Open Access Article
Open Access ArticleMechanochemical synthesis of antifungal bis(benzoxaboroles)†
Krzysztof M. Borys a,
Dorota Wieczorek
a,
Dorota Wieczorek b,
Magdalena Tarkowska
b,
Magdalena Tarkowska a,
Agnieszka Jankowskaa,
Jacek Lipokb and
Agnieszka Adamczyk-Woźniak
a,
Agnieszka Jankowskaa,
Jacek Lipokb and
Agnieszka Adamczyk-Woźniak *a
*a
aFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: agnieszka@ch.pw.edu.pl
bFaculty of Chemistry, University of Opole, Oleska 48, 45-052 Opole, Poland
First published on 7th October 2020
Abstract
Several piperazine bis(benzoxaboroles) have been obtained both in solution as well as in the solid state. The environmentally friendly mechanochemical approach – hitherto not applied for the preparation of benzoxaboroles – was particularly beneficial in the case of two products afforded in low yields in solution. The in vitro studies showed high potential of the studied bis(fluorobenzoxaboroles) as antifungal agents, highlighting also the influence of the fluorine substituent position on their microbiological activity. The highest activity against A. niger, A. terreus, P. ochrochloron, C. tenuis and C. albicans was displayed by the analogue of the known benzoxaborole antifungal drug Kerydin® (Tavaborole).
Introduction
Over recent years, mechanochemistry has drawn increasing attention not only due to its environment-friendly features like limiting, or eliminating, the use of organic solvents.1 The use of mechanochemical methods often results in reduced reaction times, different reaction outcomes in terms of product selectivity, or even formation of products that could not be otherwise obtained by means of a “wet” synthesis.1–3 To date, there have been only a few examples of boronic ester formation using a mechanochemical approach. The first study describing quantitative formation of phenylboronic acid esters with diols and polyols upon ball milling was published in 2003.4 Further study by Stanetty et al. extended the scope of mechanochemical formation of pinacol and neopentyl glycol esters to aryl-, heteroaryl- and alkylboronic acids.5 Despite promising results, the developed protocols have been scarcely featured in the literature.6Benzoxaboroles emerged over the last 10 years as a novel class of biologically active compounds, with Kerydin® (Tavaborole) and Eucrisa® (Crisaborole) already in clinical practice as well as several other in clinical trials. Tavaborole is the first representative of a novel class of antifungal drugs, the action of which is based on the unique and recently discovered oxaborole-tRNA-trapping mechanism (the OBORT mechanism).7 It was shown that the presence of a fluorine substituent as well as its position influences the overall bioactivity, which was showcased comparing the antifungal action of four fluorobenzoxaboroles, varying in the position of fluorine on the benzene ring.8 The effect of fluorine on the action of drugs is complex and covers, among others, such aspects as its impact on acidity, lipophilicity as well as the influence on the mechanism of action.9 It is worth mentioning that in addition to Kerydin®, about 25% of the currently available drugs contain fluorine.10
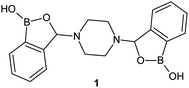
The superiority of the benzoxaborole scaffold over phenylboronic acid in terms of biological activity was confirmed by Adamczyk-Woźniak et al. 3-Aminobenzoxaboroles were found to be active against several fungal strains whereas their phenylboronic acid analogues were completely inactive.11,12 The presence of benzoxaborole system was therefore demonstrated to be pivotal for the antifungal activity of the examined compounds. The overwhelming majority of the reported benzoxaboroles contain only one benzoxaborole unit. Compounds with more than one oxaborole in their structure are considerably underdeveloped.13–16 This seems surprising as cooperative diol-binding effect of oligoboronic compounds has already been extensively exploited e.g. for the development of glucose-selective boronic-based molecular receptors.17 The first piperazine bis(benzoxaborole) (1, Fig. 1) was synthesized in 2012 and shown to display antifungal properties.18 Later on, it was also studied in terms of its adsorption on hydroxyapatite,19 as well as an organoboron receptor used for the preparation of poly(vinyl chloride) membranes for ion-selective electrodes.20 Further work on dopamine-responsive ion-selective electrodes with bis(benzoxaborole) 1 has been published recently.21
To the best of our knowledge, no mechanochemical synthesis of any benzoxaboroles has been reported to date. The aim of the study was to develop an environmentally friendly synthetic protocol for piperazine bis(benzoxaboroles), including all isomers of bis(fluorobenzoxaboroles), as well as to assess their antifungal activity.
