Open Access Article
Open Access ArticleA pH-stable Ag(I) multifunctional luminescent sensor for the efficient detection of organic solvents, organochlorine pesticides and heavy metal ions†
Jianxin Ma,
Yue Wang,
Guocheng Liu ,
Na Xu
,
Na Xu * and
Xiuli Wang
* and
Xiuli Wang *
*
College of Chemistry and Materials Engineering, Bohai University, Liaoning Professional Technology Innovation Center of Liaoning Province for Conversion Materials of Solar Cell, Jinzhou 121013, P. R. China. E-mail: xun872@bhu.edu.cn; wangxiuli@bhu.edu.cn
First published on 18th December 2020
Abstract
Developing novel luminescent materials for sensitive and rapid detection of heavy metal ions, organic solvents and organochlorine pesticides is vital for environmental monitoring. Herein, a new Ag(I) luminescent coordination polymer [Ag(3-dpyb)(H3odpa)]·H2O (LCP 1) (3-dpyb = N,N′-bis(3-pyridinecarboxamide)-1,4-butane, H4odpa = 4,4′-oxydiphthalic acid) was obtained by a hydrothermal reaction and characterized by single crystal, powder X-ray diffraction, infrared spectroscopy, thermogravimetric analysis and luminescence spectroscopy. LCP 1 is a three-dimensional (3D) supramolecular framework formed from 1D [Ag-3-dpyb-H3odpa]n chains and H-bond interactions. The luminescence sensing study of LCP 1 for recognizing organic solvents, organochlorine pesticides and heavy metal ions was performed, which demonstrated it to be a potential luminescent sensor for Hacac, NB, 2,6-DCN, Fe2+, Hg2+, and Fe3+. Fe2+, Hg2+, and Fe3+ in river water were determined using LCP 1 with satisfying recovery.
Introduction
One of the serious problems troubling the scientific community today is the increasing pollution of our environment.1 As documented in the literature, among the various pollutants, organic solvents, organochlorine pesticides, and heavy metal ions are seriously regarded as the main factors for environmental pollution. As one of the important chemical substances, small organic molecules play outstanding roles in human industrial production. However, chemicals such as acetylacetone (Hacac) and nitrobenzene (NB) are highly toxic and acutely carcinogenic, which can cause environmental pollution and threaten human health.2–5 Therefore, rapid and selective detection of organic solvents is becoming urgent for environmental safety. In addition, the heavy demand for the production of cereals and vegetables leads to excessive use of organochlorine pesticides (OCPs) during planting. However, the high toxicity and slow degradation rate of OCPs have a negative impact on soil, water, and even human health even at very low concentrations.6,7 Heavy metals are harmful to the health of virtually almost all living organisms.8 In particular, heavy metal ions, including Fe2+, Hg2+, and Fe3+, impose poisonous health effects attributed to oxidation substances formed in the body resulting in DNA damage and overall damage to the central nervous system.9,10 Hence, it is of great significance to monitor the trace amount of these harmful pollutants to avoid the risk of pollution.Currently, a variety of detection techniques have been reported, including liquid chromatography, gas chromatography, and electrochemical analysis.11–13 However, these detection methods are expensive, complicated, and time-consuming. Thus, exploring and developing new detection technology to solve the above impediments are still a valuable academic issue of practical significance.14 Recently, functional coordination polymers (CPs) have exhibited promising applications in many fields such as sensors, electronics, catalysis, and adsorption.15,16 Especially, CPs have been considered as promising detection materials for trace contaminants due to their structural diversity.17 Luminescent coordination polymers (LCPs) have attracted widespread attention because they can be employed as selective and sensitive probes for detecting pollutants. Regrettably, many LCPs can only aim to probe a single species, and it is very rare to detect multiple substances simultaneously with efficient performance.18 Among various fluorescence sensors, LCPs containing Zn2+, Cd2+ and Ag+ have emerged as a novel type of fluorescent nanoprobe in chemical analysis. Especially, Ag(I) is an ideal luminescent material in the preparation of luminescent coordination polymers due to its strong coordination ability to nitrogen-donor atoms.19 Many efforts to develop luminescent chemosensors have been reported; however, only a few successful cases of silver(I) LCP are documented. Thus, it is still highly desirable to develop novel Ag fluorescent probes with improved stability and multifunctional detection ability.
Based on the above discussion, in this work, an Ag(I) coordination polymer (LCP 1) was successfully synthesized by the hydrothermal reaction of N,N′-bis(3-pyridinecarboxamide)-1,4-butane (3-dpyb) and 4,4′-oxydiphthalic acid (H4odpa) mixed ligands. LCP 1 is a 3D supramolecular framework constructed by 1D [Ag-3-dpyb-H3odpa]n chains and H-bond interactions. Notably, LCP 1 can be used as an optical sensor for the recognition of organic solvents, organochlorine pesticides and heavy metal ions.
Experimental
Materials and methods
The 3-dpyb ligand was prepared according to the literature method.20 The reagents were commercially available and used directly without additional purification. The powder X-ray diffraction (PXRD) patterns were obtained at 40 kV, 40 mA with Cu Kα (λ = 1.5406 Å) radiation with a D/teX Ultra diffractometer and infrared spectra (IR) were acquired on a Varian 640 FTIR spectrometer with KBr discs. The thermogravimetric analysis (TGA) experiments were recorded on a Perkin-Elmer TGA analyzer. UV-vis absorption spectra were investigated through the Perkin Elmer Lambda 750. The fluorescence spectra were measured on a Hitachi F-4500 luminescence/phosphorescence spectrometer and the fluorescence lifetime curves were obtained on an FLS1000 Transient Steady-state Fluorescence Spectrometer.Synthesis of [Ag(3-dpyb)(H3odpa)]·H2O (1)
A mixture of AgNO3 (0.040 g, 0.2 mmol), 3-dpyb (0.030 g, 0.1 mmol), H4odpa (0.035 g, 0.1 mmol), H2O (9 mL) and 4 drops of HNO3 (1 M) were introduced into a Teflon-lined stainless-steel vessel (23 mL); the contents were allowed to react at 120 °C for 4 days (Scheme S1†), after which it was cooled to room temperature. The colorless and transparent blocky crystals were collected by filtration, washed with H2O and dried in air for 12 hours (yield of 69% based on Ag). Anal. calcd (%) for C32H29N4AgO12: C, 49.95; H, 3.79; N, 7.28. Found: C, 49.86; H, 3.72; N, 7.21. IR (KBr, cm−1): 3304 (s), 3072 (m), 2939 (m), 2871 (w), 2350 (w), 1662 (m), 1552 (s), 1482 (m), 1424 (w), 1370 (s), 1309 (m), 1265 (s), 1223 (s), 1135 (m), 1060 (w), 963 (s), 841 (m), 770 (s), 700 (s), 630 (m), 598 (m), 500 (w).Luminescence sensing study
The luminescence experiments of LCP 1 were proceeded via dispersing 2.5 mg solid samples in three types of analytical species to obtain suspensions by ultrasound; the fluorescence behavior of these suspensions were then observed by a luminescence spectrometer. For the first type of analytical species, LCP 1 was dispersed in the following different target organic solvents (3 mL): N,N-dimethylformamide (DMF), propyl alcohol (ProOH), N,N-dimethylacetamide (DMA), tetrahydrofuran (THF), benzene (PhH), pyridine (Py), ethanol (EtOH), cyclohexane (CyH), methanol (MeOH), acetonitrile (ACN), iso-propyl alcohol (iso-ProOH), ethylene glycol (EG), dichloromethane (DCM), 1,4-diethylene dioxide (1,4-dioxane), dimethyl sulfoxide (DMSO), acetylacetone (Hacac) and nitrobenzene (NB). For the second type, different organochlorine pesticides were used as target analytes. Here, LCP 1 was added into 10−3 mol L−1 THF solutions with separately dissolved organochlorine pesticides, namely, carbaryl, atrazine, 1,2,4,5-tetrachlorobenzene (1,2,4,5-TetraCB), 2,6-dichloro-4-nitroaniline (2,6-DCN), 1,2,3-trichlorobenzene (1,2,3-TriCB), chlorobenzene (CB) and 1,3-dichlorobenzene (1,3-DCB), and then the mixtures were dispersed ultrasonically. LCP 1 was added to aqueous solutions containing different metal ions, M(NO3)x (M = Na+, K+, Mn2+, Zn2+, Cd2+, Ba2+, Mg2+, Ca2+, Fe2+, Hg2+, and Fe3+) to obtain the third type of analytical species. The resulting concentration for the metal ions was 10−3 mol L−1 and then the mixtures containing LCP 1 and analytes were treated by ultrasonication for 1 h to form a stable suspension. Finally, the fluorescence behavior of the three types of suspensions were measured under an excitation of 345 nm for LCP 1. The fluorescence intensity of the homogeneous suspension was frequently monitored.Results and discussion
Crystal structure of LCP 1
LCP 1 crystallizes in the triclinic system with P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The asymmetric unit of LCP 1 is constituted by one Ag(I) ion, one 3-dpyb ligand, and one incompletely deprotonated H3odpa− ligand (Fig. 1a). The central Ag(I) ion is three-coordinated by two N atoms from two 3-dpyb ligands and one carboxyl oxygen atom from one H3odpa− ligand. The length of the Ag–O bond is 2.721(7) Å, which is similar to those previously reported.21
space group. The asymmetric unit of LCP 1 is constituted by one Ag(I) ion, one 3-dpyb ligand, and one incompletely deprotonated H3odpa− ligand (Fig. 1a). The central Ag(I) ion is three-coordinated by two N atoms from two 3-dpyb ligands and one carboxyl oxygen atom from one H3odpa− ligand. The length of the Ag–O bond is 2.721(7) Å, which is similar to those previously reported.21
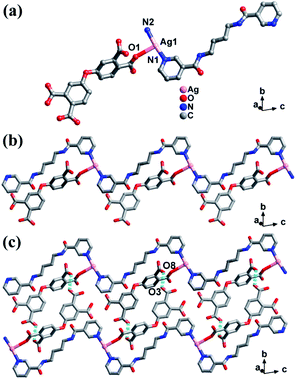 | ||
| Fig. 1 (a) The coordination geometry of Ag atom in LCP 1. (b) The 1D chain of LCP 1. (c) The 2D supramolecular layered structure of LCP 1. | ||
In LCP 1, the 3-dpyb ligands as linkers coordinated with Ag(I) ions to form a 1D [Ag-3-dpyb]n chain. Besides, incompletely deprotonated H3odpa− ligands hang on one side of the 1D [Ag-3-dpyb]n chains, which provide a prerequisite for three-dimensional (3D) supramolecular framework by H-bond interactions (Fig. 1b). Firstly, adjacent 1D chains were linked by H-bonding interactions [O(8)–H(8)⋯O(3)] with a length of 2.492 Å to form 2D supramolecular layers (Fig. 1c). The corresponding hydrogen-bonding parameters of LCP 1 are listed in Table S3.† Next, 2D layers were further connected through [O(5)–H(5)⋯O(2)] with a distance of 3.023 Å to form a 3D supramolecular structure (Fig. S1†).
The thermogravimetric analyses (TGA), infrared spectroscopy (IR), and X-ray diffraction (PXRD) studies of LCP
The TGA curve of LCP 1 is elucidated in Fig. S2† under the N2 atmosphere from 30 °C to 800 °C. LCP 1 showed a slow weight loss between 30 °C to 163 °C, followed by a weight loss of 2.33%, indicating the loss of one free H2O molecule (cal. 2.34%). From 193 °C to 325 °C, the rapid weight loss might be attributed to the decomposition of ligands. Then, with the increase of temperature, the framework collapsed and decomposed.The IR spectra are displayed in Fig. S3,† which confirms the structural composition of LCP 1. The peaks at 3304 cm−1 are the stretching vibrations of O–H bonds in water.22 The characteristic absorption peaks from 1680 to 1360 cm−1 are attributed to the COO− group. The strong absorption peaks at 841 cm−1, 770 cm−1, and 700 cm−1 are attributed to the ν(C–N) stretching vibrations of the N-heterocyclic rings.23 The strong band at about 1662 cm−1 was assigned to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibration of the amide groups.24
O) vibration of the amide groups.24
Since the stability of the fluorescent probe is critical in a sensing application, the PXRD patterns and the infrared spectrum of LCP 1 were obtained under different conditions. The PXRD patterns of the synthesized samples are exhibited in Fig. S5,† which indicated that the crystallinity and phase purity of LCP 1 were good in different conditions. The samples of LCP 1 were particularly placed in air for about 6 months to determine if the samples were stable. The results showed that the PXRD peak of LCP 1 was consistent with that of the fresh one, indicating the excellent stability of LCP 1. Moreover, the infrared spectra of LCP 1 under various conditions were further investigated. It can be seen from Fig. S4† that the absorption peaks of the samples were almost unchanged. These results collectively demonstrated that LCP 1 has great stability, which gave us reason to further conduct luminescence experiments.
Solid fluorescence spectra of LCP 1
To investigate the luminescence properties of LCP 1, the excitation and emission spectra in the solid state were measured in open-air conditions. As shown in Fig. S6,† the emission spectrum shows a peak at 405 nm under an excitation of 345 nm, which may be due to ligand-to-metal charge transfer (LMCT).25Sensing of organic solvents
Considering the high stability and obvious fluorescence properties of LCP 1 in water, we firstly explored their potential ability to sense organic solvents at room temperature and pressure. LCP 1 (2.5 mg) was dispersed in 4 mL solvents, and the suspensions were formed by ultrasonication. Herein, seventeen common organic solvents, namely, DMF, ProOH, DMA, PhH, THF, Py, EtOH, CyH, MeOH, ACN, iso-ProOH, EG, DCM, 1,4-dioxane, DMSO, Hacac and NB were selected as representatives to investigate their influence on the luminescence properties of LCP 1. Interestingly, the addition of different organic solvents induced diverse changes in the fluorescence intensity compared with the blank experiment. As shown in Fig. 2a and Fig. S11,† the luminescence intensity of LCP 1 showed a dramatic quenching effect by the addition of Hacac and NB within 15 seconds.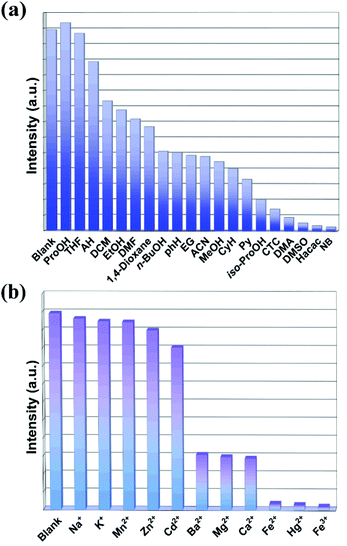 | ||
| Fig. 2 Fluorescence emission intensities of LCP 1 dispersed in different organic solvents (a), and in the presence of different metal ions (b). | ||
To further explore the sensing sensitivity of LCP 1 to Hacac and NB, the concentration gradient experiment was carried out with Hacac and NB. The concentrations were adjusted by EtOH ranging from 0 to 3.0 mM for Hacac and 0 to 2.6 mM for NB. As displayed in Fig. 3a and b, the luminescence intensities gradually decreased with an increase in Hacac and NB concentration. The luminescence-quenching efficiency was described by the Stern–Volmer (S–V) equation,26 I0/I = 1 + Ksv × [C], where I0 and I refer to the luminescence intensities of LCP 1 before and after sensing Hacac or NB, Ksv is the constant and [C] represents the concentrations of Hacac or NB (Fig. 3c and d). The correlation coefficients (R2) are 0.9965 for Hacac and 0.9993 for NB. Ksv of LCP 1 are 1.542 × 104 M−1 and 1.683 × 104 M−1 for Hacac and NB, respectively, according to the corresponding S–V plots. The detection limit is calculated with 3δ/Ksv; δ represents the blank standard deviation for ten times, as illustrated in Fig. S7.† So the detection limit for Hacac and NB are 1.517 × 10−5 M and 1.391 × 10−5 M, respectively, indicating that LCP 1 can efficiently detect Hacac and NB. The Ksv and detection limit values for LCP 1 are comparable to those of the CPs reported in the literature (Tables S4 and S5†).10,27–32
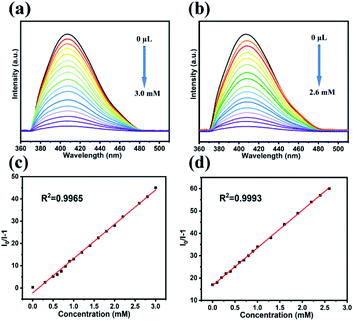 | ||
| Fig. 3 Luminescence titration of LCP 1 at different concentrations of Hacac (a) and NB (b). The S–V plot of LCP 1 at different concentrations of Hacac (c) and NB (d) in EtOH. | ||
The sensing selectivity of LCP 1 for Hacac or NB in the presence of other interferential analytes (including DMF, ProOH, DMA, PhH, THF, Py, EtOH, CyH, MeOH, ACN, iso-ProOH, EG, DCM, 1,4-dioxane, and DMSO) were evaluated. First, equal amount of interferents were mixed to form a mixture. Then, LCP 1 (2.5 mg) was dispersed in 4 mL H2O to form a suspension solution; the above interferent mixture was first added followed by Hacac or NB solution. Finally, the corresponding emissions were monitored. As shown in Fig. S8,† the emission intensity of LCP 1 was quenched by 35% or 28% in the presence of the interferent mixture. Upon introducing the mixture of Hacac or NB and the interferents, the fluorescence of the system was quenched strongly. This suggests the high selectivity of LCP 1 towards Hacac and NB despite the existence of interferents.
The recycling performance is vital for a good sensor. To further illustrate the cyclic detection performances of LCP 1 on Hacac and NB, the recycling performance were carried out. After quenching–recovery cycles, the luminescence intensity was well retained. The experimental results indicated that LCP 1 basically remained unchanged for at least five runs (Fig. S13a and b†). Besides, the PXRD pattern of the samples after five detections could still match well to that of the as-synthesized samples, showing that LCP 1 has excellent stability (Fig. S13d and e†).
Sensing of organochlorine pesticides (OCPs)
OCPs are widely used in agriculture. Although the growth of plants requires the use of pesticides, excessive use has caused an enormous detrimental effect on water and even human health. Therefore, the selective sensing of OCPs in solution at room temperature and pressure was studied in this work. Seven pesticides were selected, namely, carbaryl, atrazine, 1,2,4,5-TetraCB, 2,6-DCN, 1,2,3-TriCB, CB and 1,3-DCB. Besides, the fluorescence emission spectra of fluorophores are frequently found to be sensitive to the polarity of the solvent environment. Therefore, the impact on the emission of LCP 1 was first analyzed; an optimal solvent was identified for OCP detection after soaking LCP 1 in diverse organic solvents for 24 h. Fortunately, when dispersed in THF, the emission spectrum was basically the same as that of LCP 1 in water (Fig. S11†). The subsequent experimental methods were the same as that used for sensing the organic solvents apart from changing the solvent to THF. The resulting concentration for the OCPs was 10−3 mol L−1. Fig. 4a shows the changes in the fluorescence intensity after adding LCP 1 to the THF solvent containing seven different types of OCPs. The results showed that 2,6-DCN had a significant fluorescence quenching effect on LCP 1.The fluorescence intensities of LCP 1 for different pesticides were recorded, and is shown in Fig. 4b. After adding 2,6-DCN, the luminescence intensity was quenched in a short time (15 s) and the quenching efficiency reached 92%. However, the fluorescence intensity had little change after the addition of all OCPs except for 2,6-DCN. In order to understand the constant values, fluorimetric titrations were performed with 2,6-DCN, where the relationship between the I0/I and concentration was studied (Fig. 4c). The Ksv value is 2.028 × 105 M−1 for 2,6-DCN. The detection limit calculated by 3δ/Ksv is 1.154 × 10−6 M (Fig. S12†). The Ksv and detection limit values for LCP 1 are similar to those of the CPs reported in the literature (Table S6†).33–36
Besides, agricultural pollutants are quite complicated; there are many forms of contaminants that coexist in different pH solutions, and hence a 2,6-DCN luminescence sensor with high stability is urgently required. Bearing in mind the above considerations, we conducted the luminescence-quenching stability test of 2,6-DCN within the pH range of 2.0–12.0 (adjust the pH by dropping 0.1 M HNO3 or 0.1 M NaOH in 10−3 mol L−1 THF solution of 2,6-DCN and then add 2.5 mg of LCP 1). From Fig. 4d, it can be seen that with the change in pH values, the quenching intensity of 2,6-DCN remains good. Thus, LCP 1 can be used as a highly selective sensor for detecting 2,6-DCN in THF solutions. More importantly, the fluorescence quenching mechanism of 2,6-DCN was inactive on other pesticides present in the solution, as demonstrated by competitive experiments (Fig. S9†). Simultaneously, the recyclability study of LCP 1 on 2,6-DCN has been carried out. After five cycles, the luminescence intensity only decreased slightly compared with the original one. The PXRD patterns further proved that the initial framework was not destroyed, reflecting the good regeneration ability of LCP 1 (Fig. S13c and f†).
Sensing of metal ions
The strong emission and good water stability of LCP 1 prompted us to study its luminescence sensing characteristics. To verify the potential of LCP 1 for sensing metal ions, 2.5 mg samples of LCP 1 were firstly dispersed into 3 mL solution (1 × 10−3 M) containing Na+, K+, Mn2+, Zn2+, Cd2+, Ba2+, Mg2+, Ca2+, Fe2+, Hg2+, or Fe3+; all relevant experiments were tested at room temperature and pressure.Then, the mixed solutions were subjected to ultrasonic vibration for half an hour to form a series of suspensions; finally, the detection studies were executed.
As shown in Fig. 2b, Na+, K+, Mn2+, Zn2+ and Cd2+ had a minor effect on the luminescence intensity. Ba2+, Mg2+, and Ca2+ had a slight inhibition on the luminescence of LCP 1. Obviously, LCP 1 undergoes more intense fluorescence quenching with Fe2+, Hg2+, and Fe3+. It implies that LCP 1 can be counted as a potential candidate for probing Fe2+, Hg2+, and Fe3+. Moreover, fluorimetric titrations were performed to explore the interaction constant values. As the concentration of metal ions increases, the fluorescence intensity decreases regularly (Fig. 5). LCP 1 showed a fast quenching within 15 s towards Fe2+, Hg2+ and Fe3+ ions in the aqueous solution, and the quenching efficiency remained unchanged for 5 minutes. The quenching of the fluorescence intensity of LCP 1 was further analyzed with the help of the Stern–Volmer equation. The Ksv value for Fe2+, Hg2+, and Fe3+ were found to be 6.107 × 104 M−1, 1.005 × 105 M−1, and 1.186 × 105 M−1, respectively. The limit of detection for Fe2+, Hg2+, and Fe3+ were 3.831 × 10−6 M, 2.328 × 10−6 M, and 1.973 × 10−6 M, respectively, in water. There are reports in the literature where the detection of Fe2+, Hg2+, and Fe3+ has been recorded in both organic and water media (Tables S7–S9†).10,30,37–49 Compared with these examples reported, LCP 1 demonstrated impressive results.
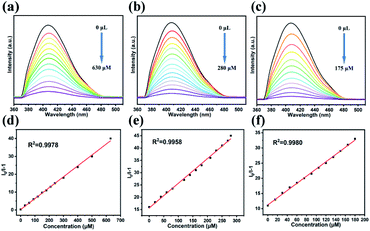 | ||
| Fig. 5 Fluorescence spectra of LCP 1 dispersed in different concentrations of Fe2+ (a), Hg2+ (b) and Fe3+ (c). The S–V plot of LCP 1 at different concentrations of Fe2+ (d), Hg2+ (e) and Fe3+ (f). | ||
Furthermore, five cycles of the Fe2+, Hg2+, and Fe3+ sensing experiments were performed. The collected results showed that LCP 1 could recover its luminescence intensity after washing several times (Fig. S14†). The competition experiments showed that the presence of other metal ions did not interfere with the fluorescence responses of Fe2+, Hg2+, and Fe3+. The results revealed that LCP 1 can detect Fe2+, Hg2+, and Fe3+ with remarkable selectivity (Fig. S10†). Thus, it can be presumed that LCP 1 can be used as a potentially stable and highly selective probe for the detection of Fe2+, Hg2+, and Fe3+ in water. To verify the practicality of LCP 1, fluorescence detection experiments on river water samples containing Fe2+, Hg2+, and Fe3+ were performed. The water samples were obtained from a local river (Table S10†). After simple filtration and separation, Fe2+, Hg2+, or Fe3+ were not detected in the river water samples. To confirm the validity of the present work, three different concentrations (5, 10 and 15 μM) of Fe2+, Hg2+, or Fe3+ ions were added to LCP 1 at three different concentrations (5, 10 and 15 μM) to the river water samples for performing the standard addition recovery experiments. The recovery values of water samples were 96.2–100.20%, 99.73–102.20%, and 100.06–104.33%, respectively. The results suggested that LCP 1 had great practicability for the determination of metal ions in the environment.
The time-dependent tests on fluorescence emission response
Time-dependent fluorescence emission response experiments were conducted by adding 50 μL of analytes (Hacac, NB, 2,6-DCN, Fe2+, Hg2+, and Fe3+) to a 3 mL dispersion of LCP 1. As shown in Fig. S16,† the intensity evidently decreased in a short time (15 s), leading to a significant increase in the quenching efficiency, which reveals that LCP 1 is sensitive and is a rapid sensor towards Hacac, NB, 2,6-DCN, Fe2+, Hg2+, and Fe3+.Sensing mechanism
The possible quenching mechanism in CPs generally includes the following points: (i) structural collapse of the CP framework, (ii) a weak coordinating interaction of analytes with the framework, and (iii) competitive adsorption between the framework and the analytes.50–52 In order to determine the possible quenching mechanism in this work, initially, we examined the coherence of the PXRD patterns between LCP 1 after luminescence research and the simulated one. The results demonstrated that the framework remained stable; so the luminescence quenching is not due to the collapse of the framework (Fig. S4†). The second possibility was also ruled out because comparing the IR spectra (Fig. S3†) before and after the sensing process, it was found that there was no obvious peak position or intensity change, that is to say, no new interactions were generated. Therefore, Forster resonance energy transfer (FRET) might play an important role in the detection of these analytes. We studied the overlap between the absorption and emission spectra for each analyte and the sensing species. The UV-vis absorption spectra of twelve analytes and the emission spectrum of LCP 1 were investigated (Fig. 6). The strong absorption bands from the UV-visible absorption spectra of 2,6-DCN, Fe2+, Hg2+, and Fe3+ show a great overlap with the emission spectrum of LCP 1; however, there is almost no obvious overlap between the emission spectrum of LCP 1 and the absorption spectra of Hacac and NB, demonstrating that these analytes are not completely gratified with the energy competition absorption. So, energy transfer may not be the only mechanism.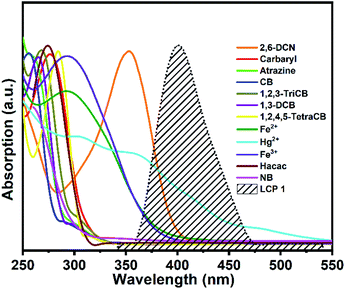 | ||
| Fig. 6 UV-vis optical absorption spectra of analytes along with the emission spectrum of LCP 1 (dotted black line). | ||
In order to further confirm the fluorescence mechanism, the fluorescence average lifetime measurements based on the suspension solution of LCP 1 were carried out before and after the addition of analytes. Notably, the lifetime decay curves of LCP 1 before and after exposure to analytes showed only an inappreciable variation, which indicated that the quenching mechanism was more inclined to static quenching (Fig. S15†). Conversely, compared with the original curve, the fluorescence lifetime had an obvious change, which belonged to a dynamic annihilation process. For LCP 1, the lifetime value was 7.948 ns. After adding analytes, the average lifetime values were 7.959 ns (adding Hacac) and 7.963 ns (adding NB). The changes in the average lifetime value indicated that static quenching was the primary pathway in the fluorescence quenching process of the organic solvents.
Conclusions
In conclusion, a novel luminescent 3D Ag(I) supramolecular framework was successfully constructed from a tetradentate carboxylate ligand (H4odpa) and flexible bis(pyridyl)bis(amide) (3-dpyb) under hydrothermal conditions. This stable framework exhibits prominent fluorescence sensing properties for the selective detection of Hacac, NB, 2,6-DCN, Fe2+, Hg2+, and Fe3+ with the advantages of low detection limit. Moreover, LCP 1 also exhibited good reproducibility and long-term stability. In the pH range of 2.0–12.0, LCP 1 could still maintain excellent sensing effect on 2,6-DCN. Thus, LCP 1 was used as a sensor for detecting Fe2+, Hg2+, and Fe3+ in river water with satisfactory recovery. Furthermore, the reason for the fluorescence quenching of LCP 1 on 2,6-DCN, Fe2+, Hg2+, and Fe3+ was related to the FRET mechanism, while Hacac and NB leading to the fluorescence quenching of LCP 1 was ascribed to static quenching. Therefore, LCP 1 can be considered as an eminently potential competitive and reliable candidate for the detection of organic solvents, organochlorine pesticides and heavy metal ions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The acknowledgements come at the end of an article after the conclusions and before the notes and references. This work was financially supported by the National Natural Science Foundation of China (No. 21971024, 21671025) and Liao Ning Revitalization Talents Program (XLYC1902011).References
- K. Remoundou and P. Koundouri, Int. J. Environ. Res. Public Health, 2009, 6, 2160–2178 Search PubMed.
- X. M. Kang, X. Y. Fan, P. Y. Hao, W. M. Wang and B. Zhao, Inorg. Chem. Front., 2019, 6, 271–277 Search PubMed.
- D. Yang, L. Lu, S. Feng and M. Zhu, Dalton Trans., 2020, 49, 7514–7524 Search PubMed.
- J. Q. Liu, Z. D. Luo, Y. Pan, A. Kumar Singh, M. Trivedi and A. Kumar, Coord. Chem. Rev., 2020, 406, 213145 Search PubMed.
- L. Esrafili, F. D. Firuzabadi, A. Morsali and M. L. Hu, J. Hazard. Mater., 2021, 403, 123696 Search PubMed.
- G. Chakraborty, P. Das and S. K. Mandal, ACS Appl. Mater. Interfaces, 2018, 10, 42406–42416 Search PubMed.
- C. L. Tao, B. Chen, X. G. Liu, L. J. Zhou, X. L. Zhu, J. Cao, Z. G. Gu, Z. Zhao, L. Shen and B. Z. Tang, Chem. Commun., 2017, 53, 9975–9978 Search PubMed.
- J. Cepeda and A. R. Diéguez, CrystEngComm, 2016, 18, 8556–8573 Search PubMed.
- G. C. Liu, Y. Li, J. Chi, N. Xu, X. L. Wang, H. Y. Lin and Y. Q. Chen, Dyes Pigm., 2020, 174 Search PubMed.
- J. X. Ma, N. Xu, Y. Liu, Y. Wang, H. Li, G. C. Liu, X. L. Wang and J. R. Li, Inorg. Chem., 2020, 59, 15495–15503 Search PubMed.
- C. Miao, Inorg. Chem. Commun., 2019, 105, 86–92 Search PubMed.
- B. Wang, X. L. Lv, D. Feng, L. H. Xie, J. Zhang, M. Li, Y. Xie, J. R. Li and H. C. Zhou, J. Am. Chem. Soc., 2016, 138, 6204–6216 Search PubMed.
- K. Ren, S. H. Wu, X. F. Guo and H. Wang, Inorg. Chem., 2019, 58, 4223–4229 Search PubMed.
- Y. Wang, S. H. Xing, F. Y. Bai, Y. H. Xing and L. X. Sun, Inorg. Chem., 2018, 57, 12850–12859 Search PubMed.
- A. J. Liu, F. Xu, S. D. Han, J. Pan and G. M. Wang, Cryst. Growth Des., 2020, 20, 7350–7355 Search PubMed.
- M. Cao, R. Pang, Q. Y. Wang, Z. Han, Z. Y. Wang, X. Y. Dong, S. F. Li, S. Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2019, 141, 14505–14509 Search PubMed.
- K. C. Stylianou, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119–4130 Search PubMed.
- C. X. Yang, H. B. Ren and X. P. Yan, Anal. Chem., 2013, 85, 7441–7446 Search PubMed.
- J. Z. Huo, X. M. Su, X. X. Wu, Y. Y. Liu and B. Ding, CrystEngComm, 2016, 18, 6640–6652 Search PubMed.
- Y. F. Hsu, C. H. Lin, J. D. Chen and J. C. Wang, Cryst. Growth Des., 2008, 8, 1094–1096 Search PubMed.
- L. Brammer, M. D. Burgard, M. D. Eddleston, C. S. Rodger, N. P. Rath and H. Adams, CrystEngComm, 2002, 4, 239–248 Search PubMed.
- X. L. Wang, B. Mu, H. Y. Lin and G. C. Liu, J. Organomet. Chem., 2011, 696, 2313–2321 Search PubMed.
- B. Dolenský, R. Konvalinka, M. Jakubek and V. Král, J. Mol. Struct., 2013, 1035, 124–128 Search PubMed.
- L. Yang, F. Wang, D. Y. Auphedeous and C. Feng, Nanoscale, 2019, 11, 14210–14215 Search PubMed.
- X. L. Wang, Y. Xiong, X. T. Sha, G. C. Liu and H. Y. Lin, Cryst. Growth Des., 2017, 17, 483–496 Search PubMed.
- J. Keizer, J. Am. Chem. Soc., 1983, 105, 1494–1498 Search PubMed.
- Q. Q. Xiao, G. Y. Dong and Y. H. Li, Inorg. Chem., 2019, 58, 15696–15699 Search PubMed.
- X. M. Kang, X. Y. Fan and P. Y. Hao, Inorg. Chem. Front., 2019, 6, 271–277 Search PubMed.
- S. L. Yao, S. J. Liu, X. M. Tian, T. F. Zheng, C. Cao, C. Y. Niu, Y. Q. Chen and H. R. Wen, Inorg. Chem., 2019, 58, 3578–3581 Search PubMed.
- B. L. Martinez, A. D. Shrode, R. J. Staples and R. L. LaDuca, Polyhedron, 2018, 151, 369–380 Search PubMed.
- X. Wang, Y. Han and X. X. Han, New J. Chem., 2018, 42, 19844–19852 Search PubMed.
- X. D. Duan, F. Y. Ge and H. G. Zheng, Inorg. Chem. Commun., 2019, 107, 107479 Search PubMed.
- G. C. Liu, S. W. Han, Y. Gao, N. Xu, X. L. Wang and B. K. Chen, CrystEngComm, 2020, 22, 7952–7961 Search PubMed.
- D. D. Feng, Y. D. Zhao, X. Q. Wang, D. D. Fang, J. Tang, L. M. Fan and J. Yang, Dalton Trans., 2019, 48, 10892–10900 Search PubMed.
- N. Xu, Q. H. Zhang, B. S. Hou, Q. Cheng and G. A. Zhang, Inorg. Chem., 2018, 57, 13330–13340 Search PubMed.
- H. He, S. H. Chen, D. Y. Zhang, R. Hao, C. Zhang, E. C. Yang and X. J. Zhao, Dalton Trans., 2017, 46, 13502–13509 Search PubMed.
- C. F. Wan, Y. J. Chang, C. Y. Chien, Y. W. Sie, C. H. Hu and A. T. Wu, J. Lumin., 2016, 178, 115–120 Search PubMed.
- N. Lashgari, A. Badiei and G. Mohammadi Ziarani, J. Fluoresc., 2016, 26, 1885–1894 Search PubMed.
- S. Çetindere and S. O. Tümay, et al., J. Fluoresc., 2016, 26, 1173–1181 Search PubMed.
- S. Pandey, A. Azam, S. Pandey and H. M. Chawla, Org. Biomol. Chem., 2009, 7, 269–279 Search PubMed.
- S. A. A. Razavi, M. Y. Masoomi and A. Morsali, Inorg. Chem., 2017, 56, 9646–9652 Search PubMed.
- M. G. Choi, Y. H. Kim, J. E. Namgoong and S. K. Chang, Chem. Commun., 2009, 24, 3560–3562 Search PubMed.
- K. Xu, Y. Li, Y. Si, Y. He, J. Ma, J. He, H. Hou and K. A. Li, J. Lumin., 2018, 204, 182–188 Search PubMed.
- R. M. Tivier, I. Leray, B. Lebeau and B. Valeurab, J. Mater. Chem., 2005, 15, 2965–2973 Search PubMed.
- Z. Zhan, X. Liang, X. Zhang, Y. Jia and M. Hu, Dalton Trans., 2019, 48, 1786–1794 Search PubMed.
- X. Y. Guo, Z. P. Dong, F. Zhao, Z. L. Liu and Y. Q. Wang, New J. Chem., 2019, 43, 2353–2361 Search PubMed.
- Y. Li, Z. Chang, F. Huang, P. Wu, H. Chu and J. Wang, Dalton Trans., 2018, 47, 9267–9273 Search PubMed.
- M. Zheng, H. Tan, Z. Xie, L. Zhang, X. Jing and Z. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 1078–1083 Search PubMed.
- B. Wang, Q. Yang, C. Guo, Y. Sun, L. H. Xie and J. R. Li, ACS Appl. Mater. Interfaces, 2017, 9, 10286–10295 Search PubMed.
- Y. K. Fan, W. Y. Zhang, S. S. Zhang, Y. T. Yan, K. Zhong, Y. Zhang, G. P. Yang and Y. Y. Wang, J. Solid State Chem., 2019, 269, 386–395 Search PubMed.
- Y. T. Yan, W. Y. Zhang, F. Zhang, F. Cao, R. F. Yang, Y. Y. Wang and L. Hou, Dalton Trans., 2018, 47, 1682–1692 Search PubMed.
- S. Y. Zhu and B. Yan, Dalton Trans., 2018, 47, 11586–11592 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S16 and Tables S1–S11 (PDF). CCDC 2035795. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra08991e |
| This journal is © The Royal Society of Chemistry 2020 |