Open Access Article
Open Access ArticleElderberry (Sambucus nigra L.) juice as a novel functional product rich in health-promoting compounds
Milena Vujanović *a,
Tatjana Majkićb,
Gökhan Zenginc,
Ivana Bearab,
Vladimir Tomovića,
Branislav Šojića,
Saša Đurovićd and
Marija Radojkovića
*a,
Tatjana Majkićb,
Gökhan Zenginc,
Ivana Bearab,
Vladimir Tomovića,
Branislav Šojića,
Saša Đurovićd and
Marija Radojkovića
aFaculty of Technology, University of Novi Sad, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia. E-mail: milenavujanovic@uns.ac; Tel: +381 21 485 3716
bFaculty of Sciences, University of Novi Sad, Trg Dositeja Obradovića 3, 21000 Novi Sad, Serbia
cDepartment of Biology, Science Faculty, Selcuk University, Campus, Konya, Turkey
dInstitute of General and Physical Chemistry, Studenstki trg 12, 11158 Belgrade, Serbia
First published on 21st December 2020
Abstract
The medicinal herbs of the Balkan Peninsula are highly represented in traditional medicine. The connection between traditional and modern life and health is reflected in the creation of new food products with added value. In this study, the plant species Sambucus nigra L. was used to obtain freshly pressed juice, whose chemical composition and various biological activities were evaluated. The most abundant compounds were phenolic acids: protocatechuic and chlorogenic acid, as well as flavonoids: quercetin-3-O-hexoside, quercetin, and rutin. The analyzed juice was very rich in total phenolic compounds (1945 mg GAE per mL juice), and a significant anthocyanin concentration was observed (30.85 mg Cy-3-GE per mL juice). Bioactivity testing revealed that elderberry juice was an extremely potent agent in the process of neutralizing NO free radicals (53.06 g TE per L juice), while in reducing over-enzyme activity, the best result was achieved in the inhibition of tyrosinase enzyme (54.70 mg KAE per g of juice).
Introduction
Research in the field of food technology shows that functional food is becoming increasingly popular around the world and occupies an important place in human nutrition.1 The global functional food market has expanded dramatically over the last decade and is estimated to grow steadily to reach $255.10 million by 2024,2 increasing the competition among producers to make the consumer demand even more intense.3 This growth has been induced not only by innovation in the food industry, but also by increasing consumer awareness of their own health and the benefits of food products. Innovations within the food industry include obtaining new and improving and enhancing the quality of existing products by applying alternative solutions that are mostly related to the use of plants, which are easily available on the Balkan Penninsula.4 Wild-growing plant species of the Balkan Peninsula have exceptional natural potential and have long been used to treat flu, colds, inflammation, and similar ailments. Numerous studies are based on the examination of the antioxidant potential of wild-growing plants in order to regulate metabolic processes.5 In addition to the antioxidant potential, researches on various plant species also aim to examine the regulation of enzyme overactivity. Excessive activity of enzymes leads to disorders in the functioning of the organism and causes the development of neurodegenerative diseases, melanoma, and diabetes.6 Biologically active compounds present in wild-growing plant species are carriers of biological activity, especially phenolic compounds, minerals, phytosterols, terpenes. Wild-growing berry fruit is a very good source of natural molecules that show neuroprotective, anti-inflammatory, antioxidant, antidiabetic, and antimicrobial properties.7 Sambucus nigra L. is a challenge for the food industry because there are still no commercially available food products based on the elderberry. Thanks to its healing properties, the elderberry is very attractive for scientific research, which is why it was selected for the topic of this study.Elderberry is a wild-growing plant, widely distributed in Europe, Africa, Asia, and North America.8 The development of products based on berry fruits increasingly attracts the attention of different research groups. Therefore, we put the focus of our research on the health and industrial potential of wild-growing fruit of elderberry (family Adoxaceae), insufficiently explored and unexploited plant material in the food industry.
Elderberry fruits are small, black berries, whose color and extent resemble aronia berries and blueberries. The content of primary metabolites (sugars and organic acids) gives elderberry the typical sweet and astringent taste, while its characteristic aroma is related to the presence of different volatile and phenolic compounds.9 The purple color of fruit originates from anthocyanins and color pigments, so it could be used in many food commodities and nutritional supplements. The use of elderberry fruits is sometimes limited due to the presence of cyanogenic glycosides in this plant species. However, ripe elderberry fruits are safe for consumption and processing, due to the fact that an increasing degree of fruit ripeness causes, the content of these compounds in the fruit to decrease, thus not causing decreases toxic effects on the body.10 Senica et al. have shown that the ripe fruits contains the least amount of cyanogenic glycosides compared to the flower and leaves.10 Berry fruits are very attractive for juice production, due to the high amount of phytochemicals and natural color. The use of natural juices as food beverages occupies a high position in human nutrition, as they are very good sources of nutrients. The processes of obtaining juices are different, but they are mainly based on the application of different temperature treatments, which greatly affects their quality, i.e. the degradation of biomolecules that are not resistant to higher temperature.11 In a study conducted by Senica et al.,12 elderberry juice was obtained by the influence of temperature, while Busso Casati et al. obtained juice from berries dried at low temperatures.13 In our research, the juice was obtained by traditional pressing, without temperature treatment of ripened elderberry fruit.14 Therefore, the main objectives of this study were analysis of biochemical profile, biological abilities, and sensory characteristics of the traditionally obtained juice as a novel functional product rich in health-promoting compounds. The results of this research are of practical importance since they provide data on biopotential and sensory characteristics of the examined product, which can be a rational basis for elderberry usage at an industrial level.