Results and discussion
The “in solution” method for preparation of the first piperazine bis(benzoxaborole) 1 as well as its fluorine-containing analogue 3 was reported by Adamczyk-Woźniak et al.22 Compound 1 was synthesized in the reaction of 2-formylphenylboronic acid with piperazine, carried out in a hazardous solvent – diethyl ether (Et2O).23 The solution of piperazine in Et2O was added dropwise to the solution of the starting aldehyde dissolved in Et2O, resulting in precipitation of the crude product. After 24 hours at room temperature, the crude solid was purified by filtration to afford piperazine bis(benzoxaborole) 1 in 94% yield. In case of piperazine bis(5-fluorobenzoxaborole) 3, the same synthetic approach was taken but a different solvent system used. Compared to its non-fluorinated counterpart, 4-fluoro-2-formylphenylboronic acid has significantly lower solubility in Et2O. To avoid using copious amounts of Et2O for preparation of its solution, a mixture of Et2O and THF (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was applied. The desired product also precipitated out of solution, and after 24 hours at room temperature the filtration gave piperazine bis(5-fluorobenzoxaborole) 3 in 86% yield.22 Similar approach was reported for, 6 resulting in 73% yield.24
1, v/v) was applied. The desired product also precipitated out of solution, and after 24 hours at room temperature the filtration gave piperazine bis(5-fluorobenzoxaborole) 3 in 86% yield.22 Similar approach was reported for, 6 resulting in 73% yield.24
It is worth mentioning that tetrahydrofuran is also a problematic solvent,23 and so is the application of a mixture of ethereal solvents. Following the literature procedure, position isomers of 3 (compounds 2, 4 and 5) were planned to be obtained to complete the series. Fluorine-containing 2-formylphenylboronic acids were accordingly reacted with piperazine in an Et2O/THF mixture for 24 hours at room temperature (Fig. 2).
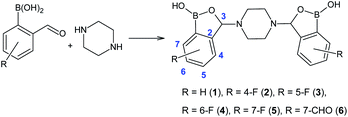 | ||
| Fig. 2 Synthetic method for the preparation of bis(benzoxaboroles) 1–6, with atoms numbering scheme. | ||
First, 5-fluoro-2-formylphenylboronic acid was reacted with piperazine, affording bis(6-fluorobenzoxaborole) 4 in 59% yield. The yield was lower than the one reported for the preparation of bis(5-fluorobenzoxaborole) 3 (86%).22 The remaining ortho-formyl substrates were more challenging due to an increased steric hindrance in the neighbourhood of either boronic or formyl group. Bis(7-fluorobenzoxaborole) 5 was isolated in 45% yield, nearly two-fold lower than in case of bis(6-fluorobenzoxaborole) 4. The last isomer, bis(4-fluorobenzoxaborole) 2, was obtained in only 6% yield. Although the “in solution” protocol delivered all the desired bis(benzoxaboroles), the yields for 2, 4 and 5 were unsatisfactory compared to the >80% yields for bis(benzoxaboroles) 1 and 3. Moreover, the use of Et2O and THF could be considered a significant drawback, especially when scaling the reaction up. In addition to the environmental impact, the tendency of THF to get oxidized upon storage leads to the formation of its peroxides. Peroxides, in turn, are known to react readily with phenylboronic species, oxidizing them to the corresponding phenols.25 The formation of any side products could lead to their co-precipitation with the desired bis(benzoxaborole), which might pose a major problem given that the crude product is purified solely by filtration.
In search for alternative methods for bis(benzoxaboroles) preparation, mechanochemical approach appeared as an attractive option. Taking the aforementioned challenges into account, mechanochemical synthesis of piperazine bis(benzoxaboroles) was attempted. The reactions were carried out in a vibrational ball mill. The unsubstituted 2-formylphenylboronic acid was used as a model substrate in the formation of 1. The optimization steps carried out for a reaction on a 1 mmol reaction scale are presented in Table 1.
| Entry | Reaction time [h] | Molar ratio of reagents | Isolated yield [%] |
|---|---|---|---|
| 1 | 0.5 | 2 | 0 |
| 2 | 1 | 2 | 0 |
| 3 | 2 | 2 | 21 |
| 4 | 3 | 2 | 51 |
| 5 | 4 | 2 | 79 |
| 6 | 4 | 4 | 73 |
| 7 | 6 | 2 | 74 |
In order to check the crude material for the formation of bis(benzoxaborole), 1H NMR analyses using a 60 MHz benchtop spectrometer were carried out. The criterion confirming the product formation by 1H NMR in CD3OD was the appearance of a signal at δ 5.90 ppm, attributed to the benzylic proton of an oxaborole system in piperazine bis(benzoxaborole) 1.22 Supplementary analytical method based on Thin Layer Chromatography was developed. It is worth noting that no TLC analyses have been hitherto reported for monitoring the formation of bis(benzoxaboroles), so an appropriate solvent system and visualization conditions had to be found (Table 2). The solvent system of AcOEt/MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, allowed to reliably distinguish the spot of piperazine bis(benzoxaborole) 1 from the spots of 2-formylphenylboronic acid and piperazine. The UV radiation (254 nm) allowed to discriminate between piperazine and the aromatic starting material. Staining the developed TLC plate with a basic aqueous solution of potassium permanganate did visualize all the spots but did not allow to distinguish between them. An acidic ethanolic solution of curcumin, reported for its highly selective TLC staining of boron-containing species,26 allowed to distinguish the boronic compounds from piperazine. Also, it indicated the presence of boron in the product (Table 2).
