the application phase, the students apply the concept to other phenomena. It requires using new knowledge in exercises, problems and research situations. (Abraham, 2005; Hanson, 2006).
The POGIL Project has defined four core characteristics critical for the POGIL implementation (Moog et al., 2019; “What is POGIL?” 2019):
1. Students work in teams of three or four collaboratively.
2. The students use POGIL activities, specifically designed guided-inquiry activities for POGIL applications.
3. The students complete the activity during the class time with a facilitator present.
4. The instruction is not lecture or instructor-centred; the instructor performs as a facilitator of student learning.
Moreover, The POGIL Project has described some common characteristics of many POGIL classroom implementations that have been found to be very helpful when combined with the core characteristics listed above:
5. Roles are assigned to the students within their teams.
6. The activity is designed to be the first introduction to the topic.
7. The students do not need to have worked on any part of the activity before the class time.
8. The teams are required to complete all the critical thinking questions during class. There may be additional exercises or problems expected to be answered outside of class.
In this study, the implementation in the POGIL group fulfilled all of these eight characteristics above. However, the activities were specially designed by the researchers by being inspired by POGIL instead of using POGIL activities available because they are not suitable for middle school graders.
POGIL in teaching matter
Chemistry is the science of matter and the PNM can be used to explain the states of matter and behaviour of particles involved in phase changes. Several studies have reported on students’ conceptual difficulties with these concepts at the submicroscopic level (Novick and Nussbaum, 1978; Novick and Nussbaum 1981; Lee et al., 1993; Özalp and Kahveci, 2015) even after they receive formal instruction in science classes (Smith and Villarreal, 2015; Vilardo et al., 2017). Many possible causes have been put forward for pupils’ experiencing some difficulties in understanding PNM by some researchers. One critical reason is the fact that many students do not understand the meaning of three levels of chemistry: macroscopic, symbolic and submicroscopic and cannot not easily link and switch from one level to another. Some pupils designate matter as a continuous substance, containing the particles of the substance. Since some aspects of the particle model mismatch with pupils’ sensory perception of matter (such as the vacuum concept, particle motion and interaction between particles), they could not be conceived of and internalized (Novick and Nussbaum, 1981).Besides PNM, the national curriculum for science instruction in Turkey places the content of physical and chemical change and density as a part of the “PNM Unit” at sixth grade. On the other hand, these concepts are taught without the notion of the particle concept. Andersson (1990) conducted a literature review of students’ conceptions of matter as well as its transformations and highlighted that the largest difficulty in understanding physical and chemical changes is students’ view of matter as continuous and static. Density is also a difficult concept to understand because it requires students to use proportional reasoning (Gennaro, 1966). Some researchers have also thought that the concept of density raises challenges for students because they have already developed alternative conceptual framework for matter that uses an undistinguished weight-density concept (Hewson, 1986; Smith et al., 1992). It is recommended that three levels of chemistry (macroscopic, particle and symbolic) need specifically to be addressed while teaching this concept (Hitt, 2005). Concordantly, Skamp (1999) showed that elementary school children could be taught about the PNM meaningfully. Actually, because shifting from a primitive continuous to a submicroscopic particulate model is a critical change in a students’ viewpoint on the physical world, it seems important to find out whether such a shift is really accomplished by the students when they first encounter the PNM in the formal learning environment (Novick and Nussbaum, 1978).
Guided-inquiry learning supports the learners to connect their understandings of macroscopic and symbolic level to their submicroscopic representations (Vilardo et al., 2017). There are some research studies that have investigated the effectiveness of an inquiry-oriented approach and the particulate-level instruction together. Indeed, these studies have usually focused on high school and university students (Bridle and Yezierski, 2012; Becker et al., 2015; Vilardo et al., 2017).
POGIL provides a collaborative guided-inquiry learning environment in which the students construct and defend their positions. They participate in collaborative discourse that provides opportunities to engage in a stage of co-construction of scientific explanations, to argue relationships between different types of evidence and to experience making evidence-based claims about science content, which may in turn support students’ individual construction of knowledge (Becker et al., 2013). There are numerous studies reporting positive effects of POGIL on students’ learning. For example, Villagonzalo (2014) used the Particulate Nature of Matter Assessment Version 2 (ParNoMA2) developed by Yezierski and Birk (2006) to evaluate the effectiveness of POGIL on high school students’ academic performance in PNM. ParNoMA Version 2 is a 20-item test to target alternative conceptions in the chemical education literature about the topics of phases of matter and phase change, using particle representations. Villagonzalo concluded that POGIL is an effective strategy to teach PNM to high school students. Likewise, Barthlow (2011) explored the impact of POGIL to reduce alternative conceptions of high school students related to PNM and revealed that this pedagogy, as opposed to traditional lecturing method, resulted in fewer alternative conceptions related to PNM. He claimed that in the POGIL lessons, the discussion between the students and the scaffolding offered by the teacher provided the appropriate level of support for students to operate at their optimal level. Several other studies have explored the impact of POGIL on improving students’ content knowledge of chemistry mostly at university level courses (Hanson and Wolfskill, 2000; Schroeder and Greenbowe, 2008; Hein, 2012; Becker et al., 2013; Warfa et al., 2014; Becker et al., 2015; De Gale and Boisselle, 2015; Qureshi et al., 2017).
Although there are no studies using POGIL to teach the nature of matter to middle school students, guided-inquiry, the main component of POGIL, has already been implemented in some studies. Guided-inquiry learning promotes the students to explore concepts, ideas and phenomena before the teacher or peers provide formal explanations. Particularly, significant positive correlations were reported between students’ science performance and aspects of inquiry like making inferences from data and conducting scientific investigations (Marshall et al., 2016). Concordantly, Kawasaki et al. (2004) implemented a cooperative guided-inquiry approach to examine the evolution of third and fourth grade level gifted students’ theory building and modelling over a unit of study on density. They adopted a “crowdedness model” as a way to help students explain density at a macroscopic level that does not require an understanding of the particulate theory of matter. Through the examination of changes in student participation into discussions and by analyzing the quality and content of these discussions, they argue that the students are developing a powerful epistemology of science that is more commensurate with the work of actual scientists. The support and guidance from the teacher as well as the inquiry-oriented curriculum as a whole led students to develop a sophisticated epistemology of science. Almuntasheri et al. (2016) used guided-inquiry to teach the density concept to sixth grade Saudi students. They revealed that in comparison to the teacher-directed conditions, the students in a guided-inquiry environment demonstrated significant improvements in both conceptual understanding and their levels of explaining the concept of density. Almuntasheri et al. attributed this improvement to challenging the students’ prior knowledge, connecting them with the new learning experiences and encouraging the students to collect their own data that supported them in construction of their own ideas before providing their own explanations. There are some other studies reporting the improvement in student performance on content knowledge, inquiry skills and understanding of nature of science in a guided-inquiry learning environment in the context of nature of matter (Blanchard et al., 2010; Chang et al., 2010; Vlassi and Karaliota, 2013; Zoupidis et al., 2016). However, very little is known about the impact of POGIL on middle school students’ learning performances in science so there seems to be a need to investigate it.
While elementary students learn the nature of matter at early stages in Turkey, the “PNM Unit” is presented at sixth grade for the first time. In this study, we generate activities inspired by POGIL to teach this topic of nature of matter to sixth graders since the existing POGIL materials used in college and high school levels for chemistry instruction are not suitable for a middle school level. Thus, we made use of materials designed in accordance with learning cycle or the 5E model for middle school science lessons. This research extends the previous studies emphasizing the impact of POGIL on students’ learning in middle school chemistry lessons in comparison to “whole-class instruction”. Lou et al. (1996) explain whole-class instruction in this way: ‘‘whole-class instruction means that students are taught as a single, large group. In whole-class instruction, there is an emphasis on the uniformity of instruction rather than on the diversity of instruction’’ (p. 424). The teacher explains the material in detail to the class, manages whole-class discussions, and sometimes gives an assignment or individual work. This is opposed to collaborative inquiry where the students are expected to reach the explanations and generalizations by themselves through interacting and discussing with the group members (Lou et al., 1996).
Based on the theoretical principles presented in this section, we seek to understand the impact of POGIL on middle school students’ learning about the nature of matter in the context of the PNM Unit. Correspondingly, the research question that guided this study was:
• How does the POGIL approach affect 6th grade students’ academic performance in the context of the “PNM Unit” including PNM, physical and chemical change and density topics?
