Open Access Article
Open Access ArticleSelective complexation and efficient separation of cis/trans-1,2-dichloroethene isomers by a pillar[5]arene†
Bin Li ab,
Kaidi Xua,
Yiliang Wang
ab,
Kaidi Xua,
Yiliang Wang a,
Hang Sua,
Lei Cuia and
Chunju Li
a,
Hang Sua,
Lei Cuia and
Chunju Li *ab
*ab
aCollege of Science, Center for Supramolecular Chemistry and Catalysis, Shanghai University, Shanghai 200444, P. R. China. E-mail: cjli@shu.edu.cn
bKey Laboratory of Inorganic-Organic Hybrid Functional Material Chemistry, Ministry of Education, Tianjin Key Laboratory of Structure and Performance for Functional Molecules, College of Chemistry, Tianjin Normal University, Tianjin 300387, P. R. China
First published on 21st December 2020
Abstract
The complexation and separation of industrially important cis- and trans-1,2-dichloroethene (cis- and trans-DCE) isomers using perethylated pillar[5]arene (EtP5) are described. EtP5 exhibits considerable binding capability for the trans-DCE isomer over the cis-DCE in organic solution. Furthermore, nonporous adaptive crystals (NACs) of EtP5 can efficiently separate trans-DCE from a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) cis/trans-isomer mixture.
50 (v/v) cis/trans-isomer mixture.
1,2-Dichloroethenes (DCEs), including cis-DCE and trans-DCE isomers, are high-value chemicals with a variety of uses in synthetic chemistry and chemical industry.1 They are widely applied as low-temperature extraction solvents for heat sensitive substances and feedstocks for synthesizing copolymer materials with other unsaturated monomers.2 trans-DCE and cis-DCE can be also used as an environment-friendly refrigerant and foaming additive agent, respectively. In the industrial production process, they are produced as a mixture of cis and trans-DCE isomers by direct chlorination of acetylene or by the pyrolytic dehydrochlorination of 1,1,2-trichloroethane.3 In most cases, the two isomers must be used separately. However, the separation of cis- and trans-DCE isomers is difficult due to their similar molecular sizes and close boiling points. Industrially, although both isomers can be separated by fractional distillation with very high columns, this process is high energy-consumption and environmentally unfriendly.
Crystalline porous materials such as zeolites,4,5 metal–organic frameworks (MOFs)6–9 and covalent organic frameworks (COFs)10 have been described as promising adsorbents for the economical and energy-efficient separation of hydrocarbons (e.g., alkanes, alkenes and benzene derivatives). However, the examples of the physisorptive separation of cis- and trans-isomers are relatively scarce.11–14 Recently, a novel class of macrocycle-based crystalline materials, termed as nonporous adaptive crystals (NACs) of pillar[n]arenes, has shown interesting adsorption/separation properties.15–21 Subsequently, some new NACs based on other important macrocycles22 such as biphen[n]arene,23 leaning towerarene,24,25 tiararene,26 geminiarene,27 hybrid[3]arene,28 naphthotubes29 and cucurbit[6]uril30 have been developed. In comparison with traditional porous materials, which usually possess large surface area, NACs are nonporous and structurally adaptive in the initial crystalline state. The intrinsic or extrinsic porosity inside macrocycle crystals could be opened by capturing preferable vaporized molecules, forming corresponding host–guest crystal structures along with a solid-state structural transformation. This unique feature enables NACs to work as adsorptive materials in adsorption and separation at the solid-vapor phase.
