Students’ competence in translating between different types of chemical representations
Vasiliki
Gkitzia
 ,
Katerina
Salta
,
Katerina
Salta
 and
Chryssa
Tzougraki
and
Chryssa
Tzougraki
 *
*
Department of Chemistry, National and Kapodistrian University of Athens, Athens, Greece. E-mail: tzougraki@chem.uoa.gr
First published on 19th October 2019
Abstract
Meaningful understanding of chemistry, among others, includes the ability of an individual to think simultaneously at macroscopic, submicroscopic and symbolic levels, and this presupposes the competence to translate between different types of chemical representations. In this study, we investigated 11th grade Greek students’ and 3rd year undergraduate chemistry students’ ability to translate chemical representations from one level of chemistry (e.g., submicroscopic) into another (e.g., symbolic) concerning the basic chemical concepts: “chemical element”, “chemical compound”, “aqueous solution” and “solid state of matter”, which have already been taught in earlier grades. We followed a mixed method design in which both quantitative and qualitative research instruments were developed and used. These instruments consisted of multiple choice and open-ended questions, which included real pictures (macroscopic), symbolizations and submicroscopic diagrams. Various representations of the three types were given to the students and they were asked to choose or to construct an equivalent one of a different type. Our results showed that the 11th grade students’ ability to move across the three levels of chemistry is very low, while the 3rd year undergraduate chemistry students’ performance is higher but not satisfactory. In addition, the results obtained from the application of “translation questions” between the three levels of chemistry highlighted many students’ alternative conceptions, some of which still persist among the undergraduate students. The students showed lower performance in translations concerning the concepts “chemical compound” and “aqueous solution” than those concerning the concepts “chemical element” and “solid state of matter”. The students also showed the lowest level of performance in translating the submicroscopic representations into the symbolic ones. Generally, our results indicate that translating between different types of chemical representations is a very challenging task, which depends on students’ conceptual understanding.
1. Introduction
Chemistry studies phenomena, such as interactions between atoms, molecules, and ions, and the dissolution of a substance in a solvent, which cannot be directly perceived by human senses. However, the understanding of chemistry is based on giving meaning to the unseen and to the untouched and on creating mental images for the corresponding molecular phenomena. Chemists in order to describe, to explain, and to visualize chemical phenomena and to facilitate their communication have invented specialized symbol systems to represent these phenomena (Hoffmann and Laszlo, 1991; Mathewson, 2005). The variety of these symbolic systems, such as molecular formulas, chemical equations, molecular models, and Fischer projections, is very wide and is continuously expanding and exploiting the potential of new technologies.Alex Johnstone, 30 years ago (1982, 1993), introduced the notion that chemical knowledge has three basic components or levels – often labeled as the macroscopic, which refers to what is tangible, edible and visible; the submicroscopic, which refers to molecular, atomic and kinetic; and the symbolic, which refers to symbols, equations, stoichiometry and mathematics. Johnstone (1982) illustrated his point with a triangle in which each vertex corresponds to a chemistry level: “macro”, “submicro” and “symbolic” respectively. In addition, Johnstone (1991, 1993) emphasized the “multilevel thought” required in learning chemistry, arguing that expert chemists can easily move between the three levels, but novice learners experience great difficulties in dealing simultaneously with the macroscopic, submicroscopic and symbolic levels, and thus, they mainly operate at the macro level, struggling to meaningfully relate to the other two.
“Almost in one breath the teacher ranges across this diagram, but the pupil can be stranded at the ‘macro’ corner.” (Johnstone, 1991, p. 78)
Many research studies have shown students’ great difficulty in abandoning the continuous model of matter, to adopt the scientifically accepted particulate model and, therefore, to visualize the particulate nature of matter (Ben-Zvi et al., 1986; Gabel et al., 1987; Nakhleh, 1992; Kozma and Russell, 1997; Treagust et al., 2003; Akaygun, 2016; Zarkadis et al., 2017).
Since then, this notion has become highly influential in the field of chemical education and has served both as the base of theoretical frameworks to guide research and as a central idea in various curriculum projects. It is commonly accepted that meaningful understanding in chemistry involves the ability not only to comprehend chemical phenomena at all three levels of chemistry, but also to move easily between them. Johnstone's triangle opened a new page in chemical education and it was the springboard for an extensive discussion about chemistry's nature that still continues nowadays. Since its initial introduction, new extensions have been given, for example, Gilbert and Treagust (2009a) referred to “the three types of representation in chemistry”, and new perspectives have been included such as the term “chemistry triplet” (Talanquer, 2011).
Talanquer (2011) pointed out that chemical knowledge actually exists in a more complex, multi-dimensional knowledge space and he expanded and enriched the original idea about what the three major levels of chemistry represent and encompass. He suggested that the chemistry knowledge can be characterized as being of three main types: Experiences: they refer to the actual empirical knowledge that we have or gather about chemical systems. Models: they refer to the theoretical models (i.e., theoretical entities, such as chemical elements and chemical compounds) that chemists have developed to make sense of the experienced world. Visualizations: they encompass the static and dynamic visual signs (from symbols to icons, such as particulate drawings) developed to facilitate qualitative and quantitative thinking and communication about both experiences and models in chemistry. For each type of knowledge Talanquer distinguished different length scales, from the macroscopic to the subatomic, and three major dimensions: composition/structure, energy and time. Talanquer argued that the experience and the model are two clearly distinctive types of knowledge that students will need considerable help and support in learning how to translate from one to the other and he, also, supported that students may not only have problems connecting or translating ideas between the three major levels, but are also likely to experience difficulties whenever they move from one scale to another in which interactions between the particles arise. Therefore, meaningful chemistry learning requires students to be able to translate within and across knowledge types, scales, dimensions, and approaches.
Skepticism has also been expressed concerning the correlation between the nature of chemistry and the science of learning. Taber (2013) suggested that novice learners find difficulties even in the macroscopic level of chemistry. As he explained, although chemistry is essential to understanding and developing materials, introductory chemistry often makes limited call upon most of the materials that learners are familiar with. Chemistry at middle and high school is primarily about pure substances – elements and compounds – which are seldom met in students’ everyday life. Thus, macroscopic phenomena make sense for most learners only if they are reconceptualised and matched to the corresponding formal descriptions (e.g., substances, elements, chemical reactions) and if they are considered in light of the relevant submicroscopic theoretical models, such as Bohr's model and atomic theory of matter (Taber, 2013).
Furthermore, Taber (2013) also expressed skepticism about the symbolic level. He argued that symbolic language is not helpful to be considered as a discrete “level” of chemical knowledge that is one element of an ontological triad of macroscopic–submicroscopic–symbolic levels. He explained that some symbolic systems are related largely to the macroscopic level, such as the standard ways to represent bench apparatus set-ups; some others are largely related to the submicroscopic level, such as the models for orbitals, for electron shells, and for bonding; while some others are ambiguous in terms of whether they refer to the macroscopic or submicroscopic level. For example, molecular formulas are used to symbolize both pure substances (macroscopic) and molecules (submicroscopic). Taber (2013) concluded to a revision of Johnstone's triangle, proposing a new schema in order to present the required, underlying thinking in chemical education (Fig. 1). In this schema, the symbolic level is not separated from the macroscopic and submicroscopic domains on the ground that it concerns the representations and the communication of the concepts and the models developed at those two levels.
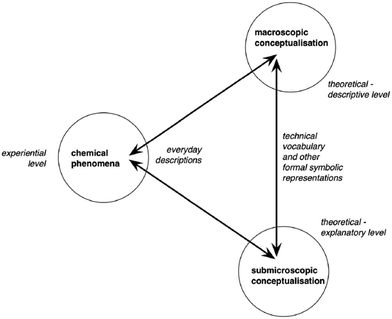 | ||
| Fig. 1 Learning chemistry involves re-descriptions (represented by the arrows) between the everyday language of direct experience and formal representations of the conceptualization of the subject at two distinct levels (reproduced from Taber, 2013, p. 165, with permission from the Royal Society of Chemistry). | ||
Despite the discussion about the nature of chemistry and the relevant levels, the major challenge for chemical education remains the same: the attainment of meaningful understanding, which undeniably involves the ability of an individual to think at the submicroscopic level (to imagine what happens to the particles of the substances) in order to explain phenomena at the macroscopic level (what one observes in the laboratory) and to use scientific symbols to describe them. Thus, full and conceptual understanding of chemical concepts and phenomena requires individuals to simultaneously engage the three levels and to move fluently between them (Johnstone, 1993; Gabel, 1999; Taber, 2013).
In this paper, we will be referring to chemistry's three levels and to the corresponding levels of chemical representations as follows: macro, submicro and symbolic representations.† We consider that macro representations depict phenomena according to the human visual sense. These phenomena are direct experiences produced by laboratory experiments or by everyday life (Treagust et al., 2003). Submicro representations depict the structure and movement of the real, but too tiny to be observed particles of matter (atoms, molecules, ions, electrons, etc.) (Wu and Shah, 2004). Symbolic representations include symbols, letters, numbers and signs that are used to represent atoms, molecules, ions, substances and chemical phenomena (Wu and Shah, 2004).
Translating between various chemical representations
In the context of the increased interest for assessing conceptual understanding in chemistry, translating representations of one type into another was a study subject for many researchers. Kozma and Russell (2005) proposed the term representational competence and described it as a set of skills and practices that allow a person to use various representations or visualizations, to think about, communicate, and act on chemical phenomena. Translating chemical representations is considered as a part of representational competence (Heitzman and Krajcik, 2005; Kozma and Russell, 2005) and includes the following skills:• The ability to interpret representations by obtaining appropriate information from them.
• The ability to move among the various representations of a concept.
In addition, Heitzman and Krajcik (2005) described students’ moving among representations by either providing other representations to express the same information or identifying the similar and different information that the representations depict. Generally, translation between different types of representation is an information processing task, requiring understanding of the underlying concept to the extent that the individual can interpret the information provided by an initial representation and infer the details required either to construct or to interpret the target representation. Therefore, representations’ translation constitutes an index of conceptual understanding (Keig and Rubba, 1993).
Students’ ability to translate representations of one type into another was studied for various concepts, such as ionic reactions (Ye et al., 2018), chemical equilibrium (Kozma and Russell, 1997; Ramnarain and Joseph, 2012), heat capacity, thermal conductivity and gas behavior (Rappoport and Ashkenazi, 2008), and diffusion and osmosis (Cook et al., 2008). In the present study, we intended to investigate the ability of school and undergraduate students to navigate between the three levels of chemistry (symbolic, macroscopic and submicroscopic) for the fundamental concepts “chemical element”, “chemical compound”, “aqueous solution” and “solid state of matter”. We focused on concepts that are prerequisite knowledge for the subsequent subjects, and thus, any possible alternative conceptions or insufficient understanding would hinder further learning of more complex concepts. Furthermore, we focused on translations among the above three types of representations, expecting the students to be familiar with, since such representations are commonly used in secondary education. In particular, we examined how and to what extent students understand whether different representations depict the same material. Asking students to translate between different representations of the same material is a good test of whether they fully understand the underlying chemical concepts or they carry several alternative conceptions (Johnstone, 1993; Gabel, 1999; Taber, 2013).
This study was guided by the following research questions:
1. How do 11th grade students respond and what difficulties do they encounter when attempting to translate representations from one level of chemistry (e.g., submicroscopic) into another (e.g., symbolic) concerning the basic chemical concepts “chemical element”, “chemical compound”, “aqueous solution” and “solid state of matter”?
2. How do undergraduate students respond and what difficulties do they encounter when attempting to translate representations from one level of chemistry (e.g., submicroscopic) into another (e.g., symbolic) concerning the above mentioned chemical concepts?
3. What are the differences between high school students and undergraduate students in translating between different types of representations?
2. Methodology
We followed a mixed method design in which both quantitative and qualitative research instruments were used (Greene et al., 1989). The data collection was completed in two main phases: (a) first, by submitting a questionnaire to all students, and (b) second, by interviewing 16 of them. Details for each of these phases are presented in the following paragraphs.2.1 Sample
In the quantitative phase the research sample consisted of:(A) 466 upper secondary school students in the 11th grade, 16–17 years old (195 males; 41.8%, 269 females; 57.7%, 2 students did not denote their gender; 0.4%). Intact classes of students drawn from 10 high schools in 6 towns in Greece (7 urban and 3 provincial high schools) served as subjects. The schools were selected so that the sample would be representative of the population's distribution in Greece and on the basis of the willingness of the teaching staff to participate in the study. The sample consisted of 130 students specialized in science and medicine, 209 in humanities, and 123 in engineering studies and 4 students did not denote their specialization.
(B) 86 undergraduate students (22 males; 25.6%, 62 females; 72.1%, 2 students did not denote their gender; 2.3%) in the 6th semester of their studies in the Chemistry Department of the National and Kapodistrian University of Athens.
In the qualitative phase the research sample consisted of 16 students of 11th grade from 2 high schools (1 urban and 1 provincial), who also participated in the quantitative part of the research.
We chose the 11th grade because the instruction of the concepts under study (i.e., chemical element, chemical compound, aqueous solution and states of matter) has been taught in the 8th grade; thus, we could investigate students’ competence in moving across the three levels of chemistry at basic concepts. Eleventh graders have covered a variety of chemical concepts, which in more detail are presented in Appendix I. Concerning undergraduate students, in the 6th semester they had moved deeply into chemistry issues and they had attended advanced lessons in all chemistry fields such as general, theoretical, inorganic, analytical, organic, physical, and thermochemistry. Therefore, in the 6th semester undergraduates’ education had been advanced to a large extent so we could evaluate the development of their translation competence.
