Open Access Article
Open Access ArticleSelf-optimising processes and real-time-optimisation of organic syntheses in a microreactor system using Nelder–Mead and design of experiments†
Verena
Fath
 ab,
Norbert
Kockmann
ab,
Norbert
Kockmann
 a,
Jürgen
Otto
c and
Thorsten
Röder
a,
Jürgen
Otto
c and
Thorsten
Röder
 *b
*b
aDepartment of Biochemical and Chemical Engineering, Equipment Design, TU Dortmund University, Emil-Figge-Str. 70, 44227 Dortmund, Germany
bInstitute of Chemical Process Engineering, Mannheim University of Applied Sciences, Paul-Wittsack-Str. 10, 68163 Mannheim, Germany. E-mail: t.roeder@hs-mannheim.de; Tel: +49 621 292 6800
cInstitute for Applied Thermo- and Fluid Dynamics, Mannheim University of Applied Sciences, Paul-Wittsack-Str. 10, 68163 Mannheim, Germany
First published on 15th June 2020
Abstract
Optimisation problems are abundant both in lab and industrial chemistry, where the determination of ideal reaction conditions poses particular challenges. This work contributes to the extant research on self-optimisation by developing a modular, autonomous platform that performs multi-variate and multi-objective optimisations in real-time, thereby generating cost and time savings. The platform consists of a fully-automated microreactor setup that is equipped with real-time reaction monitoring (through inline FT-IR spectroscopy) and a self-optimisation procedure. To demonstrate its flexibility (which extends to industrial production settings), the performances (in terms of identifying optimal reaction conditions) of two common optimisation strategies, modified simplex algorithm and model-free design of experiments, are subsequently compared. Besides enabling model-free autonomous optimisation, this novel system also permits the simultaneous collection of kinetic data to gain further insights into the involved chemical processes. In a second step, the system is enhanced to become capable of providing real-time responses to disturbances to the chemical process. Thus, this work assists researchers and production engineers alike in selecting the most suitable strategy for a given optimisation scenario, while also counteracting potential malfunctions in chemical production processes.
Introduction
Optimisation strategies are of high significance for chemical process development, both in lab and in industrial applications. They aim at improving the performance of a system, a process, a procedure, or a product. Moreover, they minimize the effort necessary to achieve these improvements, for instance through reducing the extent of human intervention or the required number of experiments.1,2 Such optimisation procedures can be performed in every stage of process development. Their high industrial significance can be attributed to economic reasons, as they guarantee a consistent quality of chemical products while simultaneously saving time and costs. During optimisation, the search for a maximum or minimum value of an objective function is performed. This is executed by systematic variation of parameters that influence the objective function.3 For instance, the yield of a chemical reaction depends on several variables, such as stoichiometries of substrates, temperature, or residence time. The number of iterations required to find the global optimum constitutes a key parameter of optimisation problems, since it correlates with the needed laboratory effort.2Although academic research has studied multidimensional, systematic optimisation strategies in detail,4–13 in industrial settings, optimisation often proceeds through changing one variable at a time (OVAT).14,15 Notwithstanding OVAT's ease of use, multidimensional approaches, which optimise all conditions simultaneously, are considerably more efficient. Two of the main advantages of multidimensional optimisation are its ability to explain interactions between reaction parameters, and a smaller number of required experiments, hence resulting in lower costs of reagents and less time consumption.16–19
Several optimisation methods exist,2,3 including the modified Nelder–Mead simplex algorithm and design of experiments (DoE). Regarding the former,20 simplexes are generated and iteratively replaced, thus leading the process to a local optimum.1,2,21–23 Simplex algorithms are mainly applied in continuous flow, with greater use of online analysis.24–34 The analysis is mostly conducted through online HPLC,25–27,33,34 but online MS,32 online NMR,30 and inline IR29,32 are also utilized.
Conversely, DoE,15,16,19 constitutes a method that relies on characterizing a chemical reaction's experimental space through a surface response model. The model is described by a simple mathematical function with one optimum and results from a multivariate and broad screening of reaction parameters according to a systematic experimental plan.16,17,35,36 From this, parameter effects can be evaluated, and optimal reaction conditions can be found.15,17 Design of experiments is mainly used in the optimisation of batch processes, identifying optimal parameters for organic syntheses in lab contexts.37–56 In continuous flow, design of experiments is used less frequently.57–70 In these rare applications, design of experiments is predominantly combined with offline analysis.62,65,67,70 To a lesser extent, online HPLC is used for reaction monitoring,66,68 with online MS or inline IR being used only in exceptional cases.58,61
Such continuous processes, however, bear the advantage of being easily automatable.5,71,72 Compared to batch processes, where many individual process steps have to be executed, continuous processes are therefore more suitable for optimisation procedures.73–76 Microstructured flow devices, in particular, benefit from high reproducibility, efficient heat and mass transfer, and enhanced space–time yield.77–87 This allows for rapid and controlled screening of reaction parameters. In combination with online analysis, real-time reaction monitoring becomes possible, where intermediates and by-products can be monitored as well.10 Moreover, the use of microreactor technology enables reaction characterisation even at conditions that would pose safety risks if being carried out through a batch procedure.88–92
While the extant literature has examined design of experiments4,66,70 as well as the modified simplex algorithm24,25,27,31 only in isolation, this work extends the prior ones by presenting a detailed comparison of each method's performance as model-free autonomous optimisation strategy. For this purpose, an automated flow microreactor system, combined with online analytics and self-optimisation, is developed. Experimental data is collected in real-time and used as feedback for rapid reaction development, while optimal reaction conditions are identified without the need for human intervention. This completely automated self-optimising system constitutes a modular concept that is compatible with industrial manufacturing conditions and can conveniently be transferred from lab to production scale. Different optimisation goals, including product concentration, production quantity, and costs, are experimentally evaluated. Besides enabling model-free autonomous optimisation to identify optimal reaction conditions, the microreactor system furthermore allows for simultaneous collection of kinetic data, granting additional insights into the chemical process. As proof of concept, imine synthesis is optimised using the developed setup. The underlying kinetics had already been verified by another work93 (using a microreactor setup as well30,94), thus permitting to judge the accuracy of the results obtained by the work at hand.
A further novelty featured in this work is the development of a model-free, real-time response of a simplex algorithm to a disturbance of the chemical process. This approach is of high industrial significance, as fluctuations in the concentration or an inaccurate dosage of starting materials, or a breakdown of temperature control, may not always be prevented in real industrial applications. Such process errors are prone to cause severe economic losses. Besides identifying optimal reaction conditions, simplex algorithms may also be modified to react to these disturbances during the reaction progress, and to compensate for them, thus preventing deteriorations of product quality.
Materials and methods
Reaction
Experimental setup
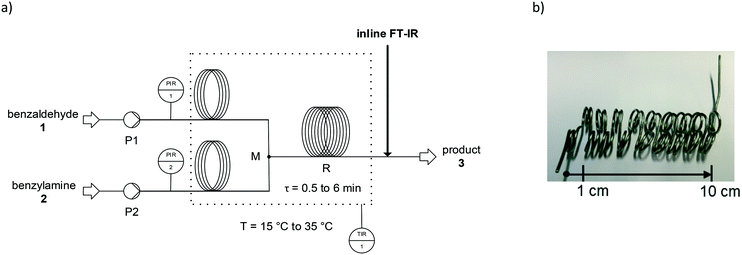 | ||
| Fig. 1 Microreactor setup for optimisation experiments, process flow chart (a), coiled capillary microreactor (b). | ||
A dosage of starting materials within 1 mL glass syringes was accomplished by continuously working syringe pumps (SyrDos2, HiTec Zang GmbH, Germany). Temperature and flow rates were controlled by a laboratory automation system (HiTec Zang GmbH, Germany).
The use of an inline FT-IR spectrometer (Bruker ALPHA, United States) allowed for real-time reaction tracking (see previous publication for details102). Infrared spectra (500–1700 cm−1) were collected with an optical wavelength resolution of 4 cm−1 through single reflection ATR (diamond crystal). Analytical IR spectra and details on the integration method are provided in ESI† A.1. Benzaldehyde 1 was identified by means of a characteristic IR band at 1680 cm−1 to 1720 cm−1. During the reaction progress, this band decreased. On this basis, the conversion of the starting material was calculated. This was done based on a previously determined calibration curve. The reaction product n-benzylidenebenzylamine 3 was identified by means of a characteristic IR band at 1620 cm−1 to 1660 cm−1. During the reaction progress, this band increased. The product yield was calculated based on a previously determined calibration curve.
Optimisation procedure
Results
Numerical evaluation of optimisation methods
The performance of the modified simplex algorithm and of design of experiments as optimisation strategies was evaluated using a theoretical chemical model reaction (eqn (1)).| cA + cB → cP → cSP | (1) |
Reaction temperature, residence time, and stoichiometric ratio of starting materials were chosen as variable parameters, resulting in a two- (temperature and time) respectively three-dimensional (temperature, time, and stoichiometric ratio) optimisation problem aiming at maximising product yield.
The described numerical approach benefits from obtaining a large number of data points within a short span of time. It can be used to evaluate optimisation strategies' performance in an efficient manner, taking various aspects into consideration. Numerical evaluation was performed using programs that were fully coded in MATLAB.
1) Maximum start simplex: a start simplex that was placed over the whole reaction space. With two variable parameters, maximum start simplex results in four possible configurations. In contrast, 16 possibilities arise with three variable parameters. Four of them were evaluated, as described in ESI† B.2.
2) Start simplex set to axes: a start simplex that was set to random values located on each axis. The last corner point was always randomly chosen within the reaction space.