Very recently, our group used NACs of 2,2′,4,4′-biphen[3]arene (MeBP3α) to separate cis-DCE from trans-DCE with a purity of 96.4% in the solid-vapor state.23 Crystal structure of cis-DCE@MeBP3 reveals that cis-DCE molecules are not encapsulated into macrocycle cavities, but located in self-assembled extrinsic porosity. Here, we present that perethylated pillar[5]arenes (EtP5) display a totally opposite selectivity to preferentially bind trans-DCE over its cis-isomer not only in solution but also in the solid state. The selectivity comes from the suitability of size/shape between EtP5's cavity and trans-DCE. NACs of EtP5 (EtP5α) efficiently separate trans-DCE from the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) mixture of cis/trans-DCE isomers with 94.94% purity (Fig. 1), accompanied by adsorption induced crystalline structure transformation. More intriguingly, when EtP5α and MeBP3α are exposed to cis- and trans-DCE mixed vapor, they exhibit self-sorting adsorption behavior for the two isomers, i.e. producing trans-DCE@EtP5 and cis-DCE@MeBP3.
50 (v/v) mixture of cis/trans-DCE isomers with 94.94% purity (Fig. 1), accompanied by adsorption induced crystalline structure transformation. More intriguingly, when EtP5α and MeBP3α are exposed to cis- and trans-DCE mixed vapor, they exhibit self-sorting adsorption behavior for the two isomers, i.e. producing trans-DCE@EtP5 and cis-DCE@MeBP3.
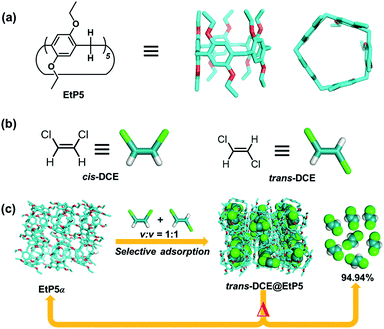 | ||
| Fig. 1 Chemical structures of (a) EtP5 and (b) cis-DCE and trans-DCE. (c) Schematic representation of the cis/trans-DCE isomers separation and the structural transformation. | ||
As we know, pillar[n]arenes, as important family of supramolecular macrocycles with prism-like geometries and π-rich cavities, have shown interesting cavity host–guest properties towards a variety of guest molecules.31–41 The most peculiar complexation behavior of pillar[n]arenes is that pillar[5]arenes can strongly bind suitable neutral molecules in organic solution compared with other popular macrocycles.42–46
First, we tested the host–guest complexation between cis- or trans-DCE and EtP5 in solution by 1H NMR spectroscopy (Fig. S1 and S2, ESI†). As shown in Fig. 2, when equimolar EtP5 was added into an o-xylene-d10 solution containing cis- or trans-DCE (5.0 mM), the signal related to the protons on trans-DCE exhibits a very significant upfield shift of −0.52 ppm as well as an extensive broadening effect compared with the free trans-DCE. The reason is that trans-DCE molecule was encapsulated in the cavity of EtP5 forming a threaded structure. Simultaneously, the aromatic protons on EtP5 moved downfield with slight chemical shift as a result of the interactions with trans-DCE. However, no obvious signal changes were observed when cis-DCE and EtP5 were mixed in o-xylene-d10, suggesting that cis-DCE can not be contained in the cavity of EtP5. trans-DCE molecule with stretched structure is more suitable for the cavity of EtP5.45 It was observed that the inclusion-induced upfield shift of trans-DCE (Δδ = −0.25 ppm for Ha) in CDCl3 was smaller than those observed in o-xylene-d10 (Fig. S3 and S4, ESI†), suggesting that stronger host–guest interactions occurred in the non-polar o-xylene-d10 solution. For comparison purpose, the interaction between perethylated pillar[6]arene (EtP6) and cis- or trans-DCE was also investigated. No obvious complexation was detected (Fig. S5 and S6, ESI†), which is reasonable that the guests are too small in comparison with EtP6's cavity. To determine the binding affinity of EtP5 to trans-DCE, 1H NMR titration methods were employed with the concentration of EtP5 kept constant at 0.50 mM and that of trans-DCE varied from 0 to 33.8 mM in o-xylene-d10 (Fig. S7, ESI†). The association constant (Ka) was determined to be (1.03 ± 0.12) × 102 M−1 by a nonlinear curve-fitting method, which is larger than that in CDCl3 (31.5 ± 4.1 M−1, Fig. S8, ESI†).
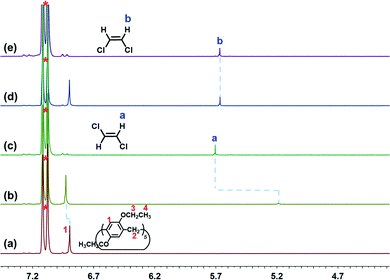 | ||
| Fig. 2 Partial proton 1H NMR spectra (500 MHz) of (a) EtP5, (b) EtP5 + trans-DCE, (c) trans-DCE, (d) EtP5 + cis-DCE, and (e) cis-DCE at 5.0 mM in o-xylene-d10. “*” indicates the solvent peaks. | ||
X-ray crystallography further confirmed the formation of inclusion complex. Crystals of trans-DCE@EtP5 were successfully obtained by slow evaporation of a solution of EtP5 in trans-DCE (Table S1, ESI†). The crystal structure of trans-DCE@EtP5 is shown in Fig. 3a and S9.† One trans-DCE molecule threads through the cavity of EtP5 to form an inclusion complex in the solid state, which is stabilized by triple C–H⋯π forces and triple weak C–H⋯Cl interactions (Fig. S10, ESI†). In addition, in the stacking mode of trans-DCE@EtP5, we find extra trans-DCE molecules lie in the channel formed by two adjacent EtP5 molecules (Fig. S9, ESI†). Although no host–guest interactions were found between EtP5 and cis-DCE in solution, we attempted to grow the crystals of cis-DCE@EtP5. Interestingly, when EtP5 was crystallized from cis-DCE solution, the obtained crystal structure of EtP5 did not contain cis-DCE molecules and formed a new structure (Fig. 3b and Table S2†). The experimental and simulated powder X-ray diffraction (PXRD) pattern of EtP5 crystals crystallized in cis-DCE was a perfect match for EtP5α (Fig. S11, ESI†), suggesting that EtP5α has the same structure as EtP5 crystallized in cis-DCE.
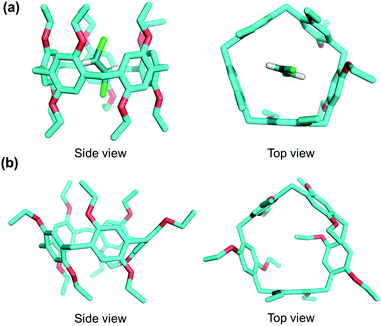 | ||
| Fig. 3 Molecular structures of (a) trans-DCE@EtP5 and (b) cis-DCE–free EtP5 obtained from cis-DCE solution in the solid-state. | ||
Based on the above highly selective host–guest complexation both in solution and in the solid state, we considered the selective adsorption of cis- and trans-DCE isomers vapor by EtP5α. Single-component time-dependent solid-vapor sorption experiments were examined to test the adsorption capacity using crystalline EtP5α by 1H NMR spectroscopy. As shown in Fig. S12,† EtP5α can rapidly capture trans-DCE vapor with an adsorption amount of about one trans-DCE/EtP5 (Fig. S14, ESI†). By contrast, EtP5α did not take up cis-DCE vapor (Fig. S13 and S15, ESI†). Thermogravimetric analysis (TGA) measurement was further confirmed the uptake amount of trans-DCE (Fig. S16, ESI†). However, the single-crystal structure of trans-DCE@EtP5 shows two host molecules and four trans-DCE, which is different from the results of vapor adsorption. This could be reasonable that during the removal of physical surface adsorption before NMR and TGA measurements (for details see ESI†), the guest molecules located at the outside of EtP5 were easily desorbed, while those stabilized in the cavities remained.47
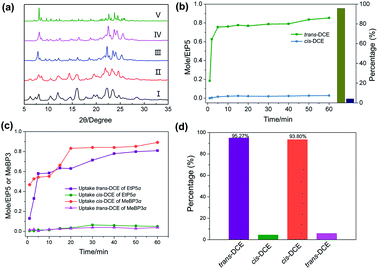
In order to investigate the mechanism for the uptake of trans-DCE vapor by EtP5α, PXRD experiments were carried out. The PXRD pattern of EtP5α after adsorption of trans-DCE was different from that of the EtP5α but almost identical to that of the simulated pattern determined from trans-DCE@EtP5 (Fig. 4a), suggesting that the uptake of trans-DCE induces the structural transformation of EtP5α into trans-DCE@EtP5. As expected, exposing EtP5α to cis-DCE vapor did not result in any structure changes (Fig. 4a).
Considering the remarkable adsorption selectivity in the single-component sorption experiments, we wondered whether crystalline EtP5α materials would be able to separate trans-DCE from cis-DCE. Therefore, a time-dependent EtP5α solid-vapor sorption experiment for the cis-/trans-DCE (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) mixture was performed. As shown in Fig. 4b, the adsorption of trans-DCE in EtP5α was very fast. Meanwhile, the uptake amount of cis-DCE by EtP5α was tiny. According to gas chromatography (GC), the adsorption percentages of trans-DCE is up to 94.94% at the saturated adsorption point (Fig. S17, ESI†). These results indicate that EtP5α favors to capture trans-DCE over cis-DCE vapor from their isomers mixture. Then, we measured the recycling adsorption capacity of EtP5α. After five adsorption–desorption cycles, no distinct loss of performance in the selective of the trans-DCE uptake was observed (Fig. S18 and S19, ESI†), showing that EtP5α crystals have excellent separation abilities.
50 v/v) mixture was performed. As shown in Fig. 4b, the adsorption of trans-DCE in EtP5α was very fast. Meanwhile, the uptake amount of cis-DCE by EtP5α was tiny. According to gas chromatography (GC), the adsorption percentages of trans-DCE is up to 94.94% at the saturated adsorption point (Fig. S17, ESI†). These results indicate that EtP5α favors to capture trans-DCE over cis-DCE vapor from their isomers mixture. Then, we measured the recycling adsorption capacity of EtP5α. After five adsorption–desorption cycles, no distinct loss of performance in the selective of the trans-DCE uptake was observed (Fig. S18 and S19, ESI†), showing that EtP5α crystals have excellent separation abilities.
Owing to the opposite adsorption selectivity for cis-/trans-DCE isomers of EtP5α and MeBP3α,23 we considered the use of co-adsorbents to achieve the self-sorting discrimination of the two isomers. We then conducted the self-sorting uptake experiments (details see ESI†). As expected, when exposing EtP5α and MeBP3α in the saturated vapor of cis- and trans-DCE (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) mixture (Fig. 4c), EtP5α selectively took up trans-DCE, and MeBP3α tended to adsorb cis-DCE (Fig. S20 and S22†), giving the uptake ratios of 95.27% and 93.80%, respectively (Fig. 4d, S21 and S23†).
50 v/v) mixture (Fig. 4c), EtP5α selectively took up trans-DCE, and MeBP3α tended to adsorb cis-DCE (Fig. S20 and S22†), giving the uptake ratios of 95.27% and 93.80%, respectively (Fig. 4d, S21 and S23†).
In summary, we have demonstrated the cis-/trans-selective complexation of industrially important DCE isomers by EtP5 macrocycle both in solution and in the solid state. Its adaptive crystals, EtP5α, are able to efficiently separate trans-DCE from a cis- and trans-DCE isomers mixture. The selectivity derives from the size/shape-fit host–guest complexation and the vapor-induced crystalline structure transformation. Interestingly, a self-sorting adsorption method to simultaneously separate cis- and trans-isomers by crystalline EtP5α and MeBP3α materials has been presented. Future work will attempt to achieve more separation of configurational isomers using extended biphen[n]arene48 and functional macrocycles.49
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NNSFC (No. 21971192 and 21772118), and funds provided by Tianjin Normal University.Notes and references
- K. A. Marshall, Chlorocarbons and Chlorohydrocarbons, Survey, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley-Interscience, New York, 2003 Search PubMed.
- F. Richter, Beilsteins Handbuch der organischen Chemie, EIII, 1958, vol. 1, p. 652 Search PubMed.
- J. W. Scroggins, US20070191653, 2007.
- M. Yu, R. D. Noble and J. L. Falconer, Acc. Chem. Res., 2011, 44, 1196–1206 Search PubMed.
- Y. Yang, P. Bai and X. Guo, Ind. Eng. Chem. Res., 2017, 56, 14725–14753 Search PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 Search PubMed.
- H. Wang and J. Li, Acc. Chem. Res., 2019, 52, 1968–1978 Search PubMed.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 Search PubMed.
- Z. R. Herm, E. D. Bloch and J. R. Long, Chem. Mater., 2014, 26, 323–338 Search PubMed.
- Y. Zhao, Chem. Mater., 2016, 28, 8079–8081 Search PubMed.
- L. Alaerts, M. Maes, M. A. van der Veen, P. A. Jacobs and D. E. De Vos, Phys. Chem. Chem. Phys., 2009, 11, 2903–2911 Search PubMed.
- H. Liu, Y. He, J. Jiao, D. Bai, D. Chen, R. Krishna and B. Chen, Chem.–Eur. J., 2016, 22, 14988–14997 Search PubMed.
- A. Luna-Triguero, J. M. Vicent-Luna, A. Poursaeidesfahani, T. J. H. Vlugt, R. Sánchez-de-Armas, P. Gómez-Álvarez and S. Calero, ACS Appl. Mater. Interfaces, 2018, 10, 16911–16917 Search PubMed.
- M. Maes, L. Alaerts, F. Vermoortele, R. Ameloot, S. Couck, V. Finsy, J. F. M. Denayer and D. E. De Vos, J. Am. Chem. Soc., 2010, 132, 2284–2292 Search PubMed.
- K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064–2072 Search PubMed.
- T. Ogoshi, R. Sueto, K. Yoshikoshi, Y. Sakata, S. Akine and T.-a. Yamagishi, Angew. Chem., Int. Ed., 2015, 54, 9849–9852 Search PubMed.
- M. Wang, J. Zhou, E. Li, Y. Zhou, Q. Li and F. Huang, J. Am. Chem. Soc., 2019, 141, 17102–17106 Search PubMed.
- T. Ogoshi, K. Saito, R. Sueto, R. Kojima, Y. Hamada, S. Akine, A. M. P. Moeljadi, H. Hirao, T. Kakuta and T. Yamagishi, Angew. Chem., Int. Ed., 2018, 57, 1592–1595 Search PubMed.
- K. Jie, M. Liu, Y. Zhou, M. A. Little, S. Bonakala, S. Y. Chong, A. Stephenson, L. Chen, F. Huang and A. I. Cooper, J. Am. Chem. Soc., 2017, 139, 2908–2911 Search PubMed.
- Y. Zhou, K. Jie, R. Zhao, E. Li and F. Huang, J. Am. Chem. Soc., 2020, 142, 6957–6961 Search PubMed.
- X. Sheng, E. Li, Y. Zhou, R. Zhao, W. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 6360–6364 Search PubMed.
- J.-R. Wu and Y.-W. Yang, Angew. Chem., Int. Ed., 2020, 59 DOI:10.1002/anie.202006999.
- Y. Wang, K. Xu, B. Li, L. Cui, J. Li, X. Jia, H. Zhao, J. Fang and C. Li, Angew. Chem., Int. Ed., 2019, 58, 10281–10284 Search PubMed.
- J.-R. Wu, B. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2020, 59, 2251–2255 Search PubMed.
- J.-R. Wu and Y.-W. Yang, CCS Chem., 2020, 2, 836–843 Search PubMed.
- W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 2–8 Search PubMed.
- J.-R. Wu and Y.-W. Yang, J. Am. Chem. Soc., 2019, 141, 12280–12287 Search PubMed.
- J. Zhou, G. Yu, Q. Li, M. Wang and F. Huang, J. Am. Chem. Soc., 2020, 142, 2228–2232 Search PubMed.
- H. Yao, Y.-M. Wang, M. Quan, M. U. Farooq, L. P. Yang and W. Jiang, Angew. Chem., Int. Ed., 2020, 59, 19945–19950 Search PubMed.
- Q. Li, K. Jie and F. Huang, Angew. Chem., Int. Ed., 2020, 59, 5355–5358 Search PubMed.
- T. Ogoshi, S. Kanai, S. Fujinami, T.-a. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 Search PubMed.
- D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 Search PubMed.
- T. Ogoshi, T.-a. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 Search PubMed.
- N. Song, T. Kakuta, T.-a. Yamagishi, Y.-W. Yang and T. Ogoshi, Chem, 2018, 4, 2029–2053 Search PubMed.
- K. Wang, J. H. Jordan, K. Velmurugan, X. Tian, M. Zuo, X.-Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202010150.
- G. Yu, J. Zhou, J. Shen, G. Tang and F. Huang, Chem. Sci., 2016, 7, 4073–4078 Search PubMed.
- B. Li, Z. Meng, Q. Li, X. Huang, Z. Kang, H. Dong, J. Chen, J. Sun, Y. Dong, J. Li, X. Jia, J. L. Sessler, Q. Meng and C. Li, Chem. Sci., 2017, 8, 4458–4464 Search PubMed.
- J. Chen, H. Ni, Z. Meng, J. Wang, X. Huang, Y. Dong, C. Sun, Y. Zhang, L. Cui, J. Li, X. Jia, Q. Meng and C. Li, Nat. Commun., 2019, 10, 3546 Search PubMed.
- X.-B. Hu, Z. Chen, G. Tang, J. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384–8387 Search PubMed.
- Q. Hao, Y. Chen, Z. Huang, J.-F. Xu, Z. Sun and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 5365–5372 Search PubMed.
- S.-H. Li, H.-Y. Zhang, X. Xu and Y. Liu, Nat. Commun., 2015, 6, 7590 Search PubMed.
- X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967–2969 Search PubMed.
- C. Li, Chem. Commun., 2014, 50, 12420–12433 Search PubMed.
- Y. Wang, G. Ping and C. Li, Chem. Commun., 2016, 52, 9858–9872 Search PubMed.
- X. Zhang, X. Wang, B. Wang, Z.-J. Ding and C. Li, Chin. Chem. Lett., 2020 DOI:10.1016/j.cclet.2020.02.037.
- B. Li, S. Li, B. Wang, Z. Meng, Y. Wang, Q. Meng and C. Li, iScience, 2020, 23, 101443 Search PubMed.
- W. Zhu, E. Li, J. Zhou, Y. Zhou, X. Sheng and F. Huang, Mater. Chem. Front., 2020, 4, 2325–2329 Search PubMed.
- B. Li, B. Wang, X. Huang, L. Dai, L. Cui, J. Li, X. Jia and C. Li, Angew. Chem., Int. Ed., 2019, 58, 3885–3889 Search PubMed.
- K. Xu, Z.-Y. Zhang, C. Yu, B. Wang, M. Dong, X. Zeng, R. Gou, L. Cui and C. Li, Angew. Chem., Int. Ed., 2020, 59, 7214–7218 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra, NMR titration curves, single crystal data and vapor adsorption. CCDC 2025010 and 1905571. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra09307f |
| This journal is © The Royal Society of Chemistry 2020 |