2.2 Instruments
To conduct the quantitative research we constructed a questionnaire consisting of 11 multiple choice questions, which included real pictures (macroscopic), molecular formulas (symbolic) and submicroscopic diagrams. Each question contained a chemical representation at a chemistry level (e.g., submicroscopic) and students were asked to choose an equivalent one at another level (e.g., symbolic) for a set of representations (see, for example, Fig. 2). The above questionnaire resulted from a preliminary investigation using open-ended questions, followed by a pilot study which was conducted using a target group of 91 students in the 11th grade from 2 urban high schools in Athens. The content validity of the questionnaire was tested by a group of experts (in-service chemistry teachers and chemistry education researchers). The group of experts evaluated the clarity of each question of the questionnaire. They also provided verification that the 11 questions adequately examine secondary students’ competence in translating between different types of chemical representations.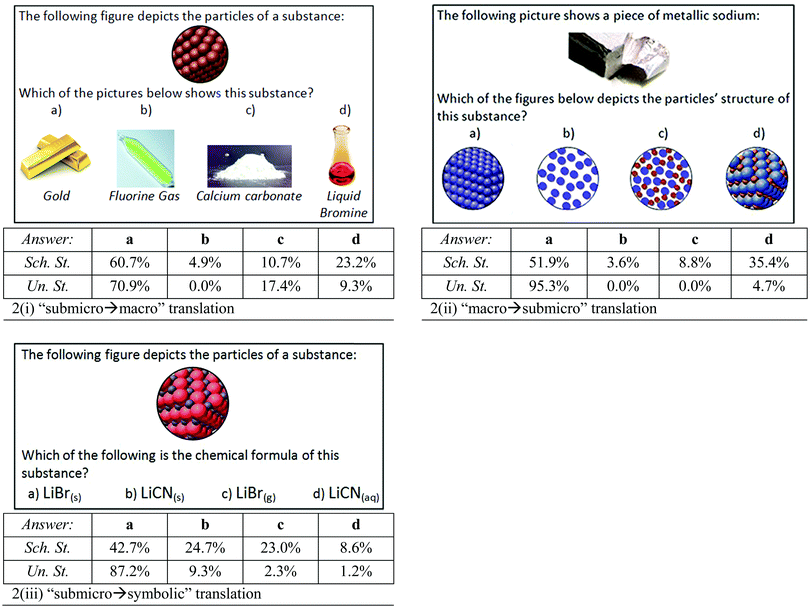 | ||
| Fig. 2 Questions asking for translation between various representations concerning both chemical substances and states of matter. | ||
In order to conduct our research we received permission from the Greek Ministry of Education, Research and Religious Affairs and we also informed the school administrations, who approved the use of the data for our study. In addition, we informed the students and their parents about the goals of the present study and that their responses to the test questions would be used in the study. The questionnaire was given to the students by their teachers and it was completed by those who agreed to participate. The coordinating researcher collected the data from the teachers. It should be noted that only students’ gender and specialization were reported and not their names (Taber, 2014). A similar process was followed for undergraduates concerning distribution and collection of the questionnaire.
Since fixed response questions do not give much insight into why certain answers were chosen by the students (Johnstone and Ambusaidi, 2000), the quantitative research was followed by a qualitative one using the semi-structured interview technique. Semi-structured interviews are well-established as the basic technique in research of exploring thinking in-depth (Gilbert et al., 1985). Our interview-guide was based on Ausubel meaningful learning theory (1968) and Johnstone's multiple levels of representations (Johnstone, 1991; Johnstone, 2010).
Meaningful learning was described by Ausubel (1963) as the formulation of no arbitrary relationships between ideas in the learner's mind. Ausubel (1968) also suggested that meaningful learning promotes deep or meaningful understanding of scientific concepts. The meaningful understanding of various chemistry concepts is mainly based on giving meaning to the unseen and to the untouched molecular phenomena (Claesgens et al., 2009). The representations of these phenomena allow chemists to communicate and visualize chemistry (Gilbert and Treagust, 2009b). They also serve as tools to help the learner to construct mental models (Vachliotis et al., 2014).
Accordingly, in order to elicit students’ mental models about the target concepts (chemical element, chemical compound, aqueous solution, mixture and solid state of matter), first we showed to the students several representations of the concepts (see Appendix II) and asked them to interpret and/or construct the corresponding ones. Afterwards, we asked them to describe their thought process in making each representation.
According to Johnstone, as students gain knowledge in chemistry, they are expected to form connections among the symbolic, submicroscopic, and macroscopic levels of chemistry (Johnstone, 1991; Johnstone, 1997; Johnstone, 2010). However, making connections among these levels was a much challenged task for students (Johnstone, 1991). Therefore, the interview guide attempted to explore the extent to which students could articulate not only a meaningful understanding of the focused concepts represented at macroscopic, submicroscopic, and symbolic levels, but also their understanding of the nature of the connections between these levels. The semi-structured interviews consisted of three steps.
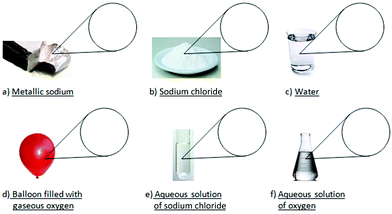
In the first step of the interviews, pictures of 6 materials were shown to the students (see Appendix II – Card 1) and the students were asked to construct one submicroscopic and one symbolic representation for each material. Afterwards, the students were asked to explain their thought process and provide description of their representations’ features.
In Step 2, five submicroscopic representations (see Appendix II – Card 2) were shown to the students, and they were asked to characterize which one represents a chemical element, which one a chemical compound and which one a mixture, and then to describe their reasoning. Afterwards, the students were asked to choose which one of these five submicroscopic representations is likely to depict the particles of a gaseous substance symbolized as “HI”, explaining their way of thinking.
In Step 3, a submicroscopic representation of an aqueous solution (see Appendix II – Card 3a) was shown to the students and they were asked to choose the corresponding one out of the four provided macroscopic representations. The students were also asked to describe the main features of that submicroscopic representation and to explain on which features they were based to make the correlation with the macroscopic one. Finally, the students were informed the name of the above aqueous solution and they were asked to choose one out of the four symbolic representations that corresponds to this name (see Appendix II – Card 3b), explaining their reasoning.
In the interviews, only volunteers participated for both methodological reasons (i.e., to ensure the validity of responses during extended interviews) and on ethical grounds (Limerick et al., 1996; Taber, 2002). All interviewing was undertaken by the first author, a few weeks after the completion of the qualitative research. The students and their parents were informed about and consented to the content, the purpose and the schedule of the interview. Each interview lasted for approximately 40 minutes and it was conducted and transcribed in Greek. The interview transcripts were analyzed by the process of open coding (Strauss and Corbin, 1990). Common mistakes and common patterns concerning translation competence were identified in students’ responses which are discussed below.
The questionnaire was composed in Greek and interviews were conducted in Greek as well. All data reported here (questions, figures, students’ drawings and interview quotes) use English translations prepared by the three researchers, who have high levels of fluency in both Greek and English. Each researcher made an independent translation of the items focusing on the meaning of each item rather than on literal word-for-word translation. After individual translations the researchers discussed the inconsistencies and decided on the most appropriate translation of the items. Finally, back translation from the target language into the source language was used, so that readers can be confident that all items are accurately translated (Taber, 2018).
3. Results and discussion of the quantitative research
The results obtained from the application of the questionnaire are presented in detail below for each chemical concept. To ascertain whether a difference was present between eleventh-graders and undergraduate students, a Pearson chi-square test was conducted for each answer.3.1 Chemical substances and the solid state of matter
Four questions (Fig. 2 and 3) were given to the students for translation between various representations concerning two chemical substances (chemical elements and chemical compounds) and the solid state of matter. For the concept “chemical element” we examined the “submicro → macro”‡ translation [Fig. 2(i)], and the “macro → submicro” translation [Fig. 2(ii)]. Our target was to investigate whether students hold the scientific conception that chemical elements consist of one type of atom. The majority of the school students (89.3%) and undergraduates (80.2%) were able to identify that a substance depicted by one type of atom corresponds to a chemical element and thus they chose either gold or fluorine or bromine [answers a, b and d in Fig. 2(i)]. However, it is notable that 10.7% of the school students and 17.4% of the undergraduate students chose incorrectly the picture of calcium carbonate [answer c in Fig. 2(i)]. According to the Pearson chi-square test there is no statistically significant difference between school and undergraduate students in answer c [x2(1) = 2.91, p > 0.05]. The identification of the correct state of matter will be discussed below, in the relevant concept “solid state of matter”.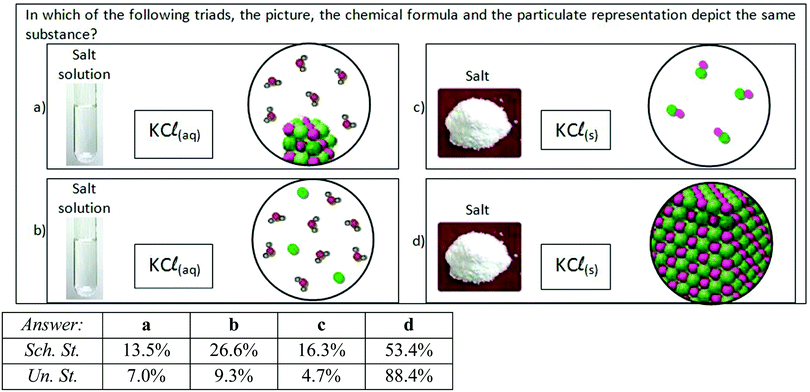 | ||
| Fig. 3 Question requiring students to choose the correct triad of representations which depict the same substance. | ||
In the “macro → submicro” translation, only half (51.9%) of the school students matched correctly the macroscopic representation of metallic sodium to the submicroscopic one consisting of one type of atom [answers a and b in Fig. 2(ii)]. It is remarkable that 44.2% of the school students assigned metallic sodium to a submicroscopic representation consisting of two types of particles [answers c and d in Fig. 2(ii)]. The reason for this notably high percentage will be further examined in the qualitative part. Undergraduate students’ performance in the same translation was fairly high, with 95.3% of them choosing the correct answer [Fig. 2(ii)]. The differences between school and undergraduate students were found to be statistically significant; for answers a [x2(1) = 56.45, p < 0.001], c [x2(1) = 8.17, p < 0.05] and d [x2(1) = 32.33, p < 0.001].
The question shown in Fig. 2(iii) involved a “submicro → symbolic” translation concerning an ionic compound. Successful translations were achieved by 65.7% of the eleventh-graders and 89.5% of the undergraduates, who chose the correct molecular formula “LiBr” as the equivalent of a submicroscopic representation of a crystal lattice consisting of two types of particles [answers a and c in Fig. 2(iii)]. However, a remarkable 33.3% of the eleventh-graders and 10.5% of the undergraduates chose the formula “LiCN” [answers b and d in Fig. 2(iii)], assigning the symbol “CN” as one type of particle. The differences in all the answers were found to be statistically significant between the two groups: for answer a: x2(1) = 57.53, p < 0.001, for answer b: x2(1) = 9.91, p < 0.05, for answer c: x2(1) = 19.51, p < 0.001 and for answer d: x2(1) = 5.81, p < 0.05.
The above three questions [Fig. 2(i)–(iii)] also involved the concept “solid state of matter”, in which both groups of students demonstrated good performance. Specifically, 71.4% of the school students and 88.3% of the undergraduate students matched the close arrangement of particles in Fig. 2(i) with a solid material; either gold or calcium carbonate [answers a and c in Fig. 2(i)]. However, a significant proportion of school students (23.2%) chose liquid bromine [answer d in Fig. 2(i)]. The causes of this high percentage need further examination, since they may involve not only misconceptions about particles’ structure in the solid state, but also misconceptions arising from the correlation between the color of the substance and the color of its representation's particles, ignoring the qualitative composition and the arrangement of particles (Griffiths and Preston, 1992; Chandrasegaran et al., 2007). Among the undergraduate students only 9.3% chose liquid bromine, which percentage is statistically significantly different from that of the school students [for answer d: x2(1) = 8.42, p < 0.05)].
The reverse translation “macro → submicro” [Fig. 2(ii)], concerning only the close arrangement of particles, was achieved by 87.3% of the school students and by 100.0% of the undergraduate students [answers a and d in Fig. 2(ii)].
Regarding the correlation between the submicroscopic and symbolic levels [Fig. 2(iii)], 67.4% of the eleventh-graders and 96.5% of the undergraduates chose the correct subscript “s” in a molecular formula in order to symbolize a substance with a close arrangement of particles [answers a and b in Fig. 2(iii)]. However, a remarkable percentage of school students (23.0%) chose the subscript “g” [answer c in Fig. 2(iii)], while fewer (8.6%) chose the subscript “aq” (answer d in Fig. 2(iii). These results show that some school students have difficulties and/or knowledge deficiencies concerning the state of matter's symbols or they do not pay attention to the phase of matter; this issue will be further explored in the qualitative part. The overriding majority of undergraduate students didn’t have any problem with the aforementioned symbols.
In another question, four triads were given to the students; each one consisted of macroscopic, symbolic and submicroscopic representations. The students had to choose the triad in which the three representations depicted the same substance (“macro–submicro–symbolic” correlation) (Fig. 3). This correlation of the three levels of chemistry was achieved by 53.4% of the school students and 88.4% of the undergraduate students (answer d in Fig. 3) [statistically significant difference: x2(1) = 57.53, p < 0.001].