Method
Research design
A quasi-experimental non-equivalent control group design was employed to determine academic performance of sixth grade students in understanding PNM Unit taught with the POGIL approach and if it is superior to that of a control group comprising similar students who were taught with whole-class instruction. Random assignment of the participants to the groups was not possible because the school's administration had already constituted the classrooms at the beginning of the semester. Instead, one class was randomly assigned as a control group while another was chosen as the experimental one. Science course was targeted by the treatment. It consisted of four 40 min class hours per week. Language of instruction and the instruments were in Turkish. To minimize validity threats of history, maturation, regression, and selection, an achievement post-test was given approximately 5 weeks after the pre-test to both the experimental and control groups.Participants
Several teachers from different schools were contacted to invite them to participate in this study. From a public middle school, the teacher and the students who volunteered to participate were included. Therefore, convenient sampling was used. Institutional Review Board approval for this research was obtained from the Çukurova University Board of Social Sciences Research and Publication Ethics on December 30, 2016 to protect human subjects prior to data collection and analysis. All students provided informed consent to be research participants. Two sixth grade elementary classes (N = 71, aged 11–12) were used to form POGIL (n = 38) and whole-class discussion (n = 33) groups. The classes were both conducted at the same school, located in Adana, a city in southern Turkey. Since the individuals who did not participate in any one of the tests were removed from the sample, 34 students (18 female and 16 male) from the experimental group and 31 students (15 female and 16 male) from the control group were included in the analyses. The first author of this study, who is also a 5 year-science teacher, taught PNM Unit to both classes while the original teacher was present in the classroom during this instruction.Nature of matter achievement test (NoMAT)
NoMAT was developed by the researchers to assess 6th grade students’ achievement in the Particular Nature of Matter Unit. After specifying the objectives of the 6th grade PNM Unit that are presented in the Turkish National Science teaching curriculum, a table of specifications was developed to align these objectives, instruction, and assessment. Test items were developed in Turkish through a review of related literature (Karaduman, 2008; Pelen Değirmenci, 2009; Tuncel, 2009; Kaçar, 2012) and investigation of previous Turkish high school entrance exams, Turkish science textbooks and several middle school text books. No items were translated from another language. At first, a four-alternative multiple choice test with 46 items was constructed. The items were reviewed by two science educators and a science teacher in order to assess the test's content validity as well as the appropriateness of its items to the cognitive domain taxonomy and the instructional objectives, accuracy, and technical quality of test items. After revising and deleting some questions based on the reviewers’ suggestions, a 42-item test was piloted at the beginning of the semester to 410 seventh grade students who had been already taught the PNM Unit. The maximum possible score was 42. Item analysis was conducted to calculate the values of discrimination and difficulty indices. Depending on these values, some items were revised and some were deleted, and thus a 36 item-form was established. This test was administered to 400 students of grade 7 studying in another middle school. Although the 36-item-test was used in the experiment, 2 items were also removed from the analyses due to further validity checks with experts. The design of NoMAT questions was based on three concepts: (a) particulate nature of matter, (b) physical and chemical change and (c) density. These concepts were generated by considering the topics covered by the unit. The distributions of NoMAT items according to instructional objectives and concepts are given in Table 1.| Concept | Instructional objective | Item | Number of questions |
|---|---|---|---|
| Particulate nature of matter | Explain that matter is made up of very small particles which are in motion with empty space between them | 1, 2, 3, 4 | 4 |
| Describe the characteristics of the three states of matter considering the motion of and space between the particles | 5, 6, 7, 8, 9 | 5 | |
| Physical and chemical change | Explain the difference between physical and chemical change by making observations | 10, 11, 12, 13, 14, 15, 16, 17 | 8 |
| Density | Define density and state the unit of density. | 18, 19, 20 | 3 |
| Calculate the densities of solids and liquids by performing measurements. | 21, 22, 23, 24, 25, 26 | 6 | |
| Compare the densities of liquids that do not dissolve in each other through experiments. | 27, 28, 29, 30, 31 | 5 | |
| Comparing the densities of ice and water, explain the water's unique properties that support life on Earth. | 32, 33, 34 | 3 |
The maximum total score, which can be obtained from the test, calculated by scoring each correct answer as 1 and false answer as 0, is 34. Specifically, as indicated by Table 1, the maximum possible scores for the particulate nature of matter, physical and chemical change and density concepts are 9, 8 and 17, respectively. The reliability coefficient of the 34-item-test by the KR-20 Formula was found to be 0.87.
Although the NoMAT was applied in Turkish, it was translated into English by the second author for this article. A specialist in linguistics and a professor in chemistry who did his doctorate in an English speaking country examined these translations and compared both versions. It is given in Appendix A.
Procedure
The PNM unit is a part of the middle school science curriculum taught to both experimental group and control group students over 5 weeks. The unit covers topics relating to: PNM, particulate nature of solid, liquid and gaseous substances, phase transition and the particulate structure, physical and chemical changes, density and importance of density of water for life.• Manager: Ensures that all members of the group engage in the task, distributes work and responsibilities, resolves conflicts and ensures that all members understand.
• Spokesperson: Ensures that he/she understands the consensus answers for each question and communicates that answer to the other groups or the instructor orally or by writing on the board.
• Recorder: Writes the answers on worksheets, prepares a brief report on the discussions performed in consultation with the others.
• Reflector: Identifies strategies for problem solving, diagnoses strengths and what needs to be modified for the group's performance in consultation with the other members and prepares a report to reflect his/her group's progression. In the present study, a Group Self-Evaluation Form was given to each group at the end of each week to be filled in by the reflector in order for the group to assess and improve its own performance each week. This form was graded by both the group and the instructor.
These roles were rotated among members weekly. The role assignment provides positive interdependence which means that the students in a cooperative group must depend on each other in such a way that no one can succeed unless all succeed. They must depend on each other to complete the task and at the end, share the reward equally (Hanson, 2006). In addition, it was explained to the students that they had two responsibilities: to understand the material and to ensure that all members of the team learned the material since each group would submit one report of what they had carried out. This report was graded each time and each member received the same grade. If one member is absent from a session (two lesson hours made a session), that person took a grade of zero for that session. Thus, the first core criterion for POGIL implementation defined by The POGIL Project was met (working in groups of four collaboratively).
The students were given a one or two question quiz (closed book, completed by individuals, not teams) on the previous session's material at the beginning of every POGIL session to be answered in five minutes. Those who come late and missed the quiz took zeros so that they were encouraged to come on time. Individual exams, activity reports, homework and quizzes were intended to promote individual accountability which assures that each member of the group understands the material and develops his/her process skills.
After the quiz, each team started to work on the worksheets that included critical thinking questions. The students were not expected to have worked on any part of the activity prior to the class meeting time as recommended by The POGIL Project (Moog et al., 2019). Information about POGIL materials for high school chemistry, general chemistry, organic chemistry, and physical chemistry are available online (POGIL, 2019). On the other hand, there is no POGIL material developed and specifically addressing the nature of matter concept for middle school students. Therefore, activities inspired by POGIL to teach the PNM Unit to sixth grade students were designed by the researchers through an examination of POGIL materials available (Hanson, 2011; Moog and Farrell, 2011) as it was necessary to understand the organization of activity design. In addition, the textbooks being used in the middle school science course contained inquiry-based activities which were also used in preparing the worksheets (MoNE, 2016). Besides the coursebook, the American Chemical Society (ACS) provides 5E model-based middle school chemistry activities related to the topics under the PNM Unit taught in Turkey (ACS, 2020c). The activities were translated into Turkish, revised and organized to fit POGIL activity design. The translations were made by the second author. A specialist in linguistics and a professor in chemistry who did his doctorate in an English speaking country examined these translations in terms of its correspondence with English, grammatical aspects and intelligibility. The POGIL Project identifies that using specifically designed POGIL activities is a core characteristic of a POGIL implementation. It is also suggested that the activities were designed to be the first introduction to the topic (Moog et al., 2019). Although POGIL activities already available for high school and university level students could not be used in the experimental classroom; the activities used were designed by the researchers especially within the frames of POGIL and this was the first introduction to the topic.
Main concepts of the “PNM Unit” are PNM, physical and chemical change and density. The PNM concept includes particulate-level behaviour of matter, characteristics of solids, liquids and gases and phase transitions on a particle level (the motion of and space between the particles). One of the activities to teach the particulate nature of matter concept to the POGIL group is Change of State and Particle Motion Activity (ACS, 2020b). It begins with a question to investigate. The students conduct an experiment in which they control some variables to discover the particle motion at different temperatures. They are expected to interpret their observations at particulate level through a model in which they examine an animation of particle motion of water at different temperatures; therefore, they relate knowledge to the macroscopic level and the submicroscopic level. In another model, the students use a simulation in which they change the temperature of different substances so that they can observe the particle motion and arrangement during change of state in each substance to discover the characteristics of matter changing during change of state at a particulate level. They answer the critical thinking questions following the model to invent and apply the concepts. In general, some problems from the application part were given as group homework and each member of the team received the same grade from the homework. An activity implemented in the POGIL group, Activity 2: Change of State and Particle Motion is given in Appendix B. It was copied with permission from middleschoolchemistry.com.
The physical and chemical change concept involves characteristics of physical and chemical changes at macroscopic and microscopic levels. The experimental group students explored different visual and verbal representations of physical and chemical changes to predict the definition and indicators of the types of changes, by observing them, and compared the features of physical and chemical change. They also worked on the visual representations of these changes at a particle level in their teams. They were expected to predict which change would occur by examining particulate models.
