Open Access Article
Open Access ArticleNH2-MIL-88B (FeαIn1−α) mixed-MOFs designed for enhancing photocatalytic Cr(VI) reduction and tetracycline elimination†
Chunhua Xu*a,
Mingjun Baob,
Jiawen Renb and
Zhiguang Zhang *b
*b
aDalian Vocational & Technical College (Dalian Radio and TV University), Dalian 116035, PR China. E-mail: xuchunhua0331@163.com; Tel: +86 411-62614316
bSchool of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, PR China. E-mail: zgzhang@lnnu.edu.cn; Tel: +86 411-82158309
First published on 25th October 2020
Abstract
Aiming at solving the issue of wastewater purification, this work synthesized NH2-MIL-88B (FeαIn1−α) photocatalysts by a simple one-pot method, which was employed for photocatalytic reduction of Cr(VI) and oxidation of TC-HCl. Compared with traditional NH2-MIL-88B (Fe) photocatalysts, NH2-MIL-88B (Fe0.6In0.4) displayed excellent photocatalytic performance; the photocatalytic redox rate for Cr(VI) and TC-HCl reached 86.83% and 72.05%, respectively. The good photocatalytic performance might be attributed to the metal-to-metal charge transition (MMCT) between Fe–O clusters and In–O clusters formed by doping In(III) into NH2-MIL-88B (Fe), which provides effective active sites for the photocatalytic reduction and oxidation routes. Besides, the synergistic effect of the ligand-to-metal charge transition (LMCT) and MMCT expands the separation and transfer of photogenerated carriers and inhibits the recombination of electron–hole pairs, thus effectively improving the photocatalytic performance. Therefore, this work could provide a new method for the construction of mixed metal MOFs for the photocatalytic degradation of pollutants.
Introduction
Heavy metals and residual antibiotics in wastewater have become a serious threat to living creatures and the environment.1–4 Hexavalent chromium (Cr(VI)), as a heavy metal ion pollutant in wastewater with high toxicity and mobility, has seriously threatened human health and the natural environment.5,6 If a large amount of Cr(VI) existed in the drinking water system, it would cause damage to the human stomach, kidneys, liver, and retinas and also serious environmental concern.7,8 Therefore, it is urgent to remove Cr(VI) from wastewater. There are many existing solutions such as chemical precipitation,9 membrane separation,10 ion exchange,11 activated carbon adsorption12 and photocatalytic reduction.13–15 Among them, the reduction of Cr(VI) to Cr(III) by semiconductor photocatalysis is a very effective strategy due to the low toxicity of Cr(III).16–18 Furthermore, tetracycline hydrochloride (TC-HCl), as one of the most common antibiotics, is widely used in various industries such as medicine, agriculture, and animal husbandry. TC-HCl is abundant in the environment due to its stable chemical structure and non-biodegradation, which has already imperiled the ecosystem and human health.19–21 There are many current ways to remove TC-HCl, including adsorption,22 advanced oxidation processes (AOPs),21 biological degradation,23 electrolysis,24 membrane filtration,25 ion exchange26 and photocatalytic degradation.27,28 Photocatalytic degradation is widely used in recent years because of mild reaction conditions, high efficiency, low cost and greenly.20,29–31Metal–organic frameworks (MOFs), as a class of crystalline inorganic–organic hybrid materials, are composed of the interconnection between metal centers and multidentate organic ligands.32,33 MOFs have been valued by researchers in the field of photocatalysis34,35 due to the inherent large specific surface area, uniform and adjustable pore structure, and abundant active sites.36 Under the irradiation of incident light, the photogenerated electrons are excited from the valence band (VB) and transferred to the conduction band (CB) through the ligand-to-metal charge transition (LMCT) process. The separated photogenerated electrons–holes pairs can perform the reduction and oxidation reaction with the reactants adsorbed on the catalyst surface.37 Traditional MOFs existed some shortcomings, such as small photoresponse range and high electrons–holes recombination rate.38 It is undoubtedly a good way to change the photoresponse range and photocatalytic performance of MOFs by adjusting the central metal or organic ligands.39 The strategy of combining two or more transition metals into the same framework to form mixed-metal MOFs has aroused the interest of researchers.40