Two other questions (Fig. 4) concern translation between submicroscopic and symbolic representations concerning chemical compounds. Fig. 4(i) presents a question that asks students to choose a chemical formula for a submicroscopic representation depicting three molecules of a molecular compound, namely a “submicro → symbolic” translation. Students had to recognize the qualitative composition of each molecule ( ) and thus to choose the correct formula “NO”. Surprisingly, only 14.2% of the eleventh-graders could achieve this translation. Undergraduate students’ performance was better, since 59.3% of them answered correctly (statistically significant difference: x2(1) = 88.56, p < 0.001), but this percentage is not satisfactory. The remaining eleventh graders took into account the depicted number of molecules, choosing either the formula “(NO)3” (31.8%) or “N3O3” (25.1%) or “3 NO” (28.1%). These incorrect answers may represent fundamental difficulties with learning or understanding the meaning and the use of subscripts and coefficients in molecular types, and will be further examined. Regarding the undergraduates, 11.6% chose “(NO)3” [statistically significant difference: x2(1) = 11.40, p < 0.001], 0.0% chose “N3O3” [statistically significant difference: x2(1) = 17.40, p < 0.001], but 29.1% chose “3 NO” [not statistically significant difference: x2(1) = 0.33, p > 0.05]. So, it seems that undergraduate students show better performance in this translation, but a significant percentage chose a molecular formula containing a coefficient.
) and thus to choose the correct formula “NO”. Surprisingly, only 14.2% of the eleventh-graders could achieve this translation. Undergraduate students’ performance was better, since 59.3% of them answered correctly (statistically significant difference: x2(1) = 88.56, p < 0.001), but this percentage is not satisfactory. The remaining eleventh graders took into account the depicted number of molecules, choosing either the formula “(NO)3” (31.8%) or “N3O3” (25.1%) or “3 NO” (28.1%). These incorrect answers may represent fundamental difficulties with learning or understanding the meaning and the use of subscripts and coefficients in molecular types, and will be further examined. Regarding the undergraduates, 11.6% chose “(NO)3” [statistically significant difference: x2(1) = 11.40, p < 0.001], 0.0% chose “N3O3” [statistically significant difference: x2(1) = 17.40, p < 0.001], but 29.1% chose “3 NO” [not statistically significant difference: x2(1) = 0.33, p > 0.05]. So, it seems that undergraduate students show better performance in this translation, but a significant percentage chose a molecular formula containing a coefficient.
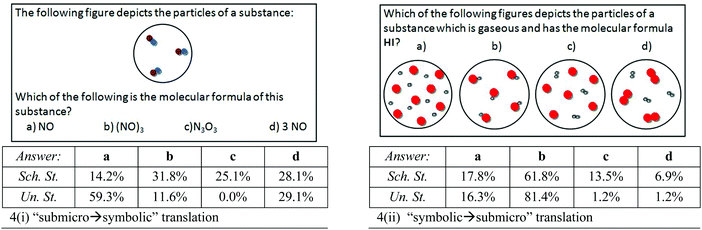 | ||
| Fig. 4 Questions asking for translation between various representations concerning chemical substances. | ||
In the reverse type of translation, the students were asked to assign the molecular formula “HI” of a gaseous compound to the appropriate type of particle [Fig. 4(ii)]. The results showed that 61.8% of the school students and 81.4% of the undergraduates succeeded in this “symbolic → submicro” translation [statistically significant difference: x2(1) = 12.23, p < 0.001]. However, the choice of answer a [Fig. 4(ii)] by 17.8% of the school students and by 16.3% of the undergraduates reflects the misconception that in the gaseous state the atoms of a chemical compound are not bonded together (Adbo and Taber, 2009) and will be further investigated in the interviews following. It seems that this likely misconception still exists among the undergraduate students [not statistically significant difference: x2(1) = 0.12, p > 0.05]. Furthermore, 13.5% of the school students chose a mixture of diatomic and monoatomic particles, answer c, and 6.9% chose a mixture of two diatomic particles, answer d. These alternative answers were found to be statistically significantly reduced among the undergraduates [for answer c: x2(1) = 10.82, p < 0.05, and for answer d: x2(1) = 4.20, p < 0.05].
3.2 Aqueous solutions
In translation questions involving aqueous solutions students faced significant difficulties. For the “submicro → macro” translation [Fig. 5(i)], a submicroscopic representation of an element's aqueous solution, consisting of water molecules and diatomic molecules, was given to students and they were asked to choose the correct picture from those of the four different materials. Only 29.4% of the eleventh-graders chose the correct material [answer b in Fig. 5(i)]. The respective percentage for the undergraduate students was increased to 67.4% [statistically significant difference: x2(1) = 45.37, p < 0.001], but it is not satisfactory concerning their education level. More than half of the eleventh-graders (51.3%) assigned the submicroscopic representation to a mixture of sulfur and iron [answer c in Fig. 5(i)], without considering that both sulfur and iron are pure chemical elements and thus each one should be depicted by one type of particle/circle. Among the undergraduates, the percentage that chose the wrong answer c was reduced (20.9%), but not eliminated [statistically significant difference: x2(1) = 26.89, p < 0.001]. Furthermore, 10.5% of the eleventh-graders assigned the submicroscopic representation with the ionic compound iron sulfate [answer d in Fig. 5(i)], ignoring that the representation consisted of two different types of molecules, and 7.9% of them assigned it with gaseous iodine [answer a in Fig. 5(i)], without considering that a pure element should be depicted by one type of particle. These answers [a and d in Fig. 5(i)] were chosen by fewer undergraduates; however, the differences were not statistically significant. The above results may be associated with various misconceptions, such as a likely correlation between the color of the particles in the submicroscopic representations and the macroscopic color of the substance (yellow color in this case) or the differentiation between pure substances and mixtures. In order to explore the presence of such likely misconceptions and explain the above statistical findings, we examined a properly modified question further down, in the qualitative part, where students analyzed their thought process (see Appendix II – Card 3a). At this point, we should point out a limitation of the used submicroscopic representation; for clarity reasons, we depicted the molecules in bigger distances than the real ones in a liquid material. So, this could be confusing for students and it might lead them to choose gaseous iodine. However, both the small percentage of students who chose answer a and the students’ explanations in the subsequent interviews allow us to assume that this limitation did not disorientate them from answering this question.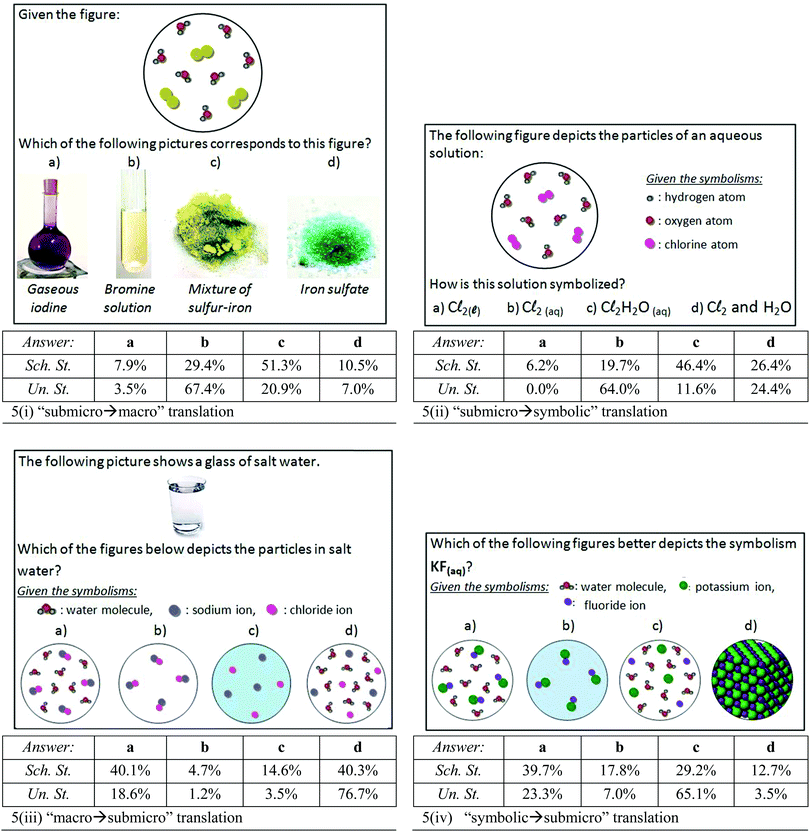 | ||
| Fig. 5 Questions asking for translation between various representations concerning aqueous solutions. | ||
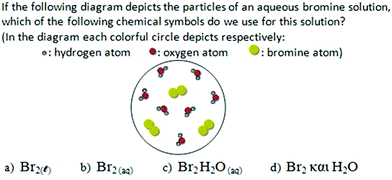
For the “submicro → symbolic” translation [Fig. 5(ii)] a submicroscopic representation of an element's aqueous solution, consisting of water molecules and diatomic chlorine molecules, was given to students. The correct symbolization (“Cl2(aq)”) was chosen by 64.0% of the undergraduates and only 19.7% of the eleventh-graders [statistically significant difference: x2(1) = 72.63, p < 0.001]. Almost half of the eleventh-graders (46.4%) symbolized the submicroscopic representation as “Cl2H2O(aq)”, ignoring that the subscript “aq” includes the information about the presence of water. Respectively, this answer was selected by 11.6% of the undergraduates [statistically significant difference: x2(1) = 36.20, p < 0.001]. Furthermore, 26.4% of the eleventh-graders used the symbolization “Cl2 and H2O”, which shows that they ignored the use of the subscript “aq”. A similar percentage of undergraduates (24.4%) selected the same symbolization [not statistically significant difference: x2(1) = 0.15, p > 0.05], which shows that the issue of aqueous solutions’ symbolization has not been fully clarified among the 3rd year university students. Finally, 6.2% of the eleventh-graders used the subscript “l” by choosing the molecular type “Cl2(l)”, which implies either confusion between the concepts “liquid state of matter” and “aqueous solution” or confusion about the corresponding subscripts “l” and “aq”. These confusions have been eliminated among the undergraduates [statistically significant difference: x2(1) = 5.65, p < 0.05]. Students’ way of thinking in this question and the reasons for their performance will be further clarified in the qualitative part (see Appendix II – Card 3b).
Fig. 5(iii) presents a question asking students to translate the picture of a salt solution to an equivalent submicroscopic representation. The correct answer d was chosen by 40.3% of the eleventh-graders, while 76.7% of the undergraduates showed a statistically significantly better performance. Furthermore, 40.1% of the school students chose answer a, which depicts water and salt molecules. Among the undergraduate students, this wrong answer was statistically significantly decreased (18.6%), but not eliminated [x2(1) = 14.47, p < 0.001]. Finally, 14.6% of the school students took into account salt's dissociation into ions, but they did not recognize the presence of water molecules (answer c) and few others (4.7%) were not aware of either salt's dissolution or water molecules (answer b). These wrong answers (c and d) were much decreased among the undergraduate students.
The final question [Fig. 5(iv)] asks students to translate the symbolism “KF(aq)” to an equivalent submicroscopic representation consisting of water molecules and two types of ions. The correct answer [answer c in Fig. 5(iv)] was chosen by 29.2% of the eleventh-graders and 65.1% of the undergraduates [statistically significant difference: x2(1) = 41.32, p < 0.001]. Furthermore, the fact that 39.7% of the eleventh-graders chose answer a [Fig. 5(iv)] shows their unawareness of salt's dissociation into ions during its dissolution in water. It is remarkable that also 23.3% of the undergraduates chose the same answer, although the difference between the two groups is statistically significant [x2(1) = 8.41, p < 0.05]. Moreover, 17.8% of the eleventh-graders chose answer b [Fig. 5(iv)], which includes only salt molecules, and 21.7% chose answer d [Fig. 5(iv)], which depicts a crystal lattice. These answers may indicate that eleventh-graders do not interpret correctly the subscript (aq). The corresponding percentages among the undergraduates for the above wrong answers are statistically significantly decreased. Students’ notions about aqueous solutions, the subscript “aq” and salt's dissociation were thoroughly investigated in the qualitative part of the research and will be discussed later [see Appendix II – Card 1(e) and (f)].
A one-way analysis of variance (ANOVA) showed that there was a statistically significant difference depending on the study specialization that school students follow in high school [F = 67.435, df(2, 459), p < 0.001]. Specifically, students specializing in science and medicine showed the best performance (p < 0.001), while no significant difference (p = 0.212) was found between students specializing in humanities and engineering studies. A statistically significant difference between the two genders was found by one-way ANOVA, with the males showing better performance (F = 7.666, df = 464, p < 0.05).
4. Results and discussion of the qualitative research
The quantitative part was followed by the qualitative one carried out with 16 11th grade upper secondary school students, who had also participated in the quantitative research, using the semi-structured interview technique.In the first step of the interviews, we showed pictures of 6 materials to students (see Appendix II – Card 1) and we asked them to construct one submicroscopic representation and one symbolic representation for each material. Afterwards, we asked them to explain their thought process and provide description of their representations’ features.