The density concept, on the other hand, involves what density is, the calculation of density of solids and liquids, comparing the densities of liquids that do not dissolve in each other and water's unique properties that support life on Earth considering the densities of ice and water. The POGIL group students observed a chromium and an aluminium cube of the same volume placed in balance and saw that the chromium had a greater mass. They were expected to develop an explanation with their peers, on the particle level, for how this could be. Students were then given cubes of different materials that all had the same volume. They determined the density of each cube and identified the substance the cube was made from. In the following activities, they calculated densities of solids and liquids and compared the densities of the liquids that did not dissolve in each other by conducting experiments and answering critical thinking questions with their teammates. They also discussed the special case of water related to density of ice and water and tried to explain some phenomena in nature by using it.
Adequate time was allocated for group work and the students were told to finish within the predetermined time limit. As they worked, the instructor walked among the groups to monitor and to assess individual and team performance, and facilitated critical thinking by asking questions instead of giving answers to students’ questions in order to encourage them to discover answers on their own. She intervened to help the teams figure out why they might be having difficulty and what they needed to make progress. By monitoring, assessing, and facilitating, the instructor ensured that each team member was fulfilling the assigned role and that everyone understood the material. This kind of intervention was intended to supply feedback and reinforcement, to teach process skills including academic and collaborative skills, to encourage argumentation and to guide the students. Likewise, The POGIL Project has defined the core characteristics of a POGIL implementation to be the fact that the dominant mode of instruction is not lecture or instructor-centered, but the instructor is a facilitator of student learning (“What is POGIL?” 2019). At the end of the time allocated for group work, the instructor led a whole class discussion with critical thinking questions by firstly asking the spokesperson to share the group's reasoning with the entire class. If a new concept was to be defined, the students wrote their definition on the board, and the class discussed together to reach a consensus about the definition of that new concept. Whole class discussions typically functioned as times when small groups communicated their reasoning through critical thinking questions and engaged in argumentation, and the instructor clarified reasoning to these questions. Finishing the whole class discussion, the groups were allowed to complete their reports including their answers to the critical thinking questions in five minutes. At the last lesson of each week, the groups were given time to discuss and complete the Group Self-Evaluation Form. Then each group shared and discussed their self-evaluation with the entire class. Finally, they submitted their reports with their answers to the critical thinking questions and the Group Self-Evaluation Form to the instructor. As suggested by The POGIL Project, teams completed all the critical thinking questions during class with a facilitator present and they worked as a team outside of class on some problems assigned as homework (Moog et al., 2019). Within this study, in general, the characteristics defined by The POGIL Project for a POGIL implementation were fulfilled in the experimental group (Moog et al., 2019).
The instructor taught the concept of physical and chemical change by explaining the features of the changes at macroscopic and microscopic levels first and then provided several examples verbally and visually. She further expected the students to give more examples to these changes. Although physical and chemical changes were described at macroscopic and microscopic levels in the textbook, there were no particle models or representations of these changes. Thus, the instructor was not able to show any particulate models of physical and chemical changes to the control group students.
When teaching the density concept, the instructor first defined density and presented the formula with the units. Then she calculated densities of different substances by using the volume and mass values given. She also expected the students to make similar calculations. She described the density of solids, liquids, and gases. She made a demonstration with a mixture of nondissolving liquids and expected the students to compare their densities. The students mostly solved problems related to density individually. Finally, the instructor explained the importance of water's unique properties that support life on Earth considering the densities of ice and water. As in the textbook, this concept was not associated with the particulate nature of matter.
The instructor used a deductive approach, which involves confirming a concept in contrast to what is in the experimental group, where the same unit was taught through an inductive approach, leading to the development of theoretical construction. In addition, the control group students gave thought and studied individually, and no collaborative teamwork was performed. The instruction was performed in an equal amount of time with the experimental group.
Actually, middle school students in Turkey are still taught with a teacher-centred approach even though the science curriculum has been revised three times (in 2005, 2013 and 2017) in accordance with the constructivist approach to increase scientifically literate citizens. This may be because of the fact that a general failure has been experienced in putting desired reform into practice. (Hazır Bıkmaz 2006; Doğan, 2010; Atila and Sözbilir, 2016). Therefore, in the control group, a teacher-centred whole-class instruction which reflects the approach being implemented today in middle school science education throughout Turkey was maintained although the curriculum currently in effect at the time this study was conducted adopted the inquiry-based approach (MoNE, 2013).
Data analysis
Statistical analyses of the data were performed through a statistical software package, SPSS 20. The students who did not take any one of the tests were removed from the data set. After handling the missing values, statistical analyses were conducted to ensure that assumptions were met for the statistical tests employed in the analyses of the data. Pre- and post-NoMAT scores of the students in the control and experimental group were analyzed by using one-way ANOVA. One-way ANOVA was preferred to independent-samples’ t-test in order to obtain a partial eta square value as an effect size. Furthermore, students’ performances on the concepts of PNM Unit including PNM, physical and chemical change and density for the pre- and post-tests were compared through ANOVA to provide detailed information about students’ understanding.Results
One-way ANOVA for pre-NoMAT scores revealed that there was not a significant difference between the groups with respect to their previous knowledge in the PNM Unit and the strength of the relationship between the means, as indicated by the partial η2 index, η2 = 0.004, was very small, which showed that the comparison groups were similar in terms of previous knowledge, F(1,63) = 0.248, p = 0.620. One-way ANOVA results for post-NoMAT indicated that the POGIL group displayed significantly better understanding of the PNM Unit [F(1,63) = 10.055, p = 0.002, partial η2 = 0.138]. Partial η2 of 0.138 represents a large relationship between treatment and students’ achievement, implying that 13.8% of variance on student achievement was attributed to treatment (Green et al., 2000). Descriptive statistics for the pre- and post-tests for NoMAT are presented in Table 2.Furthermore, the students’ pre- and post-performance on the concepts implemented in NoMAT, specifically on the items about “PNM”, “physical and chemical change” and “density” were compared to provide a detailed description of their understanding about the PNM Unit. When the pretest of the experimental and control groups for the concepts of NoMAT were compared with each other, there were no significant differences. The results of one-way ANOVA for the concepts of pre- NoMAT are given in Table 3. As shown in Table 3, effect sizes for all concepts before the treatment were very small, which means that the groups were similar in terms of previous knowledge in these concepts.
| Concept | df | F | Significance | Partial η2 |
|---|---|---|---|---|
| PNM | 1 | 0.536 | 0.467 | 0.008 |
| Physical and chemical changes | 1 | 0.385 | 0.537 | 0.006 |
| Density | 1 | 0.004 | 0.947 | 0.000 |
However, for the post-test, significant differences in the PNM concept and density concept were found. The experimental group students performed better on the items related to the PNM concept [F(1,63) = 9.0.82, p = 0.004, partial η2 = 0.126] and density concept [F(1,63) = 10.848, p = 0.002, partial η2 = 0.147] while there was no significant difference between the groups in terms of their scores calculated from the answers of the items related to physical and chemical change concept [F(1,63) = 0.485, p = 0.489, partial η2 = 0.008]. Effect size values for the PNM concept and density concept implied that the magnitude of the difference between the groups with respect to their achievements in these concepts was not small. Descriptive statistics for the concepts of pre- and post-NoMAT are presented in Table 4.
| Concept | Pre-test | Post-test | ||||||
|---|---|---|---|---|---|---|---|---|
![[X with combining macron]](https://www.rsc.org/images/entities/char_0058_0304.gif) |
SD | ![[X with combining macron]](https://www.rsc.org/images/entities/char_0058_0304.gif) |
SD | |||||
| EG | CG | EG | CG | EG | CG | EG | CG | |
| PNM | 4.41 | 4.09 | 1.87 | 1.55 | 6.55 | 5.25 | 1.61 | 1.86 |
| Physical and chemical changes | 2.94 | 2.70 | 1.61 | 1.37 | 4.47 | 4.12 | 1.89 | 2.06 |
| Density | 6.44 | 6.38 | 3.56 | 2.85 | 12.11 | 9.48 | 3.04 | 3.45 |
One-way ANOVA results for the concepts of post-NoMAT are given in Table 5.