Materials and methods
Plant material
Plant material of S. nigra was collected in August 2017 in Pljevlja (Republic of Montenegro). The specimen voucher was prepared and identified by Dr Milica Rat and deposited at the Herbarium of the Department of Biology and Ecology (BUNS Herbarium), University of Novi Sad, Faculty of Sciences, Republic of Serbia. The number of specimen voucher of S. nigra was 2-1512.The juice was made by hand pressing of 1 kg of berries. After pressing, 0.5 L of mother elderberry juice was obtained, and the juice was filtered through sterile gauze to remove residual impurities. Gravimetric dry matter measurement of the juice was performed by heating the juice in an oven at 105 ± 0.5 °C until the constant mass was formed. The dry matter content expressed as a percentage in the original juice sample was 7.70%. The degree of ripeness of the fruits was assessed by determining the sugar content of the refractometer, using an Oechsle scale.
The sugar content for the degree of ripeness of the fruit was calculated according to the formula: (Oe° × 0.266) − 3. Read value at the Oechsle scale was 50 Oe°, and the content of sugar was 10.3%, indicating a high degree of fruits maturity.
Chemical composition of elderberry juice
The content of organic acids was determined using Waters Alliance HPLC system with a Waters 2414 refractive index detector. The column was “Carbohydrate analysis column” – Waters (3.9 × 300 mm) with an ambient temperature of the oven. The mobile phase was ACN/H2O (80/20%) with a flow of 1.5 mL min−1. Final sugar content was expressed as gram per liter of juice (g L−1 juice). The content of sugars was determined using Waters Alliance HPLC system with a UV/VIS detector. The column was Atlantis C18 (4.6 × 150 mm, 5 μm) with the oven temperature of 45 °C. The mobile phase was NaH2PO4 × H2O (20 mmol L−1) with a flow of 0.5 mL min−1, while the UV detector was set at 210 nm. The volume of the sample was 10 μL. The final content was expressed as gram per liter of juice (g L−1 juice).pH was determined at room temperature with a pH meter (Metrohm, Switzerland).
Nutritional profile
The carbohydrate content was determined by the method described by Trajković et al.15 The content of fat in elderberry juice was determined by applying the method prescribed by the Regulation,16 according to which the extraction of fat is carried out by applying an organic solvent on an apparatus according to Soxhlet, while the protein content was determined by the method described by Regulation.17 The energy value was calculated based on the obtained content of protein, fat, and carbohydrate.Mineral composition
The mineral composition was established using a previously described method (Đurović et al.18) by ICP-OES analytical technique. The content of each element was expressed as milligram per liter of juice (mg L−1 juice).Determination of bioactive components
Total phenolic (TPC) and total flavonoid (TFC) contents were determined using the previously described methods.19 Final contents for TPC was expressed as milligram of gallic acid equivalents per L of juice (mg GAE per L juice), while results for TFC were expressed as milligrams of catechin equivalents per L of juice (mg CE per L juice).Determination of total monomeric anthocyanins
Anthocyanins were determined according to the previously described procedure (Majkić et al.20) using the pH differential method. Total anthocyanins content (TAC) was expressed as mg equivalents cyanidin-3-O-glucoside per L of juice (mg Cy-3-GE per L juice).Determination of total tannins
The content of total tannins (TTC) in elderberry was determined spectrophotometrically, using the characteristic of polyvinylpolypyrrolidone (PVPP) to bind tannins. The total tannins content was expressed as mg catechin per L of juice (mg CE per L juice).21HPLC-MS/MS analysis of phenolic compounds
The quantification of the 44 phenolic compounds and quinic acid in elderberry juice was carried out using the LC-MS/MS method published by Orčić et al.22 with additional compounds: morin, ellagic, and ursolic acid.Determination of biological activity
Enzyme inhibitory activity
In this study, the inhibitory activity of elderberry juice towards acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, α-amylase, and α-glucosidase enzymes involved in some key metabolic pathways were investigated using the previously described methods.19 The inhibitory effects were expressed as standard inhibitor equivalents (galanthamine (mg GALE per g juice) for AChE and BChE; kojic acid (mg KAE per g juice) for tyrosinase; and acarbose (mmol ACAE per g juice) for α-amylase and α-glucosidase).Color of elderberry juice
Instrumental color parameters (eight replicates): L* (lightness), a* (redness), b* (yellowness), C* (chroma – saturation index), h (hue angle), and λ [dominant wavelength (nm)] were determined by using a Konica Minolta Chroma Meter CR-400, using glass cell 20 mm CM-A99, D-65 lighting, a 2° standard observer angle and an 8 mm aperture in the measuring head. Also, the elderberry juice was subjected to visual color evaluation by 10 trained sensory panelists using the NCS color atlas.23Sensory profile of elderberry juice
The elderberry juice was sensory evaluated by quantitative descriptive analysis. The sensory analysis was carried out by a trained panel consisting of 12 members, per two sessions. The panelists were trained according to methods described in ISO 8586,24 in a sensory laboratory equipped according to ISO 8589.25 During calibration sessions, a panel discussion was held to develop a consensus vocabulary for sensory attributes for elderberry juice. The panelists were asked to identify references that would serve for each attribute term ISO 11035.26 A sensory lexicon of the elderberry juice was created based on the languages generated from calibration sessions (Table 4). Individual elderberry juice was accessed for the intensity of the studied sensory attributes using a 100 mm-long line with line anchors of 0 – no intensity or extremely low intensity and 10 – extremely high intensity.The elderberry juice sensory acceptance test was performed by 70 students of the Faculty of Technology Novi Sad, Serbia. Consumer acceptability was measured on a 9-point hedonic scale anchored as 1 being ‘dislike extremely’ and 9 being ‘like extremely’. The panelists were asked to evaluate appearance liking, flavor liking, mouthfeel liking, aftertaste liking, and overall liking.
Sensory evaluation of the color of the elderberry juice was also performed by trained evaluators (a panel of 12 evaluators) using NCS color atlas Natural Color System®© – The international language of colour communication™, Scandinavian Colour Institute AB, Stockholm, Sweden, http://www.ncscolour.com.