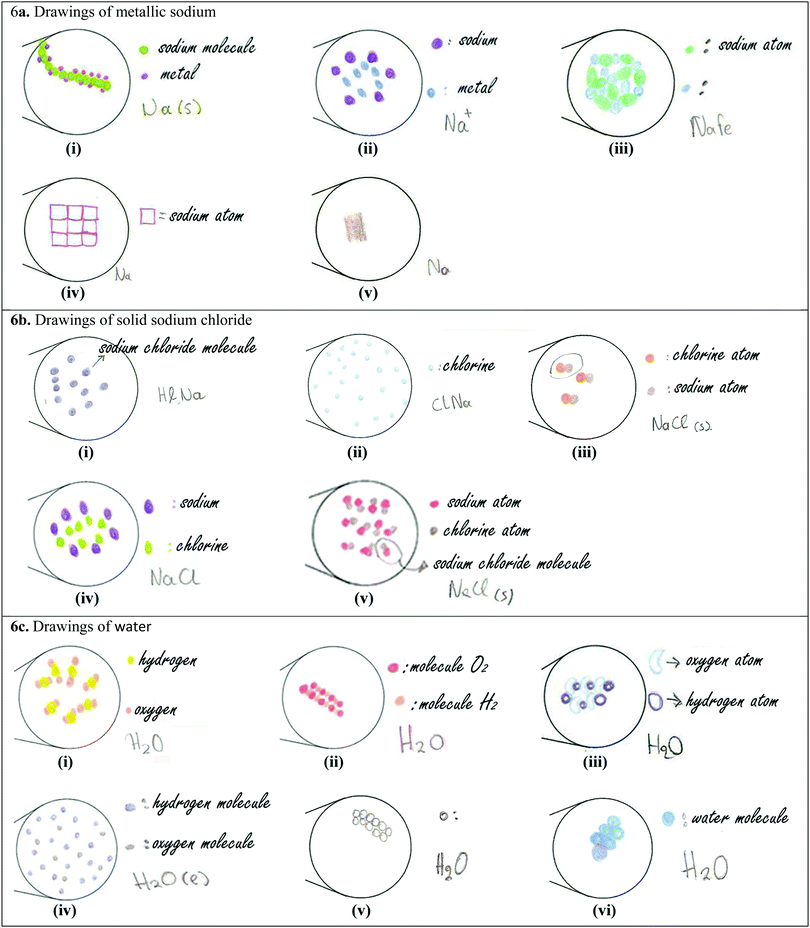
The 1st material [Appendix II, Card 1(a)] that we examined was metallic sodium, using the same picture with that of the quantitative part of the research [Fig. 2(ii)]. The equivalent submicroscopic representation should have two basic characteristics: (a) it should consist of one type of particle/circle and (b) the particles/circles should have a close arrangement. Only 6 students, out of the 16, created a correct representation containing the above characteristics. The remaining students made the following mistakes in their drawings: (a) they depicted metallic sodium using two or three types of circles [Fig. 6a(i)–(iii)]. The interviews revealed that these students had the misconception that the word “metallic” denotes the presence of a second component in the material, which gives the metallic properties to sodium. For example, Angela reasoned her drawing [Fig. 6a(i)] as: “Sodium's molecules are in green, they are circular and since it is metallic sodium I also drew these circles in purple for the metal and they are connected to each other. I thought that it (the material) consists of sodium and metal and I connected the two substances.” Furthermore, Emmanuel argued about his drawing [Fig. 6a(ii)]: “I drew purple spherical particles which correspond to sodium and each purple particle corresponds to a blue one which I think is the metal. […] They do not touch each other. […] They have some distance.” Nick stated about his drawing [Fig. 6a(iii)]: “We will have sodium in small particles which are connected to something else that makes it metallic. This something else I don’t know what can be […] I wouldn’t say that the particles are glued together, but they are very close.” This tendency to substantialize some properties of matter (i.e., they see the metallic property as the presence of a separable “material” which gives the metallic property to the material) is likely to hinder students’ ability to differentiate a substance from a mixture (Ngai et al., 2014). (b) Bill drew square atoms, [Fig. 6a(iv)], stating: “I drew sodium's atom square to differentiate it from chlorine atom [he is referring to his drawing of sodium chloride], randomly. But I believe […] they will definitely have a different shape.” (c) Mary drew a compact surface instead of particles [Fig. 6a(v)], explaining: “[We would see] a smooth surface, maybe with some small circles which are metallic sodium's particles.” This statement denotes the student's weakness to visualize the particulate nature of matter (Ben-Zvi et al., 1986; Nakhleh, 1992). (d) The six students drew sodium particles connected to each other, like in a chemical compound [e.g., Fig. 6a(i)], which echoes the misconception that atoms are always connected to each other in the solid state of matter (Adbo and Taber, 2009). (e) Emmanuel drew sodium particles in sparse distribution, such as in a gas state [Fig. 6a(ii)].
Concerning the symbolic representation “Na(s)” only 6 students, out of the 16, wrote the full symbolization, while 5 students wrote only “Na”, declaring that they don’t know the symbolization for the solid state. The remaining students wrote wrong symbols: (a) Christianna wrote “Na↓”, arguing: “the solid state of a material is symbolized by an arrow pointing down.” (b) Emmanuel and Rita wrote “Na+”. When Emmanuel was asked by the interviewer to explain what does the “+” mean, he responded: “…that (metallic sodium) is cation.” Rita initially wrote “Na” and the interviewer asked:
Interviewer: “Do you know any way to symbolize the physical state of this solid material?”
Rita: “By plus.”
Interviewer: “Can you write it?”
Rita wrote “Na+”.
Interviewer: “You put a plus like a superscript. What does it symbolize?”
Rita: “That it is solid? Either plus or minus.”
(c) Nick wrote “NaFe”, explaining: “I put sodium (Na) and metallic with ferrous (Fe).”
The 2nd material that we showed to students was solid sodium chloride [see Appendix II, Card 1(b)]. Only one student drew a correct submicroscopic representation containing two types of circles – one for sodium ions and one for chloride ions – in a close-packed structure. The remaining 15 students made various mistakes; the most important of them are the following: (a) five students drew only one type of particle/circle, as, for example, shown in Fig. 6b(i) and (ii), which they named it a “molecule of sodium chloride” or “particle of sodium chloride” or “sodium molecule” or “chlorine” or “small granule”. So, these students do not hold the scientific conception that chemical compounds are created by the connection of different atoms or ions, making no distinction between molecules and atoms/ions. For example, Maria argued about her drawing [Fig. 6b(i)]:
Maria: “It (The drawing of sodium chloride's particles) is similar to sodium's (drawing). But these (particles) are a bit sparser comparing to those of metallic sodium.”
Interviewer: “Describe in words what you drew.”
Maria: “Again molecules of sodium chloride. They are sparser because it is in powder form.”
Interviewer: “So, if we could see the particles we would see circles, namely small spheres?”
Maria: “Yes.”
Interviewer: “What were your thoughts in drawing this?”
Maria: “I thought that if we magnify it, it would look like this. That is the sense that I have.”
Adbo and Taber (2009) also reported students’ lack of understanding how a material comprised of different types of atoms could be a single substance. (b) Eight students used two types of particles, but they drew molecules consisting of sodium and chlorine atoms [Fig. 6b(iii)]. This is consistent with Taber's finding (1997), who also reported that students hold “molecular models” for ionic compounds. (c) Some others drew scattered circles for sodium and chlorine [Fig. 6b(iv)], or closely packed structures of sodium and chlorine atoms but in a random ratio. The above mistakes indicate how demanding it is for students to combine and understand multiple information at the same time (i.e., the number of elements, the type of the compound (molecular or ionic), the distance between particles, the ratio between particles, etc.). As Johnstone (2000) and Taber (2009) also reported, the visualization of the particulate nature of matter and the transition from one level of chemistry into another are very challenging tasks for students. (d) Furthermore, 7 students were confused by the form of granules, arguing:
Irene [Fig. 6b(ii)]: “I put the particles of sodium chloride sparser because it is not exactly solid, it is in powder, so the elements are not so much connected.”
Interviewer: “The distances between the particles in this case are the same as in metallic sodium?”
Irene: “No, the particles are sparser in sodium chloride, because it is not as solid as metallic sodium.”
Danae [Fig. 6b(iii)]: “About sodium chloride's particles, I’m not absolutely sure. I thought that sodium chloride is basically chlorine and sodium and that one chlorine atom is connected to one sodium atom. However, it is in a solid form, but there are small, small (she said small twice) granules and it is not a compact thing. I drew it like this and chlorine and sodium are connected here.”
Interviewer: If it was a compact thing what would you draw differently?
Danae: “Probably, I would draw these (particles) in line, one next to the other, to touch each other, like I drew them here (she shows metallic sodium).”
Emmanuel [Fig. 6b(iv)]: “I believe that they (particles) touch each other, because as I can see in the picture it is in powder, so they are granules, so as I imagine it, there is not a chance that they are not connected to each other.”
This confusion about the form of granules has been previously reported in the literature. Taber (2009) mentioned that the term particle is itself misleading for some younger students, who have been shown to consider that grains of salt or sugar are the particles that their teachers refer to, rather than hypothetical particles at a considerably smaller scale. We should also point out that although some students had a sufficient theoretical background, they could not construct a correct submicroscopic representation. For example, Anastasis knew that sodium chloride has a crystalline form, but he drew molecules instead of ions [Fig. 6b(v)]. In addition, he attributed macroscopic characteristics such as hardness to the particles:
Anastasis [Fig. 6b(v)]: “At first we know that sodium chloride is the table salt, which is also solid, but it is in crystals, so I don’t think that it is exactly as metallic sodium, but despite this, the bonds will be very strong.”
Interviewer: “The fact that the bonds are strong what effect does it have to how particles would be?”
Anastasis: “They will be solid and quite hard, like a crystal salt. This is how I think about it.”
Interviewer: “Explain to me your way of thinking. How did you think to make this drawing?”
Anastasis: “I thought that I know the chemical formula of sodium chloride, which if I am correct is one sodium atom and one chlorine atom. […] So I put in pairs one atom of one color and one atom of another color. I put them in pairs and very close to each other.”
Interviewer: “Why the pairs are very close to each other?”
Anastasis: “Because it is in solid form.”
The above finding about students’ confusion concerning particles’ properties is consistent with previous research reports, according to which students fail to fully appreciate how the particles exhibit different properties in relevant materials and they commonly adopt a type of pseudo-explanation (Taber, 2009). So, as Ben-Zvi et al. (1986) and Chandrasegaran et al. (2007) also reported, students consider that the particles that make up the materials are hard or soft, warmer or cooler, sharp, conducting, square, etc.
Concerning sodium chloride's symbolic representation, only 4 students wrote the correct symbolization “NaCl(s)”. The remaining students had problems for both (molecular formula and solid state's symbol), writing: (a) “NaCl ↓”, symbolizing the solid state by “↓”, (b) “ClNa”, (c) “NaCl2(s)”, arguing: “…subscript “2” symbolizes sodium's oxidation number”, and (d) “Cl, Na”, claiming, “…chemical compounds are symbolized by dividing the chemical symbols by a comma.”
The 3rd material [Appendix II, Card 1(c)] that we examined was water. Although students had extensively studied water in previous years, only 5 of them could generate a correct submicroscopic representation and only 3 of these 5 could precisely name the involved particles. In students’ drawings the following mistakes were recorded: (a) Emmanuel drew molecules in a reverse ratio of hydrogen and oxygen atoms [Fig. 6c(i)]. (b) Angela depicted particles in close arrangement like in a crystal lattice [Fig. 6c(ii)], explaining: “I know that water consists of hydrogen and oxygen … I connected hydrogen and oxygen.” This statement indicates that she does not understand the liquid state of matter (Adbo and Taber, 2009). (c) In some cases the particles were not circular, like in the drawing of Bill [Fig. 6c(iii)], who explained:
Bill “… We have analogy 1 oxygen atom for 2 hydrogen atoms. Hydrogen atoms are by dark blue circles and oxygen atoms are by light blue with the shape of a bean. […] It is like a chain, one next to the other, in a line, they are joined together in the line. It is a chain where each time we have 2 hydrogens and 1 oxygen. An oxygen never touches another oxygen, it is always in the middle with a hydrogen bond […]. Below there is another line and another line.”
(d) Georgia drew two types of circles sparsely arranged to each other like being a mixture [Fig. 6c(iv)]. This is an excerpt from the interview with Georgia:
Interviewer: “We are talking about water's particles. What kind of particles does water consist of?”
Georgia: “Hydrogen and oxygen.”
Interviewer: “And how are these particles arranged?”
Georgia: “They are not totally sparse, as they were in the previous one (she means sodium chloride), but they are more condensed, closer to each other.”
Interviewer: “Are they closer or connected to each other?”
Georgia: “No, they are just closer.”
Interviewer: “Explain to me what each particle that you drew is.”
Georgia: “Each blue dot is 1 hydrogen molecule and each grey dot is 1 oxygen molecule”. […].“Hydrogen's atoms are more than oxygen's. “Everything are very close to each other, but not joined together.”
(e) They drew one type of circle [Fig. 6c(v) and (vi)], which implies that they match each substance with one type of circle. (e) They sketched macroscopic characteristics such as drops or bubbles. (f) They didn’t draw anything. For example, Mary argued: “…even if we used a magnifying glass to see the particles, we would not see anything.” The latter two mistakes show students’ difficulty in adopting the particulate model of matter, and have been reported in the early literature (Ben-Zvi et al., 1986; Nakhleh, 1992).