1 v/v, allowed to reliably distinguish the spot of piperazine bis(benzoxaborole) 1 from the spots of 2-formylphenylboronic acid and piperazine. The UV radiation (254 nm) allowed to discriminate between piperazine and the aromatic starting material. Staining the developed TLC plate with a basic aqueous solution of potassium permanganate did visualize all the spots but did not allow to distinguish between them. An acidic ethanolic solution of curcumin, reported for its highly selective TLC staining of boron-containing species,26 allowed to distinguish the boronic compounds from piperazine. Also, it indicated the presence of boron in the product (Table 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v; 80 mm SiO2 plate) of the reaction mixture components in the synthesis of bis(benzoxaborole) 1
1 v/v; 80 mm SiO2 plate) of the reaction mixture components in the synthesis of bis(benzoxaborole) 1
| Compound | Rf | Visualization method | ||
|---|---|---|---|---|
| UV | KMnO4 | Curcumin | ||
| 2-Formylphenylboronic acid | 0.28 | + | + | + (yellow stain) |
| Piperazine | 0.00 | − | + | + (white stain) |
| 1 | 0.48 | + | + | + (yellow stain) |
In all optimization steps in which the product was formed (Table 1, entries 3–7), residual aldehyde was found to contaminate the crude product. Washing with Et2O was attempted as the method to purify bis(benzoxaborole), yet had to be adapted to the mechanochemical reaction setup and implemented in such a way that its environmental impact was reduced. The first approach to the work-up was scraping the crude solid off from the mortar and the ball, transferring it onto a fritted filter funnel and washing with Et2O. However, the crude material obtained in the result of grinding was often so fine that it easily got through the frit to the filtrate, even when frits with low pore size (G-4) were used. Filtration through a Büchner funnel with a paper filter was then tried, successfully stopping most of the solid from getting to the filtrate. However, when the washed solid was checked for its purity, some aldehyde was still present in the product. Removing the starting material was only possible using significant amounts of Et2O, which would be in strong opposition to the idea of limiting solvent use in mechanochemistry. Hence, another work-up procedure had to be developed.
Same as in the previous work-ups, as much of the crude material as possible was scraped off from the mortar and the ball and transferred to a 15 mL Falcon centrifuge tube. A small portion of Et2O (up to 10 mL) was then added to the mortar and the ball, washing most of the remaining solid off them. The suspension was then sonicated for 5 minutes at room temperature, allowing to dissolve the remaining starting material and purify the ether-insoluble product. The suspensions were then centrifuged for 5 minutes. The supernatants were decanted, followed by another addition of Et2O (5 mL, half of the first portion), sonication, centrifugation and decantation. While still in the Falcon tube, the resulting solid was dried under vacuum, and then transferred onto a Petri dish to be air-dried overnight. The developed protocol delivered pure bis(benzoxaborole) 1 in all cases from entry 3 to 7 (Table 1). Concerning the optimization of the mechanochemical reaction, extending the reaction time above 2 hours was found beneficial to the yield of the product (Table 1). Accordingly, the yield could be increased from rather low (21%) after 2 h of ball milling to moderate (51%) after 3 h and high (79%) after 4 h. However, further extension of the reaction time up to 6 hours did not improve the yield further. Also, increasing the molar ratio of aldehyde to piperazine (from 2.00 to 4.00, entry 6) was attempted in order to increase the conversion of the limiting substrate, i.e., piperazine. However, such a change was not beneficial to the yield, increasing the number of Et2O washings needed to remove the remaining aldehyde from the crude material. The optimized conditions were then settled as in entry 5 (Table 1): reaction time of 4 hours, with the stoichiometric ratio of the starting aldehyde to piperazine, i.e., 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
With the optimized conditions at hand, the preparation of bis(fluorobenzoxaboroles) 2–5 was carried out (Table 3). The same batches of starting materials were used for the mechanochemical approach as in the solution studies. In all cases, washing with small portions of Et2O was employed to isolate the pure products. The work-up protocol was the same as the one developed for the mechanochemical synthesis of piperazine bis(benzoxaborole) 1.