Results and discussion
Chemical composition of elderberry juice
The content of organic acids, sugars, pH values, nutritional profile, and mineral composition of elderberry juice are shown in Table 1. Malic acid was the most abundant organic acid in the juice (3.75 g L−1), followed by citric acid (1.63 g L−1), while the tartaric acid content was 0.50 g L−1. As expected, glucose was the predominant sugar (29.12 g L−1), while the fructose content was 22.60 g L−1, which is in agreement with literature data.13,27 Also, the obtained results were comparable with the results of different research group, which reported the dominance of glucose over fructose in S. nigra.13 In the previous study,28 sucrose was also detected, but in a significantly lower concentration than glucose and fructose, which differs from our research, since we did not recorded its presence in our sample. The pH of the juice was 4.11, which was higher than the pH of blueberry, elderberry, and blackcurrant juices.13 This could be a consequence of the uneven degree of ripeness of the fruit, different content of organic acids and sugars in the juices, as well as different treatments in juice preparation, which all affect the acceptability of the product by consumers. An assessment of the nutritional composition showed that elderberry juice had an extremely low fat and protein content (0.02 g per 100 g), and up to total sugar content of 3.74 g per 100 g. The calorific value of 15.22 was significantly lower than that of commercial juices. It is assumed that this result is a consequence of the application of a traditional juice production technique that did not involve temperature treatment to assist the extraction of primary metabolites from the plant matrix.| Nutritional profile | |||
|---|---|---|---|
| Carbs (g/100 g) | Fat (g/100 g) | Proteins (g/100 g) | Calories (kcal/100 g) |
| 3.74 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 | 15.22 ± 0.21 |
| Mineral composition | |||||
|---|---|---|---|---|---|
| Macro elements | Contentc (mg L−1 juice) | Trace elements | Contentc (mg L−1 juice) | Toxic elements | Contentc (mg L−1 juice) |
| Na | 7.69 ± 0.18 | Fe | 1.27 ± 0.01 | Pb | <0.001 |
| K | 3956 ± 2.56 | Cu | 0.13 ± 0.01 | Cd | <0.001 |
| Mg | 280.40 ± 0.91 | Mn | 1.16 ± 0.02 | Hg | <0.001 |
| Ca | 163.80 ± 2.01 | Zn | 0.48 ± 0.01 | As | <0.001 |
| — | — | Cr | 0.01 ± 0.00 | — | — |
| — | — | Ni | 0.01 ± 0.00 | — | — |
Berries, such as strawberries, blueberries, blackberries, raspberries, and cranberries, are low in calories (∼0.3 to 0.6 kcal g−1) and are a very good source of nutrients such as vitamin C, potassium, and manganese, as well as other vitamins and minerals.29
As it can be seen from the results, the juice of matured elderberry berries contains a particularly high concentration of macroelements K and Mg (3955.56 and 280.40 mg L−1, respectively), while Ca and Na are present at somewhat lower amounts (163.80 and 7.69 mg L−1, respectively). The relatively large content of K and Mg is characteristic of the plant species S. nigra, and thus their large presence in elderberry fruit was expected.27 The content of trace elements in the examined elderberry juice was in the range of 0.01–1.27 mg L−1, where Fe and Mn were present in the highest amounts (1.27 and 1.16 mg L−1, respectively), while Cr and Ni were quantified in the lowest concentrations (0.01 mg L−1). The safety of the produced juice was analysed by utilizing toxic elements presence: Pb, Cd, Hg and As were not detected in elderberry juice (the amount in the juice was below the detection limit). The results of the toxic element analysis pointed out that the examined elderberry was growing in an ecological area and non-contaminated soil.
The results of the research conducted by Konić-Ristić et al. confirmed that minerals K and Fe are dominant in the juice of different berry fruits.30 The content of the minerals in the elderberry juice was higher than in the fruit juices that were examined by Konić-Ristić et al.30 The assessment of the content of macro and trace elements is particularly important in the case of Fe and K since these elements are significant factors in the prevention of various diseases. The Recommended Dietary Allowances of K for men is 3400 mg, while for women it is somewhat lower – 2600 mg. The content of K in the analysed juice is a bit greater than the Recommended Dietary Allowances, so a 1 dL glass of elderberry juice is sufficient to provide the daily doses of K. The recommended daily doses of Fe for men are 8 mg and 18 mg for women.30 The content of Fe in elderberry juice in a 1 dL glass is not sufficient to provide the daily doses Fe for the organism, but can be used as a source of this trace element. Overall, the results of this study indicate that elderberry juice is a very potent source of minerals.