With regard to the symbolic representation of water, only 6 students wrote correctly the full symbol “H2O(l)”, while 9 students wrote only the molecular formula “H2O”, because as they stated they didn’t know the symbol for the liquid state. Interviews revealed that many students could not explain what the formula “H2O” they had written expresses, declaring that they had just memorized it, while some others had the misconception that the subscript “2” refers to oxygen. We should point out that there were cases where although students could interpret the molecular formula, they couldn’t draw the equivalent molecules! For example, out of the 11 students who mentioned that the subscript “2” refers to hydrogen, only 5 of them generated the equivalent molecules, while the others either drew molecules in a reverse ratio of hydrogen and oxygen or they did not draw molecules at all. As emerged from the interviews, the dissimilarity between students’ ability to symbolize water and their ability to construct a submicroscopic representation for it, among others, is due to the fact that students memorize chemical symbols without conceptually understanding their meaning. Below, we present some characteristic examples:
About Emmanuel's Fig. 6c(i):
Interviewer: “What does each of these symbols symbolize?”
Emmanuel: “H symbolizes hydrogen and O symbolizes oxygen.”
Interviewer: “What about number 2?”
Emmanuel: “2 is oxygen's anion (he means oxygen's charge) which goes to hydrogen.”
Interviewer: “Does it mean something or we just put it to make the formula? Does it have any meaning when we put it at the formula?”
Emmanuel: “We have two hydrogens.”
About Bill's drawing, Fig. 6c(iii):
Interviewer: “What does each symbol that you used symbolize? (Bill wrote H2O.)”
Bill: “H symbolizes hydrogen's atom, 2 (symbolizes) that there are two (hydrogen atoms) and O (symbolizes) oxygen, which is one.”
Interviewer: “Do you know any way to symbolize the physical state, i.e. which is solid, which is liquid and which is gaseous? […] That this is a liquid material?”
Bill: “[…] It doesn’t come to my mind.”
About Rita's Fig. 6c(v):
Interviewer: “Explain to me analytically what each symbol that you used symbolizes.”
Rita: “H symbolizes hydrogen and O symbolizes oxygen.”
Interviewer: “What about 2?”
Rita: “2 comes out of types/formulas, but I don’t know what it symbolizes. It comes out of formula.”
About Stephanie's Fig. 6c(vi):
Interviewer: “What does each of these (symbols) symbolize?”
Stephanie: “Basically I have learnt it from chemistry.”
Interviewer: “Can you assume what each of these (symbols) is?”
Stephanie: “That two hydrogens are contained in oxygen? Something like that?”
The 4th material [Appendix II, Card 1(d)] that we examined was gaseous oxygen. Only 5 out of the 16 students created a correct submicroscopic representation and only 4 of them named precisely the involved particles. Other students’ representations enclosed the following mistakes: (a) they drew oxygen atoms scattered. For example, Georgia [Fig. 7a(i)] explained: “The particles are very sparse between them. One is far away from the other and they are oxygen's molecules.” Emmanuel [Fig. 7a(ii)] supported: “In the balloon we would see some spherical particles, white, which would symbolize oxygen. They wouldn’t be joined together. […] I believe that if they join together it will be either solid or liquid. So, since it is gas the particles are scattered.” The students’ statements demonstrate that they do not have a sound understanding of matter in the three different states and this finding is in agreement with Adbo and Taber's findings (2009). (b) Angela [Fig. 7a(iii)] sketched two types of circles sparsely to each other, like in a mixture, arguing: “I put oxygen's molecules. […] I just know that in atmosphere there is also nitrogen and I also put nitrogen.” This confusion is in agreement with reports that some students confuse gaseous oxygen with atmospheric air, or they believe that among the oxygen particles there is matter, i.e., air (cf.Griffiths and Preston, 1992). (c) Some students drew oxygen particles in close arrangement. For example, Bill [Fig. 7a(iv)] explained:
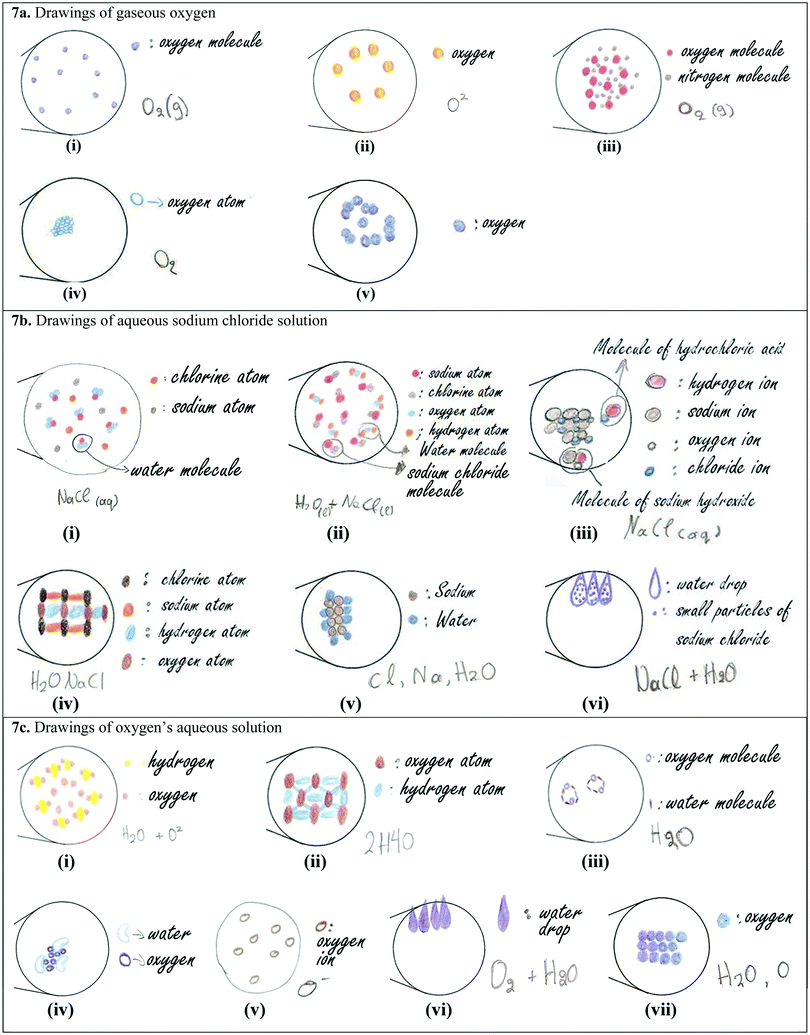 | ||
| Fig. 7 Examples of students’ generated drawings and symbolic representations for a gaseous material and two aqueous solutions. | ||
Bill:“(Oxygen's particles) will be like small balls glued to each other.”
Interviewer: “How did you think to make this drawing?”
Bill: “All the symbolisms that I have seen for water, usually are with circles and for air are with small balls. So, I imagined it like this and I believe that since they (particles) are in a balloon, in a specific space, they will be compressed and thus the one will be on the other.”
Bill's explanation shows that he does not have accurate notions about particles’ distances when a gas is under higher pressure than the atmospheric one. It also shows that he transfers the macroscopic property of the pressure to the particles, considering that they are “compressed” (Ben-Zvi et al., 1986; Taber, 2009).
(d) Stephanie stated for her drawing [Fig. 7a(v)]: “There would be caged molecules and particles in a specific space.”
Interviewer: “How many types of particles do we have?”
Stephanie: “One.”
Interviewer: “I see that some circles are separate, some are joined per two, and some are joined per three.” “Can you explain this to me?”
Stephanie: “Basically I imagine that is very difficult to see the particles.”
Interviewer: “It is impossible to see them.”
Stephanie: “So, I drew them like this (she means separately), or joined per two, or joined per three.”
Interviewer: “Did the fact that the material is gaseous give you any information?”
Stephanie: “The blue, basically their (particles’) color.”
Interviewer: “All gases are blue?”
Stephanie: “No. Basically the oxygen.”
(e) Three students stated that they cannot imagine the particles or that even by using a super magnifying glass they wouldn’t see anything.
Regarding oxygen's symbolic representation, 6 students successfully wrote the full symbol “O2(g)”, while 4 students wrote only “O2”, not knowing the symbol for the gaseous state. The remaining students used symbols such as “O2 ↑”, “O”, “O ↑” or “O2”. We should point out that only 6 out of the 12 students who wrote correctly the formula “O2” could interpret accurately the meaning of the subscript “2”. The majority of the remaining students declared that they didn’t know the meaning of it.
The 5th material that we showed to students was aqueous sodium chloride solution [Appendix II, Card 1(e)]. The visualization and the construction of an equivalent submicroscopic representation proved to be significantly difficult for the students, as expected according to the quantitative results. It is indicative that no student managed to generate a correct particulate drawing. It is also remarkable that only 7 out of the 16 students recognized the presence of water as a component in the solution. The most significant mistakes made by students are the following: (a) Danae [Fig. 7b(i)] drew water molecules, sodium atoms and chlorine atoms. She argued: “we will have water molecules which are everywhere and since it is sodium chloride solution, we will have chlorine and sodium atoms, but they won’t be joined together, they will be scattered in the water.” (b) Some students claimed that in the aqueous solution there are sodium chloride molecules. For example, Anastasis [Fig. 7b(ii)] supported: “[…] since we have aqueous solution it means that sodium chloride is dissolved in water. Simultaneously, it means that it is in liquid state, so comparing to the previous case, where we had pure sodium chloride, here, they will be sodium chloride molecules in little more distance between them and water molecules.” (c) Efthimis [Fig. 7b(iii)] depicted molecules of hydrochloric acid and molecules of sodium hydroxide, believing that during dissolution a chemical reaction takes place. He explained: “Sodium chloride is a solid material. Here, this (solution) is transparent. This means that sodium chloride dissolved in the water after some time. So, (at first) we had these crystals and gradually the water took some pieces. Let's say that hydrogen took the chlorine to form hydrochloric acid, i.e. a hydrogen is released from the water molecule and it goes and attracts chlorine to form hydrochloric acid, while one hydroxide leaves another water molecule and takes sodium to form sodium hydroxide.” (d) Nick [Fig. 7b(iv)] drew a grid of sodium, chlorine, hydrogen and oxygen atoms, claiming that during dissolution water molecules and solute's molecules create a grid. Specifically, he stated: “I put sodium chloride again, and in between I put water, i.e. it will be like layers. […] The particles will be mixed. […] Sodium chloride dissolves in water, so it will lose its form and it will be a mixing.” All the above mistakes show that students have significant knowledge deficiencies and alternative notions about the phenomenon of dissolution in water. As shown by the students’ explanations mentioned above, they use various modes of reasoning while they are struggling to correlate the macroscopic phenomenon of dissolution with the particulate model of matter. (e) Stephanie initially pictured one type of circle for the sodium, but during the interview she added a second one for the water. She drew the particles in close arrangement and she attributed macroscopic characteristics to them influenced by the solid sodium's color and the container's shape [Fig. 7b(v)]. Stephanie's conversation with the interviewer is as follows:
Stephanie: “(I drew) some particles connected to each other in grey color.”
Interviewer: “How did you think that?”
Stephanie: “From the shape that it has in the picture. […] The shape (of the container) is somehow rectangular, so they (particles) take the shape of the container.”
Interviewer: “And the color?”
Stephanie: “From sodium (she showed the picture of solid sodium).”
Interviewer: “Are the particles connected to each other or they just touch each other?”
Stephanie: “They touch each other.”
Interviewer: “How do you know this?”
Stephanie: “Because it's liquid.”
Interviewer: “What kind of particle do you think that there are in this (sodium chloride solution)?”
Stephanie: “Sodium and water?”
Interviewer: “As I can see you drew only sodium's particles.”
Stephanie: “Now that I’m looking better at it, it is liquid and transparent, so it will contain water.”
Interviewer: “Can you draw water's particles?”
Stephanie: (she added blue circles) “One blue circle completes the other (she means the grey). They will be together.”
(f) Christiana sketched tangential water drops containing one type of particle that correspond to sodium chloride particles [Fig. 7b(vi)]. She explained: “There are water drops and into these drops there are small salt's particles, sodium chloride's particles. […] (I thought that) in order to make salt water we put water with salt and I imagined that it (salt) cannot be totally dissolved and some small particles will remain.” Christiana's explanation reflects the notion that atoms are embedded in matter. This is in agreement with the literature report that the students adopt a hybrid model of matter at the sub-microscopic level, where atoms supplement rather than constitute the basis of what appears as a continuous matter at the macroscopic level (Renström et al., 1990).
Regarding the symbolic representation of aqueous sodium chloride solution, only 2 students wrote the correct symbolization “NaCl(aq)”. The remaining students wrote symbolizations such as: (a) “NaCl(l)” using the symbol “l”. For example, Georgia stated: “(I put) subscript ‘l’ because the solution is liquid”, and (b) Irene wrote “ClNa↓”, supporting: “symbol ‘↓’ is for liquid.” (c) Vassilis wrote “Na+, Cl−, explaining: “[…] there are dissolved ions, so I put ‘+’ in the one and ‘−’ in the other, so when they combine they will be neutralized. (d) Anastasis wrote “H2O(l) + NaCl(l)” and (e) Nick wrote “H2ONaCl”.