| Concept | df | F | Significance | Partial η2 |
|---|---|---|---|---|
| PNM | 1 | 9.082 | 0.004 | 0.126 |
| Physical and chemical changes | 1 | 0.485 | 0.489 | 0.008 |
| Density | 1 | 10.848 | 0.002 | 0.147 |
Discussion
In the present study, the implementation of the POGIL pedagogy in the middle school science classroom has been shown to positively influence student learning of the “PNM Unit” with a large effect size (η2 = 0.138) when compared to teacher-based whole-class instruction. This is most probably because the students were engaged in constructing evidence-based explanations through collaborative inquiry, contrasted with simply receiving or affirming scientists’ explanations of chemical phenomena. Studies of students’ understanding of science concepts offer clear evidence that traditional, didactic teaching methods are incompetent in generating productive changes in students’ conceptions (Gunstone and White, 1981; Gabel et al., 1987; Bodner, 1991; Nakhleh, 1992; Smith and Metz, 1996). While teacher-based instruction can be effective in teaching the facts, rules, algorithms and procedures of a subject, it is not successful in encouraging student reflection and the engagement of students in processes that promote better understanding. This is partially because traditional methods neither require nor encourage metacognition (Rickey and Stacy, 2000). On the other hand, POGIL uses metacognition in such a way that the students monitor their own learning and learning teams assess their strategies and the strategies used by the other teams to specify their strengths, aspects to be improved and visions about problem solving. The yields of this assessment are then shared within group and with the other groups to improve everyone's understanding of problem solving and how it can be applied to very rich problems. Metacognition also increases transfer since the students, in their teams, discuss their objectives, and strategies to reach them in different contexts and reflect upon how those strategies can be developed (Bransford et al., 2000; Hanson, 2006). Likewise, the participants of this study monitored and assessed their learning by discussing the questions in the Group Self-Evaluation Form and took feedback from the other teams and the instructor each week. Correspondingly, some studies provide evidence that POGIL enhances metacognitive skills (Vrieling et al., 2010; Şen and Yılmaz, 2015).In addition to metacognition, POGIL pedagogy utilizes critical thinking questions to promote higher order thinking skills (or process skills) such as critical thinking, problem solving and analytical thinking. Students who are required to use higher order thinking skills attain better results in achievement tests both in problem solving and understanding the concepts (Dori et al., 2003; Ramos et al., 2013). In POGIL classrooms, the students develop understanding by examining models and exploring the patterns in the models. They apply their self-constructed knowledge in exercises and unfamiliar problem situations, reflect on and explain what they have learned and evaluate their and others’ performances. They take responsibility for their own learning and justify their use of evidence along with their conclusions (Hanson, 2006).
There are many ways to utilize POGIL pedagogy in a classroom. Hanson (2006), in his Instructor's Guide to Process-Oriented Guided-Inquiry Learning, suggested the use of quizzes on previous session's material at the beginning of each POGIL session to assure individual accountability which is a critical component of cooperative learning. Concordantly, there are many studies involving quizzes as a part of POGIL implementation in the classroom (Farrell et al., 1999; Mewhinney and Zükerman, 2008; Kulatunga and Lewis, 2013; Simonson and Shadle, 2013; Qureshi et al., 2017). As Brown et al. (2014) stated, a simple quiz provides a retrieval practice and so improves learning because it provides recalling facts or concepts from memory. Although this is not the primary reason to use quizzes in the POGIL environment, it might have contributed to learning. In any case, improvement in learning in the experimental group can be attributed to the POGIL implementation since the quizzes were used intentionally in this environment.
The results of further analysis of the NoMAT scores considering the concepts of the unit revealed that the difference between the two groups of participants was in the “PNM” and “density” concepts. It is more difficult for middle school students to explain the properties of matter on the microscopic level of atoms and molecules compared to explaining them on the macroscopic level. They are more likely to comprehend observable and familiar phenomena. However, inquiry-based learning is a successful strategy to relate observable properties with particulate behaviour of matter. The students in this study developed and discussed their particle models. Likewise, Harrison and Treagust (2000) suggest that part of the instructional process must require exploring and discussing the advantages and limitations of a particular model so that the students are able to differentiate between what a model is intended to show about a situation and the limitations of the model. In some activities, students used particle model animations and simulations to answer the critical thinking questions. Animations and simulations were reported as effective techniques to help students better visualize particle behaviour and to increase students’ understanding of chemical and physical phenomena (Sanger et al., 2000; Bunce, 2001; Sanger and Badger, 2001; Yezierski and Birk, 2006; Williamson et al., 2012). Accordingly, Baker and Talley (1972) suggested that success in chemistry is more strongly related to visualization abilities than to general academic ability. Although the same animations and simulations were displayed to the control group students, they did not have a platform for discussing the particle behaviour with their peers. Besides discussing the particle models in the animations and simulations, experimental group students also had more chance to draw and argue their own particle-level mental models with their peers through critical thinking questions as well.
POGIL group students achieved a significantly better acquisition of knowledge related to the density concept than the teacher-centred whole class instruction group with a large magnitude of difference (η2 = 0.147). This concept was taught to the POGIL group by means of particle level characteristics of different substances. Illustrations were used to show particle arrangement of different substances and they explained density with these particulate characteristics of the substances. They also learned to calculate volumes of different geometrical objects by taking measurements by themselves. They calculated densities of different substances by measuring. Likewise, Hitt (2005) suggests combining three levels (macroscopic, submicroscopic and symbolic) in teaching density concept to the students. On the other hand, in the control group, the density concept was not associated with particle level characteristics of the matter. The teacher used experiments as a demonstration and the students calculated the densities of different substances by using values of mass and volume given by the teacher.
Thus, as Johnstone (1993) suggested, in this study, guided-inquiry activities encouraged the students to connect what they have seen (macroscopic level); symbols, equations and mathematics they used (symbolic level) and the particle behaviour (submicroscopic level). They had chance to make observations, collect data and explain the pattern in the phenomena by using particulate nature of matter both in their teams and in whole-class discussions supported by the instructor facilitation. Correspondingly, Becker et al. (2015) revealed that “in whole-class discussion, instructor facilitation contributed to greater elaboration of relationships among macro, submicro, and symbolic-level information than typically occurred in small group reasoning” (p. 779).
POGIL-style activities require collaboration for success. The students in this study exercised their skills in learning, thinking, and problem solving by explaining concepts and strategies to their teammates. They were dependent on each other to complete the task. In a collaborative learning environment, the peers either elaborate or critique each other's contribution, which results in co-construction. The students in the learning teams listened to one peer self-explain. Self-explanation has been reported as a technique for improvement of problem-solving strategies (Chi et al., 1989; Cooper et al., 2008). These experiences might possibly enhance students’ academic performance in the present study. The literature also supports that POGIL positively influences student learning of PNM at secondary and university levels (Barthlow and Watson, 2014; Villagonzalo, 2014; Becker et al., 2015). While there aren’t any studies using POGIL to teach matter at middle school level, inquiry-based learning has been found to be effective (Kawasaki et al., 2004; Blanchard et al., 2010; Chang et al., 2010; Vlassi and Karaliota, 2013; Almuntasheri et al., 2016).
The performances of the students in both groups related to physical and chemical change were similar in post-NoMAT. Inquiry-based particulate level activities did not make any difference among the groups in the context of physical and chemical change. This may be because of the fact that they mostly required the students to associate the type of change with various daily events by considering the properties of the changes. Most of these examples (or similar ones) were used in the classroom in both groups. In addition, there is no item in the test related to visual representation of the particles during these changes.
Limitations
This study compared two groups, however; the individuals were not randomly assigned to the groups compared since the school principle had already formed the classrooms and did not allow reconstituting them. While 71 students participated in the study, 65 of them were included in the analysis due to some students’ not being involved in some tests. The length of time during which the instructor and the students used the POGIL method was limited to less than one semester of the school year and to teaching the concepts related to the PNM Unit only. The full impact of the POGIL approach would have been better measured if students in the experimental group had been taught the entire school year, being exposed to POGIL pedagogy over all science topics. The small sample size, limited length of implementation and lack of longitudinal assessment have limited the conclusions that could be drawn from this study. It was also limited to a single school in a Turkish educational context, which may not constitute a representative sample of the global population of all middle school students. Moreover, the instructor was not the original teacher of the students although their teacher was present in the class during POGIL implementation. Since the original teacher is not experienced in POGIL, cooperative learning or inquiry-based learning, the first author, who is also an experienced science teacher and was working on her master thesis on POGIL at that time, conducted the lessons. Therefore, the students’ behaviours might have been affected because they were instructed by a different science teacher. However, since the control group students were also in the same position, this threat has been probably controlled. Still, a similar study where the usual teacher of the students teaches the subject with POGIL may lead to more generalizable results. One possible concern about the external validity of the experiment is the possible experimenter bias effect. The instructor for the experiment was one of the authors of this study, thus might be unconsciously biased to favour the students in the experimental group. Nevertheless, she tried to behave similarly to the participants of each group. Another limitation of the study was the lack of training and knowledge in developing POGIL-like activities. Although we examined many materials developed for high school and college students, we did not attend any POGIL workshops and no material in chemistry is available for middle school students. Nevertheless, learning cycle (or 5E model) materials were very helpful to develop the worksheets inspired by POGIL.Conclusions and implications
The present study provides evidence supporting POGIL being an effective pedagogy to teach science to middle school students. Specifically, this study used NoMAT to examine Turkish middle school students’ achievement in the PNM Unit. The results suggest that middle school students’ achievement improved positively by POGIL.The outcomes of this research present useful information to the practitioners of inquiry-based learning especially at middle school grade. Though the effectiveness of POGIL on high school and college students has been well documented, its impacts for middle school level need to be investigated. Furthermore, more POGIL materials for middle school science need to be developed. The researchers may not have focused on POGIL implementation in middle school since there are only a few sets of POGIL materials available for middle school students that are related to Earth and Space (Flinn Scientific, 2020a) and Life Science (Flinn Scientific, 2020b). This study may open the road for future investigations. It supports the claim that a new pedagogical intervention like POGIL is one way forward to prepare middle school Turkish students academically for the challenging real world. Considering the current Turkish science curriculum adopting an inquiry-based approach, this study also revealed that the learning objectives concerning the “PNM Unit” at sixth grade can be successfully taught in the period suggested by the curriculum without reducing content coverage.