Mixed-metal organic framework (MM-MOFs), containing two or more metal centers, are periodically arranged with a single ligand in the whole framework.41,42 There is a metal-to-metal charge transition (MMCT) process between multiple metals in MM-MOFs, which facilitates the transfer of photogenerated electrons and inhibits the recombination of electrons–holes pairs. Compared with single-metal MOFs, the synergistic effects between LMCT and MMCT of MM-MOFs can effectively improve the photocatalytic activity in various applications.43 Also, MM-MOFs can effectively control the electronic structure and bandgap structure while increasing the metal active sites.44–46 For instance, Vu et al. synthesized Fe-Cr-MIL-101 for dye degradation by the hydrothermal method. Mixed-metal MOFs showed better adsorption capacity and higher photocatalytic activity and stability in comparison with Cr-MIL-101.47 Maite P. et al. studied the synthesis of multimetal MOFs doped with Zn(II), Ru(III) and Pd(II) into Cu-MOF and applied to electrochemical reduction of CO2.48 In our previous report, we successfully synthesized NH2-MIL-68 (InαFe1−α) that displayed good photocatalytic activity.49 According to reports, NH2-MIL-88B (Fe) has a flexible pore structure and a large open structure channel due to its 3D porous structure containing hexagonal channels and bipyramidal cages.50 The wide and flexible pore structure facilitated other metal ions to smoothly enter the 3D structure to replace the original central metal ions, and can provide adjustment space for structural changes that may be caused by the introduction of other central metal ions, thereby improving related performance.
Based on the above analysis, a novel MM-MOFs material NH2-MIL-88B (FeαIn1−α) was constructed and synthesized via the one-pot method directly, which used NH2-MIL-88B (Fe) as the structural framework. Under visible light irradiation, the activity of photocatalytic treatment of pollutants in water was tested. The results showed that the efficiency of Cr(VI) reduction and TC-HCl oxidation of NH2-MIL-88B (FeαIn1−α) was 1.7 and 1.6 times that of NH2-MIL-88B (Fe). Moreover, a possible photocatalytic mechanism was proposed through characterization and performance and active species testing. This work provides an effective way for the construction of MM-MOFs and the photocatalytic degradation of various pollutants in wastewater.
Experimental sections
The chemicals and characterization details could be found in ESI.†Synthesis of NH2-MIL-88B (Fe)
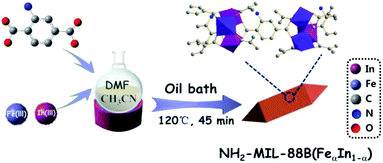
The NH2-MIL-88B (Fe) sample was prepared via a one-pot method. 2-Aminoterephthalic acid (NH2-BDC) (0.54 mmol) and Fe(NO3)3·9H2O (0.48 mmol) were dissolved in DMF (12 mL), added CH3CN (12 mL) after mixed well. Then, and the mixture was transferred into a flask, oil bath at 120 °C for 45 min. The suspension was cooled to room temperature naturally and washed with methanol. The sample was dried at 70 °C for 5 h, then NH2-MIL-88B (Fe) was gained.Synthesis of In-doped NH2-MIL-88B (Fe)
In-doped NH2-MIL-88B (Fe) was synthesized through the above similar process, except that pure Fe(NO3)3·9H2O was replaced with different ratios Fe(NO3)3·9H2O and In(NO3)3·xH2O. The products were named as NH2-MIL-88B (FeαIn1−α), and the synthesis diagram was shown in Scheme 1.Evaluation of photocatalytic performance