3) Random start simplex with defined size: a start simplex that was stretched around a randomly chosen point within the reaction space. Simplex size is defined through a fixed value delta. Hence, the delta is multiplied with the maximum value of every single variable parameter to include the whole reaction space and is then subsequently transferred to the respective start simplex. Three different values for delta were tested: 0.1 (size of start simplex corresponding to 10% in each parameter direction), 0.2, and 0.4.
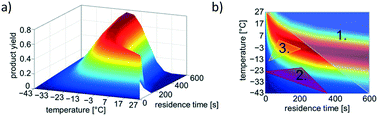
For an optimisation problem with two variable parameters (reaction temperature and residence time), Fig. 2 displays the three main possibilities to generate a start simplex and the contour plot of the model reaction resulting from the defined reaction kinetics.
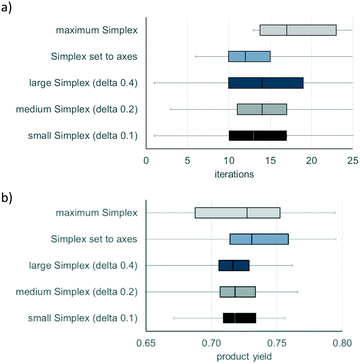
All five possibilities to generate a start simplex were evaluated independently regarding their number of iterations needed to surpass the quality threshold of at least 70% product yield (the highest possible yield – as defined by the kinetic model – amounts to 0.79). Each optimisation run ended as soon as one out of three conditions was met: a) surpassing the quality threshold of 70%, b) no improvement in product yield for five consecutive iterations, or c) after having performed 30 iterations.
Each of the five methods was calculated and evaluated 100 times, both for two and three parameter optimisation each. This enabled the simulation of various starting points.
Fig. 3 compares the performance of the described approaches in generating a start simplex in terms of their number of iterations needed to reach the quality criterion of at least 70% product yield, and furthermore outlines the corresponding optimal product yield achieved after simplex optimisation.
As demonstrated by Fig. 3, the approach using start simplex that was set to axes, on average, required the fewest iterations to surpass the quality requirement. Moreover, this approach delivered the best performance regarding maximum product yield after simplex optimisation. As reducing the number of iterations necessary to find optimal reaction conditions is especially crucial for an experimental application of simplex optimisation, the approach with start simplex that was set to axes was chosen for the following experimental optimisation procedures. However, it should be stated that the other methods of generating start simplexes (with the exception of maximum start simplex) performed almost equally well. Therefore, an advantage of the simplex algorithm methodology is that its effectiveness is hardly affected by initial simplex size, implying that optimisation can be performed even without detailed foreknowledge about the investigated reaction.
Every design was evaluated with and without consideration of interactions between variable parameters. This resulted in different polynomial equations used to describe the surface response model, see ESI† A.4.
Optimisation always started with a first experimental plan that was placed over the reaction space in order to screen it in its entirety. Based on these results, a surface response model was calculated. Its corresponding optimum (in other words, the parameter combination that resulted in the highest product yield), was then determined. On this basis, a second experimental plan was built, containing the optimum of the first DoE run as central point. The size of the second DoE run was defined through a variable delta that limited the plan to 20% of the size of the first DoE run. Consequently, the second DoE run led to a surface response model that lied closer to the global optimum.
Afterwards, the quality of all calculated optimal solutions provided by the surface response models was examined: these (from a mathematical perspective) optimal results were compared with 1) maximum possible product yield (amounting to 0.79; Fig. 5 illustrates how these terms are related) and 2) product yield as predicted by the simulated kinetic model (based on the optimal combination of parameters – temperature, residence time, and stoichiometric ratio of starting materials – as specified by the respective surface response model).
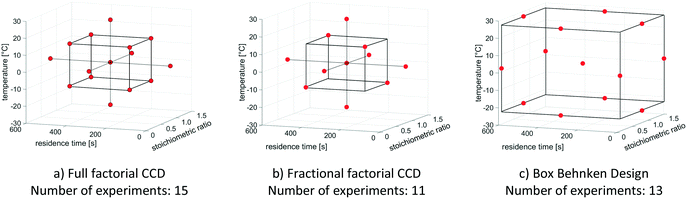
Table 1 outlines the results for all three investigated experimental designs.
| Experimental design | 0) Optimum product yield as predicted by DoE | 1) Maximum possible yield | Deviation between 0) and 1) [%] | 2) Product yield predicted by simulated kinetic model | Deviation between 0) and 2) [%] |
|---|---|---|---|---|---|
| a Quality criterion (product yield >70%) is reached. Note: optimum product yields (as predicted by DoE) greater than the maximum possible yield of 0.79 indicate that the respective surface response model has been subject to excessive extrapolation. | |||||
| 1st DoE run full factorial CCD | 0.60 | 0.79 | −23 | 0.75 | −19 |
| 2nd DoE run full factorial CCD | 0.82a | 0.79 | +6 | 0.71 | +16 |
| 1st DoE run fractional factorial CCD | 0.59 | 0.79 | −24 | 0.74 | −19 |
| 2nd DoE run fractional factorial CCD | 0.84a | 0.79 | +7 | 0.73 | +15 |
| 1st DoE run Box Behnken design | 0.52 | 0.79 | −33 | 0.62 | −16 |
| 2nd DoE run Box Behnken design | 0.67 | 0.79 | −14 | 0.72 | −6 |
Relevance and meaningfulness of the inclusion of parameter interactions are discussed in ESI† B.3. The ESI† demonstrates that, especially regarding chemical reactions, it is preferable to exclude parameter interactions. Neither physical nor chemical considerations argue in favour of including parameter interactions within response surface models.
Without consideration of parameter interactions, fractional factorial CCD performs well in predicting optimal and reasonable values for product yield, while simultaneously reducing the number of experiments needed. The quality criterion (product yield >70%) is exceeded after the second DoE run, while the deviations from both the maximum possible yield (amounting to 0.79) and from the actual yield (indicated by the kinetic model) are quite low. Hence, a fractional factorial CCD is well-suited for optimising the theoretical model reaction.
In the following experimental implementation of design of experiments in the context of reaction optimisation, CCD is used as experimental design. This results in 9 experiments per DoE run in terms of optimisation of two variable parameters, and in 11 experiments per fractional factorial DoE run in terms of optimisation of three variable parameters.
Optimisation using simplex algorithm
As proof of concept, the reaction of benzaldehyde 1 with benzylamine 2 to form n-benzylidenebenzylamine 3 was experimentally investigated. As selectivity always amounts to nearly 100%, yield is determined by conversion. The underlying kinetics are already known, see previous publication93 for details. Therefore, this organic synthesis is well-suited for acting as model reaction to test the presented optimisation workflow and compare performances of different optimisation procedures.Over the course of optimisation of two variable parameters, stoichiometric ratio and residence time were optimised. The stoichiometric ratio was varied in the range between 0.1 and 2 (benzaldehyde 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzylamine 2), thus, both starting materials were provided either in excess or in shortage. Residence time was varied between 0.5 and 6 min, ensuring conversion of nearly 100% at its peak, see determined kinetics of model reaction in previous publication.93 Reaction temperature was always held constant at 25 °C.
benzylamine 2), thus, both starting materials were provided either in excess or in shortage. Residence time was varied between 0.5 and 6 min, ensuring conversion of nearly 100% at its peak, see determined kinetics of model reaction in previous publication.93 Reaction temperature was always held constant at 25 °C.
Fig. 6 exemplarily displays the simplex progress as a function of stoichiometric ratio and residence time, and the progress of the objective function over the number of iterations when maximum product concentration serves as optimisation goal.
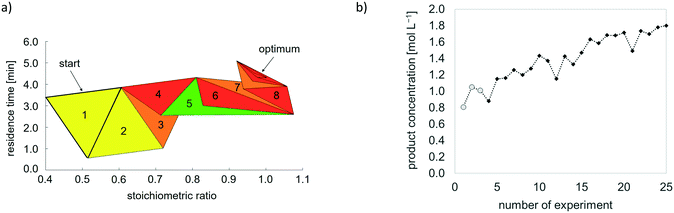 | ||
| Fig. 6 Simplex optimisation of two variable parameters with product concentration as objective function. a) Exemplary simplex progress. Legend: Colouring of simplexes corresponds to simplex movement: yellow – reflection, green – expansion, red – contraction, orange – contraction with change of movement direction (see details on this procedure in ESI† A.2). b) Exemplary progress of objective function over number of experiments. Legend: ○ values of start simplex. | ||
This example (Fig. 6) outlines the functioning of simplex optimisation: a randomly chosen initial simplex, which was set to axes, is iteratively replaced until the simplexes finally converge to a local optimum, where the value of the objective function does not vary anymore. Hence, optimal conditions are identified before the maximum number of experiments, set to 30, is reached.
During multidimensional optimisation of three variable parameters, stoichiometric ratio, residence time, and reaction temperature were optimised. The stoichiometric ratio was again varied between 0.1 and 2 (benzaldehyde 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzylamine 2) and the residence time between 0.5 and 6 min. Moreover, reaction temperature was varied in the range between 15 and 35 °C. The model reaction's temperature dependence has already been investigated during prior kinetic studies, as described in a previous publication.93 It was shown that higher temperatures slightly accelerate the reaction. The activation energy amounted to 40 kJ mol−1. Using the example of maximum product concentration as optimisation goal, the simplex progress and the progress of the objective function over the number of iterations (in case of three-parameter optimisation) are displayed in ESI† C.2.
benzylamine 2) and the residence time between 0.5 and 6 min. Moreover, reaction temperature was varied in the range between 15 and 35 °C. The model reaction's temperature dependence has already been investigated during prior kinetic studies, as described in a previous publication.93 It was shown that higher temperatures slightly accelerate the reaction. The activation energy amounted to 40 kJ mol−1. Using the example of maximum product concentration as optimisation goal, the simplex progress and the progress of the objective function over the number of iterations (in case of three-parameter optimisation) are displayed in ESI† C.2.