Findings from this study suggest that continued research to define the value of the POGIL approach in middle school science education would be beneficial. For the future studies, the impact of POGIL on different concepts of middle school science and even disciplines other than science are recommended to be investigated. Besides content knowledge, its impact on middle school students’ process skills such as problem solving, metacognition, science process skills and formal reasoning ability may be investigated.
Conflicts of interest
There are no conflicts to declare.Appendix A. Nature of matter achievement test (NoMAT)
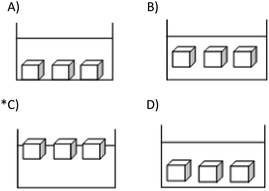
1. A small piece of solid iodine is added to the alcohol in the test tube below. After a while, the mixture looks like what is in the tube rightmost in the figure below.Based on this observation, which of the following cannot be said about the structure of matter?
A) Matter is made of tiny particles.
*B) Matter can easily be compressed.
C) Particles of matter are in motion.
D) There is empty space between the particles of matter.
2. Which of the following can explain the fluidity of liquids and gases?
A) Their physical state can be changed.
*B) Their molecules can make translational movement.
C) There is a big space between the particles.
D) They have a great number of particles
3. – Dispersion of ink dropped into water
– Dissolution of salt in water
– The spread of the smell of food being cooked in the kitchen
Which characteristic of matter can the cases given above be explained by?
A) The number of particles does not change when the matter changes state.
B) The particles forming a matter are different from one another.
*C) The particles forming the matters are moving.
D) The particles forming the matters are of different size.
4.
The movements of the particles forming the Substances A, B and C are shown in the diagram above. Accordingly, which of the following best represents the physical state of the substances?
*A) Liquid Gas Solid
B) Liquid Solid Gas
C) Solid Gas Solid
D) Gas Liquid Liquid
5.
Which of the following represents the physical state of the substances illustrated at particulate level in the closed containers?
A) Liquid Liquid Gas
B) Gas Solid Liquid
*C) Gas Liquid Solid
D) Solid Gas Liquid
6. When a substance is changing state as a result of the process applied; it is observed that;
– the particles of it move faster.
– the distance between the particles increases.
– the volume of it increases.
Accordingly, which of the phase transitions below has the matter experienced?
A) from gas to liquid *B) from liquid to gas
C) from liquid to solid D) from gas to solid
7. When a substance is heated, which of the following does not occur?
A) Particles move faster.
B) The distance between the particles increases.
C) Temperature of the substance increases unless there is a phase transition
*D) Particles that form the substance expand.
8. During the transition of a liquid substance into gas, which one or ones of the following occur?
I. the distance between the particles increases.
II. the substance undergoes chemical change.
III. the movement of the particles increases.
A) Only I B) Only III
*C) I and III D) I, II and III
9. When naphthalene evaporates,
I. the particles of it make translational movement.
II. the distance between the particles decreases.
III. the particles of it become disordered.
Which of the statements above are
 ?
?
A) I and II *B) I and III
C) II and III D) I, II and III
10. Which of the following is an example of a case in which the identity of the substance changes?
A) Breaking an almond
B) Folding of paper
C) Making paper-shaped plates from play dough
*D) Keeping a metal in acid
11. Four students want to change the structure of the wood by applying the following processes to each piece of wood.
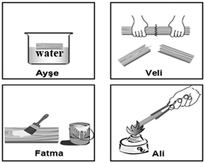
Which student changes the structure of the wood as a result of the process s/he follows?
A) Ayşe B) Veli C) Fatma *D) Ali
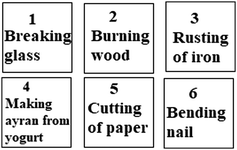
12.
Which events on the cards above have only changed the appearance of the matter?
A) 2, 3, 5, 6 B) 1, 2, 4, 5
C) 1, 3, 5, 6 *D) 1, 4, 5, 6
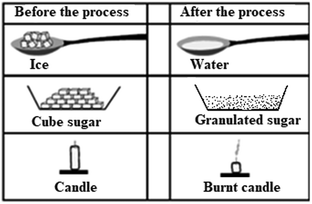
13. The appearances of some materials before and after a process is applied are given below.
Which material(s) has/have undergone a chemical change?
*A) Only candle
B) Only ice
C) Ice and cube sugar
D) Ice, candle and cube sugar
14.
According to the procedure above, which of the following best classifies the cases taking place in processes I and II?
A) Physical change Physical change
B) Chemical change Chemical change
*C) Physical change Chemical change
D) Chemical change Physical change
15. Which of the following are examples of chemical change?
I. Sublimation of carbon dioxide
II. The conversion of wine to vinegar
III. Grinding wheat into flour
IV. Burning of nutrients in the body
A) I and II B) I and IV
C) II,III and IV *D) II and IV
16.
I – Only changes in the appearance of matter are called physical changes.
II – By physical change, the speed and the arrangement of hollow structure of the particles of matter change.
III – Chemical changes are changes in the internal structure of matter.
Which one(s) of the statements above is/are
 ?
?
A) Only I B) I and II C) Only II *D) I, II and III
17. Events in which the identity of the substance changes are ……………… changes such as ………………….
Which of the following should be used to complete the definition above correctly?
A) Physical, coal combustion
B) Chemical, dissolution of sugar in water
C) Physical, slicing apple
*D) Chemical, toasting bread
18. Which of the following is a distinguishing property of all matters?
A) Mass
B) Volume
C) Weight
*D) Density
19. Which of the following expressions is the unit of density?
A) mL g−1 *B) g cm−3
C) kg m−1 D) kcal
20. Mete and Melis drop the ball into a pool while they are playing by the pool. They are surprised to see that the ball remains on the surface of the pool. Which property of the ball explains this situation?
A) Weight
B) Volume
*C) Density
D) Mass
21. Volume and mass values of some substances are given in the table below.
| Substance | Volume (cm3) | Mass (g) |
|---|---|---|
| K | 5 | 20 |
| L | 6 | 24 |
| M | 5 | 30 |
| N | 7 | 28 |
Accordingly, which of the substances is different from the others?
A) K B) L *C) M D) N
22. Values for the mass and volumes of different objects are given in the table below.
| Object | Mass (g) | Volume (cm3) |
|---|---|---|
| K | 20 | 20 |
| L | 20 | 10 |
| M | 10 | 10 |
| N | 5 | 20 |
Accordingly, which object has the highest density?
A) K *B) L C) M D) N
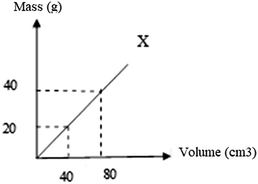
23.
Consider the graph above, what is the density of X in g cm−3?
*A) 0.5 B) 1 C) 2 D) 4
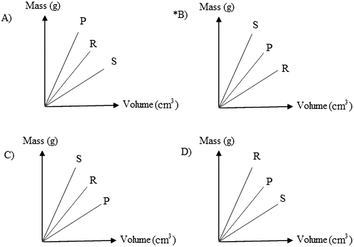
24. The densities of P, R, S are given below.
P 2.00
R 1.28
S 3.20
Which of the following can be the mass-volume graph of these substances?
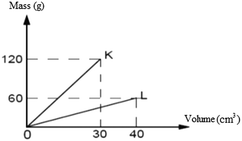
25. The mass-volume values of pure K and L substances at room temperature are given in the graph.
Accordingly, which of the following judgments is
 ?
?
*A) Having equal mass, the volume of K is greater than that of L.
B) K and L are two different substances.
C) The density of the substance K is 4 g cm−3.
D) Having equal volume, the mass of K is greater than that of L.
26. Given that the density of iron is 7.86 g cm−3, what is the mass of 100 cm3 iron?
A) 0.786
B) 0.0786
C) 78.6
*D) 786
27.
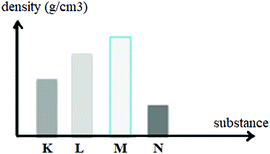
A bar graph demonstrating the densities of immiscible liquids of K, L, M and N is given above. Accordingly, which of the following is observed when all are put into the same container?
28.
| Liquid | Density (g cm−3) |
|---|---|
| K | 1.8 |
| L | 4.2 |
| M | 0.9 |
| N | 1.6 |
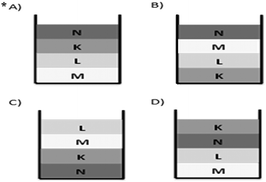
The table above presents the densities of liquids K, L, M, N, which are insoluble in each other. Which one of the following best represents the situation in which all of the liquids with equal volumes are put into one container?