The photocatalytic performance of as-obtained samples was evaluated by removing Cr(VI) and TC-HCl. A certain amount of synthesized NH2-MIL-88B (FeαIn1−α) was added into the Cr(VI) solution and stirring sufficiently. Simultaneously, the pH value of the Cr(VI) solution was adjusted to 2 with HCl solution. Then proceed to photocatalytic degradation. During the experiment, pay attention to the concentration of Cr(VI) in the system. The suspension was stirred for some time under dark conditions. When the concentration of Cr(VI) no longer changed, switch to Xe lamp irradiation. The suspensions taken out regularly are centrifuged and filtered with a 0.22 μm syringe filter. The determination of Cr(VI) was realized by the DPC method with a UV-visible spectrophotometer. The photocatalytic oxidation of TC-HCl is similar to the above process. Where the pH value of the TC-HCl solution was adjusted to 9 with NaOH solution and the determination was realized by the UV-visible spectrophotometer method. The removal adsorption reduction/oxidation efficiency of pollutant is calculated using Lambert Beer's law and the following formula:| Efficiency (%) = C/C0 × 100% | (1) |
Results and discussions
Structure and morphology analysis
As revealed in Fig. 1a, the crystalline structure of NH2-MIL-88B (Fe) and In-doped NH2-MIL-88B (FeαIn1−α) are confirmed by the XRD pattern. The obvious characteristic peaks of NH2-MIL-88B (Fe) located at 9.21°, 10.25°, 13.07°, 16.65°, 18.53°, 20.69°, consistent with the literature reported,15 verified the successful preparation of NH2-MIL-88 (Fe). The doped amounts of In on NH2-MIL-88B (Fe) determined by the ICP-AES analysis as shown in Table S1.† Concomitantly, the main characteristic peaks of NH2-MIL-88B (FeαIn1−α) appeared in the same position as NH2-MIL-88B, which preliminarily proved that NH2-MIL-88B (FeαIn1−α) was successfully prepared. The as-prepared MM-MOFs mainly exist combination and crystal lattice in the form of NH2-MIL-88B (Fe). As the molar ratio of In(III) ions increases, the characteristic peak at 9.21° shifts to 9.02°. The deviation of the characteristic peak might be due to that the ion size of In(III) (94.0 pm) in the crystal structure was significantly larger than the ion size of Fe(III) (78.5 pm), thereby the size of the trimer composed of InO6 octahedron was larger than that of FeO6 octahedron in the crystal structure of NH2-MIL-88B (FeαIn1−α). Therefore, the peak corresponding to the characteristic peak in the spectrum could shift to a lower angle due to the increase of the unit cell parameters.51To further determine the chemical structure and investigate the doped of In(III) of the prepared samples, the FT-IR spectrum is employed to analyze. As presented in Fig. 1b, the FT-IR spectrum of NH2-MIL-88B (Fe) is consistent with the previous literature.52 By comparison, after indium doping, a new characteristic absorption peak generation, which could be attributed to the In–O bond appears at 663 cm−1, indicating that In(III) is successfully introduced and the In–O bond is formed. Except for the appearance of new characteristic absorption peaks, the characteristic absorption peaks corresponding to other chemical bonds have not been changed, indicating that the introduction of In(III) does not affect the chemical structure of NH2-MIL-88B (Fe), NH2-MIL-88B (FeαIn1−α) was successfully prepared.
Fig. 2a–f displays representative SEM images and length comparison of NH2-MIL-88B (Fe) and In-doped NH2-MIL-88B (FeαIn1−α). As shown in Fig. 2a, NH2-MIL-88B (Fe) performed a standard spindle with uniform morphology, in which the length is 300–500 nm. Additionally, the spindle is continuously elongated in the longitudinal direction with the increase of In(III) ion content as shown in Fig. 2b–f. However, the overall morphology of NH2-MIL-88B (FeαIn1−α) is unchanged, forming a six-sided double pyramid with a length in the range of 500–1000 nm. The cause of the morphological change may be ascribed to In(III) ions replacing Fe(III) into the NH2-MIL-88B (Fe) lattice, which increases the lattice spacing in the crystal.51 The BET specific surface area test results showed that the BET specific surface areas of NH2-MIL-88B (Fe0.6In0.4) is 204 m2 g−1 less than that of NH2-MIL-88B (Fe), as Table S2† in details. The pore structure of NH2-MIL-88B (Fe) was narrowed after the introduction and the specific surface area was relatively reduced because of the large ion size of In(III).