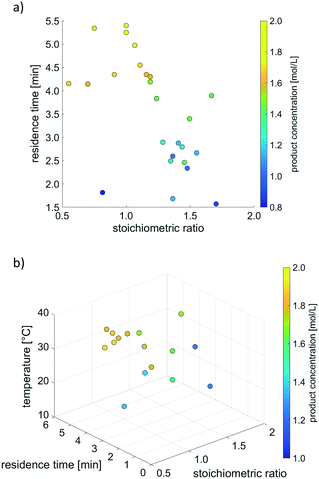
Fig. 7 illustrates the results of simplex optimisation with product concentration as objective function, both for two and three variable parameters. The investigated experimental conditions are displayed as coloured dots, with the colours indicating the corresponding values of the objective function.
Both examples displayed in Fig. 7 result in a maximum product concentration of 1.8 mol L−1. This constitutes a plausible value and is in accordance with the reaction kinetics that had previously been obtained.93
For all three objective functions, i.e. maximum product concentration, maximum production quantity, and minimum costs per kg of product, Table 2 provides an overview of the results of simplex optimisation, both for the case of two and of three variable parameters.
| Optimisation of two variable parameters | |||
|---|---|---|---|
| Parameters | Product concentration | Production quantity | Costs |
| (Experimentally obtained) optimal result | 1.8 mol L−1 | 5.3 mmol min−1 | 170 € per kg |
| Stoichiometric ratio | 1.0 | 1.0 | 1.2 |
| Residence time [min] | 5.4 | 0.7 | 0.8 |
| Temperature [°C] | 25 | 25 | 25 |
| Number of experiments | 25 | 26 | 22 |
| Product concentration [mol L−1] | — | 1.4 | 1.6 |
| Production quantity [mmol min−1] | 4.3 | — | 5.0 |
| Costs [€ per kg] | 364 | 183 | — |
| Optimisation of three variable parameters | |||
|---|---|---|---|
| Parameters | Product concentration | Production quantity | Costs |
| (Experimentally obtained) optimal result | 1.8 mol L−1 | 5.3 mmol min−1 | 155 € per kg |
| Stoichiometric ratio | 1.0 | 1.1 | 1.2 |
| Residence time [min] | 4.2 | 1.7 | 0.8 |
| Temperature [°C] | 35 | 33 | 20 |
| Number of experiments | 20 | 27 | 22 |
| Product concentration [mol L−1] | — | 1.7 | 1.5 |
| Production quantity [mmol min−1] | 4.6 | — | 4.9 |
| Costs [€ per kg] | 297 | 173 | — |
As far as optimisation aiming at maximising product concentration is concerned, results at a stoichiometric ratio of 1.0 and a high residence time meet expectations, since the model reaction proceeds with a selectivity of nearly 100%. Therefore, no formation of by-products is expected, and the highest conversion occurs at the highest possible residence time, according to a second order reaction. If additionally taking the reaction temperature into account as variable parameter, the simplex algorithm drives the process to a high temperature. This is also in line with expectations, see reaction kinetics in previous publication.93 However, it should be noted that, in contrast to the other two parameters, temperature exerts only a minor influence on the reaction progress.
Maximum production quantity is also accomplished at a stoichiometric ratio of 1.0, but at lower residence times. Optimal conditions are found as compromise between a high product yield and a concurrently high productivity. The results for two-parameter optimisation are hence aligned with theoretical expectations (see ESI† C.1). Note that, when comparing the results of two- and three-parameter optimisation regarding production quantity, however, a drawback to the simplex methodology becomes apparent: theoretically, the optimal solution identified by three-parameter optimisation should involve a lower residence time compared to the optimal solution identified by two-parameter optimisation. The obtained data displays a reverse effect, however. This is due to the fact that the optimum constitutes a corner point solution, which (owing to inherent methodological limitations) the simplex algorithm occasionally fails to identify.¶
Costs are determined by a cost function consisting of a variable and a fixed portion. This cost function serves as an approximation of actual costs per kg of production output incurred in industrial applications. The variable portion indicates costs per kg of starting materials. Conversely, fixed costs (i.e., costs of operation) are assumed to amount to 30% of the variable costs arising at a predefined output level (see ESI† C.1 for detailed information). The cost function predicts that total costs are minimised at a stoichiometric ratio of 1.0 and a residence time of 0.6 min. For residence times smaller than 0.6 min, high volumetric flow rates occur, resulting in high costs for starting materials, whereas residence times considerably greater than 0.6 min cause the overall costs to rise again. The obtained results correspond closely to theoretical expectations, as outlined in ESI† C.1.
Based on these studies, it was determined that the influence of start simplexes on the optimisation result is negligible. The optimal stoichiometric ratio was always close to 1.0, whereas the optimal residence time always amounted to roundabout 5 min. After six repetitions, the relative standard deviation of the stoichiometric ratio amounted to 4%, and the one of residence time to 5%. Details on the investigated start simplexes and all individual results are provided in ESI† C.3.
Optimisation using design of experiments
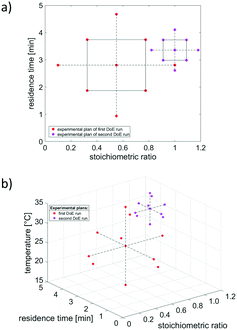
Again, the reaction of benzaldehyde 1 with benzylamine 2 was used as proof of concept to experimentally investigate design of experiments as optimisation strategy, and to finally compare its performance with that of simplex optimisation. During multidimensional optimisation of two respectively three variable parameters, two different optimisation goals were examined: maximum product concentration and lowest costs per kg of product.Based on the results of the numerical evaluation of DoE as optimisation method, CCD was chosen as experimental design, both for two and three variable parameters. It should be noted that a full factorial approach was applied for optimisation with two variable parameters, since, otherwise, too much information loss would have occurred. In case of three-parameter optimisation, a fractional factorial CCD was used, reducing the number of required experiments. During each optimisation, two experimental runs were executed. The first run was utilized to screen the whole experimental space, whereas the second run refined the search for a global optimum. The size of the second DoE run amounted to 20% of the size of the first DoE run, while containing the optimum of the first DoE run as central point. The resulting experimental plans for both DoE runs, with product concentration as objective function, and for two respectively three variable parameters, are displayed in Fig. 8.
In case of optimisation with two variable parameters, nine experiments were required for every DoE run, resulting in a total of 18 experiments. In contrast, three-parameter optimisation necessitated 11 experiments for each DoE run, leading to an overall number of 22 experiments.
In terms of product concentration as objective function, the surface response models obtained by the first and second DoE run (while optimising two variable parameters: stoichiometric ratio and residence time), are exemplarily provided in Fig. 9. Experimental data points are displayed as red dots from which the respective surface response model was built as best fit to all experimental data.
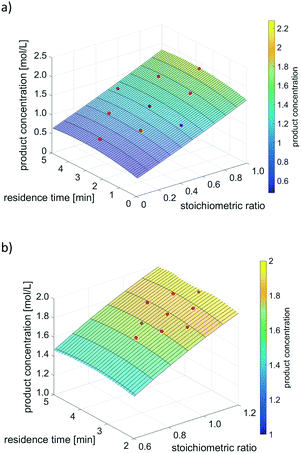 | ||
| Fig. 9 Optimisation of product concentration with two variable parameters using DoE. a) First DoE run for screening of whole experimental space. b) Second DoE run to refine optimisation. | ||
As displayed in Fig. 9, both DoE runs result in surface response models that represent the experimental data well. The visualization indicates where to expect optimal reaction conditions (and thus the highest product concentration), namely at a stoichiometric ratio near 1.0 and a high residence time.
Surface response models derived for the optimisation of three variable parameters in order to pursue the costs per kg of product as objective function can be found in ESI† C.4.
Table 3 summarizes the obtained results for both optimisation goals (maximum product concentration and minimum costs per kg of product), and for two, respectively three variable parameters. For every case, optimal reaction conditions for the first and second DoE run are displayed, which were calculated based on the corresponding surface response model. Moreover, the average deviation of the experimental data points from the associated surface response model was determined, allowing to assess each optimisation's accuracy.
| First DoE run | ||||
|---|---|---|---|---|
| Parameters | Optimisation of two parameters | Optimisation of three parameters | ||
| Product concentration | Costs | Product concentration | Costs | |
| (Experimentally obtained) optimal result | 1.8 mol L−1 | 98 € per kg | 1.7 mol L−1 | 96 € per kg |
| Stoichiometric ratio | 1.0 | 0.9 | 1.0 | 0.9 |
| Residence time [min] | 3.4 | 0.2 | 3.5 | 0.1 |
| Temperature [°C] | 25 | 25 | 35 | 20 |
| Average deviation experimental data points – surface response model [%] | 1.9 | 3.1 | 3.5 | 1.6 |
| Number of experiments | 9 | 9 | 11 | 11 |
| Product concentration [mol L−1] | — | 0.7 | — | 0.4 |
| Costs [€ per kg] | 256 | — | 259 | — |
| Second DoE run | ||||
|---|---|---|---|---|
| Parameters | Optimisation of two parameters | Optimisation of three parameters | ||
| Product concentration | Costs | Product concentration | Costs | |
| (Experimentally obtained) optimal result | 2.0 mol L−1 | 136 € per kg | 1.9 mol L−1 | 130 € per kg |
| Stoichiometric ratio | 1.0 | 1.1 | 1.0 | 1.1 |
| Residence time [min] | 4 | 0.5 | 4.5 | 0.7 |
| Temperature [°C] | 25 | 25 | 35 | 20 |
| Average deviation experimental data points – surface response model [%] | 0.7 | 1.4 | 0.4 | 0.9 |
| Number of experiments | 9 | 9 | 11 | 11 |
| Product concentration [mol L−1] | — | 1.3 | — | 1.2 |
| Costs [€ per kg] | 288 | — | 314 | — |
The results of DoE optimisation are comparable to those of simplex optimisation. Highest product concentration is reached at a stoichiometric ratio of 1.0 and a high residence time. Conversely, lowest costs are incurred at a stoichiometric ratio near 1.0 and a considerably lower residence time. These results are in accordance with theoretically expected values that were calculated based on the kinetic model, see ESI† C.1. Moreover, comparing simplex and DoE optimisation, the results in terms of costs per kg of product are discussed in ESI† C.1.