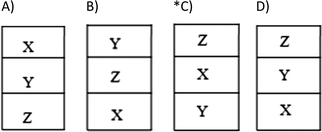
29. The equilibrium state of the immiscible X, Y, Z fluids in a container is shown below.
Accordingly, which of the following best represents the mass and volume values of these liquids?
A)
| Object | Mass (g) | Volume (cm3) |
|---|---|---|
| X | 100 | 10 |
| Y | 200 | 20 |
| Z | 150 | 30 |
B)
| Object | Mass (g) | Volume (cm3) |
|---|---|---|
| X | 60 | 30 |
| Y | 80 | 40 |
| Z | 100 | 50 |
C)
| Object | Mass (g) | Volume (cm3) |
|---|---|---|
| X | 100 | 10 |
| Y | 100 | 20 |
| Z | 100 | 50 |
*D)
| Object | Mass (g) | Volume (cm3) |
|---|---|---|
| X | 60 | 20 |
| Y | 80 | 20 |
| Z | 100 | 20 |
30.
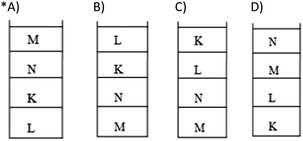
X, Y and Z with densities Y > X > Z are put in identical containers. If these immiscible liquids are poured into the same container, which of the following shows their final condition in the container?
31. Yasemin learns that an oil tanker is stranded on the coast where she goes on vacation and that oil is seeping from the tanker into the sea. When she goes to the sea to see the situation, she observes that the oil is collected on the sea surface.
Which of the followings explain her observation?
I. Amount of oil is less than water.
II. Seawater and oil are insoluble in each other.
III. Density of oil is less than seawater
A) I and II B) I and III *C) II and III D) I, II and III
32. If ice was denser than water, which of the following would occur?
A) The ice would float on water.
B) The ice layer would keep the water warm like a dress.
*C) It would sink and so affect the lives of plants and animals negatively.
D) It would keep the temperature of water at the level that living things could survive.
33. Ice is the solid state of water. However, the densities of ice and water are different. The density of ice is less than the density of water.
Based on this information, which of the following best represents the position of the ice cubes in water?
34. Ela: Freezing of water first from its surface is vital for living things.
Seda: If ice was denser than water, no living creature could live under water.
Ela and Seda make the explanations above in a science class. Which of the following is true about these statements?
*A) Both are correct.
B) Both are incorrect.
C) Eda is incorrect, Seda is correct.
D) Eda is correct, Seda is incorrect.
Appendix B. An activity implemented in the POGIL group
Activity 2: Change of State and Particle MotionExploration-1
Question to investigate
Is the speed of water particles different in hot and cold water?
Materials for each group
• Hot water in a clear plastic cup
• Cold water in a clear plastic cup
• Food coloring (yellow and blue)
• 4 droppers
Procedure
1. With the help of your partners, use droppers to carefully place 1 drop of yellow and 1 drop of blue food coloring into the hot and cold water at the same time.
2. Allow the colors to mix on their own as you watch them for a couple of minutes.
What did you observe?
1.1. Describe what the colors looked like and how they moved and mixed in the cold water.
1.2. Describe what the colors looked like and how they moved and mixed in the hot water.
1.3. What does the speed of the mixing colors tell you about the speed of the particles in hot and cold water?
1.4. There were several variables in this experiment:
• Amount of water in each cup
• Type of cup used
• Number of drops of food coloring
• When the coloring was added to the water
Pick one of these variables and explain why you made sure it was kept the same in the two cups.
Concept Invention-1
Model 1: Water molecules at different temperatures
Watch the animation of water particles at different temperatures: Heating and Cooling a Liquid.
[Particle model animation is shown from ACS website (ACS, 2020a)].
1.5. Are the particles moving faster in cold or hot water?
1.6. How does this match with your observations with the food coloring?
1.7. Look closely at the space between the particles in cold and hot water. Is there more space in between the particles in hot water or in cold water? Is it a lot of space?
1.8. Based on your observations and the animations, fill in the blanks with the words “increases” or “decreases”.
a. Heating a substance ______________________particle motion.
b. Cooling a substance ______________________particle motion.
c. As particle motion increases, the space between particles____________.
d. As particle motion decreases, the space between particles ___________.
Now you can draw your own particle model:
1.9. Using circles to represent water particles draw a model of the particles in cold and hot water.
• Use motion lines to show the speed of the particles.
• Consider the space between particles in each temperature of water.
Model 2: Change of State
Watch the animation: States of Matter.
[Particle model animation is shown from PhET website (University of Colorado, 2020)].
Exploration-2
2.1. Based on the simulation you saw, explain what happens when the matter freezes from liquid into solid by considering particles’ motion and arrangement.
2.2. Based on the simulation you saw, explain what happens when the matter melts from solid into liquid by considering particles’ motion and arrangement.
2.3. Based on the simulation you saw, explain what happens when the matter vaporizes from liquid into gas by considering particles’ motion and arrangement.
2.4. During the change of state, which properties of the particles change?
2.5. Draw pictures that show the difference in the arrangement and motion of particles for the liquid state and the gas state considering your observations from the simulation.
Concept Invention-2
2.6. Suppose that you have an ice cube in your hand. You see that it melts and vaporize over time. Which of the followings shows this phenomenon best? Explain your reasoning.
2.7. Agree or disagree with the statements below, and explain your reasoning.
a. When a substance changes state, its constituent particles also change state.
b. When a substance changes state, the speed of the particles that make up the substance changes as well.
c. When the liquids freeze, translation movement of the particles stops.
d. When the solids melt, their particles move faster.
e. When a substance is heated, the speed of vibration motion of its particles decreases while the speed of translation movement of them increases.
Application
1. Let's say that you measure exactly 100 millilitres of water in a graduated cylinder. You heat the water to 100 °C and notice that the volume increases to 104 millilitres.
Using what you know about the attractions between water particles and the way heat affects particles’ motion; explain why the volume of water in the cylinder increases when it is heated.
2. One common place you see the results of condensation is in the weather. Water vapour in the air (humidity), clouds, and rain are all the result of evaporation and condensation. Using what you know about evaporation and condensation, explain what causes rain.
References
- Abell T. N. and Bretz S. L., (2018), Dissolving salts in water: Students’ particulate explanations of temperature changes, J. Chem. Educ., 95(4), 504–511.
- Abraham M. R., (2005), Inquiry and the learning cycle approach, in Pienta N. J., Cooper M. M. and Greenbowe T. J. (ed.), Chemists’ guide to effective teaching, Upper Saddle River, NJ, Pearson Prentice Hall, pp. 41–52.
- ACS, (2020a), Middle school chemistry: chapter 1, lesson 2 multimedia. Retrieved from https://www.middleschoolchemistry.com/multimedia/chapter1/lesson2#heating_and_cooling.
- ACS, (2020b), Middle school chemistry: Lesson 1.2 Molecules in motion. Retrieved from https://www.middleschoolchemistry.com/lessonplans/chapter1/lesson2.
- ACS, (2020c), Middle school chemistry: Lesson plans. Retrieved from https://www.middleschoolchemistry.com/lessonplans/.
- Ahtee M. and Varjola I., (1998), Students’ understanding of chemical reaction, Int. J. Sci. Educ., 20(3), 305–316.
- Almuntasheri S., Gillies R. M. and Wright T., (2016), The effectiveness of a guided inquiry-based, teachers' professional development programme on Saudi students' understanding of density, Sci. Educ. Int., 27(1), 16–39.
- Anderson R. D., (2007), Inquiry as an organizing theme for science curricula, in Abell S. K. and Lederman N. G. (ed.), Handbook of research on science education, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 807–830.
- Andersson B., (1990), Pupils, conceptions of matter and its transformation (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Atila M. E. and Sözbilir M., (2016), Fen ve teknoloji dersi öğretim programındaki yapılandırmacılığa dayalı öğelerin öğretmenler tarafından uygulanışı: Nitel bir çalışma. [Application of constructivist principles in science and technology curriculum into practice by teachers], Erz. Üni. Eğit. Fak. Der., 18(2), 1418–1457.
- Ayyıldız Y. and Tarhan L., (2013), Case study applications in chemistry lesson: gases, liquids, and solids, Chem. Educ. Res. Pract., 14(4), 408–420.
- Baker S. R. and Talley L., (1972), The relationship of visualization skills to achievements in freshman chemistry, J. Chem. Educ., 49(11), 775.
- Barthlow M. J., (2011), The Effectiveness of process oriented guided inquiry learning to reduce alternate conceptions in secondary chemistry, Doctoral Dissertation, Lynchburg, VA: Liberty University.
- Barthlow M. J. and Watson S. B., (2014), The effectiveness of process-oriented guided inquiry learning to reduce alternative conceptions in secondary chemistry, Sch. Sci. Math., 114(5), 246–255.