The 6th and final material [Appendix II, Card 1(f)] that we examined was oxygen's aqueous solution. Three out of the 16 students constructed a correct submicroscopic representation, while only 8 depicted particles presenting the water. The most considerable mistakes that we recorded from the explanations of the students are the following: (a) Emmanuel [Fig. 7c(i)] depicted oxygen particles by one circle, considering it as a monoatomic substance. (b) Some students drew structures in which the water molecules were joined with oxygen atoms, because they believe that during dissolution water is joined to oxygen [Fig. 7c(ii)–(iv)]. Specifically, Nick [Fig. 7c(ii)] explained: “[…] in water molecules we will have more oxygen, i.e. there may be 2 hydrogens and 4 oxygens, or 1 hydrogen atom with 3 oxygen atoms or another combination.” Maria [Fig. 7c(iii)] argued: “I drew oxygen's molecules with water because it is in liquid form. […] They are connected to each other.” Bill [Fig. 7c(vi)] explained: “[…] it is a connection between water and oxygen […] one water molecule is connected with 2 or 3 oxygen's circles.” (c) Vassilis sketched oxygen ions [Fig. 7c(v)], believing that during dissolution oxygen molecules dissociate into ions. In particular, he stated: “[…] we put gaseous oxygen in water and it was dissolved. […] So, oxygen's bonds will be broken and there will be free atoms.” (d) Christiana drew only water drops close to one another [Fig. 7c(vi)], stating: “[…] I thought that oxygen is gaseous so there will be no change to the form of water. There will be water maybe with a little different color or taste or something like that.” (e) Mary declared: “…even if we used a magnifying glass to see the particles, we would not see anything” (Novick and Nussbaum, 1981; Gabel et al., 1987; Treagust et al., 2003; Nyachwaya et al., 2011). (f) Stephanie [Fig. 7c(vii)] attributed macroscopic characteristics to the particles, influenced by the color of the substance and the shape of the container (Ben-Zvi et al., 1986; Griffiths and Preston, 1992). Specifically, she argued: “Again, (I make the drawing) in regard to the shape of the container … Maybe, I wouldn’t make them (particles) grey (initially she had drawn grey circles), I would make them blue because now that I’m noticing better the caption that says oxygen's solution (she changed the color of the circles to blue). In the beginning I put grey color because I saw the picture which is a bit dark … I’m trying to form container's shape.” The latter two students’ explanations indicate that they have not adopted yet the particulate model of matter.
Concerning the symbolic representations of oxygen's aqueous solution, only 2 students wrote the complete symbolization “O2(aq)”. The remaining students wrote incorrect symbols such as “H2O(l) + O2(l)”, “H2O + O2”, “H2O + O”, “H2O + O2”, “O2(l)”, “O↓”, and “H2O” (Fig. 7c).
To sum up the results found regarding the phenomenon of dissolution, the data of the qualitative study confirm those of the quantitative one, which reveal that students face many difficulties: some students do not recognize the presence of water molecules in aqueous solutions, and some others believe that during dissolution water molecules are joined to solute's molecules forming complex structures. Some students think that solute's molecules break down into atoms, while others believe that they break down into ions. There are students that fail to differentiate the liquid state of matter from aqueous solutions. In particular, in the case of salts’ dissolution, students’ translations of representations concerning aqueous ionic solutions reveal that they hold “molecular models” for ionic compounds as Taber also concluded (1997).
A common finding from the whole qualitative research is that when we asked students to symbolize the given materials they had great difficulties in generating symbolic representations. Moreover, they have significant knowledge gaps concerning the meaning of molecular formulas and the symbolization of the physical states of matter and the aqueous solutions; that is, they were not aware of the symbols’ conventions. When translating symbolic representations, if the students recognize a chemical substance and they know its particulate structure, then they choose the correct submicroscopic representation. In the opposite case, they cannot infer the chemical composition from the symbolic representation. In many cases, it was shown that students memorize molecular formulas (e.g., H2O, O2) without understanding their meaning. This explains the fact that often there was no correlation between the symbolic representations (e.g., molecular formulas) and the submicroscopic ones (e.g., relevant molecules) drawn by the students. In some cases students’ difficulties were coming from holding misconceptions, confusion or deficient understanding of the relevant concepts, e.g., the phenomenon of dissolution in water, and the differentiation between chemical elements, chemical compounds and mixtures. That is fully aligned with a literature report (Taber, 2009), according to which: “Not understanding the ideas, or holding alternative conceptions for basic chemical concepts, makes it easy to misinterpret what is represented through the language. Not understanding the subtleties of the symbolic language makes it difficult to learn the ideas.”
Step 2 of the interview
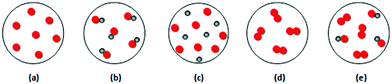
In order to further clarify some of the quantitative results and to explore students’ notions and their way of thinking during translating submicroscopic representations, we gave the 5 submicroscopic representations shown in Fig. 8 to the 16 students and asked them to characterize which one represents a chemical element, which one a chemical compound and which one a mixture, and to explain the reasoning behind their decision.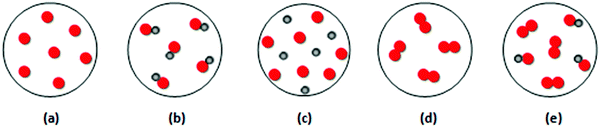 | ||
| Fig. 8 Submicroscopic representations that students should characterize each one as a chemical element, or a chemical compound, or a mixture. | ||
The students’ performance in interpreting representations was much better than that in constructing ones. However, some important misconceptions were recorded regarding the concept “chemical compound”. For example, Georgia stated: “Fig. 8dis a chemical compound because the circles are joined together.” Mary argued: “Fig. 8eis chemical compound because gray little balls are joined to orange little balls and this shows a compound.” Emmanuel supported: “Fig. 8eis chemical compound because there are connections between similar and between different atoms.”
It is obvious that some students believe that: (a) when atoms are joined together, regardless of whether they are of different kind or not, the material is a chemical compound (e.g., Fig. 8d and e), and (b) if chemical compounds coexist, then the material is also characterized as a chemical compound (e.g., Fig. 8e).
Regarding the concept “chemical element” some students argued that when atoms are not joined together, even if they are of different types, the represented material is a chemical element (e.g., Fig. 8c). For example, Nick supported: “Fig. 8cis a chemical element because the particles are not joined together, there are two different types (of particles) which coexist.” As for the concept “mixture” the most important misconception found was that when two different types of atoms coexist, regardless of whether they are joined together or not, the material is a mixture (e.g., Fig. 8b). As Irene explained, “Fig. 8bis a mixture because it contains an additional chemical element (she means comparing toFig. 8a). I saw that from the black circles, which are joined to the red circles.”
These findings contribute to previous reports on students’ difficulties in differentiating between the concepts “element”, “compound”, and “mixture”. Stains and Talanquer (2007a; 2007b) reported that students associate identical isolated atoms with elements, and bonded particles (molecules) with compounds. Adbo and Taber (2009) reported that students do not appreciate the difference between atoms and molecules, arguing that an understanding of the nature of pure substances at the sub-microscopic level requires an appreciation of the relationship between atoms and molecules or ions. When students lack this understanding, they are not able to appreciate how a material composed of different types of atoms could be a single substance (Briggs and Holding, 1986, in Adbo and Taber, 2009).
To investigate the reason for the high percentage of students’ wrong responses to the question in Fig. 4(ii) of the quantitative part we asked the 16 students to choose which one of the 5 representations in Fig. 8 is likely to depict the particles of a gaseous substance symbolized as “HI” and to explain their thought process. Ten students answered correctly representation b, while the remaining 6 students chose the wrong representation c. The interviews revealed that these students hold the misconception that in a gaseous substance the atoms are not joined together. For example, Angela argued: “HI corresponds toFig. 8cbecause, if we assume that red (particles) are hydrogen and grey iodine, since it is gaseous, they are not joined together.” This misconception is consistent with Abdo and Taber's finding (2009) who also reported that students did not seem able to conceptualize that a gas could comprise of molecules and they rather consider the gaseous state as an atomic state, where there is no bonding between the atoms.
Step 3 of the interview
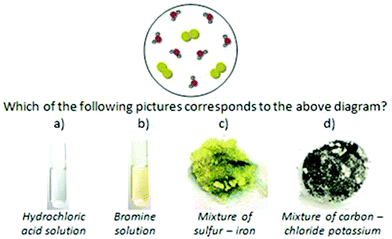
In order to further investigate the reasons for the high percentages of students’ wrong answers to the questions in Fig. 5(i) and (ii) of the quantitative research and the students’ way of thinking, we carried out Step 3. We showed to students Fig. 9(i) and asked them to describe the main features of the submicroscopic representation and explain on which features they were based in order to make the correlation with the macroscopic one. The fact that 9 students (out of the 16) chose wrongly either c or d in Fig. 9(i) supports the hypothesis that we have made from the quantitative analysis that some students associate the color of the particles with the color of the materials. Another conclusion recorded in the previous quantitative phase that students have no sound understanding of matter in the three different states was also confirmed, with the students claiming that the material must be either solid or liquid, because the atoms in the representation are joined together. Adbo and Taber (2009) also reached the above conclusion. Here are excerpts from interviews with 2 students.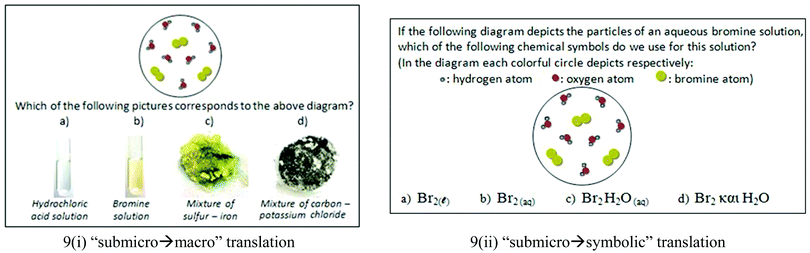 | ||
| Fig. 9 Questions asking for translation between various representations concerning aqueous solutions. | ||
Christiana: “The correct answer to question shown inFig. 9(i) is c.”
Interviewer: “What do you think that colorful circles symbolize?”
Christiana: “The yellow (particles) is for sulfur because it is yellow and the red joined with two grey (particles) is for iron. Because iron … I don’t know, it fits better.”
Interviewer: “Which characteristic helped you to answer (the question)?”
Christiana: “That is yellow and solid.”
Interviewer: “Why the drawing with these circles corresponds to a solid? How do you understand this?”
Christiana: “Because they (particles) are not scattered, they are in pairs or per three.”
Georgia answered: “It's (b).”
Interviewer: “How did you think to choose (b)?”
Georgia: “Because it (drawing) has these yellow circles, which have the color of the bromine solution and there are water molecules which are three (atoms) joined together and they make the solution.”
Interviewer: “Which characteristic helped you to choose your answer?”
Georgia: “The fact that it is water, basically it is solution, it is liquid and at the drawing there are water molecules and some other molecules, which have the same color to the one in the picture for bromine.”
Interviewer: “Answer (a) also shows a solution. Why didn’t you choose this one?”
Georgia: “Because the drawing has the color.”
Finally, interviews revealed an alternative mode of reasoning in making a translation; some students chose picture d in Fig. 9(i), because its caption contains three different names of elements as three is the number of different circles in the submicroscopic representation. That is, their criterion to make the translation is the number of elements’ names, regardless of the chemical composition. We should point out that a common characteristic in all the above modes of reasoning is that students use only one criterion/variable in order to make the requested translation and, usually, this criterion is a meaningless extraneous feature, as has also reported by several other authors (Driver et al., 1985; Viennot, 2001; Stains and Talanquer, 2007b; Talanquer, 2018). This is obvious from Angela's explanation for her choice.
Angela: “It (the correct answer) is d.”
Interviewer: “What do you think that colorful circles symbolize?”
Angela: “There are three different (circles), carbon, chlorine and potassium.”
Interviewer: “How did you think to choose your answer? From where did you start: from the pictures or from the drawing?”
Angela: “I saw that the drawing has 3 different elements. Picture (a) has hydrochloric acid, i.e. water and chlorine, while the drawing has 3 (elements). Bromine solution is one element and that's why I rejected it. And the mixture of sulfur-iron has 2 elements, while d has 3.”
Afterwards, we examined the question shown in Fig. 9(ii). Specifically, students were informed with the name of the aqueous solution depicted by the submicroscopic representation and they were asked to choose one out of the four symbolic representations that correspond to this name, explaining their reasoning. Some examples from students’ explanations follow:
Maria: “Br2(l)and Br2(aq)have only bromine. But, it (the drawing) consists of hydrogen and oxygen atoms, so here [she points Br2H2O(aq)] it has both hydrogen and oxygen. Answer d (Br2and H2O) means that it is a compound, they are together.”
Interviewer: “Do subscripts (l) and (aq) play any role?”
Maria: “Yes, they show us their form. I think that (aq) is for liquid state.”
Angela supported: “It's (c), because (a) has only bromine, while we see that the drawing also has oxygen and hydrogen atoms. For the same reason I rejected (b). Answer (d) has hydrogen and oxygen atoms, but it doesn’t have (aq) which is the characteristic for aqueous solution.”
Georgia explained: “It's (a) because Br2is for bromine and there is (l) for liquid. So, I don’t think that we need to put something more, such as the water, that there is in the rest (symbolizations).”
Interviewer: “What does subscript (l) symbolize?”
Georgia: “The liquid.”
Interviewer: “Do you know what subscript (aq) symbolizes?”
Georgia: “No.”
Interviewer: “Do you think that this material is liquid?”
Georgia: “Yes.”
Interviewer: “Why? How do you understand this?”
Georgia: “Because it says aqueous solution.”