| Entry | Substituent | Yield in solid state [%] | Yield in solution [%] |
|---|---|---|---|
| 1 | 4-F (2) | 74 | 6 |
| 2 | 5-F (3) | 59 | 86 (ref. 22) |
| 3 | 6-F (4) | 57 | 59 |
| 4 | 7-F (5) | 71 | 45 |
The mechanochemical approach provided all four piperazine bis(fluorobenzoxaboroles) 2–5 in moderate to good yields. It is worth noting that compounds 2 and 5 were obtained in >70% yields. Compound 4 was prepared in a comparable yield to the solution method (57% mechanochemical yield vs. 59% yield in solution), while compound 3 was prepared in ca. 30% lower yield than the literature synthesis in solution.22
The hitherto unreported bis(fluorobenzoxaboroles) 2, 4 and 5 were characterized to confirm their structure and purity. Compounds 2 and 4 were analyzed by means of 1H, 13C and 19F NMR in DMSO-d6. In both cases, the 1H NMR showed signals that were attributed to the benzylic protons of the oxaborole rings (δ 6.00–5.80 ppm) and no signal of the formyl proton of the starting aldehyde (δ 10.30 ppm). 19F NMR spectra demonstrated the presence of only one fluorine-containing species in each sample, confirming that the products were free of the starting fluorinated aldehydes. In addition to the NMR studies, FTIR spectra of compounds 2 and 4 were also obtained and the characteristic bands matched the ones reported for bis(5-fluorobenzoxaborole).22 Bis(7-fluorobenzoxaborole) (5) turned out to be problematic in terms of characterization. The compound was insoluble in DMSO, methanol, acetonitrile, acetone, chloroform and water at the concentration as low as 5 mg mL−1, even at an elevated temperature (>50 °C) or upon prolonged (4 h) sonication. Therefore, its spectroscopic characterization was limited to FTIR. The FTIR spectrum showed a set of bands that occurred for bis(benzoxaboroles) 2 and 4, additionally in spectrum of 6 the characteristic signal of a formyl group at 1685 cm−1 was present.
Regardless of the method for samples preparation – solution-based or mechanochemical – all products gave satisfactory results of elemental analyses, unequivocally confirming their high purity.
Microbiological evaluation of piperazine bis(benzoxaboroles)
The first report of high antifungal activity of 1 dates back to 2012.18 In 2014, Adamczyk-Woźniak et al. compared the antifungal activity of piperazine bis(benzoxaborole) 1 with its bis(phenylboronic acid) analogue.11 The evaluation was carried out by the agar diffusion method against five filamentous fungi: Aspergillus terreus, Fusarium dimerum, Fusarium solani, Penicillium ochrochloron and Aspergillus niger. Compound 1 had the inhibitory activity even higher than the model WHO-listed antifungal drug amphotericin B, while the corresponding bis(phenylboronic acid) was found to be completely inactive.11Microbiological tests of the obtained bis(fluorobenzoxaboroles) 2–4 were run against a series of fungi: Aspergillus niger, Aspergillus terreus, Fusarium dimerum, Fusarium oxysporum, Fusarium solani, Penicillium ochrochloron, Candida tenuis and Candida albicans. Due to the insolubility of bis(7-fluorobenzoxaborole) 5 in DMSO, which was used in the study, compound 5 was excluded from the evaluation. The series was extended with bis(7-formylbenzoxaborole) 6. The study was invariably based on the agar diffusion method, one of the most common method for preliminary antifungal screening of organic species. It allows for a comparative evaluation of fungicidal activity, based on the values of diameters of limited or inhibited growth of the fungus under the influence of a given amount of the substance tested, usually within the range from 1 to 100 μg. The obtained results are shown in Table 4.
| Fungus | Amount | ||||
|---|---|---|---|---|---|
| 10 μg | 25 μg | 50 μg | 100 μg | ||
| a Diameter of the zone of the totally inhibited growth of the fungus (no mycelium within the growth medium) is shown in parentheses. The values beyond parentheses relate to the diameter of the zone of both limited and totally inhibited growth of the fungus; n/d – not determined. | |||||
| 2 | A. niger | (19) | (27) | (32) | (39) |
| A. terreus | 4 | 12 | 24 | (33) | |
| F. dimerum | 5 | 12 | 19 (11) | 30 (23) | |
| F. oxysporum | 20 | 28 | nd | 42 (32) | |
| P. ochrochloron | 0 | 0 | 11 | 18 | |
| C. albicans | (13) | (20) | (23) | (29) | |
| 3 | A. niger | (26) | (32) | (36) | (41) |
| A. terreus | (30) | (35) | (42) | (46) | |
| F. dimerum | 0 | 0 | 6 | 9 | |
| F. oxysporum | 10 | 12 | 16 | 26 | |
| F. solani | 0 | 8 | 12 | 27 (12) | |
| P. ochrochloron | 22 | (29) | (36) | (41) | |
| C. tenuis | (11) | (20) | (24) | (26) | |
| C. albicans | (24) | (28) | (31) | (35) | |
| 4 | A. niger | 0 | 0 | 11 | 19 (9) |
| A. terreus | 0 | 13 | 20 | 28 (10) | |
| F. dimerum | 0 | 0 | 7 | 9 | |
| F. oxysporum | 0 | 0 | 8 | 10 | |
| F. solani | 0 | 0 | 6 | 8 | |
| P. ochrochloron | 0 | 0 | 17 | 29 (11) | |
| C. tenuis | 0 | 7 | 12 | 21 | |
| C. albicans | 0 | 5 | 13 | 20 | |
| 6 | A. niger | 0 | 0 | 0 | 0 |
| A. terreus | 0 | 0 | 0 | 0 | |
| F. oxysporum | 0 | 0 | 0 | 0 | |