- Becker N., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R., (2013), Reasoning using particulate nature of matter: an example of a sociochemical norm in a university-level physical chemistry class, Chem. Educ. Res. Pract., 14, 81.
- Becker N., Stanford C., Towns M. and Cole R., (2015), Translating across macroscopic, submicroscopic, and symbolic levels: the role of instructor facilitation in an inquiry-oriented physical chemistry class, Chem. Educ. Res. Pract., 16(4), 769–785.
- Blanchard M. R., Southerland S. A., Osborne J. W., Sampson V. D., Annetta L. A. and Granger E. M., (2010), Is inquiry possible in light of accountability? a quantitative comparison of the relative effectiveness of guided inquiry and verification laboratory instruction, Sci. Educ., 94(4), 577–616.
- Bodner G. M., (1991), I have found you an argument: the conceptual knowledge of beginning chemistry graduate students, J. Chem. Educ., 68(5), 385–388.
- Bransford J. D., Brown A. L. and Cocking R. R., (2000), How people learn, Washington, DC: National Academy Press.
- Bridle C. A. and Yezierski E. J., (2012), Evidence for the effectiveness of inquiry-based, particulate-level instruction on conceptions of the particulate nature of matter, J. Chem. Educ., 89(2), 192–198.
- Brown P. C., Roediger III H. L. and McDaniel M. A., (2014), Make it stick, Harvard University Press.
- Bunce D. M., (2001), Does Piaget still have anything to say to chemists? J. Chem. Educ., 78 (8), 1107–1120.
- Chang H. Y., Quintana C. and Krajcik J. S., (2010), The impact of designing and evaluating molecular animations on how well middle school students understand the particulate nature of matter, Sci. Educ., 94(1), 73–94.
- Chi M. T., Bassok M., Lewis M. W., Reimann P. and Glaser R., (1989), Self-explanations: How students study and use examples in learning to solve problems, Cognit. Sci., 13(2), 145–182.
- Christian B. N. and Yezierski E. J., (2012), Development and validation of an instrument to measure student knowledge gains for chemical and physical change for grades 6–8, Chem. Educ. Res. Pract., 13(3), 384–393.
- Cooper M. M., Cox Jr C. T., Nammouz M., Case E. and Stevens R., (2008), An assessment of the effect of collaborative groups on students' problem-solving strategies and abilities, J. Chem. Educ., 85(6), 866.
- Dawkins K. R., Dickerson D. L., McKinney S. E. and Butler S., (2008), Teaching density to middle school students: preservice science teachers' content knowledge and pedagogical practices, Clear. House, 82(1), 21–26.
- De Gale S., and Boisselle L. N., (2015), The effect of POGIL on academic performance and academic confidence, Sci. Educ. Int., 26(1), 56–61.
- Doğan Y., (2010), Fen ve teknoloji dersi programının uygulanması sürecinde karşılaşılan sorunlar. [The problems encountered during the implementatıon of science and technology curriculum], Yüz. Yıl Üni. Eğit. Fak. Der., 7(1), 86–106.
- Dori Y. J., Tal R. T. and Tsaushu M. (2003), Teaching biotechnology through case studies: can we improve higher order thinking skills of nonscience majors? Sci. Educ., 87(6), 767–793.
- Farrell J. J., Moog R. S. and Spencer J. N., (1999), A guided-inquiry general chemistry course, J. Chem. Educ., 76(4), 570.
- Flinn Scientific, (2020a), POGIL® activities for earth & space science—Designed to support the NGSS. Retrieved from https://www.flinnsci.com/pogil-activities-for-earth–space-science—designed-to-support-the-ngss/ap10094/.
- Flinn Scientific, (2020b), POGIL® activities for life science—Designed to support the NGSS. Retrieved from https://www.flinnsci.com/pogil-activities-for-life-science—designed-to-support-the-ngss/fb2324/.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193.
- Gabel D. L., (1999), Improving teaching and learning through chemistry evaluation research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gabel D. L., Samuel K. V. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 64(8), 695.
- Gennaro E. D., (1966), Teaching “density”—A real problem, Sch. Sci. Math., 66(6), 559–560.
- Gennaro E. D., (1981), Assessing junior high students’ understanding of density and solubility, Sch. Sci. Math., 81, 399–404.
- Green S. B., Sulkind N. J. and Akey T. M., (2000), Using SPSS for windows: Analyzing and understanding data, 2nd edn, New Jersey: Prentice-Hall, Inc.
- Gunstone R. F. and White R. T., (1981), Understanding of gravity, Sci. Educ., 65(3), 291–299.
- Gülmez Güngörmez H., (2018), Süreç odaklı rehberli sorgulayıcı öğrenme yöntemine dahil edilen bilimin doğası etkinliklerinin 7. sınıf öğrencilerinin kavramsal değişimlerine ve bilimsel muhakeme becerilerine etkisi [The effect of nature of science activities embedded in process oriented guided inquiry learning method on seventh grade students conceptual changes and their scientific reasoning skills], Doctoral Dissertation, Adıyaman, Turkey: Adıyaman University. Retrieved from http://dspace.adiyaman.edu.tr:8080/xmlui/handle/20.500.12414/445.
- Hanson D. M., (2006), Instructor’ s Guide to Guided-Inquiry Learning by With Contributions from other POGIL project personnel: Instructor’ s Guide to Process-Oriented Guided-Inquiry Learning, Lisle, IL: Pacific Crest.
- Hanson D., (2011), General chemistry: Guided explorations, 2nd edn), CA, USA: Brooks/Cole.
- Hanson D. and Wolfskill T., (2000), Process workshops-A new model for instruction, J. Chem. Educ., 77(1), 120.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Hazır Bıkmaz F., (2006), Yeni ilköğretim programları ve öğretmenler [New elementary curricula and teachers], Ank. Uni. J. Fac. Educ. Sci., 39(1), 97–116.
- Hein S. M., (2012), Positive impacts using POGIL in organic chemistry. J. Chem. Educ., 89(7), 860–864.
- Hernández G. E., Criswell B. A., Kirk N. J., Sauder D. G. and Rushton G. T., (2014), Pushing for particulate level models of adiabatic and isothermal processes in upper-level chemistry courses: a qualitative study, Chem. Educ. Res. Pract., 15, 354–365.
- Hewson M. G., (1986), The acquisition of scientific knowledge: analysis and representation of student conceptions concerning density, Sci. Educ., 70, 159–170.
- Hitt A. M., (2005), Attacking a dense problem: a learner-centered approach to teaching density, Sci. Act., 42(1), 25–29.
- Jasperson J., (2013), The effects of guided inquiry on students' understanding of physics concepts in the middle school science classroom, Master's thesis, Retrieved from https://scholarworks.montana.edu/xmlui/handle/1/2794.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701.
- Kaçar S., (2012), Görsel sanatlarla bütünleştirilmiş probleme dayali öğrenme yönteminin öğrencilerin fen akademik başarilarina, bilimsel yaraticiliklarina ve sanat etkinlikleriyle fen öğrenme tutumlarina etkileri [The effects of problem based learning method integrated visual arts on students’ academic achievements, scientific creativities and attitudes towards science teaching with art activities], Unpublished Master's thesis, İzmir, Turkey: Dokuz Eylül University.
- Karaduman B., (2008), İlköğretim 6. sınıf fen ve teknoloji dersi “maddenin tanecikli yapısı” ünitesinin öğretiminde, bilgisayar destekli ve bilgisayar temelli öğretim yöntemlerinin, akademik başarıya ve kalıcılığa etkisi [The effects of computer-assisted and computer-based instructional methods on 6th grade science and technology course students’ achievement and retention of knowledge on the content of “particulate nature of matter”], Unpublished Master's thesis, Adana, Turkey: Çukurova University.
- Kawasaki K., Rupert Herrenkohl L. and Yeary S. A., (2004), Theory building and modeling in a sinking and floating unit: a case study of third and fourth grade students' developing epistemologies of science, Int. J. Sci. Educ., 26(11), 1299–1324.
- Kulatunga U., and Lewis J. E. (2013), Exploration of peer leader verbal behaviors as they intervene with small groups in college general chemistry, Chem. Educ. Res. Pract., 14(4), 576–588.
- Lee V. S., (2011). The power of inquiry as a way of learning, Innovative Higher Educ., 36(3), 149–160.
- Lee O., Eichinger D. C., Anderson C. W., Berkheimer G. D. and Blakeslee T. D., (1993), Changing middle school students’ conception of matter and molecules, J. Res. Sci. Teach., 30(3), 249–270.
- Liu X. and Lesniak K. M., (2005), Students' progression of understanding the matter concept from elementary to high school, Sci. Educ., 89(3), 433–450.
- Lou Y., Abrami P. C., Spence J. C., Poulson C., Chambers B. and d’Apollonia S., (1996), Within-class grouping: a meta-analysis, Rev. Educ. Res., 66, 423–458.
- Maclin D., Grosslight L. and Davis H., (1997), Teaching for understanding: a study of students' preinstruction theories of matter and a comparison of the effectiveness of two approaches to teaching about matter and density, Cogn. Instr., 15(3), 317–393.