From the students’ explanations it is clear that many students do not know the meaning of the subscript “aq”, some students do not know the difference between aqueous solution and the liquid state of matter, some students believe that the subscript “aq” corresponds to the liquid state of matter, while some students do not know the meaning of aqueous solution, i.e., that it contains water. These conclusions are in agreement with a previous report (Taber 2009).
5. Conclusions
This study was carried out to investigate 11th grade school students’ and 3rd year undergraduate chemistry students’ competence in translating different types of chemical representations. What differentiates the present study from others in the same field is that we examined a frame of four basic concepts (chemical element, chemical compound, solid state of matter and aqueous solution) and we tried to combine all three levels of chemistry (macroscopic, submicroscopic and symbolic). As far as we are aware of, other researches focus either on a specific concept (for example, chemical reaction, chemistry apparatus, atom) or on a specific combination of levels (Keig and Rubba, 1993; Kozma and Russell, 1997; Treagust et al., 2003; Onwu and Randall, 2006; Chandrasegaran et al., 2007; Chittleborough and Treagust, 2008; Cook et al., 2008; Rappoport and Ashkenazi, 2008; Gilbert and Treagust, 2009a, 2009b; Ramnarain and Joseph, 2012; Waight and Gillmeister, 2014; Lin et al., 2016; Chi et al., 2018; Ye et al., 2018). As we thoroughly describe the results presented above, during the examination of students’ competence to translate between various representations, we found many students’ difficulties and/or misconceptions known from previous research, but we also found some new ones. Such a distinctive example is that many students have the alternative conception that the metallic property of a material (i.e., sodium) denotes the presence of a separable component which gives the metallic property to it. Another example is an alternative mode of reasoning used by some students in making translations; they match directly the number of elements’ names mentioned in a material's name (i.e., bromine's solution) to the number of different types of atoms in the submicroscopic representation, regardless of the chemical composition.According to the results described above, we come to the conclusion that the group of eleventh-graders seems to have a limited ability in translating chemical representations. They do not perceive chemical phenomena equally at macroscopic, submicroscopic and symbolic levels and this is in agreement with the findings of previous research on the subject (Johnstone, 1991; Johnstone, 1993; Potgieter et al., 2005; Onwu and Randall, 2006; Chittleborough and Treagust, 2008; Talanquer, 2011; Waight and Gillmeister, 2014; Chi et al., 2018; Scalco et al., 2018; Ye et al., 2018). Regarding the group of undergraduate students, their translation ability is better compared to that of secondary students, as expected; however, it cannot be considered satisfactory given their level of education. As shown in Table 1, students’ performance depends both on the concept and on the type of translation. The school students’ highest success rate was in a “submicro → macro” translation of a chemical element [Fig. 2(i)] and the lowest one was in a “submicro → symbolic” translation of a chemical compound [Fig. 4(i)]. The undergraduate students’ highest performance was in a “macro → submicro” translation concerning the solid state of matter [Fig. 2(ii)] and their lowest performance was in a “submicro → symbolic” translation of a chemical compound [Fig. 2(i)]. The most difficult concept for both groups was “aqueous solution” and the easiest concept was the “solid state of matter” (Table 1). Cross tabulation analysis showed that students’ ability to translate a representation from one level to another does not ensure that they can achieve the reverse translation. See, for example, that for the “symbolic → submicro” translation of a molecular compound, the school students’ success rate is 61.8% [(Fig. 4(ii))], while for the reverse one it is only 14.2% (Fig. 4(i)). It is concluded that translating between different types of chemical representations is a very challenging task depending on conceptual understanding (Ausubel, 1968).
| Type of translation | Figure no. | School students’ success rate (%) | Undergraduates’ success rate (%) | |
|---|---|---|---|---|
| a Taking into account students’ performance only at the specific concept. b Taking into account students’ performance at both concepts included in the same question. | ||||
| Chemical elementa | Submicro → macro | 2(i), p. 8 | 89.3 | 80.2 |
| Macro → submicro | 2(ii), p. 8 | 55.5 | 95.3 | |
| Ionic compounda | Submicro → symbolic | 2(iii), p. 8 | 65.7 | 89.5 |
| Molecular compound | Submicro → symbolic | 4(i), p. 10 | 14.2 | 59.3 |
| Symbolic → submicro | 4(ii), p. 10 | 61.8 | 81.4 | |
| Solid statea | Submicro → macro | 2(i), p. 8 | 71.4 | 88.3 |
| Macro → submicro | 2(ii), p. 8 | 87.3 | 100.0 | |
| Submicro → symbolic | 2(iii), p. 8 | 67.4 | 96.5 | |
| Chemical element & solid stateb | Submicro → macro | 2(i), p. 8 | 60.7 | 70.9 |
| Macro → submicro | 2(ii), p. 8 | 51.9 | 95.0 | |
| Ionic compound & solid stateb | Submicro → symbolic | 2(iii), p. 8 | 42.7 | 87.2 |
| Aqueous solution of an element | Submicro → macro | 5(i), p. 12 | 29.4 | 67.4 |
| Submicro → symbolic | 5(iii), p. 12 | 19.7 | 64.0 | |
| Aqueous salt solution | Macro → submicro | 5(ii), p. 12 | 40.3 | 76.7 |
| Symbolic → submicro | 5(iv), p. 12 | 29.2 | 65.1 | |
Various mistakes were recorded from students’ answers to the translation questions, which can attributed to deficiencies in knowledge or to alternative conceptions or lack of experience in such tasks. A thorough examination of students’ mistakes reveals some patterns in their responses that permit us to infer modes of students’ reasoning during the translation tasks. A mode of reasoning observed when students translated submicroscopic representations into macroscopic ones is that they fail to take into account two or more variables of the submicroscopic representation, such as the particles’ composition, the particles’ distances, and the number of different molecules. Concerning chemical elements, compounds and the solid state of matter, some undergraduates focus on features that depict the state of matter, while eleventh-graders on meaningless features like the color of the circles that depict particles. According to the literature, in many cases common sense learners tend to reduce the number of variables to be considered when deciding (Driver et al., 1985; Viennot, 2001). This mode of reasoning is known as a one-reason decision-making heuristic (Talanquer, 2018).
The symbolic level was proven to be very challenging and demanding for 11th grade students. It is surprising that even though we examined fundamental symbolic representations (i.e., chemical symbols, molecular formulas and symbols for the states of matter and aqueous solutions, taught already in the 8th grade) the students faced remarkable difficulties in using these representations and in correlating them with submicroscopic and macroscopic representations. In many cases mistakes were made because students were not fully aware of the symbols’ conventional meaning or because they had misconceptions about the underlying concepts. These difficulties among the Greek students may be reinforced by the fact that, according to Greek curriculum, the symbolic representations for the examined concepts are briefly reviewed. Furthermore, chemical symbols may generate extra misinterpretations to novices because, often, the same symbols are used for both the macroscopic and sub-microscopic levels, as also reported by Taber. For example, H2 is used to symbolize a molecule and also a substance. As it emerged from the present study, for novices symbolic representations are another layer of complexity and, usually, they focus on the incidental aspects of the formalism used. The origin of the difficulties related to the symbolic level of chemistry was described by Taber (2009): “The symbols used in chemistry are not just labels for words, but they are closely linked to concepts. Chemistry students are similar to learners of a second language, where they are expected to be both learning the language and using the language to understand substantive material simultaneously.”
From the findings of a number of authors (Novick and Nussbaum, 1981; Gabel et al., 1987; Treagust et al., 2003; Nyachwaya et al., 2011) it is known that students face great difficulties in adopting the particulate model of matter and in abandoning the continuous one. This was clearly illustrated in the present study, as well, since some students still attribute the macroscopic characteristics of a material to its particles or they struggle to visualize the particulate nature of matter. Our results allow us to consider that students have not fully adopted the particle model of matter, but they are in a transition process from the macroscopic to the particulate view of matter. Some students are closer to the macroscopic view and some others are closer to the particulate. Previous research has also shown that even university students may not have developed the mental models needed to facilitate effective thinking about the sub-microscopic world (Chittleborough et al., 2002). An explanation for the observed results could be the shallow learning in the introductory courses. If learning for some students is based on rote memorization rather than on deep understanding of basic chemical concepts, it is likely that they will revert to their prior conceptions or knowledge later on (Stains and Talanquer, 2007b).
The present research shows up the complexity in translating various chemical representations even in very simple concepts. The moving among the different levels of chemistry proved to be a very challenging task, not only for students who had knowledge deficiencies, but also for those who had the appropriate background knowledge. Therefore, it is not assured that students can apply or transform an existing form of knowledge into a different form. Generally, in our research, it was shown that students face challenges in interpreting the given representations by obtaining appropriate information either to construct or to interpret a target representation. It seems that the students' difficulties in the identification, differentiation and translation of chemical representations still remain even after students’ training in Chemistry. Furthermore, this research gave prominence to the possibility of translation questions to highlight students’ misconceptions.
6. Implications for teaching
We believe that the findings obtained from the present study make a contribution to our knowledge in evaluating students’ competence in using the three levels of representation in chemistry and in interpreting chemical representations. Too often teachers take it for granted that students can easily grasp the meaning of the representations and they are able to easily switch back and forth between levels of representation. This study has shown that this was far from the case when students were asked to translate between different types of representations. Therefore, teachers and textbook writers when they write textbooks and other educational material, or design instructional activities, should take into account research findings in science education – such as the revealed common patterns of students’ reasoning, the tendency of common-sense learners to reduce the complexity of a task by using a single variable and the tendency to pay attention to extraneous features. Our study suggests that special emphasis should be given to clarifying the meaning of chemical representations and in assuring that learners know how to read and decode the information included/enfolded in representations. Additionally, educators should bear in mind that novice learners struggle to consolidate the particle model of matter (Novick and Nussbaum, 1981; Gabel et al., 1987; Treagust et al., 2003; Nyachwaya et al., 2011) and, thus, they should extensively use submicroscopic representations when teaching this subject and thus will help students to enlighten the particle nature of matter and shape an accurate mental model for it. They should also thoroughly explain both the symbols’ conventions and the double role of the symbolic level, as suggested by Taber (2009, 2013) as well, because symbolic representations may increase the perceived complexity, and, thus, the cognitive demand of a task. Finally, teachers and textbook writers should consider how to get students think about the connections between the three levels of chemistry, because students cannot easily infer these connections by themselves. Moreover, they should use multiple representations including submicro, symbolic and macro representations, as advised also by Chandrasegaran et al. (2009), in order to help students understand that chemistry refers to three levels and they should also highlight the connections between these representations. So, successful incorporation of chemical representations in school textbooks should fulfill a set of minimum requirements, which are not always taken into account by textbook writers, as shown by previous research (Gkitzia et al., 2011; Scalco et al., 2018).Another issue that needs attention is the models that are used for the construction of chemical representations. As we all know, teachers and textbook writers use teaching models which are simplified versions (e.g., simple physical models, teaching analogies, and standard diagrams) of the corresponding scientific ones used by chemists (Gilbert et al., 2002). It has been shown that these teaching models are usually presented as unproblematic representations of nature, with no explicit acknowledgement that what is being discussed are models (Duit, 1991; Harrison and Treagust, 2000; Justi and Gilbert, 2000; Justi and Gilbert, 2002). It has long been recognized (Carr, 1984) that this can be a source of confusion for learners in chemistry, as students often see models as reproductions of reality (Justi and Gilbert, 2002). So, teachers and textbook writers should use multiple types of models (Ainsworth, 1999; Lin et al., 2016) and they should emphasize to learners the purpose, the limitations and the roles of these models.
A limiting factor in the implementation of the above suggestions, at least at Greek schools, is the narrow time frame provided for chemistry courses. Therefore, curriculum planners should take into account research findings in order to formulate syllabuses that support gradual progression of teaching subjects at levels of increasing complexity (Taber, 2009), either by increasing the available time frame or by reducing the teaching topics. Namely, syllabuses should provide to teachers the necessary time frame to explain the relevant concepts in all three levels of chemistry, to discuss about chemical representations and limitations of the teaching models, and to emphasize symbols’ conventions. Furthermore, curriculum planners should plan a periodical repetition of the particle model of matter and the basic chemical concepts and symbols, taking into consideration novice learners’ difficulties in the above topics. Only then will student learning be promoted with understanding rather than with accumulation of knowledge that is rote-learned, and thus, it could be considered as a robust foundation for supporting further learning.
The present study has also shown that translation questions are not only a valuable teaching tool in promoting meaningful understanding, but also a reliable tool in teachers’ hands to elicit students’ alternative conceptions and provide insight into students’ understanding of the chemical concepts, which is not easily evident by asking them to solve numerical problems where students can simply apply an algorithm (Salta and Tzougraki, 2011) or by using questions requiring recall of information (Kawalkar and Vijapurkar, 2013).
In conclusion, we would like to point out our consideration that further research is needed to explore the development of students’ understanding of key scientific concepts in different contexts, as well as the types of teaching schemes that might best support student learning.
Conflicts of interest
There are no conflicts to declare.Appendix I: the chemical concepts that 11th grade Greek students have been taught
11th graders have been taught the following chemical concepts: the three states of matter, homogeneous and heterogeneous mixtures, solution's concentrations, the particle model of matter, chemical elements, molecular and ionic compounds, chemical symbols and molecular formulas, periodic table, alkali metals, carbon, silicon, hydrocarbons, acids, bases, salts, Bohr atomic model, covalent and ionic bonds, oxidation number, chemical reactions and chemical equations, relative atomic and molecular mass, mole, Avogadro number, molar volume, ideal gas equation, molar concentration, solution's dilution and condensation and stoichiometry. Our research took place in the middle of the 11th grade, where all students, at that time, were studying the structure, the properties and the reactions of organic compounds, while the students who followed science and medicine specialization were studying, in addition, thermochemistry and chemical kinetics.Appendix II: cards and research questions used in the qualitative research
Card 1
Questions addressed by the researcher about Card 1:
(1) Using colorful circles, can you draw how you visualize the particles shown in Card 1?