In case of product concentration, the first DoE run already leads to reasonable findings, from which the approximate range of optimal reaction conditions can be derived. The second DoE run then converges to theoretically expected, ideal parameter combinations, and the average deviation of experimental data points from the respective surface response model decreases to values near 1.0%.
Regarding cost minimisation, however, the surface response model derived from the first DoE run conducts an extrapolation that is too strong in magnitude, leading to cost and residence time estimates that are well below the feasible minimum. Nonetheless, the first DoE run narrows down the location of the optimal reaction conditions. Thereby, it constitutes the basis for a second run, whose surface response model improves considerably upon the first one by analysing a larger number of data points located close to the theoretically expected optimum (see ESI† C.1 and C.4).
Thus, it becomes apparent that DoE optimisation requires two runs to tackle such optimisation problems, resulting in surface response models from which optimal reaction conditions can be predicted in a reliable and reproducible manner. Details on the reproducibility of DoE optimisation are provided in ESI† C.4.
Real-time optimisation
To further increase the simplex algorithm's usefulness for industrial applications, it was enhanced to become capable of reacting autonomously to disturbances of the chemical process. This allowed for a model-free, real-time optimisation with efficient control of chemical reactions. A constant, optimum level of product yield was ensured, independent of external influences and without the need for human intervention. As soon as the algorithm detects a decrease in product yield, it autonomously reacts in order to raise product yield again. This is accomplished by searching for new optimal reaction conditions that offset the actual disturbance.Responses of the simplex to three types of disturbances were investigated: fluctuations in concentration of starting materials, inaccurate dosage of starting materials, and breakdown of temperature control. These purposefully-induced disturbances constituted realistic scenarios that cannot be ruled out entirely in actual industrial applications, and that are likely to cause considerable economic losses in case of occurrence. In all cases, the real-time optimisation strived for converging to highest possible product concentration once again.
Table 4 provides an overview of one of the investigated disturbances: fluctuations in concentration of starting materials. Such an incident can only be rectified through adjusting stoichiometric values. For all particular cases, Table 4 lists the expected new stoichiometric values that are supposed to re-establish optimal results. The results of the simplex response to a breakdown of temperature control as well as to an inaccurate dosage of starting materials are discussed in ESI† D.1 respectively D.2.
| Fluctuations in concentration of starting materials | Expected new stoichiometric value | Stoichiometric value reached after real-time optimisation | Number of experiments required to offset disturbance |
|---|---|---|---|
| a Average value of six repeated measurements, reference case of simplex optimisation without disturbance by means of fluctuation in concentration of starting materials (relative standard deviation 4%). b Average value of three repeated measurements (relative standard deviation 5%). | |||
| Reference case | 1.0 | 1.0a | — |
| Concentration of [1]: 4 mol L−1 | |||
| Concentration of [2]: 4 mol L−1 | |||
| Concentration of [1]: 5 mol L−1 | 0.8 | 0.82 | 7 |
| Concentration of [2]: 4 mol L−1 | |||
| Concentration of [1]: 2 mol L−1 | 2.0 | 2.0 | 9 |
| Concentration of [2]: 4 mol L−1 | |||
| Concentration of [1]: 4 mol L−1 | 1.25 | 1.3 | 7 |
| Concentration of [2]: 5 mol L−1 | |||
| Concentration of [1]: 4 mol L−1 | 0.5 | 0.54b | 10 |
| Concentration of [2]: 2 mol L−1 | |||
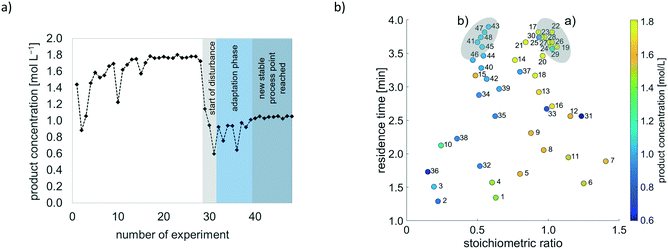
As soon as the disturbance was induced, the simplex algorithm recognized a drop in product concentration and took countermeasures. Exemplarily for a disturbance through reduction of concentration of benzylamine 2 from 4 to 2 mol L−1, Fig. 10 displays the progression of simplexes during real-time optimisation.
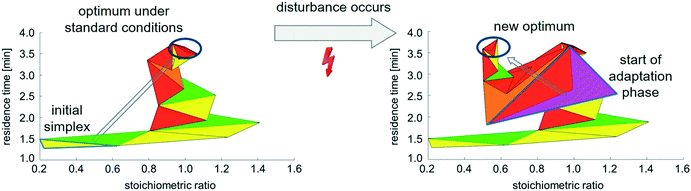 | ||
| Fig. 10 Simplex progress during real-time optimisation. a) Progress under standard conditions (initial concentrations of benzaldehyde 1 and benzylamine 2 amounting to 4 mol L−1 respectively). b) Simplex response towards fluctuation in concentration of starting materials (initial concentration of benzylamine 2 is switched to 2 mol L−1). Legend: Colouring of simplexes corresponds to simplex movement: yellow – reflection, green – expansion, red – contraction without change in movement direction, orange – contraction with change in movement direction, purple – start of adaptation phase after disturbance has occurred, see details in ESI† A.2). | ||
Once a disturbance had occurred, simplex size increased, and the algorithm subsequently converged to a new optimal stoichiometric ratio, whilst maintaining the highest possible residence time. Although a stoichiometric ratio of 1.0 yielded the best result in the initial situation, the simplex proposed a stoichiometric ratio of around 0.5 after the initial concentration of benzylamine 2 had been halved. This result is in accordance with expectations, as indicated in Table 4.
In Fig. 11, the progression of the objective function (product concentration) during real-time optimisation with halved initial concentration of benzylamine 2, and the related parameter combinations, are presented.
The progression of the objective function, as displayed in Fig. 11, indicates a sharp decrease in product concentration at the very moment of disturbance. However, the simplex algorithm quickly responds to the disturbance, causing product concentration to increase again. It should be noted that the absolute product concentration changed from around 1.8 mol L−1 (in the initial situation) to 0.9 mol L−1 as the initial concentration of benzylamine 2 was reduced to 2 mol L−1. The visualisation of product concentration as function of the stoichiometric ratio and residence time in Fig. 11 provides clear evidence that the process is driven towards a new stoichiometric ratio after occurrence of the disturbance. In the given example, a parameter combination of a stoichiometric ratio of 0.5 and a high residence time rectify undesired changes in product concentration following the disturbance.
The results of the simplex response to other disturbances of the chemical process (through fluctuations in concentration of starting materials) are provided in Table 4 as well. To summarize, real-time optimisation always succeeded, as the simplex algorithm autonomously responded to the given disturbance by adapting the stoichiometric ratio. The adapted stoichiometric ratios always corresponded to the expected values.
An inaccurate dosage of starting materials was induced through simulating a defect of the syringe pump: hence, the flow rate of one of the starting materials was halved. Once again, the disturbance was simulated on the basis of an initial situation featuring equal initial concentrations of both starting materials (benzaldehyde 1 and benzylamine 2 4 mol L−1) and accurate flow rates. Once the process had identified optimal reaction conditions (stoichiometric ratio of 1.0 and high residence time of 4 min at a constant reaction temperature of 25 °C, resulting in a maximum product concentration of 1.8 mol L−1), the inaccurate dosage of one of the starting materials was induced. Results of real-time optimisation in the event of an inaccurate dosage of starting materials are discussed in ESI† D.2. Two examples of inaccurate dosage of starting materials were evaluated: halved flow rate of benzaldehyde 1, and halved flow rate of benzylamine 2. In both cases, the simplex autonomously discovered the new optimum stoichiometric ratio, which always corresponded to the expected values (see ESI† D.2).
Discussion
Online process development
On average, 20 experiments were needed to arrive at optimal results when applying simplex optimisation, both for two and three variable parameters. The required number of experiments for various cases of product concentration and costs as objective function are provided in ESI† E. An effort of roughly 20 experiments seems justifiable for such an investigation, whereas conducting a drastically higher number of experiments would diminish optimisation efficiency. The influence of different start simplexes on the optimal result was found to be negligible. However, for a more complex reaction, it cannot a priori be ruled out entirely that the simplex might get stuck at a local optimum instead of identifying the global one. Moreover, the amount of experiments needed to identify optimal conditions is also likely to depend on the complexity of the investigated reaction.