- Marshall J. C., Smart J. B. and Alston D. M., (2016), Inquiry-Based instruction: a possible solution to improving student learning of both science concepts and scientific practices, Int. J. Sci. Math. Educ., 1–20 DOI:10.1007/s10763-016-9718-x.
- Marx R. W., Blumenfeld P. C., Krajcik J. S., Fishman B., Soloway E., Geier R. and Tal R. T., (2004), Inquiry-based science in the middle grades: assessment of learning in urban systemic reform, J. Res. Sci. Teach., 41(10), 1063–1080.
- Mewhinney C. and Zükerman E. J., (2008), Enhancing the POGIL experience with tablet personal computers: digital ink in the learner-centered classroom, in Moog R. S. and Spencer J. N. (ed.), Process Oriented Guided Inquiry Learning (POGIL), ACS Symposium Series, vol. 994, pp. 157–192.
- MoNE (Ministry of National Education), (2013), Elementary science and technology course curriculum (for 3-8. grades), Ankara, Turkey: Head Council of Education and Morality.
- MoNE, (2016), Middle school science, 6th grade, 2nd edn, Ankara, Turkey: MoNE.
- Moog R. S. and Farrell J. J., (2011), Chemistry: A guided inquiry, 5th edn, USA: John Wiley & Sons, Inc.
- Moog R. S., Dubroff M. and Simonson S. R., (2019), Preface, in Simonson S.R. (ed.), An introduction to process oriented guided inquiry learning for those who wish to empower learners, USA: Stylus Publishing, LLC.
- Nakhleh M. B., (1992), Why some students don't learn chemistry: chemical misconceptions, J. Chem. Educ., 69(3), 191.
- Nakhleh M. B. and Samarapungavan A., (1999), Elementary school children's beliefs about matter, J. Res. Sci. Teach., 36(7), 777–805.
- National Research Council, (1996), National science education standards, Washington, DC: National Academy Press.
- National Research Council, (2000), Inquiry and the National Science Education Standards: A Guide for Teaching and Learning, Washington, DC: The National Academies Press DOI:10.17226/9596.
- Novick S. and Nussbaum J., (1978), Junior high school pupils’ understanding of the particulate nature of matter: an interview study, Sci. Educ., 62(3), 273–281.
- Novick S. and Nussbaum J., (1981), Pupils’ understanding of the particulate nature of matter: a cross-age study, Sci. Educ., 65(2), 187–196.
- Özalp D. and Kahveci A., (2015), Diagnostic assessment of student misconceptions about the particulate nature of matter from ontological perspective, Chem. Educ. Res. Pract., 16(3), 619–639.
- Pelen Değirmenci A., (2009), İlköğretim 6. sınıf öğrencilerinin maddenin tanecikli yapısı ünitesindeki başarılarına, tutum ve algilamalarına çoklu zeka kuramının etkisi [The effects of multiple intelligence theory on the success, attitudes and the perception of sixth grade students in primary school for the unit of particulate nature of matter], Unpublished Master's thesis, Ankara, Turkey: Gazi University.
- POGIL, (2019), POGIL Project's web site, Retrieved from http://www.pogil.org.
- Qureshi S., Vishnumolakala V. R., Southam D. C. and Treagust D. F., (2017), Inquiry-based chemistry education in a high-context culture: a Qatari case study, Int. J. Sci. Math. Educ., 15(6), 1017–1038.
- Rahayu D. P. and Pamelasari S. D., (2018), Critical thinking ability junior high school student's with process oriented guided inquiry model, International Conference on Mathematics and Science Education of Universitas Pendidikan Indonesia, vol. 3, pp. 492–497, Retrieved from http://science.conference.upi.edu/proceeding/index.php/ICMScE/article/view/172.
- Ramos J. L. S., Dolipas B. B. and Villamor B. B., (2013), Higher order thinking skills and academic performance in physics of college students: a regression analysis, Int. J. Innovative Interd. Res., 4, 48–60.
- Rickey D. and Stacy A. M., (2000), The role of metacognition in learning chemistry, J. Chem. Educ., 77(7), 915.
- Sanger M. J. and Badger S. M., (2001), Using computer-based visualization strategies to improve students' understanding of molecular polarity and miscibility, J. Chem. Educ., 78(10), 1412.
- Sanger M. J., Phelps A. J. and Fienhold J., (2000), Using a computer animation to improve students' conceptual understanding of a can-crushing demonstration, J. Chem. Educ., 77(11), 1517.
- Schroeder J. D. and Greenbowe T. J., (2008), Implementing POGIL in the lecture and the Science Writing Heuristic in the laboratory-student perceptions and performance in undergraduate organic chemistry, Chem. Educ. Res. Pract., 9(2), 149–156.
- Şen Ş. and Yılmaz A., (2015), the Effects of Process Oriented Guided Inquiry Learning Environment on Students’ Self-Regulated Learning Skills, Prob. Educ. 21st Cent., 66, 54–66.
- Simonson S. R. and Shadle S., (2013), Implementing process oriented guided inquiry learning (POGIL) in undergraduate biomechanics: lessons learned by a novice, J. STEM Educ., 14(1), 56–64.
- Skamp K., (1999), Are atoms and molecules too difficult for primary children? Sch. Sci. Rev., 81(295), 87–96.
- Smith K. J., and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73(3), 233.
- Smith K. C. and Villarreal S., (2015), Using animations in identifying general chemistry students' misconceptions and evaluating their knowledge transfer relating to particle position in physical changes, Chem. Educ. Res. Pract., 16(2), 273–282.
- Smith C., Snir J. and Grosslight L., (1992), Using conceptual models to facilitate conceptual change: the case of weight-density differentiation, Cogn. Instr., 9(3), 221–283.
- Tuncel S., (2009), İlköğretim 6. sınıf fen ve teknoloji dersinde maddenin tanecikli yapısı ünitesinin yaratıcı drama ile öğretiminin öğrencilerin başarısına etkisi [The Effect of teaching particulate nature of matter unit by creative dramas on the achievement of students at 6 th grade science and technology lesson], Unpublished master's thesis, Konya, Turkey: Selçuk University.
- University of Colorado, (2020), PhET Interactive Simulations: Maddenin halleri [States of matter]. Retrieved from https://phet.colorado.edu/tr/simulation/legacy/states-of-matter.
- Valanides N., (2000), Primary student teachers’ understanding of the particulate nature of matter and its transformations during dissolving, Chem. Educ. Res. Pract., 1(2), 249–262.
- Vilardo D. A., MacKenzie A. H. and Yezierski E. J., (2017), Using students’ conceptions of air to evaluate a guided-inquiry activity classifying matter using particulate models, J. Chem. Educ., 94(2), 206–210.
- Villagonzalo E. C., (2014), Process oriented guided inquiry learning: an effective approach in enhancing students’ academic performance, DLSU Research congress, vol. 2, no. 1, pp. 1–6.
- Vlassi M., and Karaliota A., (2013), The comparison between guided inquiry and traditional teaching method. A case study for the teaching of the structure of matter to 8th grade Greek students, Proc.-Soc. Beh. Sci., 93, 494–497.
- Vrieling E. M., Bastiaens T. J. and Stijnen S., (2010), Process-oriented design principles for promoting self-regulated learning in primary teacher education, Int. J. Educ. Res., 49(4–5), 141–150.
- Warfa A. R. M., Roehrig G. H., Schneider J. L. and Nyachwaya J., (2014), Collaborative discourse and the modeling of solution chemistry with magnetic 3D physical models–impact and characterization, Chem. Educ. Res. Pract., 15(4), 835–848.
- What is POGIL? (2019), Retrieved from https://pogil.org/about-pogil/what-is-pogil.
- Williamson V. M., Lane S. M., Gilbreath T., Tasker R., Ashkenazi G., Williamson K. C. and Macfarlane R. D., (2012), The effect of viewing order of macroscopic and particulate visualizations on students’ particulate explanations, J. Chem. Educ., 89(8), 979–987.
- Yezierski E. J. and Birk J. P., (2006), Misconceptions about the particulate nature of matter. Using animations to close the gender gap, J. Chem. Educ., 83(6), 954.
- Zoupidis A., Spyrtou A., Malandrakis G. and Kariotoglou P., (2016), The evolutionary refinement process of a teaching-learning sequence for introducing inquiry aspects and density as materials’ property in floating/sinking phenomena. in Psillos D. and Kariotoglou P. (ed.), Iterative design of teaching-learning sequences, Dordrecht: Springer, pp. 167–199.
Footnotes |
| † A part of this study was presented at 27. International Conference on Educational Sciences (ICES-UEBK) 2018, Antalya, Turkey, April 18–22. |
| ‡ This study is a part of the first author's Master's thesis. |
| This journal is © The Royal Society of Chemistry 2020 |

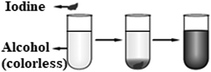
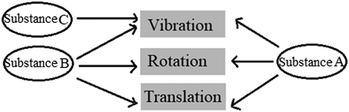

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)