Clarification in case the student cannot respond to the previous question:
(1*) What would we see if we had a super magnifying glass which could show us the particles of each material? For example, what kind of particle the metallic sodium consists of?
(2) Describe in your own words what you drew in each case.
(3) Explain your thought process in making each drawing.
(4) What is each one of the particles that you drew?
(5) How do you think a chemist would symbolize each material?
Clarification in case that the student did not/cannot respond to, or did not understand the previous question:
(5*) How is each material symbolized in chemistry?
(6) Describe your thought process prior to writing down each symbolism.
Additional question if necessary:
(6*) We explicitly ask the students to explain what each symbol and each number that he/she wrote symbolizes.
Additional question in case the student does not write a subscript for the physical state or the aqueous solution:
(6**) Do you know how the physical state of a material is symbolized? Do you know how the aqueous solution is symbolized?
Card 2
Questions addressed by the researcher about Card 2:
These drawings depict the particles of five materials.
(1) What kind of material is each one: chemical element, chemical compound or mixture?
(2) Explain your thinking process prior to choosing your answer.
Additional question if necessary:
(2*) What do you think the colorful circles symbolize?
(3) Which of the drawings’ characteristics led you to your answer?
(4) Which of the drawings would you use to depict the particles of a gaseous substance symbolized as “HI”?
(5) Explain your thinking process.
Card 3a
Questions addressed by the researcher about Card 3a:
(1) What do you think the colorful circles symbolize?
(2) Explain your thinking process prior to choosing your answer.
Additional question if necessary:
(2*) Which led you first to the correct answer, the pictures or the drawing?
(3) Which characteristic helped you find your answer?
Card 3b
Questions addressed by the researcher about Card 3b:
(1) How did you think in order to choose your answer?
Additional question if necessary:
(1*) Which led you first to the correct answer, the symbols or the drawing?
(2) Which characteristic of the card helped you to choose your answer?
References
- Adbo K. and Taber K. S., (2009), Learners’ mental models of the particle nature of matter: a study of 16-year-old Swedish science students, Int. J. Sci. Educ., 31(6), 757–786.
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ., 33, 131–152.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students’ mental models of atomic structure, Chem. Educ. Res. Pract., 17, 788–807.
- Ausubel D. P., (1963), The psychology of meaningful verbal learning, New York: Grune & Stratton.
- Ausubel D. P., (1968), Educational psychology: a cognitive view, New York: Holt, Rinehart and Winston.
- Ben-Zvi R., Eylon B. and Silberstein J., (1986), Is an Atom of Copper Malleable? J. Chem. Educ., 63 (1), 64–66.
- Carr M., (1984), Model confusion in chemistry, Res. Sci. Educ., 14, 97–103.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The Development of a Two-tier Multiple-Choice Diagnostic Instrument for Evaluating Secondary School Students’ Ability to Describe and Explain Chemical Reactions Using Multiple Levels of Representation, Chem. Educ. Res. Pract., 8(3), 293–307.
- Chandrasegaran A. L., Treagust D. F., Waldrip B. G. and Chandrasegaran A., (2009), Students’ dilemmas in reaction stoichiometry problem solving: deducing the limiting reagent in chemical reactions, Chem. Educ. Res. Pract., 10, 14–23.
- Chi S., Wang Z., Luo M., Yang Y. and Huang M., (2018), Student progression on chemical symbol representation abilities at different grade levels (Grades 10–12) across gender, Chem. Educ. Res. Pract., 19, 1055–1064.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38, 463–482.
- Chittleborough G. D., Treagust D. F. and Mocerino M., (2002), Constraints to the development of first year university chemistry students’ mental models of chemical phenomena, Paper presented at the 11th Annual Teaching and Learning Forum for Western Australian Universities, Australia: Edith Cowan University.
- Claesgens, J., Scalise, K., Wilson, M. and Stacy, A., (2009), Mapping student understanding in chemistry: the perspective of chemists, Sci. Educ., 93, 56–85.
- Cook M., Wiebe E. N. and Carter G., (2008), The influence of prior knowledge on viewing and interpreting graphics with macroscopic and molecular representations, Sci. Educ., 92, 848–867.
- Driver R., Guesne E. and Tiberghien A., (1985), Children's ideas in science, Milton Keynes, UK: Open University Press.
- Duit R., (1991), On the role of analogies and metaphors in learning science, Sci. Educ., 75 (6), 649–672.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gabel D., Samuel K. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 68 (8), 695–697.
- Gilbert J. K. and Treagust D. F., (2009a), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, Dordrecht: Springer, pp. 1–8.
- Gilbert J. K., and Treagust D. F., (2009b), Towards a coherent model for macro, submicro and symbolic representations in chemical education, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, Dordrecht: Springer, pp. 333–350.
- Gilbert J. K., Watts D. M. and Osborne R. J., (1985), Eliciting student views using an interview about-instances technique, in L. H. T. West, A. L. Pines and A. Leon (ed.), Cognitive Structure and Conceptual Change, London: Academic Press, pp. 11–27.
- Gilbert J. K., De Jong O., Jusit R., Treagust D. F. and Van Driel, J. H., (2002), Chemical education: towards research-based practice, Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Gkitzia V., Salta K. and Tzougraki C., (2011). Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12(1), 5–14.
- Greene J. C., Caracelli V. J. and Graham W. F., (1989), Toward a Conceptual Framework for Mixed-Method Evaluation Designs, Educ. Eval. Policy Anal., 11(3), 255–274.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Harrison A. G. and Treagust D. F., (2000), A typology of school science models, Int. J. Sci. Educ., 22(9), 1011–1026.
- Heitzman M. and Krajcik J., (2005), Urban seventh-graders’ translations of chemical equations: which parts of the translation process do students’ have trouble? Paper presented at the annual meeting of the National Association for Research in Science Teaching (NARST), Dallas, TX.
- Hoffmann R. and Laszlo R., (1991), Representation in chemistry, Angew. Chem., Int. Ed. Engl., 30, 1–16.
- Johnstone A. H., (1982), Macro- and micro-chemistry, Sch. Sci. Rev., 64(227), 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comp. Ass. Learn., 7, 75–83.
- Johnstone A. H., (1993), The development of chemistry teaching, J. Chem. Educ., 70, 701–705.
- Johnstone A. H., (1997), Chemistry teaching – science or alchemy? J. Chem. Educ., 74(3), 262–268.
- Johnstone A. H., (2000), Teaching of Chemistry – logical or psychological? Chem. Educ.: Res. Pract., 1(1), 9–15.
- Johnstone A. H., (2010), You can’t get there from here, J. Chem. Educ., 87(1), 22–29.
- Johnstone A. H. and Ambusaidi A., (2000), Fixed response: what are we testing? Chem. Educ.: Res. Pract., 1(3), 323–328.
- Justi R. and Gilbert J. K., (2000), History and philosophy of science through models: some challenges in the case of ‘the atom’, Int. J. Sci. Educ., 22 (9), 993–1009.
- Justi R. S. and Gilbert J. K., (2002), Modelling teachers’ views on the nature of modelling, and implications for the education of modellers, Int. J. Sci. Educ., 24 (4), 369–387.
- Kawalkar A. and Vijapurkar J., (2013), Scaffolding Science Talk: the role of teachers’ questions in the inquiry classroom, Int. J. Sci. Educ., 35(12), 2004–2027.
- Keig P. and Rubba P., (1993), Translation of representations of the structure of matter and its relationship to reasoning, gender, spatial reasoning, and specific prior knowledge, J. Res. Sci. Teach., 30, 883–903.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Kozma R. and Russell J., (2005), Students Becoming Chemists: Developing Representational Competence, in Gilbert J. K. (ed.), Visualization in Science Education. Models and Modeling in Science Education, Springer, Dordrecht, vol. 1, pp. 121–145.
- Limerick B., Burgess-Limerick T. and Grace M., (1996), The politics of interviewing: power relations and accepting the gift, Int. J. Qual. Stud. Educ., 9(4), 449–460.
- Lin Y. I., Son J. Y. and Rudd J. A., (2016), Asymmetric translation between multiple representations in chemistry, Int. J. Sci. Educ., 38(4), 644–662.
- Mathewson J. H., (2005), The visual core of science: definitions and applications to education, Int. J. Sci. Educ., 27, 529–548.
- Nakhleh M. B., (1992), Why some students don’t learn chemistry, J. Chem. Educ., 69 (3), 191–196.
- Ngai C., Sevian H. and Talanquer V., (2014), What is this substance? What makes it different? Mapping progression in students’ assumptions about chemical identity, Int. J. Sci. Educ., 36(14), 2438–2461.
- Novick S. and Nussbaum J., (1981), Pupils’ understanding of the particulate nature of matter: a cross-age study, Sci. Educ., 65(2), 187–196.
- Nyachwaya J. M., Mohamed A., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The Development of an Open-Ended Drawing Tool: An Alternative Diagnostic Tool for Assessing Students’ Understanding of the Particulate Nature of Matter, Chem. Educ. Res. Pract., 12, 121–132.
- Onwu G. O. M. and Randall E., (2006), Some aspects of student understanding of representational model of the particular nature of matters in chemistry in three different countries, Chem. Educ. Res. Pract., 7, 226–236.
- Potgieter M., Rogan J. M. and Howie S., (2005), Chemical concepts inventory of Grade 12 learners and UP foundation year students, Afr. J. Res. SMT Educ., 9, 121–134.
- Ramnarain U. and Joseph A., (2012), Learning difficulties experienced by grade 12 South African students in the chemical representation of phenomena, Chem. Educ. Res. Pract., 13, 462–470.
- Rappoport L. T. and Ashkenazi G., (2008), Connecting levels of representation: emergent versus submergent perspective, Int. J. Sci. Educ., 30, 1585–1603.
- Renström L., Andersson B. and Marton F., (1990), Students’ conceptions of matter, J. Educ. Psychol., 82(3), 555–569.
- Salta K. and Tzougraki C., (2011), Conceptual versus algorithmic problem-solving: focusing on problems dealing with conservation of matter in Chemistry, Res. Sci. Educ., 41(4), 587–609.
- Scalco K. C., Talanquer V., Kiill K. B. and Cordeiro M. R. (2018), Making sense of phenomena from sequential images versus illustrated text, J. Chem. Educ., 95 (3), 347–354.
- Stains M. and Talanquer V., (2007a), A2: element or compound?, J. Chem. Educ., 84(5), 880–883.
- Stains M. and Talanquer V., (2007b), Classification of chemical substances using particulate representations of matter: an analysis of student thinking, Int. J. Sci. Educ., 29(5), 643–661.
- Strauss A. and Corbin J., (1990), Basics of qualitative research, grounded theory procedures and techniques, Newbury Park, CA: Sage Publications Inc.
- Taber K. S., (1997), Student understanding of ionic bonding: molecular versus electrostatic thinking? Sch. Sci. Rev., 78(285), 85–95.
- Taber K. S., (2002), ‘Intense, but it's all worth it in the end’: the colearner's experience of the research process, Br. Educ. Res. J., 28(3), 435–457.
- Taber K. S., (2009), Learning at the symbolic level, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, Dordrecht: Springer, pp. 75–105.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemical education, Chem. Educ. Res. Pract., 14, 156–168.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15(2), 109–113.
- Taber K. S., (2018), Lost and found in translation: guidelines for reporting research data in an ‘other’ language, Chem. Educ. Res. Pract., 19(3), 646–652.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Talanquer V., (2018), Exploring Mechanistic Reasoning in Chemistry, in J. Yeo et al. (ed.), Science Education Research and Practice in Asia-Pacific and Beyond, Dordrecht: Springer, pp. 39–52.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
- Vachliotis T., Salta K. and Tzougraki C., (2014), Meaningful understanding and systems thinking in organic chemistry: validating measurement and exploring relationships, Res. Sci. Educ., 44(2), 239–266.
- Viennot L., (2001), Reasoning in physics: the part of common sense, Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Waight N. and Gillmeister K., (2014), Teachers and students’ conceptions of computer-based models in the context of high school chemistry: elicitations at the pre-intervention stage, Chem. Educ. Res. Pract., 44, 335–361.
- Wu H. K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
- Ye J., Lu S. and Bi H., (2018), The effects of microcomputer-based laboratories on students macro, micro, and symbolic representations when learning about net ionic reactions, Chem. Educ. Res. Pract., 20, 288–301.
- Zarkadis N., Papageorgiou G. and Stamovlasis D., (2017), Studying the consistency between and within the student mental models for atomic structure, Chem. Educ. Res. Pract., 18, 893–902.
Footnotes |
| † The terms “macro”, “submicro” and “symbolic” for the three types of chemical representations were used according to the suggestions of Gilbert and Treagust (2009a). |
| ‡ In the present paper the translations between representations will be symbolized according to the underlying levels of chemistry as follows: “symbolic → submicro”, “submicro → macro”, etc., where “symbolic”: symbolic representation, “macro”: macroscopic representation and “submicro”: submicroscopic representation. |
| This journal is © The Royal Society of Chemistry 2020 |