Total bioactive compounds and phenolic components
Polyphenols are pharmacologically active compounds and could be used as active food supplements. Flavonoids are a large group of polyphenols that are very common among plants and participate in processes such as plant pigmentation, antioxidant capacity, protection from biotic and abiotic stresses, and plant–environment interactions. Anthocyanin pigments are responsible for the orange, red, and blue colors of plants.31Berry fruits contain tannins, which are commonly found in the skin, stems, and seeds and have a significant impact on the physical, chemical and sensory characteristics of juices.8 The content of bioactive compounds is presented in Table 2.| Content of biologically active compounds | |
|---|---|
| a mg gallic acid equivalent per L of juice.b mg catechin equivalent per L juice.c μg cyaniding 3-O-glucoside equivalent per mL of juice.d μg catechin equivalent per mL of juice.e mg of detected compound per L of juice. | |
| TPCa (mg GAE per L juice) | 1945 ± 33.10 |
| TFCb (mg CE per L juice) | 906.30 ± 2.31 |
| TACc (mg Cy-3-GE per mL juice) | 30.85 ± 2.46 |
| TTCd (mg CE per mL juice) | 1189.62 ± 21.71 |
| Phenolic profile | |
|---|---|
| Compounds | Contente (mg L−1) |
| p-Hydroxybenzoic acid | 3.45 ± 0.03 |
| Protocatechuic acid | 42.7 ± 0.85 |
| p-Coumaric acid | 1.82 ± 0.01 |
| Gallic acid | 3.93 ± 0.29 |
| Chlorogenic acid | 7.52 ± 0.23 |
| Caffeic acid | 3.07 ± 0.06 |
| Quinic acid | 310 ± 1.86 |
| Quercetin | 6.17 ± 0.20 |
| Kaempferol 3-O-glucoside | 0.46 ± 0.02 |
| Quercetin 3-O-hexoside | 18.0 ± 0.90 |
| Amentoflavone | 0.36 ± 0.01 |
| Rutin | 5.11 ± 0.05 |
| Esculetin | 0.27 ± 0.02 |
The content of total phenols in the juice obtained by the traditional method of squeezing elderberry fruits was 1945 mg GAE per L juice, while the content of total flavonoids was 906.30 mg CE per mL of juice. As for the content of total monomeric anthocyanins in elderberry juice, it was 30.85 mg of CGE per mL of juice. The tannin content in the analysed juice was significantly higher: 1189.62 mg CE per mL juice. By comparing the obtained results with the results of previous study,13 where juices of blueberries, elderberry, blackcurrant, and maqui berries were analysed, a significant difference in the content of total phenols and monomeric anthocyanins was noticed. The traditional elderberry juice had a lower content of these biomolecules than the juices analysed by Busso Casati et al.13
These results could be explained by different ways of berry fruits processing and juice preparation. Juices from blueberries, elderberry, blackcurrant, and maqui berries were obtained using lyophilized fruit pulps, which were afterwards dissolved in water.13 So, lyophilization provides safe removal of water and concentration of nutrients, which results in their higher final content.
Also, the results of this study were compared to the content of total polyphenolic compounds of blueberry juice, which was determined by Siddiq et al.32 It can be seen that the total content of bioactive molecules was higher in the traditionally obtained elderberry juice. In this case, one of the reasons for the difference in the results was the method used for juice preparation. In the previous study,32 the temperature and enzyme treatment was applied to prepare the blueberry juice, and it resulted in a lower content of total polyphenolic compounds. Other reasons could be disparity in plant species, the geographical area where these plants grow, or interactions with other phytochemicals (non-phenolics) and Folin–Ciocalteu reagent used in this analysis.
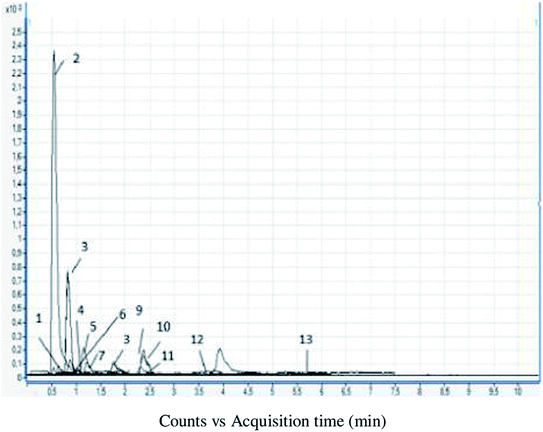
To conduct a more detailed analysis, identification and quantification of the bioactive compounds (selected phenolic acids and flavonoids) in elderberry juice were performed using LC-MS/MS technique. The results are shown in Table 2, while corresponding chromatograms are shown in Fig. 1.
In the examined juice, six phenolic acids were determined. Phenolic acids, which were present in the highest concentration, belong to the group of benzoic acids, while the phenolic acids in lower concentrations were cinnamic acid derivatives. The dominant phenolic acids in elderberry juice were protocatechuic and chlorogenic (42.70 and 7.52 mg L−1, respectively). The elderberry juice was a good source of gallic and p-hydroxybenzoic acid (3.93 and 3.45 mg L−1, respectively). The presence of cinnamic acids in elderberry juice was characterized by caffeic (3.07 mg L−1) and p-coumaric acid (1.82 mg L−1). The high content of protocatechuic and chlorogenic acid is of great importance for the biological potential of the analysed juice. It has been proven that foods rich in berries and anthocyanins reduce the risk of developing metabolic disorders, so it is widely advocated to increase the intake of berry fruits.33 Scientific studies have shown that protocatechuic and chlorogenic acid contribute to the prevention of the development of neurodegenerative diseases, show strong antioxidant, cytotoxic, antidiabetic, and anti-inflammatory activity, thus making elderberry juice a very potent product which could protect the body against various diseases.34 For example, in vivo studies showed that there was significant increase in the blood glucose level, glycosidase enzymes, and some lipids and lipid metabolites (TAG, LDL, lipid peroxidation) after 14 days treatment of Wistar rats with 38.75 mg kg−1 protocatechuic acid.35 Having in mind that examined juice is a good source of protocatechuic acid, but also that this acid is the major metabolite of cyanidin 3-O-glucoside in humans,36 which is probably also present in our sample, it can be assumed that examined juice can be a good source of protocatechuic acid and that its consumption can, at least partially, contribute to the prevention of glucose and lipid metabolism disorders.