The morphology of the NH2-MIL-88B (Fe0.6In0.4) in the TEM image is a hexagonal prism with sharp ends, as shown in Fig. 2g, which is the same as the result obtained in the SEM image. HRTEM displayed that NH2-MIL-88B (Fe0.6In0.4) has an ordered pore structure, where is difficult to find nanoparticles or metal oxide clusters outside the catalyst. Thence, In(III) has been incorporated into the framework of NH2-MIL-88B (Fe) instead of being supported on the surface. As shown in Fig. 2h, In and Fe elements were approved in the EDX images, which even demonstrated the conformation of MM-MOFs NH2-MIL-88B (FeαIn1−α).
XPS analysis
The XPS spectra of NH2-MIL-88B (Fe0.6In0.4) composite is shown in Fig. 3a. There are five characteristic peaks in the survey spectrum, which corresponding to the C 1s, N 1s, In 3d, O 1s, and Fe 2p electron orbitals, respectively. These elements confirm the results of the EDX analysis as displayed in Table S3.† Fig. 3b is the characteristic peak of C, all of them are derived from organic ligands. The characteristic peaks at 284.4, 286.2, and 288.2 eV could be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and C
C, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O respectively, which correspond to various carbon-containing covalent bonds in NH2-BDC.53 In Fig. 3c, the XPS spectrum of N 1s can be divided to 396.8 and 397.9 eV, which represents the –N
O respectively, which correspond to various carbon-containing covalent bonds in NH2-BDC.53 In Fig. 3c, the XPS spectrum of N 1s can be divided to 396.8 and 397.9 eV, which represents the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) + and –NH–+ respectively. It belonged to the amino group extending or protruding into the cavity and the positively charged. There are four characteristic peaks in the XPS spectrum of O 1s shown in Fig. 3d. The peaks at 531.7 and 530.8 eV can be attributed to –OH and C
+ and –NH–+ respectively. It belonged to the amino group extending or protruding into the cavity and the positively charged. There are four characteristic peaks in the XPS spectrum of O 1s shown in Fig. 3d. The peaks at 531.7 and 530.8 eV can be attributed to –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the organic ligand. Also, 529.9 and 529.1 eV correspond to the characteristic peaks of In–O and Fe–O respectively. The appearance of In–O clusters shows that In(III) has been doped into the catalyst and connected to the organic ligands, thus successfully constructing NH2-MIL-88B (FeαIn1−α), which is consistent with the results of TEM. Fig. 3e shows the characteristic peaks of Fe 2p, where the satellite peak of Fe is at 715.2 eV. Among the peaks of Fe 2p1/2 and Fe 2p3/2, 724.0 eV and 710.0 eV represent Fe3+, and 722.2 eV and 709.0 eV represent Fe2+.54–56 The appearance of Fe2+ is due to the combination of photogenerated electrons and Fe3+, reducing Fe3+ to Fe2+. The peak at 450.6 and 443.1 eV can be verified to In 3d3/2 and In 3d5/2 as presented in Fig. 3f, respectively. This once again proves that In is successfully doped in NH2-MIL-88B (FeαIn1−α). Contacted with the characterization results of XRD, IR, EDS, and XPS, it can be further confirmed that the In-doped NH2-MIL-88B (FeαIn1−α) bimetallic material has been successfully prepared.
O of the organic ligand. Also, 529.9 and 529.1 eV correspond to the characteristic peaks of In–O and Fe–O respectively. The appearance of In–O clusters shows that In(III) has been doped into the catalyst and connected to the organic ligands, thus successfully constructing NH2-MIL-88B (FeαIn1−α), which is consistent with the results of TEM. Fig. 3e shows the characteristic peaks of Fe 2p, where the satellite peak of Fe is at 715.2 eV. Among the peaks of Fe 2p1/2 and Fe 2p3/2, 724.0 eV and 710.0 eV represent Fe3+, and 722.2 eV and 709.0 eV represent Fe2+.54–56 The appearance of Fe2+ is due to the combination of photogenerated electrons and Fe3+, reducing Fe3+ to Fe2+. The peak at 450.6 and 443.1 eV can be verified to In 3d3/2 and In 3d5/2 as presented in Fig. 3f, respectively. This once again proves that In is successfully doped in NH2-MIL-88B (FeαIn1−α). Contacted with the characterization results of XRD, IR, EDS, and XPS, it can be further confirmed that the In-doped NH2-MIL-88B (FeαIn1−α) bimetallic material has been successfully prepared.
 | ||
| Fig. 3 XPS spectra of NH2-MIL-88B (Fe0.6In0.4) composite (a). Survey; (b) C 1s; (c) N 1s; (d) O 1s; (e) Fe 2p; (f) In 3d, respectively. | ||
Optical properties
The UV-vis DRS spectra of the samples were used to demonstrate the optical absorption properties in Fig. 4a. As reduced, the absorption intensity of NH2-MIL-88B (FeαIn1−α) gradually decreases in the visible light region. NH2-MIL-88B (Fe0.8In0.2) has the strongest absorption intensity of visible light, and NH2-MIL-88B (Fe0.6In0.4) has a slight enhance in visible light absorption intensity. Comparing with NH2-MIL-88B (Fe), both have significantly enhanced visible light absorption. Thereby, this indicates that a certain proportion of In(III) doping, which is beneficial to improve the visible light absorption capacity of NH2-MIL-88B (Fe). | ||
| Fig. 4 (a) UV-vis DRS spectrum and (b) plots of (αhν)2 versus photon energy (hν) of the NH2-MIL-88B (Fe) and the NH2-MIL-88B (FeαIn1−α) samples. | ||
The corresponding bandgap energy (Eg) values could be calculated by following the eqn (1)
| (αhv)2 = A(hv − Eg) | (2) |
As shown in Fig. S1,† the fluorescence intensity of NH2-MIL-88B (Fe0.6In0.4) is significantly lower than that of NH2-MIL-88B (Fe), which indicates that the addition of In(III) inhibits the recombination of photogenerated e−–h+, thereby allowing more photogenerated carriers to participate photocatalytic reaction.57,58
Photocatalytic activities
For verifying the photocatalytic performance of NH2-MIL-88B (FeαIn1−α) obtained from the characterization results, we conducted experiments on photocatalytic Cr(VI) reduction under different catalytic conditions. As displayed in Fig. 5a, the Cr(VI) concentration was unaltered significantly with visible light irradiation and no photocatalyst, at the same time, the small range change of Cr(VI) concentration was due to adsorption with photocatalyst and no light, which indicated that visible light irradiation played an important role in the photocatalytic reduction of Cr(VI). The photocatalytic reduction efficiency of NH2-MIL-88B (Fe0.6In0.4) for reducing Cr(VI) is 86.83%, which is significantly higher 1.7 times than that of NH2-MIL-88B (Fe) of 50.96%, thereby, which demonstrated that the doping of In(III) effectively improves the photocatalytic reduction performance. As shown in Fig. 5b, the different In(III) doped amount of NH2-MIL-88B (FeαIn1−α) was carried out to discuss the capability of photocatalytic Cr(VI) reduction. With the doping of In, the photocatalytic reduction effect of the catalyst gradually increases. Among them, NH2-MIL-88B (Fe0.6In0.4) has the highest photocatalytic reduction rate. By contrast, NH2-MIL-88B (Fe0.8In0.2) is the lowest one. With the addition of more In(III) content, the catalytic effect gradually decreases. This may be due to more In(III) doping, resulting in lower visible light absorption. And higher bandgap energy is not conducive to the excitation of electrons on VB, thereby reducing the photocatalytic activity of photocatalytic reduction of hexavalent chromium. Meanwhile, Fig. 5c shows that the photocatalytic Cr(VI) on different concentrations reduction performance of NH2-MIL-88B (Fe0.6In0.4) was investigated. With increasing the amount of catalyst, the photocatalytic performance gradually increases, reaching the maximum at 10 mg L−1. However, an excess of the catalyst leads to lower photocatalytic reduction efficiency. This may be due to excessive catalyst hindering the light irradiation of the Cr(VI) solution. Fig. 5d displays that the photocatalytic reduction efficiency has a good linear relationship with the reaction time, which conforms to the first-order reaction kinetics. The pseudo-first-order rate constant k is obtained by the following formula:| ln(C0/C) = kt | (3) |
To investigate the oxidation performance of the prepared photocatalyst, the photocatalytic degradation of TC-HCl by NH2-MIL-88B (FeαIn1−α) under different catalytic conditions was performed. Similar to the photocatalytic reduction effect of Cr(VI), the test result of Fig. 6a shows that the light and photocatalyst are crucial in the reaction system. As shown in Fig. 6b, the photocatalytic degradation efficiency of NH2-MIL-88B (Fe0.6In0.4) on TC-HCl under visible light irradiation conditions is 72.05%, which is significantly higher 1.6 times than that of NH2-MIL-88B (Fe) of 44.68%. Fig. 6c demonstrated the effect of different concentrations photocatalytic degradation efficiency of TC-HCl. Among them, as the content of TC-HCl increases, the degradation rate gradually decreases, and when the content is 10 mg, NH2-MIL-88B (Fe0.6In0.4) displays the best degradation effect. The relationship between ln(C0/C) and time shows that the photocatalytic degradation efficiency has a good linear relationship with the reaction time, as presented in Fig. 6d, which is in line with the first-order reaction kinetics. The value of k and R2 of NH2-MIL-88B (Fe) and NH2-MIL-88B (FeαIn1−α) was displayed in Tables S6 and S7.†
To detect the reactive oxygen species generation during the photocatalytic degradation of TC-HCl by NH2-MIL-88B (FeαIn1−α), electron spin resonance (ESR) technique is employed. Fig. 7 depicts the conspicuous characteristic peak signals of DMPO-˙O2− and DMPO-˙OH, which confirms the generation of ˙O2− and ˙OH radicals in the experiment. The peak intensity ratio of the quadruple characteristic peak signal of DMPO-˙OH is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.59 NH2-MIL-88B (FeαIn1−α) by the photoexcitation generates e− under visible light irradiation, which can activate dissolved oxygen in the solution that promotes oxygen molecules to become ˙O2− species and can also interact with water molecules to generate ˙OH species, simultaneously.
1.59 NH2-MIL-88B (FeαIn1−α) by the photoexcitation generates e− under visible light irradiation, which can activate dissolved oxygen in the solution that promotes oxygen molecules to become ˙O2− species and can also interact with water molecules to generate ˙OH species, simultaneously.
 | ||
| Fig. 7 ESR spectra of DMPO-˙O2− and DMPO-˙OH adducts generated NH2-MIL-88B (FeαIn1−α) after 60 seconds under visible light irradiation (λ > 400 nm) during the photocatalytic process. | ||
Mechanism
Based on the above results, the possible mechanism of NH2-MIL-88B (FeαIn1−α) photocatalytic treatment of Cr(VI) and TC-HCl in water is proposed. First, the electrons transfer process of NH2-MIL-88B (FeαIn1−α) photocatalytic reduction of Cr(VI) is as follows. Under visible light irradiation, the organic ligand of NH2-MIL-88B (FeαIn1−α) can be excited to generate electrons and holes. A part of the photogenerated electrons transfers to the surface of the catalyst through the In–O clusters through the LMCT to reduce Cr(VI) adsorbed directly. Then, the In–O clusters transfer another electron to the Fe–O cluster through MMCT, which promotes the separation of electrons and holes effectively.49 The excitation and transfer of photogenerated carriers of photocatalytic oxidation of TC-HCl are similar to the process of reduction of Cr(VI). The photogenerated electrons transfer to the surface of the catalyst combine with O2 and H2O in the solution to produce ˙O2− and ˙OH. Both the holes generated by NH2-MIL-88B (FeαIn1−α) excited by visible light and ˙O2−/˙OH can interact with TC-HCl directly and eventually generate to CO2 and H2O. Anyway, the MMCT in the NH2-MIL-88B (FeαIn1−α) expands the active sites of photocatalytic oxidation-reduction. Also, the synergistic process of LMCT and MMCT effectively inhibits the recombination of electrons and holes, thereby enhancing the performance of the NH2-MIL-88B (FeαIn1−α) (Scheme 2).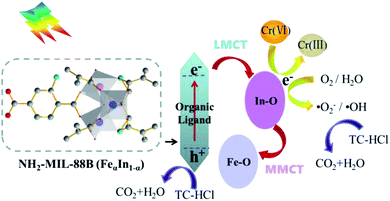 | ||
| Scheme 2 Possible mechanism of photocatalytic degradation of Cr(VI) and TC-HCl by NH2-MIL-88B (FeαIn1−α). | ||
Conclusions
In summary, a series of NH2-MIL-88B (FeαIn1−α) photocatalysts were designed and synthesized via a one-pot method. Proved by characterizations of TEM and XPS, In(III) ions successfully replaced part of Fe(III) ions in the structure, which formed mixed-metal MOFs NH2-MIL-88B (FeαIn1−α). Compared with single NH2-MIL-88B (Fe), NH2-MIL-88B (Fe0.6In0.4) shows excellent photocatalytic performance: the reduction rate of Cr(VI) and the oxidation rate of TC-HCl reach 86.83% and 72.05%, which 1.7 and 1.6 times that of NH2-MIL-88B (Fe), respectively. The improvement of photocatalytic performance is mainly attributed to the more reactive sites when In(III) doped to NH2-MIL-88B (Fe). Simultaneously, the synergistic effect of LMCT and MMCT of NH2-MIL-88B (FeαIn1−α) effectively expands the separation and transfer of photogenerated carriers and inhibits the recombination of electrons–holes pairs, thereby enhancing the performance of photocatalytic reduction of Cr(VI) and oxidative degradation of TC-HCl. This work may provide an insight to design and develop mixed-metal MOFs as photocatalysts for the wastewater treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks to the 2020 School-level Scientific Research Project of Dalian Vocational & Technical College (Dalian Radio and TV University) and the Key Project of Natural Science Foundation of Liaoning Province (No. 20170540578).References
- X. H. Yi, F. X. Wang, X. D. Du, H. Fu and C. C. Wang, Polyhedron, 2018, 152, 216–224 CrossRef CAS.
- H. Xie, J. Zhang, D. Wang, J. Liu, L. Wang and H. Xiao, Appl. Surf. Sci., 2020, 504, 144456 CrossRef CAS.
- L. Zeng, X. Li, S. Fan, M. Zhang, Z. Yin, M. Tadé and S. Liu, ACS Appl. Energy Mater., 2018, 1, 3752–3762 CrossRef CAS.
- L. Zeng, X. Li, S. Fan, Z. Yin, J. Mu, M. Qin and A. Chen, J. Power Sources, 2020, 478, 228755 CrossRef CAS.
- F. Yuan, Z. Sun, C. Li, Y. Tan, X. Zhang and S. Zheng, J. Hazard. Mater., 2020, 396, 122694 CrossRef CAS.
- D. D. Chen, X. H. Yi, C. Zhao, H. Fu, P. Wang and C. C. Wang, Chemosphere, 2020, 245, 125659 CrossRef CAS.
- S. Wu, Y. Ge, Y. Wang, X. Chen, F. Li, H. Xuan and X. Li, Environ. Technol., 2017, 39, 1937–1948 CrossRef.
- F. Wei, D. Chen, Z. Liang, S. Zhao and Y. Luo, Dalton Trans., 2017, 46, 16525–16531 RSC.
- C. Peng, H. Meng, S. Song, S. Lu and A. Lopez-Valdivieso, Sep. Sci. Technol., 2005, 39, 1501–1517 CrossRef.
- R. K. Goyal, N. S. Jayakumar and M. A. Hashim, J. Hazard. Mater., 2011, 195, 383–390 CrossRef CAS.
- R. K. Misra, S. K. Jain and P. K. Khatri, J. Hazard. Mater., 2011, 185, 1508–1512 CrossRef CAS.
- Y. J. Zhang, J. L. Ou, Z. K. Duan, Z. J. Xing and Y. Wang, Colloids Surf., A, 2015, 481, 108–116 CrossRef CAS.
- L. Shi, T. Wang, H. Zhang, K. Chang, X. Meng, H. Liu and J. Ye, Adv. Sci., 2015, 2, 1500006 CrossRef.
- R. Liang, F. Jing, L. Shen, N. Qin and L. Wu, J. Hazard. Mater., 2015, 287, 364–372 CrossRef CAS.
- L. Zeng, X. Li, S. Fan, M. Zhang, Z. Yin, M. Tadé and S. Liu, J. Power Sources, 2019, 413, 310–317 CrossRef CAS.
- C. Zhao, Z. Wang, X. Li, X. Yi, H. Chu, X. Chen and C. C. Wang, Chem. Eng. J., 2020, 389, 123431 CrossRef CAS.
- H. Xie, D. Ma, W. Liu, Q. Chen, Y. Zhang, J. Huang, H. Zhang, Z. Jin, T. Luo and F. Peng, New J. Chem., 2020, 44, 7218–7225 RSC.
- J. Ke, H. Zhou, J. Liu, Z. Zhang, X. Duan and S. Wang, J. Colloid Interface Sci., 2019, 555, 413–422 CrossRef CAS.
- H. Fakhri and H. Bagheri, Mater. Sci. Semicond. Process., 2020, 107, 104815 CrossRef CAS.
- D. Wang, F. Jia, H. Wang, F. Chen, Y. Fang, W. Dong, G. Zeng, X. Li, Q. Yang and X. Yuan, J. Colloid Interface Sci., 2018, 519, 273–284 CrossRef CAS.
- Y. Zhang, J. Zhou, X. Chen, L. Wang and W. Cai, Chem. Eng. J., 2019, 369, 745–757 CrossRef CAS.
- L. L. Yu, W. Cao, S. C. Wu, C. Yang and J. H. Cheng, Ecotoxicol. Environ. Saf., 2018, 164, 289–296 CrossRef CAS.
- X. Wen, Y. Jia and J. Li, J. Hazard. Mater., 2010, 177, 924–928 CrossRef CAS.
- T. Chen, R. Tsai, Y. Chen and K. Huang, Int. J. Electrochem. Sci., 2014, 9, 8422–8434 Search PubMed.
- K. Kosutic, D. Dolar, D. Asperger and B. Kunst, Sep. Purif. Technol., 2007, 53, 244–249 CrossRef CAS.
- K. J. Choi, H. J. Son and S. H. Kim, Sci. Total Environ., 2007, 387, 247–256 CrossRef CAS.
- Y. Gao, J. Wu, J. Wang, Y. Fan, S. Zhang and W. Dai, ACS Appl. Mater. Interfaces, 2020, 12, 11036–11044 CrossRef.
- J. Ke, C. Zhao, H. Zhou, X. Duan and S. Wang, Sustainable Mater. Technol., 2019, 19, e00088 CrossRef CAS.
- J. Liu, J. Zhang, D. Wang, D. Li, J. Ke, S. Wang, S. Liu, H. Xiao and R. Wang, ACS Sustainable Chem. Eng., 2019, 7, 12428–12438 CAS.
- J. Li, X. Li, L. Zeng, S. Fan, M. Zhang, W. Sun, X. Chen, M. O. Tade and S. Liu, Nanoscale, 2019, 11, 3877–3887 RSC.
- S. Y. Fan, X. