Open Access Article
Open Access ArticleRing-opening 1,3-arylboration of arylcyclopropanes mediated by BCl3†
Yuichi Kuboki,
Mitsuhiro Arisawa and
Kenichi Murai *
*
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamada-oka, Suita, Osaka 565-0871, Japan. E-mail: murai@phs.osaka-u.ac.jp
First published on 13th October 2020
Abstract
Herein, we report a ring-opening 1,3-arylboration of aryl cyclopropanes using BCl3 in the presence of arene nucleophiles. Formal 1,3-oxy arylation and 1,3-amino arylation of the arylcyclopropane via one-pot derivatization of the installed boron group were also achieved.
Cyclopropane derivatives are versatile building blocks in organic synthesis used frequently. Transformations utilizing their ring strain and unique reactivity have been actively studied.1–3 Among them, the ring-opening reaction is one of the important transformations that provide synthetically useful 1,3-functionalized molecules. The cyclopropane ring-opening strategy mainly utilizes donor–acceptor cyclopropanes due to their high reactivity.2 The transition metal-mediated process is also used for cyclopropanes bearing a chelating group that can coordinate to transition metals.3 On the other hand, the ring-opening 1,3-functionalization reaction of simply substituted cyclopropane, such as aryl-substituted cyclopropane, has not been studied so much. Recently, ring-opening of cyclopropane by phosphine/borane frustrated Lewis pairs,4 1,3-aminofluorination by N–F reagents,5 1,3-oxyfluorination and 1,3-difluorination by fluoroiodine reagents,6 1,3-dioxygenation by hypervalent iodine reagent,7 oxo-amination by photoredox catalysis,8 and 1,3-hydrosilylation by silylium-ion9 have been successfully reported. However, the development of new ring-opening 1,3-functionalization reactions of simply substituted cyclopropanes is still significant due to the limited variety of functional groups that can be introduced.
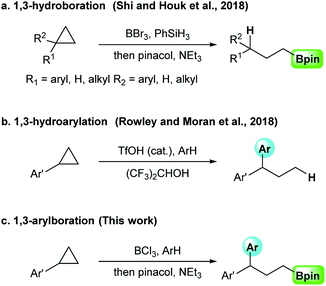
Recently, the methods using boron halides as a borane source has been actively studied and applied to metal-free electrophilic borylation reaction with arenes, heteroarenes, alkynes, and alkenes.10 In this context, Shi and Houk have successfully developed a ring-opening 1,3-hydroboration of cyclopropanes using BBr3 in the presence of PhSiH3 (Scheme 1a).11 On the other hand, ring-opening 1,3-hydroarylation of monosubstituted cyclopropanes, including simply substituted cyclopropanes, was developed by Rowley and Moran. This reaction has been accomplished using catalytic TfOH in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (Scheme 1b).12 Both methods are useful, but one functional group introduced is limited to a hydrogen atom. Inspired by these works, we sought to develop a new 1,3-functionalization of cyclopropanes. Herein, we report a ring-opening 1,3-arylboration of aryl cyclopropanes using BCl3 in the presence of arene nucleophiles (Scheme 1c).
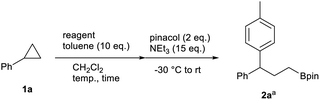
We began our study on the ring-opening 1,3-arylboration of cyclopropanes using cyclopropylbenzene (1a) and toluene (10 equiv.) as a model reaction (Table 1). The use of BCl3 in CH2Cl2 at room temperature was not effective, and allylbenzene was mainly obtained (entry 1). Allylbenzene is considered to be produced through a formation of benzyl cation via C–C bond cleavage by BCl3, a formation of homobenzyl cation by 1,2-hydride migration, and subsequent dissociation of the boron group.13 On the other hand, when the reaction was carried out at −30 °C, the formation of allylbenzene was suppressed, and the desired 1,3-arylborated product 2a was isolated in 11% yield via in situ formations of the pinacol boronate ester by treatment with pinacol and triethylamine (entry 2). Encouraged by these results, we further investigated different borane reagents for the reaction. When BBr3 was used, 2a was not produced. Instead, allylbenzene was formed even at low temperature (entry 3). The use of B-bromocatecholborane or BF3·OEt2 was also not effective (entries 4 and 5). From these results, BCl3 was found to be an optimal reagent. Then, we investigated the reaction time and reaction temperature in detail. It was found that extending the reaction time to 48 hours improves the yield to 45% (entry 6). On the other hand, further studies on the reaction temperature did not improve the yield (entries 7 and 8). Finally, it was found that the amount of BCl3 used is essential for improving the efficiency of the reaction (entry 9 and 10), and when five equivalent of BCl3 was used, compound 2a was obtained in 81% yield. Regarding the amount of nucleophile, the yield was decreased when the amount of toluene was reduced to 5 equivalent (entry 11). Therefore, the optimal reaction conditions found for the 1,3-arylboration of arylcyclopropane involved treatment with BCl3 (5 equiv.) in CH2Cl2 in the presence of toluene (10 equiv.).
| Entry | Reagent (equiv.) | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
a p![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o = >20 o = >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b Pinacol and NEt3 were not treated.c Not detected.d No reaction.e Toluene (5 equiv.). 1.b Pinacol and NEt3 were not treated.c Not detected.d No reaction.e Toluene (5 equiv.). |
||||
| 1b | BCl3 (1.1) | rt | 2 | Trace |
| 2 | BCl3 (1.1) | −30 | 5 | 11 |
| 3 | BBr3 (1.1) | −30 | 10 | N.D.c |
| 4b | B-Bromocatecholborane (1.1) | −30 | 10 | N.R.d |
| 5b | BF3·OEt2 (1.1) | −30 | 10 | N.R.d |
| 6 | BCl3 (1.1) | −30 | 48 | 45 |
| 7 | BCl3 (1.1) | −20 | 48 | 31 |
| 8 | BCl3 (1.1) | −40 | 48 | Trace |
| 9 | BCl3 (3.0) | −30 | 48 | 66 |
| 10 | BCl3 (5.0) | −30 | 48 | 81 |
| 11e | BCl3 (5.0) | −30 | 48 | 62 |
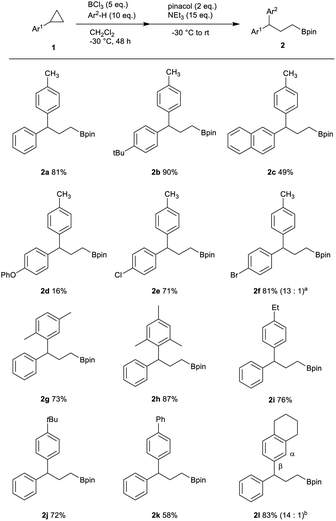
With optimized conditions in hand, we investigated the substrate scope of this transformation (Table 2). p-tBu cyclopropylbenzene and 2-cyclopropylnaphthalene gave 2b and 2c in moderate to excellent yields. In contrast, p-PhO cyclopropylbenzene gave only a 16% yield, showing that the oxygen atom in the substrate could not be preferable for the reaction, probably due to the high affinity of oxygen atom toward BCl3. On the other hand, bromo or chloro substituted aryl cyclopropanes worked well (2e, 2f). We also studied the scope of nucleophiles with 1a. The reactions using benzene were first examined, but it was found that benzene gave a complex mixture (not shown in table). It is probably because the nucleophilicity of cyclopropylbenzene was relatively higher than that of benzene. Therefore, we focused on a series of alkyl-substituted benzenes. p-Xylene, mesitylene, p-Et benzene, p-tBu benzene, and biphenyl provided the corresponding products 2g–2k in good yield. The reaction with 1,2,3,4-tetrahydronaphthalene also proceeded to give 2l regioselectively.
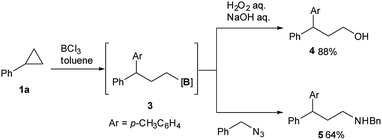
One-pot derivatization using the installed boron group was also studied in addition to converting it into pinacol boronic esters for isolation. As shown in Scheme 2, oxidative workup of the C–B bond using hydrogen peroxide under basic conditions readily furnished alcohol 4 in excellent yield from 1a. Also, the treatment of 3 with benzyl azide gave secondary amine 5 in 64% yield.14 It should be noted that 3,3-diaryl-propylamine is a structural motif found in some pharmaceuticals such as fendilline.15 These one-pot transformations can be considered as a formal 1,3-oxy arylation or 1,3-amino arylation of the arylcyclopropane, which has not been reported so far.
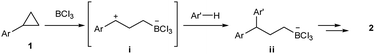
A possible reaction mechanism was depicted in Scheme 3. Given the reported paper,11 this ring-opening 1,3-arylboration is suggested to proceed in a stepwise manner. That is, the treatment of BCl3 to aryl cyclopropane could generate a zwitterionic intermediate i. The following nucleophilic addition of an arene to benzylic cation could give intermediate ii, which is transformed to pinacol borate by the reaction with pinacol and triethylamine.
In summary, we have developed a method for the 1,3-arylboration of arylcyclopropanes to provide 3,3-diaryl-propyl boronic esters for the first time. It was found that BCl3 was optimal as the boron source in the presence of arene nucleophiles. The formal 1,3-oxy arylation and 1,3-amino arylation of the arylcyclopropane via one-pot derivatization of the installed boron group were also achieved. A full account of these studies will be reported in due course.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by a Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number T17K082100 (K. M.) and by a Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from the AMED under Grant Number JP19am0101084 and JP20am0101084.References
- For reviews, see: (a) H. N. C. Wong, M. Y. Hon, C. W. Tse and Y. C. Yip, Chem. Rev., 1989, 89, 165–198 CrossRef CAS; (b) O. G. Kulinkovich, Chem. Rev., 2003, 103, 2597–2632 CrossRef CAS.
- For reviews, see: (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS; (b) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804–818 RSC; (c) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS; (d) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655–671 RSC; (e) S. J. Gharpure and L. N. Nanda, Tetrahedron Lett., 2017, 58, 711–720 CrossRef CAS; (f) P. Singh, R. K. Varshnaya, R. Dey and P. Banerjee, Adv. Synth. Catal., 2020, 362, 1447–1484 CrossRef CAS.
- For reviews, see: (a) M. H. Shaw and J. F. Bower, Chem. Commun., 2016, 52, 10817–10829 RSC; (b) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410–9464 CrossRef CAS; (c) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117–3179 CrossRef CAS.
- J. G. M. Morton, M. A. Dureen and D. W. Stephan, Chem. Commun., 2010, 46, 8947–8949 RSC.
- C. R. Pitts, B. Ling, J. A. Snyder, A. E. Bragg and T. Lectka, J. Am. Chem. Soc., 2016, 138, 6598–6609 CrossRef CAS.
- (a) N. O. Ilchenko, M. Hedberg and K. J. Szabó, Chem. Sci., 2017, 8, 1056–1061 RSC; (b) S. M. Banik, K. M. Mennie and E. N. Jacobsen, J. Am. Chem. Soc., 2017, 139, 9152–9155 CrossRef CAS.
- M. H. Gieuw, Z. Ke and Y.-Y. Yeung, Angew. Chem., Int. Ed., 2018, 57, 3782–3786 CrossRef CAS.
- L. Ge, D.-X. Wang, R. Xing, D. Ma, P. J. Walsh and C. Feng, Nat. Commun., 2019, 10, 4367 CrossRef.
- A. Roy, V. Bonetti, G. Wang, Q. Wu, H. F. T. Klare and M. Oestreich, Org. Lett., 2020, 22, 1213–1216 CrossRef CAS.
- For reviews see: (a) M. J. Ingleson, Synlett, 2012, 23, 1411–1415 CrossRef CAS; (b) A. Issaian, K. N. Tu and S. A. Blum, Acc. Chem. Res., 2017, 50, 2598–2609 CrossRef CAS; (c) Y. Li and X.-F. Wu, Angew. Chem., Int. Ed., 2020, 59, 1770–1774 CrossRef CASand references therein. Also, see: (d) A. J. Warner, J. R. Lawson, V. Fasano and M. J. Ingleson, Angew. Chem., Int. Ed., 2015, 54, 11245–11249 CrossRef CAS; (e) A. J. Warner, A. Churn, J. S. McGough and M. J. Ingleson, Angew. Chem., Int. Ed., 2017, 56, 354–358 CrossRef CAS; (f) Y. Wei, D. Liu, X. Qing and L. Xu, Asian J. Org. Chem., 2017, 6, 1575–1578 CrossRef CAS; (g) C.-H. Yang, Y.-S. Zhang, W.-W. Fan, G.-Q. Liu and Y.-M. Li, Angew. Chem., Int. Ed., 2015, 54, 12636–12639 CrossRef CAS.
- D. Wang, X.-S. Xue, K. N. Houk and Z. Shi, Angew. Chem., Int. Ed., 2018, 57, 16861–16865 CrossRef CAS.
- E. Richmond, J. Yi, V. D. Vuković, F. Sajadi, C. N. Rowley and J. Moran, Chem. Sci., 2018, 9, 6411–6416 RSC.
- Z.-Y. Zhang, Z.-Y. Liu, R.-T. Guo, Y.-Q. Zhao, X. Li and X.-C. Wang, Angew. Chem., Int. Ed., 2017, 56, 4028–4032 CrossRef CAS.
- H. C. Brown, A. M. Salunkhe and B. Singaram, J. Org. Chem., 1991, 56, 1170–1175 CrossRef CAS.
- D. I. B. Kerr, J. Ong, N. M. Puspawati and R. H. Prager, Eur. J. Pharmacol., 2002, 451, 69–77 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08151e |
| This journal is © The Royal Society of Chemistry 2020 |