In addition to phenolic acids, six flavonoid compounds were identified in elderberry juice. The dominant flavonoids were quercetin-3-O-hexoside, quercetin, and rutin (18.00, 6.17, 5.11 mg L−1, respectively). These bioflavonoids protect the brain cells from oxidative stress, which damages the tissue and leads to Alzheimer's and other neurological disorders. Quercetin could prevent allergic reactions by inhibiting the release of histamine, and consequently contributing to the treatment and prevention of asthma and bronchitis.37 The presence of quercetin-3-O-hexoside, quercetin, and rutin, as the dominant flavonoids, gives special significance to elderberry juice because of their potential in terms of health benefits.38
Considering the polyphenolic profile of the elderberry juice from this study, and study by Senica et al.,12 chlorogenic acid was present in both tested samples, but was somewhat higher in ours. In the juice11 there was no presence of protocatechuic, nor gallic and p-hydroxybenzoic acids. Also, a higher content of caffeic acid was identified in the elderberry juice in this study, while p-coumaric acid was recorded at a concentration of 6.6 mg kg−1 in the analysis of Senica et al.,12 which was about 3.6 times higher than in ours. The difference between two juice samples also reflected in the flavonoid content. Catechin and epicatechin were detected in juice analysed by Senica et al., while the presence of these flavonoids compounds was not detected in this research. The differences in the phytochemical composition that occurred between juices could be explained by the influence of environmental factors on the development of S. nigra fruits, the composition of the soil on which the plant grows, as well as the techniques and methods used for obtaining and analysing of juice. However, the results obtained in this study are in agreement with the research conducted by Natic et al.39 and Caruso et al.40
Based on this research, it could be suggested that dominant phenolic acids and flavonoids which are present in great concentrations in the elderberry juice, in synergism with another phenolic compound could positively affect the health of the consumer and the functional characteristics of the product itself.
Biological activity
| Antioxidant assays | |
|---|---|
| a g equivalent Trolox per liter of juice.b g equivalent EDTA per liter of juice.c mg galantamine equivalent per g of juice.d mg kojic acid equivalent per g of juice.e mmol acarbose equivalent per g of juice. | |
| ABTSa (g TE per L juice) | 5.38 ± 0.30 |
| DPPHa (g TE per L juice) | 2.16 ± 0.79 |
| LPa (g TE per L juice) | 0.18 ± 0.04 |
| NOa (g TE per L juice) | 53.06 ± 17.40 |
| FRAPa (g TE per L juice) | 7.26 ± 1.63 |
| CUPRACa (g TE per L juice) | 10.98 ± 0.46 |
| Phosphomolybdenuma (g TE per L juice) | 29.63 ± 2.31 |
| Metal chelatingb (g EDTAE per L juice) | 0.11 ± 0.02 |
| Attribute | Description | Reference material |
|---|---|---|
| Beet | The damp, musty/earthy, and slightly sweet aromatics commonly associated with beets | Beet juice |
| Blackberry | Sweet, sour, and fruity aromatics associated with blackberries | Blackberry juice |
| Cherry | The sour, fruity, and slightly bitter aromatics commonly associated with cherries | Cherry juice |
| Vinegar | Sour, astringent, and slightly pungent aromatics associated with vinegar | Apple vinegar |
| Grapefruit | Characteristic aromatics associated with grapefruit | Fresh grapefruit |
| Coconut | Characteristic aromatics associated with coconut | Coconut milk |
| Clove | Characteristic aromatics associated with clove | Clove – dried flower |
| Jasmine, lilac | Sweet, light, and slightly perfume impression associated with flowers | Benzyl acetate |
| Dried plums | Characteristic aromatics associated with dried plums | Dried plums |
| Bitter | The fundamental taste factor associated with a caffeine solution | Caffeine |
| Sweet | The fundamental taste factor associated with a sucrose solution | Sucrose |
| Salty | The fundamental taste factor associated with a sodium chloride solution | Sodium chloride |
| Sour | The fundamental taste factor associated with a citric acid solution | Citric acid |
| Astringent | The dry puckering mouthfeel associated with an alum solution | Alum |
| Throatetch | A sensation of abrasion and drying of the throat | Fresh quince |
| Persistency | Perception of unclean sensation within the oral cavity after swallowing sample |
Elderberry juice has extremely strong antioxidant activity, according to all applied assays. In this study, ˙NO radical scavenging ability of elderberry juice was investigated for the first time. Based on the achieved value, which was very high (53.06 g TE per L juice), it was proven that the analysed juice was a very potent agent in the ˙NO radical scavenging process. Reactive nitrogen species (RNS), as endogenous intermediates that continuously occur in living cells, play an essential role in the regulation of physiological processes, which is why different biological activities of ˙NO are conditioned by its radical character.41 The elderberry juice also achieved strong total antioxidant and metal chelating potential. Significant radical scavenging and a reducing power capability of elderberry juice were reached, and the obtained values ranged from 2.16–5.38 to 7.26–10.98 g TE per L juice. Compared to blueberry juice,32 elderberry juice exhibited stronger reduction potential.
The very good ability to chelate metal ions of elderberry juice is probably conditioned by its phytochemical (polyphenol) composition. Namely, as we determined, the examined juice is a good source of phenolics, and the ability to chelate metal ions shown by phenolic compounds is a function of their characteristic chemical structure, number, and position of hydroxyl groups.41
It is important to point out that within this research, the ability of elderberry juice to inhibit lipid peroxidation, i.e. to prevent damage to cell membranes caused by free radicals, was also examined. The ability of the juice to inhibit the process of lipid peroxidation was 0.18 g TE per L of juice. In this way, it has been implicated that elderberry juice, as a potentially new functional product, can reduce the risk of developing different diseases of modern society caused by oxidative stress.
The plant species S. nigra is characterized as a “natural laboratory” of phytopharmacological molecules that are carriers of different biological activities.42 The pronounced overall antioxidant activity of elderberry juice, which was determined by applying listed antioxidant assay, is most likely a consequence of the occurrence of various phenolic compounds, particularly dominantly present protocatechuic acid, quercetin-3-O-hexoside, chlorogenic acid, and rutin. Compared to the antioxidant potential of commercial berry fruits juice, the examined juice showed slightly lower radical scavenging activity because it has no additional enzymes or additives to achieve better bioactivity of the product.43
Enzyme-inhibitory activity of elderberry juice
Enzymes, as valuable pharmaceutical targets for controlling global health problems, were examined within the frame of this study, and the results are presented in Table 3.The development of Alzheimer's disease is associated with altered enzyme activity, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). These enzymes play a role in regulation of the levels of the neurotransmitter acetylcholine (ACh) on the synaptic gap. In this regard, inhibiting the overactivity of these enzymes is a valuable therapeutic way of alleviating Alzheimer's symptoms. The obtained results show that elderberry juice inhibits AChE and BChE well (5.08 and 1.45 mg GALAE per g, respectively). Previous studies have found that phenolic acids, or molecules with a phenolic ring and hydrophobic residues in their structure, are considered to be potential inhibitors of AChE and BChE.19 The phenolic acids identified in elderberry juice significantly contribute to the realized activity.