In case of DoE optimisation, two consecutive DoE runs were performed to search for optimal reaction conditions. The second DoE run always improved upon the first run's optimisation result. In the event of optimisation with two variable parameters, nine experiments were needed for each DoE run, resulting in a total of 18 experiments. Including three variable parameters, every DoE run required 11 experiments, and therefore overall 22 experiments had to be conducted. Hence, the number of experiments to be conducted depends on the number of variable parameters. A fractional factorial design can be used to reduce the number of experiments, but the required amount of experiments might nevertheless remain high. This is particularly the case for more complex optimisation problems with more than three variable parameters. In case of more complex circumstances, it should further be scrutinized whether replication of the experimental plan's centre point is essential, given that such a requirement would increase the number of experiments even further. Nonetheless, in contrast to the simplex approach, when using DoE, the amount of required experiments always remains constant for a given number of parameters to be optimised. A further advantage of the DoE method is that, unlike simplex, there is no risk of choosing a poor experimental starting point. Such a poor experimental starting point might cause the simplex algorithm to get stuck at a local optimum. Regarding the investigated reaction of benzaldehyde 1 with benzylamine 2, all surface response models obtained by DoE represented the experimental data well. However, for more complex reactions, for example reactions forming side products, model fit might be worse.
For both optimisation strategies, the overall measurement times needed to complete an optimisation cycle are discussed in ESI† E. All optimisation problems were successfully solved within a single working day.
The results of the simultaneously conducted kinetic modelling were compared to already existing kinetic data. The latter had been obtained during an evaluation of steady-state and nonsteady-state experiments (subsequently referred to as prior work), as described in a previous publication.93 A second order mechanism was appropriate to describe the investigated model reaction. Table 5 lists the kinetic data that had been collected by the prior work, including error limits.
| Kinetic parameters | Steady-state experiments | Nonsteady-state experiments |
|---|---|---|
| Reaction rate coefficient kref [L mol−1 s−1] (confidence level 95%, at Tref 25 °C) | 0.025 (±3.7%) | 0.028 (±2.4%) |
| Activation energy EA [kJ mol−1] (confidence level 95%) | 39.2 (±10.9%) | 40.1 (±5.3%) |
| Number of experiments | 30 | 336 |
The aforementioned prior work had relied on a structured procedure to gain kinetic data in a reliable manner.93 However, a high number of experiments might be necessary to examine the entire reaction space, including investigation of the reaction's temperature dependence and the influence of varying stoichiometric ratios (in case of more complex reaction mechanisms). Consequently, this technique (described in a prior work93) may result in high measurement times.
Combining the performance of optimisation procedures with the simultaneous collection of kinetic data renders the latter more efficient. For this reason, the experimental setup described in this article was concurrently used to gather kinetic data besides running simplex and DoE optimisation (with product concentration as objective function). For the purpose of kinetic modelling, only experimental data that had been gained during a particular optimisation cycle was considered. Based on these measurements, the concentrations of all involved components were calculated. Hence, individual data points were evaluated that were spread over the whole experimental space, depending on the simplex algorithm's route respectively the DoE's experimental plans. To summarize, this procedure resulted in experimental data that was less structured compared to the prior work, but nonetheless sufficient to perform kinetic modelling.
Table 6 provides the kinetic data that was collected while running the simplex optimisation, whereas Table 7 outlines the kinetic data gathered during DoE optimisation. It should be noted that, in case of optimisation with two variable parameters, only reaction rate coefficients can be calculated, as the reaction temperature is held constant. In contrast, in case of optimisation with three variable parameters, activation energy can be determined as well. As in the prior work, kinetic data was determined through fitting the experimental results to a kinetic model.93
| Kinetic parameters | Simplex with two-parameter optimisation | Simplex with three-parameter optimisation |
|---|---|---|
| a Average values of six independent measurements (relative standard deviation of reaction rate coefficient: 5%). b Average values of three independent measurements (relative standard deviation of reaction rate coefficient: 4%; relative standard deviation of activation energy: 3%). | ||
| Reaction rate coefficient kref [L mol−1 s−1] (confidence level 95%, at Tref 25 °C) | 0.021 (±10.5%)a | 0.024 (±14.5%)b |
| Activation energy EA [kJ mol−1] (confidence level 95%) | 0 | 40.6 (±24.9%)b |
| Number of experiments | 25 | 17 |
| Kinetic parameters | DoE with two-parameter optimisation | DoE with three-parameter optimisation |
|---|---|---|
| Reaction rate coefficient kref [L mol−1 s−1] (confidence level 95%, at Tref 25 °C) | 0.026 (±11.5%) | 0.022 (±15.2%) |
| Activation energy EA [kJ mol−1] (confidence level 95%) | 0 | 38.2 (±26.2%) |
| Number of experiments | 18 | 22 |
The kinetic data that was collected during simplex optimisation corresponds to the results obtained by the prior work. However, the error limits are higher. This is due to the fact that, during multidimensional simplex optimisation, all involved variable parameters were iterated frequently, resulting in diverse parameter combinations. Therefore, data acquisition no longer proceeded in a well-structured fashion, as it had been the case in the prior work, where only one variable had been changed at a time.93 Instead, the simplex algorithm quickly converged to parameter combinations that ensured high product concentration.
The kinetic data that was gathered during DoE optimisation also corresponds to the results obtained by the prior work. Again, the error limits are higher compared to the prior work. Moreover, they slightly succeed those of the simplex approach as well, albeit, in theory, they had been expected to be lower in the DoE scenario, since the experimental data points of a DoE run are linearly independent. Nonetheless, the confidence intervals of the kinetic data collected during simplex respectively DoE optimisation are of comparable size. Details on all three described techniques to determine kinetic data can be found in ESI† F.
Overall, the experimental setup was successful in obtaining kinetic data that matched with the prior work's results. As self-optimisation and kinetic modelling can now be combined in a single step, this newly developed approach is considerably more efficient. To conclude, while both approaches are of relevance to research and industry alike, in terms of collecting kinetic data, the newly-proposed approach is more suitable when a quick assessment is to be obtained. By accelerating the process of gathering kinetic data, this newly proposed approach assists in understanding chemical reactions at hand.
Real-time optimisation
Independent of the type of disturbance, the employed real-time optimisation reliably recognized and solved the issue. Thus, as soon as product quality began to decrease, the simplex algorithm stepped in to remedy the disturbance. For this purpose, the simplex algorithm autonomously decided which experiment to perform next, based on previous results.
All disturbances were successfully compensated after just a few experiments. For the short time during which product quality inadvertently decreases (i.e., the time needed until the simplex algorithm steps in and identifies new reaction conditions that offset the disturbance), the corrupted product output should simply be disposed by using a bypass.
To summarize, this improvement to the simplex algorithm may assist in preventing production process failures in lab and industrial applications, without the need for human intervention. The transfer from lab to production scale can easily be implemented. It is particularly useful for self-optimisation in small-scale production scenarios, and when production inputs and their ratios change frequently.
Conclusions
This article describes a fully automated, self-optimising platform that autonomously guides chemical processes towards ideal reaction conditions whilst reducing the need for human intervention. Experimental data was collected in real-time and used as feedback to decide on the next experimental conditions to steer the reaction towards its optimum.Moreover, the performance of two different optimisation strategies was measured and compared: modified simplex algorithm and model-free design of experiments. It was demonstrated that both methods can be applied as robust and reliable optimisation strategies within the derived autonomous, self-optimising system. For the investigated reaction of benzaldehyde 1 with benzylamine 2 to form n-benzylidenebenzylamine 3, globally optimal reaction conditions could always be identified. Hence, all studied optimisation problems were successfully solved within a single working day.
The derived self-optimising platform contains an automated flow microreactor system that is combined with inline FT-IR spectroscopy. The use of inline FT-IR spectroscopy circumvents waiting times between the measurement itself and the availability of its analytical result. The latter is directly transferred to a respective optimisation algorithm. Consequently, the overall waiting time between two measurements depends only on the reaction kinetics (since the system always waits for the re-establishing of steady-state reaction conditions). Data on the latter may be collected concurrently while performing the optimisation process, hence allowing further time savings.
Moreover, an enhancement to the simplex algorithm rendered the platform capable of autonomously responding to disturbances of the chemical process in real-time. The incorporation of a real-time optimisation strategy thus guaranteed consistently high product quality, irrespective of external disruptive factors.
To conclude, the derived autonomous platform enables multi-variate and multi-objective optimisations in real-time, constituting a modular and flexible system with high efficiency and of considerable industrial relevance. It is easily transferable from lab to production scale and is suited for a broad range of applications. This reduces the time needed for process development and lowers the barriers for its application. Finally, other optimisation strategies besides simplex algorithm and design of experiments can easily be integrated into the platform.
Definitions
| 1 [—] | Benzaldehyde |
| 2 [—] | Benzylamine |
| 3 [—] | n-Benzylidenebenzylamine |
| c j [mol L−1] | Concentration of compound j |
| EA,i [kJ mol−1] | Activation energy of reaction i |
| k ref [L mol−1 s−1] | Reaction rate coefficient at reference temperature |
| T ref [K] | Reference temperatures |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the German Federal Ministry of Education and Research (BMBF), programme FH Impuls – Partnership for Innovation M2Aind, project SM2all (grant No. 13FH8I01IA). The authors would like to thank Stefanie Szmais, Philipp Lau, and Christoph Greve (Merck KGaA, Darmstadt) for valuable discussions, and Jonathan Geiger, Kai Tornow, and Marius Graute (Mannheim University of Applied Sciences) for great technical support. Moreover, the authors would like to thank Scale-up Systems Ltd. (Dublin, Ireland) for providing the software DynoChem, especially Dr. Wilfried Hoffmann and Steve Cropper.References
- F. H. Walters, L. R. Parker, S. L. Morgan and S. N. Deming, Sequential simplex optimization: A technique for improving quality and productivity in research, development, and manufacturing, Chemometrics series, CRC Press, Boca Raton, 1991 Search PubMed.
- R. Wehrens and L. M. C. Buydens, in Encyclopedia of Analytical Chemistry, ed. R. A. Meyers, John Wiley & Sons, Chichester, 2006 Search PubMed.
- T. Schlick, in Reviews in Computational Chemistry, ed. K. B. Lipkowitz and D. B. Boyd, VCH Publishers, New York, 1992 Search PubMed.
- B. J. Reizman and K. F. Jensen, An Automated Continuous-Flow Platform for the Estimation of Multistep Reaction Kinetics, Org. Process Res. Dev., 2012, 16(11), 1770–1782, DOI:10.1021/op3001838.
- J. P. McMullen and K. F. Jensen, Integrated microreactors for reaction automation: new approaches to reaction development, Annu. Rev. Anal. Chem., 2010, 3, 19–42, DOI:10.1146/annurev.anchem.111808.073718.
- M. Rasheed and T. Wirth, Intelligenter Mikrofluss, Angew. Chem., 2011, 123(2), 374–376, DOI:10.1002/ange.201006107.
- D. C. Fabry, E. Sugiono and M. Rueping, Self-Optimizing Reactor Systems, Isr. J. Chem., 2014, 54(4), 341–350, DOI:10.1002/ijch.201300080.
- S. V. Ley, D. E. Fitzpatrick, R. M. Myers, C. Battilocchio and R. J. Ingham, Machine-Assisted Organic Synthesis, Angew. Chem., Int. Ed., 2015, 54(35), 10122–10136, DOI:10.1002/anie.201501618.
- C. Houben and A. A. Lapkin, Automatic discovery and optimization of chemical processes, Curr. Opin. Chem. Eng., 2015, 9, 1–7, DOI:10.1016/j.coche.2015.07.001.
- D. C. Fabry, E. Sugiono and M. Rueping, Online monitoring and analysis for autonomous continuous flow self-optimizing reactor systems, React. Chem. Eng., 2016, 1(2), 129–133, 10.1039/c5re00038f.
- V. Sans and L. Cronin, Towards dial-a-molecule by integrating continuous flow, analytics and self-optimisation, Chem. Soc. Rev., 2016, 45(8), 2032–2043, 10.1039/c5cs00793c.
- K. F. Jensen, Flow chemistry - Microreaction technology comes of age, AIChE J., 2017, 63(3), 858–869, DOI:10.1002/aic.15642.
- A.-C. Bédard, A. Adamo, K. C. Aroh, M. G. Russell, A. A. Bedermann, J. Torosian, B. Yue, K. F. Jensen and T. F. Jamison, Reconfigurable system for automated optimization of diverse chemical reactions, Science, 2018, 361(6408), 1220–1225, DOI:10.1126/science.aat0650.
- O. W. Gooding, Process optimization using combinatorial design principles: parallel synthesis and design of experiment methods, Curr. Opin. Chem. Biol., 2004, 8(3), 297–304, DOI:10.1016/j.cbpa.2004.04.009.
- P. M. Murray, F. Bellany, L. Benhamou, D.-K. Bučar, A. B. Tabor and T. D. Sheppard, The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry, Org. Biomol. Chem., 2016, 14(8), 2373–2384, 10.1039/c5ob01892g.
- R. Leardi, Experimental design in chemistry, Anal. Chim. Acta, 2009, 652(1–2), 161–172, DOI:10.1016/j.aca.2009.06.015.
- R. Carlson and J. E. Carlson, Design and optimization in organic synthesis, Data handling in science and technology, Elsevier, Amsterdam, 2005 Search PubMed.
- D. R. Pilipauskas, in Process Chemistry in the Pharmaceutical Industry, ed. K. Gadamasetti, Marcel Dekker Inc., New York, 1999 Search PubMed.
- G. E. P. Box, J. S. Hunter and W. G. Hunter, Statistics for experimenters: Design, innovation, and discovery, Wiley, Weinheim, 2005 Search PubMed.
- J. A. Nelder and R. Mead, A Simplex Method for Function Minimization, Comput. J., 1965, 7(4), 308–313, DOI:10.1093/comjnl/7.4.308.
- P. Gans, Numerical methods for data- fitting problems, Coord. Chem. Rev., 1976, 19(2), 99–124, DOI:10.1016/S0010-8545(00)80313-6.
- A. D. Clayton, J. A. Manson, C. J. Taylor, T. W. Chamberlain, B. A. Taylor, G. Clemens and R. A. Bourne, Algorithms for the self-optimisation of chemical reactions, React. Chem. Eng., 2019, 4(9), 1545–1554, 10.1039/c9re00209j.
- V. Cerdà, J. L. Cerdà and A. M. Idris, Optimization using the gradient and simplex methods, Talanta, 2016, 148, 641–648, DOI:10.1016/j.talanta.2015.05.061.
- M. Fernanda Giné, R. L. Tuon, A. A. Cesta, A. Paula Packer and B. F. Reis, Real-time simplex optimization of flow-injection systems for chemical analysis, Anal. Chim. Acta, 1998, 366(1–3), 313–318, DOI:10.1016/S0003-2670(98)00158-5.
- J. P. McMullen and K. F. Jensen, An Intelligent Microreactor System for Real-Time Optimization of a Chemical Reaction, Proc. Int. Conf. Miniat. Syst. Chem. Life Sci., 2008, 12, 1907–1909 Search PubMed.
- J. P. McMullen and K. F. Jensen, An Automated Microfluidic System for Online Optimization in Chemical Synthesis, Org. Process Res. Dev., 2010, 14(5), 1169–1176, DOI:10.1021/op100123e.
- J. P. McMullen, M. T. Stone, S. L. Buchwald and K. F. Jensen, An integrated microreactor system for self-optimization of a Heck reaction: from micro- to mesoscale flow systems, Angew. Chem., Int. Ed., 2010, 49(39), 7076–7080, DOI:10.1002/anie.201002590.
- D. N. Jumbam, R. A. Skilton, A. J. Parrott, R. A. Bourne and M. Poliakoff, The Effect of Self-Optimisation Targets on the Methylation of Alcohols Using Dimethyl Carbonate in Supercritical CO2, J. Flow Chem., 2012, 2(1), 24–27, DOI:10.1556/jfchem.2012.00019.
- R. A. Skilton, A. J. Parrott, M. W. George, M. Poliakoff and R. A. Bourne, Real-time feedback control using online attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy for continuous flow optimization and process knowledge, Appl. Spectrosc., 2013, 67(10), 1127–1131, DOI:10.1366/13-06999.
- V. Sans, L. Porwol, V. Dragone and L. Cronin, A self optimizing synthetic organic reactor system using real-time in-line NMR spectroscopy, Chem. Sci., 2015, 6(2), 1258–1264, 10.1039/c4sc03075c.
- D. Cortés-Borda, K. V. Kutonova, C. Jamet, M. E. Trusova, F. Zammattio, C. Truchet, M. Rodriguez-Zubiri and F.-X. Felpin, Optimizing the Heck–Matsuda Reaction in Flow with a Constraint-Adapted Direct Search Algorithm, Org. Process Res. Dev., 2016, 20(11), 1979–1987, DOI:10.1021/acs.oprd.6b00310.
- D. E. Fitzpatrick, C. Battilocchio and S. V. Ley, A Novel Internet-Based Reaction Monitoring, Control and Autonomous Self-Optimization Platform for Chemical Synthesis, Org. Process Res. Dev., 2016, 20(2), 386–394, DOI:10.1021/acs.oprd.5b00313.
- D. Cortés-Borda, E. Wimmer, B. Gouilleux, E. Barré, N. Oger, L. Goulamaly, L. Peault, B. Charrier, C. Truchet, P. Giraudeau, M. Rodriguez-Zubiri, E. Le Grognec and F.-X. Felpin, An Autonomous Self-Optimizing Flow Reactor for the Synthesis of Natural Product Carpanone, J. Org. Chem., 2018, 83(23), 14286–14299, DOI:10.1021/acs.joc.8b01821.
- E. Wimmer, D. Cortés-Borda, S. Brochard, E. Barré, C. Truchet and F.-X. Felpin, An autonomous self-optimizing flow machine for the synthesis of pyridine–oxazoline (PyOX) ligands, React. Chem. Eng., 2019, 4(9), 1608–1615, 10.1039/c9re00096h.
- L. Eriksson, E. Johansson, N. Kettaneh-Wold, C. Wikström and S. Wold, Design of experiments: Principles and applications, Umetrics Academy - training in multivariate technology, Umetrics AB, Umea, 2000 Search PubMed.
- R. L. Mason, R. F. Gunst and J. L. Hess, Statistical design and analysis of experiments: With applications to engineering and science, Wiley, Weinheim, 2003 Search PubMed.
- V. K. Aggarwal, A. C. Staubitz and M. Owen, Optimization of the Mizoroki−Heck Reaction Using Design of Experiment (DoE), Org. Process Res. Dev., 2006, 10(1), 64–69, DOI:10.1021/op058013q.
- A. R. Alimardanov, M. T. Barrila, F. R. Busch, J. J. Carey, M. A. Couturier and C. Cui, Use of DOE for Rapid Development of a Red-Al Reduction Process for the Synthesis of 3,4-Isopropylidenedioxypyrrolidine Hydrotosylate, Org. Process Res. Dev., 2004, 8(6), 834–837, DOI:10.1021/op040204q.
- Y. Bayat, H. Ebrahimi and F. Fotouhi-Far, Optimization of Reductive Debenzylation of Hexabenzylhexaazaisowurtzitane (the Key Step for Synthesis of HNIW) Using Response Surface Methodology, Org. Process Res. Dev., 2012, 16(11), 1733–1738, DOI:10.1021/op300162d.
- J. J. Chen, T. C. Nugent, C. V. Lu, S. Kondapally, P. Giannousis, Y. Wang and J. T. Wilmot, Rapid Improvement of a Reductive Sulfonylation Using Design of Experiment Methods, Org. Process Res. Dev., 2003, 7(3), 313–317, DOI:10.1021/op034018g.
- A. Ekebergh, C. Lingblom, P. Sandin, C. Wennerås and J. Mårtensson, Exploring a cascade Heck-Suzuki reaction based route to kinase inhibitors using design of experiments, Org. Biomol. Chem., 2015, 13(11), 3382–3392, 10.1039/c4ob02694b.
- D. F. Emiabata-Smith, D. L. Crookes and M. R. Owen, A Practical Approach to Accelerated Process Screening and Optimisation, Org. Process Res. Dev., 1999, 3(4), 281–288, DOI:10.1021/op990016d.
- M. D. Evans, J. Ring, A. Schoen, A. Bell, P. Edwards, D. Berthelot, R. Nicewonger and C. M. Baldino, The accelerated development of an optimized synthesis of 1,2,4-oxadiazoles: application of microwave irradiation and statistical design of experiments, Tetrahedron Lett., 2003, 44(52), 9337–9341, DOI:10.1016/j.tetlet.2003.10.055.
- A. L. García-Cabeza, R. Marín-Barrios, R. Azarken, F. J. Moreno-Dorado, M. J. Ortega, H. Vidal, J. M. Gatica, G. M. Massanet and F. M. Guerra, DoE (Design of Experiments) Assisted Allylic Hydroxylation of Enones Catalysed by a Copper-Aluminium Mixed Oxide, Eur. J. Org. Chem., 2013, 2013(36), 8307–8314, DOI:10.1002/ejoc.201301145.
- O. W. Gooding, L. Vo, S. Bhattacharyya and J. W. Labadie, Use of Statistical Design of Experiments in the Optimization of Amide Synthesis Utilizing Polystyrene-Supported N -Hydroxybenzotriazole Resin, J. Comb. Chem., 2002, 4(6), 576–583, DOI:10.1021/cc0200282.
- G. Guercio, A. Perboni, F. Tinazzi, L. Rovatti and S. Provera, The Synthesis of GV143253A: A Case Study for the Use of Analytical and Statistical Tools to Elucidate the Reaction Mechanism and to Optimize the Process, Org. Process Res. Dev., 2010, 14(4), 840–848, DOI:10.1021/op100097c.
- V. Hajzer, P. Alexy, A. Latika, J. Durmis and R. Šebesta, Optimization of stereoselective Michael addition of 2-(pentan-3-yloxy)acetaldehyde to N-[(Z)-2-nitroethenyl]acetamide with the aid of design of experiments, Monatsh. Chem., 2015, 146(9), 1541–1545, DOI:10.1007/s00706-015-1486-8.
- P. A. Hopes, A. J. Parker and I. Patel, Development and Optimisation of an Unsymmetrical Hantzsch Reaction for Plant-Scale Manufacture, Org. Process Res. Dev., 2006, 10(4), 808–813, DOI:10.1021/op060057r.
- C. Jamieson, M. S. Congreve, D. Emiabata-Smith and S. V. Ley, A Rapid Approach for the Optimisation of Polymer Supported Reagents in Synthesis, Synlett, 2000, 11, 1603–1607 Search PubMed.
- C. Jamieson, M. S. Congreve, D. F. Emiabata-Smith, S. V. Ley and J. J. Scicinski, Application of ReactArray Robotics and Design of Experiments Techniques in Optimisation of Supported Reagent Chemistry, Org. Process Res. Dev., 2002, 6(6), 823–825, DOI:10.1021/op010108e.
- J. T. Kuethe, D. M. Tellers, S. A. Weissman and N. Yasuda, Development of a Sequential Tetrahydropyran and Tertiary Butyl Deprotection: High-Throughput Experimentation, Mechanistic Analysis, and DOE Optimization, Org. Process Res. Dev., 2009, 13(3), 471–477, DOI:10.1021/op8002739.
- H. Tye and M. Whittaker, Use of a Design of Experiments approach for the optimisation of a microwave assisted Ugi reaction, Org. Biomol. Chem., 2004, 2(6), 813–815, 10.1039/b400298a.
- R. W. Wagner, F. Li, H. Du and J. S. Lindsey, Investigation of Cocatalysis Conditions Using an Automated Microscale Multireactor Workstation: Synthesis of meso -Tetramesitylporphyrin, Org. Process Res. Dev., 1999, 3(1), 28–37, DOI:10.1021/op9800459.
- L. Synoradzki, D. Jańczewski and M. Włostowski, Optimisation of Ethyl(2-phthalimidoethoxy)acetate Synthesis with the Aid of DOE, Org. Process Res. Dev., 2005, 9(1), 18–22, DOI:10.1021/op030029y.
- L. Vo, J. Ciula and O. W. Gooding, Chemical Development on the Chiral Auxiliary (S )-4-(Phenylmethyl)-2-oxazolidinone Utilizing Automated Synthesis and DoE, Org. Process Res. Dev., 2003, 7(4), 514–520, DOI:10.1021/op034033l.
- S. Stone, T. Wang, J. Liang, J. Cochran, J. Green and W. Gu, Application of design of experiments (DoE) optimization to the one-pot synthesis of 4,6-dihydropteridinones, Org. Biomol. Chem., 2015, 13(42), 10471–10476, 10.1039/c5ob01154j.
- O. Benali, M. Deal, E. Farrant, D. Tapolczay and R. Wheeler, Continuous Flow Microwave-Assisted Reaction Optimization and Scale-Up Using Fluorous Spacer Technology, Org. Process Res. Dev., 2008, 12(5), 1007–1011, DOI:10.1021/op700225u.
- R. J. Ingham, C. Battilocchio, J. M. Hawkins and S. V. Ley, Integration of enabling methods for the automated flow preparation of piperazine-2-carboxamide, Beilstein J. Org. Chem., 2014, 10, 641–652, DOI:10.3762/bjoc.10.56.
- S. Mostarda, P. Filipponi, R. Sardella, F. Venturoni, B. Natalini, R. Pellicciari and A. Gioiello, Glucuronidation of bile acids under flow conditions: design of experiments and Koenigs-Knorr reaction optimization, Org. Biomol. Chem., 2014, 12(47), 9592–9600, 10.1039/C4OB01911C.
- B. J. Reizman and K. F. Jensen, Simultaneous solvent screening and reaction optimization in microliter slugs, Chem. Commun., 2015, 51(68), 13290–13293, 10.1039/c5cc03651h.
- N. Holmes, G. R. Akien, R. J. D. Savage, C. Stanetty, I. R. Baxendale, A. J. Blacker, B. A. Taylor, R. L. Woodward, R. E. Meadows and R. A. Bourne, Online quantitative mass spectrometry for the rapid adaptive optimisation of automated flow reactors, React. Chem. Eng., 2016, 1(1), 96–100, 10.1039/c5re00083a.
- A. Echtermeyer, Y. Amar, J. Zakrzewski and A. Lapkin, Self-optimisation and model-based design of experiments for developing a C-H activation flow process, Beilstein J. Org. Chem., 2017, 13, 150–163, DOI:10.3762/bjoc.13.18.
- M. M. E. Delville, P. J. Nieuwland, P. Janssen, K. Koch, J. C. M. van Hest and F. P. J. T. Rutjes, Continuous flow azide formation: Optimization and scale-up, Chem. Eng. J., 2011, 167(2–3), 556–559, DOI:10.1016/j.cej.2010.08.087.
- P. J. Nieuwland, R. Segers, K. Koch, J. C. M. van Hest and F. P. J. T. Rutjes, Fast Scale-Up Using Microreactors: Pyrrole Synthesis from Micro to Production Scale, Org. Process Res. Dev., 2011, 15(4), 783–787, DOI:10.1021/op100338z.
- K. Koch, B. J. A. van Weerdenburg, J. M. M. Verkade, P. J. Nieuwland, F. P. J. T. Rutjes and J. C. M. van Hest, Optimizing the Deprotection of the Amine Protecting p -Methoxyphenyl Group in an Automated Microreactor Platform, Org. Process Res. Dev., 2009, 13(5), 1003–1006, DOI:10.1021/op900139u.
- J. P. McMullen and K. F. Jensen, Rapid Determination of Reaction Kinetics with an Automated Microfluidic System, Org. Process Res. Dev., 2011, 15(2), 398–407, DOI:10.1021/op100300p.
- P. J. Nieuwland, K. Koch, N. van Harskamp, R. Wehrens, J. C. M. van Hest and F. P. J. T. Rutjes, Flash chemistry extensively optimized, Chem. – Asian J., 2010, 5(4), 799–805, DOI:10.1002/asia.200900705.
- B. J. Reizman, Y.-M. Wang, S. L. Buchwald and K. F. Jensen, Suzuki-Miyaura cross-coupling optimization enabled by automated feedback, React. Chem. Eng., 2016, 1(6), 658–666, 10.1039/c6re00153j.
- B. J. Reizman and K. F. Jensen, Feedback in Flow for Accelerated Reaction Development, Acc. Chem. Res., 2016, 49(9), 1786–1796, DOI:10.1021/acs.accounts.6b00261.
- D. Sleveland and H.-R. Bjørsvik, Synthesis of Phenylboronic Acids in Continuous Flow by Means of a Multijet Oscillating Disc Reactor System Operating at Cryogenic Temperatures, Org. Process Res. Dev., 2012, 16(5), 1121–1130, DOI:10.1021/op3000493.
- J. S. Moore and K. F. Jensen, in Microreactors in organic chemistry and catalysis, ed. T. Wirth, Wiley-VCH, Weinheim, 2013 Search PubMed.
- N. Kockmann, Digital methods and tools for chemical equipment and plants, React. Chem. Eng., 2019, 4(9), 1522–1529, 10.1039/C9RE00017H.
- J. Wegner, S. Ceylan and A. Kirschning, Ten key issues in modern flow chemistry, Chem. Commun., 2011, 47(16), 4583–4592, 10.1039/C0CC05060A.
- L. Vaccaro, D. Lanari, A. Marrocchi and G. Strappaveccia, Flow approaches towards sustainability, Green Chem., 2014, 16(8), 3680–3704, 10.1039/C4GC00410H.
- B. Gutmann, D. Cantillo and C. O. Kappe, Continuous-flow technology - a tool for the safe manufacturing of active pharmaceutical ingredients, Angew. Chem., Int. Ed., 2015, 54(23), 6688–6728, DOI:10.1002/anie.201409318.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, The Hitchhiker's Guide to Flow Chemistry, Chem. Rev., 2017, 117(18), 11796–11893, DOI:10.1021/acs.chemrev.7b00183.
- J.-I. Yoshida, Flash chemistry: Fast organic synthesis in microsystems, Wiley, Weinheim, 2008 Search PubMed.
- D. M. Roberge, L. Ducry, N. Bieler, P. Cretton and B. Zimmermann, Microreactor Technology: A Revolution for the Fine Chemical and Pharmaceutical Industries?, Chem. Eng. Technol., 2005, 28(3), 318–323, DOI:10.1002/ceat.200407128.
- T. Wirth, Novel Organic Synthesis through Ultrafast Chemistry, Angew. Chem., Int. Ed., 2017, 56(3), 682–684, DOI:10.1002/anie.201609595.
- J.-I. Yoshida, A. Nagaki and T. Yamada, Flash chemistry, Chem. – Eur. J., 2008, 14(25), 7450–7459, DOI:10.1002/chem.200800582.
- V. Hessel, D. Kralisch and N. Kockmann, Novel Process Windows: Innovative Gates to Intensified and Sustainable Chemical Processes, Wiley, Weinheim, 2015 Search PubMed.
- V. Hessel, D. Kralisch, N. Kockmann, T. Noël and Q. Wang, Novel process windows for enabling, accelerating, and uplifting flow chemistry, ChemSusChem, 2013, 6(5), 746–789, DOI:10.1002/cssc.201200766.
- K. F. Jensen, Microreaction engineering, Chem. Eng. Sci., 2001, 56(2), 293–303, DOI:10.1016/S0009-2509(00)00230-X.
- C. Wiles and P. Watts, Micro Reaction Technology in Organic Synthesis, CRC Press, Boca Raton, 2016 Search PubMed.
- S. Schwolow, J. Y. Ko, N. Kockmann and T. Röder, Enhanced heat transfer by exothermic reactions in laminar flow capillary reactors, Chem. Eng. Sci., 2016, 141, 356–362, DOI:10.1016/j.ces.2015.11.022.
- N. Kockmann, M. Gottsponer, B. Zimmermann and D. M. Roberge, Enabling continuous-flow chemistry in microstructured devices for pharmaceutical and fine-chemical production, Chem. – Eur. J., 2008, 14(25), 7470–7477, DOI:10.1002/chem.200800707.
- N. Kockmann, Transport Phenomena in Micro Process Engineering, Heat and mass transfer, Springer-Verlag, Berlin, 2008 Search PubMed.
- R. L. Hartman, J. P. McMullen and K. F. Jensen, Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis, Angew. Chem., Int. Ed., 2011, 50(33), 7502–7519, DOI:10.1002/anie.201004637.
- K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Chemistry in microstructured reactors, Angew. Chem., Int. Ed., 2004, 43(4), 406–446, DOI:10.1002/anie.200300577.
- M. Movsisyan, E. I. P. Delbeke, J. K. E. T. Berton, C. Battilocchio, S. V. Ley and C. V. Stevens, Taming hazardous chemistry by continuous flow technology, Chem. Soc. Rev., 2016, 45(18), 4892–4928, 10.1039/C5CS00902B.
- J.-I. Yoshida, Y. Takahashi and A. Nagaki, Flash chemistry: flow chemistry that cannot be done in batch, Chem. Commun., 2013, 49(85), 9896–9904, 10.1039/c3cc44709j.
- N. Kockmann, P. Thenée, C. Fleischer-Trebes, G. Laudadio and T. Noël, Safety assessment in development and operation of modular continuous-flow processes, React. Chem. Eng., 2017, 2(3), 258–280, 10.1039/c7re00021a.
- V. Fath, P. Lau, C. Greve, N. Kockmann and T. Röder, Efficient Kinetic Data Acquisition and Model Prediction: Continuous Flow Microreactors, Inline FT-IR Spectroscopy, and Self-modeling Curve Resolution, Org. Process Res. Dev., 2020 DOI:10.1021/acs.oprd.0c00037.
- P. J. Kitson, M. H. Rosnes, V. Sans, V. Dragone and L. Cronin, Configurable 3D-Printed millifluidic and microfluidic 'lab on a chip' reactionware devices, Lab Chip, 2012, 12(18), 3267–3271, 10.1039/C2LC40761B.
- C. Grosjean, The strecker reaction of benzaldehyde, amines and cyanide: some mechanistic and synthetic studies, Doctoral thesis, Durham University, http://etheses.dur.ac.uk/3043/, 2004 Search PubMed.
- J. Krupka, L. Dluhoš and L. Mrózek, Evaluation of Benzylamine Production via Reductive Amination of Benzaldehyde in a Slurry Reactor, Chem. Eng. Technol., 2017, 40(5), 870–877, DOI:10.1002/ceat.201600538.
- R. W. Layer, The Chemistry of Imines, Chem. Rev., 1963, 63(5), 489–510, DOI:10.1021/cr60225a003.
- M.-K. Lee, H.-S. Kim, H.-J. Rhee and J.-B. Choo, Reaction Monitoring of Imine Synthesis Using Raman Spectroscopy, Bull. Korean Chem. Soc., 2003, 24(2), 205–208, DOI:10.5012/bkcs.2003.24.2.205.
- A. T. Nielsen, R. A. Nissan and D. J. Vanderah, Polyazapolycyclics by Condensation of Aldehydes with Amines, J. Org. Chem., 1990, 55(5), 1459–1466 CrossRef CAS.
- A. Simion, C. Simion, T. Kanda, S. Nagashima, Y. Mitoma, T. Yamada, K. Mimura and M. Tashiro, Synthesis of imines, diimines and macrocyclic diimines as possible ligands, in aqueous solution, J. Chem. Soc., Perkin Trans. 1, 2001,(17), 2071–2078, 10.1039/b102749m.
- H. Wensink, F. Benito-Lopez, D. C. Hermes, W. Verboom, H. J. G. E. Gardeniers, D. N. Reinhoudt and A. van den Berg, Measuring reaction kinetics in a lab-on-a-chip by microcoil NMR, Lab Chip, 2005, 5(3), 280–284, 10.1039/b414832k.
- V. Fath, N. Kockmann and T. Röder, In-situ Reaction Monitoring of Unstable Lithiated Intermediates through Inline FT-IR Spectroscopy: A Mechanistic Investigation, Chem. Eng. Technol., 2019, 42(10), 2095–2104, DOI:10.1002/ceat.201900074.
- M. A. Bezerra, Q. O. dos Santos, A. G. Santos, C. G. Novaes, S. L. C. Ferreira and V. S. de Souza, Simplex optimization: A tutorial approach and recent applications in analytical chemistry, Microchem. J., 2016, 124, 45–54, DOI:10.1016/j.microc.2015.07.023.
- S. L. C. Ferreira, R. E. Bruns, H. S. Ferreira, G. D. Matos, J. M. David, G. C. Brandão, E. G. P. da Silva, L. A. Portugal, P. S. dos Reis, A. S. Souza and W. N. L. dos Santos, Box-Behnken design: an alternative for the optimization of analytical methods, Anal. Chim. Acta, 2007, 597(2), 179–186, DOI:10.1016/j.aca.2007.07.011.
- K. Siebertz, D. van Bebber and T. Hochkirchen, Statistische Versuchsplanung: Design of Experiments (DoE), Vieweg, Berlin, 2017 Search PubMed.
- S. A. Weissman and N. G. Anderson, Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications, Org. Process Res. Dev., 2015, 19(11), 1605–1633, DOI:10.1021/op500169m.
- A. Gioiello, V. Mancino, P. Filipponi, S. Mostarda and B. Cerra, Concepts and optimization strategies of experimental design in continuous-flow processing, J. Flow Chem., 2016, 6(3), 167–180, DOI:10.1556/1846.2016.00012.
Footnotes |
| † Electronic supplementary information (ESI) available: A closer look at the experimental procedure, including analytical method, additional description of optimisation workflow, details on optimisation strategies, and further results, is provided in the supporting information section. See DOI: 10.1039/d0re00081g |
| ‡ Both optimisation strategies are subordinate to the implemented safety measures. Thus, if, for instance, incorrect boundaries (regarding the experimental space) were set for a start simplex, thereby threatening operational safety, the safety measures would intervene, (re-)establishing safe operating conditions. In this case, the self-optimising process would come to a halt. |
| § Note that both programmed algorithms (simplex and DoE) work in a model-free manner. Thus, commencing the self-optimisation process requires solely information on the permissible boundaries to the experimental space. No prior knowledge on the underlying kinetics is required. ESI† A.2 provides further information on this matter. |
| ¶ Whether a given corner point solution is successfully identified by a simplex algorithm depends on the geometric location of the last iteration that can still be carried out experimentally. Further details are given in the ESI† A.2. |
| This journal is © The Royal Society of Chemistry 2020 |