| P. ochrochloron | 0 | 0 | 0 | 0 | |
| C. albicans | 0 | 0 | 0 | 0 | |
Compounds 2–4 were found to be differently active against all the investigated strains. The most potent compound proved to be piperazine bis(5-fluorobenzoxaborole) 3, which is an analogue of Tavaborole. It showed considerable antifungal activity towards all seven strains at 100 μg and 50 μg quantities, against six strains at 25 μg and five strains at the amount as low as 10 μg. The activity of compound 3 was most appreciable towards both Aspergillus strains and P. ochrochloron. Among the strains studied, it remained least active against two Fusarium strains: F. dimerum and F. solani. Importantly, bis(benzoxaborole) 3 contains two 5-fluorobenzoxaborole units characteristic for the US FDA-approved drug Tavaborole. The activity of 3 was however lower than that the one reported for Tavaborole in the literature study that used exactly the same protocol as the one herein.8,28 Bis(4-fluorobenzoxaborole) 2 also showed noteworthy activity against the tested microorganisms. Compound 2 inhibited the fungal growth less than compound 3, and only in case of Fusarium strains the activity was significantly higher. Bis(6-fluorobenzoxaborole) 4 was considerably less active than 3 towards all the investigated strains at the amounts of 100 μg and 50 μg. At 25 μg, it remained active only against A. terreus and C. tenuis, while at 10 μg it did not show any activity whatsoever. Compound 6 containing formyl group in the proximity of the benzoxaborole ring displayed no activity against A. niger, A. terreus, F. oxysporum, P. ochrochloron and C. albicans. Comparing the results obtained for 50 μg of compounds 2 and 3 with the activity of the same dose of antibiotic – amphotericin B – presented in the previous study27 and obtained following the same protocol, it may be concluded that the antifungal activity of bis(benzoxaboroles) was comparable (compound 2 against P. ochlochloron, compound 3 against F. dimerum and F. solani) or higher than that of the standard antibiotic.
Experimental
General procedure – solution method
To a stirred solution of fluoro-2-formylphenylboronic acid (0.1 M, 2.00 eq.) in Et2O/THF (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), a solution of piperazine (0.1 M, 1.00 eq.) was added dropwise at RT. The addition resulted in the formation of a white precipitate. The resulting suspension was left stirring for 24 h at RT. The precipitate was filtered off and dried in air overnight.
1 v/v), a solution of piperazine (0.1 M, 1.00 eq.) was added dropwise at RT. The addition resulted in the formation of a white precipitate. The resulting suspension was left stirring for 24 h at RT. The precipitate was filtered off and dried in air overnight.
General procedure – mechanochemical method
The starting 2-formylphenylboronic acid (2.00 eq.) and piperazine (1.00 eq.) were added as solids to the mortar of vibrational ball mill. The solids were ground with a milling ball for 4 h without external heating. As much of the white crude material as possible was scraped off the mortar and ball and transferred into a 15 mL Falcon centrifuge tube. The mortar and ball were washed with Et2O (up to 10 mL) and the resulting suspension added to the solid in the Falcon tube. The suspension was sonicated for 5 min at RT, and centrifuged for 5 min at 6000 rpm. The supernatant was decanted, followed by another addition of Et2O (5 mL), sonication for 5 min at RT, centrifugation for 5 min at 6000 rpm and decantation. The obtained solid was dried first under vacuum while still in the Falcon tube, and then transferred onto a Petri dish for drying in air overnight.3,3′-(Piperazine-1,4-diyl)bis(benzo[c][1,2]oxaborol-1(3H)-ol) (1)
The title compound was prepared mechanochemically starting from 2-formylphenylboronic acid (300 mg, 2.00 mmol, 2.00 eq.) and piperazine (86 mg, 1.00 mmol, 1.00 eq.). The product was obtained as a white powder (277 mg, 0.79 mmol, 79%).TLC (SiO2; AcOEt/MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v; UV, KMnO4 or curcumin stain) Rf 0.48. 1H NMR (500 MHz, CD3OD) δ 7.64–7.13 (m, 8H), 5.90 (s, 2H), 3.14–2.76 (br, 8H). 11B NMR (160 MHz, CD3OD) δ 14.4. Mp 210–218 °C (degradation). The data are in agreement with the ones previously reported.22
1 v/v; UV, KMnO4 or curcumin stain) Rf 0.48. 1H NMR (500 MHz, CD3OD) δ 7.64–7.13 (m, 8H), 5.90 (s, 2H), 3.14–2.76 (br, 8H). 11B NMR (160 MHz, CD3OD) δ 14.4. Mp 210–218 °C (degradation). The data are in agreement with the ones previously reported.22
3,3′-(Piperazine-1,4-diyl)bis(4-fluorobenzo[c][1,2]oxaborol-1(3H)-ol) (2)
The title compound was prepared in solution starting from 3-fluoro-2-formylphenylboronic acid (700 mg, 4.16 mmol, 2.00 eq.) and piperazine (180 mg, 2.08 mmol, 1.00 eq.). The product was obtained as a white powder (48 mg, 0.125 mmol, 6%).The title compound was prepared mechanochemically starting from 3-fluoro-2-formylphenylboronic acid (500 mg, 2.98 mmol, 2.00 eq.) and piperazine (128 mg, 1.49 mmol, 1.00 eq.). The product was obtained as a white powder (414 mg, 1.10 mmol, 74%).
1H NMR (500 MHz, DMSO-d6) δ 9.34 (s, 1H), 9.32 (s, 1H), 7.52 (m, 1H), 7.49–7.40 (m, 3H), 7.29–7.20 (m, 2H), 6.02 (s, 1H), 5.98 (s, 1H), 2.66–2.54 (br m, 4H), 2.48–2.34 (br m, 4H). 13C NMR (126 MHz, DMSO-d6) δ 158.3, 156.3, 137.3, 137.2, 136.4, 131.1, 126.2, 117.9, 117.7, 93.4, 46.4. 19F NMR (470 MHz, DMSO-d6) δ −118.96 (m). FTIR (KBr) vmax 3358 (br), 3072, 2961, 2846, 1690, 1578, 1463, 1343, 1238, 1158, 1011, 892, 792, 724, 667, 606. Mp 196–205 °C (degradation). Elemental analysis: calculated for C18H18B2F2N2O4: C, 56.01; H, 4.70; N, 7.26. Found: C, 55.79; H, 4.35; N 6.87.
3,3′-(Piperazine-1,4-diyl)bis(5-fluorobenzo[c][1,2]oxaborol-1(3H)-ol) (3)
The title compound was prepared mechanochemically starting from 4-fluoro-2-formylphenylboronic acid (336 mg, 2.00 mmol, 2.00 eq.) and piperazine (86 mg, 1.00 mmol, 1.00 eq.). The product was obtained as a white powder (229 mg, 0.59 mmol, 59%).1H NMR (500 MHz, CD3OD) δ 7.53–7.41 (m, 2H), 7.04–6.93 (m, 3H), 5.86 (s, 2H), 3.31–2.70 (m, 8H). 11B NMR (160 MHz, CD3OD) δ 14.1. Mp 211–220 °C (degradation). The data are in agreement with the ones previously reported.22
3,3′-(Piperazine-1,4-diyl)bis(6-fluorobenzo[c][1,2]oxaborol-1(3H)-ol) (4)
The title compound was prepared in solution starting from 5-fluoro-2-formylphenylboronic acid (1.30 g, 7.74 mmol, 2.00 eq.) and piperazine (0.333 g, 3.87 mmol, 1.00 eq.). The product was obtained as an off-white powder (0.888 g, 2.30 mmol, 59%).The title compound was prepared mechanochemically starting from 5-fluoro-2-formylphenylboronic acid (672 mg, 4.00 mmol, 2.00 eq.) and piperazine (172 mg, 2.00 mmol, 1.00 eq.). The product was obtained as a white powder (440 mg, 1.14 mmol, 57%).
1H NMR (500 MHz, DMSO-d6) δ 9.15–8.80 (br s, 2H), 7.41–7.13 (m, 6H), 5.81 (s, 1H), 5.76 (s, 1H), 2.76–2.40 (br s, 8H). 13C NMR (126 MHz, DMSO-d6) δ 158.3, 156.3, 137.3, 137.2, 136.4, 131.1, 126.2, 117.7, 93.4, 46.4. 11B NMR (160 MHz, CD3OD) δ 14.2. 19F NMR (470 MHz, DMSO-d6) δ −115.02 (m). FTIR (KBr) vmax 3293 (br), 2954, 2851, 1616, 1586, 1453, 1349, 1266, 1213, 1016, 961, 913, 826, 714, 634, 572. Mp 193–199 °C (degradation). Elemental analysis: calculated for C18H18B2F2N2O4: C, 56.01; H, 4.70; N, 7.26. Found: C, 56.03; H, 4.98; N, 7.32.
3,3′-(Piperazine-1,4-diyl)bis(7-fluorobenzo[c][1,2]oxaborol-1(3H)-ol) (5)
The title compound was prepared in solution starting from 6-fluoro-2-formylphenylboronic acid (302 mg, 1.80 mmol, 2.00 eq.) and piperazine (78 mg, 0.90 mmol, 1.00 eq.). The product was obtained as an off-white powder (156 mg, 0.41 mmol, 45%).The title compound was prepared mechanochemically starting from 6-fluoro-2-formylphenylboronic acid (300 mg, 1.79 mmol, 2.00 eq.) and piperazine (77 mg, 0.89 mmol, 1.00 eq.). The product was obtained as a white powder (243 mg, 0.63 mmol, 71%). FTIR (KBr) vmax 3342, 2944, 2851, 1623, 1579, 1469, 1239, 1185, 1032, 958, 912, 838, 792, 630, 607. Mp 182–186 °C (degradation). Elemental analysis: calculated for C18H18B2F2N2O4: C, 56.01; H, 4.70; N, 7.26. Found: C, 56.18; H, 4.65; N, 7.15.
3,3′-(Piperazine-1,4-diyl)bis(7-formylbenzo[c][1,2]oxaborol-1(3H)-ol) (6)
The title compound was prepared in solution starting from 2,6-diformylphenylboronic acid (2.18 g, 12.3 mmol, 2.00 eq.) and piperazine (0.527 g, 6.12 mmol, 1.00 eq.). The product was obtained as a white powder (2.07 g, 5.1 mmol, 82%).The title compound was prepared mechanochemically from 2,6-diformylphenylboronic acid (114 mg, 0.64 mmol, 2.00 eq.) and piperazine (27 mg, 0.32 mmol, 1.00 eq.). The product was obtained as a white powder (57 mg, 0.14 mmol, 44%).
1H NMR (500 MHz, DMSO-d6) δ 10.40 (s, 1H), 10.35 (s, 1H, CHO), 9.28 (s, 1H) 9.24 (s, 1H), 7.93 (m, 1H), 7.87 (m, 1H), 7.74 (t, 1H), 7.70–7.67 (m, 2H), 7.64 (m, 1H), 6.01 (s, 1H), 5.96 (s, 1H), 2.66 (br s, 4H), 2.46 (br s, 4H). 11B NMR (CD3OD, 64 MHz) δ 12.0. 13C NMR (126 MHz, DMSO-d6) δ 193.2, 153.6, 138.6, 138.5, 131.9, 131.8, 128.3, 127.0, 96.0, 46.4. FTIR vmax 3420, 2944, 2836, 1685, 1598, 1578, 1336, 1249, 1134, 1057, 980, 961, 837, 676, 631, Mp 220–240 °C (degradation). Elemental analysis: calculated for C20H20B2N2O4: C, 59.17; H, 4.97; N, 6.90. Found: C, 59.26; H, 4.75; N, 6.81.
Evaluation of the antifungal activity of bis(benzoxaboroles) by agar diffusion method
An inoculum (0.5 mL) containing 106–107 spores or cells was spread on the surface of the solidified Czapek, potato dextrose or YPD medium and allowed to dry. The amounts of 100, 50, 25 and 10 μg of the tested compounds dissolved in DMSO were placed in 2 mm diameter holes, which were cut in the solidified media. The holes in control runs were filled with DMSO. The duration of fungi incubation was dependent on the vigour of their growth and was established as 48 h for Candida and Aspergillus strains and 72 h for other strains. The optimal temperature for the incubation was 27 °C for Candida and Fusarium strains, and 30 °C for other strains. Each experiment, including control, was carried out in at least three repetitions. The antifungal activity was evaluated by the diameter of the clear zone surrounding the holes, whereas a halo indicated partial inhibition of growth.Conclusions
The mechanochemical approach delivered several bis(benzoxaboroles) 2–6 in moderate (3, 4 and 6) to good yields (2 and 5). In case of compound 2, the mechanochemical yield was almost 70% higher than the yield in solution. To the best of our knowledge, the developed protocol constitutes the first mechanochemical synthesis of benzoxaboroles. The preliminary microbiological studies with the agar diffusion method showed that the antifungal activity is affected by the position of the fluorine atoms. Bis(fluorobenzoxaboroles) 2–4 were found to be active against all the investigated strains, while compound 6 was proved to be completely inactive. The highest activity against A. niger, A. terreus, P. ochrochloron, C. tenuis and C. albicans was displayed by the analogue of the known benzoxaborole antifungal drug Kerydin® (Tavaborole), showing pivotal role of the position of fluorine atom on the activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The support of the National Science Centre of Poland (NCN, grant no. 2016/23/B/ST5/02847) is kindly acknowledged. K. M. B. received financial support from the National Science Centre of Poland within an ETIUDA doctoral scholarship (NCN, project no. 2017/24/T/ST5/00298).Notes and references
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- W. Jones and M. D. Eddleston, Faraday Discuss., 2014, 170, 9–34 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- G. Kaupp, M. R. Naimi-Jamal and V. Stepanenko, Chem.–Eur. J., 2003, 9, 4156–4161 CrossRef CAS.
- M. Schnürch, M. Holzweber, M. D. Mihovilovic and P. Stanetty, Green Chem., 2007, 9, 139–145 RSC.
- A. Adamczyk-Woźniak, M. Jakubczyk, A. Sporzyński and G. Zukowska, Inorg. Chem. Commun., 2011, 14, 1753–1755 CrossRef.
- F. L. Rock, W. Mao, A. Yaremchuk, M. Tukalo, T. Crépin, H. Zhou, Y. K. Zhang, V. Hernandez, T. Akama, S. J. Baker, J. J. Plattner, L. Shapiro, S. A. Martinis, S. J. Benkovic, S. Cusack and M. R. K. Alley, Science, 2007, 316, 1759–1761 CrossRef CAS.
- A. Adamczyk-Woźniak, P. M. Cabaj, M. K. Dominiak, P. Gajowiec, B. Gierczyk, J. Lipok, A. Sporzyński, Ł. Popenda, G. Schroeder, E. Tomecka, P. Urbański and D. Wieczorek, Bioorg. Chem., 2015, 60, 130–135 CrossRef.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- Y. Yu, X. Dai, X. Wei, X. Dai, C. Yu, X. Duan, X. Zhang and C. Li, Chem. Mater., 2018, 30, 8795–8803 CrossRef CAS.
- D. Wieczorek, J. Lipok, K. M. Borys, A. Adamczyk-Woźniak and A. Sporzyński, Appl. Organomet. Chem., 2014, 28, 347–350 CrossRef CAS.
- K. M. Borys, A. Matuszewska, D. Wieczorek, K. Kopczyńska, J. Lipok, I. D. Madura and A. Adamczyk-Woźniak, J. Mol. Struct., 2019, 1181, 587–598 CrossRef CAS.
- A. Pal, M. Berube and D. G. Hall, Angew. Chem., Int. Ed., 2010, 49, 1492–1495 CrossRef CAS.
- D. Claes, M. Holzapfel, N. Clausen and W. Maison, Eur. J. Org. Chem., 2013, 2013, 6361–6371 CrossRef CAS.
- A. Larcher, A. Nocentini, C. T. Supuran, J.-Y. Winum, A. van der Lee, J.-J. Vasseur, D. Laurencin and M. Smietana, ACS Med. Chem. Lett., 2019, 10, 1205–1210 CrossRef CAS.
- C. S. Guy, K. Murray, M. I. Gibson and E. Fullam, Org. Biomol. Chem., 2019, 17, 9524–9528 RSC.
- X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037 CrossRef CAS.
- A. Adamczyk-Woźniak, O. Komarovska-Porokhnyavets, B. Misterkiewicz, V. P. Novikov and A. Sporzyński, Appl. Organomet. Chem., 2012, 26, 390–393 CrossRef.
- M.-A. A. Pizzoccaro, O. Nikel, S. Sene, C. Philippe, P. H. Mutin, S. Bégu, D. Vashishth and D. Laurencin, Acta Biomater., 2016, 41, 342–350 CrossRef CAS.
- M. Jańczyk, K. M. Borys, A. Sporzyński and W. Wróblewski, Procedia Eng., 2014, 87, 568–571 CrossRef.
- M. Durka, K. Durka, A. Adamczyk-Woźniak and W. Wróblewski, Sensors, 2019, 19, a283 CrossRef.
- A. Adamczyk-Woźniak, K. M. Borys, I. D. Madura, S. Michałek and A. Pawełko, Tetrahedron, 2013, 69, 8936–8942 CrossRef.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- A. Adamczyk-Woźniak, K. Ejsmont, B. Gierczyk, E. Kaczorowska, A. Matuszewska, G. Schroeder, A. Sporzyński and B. Zarychta, J. Organomet. Chem., 2015, 788, 36–41 CrossRef.
- A. R. Lippert, G. C. Van de Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS.
- K. Lawrence, S. E. Flower, G. Kociok-Kohn, C. G. Frost and T. D. James, Anal. Methods, 2012, 4, 2215–2217 RSC.
- K. M. Borys, D. Wieczorek, K. Pecura, J. Lipok and A. Adamczyk-Woźniak, Bioorg. Chem., 2019, 91, a103081 CrossRef.
- A. Adamczyk-Woźniak, J. T. Gozdalik, D. Wieczorek, I. D. Madura, E. Kaczorowska, E. Brzezińska, A. Sporzyński and J. Lipok, Molecules, 2020, 799, 1–17 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13NMR spectra of novel compounds, photos of chosen results of the antifungal activity studies. See DOI: 10.1039/d0ra07767d |
| This journal is © The Royal Society of Chemistry 2020 |