Tyrosinase is a key enzyme in the synthesis of melanin and some other pigments. An increased concentration of this enzyme in the body could lead to melanoma. Therefore, tyrosinase inhibition can be very important for controlling various forms of skin disorders, especially pigmentation disorders. Hence, the need to find and isolate the natural inhibitors of this enzyme has been imposed.44
The inhibitory power of elderberry juice was also tested on the tyrosinase enzyme. Analyses have shown that examined juice was a very potent tyrosinase inhibitor (juice 54.70 mg KAE per g). Antityrosinase potential of the examined juice could be explained by the extremely rich phytochemical composition. The most abundant compounds, such as protocatechuic acid, quercetin, quercetin-3-O-hexoside, chlorogenic acid, and rutin can contribute to the strong antityrosinase potential of elderberry juice.19 Namely, flavanones and flavanonols have the ability to inhibits tyrosinase reversibly and competitively. Flavanones are copper chelators which disrupt tyrosinase structure by hydrophobic interactions and chelate a copper ion, coordinating with 3 histidine residues (HIS61, HIS85, and HIS259).45 So, it can be concluded that compounds with flavonoid structure, detected in the elderberry juice, could affect the chelation of copper ions and thus reduce the overactivity of tyrosinase, which further reduces the possibility of various diseases development. Also, this ability is supported by previously discussed ability of phenol-rich elderberry juice to chelate metal ions (Table 3).
α-Amylase and α-glucosidase are enzymes involved in carbohydrate metabolism, and the overactivity of these enzymes leads to the accumulation of glucose in the blood, which is an initial step in the development of diabetes, one of the most widespread diseases of modern society. Inhibition of α-amylase and α-glucosidase is considered as an effective way of controlling blood glucose levels in patients with diabetes.46 Elderberry juice inhibited both enzymes at concentrations of 0.12 and 0.05 mmol ACAE per g juice, respectively. These results indicated that elderberry juice obtained by the traditional pressing technique is a product that could potentially interfere with the evolution of diabetes.47
The results of this research support the concept of functional food – the food which provides not only essential nutrients but also compounds that contribute to normal function and can help prevent/alleviate diseases.48 Thus, through molecular and physiological mechanisms, the potential health benefits of elderberry juice can be linked to the chemical characteristics of present phenolic acids and flavonoids and their ability to remove free radicals and/or chelate redox-active metals or to interact with specific enzymes included in different metabolic processes. Elderberry as a wild-growing plant that has not been technologically changed could be characterized as a plant ‘from the field to health’.49
Color of elderberry juice
Average values of lightness (L*), redness (a*), yellowness (b*), chroma – saturation index (C*), hue angle (h) and dominant wavelength (λ) for elderberry juice are shown in Table 5. According to the NCS color atlas (the consensus method), the color of the elderberry juice was visually identified as the color of the S 5040-R20B color card (Fig. 2). The color of elderberry juice was compared with the color of blueberry juice examined by Siddiq et al.30 In this study, it was determined that elderberry juice was darker, while the redness of blueberry juice was higher. The biggest difference was observed in the value of the angle of hue, which is much higher in elderberry juice, 69.84, while in blueberry juice it was only 6.88. The results were also compared to the results of the Busso-Casati et al. study,13 where the color of juices and extracts obtained from various berries (blueberry, elderberry, black currant, and maqui berry) was determined. An extensive analysis showed that the juice tested in this study has a lower color intensity than the juices and extracts tested by Busso-Casati et al.,13 which is a consequence of the application of different methods of product preparation, as well as production techniques. In the mentioned study, after collection, the elderberries were lyophilized, and methanol was used as a solvent to obtain extracts, which greatly influenced the final result.| L* | a* | b* | C* | h | λ |
|---|---|---|---|---|---|
| 19.68 ± 0.11 | 0.63 ± 0.38 | 1.66 ± 0.09 | 1.81 ± 0.17 | 69.84 ± 10.58 | 581.71 ± 3.38 |
It is important to point out that Porras-Mija28 research group performed color determination of elderberry fruits ethanol extracts too. Also, in this case, a slightly stronger shade of color was observed in relation to the color of the juice examined in our study. The color differences between the two juices obtained from the berries come from the degree of ripeness of the fruits, and the content of plant pigments that is responsible for the color of the juices. The extraction of plant pigments anthocyanins depends on the applied technological procedure during product preparation. In the first step, the application of lyophilisation as a modern drying technique influenced the preservation of the chemical composition of berries, and secondly, the extraction with methanol and ethanol provided higher amounts of pigments and consequently a darker color of the products.13,28 Our traditional way of obtaining juice, without the influence of temperature, ensured high quality of the product, and on the other hand, it led to incomplete isolation of anthocyanins, due to which the examined juice had lighter color.
Sensory profile and consumer's appearance of elderberry juice
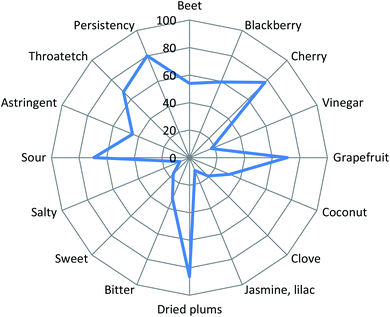
The mean scores for 16 identified sensory attributes are shown in Fig. 3. The elderberry juice had the highest intensities of dried plums (86.5), cherry (77.3), grapefruit (70.9), sour (69.3) and throatetch (67.6) notes. Further, the elderberry juice had a blackberry (59.6), beet (54.0) and astringent (44.7) note. Finally, the juice had lower notes of bitter (32.2), coconut (31.7), clove (18.8), vinegar (17.3), sweet (16.7), jasmine (lilac) (10.1) and salty (7.5). The persistence of the elderberry juice was 80.1.The mean scores for appearance liking, flavor liking, mouthfeel liking, aftertaste liking, and overall liking are shown in Table 6 and values were 7.4 (between ‘like moderately’ and ‘like very much’), 5.7 (between ‘neither like nor dislike’ and ‘like slightly’), 6.1 (slightly higher than ‘like slightly’), 5.9 (slightly lower than ‘like slightly’) and 6.1 (slightly higher than ‘like slightly’), respectively.
| Appearance liking | Flavour liking | Mouthfeel liking | Aftertaste liking | Overall liking |
|---|---|---|---|---|
| 7.40 | 5.70 | 6.10 | 5.90 | 6.10 |
Conclusions
We investigated elderberry juice, obtained by a traditional pressing method, to validate its potential as a novel functional product, which is not present on the market. For the first time, the nutritional composition of the elderberry juice, its neuroprotective, antityrosinase and antidiabetic activities, as well as sensory properties were examined, which gives particular value to this research.The results obtained by analysing the polyphenol profile of elderberry juice showed that a significant amount of primary and secondary metabolites is present in the juice. The analysis demonstrated the great presence of protocatechuic acid and quercetin-3-O-hexoside as a dominant phenolics. Elderberry juice expressed strong biological potential, especially the antioxidant and antityrosinase activity. Moreover, the evaluated juice exhibited very good neuroprotective ability. Consumption of this juice could be of importance for adequate daily intake of the micronutrients, especially K and Fe, because these minerals are present in immense concentration. Also, a positive assessment of the acceptability of this product by consumers is of great importance. The obtained results could be used as guidelines and selection criteria for the further use of elderberry juice as a new potential functional product that could become concurrent with existing juices on the market.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors of this study are grateful to Dr Milica Rat, University of Novi Sad Faculty of Sciences, Department of Biology and Ecology, Republic of Serbia, for the support in terms of confirmation and deposition of Sambucus nigra L. at the BUNS Herbarium. This research study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Projects No. TR31013 and Grant No. 451-03-68/2020-14/200125). The authors of this study are also grateful to Suzana Pronić, Sofija Rankov, Saša Šorgić, and Saša Popov, Oenological Laboratory, Vršac, Serbia for the support in the determination of primary metabolites and minerals.References
- M. Kaur and S. Singh, Int. J. Food Prop., 2016, 11, 2432–2442 Search PubMed.
- Food Glazing Agents Market Size & Trend Analysis By Ingredient (Carnauba Wax, Candelilla Wax), By Function (Coating Agent, Surface Finishing Agent), By Application, And Segment Forecasts To 2024: new report by Grand View Research, Inc., 2016 Search PubMed.
- D. Cianciosi, J. Simal-Gandara and T. Y. Forbes-Hernandez, Mediterr. J. Nutr. Metabol., 2019, 12, 335–340 Search PubMed.
- M. Vujanović, G. Zengin, S. Đurović, P. Mašković, A. Cvetanović and M. Radojković, S. Afr. J. Bot., 2019, 120, 213–218 Search PubMed.
- M. Maleki, N. Khelghati, F. Alemi, M. Bazdar, Z. Asemi, M. Majidinia, A. Sadeghpoor, A. Mahmoodpoor, F. Jadidi-Niaragh, N. Targhazeh and B. Yousefi, Life Sci., 2020, 259, 118341 Search PubMed.
- A. H. Barrett, N. F. Farhadi and T. J. Smith, LWT–Food Sci. Technol., 2018, 87, 394–399 Search PubMed.
- I. A. Neri-Numa, R. A. Soriano Sancho, A. P. Aparecida Pereira and G. M. Pastore, Food Res. Int., 2018, 103, 345–360 Search PubMed.
- R. Domínguez, L. Zhang, G. Rocchetti, L. Lucini, M. Pateiro, P. E. S. Munekata and J. M. Lorenzo, Food Chem., 2020, 330, 127266 Search PubMed.
- S. Ferreira, P. Silva, A. Silva and F. Nunes, Food Chem., 2020, 302, 125366 Search PubMed.
- M. Senica, F. Stampar and M. Mikulic-Petkovsek, J. Berry Res., 2019, 9, 395–404 Search PubMed.
- E. Ephrem, A. Najjar, C. Charcosset and H. Greige-Gerges, J. Funct. Foods, 2019, 48, 65–84 Search PubMed.
- M. Senica, F. Stampar, R. Veberic and M. Mikulic-Petkovsek, LWT–Food Sci. Technol., 2016, 72, 182–188 Search PubMed.
- C. Busso Casati, R. Baeza and V. Sanchez, J. Berry Res., 2019, 9, 431–447 Search PubMed.
- A. Sidor and A. Gramza-Michałowska, J. Funct. Foods, 2015, 18, 941–958 Search PubMed.
- J. Trajković, M. Mirić, J. Baras and S. Šiler, Food Analysis, Faculty of Technology and Metallurgy, Beograd, Serbia, 1983 Search PubMed.
- Regulation of methods of physical and chemical analyses, SFRY, 74/1988 Search PubMed.
- Regulation of methods of sampling and methods of performing chemical and physical analysis, SFRY, 41/1985 Search PubMed.
- S. Đurović, B. Pavlić, S. Šorgić, S. Popov, S. Savić, M. Petronijević, M. Radojković, A. Cvetanović and Z. Zeković, J. Funct. Foods, 2017, 32, 18–26 Search PubMed.
- G. Zengin, S. Uysal, R. Ceylan and A. Aktumsek, Ind. Crops Prod., 2015, 70, 1–6 Search PubMed.
- T. M. Majkić, L. D. Torović, M. M. Lesjak, D. D. Četojević-Simin and I. N. Beara, Food Res. Int., 2019, 121, 151–160 Search PubMed.
- FAO, Quantification of tannins in tree foliage, IAEA, Vienna, 2000, vol. 49, p. 6221 Search PubMed.
- D. Orčić, M. Francišković, K. Bekvalac, E. Svirčev, I. Beara, M. Lesjak and N. Mimica-Dukić, Food Chem., 2014, 143, 48–53 Search PubMed.
- Natural Color System®© - The international language of colour communication™, Scandinavian Colour Institute AB, Stockholm, Sweden, http://www.ncscolour.com/ Search PubMed.
- ISO 11035, 1994, Sensory analysis - Identification and selection of descriptors for establishing a sensory profile by a multidimensional approach. International Organization for Standardization, Geneva, Switzerland Search PubMed.
- ISO 8586, 2012, Sensory analysis - General guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors. International Organization for Standardization, Geneva, Switzerland Search PubMed.
- ISO 8589, 2007, Sensory analysis - General guidance for the design of test rooms. International Organization for Standardization, Geneva, Switzerland Search PubMed.
- J. J. Vulić, L. O. Vračar and Z. M. Šumić, Acta Period. Technol., 2008, 39, 85–90 Search PubMed.
- I. Porras-Mija, A. Aguilar-Galvez, C. Huaman-Alvino, R. Pedreschi and D. Campos, J. Berry Res., 2019, 10, 1–16 Search PubMed.
- B. M. Burton-Freeman, P. M. Guenther, M. Oh, D. Stuart and H. H. Jensen, Food Funct., 2018, 9(2), 1009–1016 Search PubMed.
- A. Konić-Ristić, K. Šavikin, G. Zdunić, T. Janković, Z. Juranic, N. Menković and I. Stanković, Food Chem., 2011, 125, 1412–1417 Search PubMed.
- F. Giampieri, M. Gasparrini, T. Y. Forbes-Hernandez, L. Mazzoni, F. Capocasa, S. Sabbadini and B. Molesini, et al., J. Agric. Food Chem., 2018, 66(3), 581–592 Search PubMed.
- S. M. T. Gharibzahedi and S. M. Jafari, Trends Food Sci. Technol., 2017, 62, 119–132 Search PubMed.
- P. Chandra, A. S. Rathore, K. L. Kay, J. L. Everhart, P. Curtis, B. Burton-Freeman and C. D. Kay, et al., Molecules, 2019, 4(23), 4220 Search PubMed.
- M. Siddiq, K. D. Dolan, P. Perkins-Veazie and J. K. Collins, LWT–Food Sci. Technol., 2018, 92, 127–132 Search PubMed.
- M. Miao and L. Xiang, Pharmacological Advances in Natural Product Drug Discovery, 2020, p. 71 Search PubMed.
- I. A. Adedara, O. B. Fasina, M. F. Ayeni, O. M. Ajayi and E. O. Farombi, Food Chem. Toxicol., 2019, 25, 170–181 Search PubMed.
- P. Vitaglione, G. Donnarumma, A. Napolitano, F. Galvano, A. Gallo, L. Scalfi and V. Fogliano, J. Nutr., 2007, 137, 2043–2048 Search PubMed.
- B. Gullón, T. A. Lú-Chau, M. T. Moreira, J. M. Lema and G. Eibes, Trends Food Sci. Technol., 2017, 67, 220–235 Search PubMed.
- M. Natić, A. Pavlović, F. L. Bosco, N. Stanisavljević, D. D. Zagorac, M. F. Akšić and A. Papetti, Eur. Food Res. Technol., 2019, 245, 469–478 Search PubMed.
- M. C. Caruso, F. Galgano, R. Tolve, M. Pecora, I. Tedesco, F. Favati and N. Condelli, J. Berry Res., 2016, 6, 321–332 Search PubMed.
- O. Shadyro and A. Lisovskaya, Chem. Phys. Lipids, 2019, 221, 176–183 Search PubMed.
- K. Mlynarczyk, D. Walkowiak-Tomczak, H. Staniek, M. Kidon and G. P. Lysiak, Molecules, 2020, 25, 876 Search PubMed.
- X. Zhang, J. K. C. Ahuja and B. M. Burton-Freeman, Nutr. Healthy Aging, 2019, 5, 225–236 Search PubMed.
- M. J. Bermúdez-Soto and F. A. Tomás-Barberán, Eur. Food Res. Technol., 2004, 219, 133–141 Search PubMed.
- S. Zolghadri, A. Bahrami, M. T. H. Khan, J. Munoz-Munoz, F. Garcia-Molina, F. Garcia-Canoras and A. A. Sabour, J. Enzyme Inhib. Med. Chem., 2019, 34, 279–309 Search PubMed.
- L. Gou, J. Lee, J. M. Yang, Y. D. Park, H. M. Zhou, Y. Zhan and Z. R. Lü, Int. J. Biol. Macromol., 2017, 105, 1654–1662 Search PubMed.
- L. Domínguez Díaz, V. Fernández-Ruiz and M. Cámara, J. Funct. Foods, 2020, 68, 103896 Search PubMed.
- M. Battino, T. Y. Forbes-Hernández, M. Gasparrini, S. Afrin, D. Cianciosi, J. Zhang and B. Mezzetti, et al., Crit. Rev. Food Sci. Nutr., 2019, 59(6), 893–920 Search PubMed.
- L. Mazzoni, P. Perez-Lopez, F. Giampieri, J. M. Alvarez-Suarez, M. Gasparrini, T. Y. Forbes-Hernandez and M. Battino, et al., J. Sci. Food Agric., 2016, 96(2), 365–371 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |