Open Access Article
Open Access ArticleRuddlesden–Popper phases of lithium-hydroxide-halide antiperovskites: two dimensional Li-ion conductors†
Anucha Koedtruada,
Midori Amano Patino a,
Yu-Chun Chuang
a,
Yu-Chun Chuang b,
Wei-tin Chen
b,
Wei-tin Chen c,
Daisuke Kan
c,
Daisuke Kan a and
Yuichi Shimakawa
a and
Yuichi Shimakawa *a
*a
aInstitute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan. E-mail: shimak@scl.kyoto-u.ac.jp; Fax: +81-774-38-3118; Tel: +81-774-38-3110
bNational Synchrotron Radiation Research Center, 101 Hsin-Ann Road, Hsinchu Science Park, Hsinchu 30076, Taiwan
cCenter for Condensed Matter Sciences, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 10617, Taiwan
First published on 17th November 2020
Abstract
Lithium-oxide-halide and lithium-hydroxide-halide antiperovskites were explored for potential electrolytes in all-solid Li-ion batteries. A single-phase sample of the Ruddlesden–Popper (RP) series of compounds, LiBr(Li2OHBr)2 with double antiperovskite Li2OHBr layers and rigid rock-salt type LiBr layers, was obtained. Li+-ion vacancies are introduced in the double antiperovskite Li2OHBr layers but not in the LiBr layers and induce two-dimensional Li-ion conduction with low activation energy by mediating Li-ion hopping. In contrast to the Br-containing RP phase, Cl-containing Li-oxide-halide and Li-hydroxide-halide RP phases cannot be crystallized due to the structural mismatch between the antiperovskite layers and rigid LiCl layers.
Introduction
All-solid Li-ion batteries are attracting interest due to their high energy densities and high voltage capabilities. The safe and thermally stable properties of solid electrolytes are also great advantages when compared with the properties of liquid electrolytes used in conventional Li-ion batteries.1–4 Significant efforts have thus been made to develop solid-state Li-ion conducting electrolytes. The intensively studied electrolyte materials include oxides, sulfides, and polymers. However, there are still many obstacles for their practical implementations.5–8Li-oxide halides and Li-hydroxide halides (Li3−pOHpX, p = 0–1, X = Cl and Br) with antiperovskite structures attract much attention recently as promising solid electrolytes. Although the Li-ion conductivity of the simple Li-oxide-halides antiperovskites was found to be low (10−9–10−7 S cm−1 at room temperature), chemical manipulation by substitution with hydroxides increased the conductivity significantly.9–12 For example, solid solution of Li3OCl1−xBrx and partial substitution of the constituent halide X ions with BH4− and F− were also reported to increase the Li-ion conductivity.13–16 Additional improvement in electrochemical window up to 9 V versus Li/Li+ was found in F− doped Li2OHX (X = Cl and Br).17
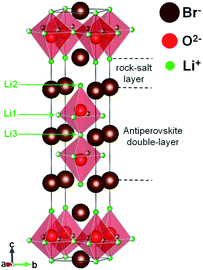
We here focus on structural manipulation of the Li-oxide-halide and Li-hydroxide-halide antiperovskites by making layered-structure compounds. Ruddlesden–Popper (RP) series are known layered-structure phases based on the perovskite-structure materials, and many RP phases in oxides have been found.18,19 We thus aim to synthesize the RP phases of the antiperovskites containing Li-oxide halides and Li-hydroxide halides. An RP phase of the antiperovskite, LiX(Li3OX), was recently computationally proposed to be a good Li-ion conductor.20 The calculation suggested that an optimised LiCl(Li3OCl) structure included enlarged bottleneck space to facilitate the Li-ion hopping with a low barrier energy. And the molecular dynamics simulation indeed gave a high Li-ion diffusion coefficient. However, this phase has never been obtained experimentally. Although attempts were made to prepare LiBr(Li3OBr)2 and a minor phase of LiBr(Li3OBr)2 was found to be included in the Li3OBr composition sample, it was difficult to isolate the pure RP phase.21,22 In this study, we prepared a single-phase Li-hydroxide bromide with the RP structure and found that it shows high Li-ion conduction. Details of its crystal structure and Li-ion conductivity are discussed.
Experimental
The RP phase structures of the antiperovskites are considered as intergrowth layered structures consisting of LiX-rock-salt layers and the Li-oxide-halide-antiperovskite layers. The general formula is given as LiX(Li3OX)n, where n represents the number of antiperovskite layers stacked between LiX layers. Because Li-hydroxide halides (Li3−pOHpX) exhibit an enhanced Li-ion conductivity, RP phases with the Li-hydroxide-halide layers are also expected to be Li-ion conductors. We thus synthesized samples with compositions of LiX(Li3−pOHpX)n with X = Br and Cl, n = 1, 2, and 3, and p = 0, 0.5, and 1 by solid-state reaction. The compositions cover the area shown in a ternary-phase diagram in Fig. 1.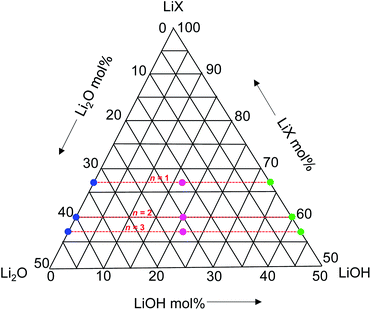 | ||
| Fig. 1 Synthesized LiX(Li3−pOHpX)n compositions with X = Br and Cl, n = 1, 2, and 3, and p = 0, 0.5, and 1, presented in a ternary phase diagram. | ||
The stoichiometric amounts of LiOH (98%, Alfa Aesar), Li2O (>98%, Fujifilm Wako), and LiCl (>99%, Fujifilm Wako) or LiBr (>99.9%, Kojundo Chemical) were weighed and mixed well in a glovebox under an Ar atmosphere. Powder samples of the mixtures were placed in a glass tube and heated at 210–225 °C for 15 h. After cooling to room temperature, the samples were ground and reheated in the same condition for two times.
The phases of the synthesized samples were identified by a conventional X-ray diffraction (XRD) method with Cu-Kα radiation using the Bruker D8 ADVANCE diffractometer. Crystal structures were analyzed by using synchrotron X-ray diffraction (SXRD) data. The SXRD measurements were performed at the TPS09A beamline in Taiwan Photon Source with a wavelength of 0.82656 Å. The powder samples were packed into sealed glass capillary tubes and were rotated during the measurement to obtain better averaging of the observed intensities. Diffraction data were collected at temperatures from −153 to 207 °C. The obtained data were analyzed with the Rietveld method using the program RIETAN-VENUS.23 The conductivity of the samples was measured by AC impedance spectroscopy using the Solatron1260 impedance analyzer and by the DC polarization method using the Keithley 2450 source-measure unit. The samples were pelletized into discs 10 mm in diameter and 3 mm thick, and for the conductivity measurements they were sandwiched by graphite electrodes. The AC impedance spectra at temperatures from room temperature to 80 °C were collected from 10 MHz to 1 kHz with an applied voltage of 0.1 V under N2 gas flow. The DC polarization at room temperature was measured by applying a voltage of 0.1 V to complete polarization. The current as a function of time was collected.
Results and discussion
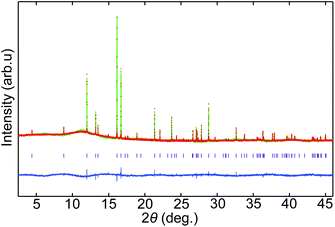
In the XRD patterns of prepared samples with X = Br (Fig. S1†), a characteristic diffraction peak originating from long-range layered order with d ≈ 5.39 Å (2θ ≈ 16.5°) can be observed. This peak can be indexed as (0,0,4) and the d value corresponds to one fourth of the c axis length of the n = 2 RP phase (see the crystal structure in Fig. 2). Some of the diffraction peaks such as (1 0 5) at 2θ ≈ 30.5° and (1 1 0) at 2θ ≈ 31.5° in the patterns can also be indexed with the n = 2 RP phase. Although the diffraction peaks of the simple antiperovskite Li3−pOHpBr were also included in the patterns except that of the LiBr(Li2.5OH0.5Br) (n = 1 and p = 0.5) composition sample, characteristic diffraction peaks of the n = 1 and 3 RP layered structures were not observed. The results suggest that the n = 2 RP phase with X = Br can be crystalized but the other n = 1 and 3 RP phases are difficult to obtain under the present synthesis conditions. On the other hand, characteristic diffraction peaks of the RP layered structures were not seen in the patterns of the samples with X = Cl (Fig. S2†), indicating that none of the RP layered phases for the Cl-containing samples can be crystalized under the present synthesis conditions. The results are consistent with previous reports where only the n = 2 RP phase with Br was reported in multiphase samples and no Cl-containing RP phases have been reported.21,22Importantly, from the diffraction pattern, the LiBr(Li2.5OH0.5Br) composition appears to produce a single phase with no evident additional diffraction peaks from secondary phases. Then the crystal structure of the sample was analyzed with the SXRD data (Fig. 3). The observed diffraction pattern is essentially reproduced with the tetragonal (I4/mmm) structure model of the n = 2 RP phase (Fig. 2). Because H is insensitive to XRD, the initial model was thus set to be LiBr(Li3OBr)2, where double layers of the Li3OBr antiperovskite are intercalated with rock-salt type LiBr layers. Given the O(4e) sites at the center of the Li octahedra are fully occupied, the refined occupancies for the Br (2a and 4e) sites are also found to be fully occupied, and the occupancies for the sites were fixed to be 1.0. The refined occupancies for Li at the 8g, 4e, and 2a sites are respectively 0.59(4), 1.0, and 0.68(7). The final refinement results are listed in Table 1. The refinement results give a composition of Li5O2Br3. Note that the Li occupancies for the Li1(8g) and Li3(2a) are significantly less than unity. Even though Li is also challenging to detect by XRD, these results suggest that some of the octahedra are Li deficient and instead H is introduced for making the Li-hydroxide-halide antiperovskite layers. Taking into account the charge neutrality condition, the most probable composition of the obtained single-phase sample would be LiBr(Li2OHBr)2, which is slightly different from, but close to, the target stoichiometry of the prepared sample. It is also interesting to note that the Li-ion vacancies at the octahedra are introduced not randomly but selectively and that rock-salt type LiBr layers are rigid.
| Atom | Site | x | y | z | g | B (Å2) |
|---|---|---|---|---|---|---|
| a g is a site occupancy and B is an isotropic thermal parameter. The refined occupancies give a composition of LiBr(Li2OBr)2. Crystal structure; tetragonal, I4/mmm space group, a = 4.02363(3) Å, c = 21.5736(2) Å, V = 349.269(5) Å3, Rwp = 1.826%, and Rp = 1.431%. | ||||||
| Li1 | 8g | 0 | 0.5 | 0.079(3) | 0.59(4) | 5.9(7) |
| Li2 | 4e | 0 | 0 | 0.199(2) | 1.0 | 5.9(7) |
| Li3 | 2a | 0 | 0 | 0 | 0.68(7) | 5.9(7) |
| O | 4e | 0 | 0 | 0.0994(7) | 1.0 | 2.6(3) |
| Br1 | 2b | 0 | 0 | 0.5 | 1.0 | 1.06(5) |
| Br2 | 4e | 0 | 0 | 0.3123(1) | 1.0 | 1.06(5) |
As shown in the temperature-dependent SXRD (Fig. S3†), the n = 2 RP structure is stable at temperatures between −153 and 207 °C. No structural transition is observed in the measured temperature range.
The rigid rock-salt type LiBr layers play an important role in stabilizing the RP phase and also explain why the Cl-containing RP phases were difficult to obtain. The large Br− ions can easily be stabilized by the modified 9-fold coordination with the Li+ ions in the rock-salt type layers. Additionally, the rock-salt type LiBr layer fits well to the Li-hydroxide (oxide) halide antiperovskite octahedra, as suggested by its tolerance factor t = (rCl/Br + rLi)/√2(rO + rLi) = 0.90, where r is an ionic radius for each constituent ion. In the Cl-containing compounds, on the other hand, Cl− ions are too small to be located at the highly coordinated sites. The structural misfit between the rock-salt type layer and the antiperovskite layer (t = 0.85) makes the RP phases with Cl hard to synthesize.
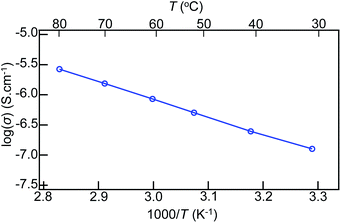
Like the Li-hydroxide-halide antiperovskite, the present n = 2 RP phase LiBr(Li2OHBr)2 can be a Li-ion conductor. Although the conduction due to hopping of H+ or OH− can also be considered, the Li+ conduction would be the most likely, as suggested in a previous report.12 The conducting properties of the sample with the Nyquist plots of the impedance spectra are displayed in Fig. 4. The impedance, Z = Z′ − iZ′′, shows typical semi-circle behaviors at various temperatures, and the total conductivity (σ) is calculated from intersections at the real-part axes. The obtained conductivity at 30 °C is 1.27 × 10−7 S cm−1, which is comparable to that of the simple antiperovskite Li2OHBr (1.33 × 10−7 S cm−1).24 A DC polarization measurement at the same temperature (Fig. S4†) reveals that the electronic conductivity is of the order of 10−11 S cm−1, which is about four orders of magnitude lower than the total conductivity. It is thus concluded that the electronic contribution to the conducting properties is very small and that the ionic conduction is dominant in the present n = 2 RP phase LiBr(Li2OHBr)2. The Arrhenius plot of temperature-dependent ionic conductivity shown in Fig. 5 gives the activation energy Ea = 0.57 eV by using the equation σ = σ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/kT), where σ0 is the conductivity pre-exponential factor, k is the Boltzmann constant, and T is the absolute temperature. This activation energy is similar to that of Li2OHBr (0.55 eV).24
exp(−Ea/kT), where σ0 is the conductivity pre-exponential factor, k is the Boltzmann constant, and T is the absolute temperature. This activation energy is similar to that of Li2OHBr (0.55 eV).24
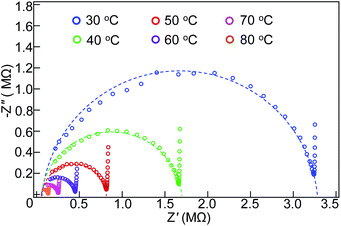 | ||
| Fig. 4 Nyquist plots from impedance spectroscopy measurements of the n = 2 RP phase LiBr(Li2OHBr)2 antiperovskite at various temperatures. | ||
We note again that the Li vacancies are introduced in the Li1 and Li3 sites and that the rock-salt type LiBr layer is rigid. This implies that Li-ion hopping conduction through the vacant sites are confined within the two-dimensional double antiperovskite layers. Interlayer hopping between the rigid LiBr layers should need high activation energies and thus are less likely to occur, leading to almost no Li-ion conduction along the stacking layer. The observed low activation energy for the present n = 2 RP antiperovskite phase, which is comparable to that of the simple antiperovskite Li2OHBr, is also consistent with the conduction only of the antiperovskite layers. Because the present Li-ion conductivity was measured in a polycrystalline sample, the actual (intrinsically two-dimensional) ionic conductivity within the antiperovskite layers in the RP phase is expected to be much larger than that measured here.
Conclusions
We synthesized the Li-oxide-halide and Li-hydroxide-halide antiperovskites LiX(Li3OX)n and LiX(Li3−pOHpX)n (X = Br and Cl) by solid-state reactions. We identified the phases and analyzed their crystal structures by SXRD. We found that the n = 2 layered RP phase of Li-hydroxide-bromide antiperovskite LiBr(Li2OHBr)2 crystallizes in the I4/mmm tetragonal structure, which consists of double layers of the Li2OHBr antiperovskite between rock-salt-type LiBr layers. Li+-ion vacancies were introduced selectively in antiperovskite layers, and the rock-salt type LiBr layers were rigid. The RP structure is stabilized by the large Br− ions coordinated with 9 Li+ ions and by the lattice match between the rock-salt LiBr type layer and the Li-hydroxide-bromide antiperovskite layer. The RP phases with Br, n = 1, 3, and Cl, n = 1, 2, 3, were not stable.The Li+ vacancies revealed in the n = 2 RP phase LiBr(Li2OHBr)2 promote the Li-ion conduction and the compound shows ionic conductivity of 1.27 × 10−7 S cm−1 at 30 °C with an activation energy of 0.57 eV. Because the rigid rock-salt type LiBr layers contribute less in the Li-ion hopping, Li+ ions primarily migrate within the double antiperovskite layers, leading to the two-dimensional ion conduction. The intrinsic two-dimensional Li+-ion conductivity may be much higher than that observed. The present results demonstrate that the novel RP phases of antiperovskite halides have great potential for developing highly conductive Li-ion conductors with thermally stable properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Masato Goto, Zhenhong Tan and Yoshihisa Kosugi for help in experiments. The synchrotron radiation experiments were performed at the National Synchrotron Radiation Research Center (proposal No. 198). This work was partly supported by Grants-in-Aid for Scientific Research (No. 16H02266, 17K19177, 19H05816, 19K23650, and 20H00397) and by grants for the Integrated Research Consortium on Chemical Sciences and the International Collaborative Research Program of Institute for Chemical Research in Kyoto University from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. This work was also supported by the Japan Society for the Promotion of Science Core-to-Core Program (A) Advanced Research Networks.References
- Q. Li, J. Chen, L. Fan, X. Kong and Y. Lu, Green Energy Environ, 2016, 1, 18–42 CrossRef.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2(4), 16103 CrossRef CAS.
- T. Framprikis, P. Canepa, J. A. Dawson, M. S. Islam and C. Masquelier, Nat. Mater., 2019, 18, 1278–1291 CrossRef.
- Q. Yang and C. Li, Energy Storage Materials, 2018, 14, 100–117 CrossRef.
- P.-J. Lian, B.-S. Zhao, L.-Q. Zhang, N. Xu, M.-T. Wu and X.-P. Gao, J. Mater. Chem. A, 2019, 7, 20540–20557 RSC.
- Z. Gao, H. Sun, L. Fu, F. Ye, Y. Zhang, W. Luo and Y. Huang, Adv. Mater., 2018, 30, 1705702 CrossRef.
- S. Ohno, A. Banik, G. F. Dewald, M. A. Kraft, T. Krauskopf, N. Minafra, P. Till, M. Weiss and W. G. Zeier, Prog. Energy, 2020, 2, 022001 CrossRef.
- X. Li, J. Liang, X. Yang, K. R. Adair, C. Wang, F. Zhao and X. Sun, Energy Environ. Sci., 2020, 13, 1429–1461 RSC.
- X. Lü, G. Wu, J. W. Howard, A. Chen, Y. Zhao, L. L. Daemen and Q. Jia, Chem. Commun., 2014, 50, 11520–11522 RSC.
- I. Hanghofer, G. J. Redhammer, S. Rohde, I. Hanzu, A. Senyshyn, H. M. R. Wilkening and D. Rettenwander, Chem. Mater, 2018, 30, 8134–8144 CrossRef CAS.
- A.-Y. Song, Y. Xiao, K. Turcheniuk, P. Upadhya, A. Ramanujapuram, J. Benson, A. Magasinki, M. Olguin, L. Meda, O. Borodin and G. Yushin, Adv. Energy Mater., 2018, 8, 1700971 CrossRef.
- J. A. Dawson, T. S. Attari, H. Chen, S. P. Emge, K. E. Johnston and M. S. Islam, Energy Environ. Sci., 2018, 11, 2993–3002 RSC.
- Y. Zhao and L. L. Daemen, J. Am. Chem. Soc., 2012, 134, 15042–15047 CrossRef CAS.
- Z. Deng, B. Radhakrishnan and S. P. Ong, Chem. Mater., 2015, 27, 3749–3755 CrossRef CAS.
- H. Fang, S. Wang, J. Liu, Q. Sun and P. Jena, J. Mater. Chem. A, 2017, 5, 13373–13381 RSC.
- H. Fang and P. Jena, Proc. Natl. Acad. Sci. U.S.A., 2017, 114, 11046–11051 CrossRef CAS.
- Y. Li, W. Zhou, S. Xin, S. Li, J. Zhu, X. Lu, Z. Cui, Q. Jia, J. Zhou, Y. Zhao and J. B. Goodenough, Angew. Chem., Int. Ed., 2016, 55, 9965–9968 CrossRef CAS.
- B. V. Beznosikov and K. S. Aleksandrov, Crystallography Report, 2000, 45, 792–798 CrossRef.
- G. Nirala, D. Yadav and S. Upadhyay, J. Adv. Ceram., 2020, 9, 129–148 CrossRef CAS.
- Z. Lu, J. Liu and F. Ciucci, Energy Storage Materials, 2020, 28, 146–152 CrossRef.
- J. Zhu, S. Li, Y. Zhang, J. W. Howard, W. Lu, Y. Li, Y. Wang, R. S. Kumar, L. Wang and Y. Zhao, Appl. Phys. Lett., 2016, 109, 101904 CrossRef.
- K. Friese, A. Honnerscheid and M. Jansen, Z. Kristallogr., 2003, 218, 536–541 CAS.
- F. Izumi and K. Momma, Solid State Phenom, 2007, 130, 15–20 CAS.
- A. Koedtruad, M. A. Patino, N. Ichikawa, D. Kan and Y. Shimakawa, J. Solid State Chem., 2020, 286, 121263 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07803d |
| This journal is © The Royal Society of Chemistry 2020 |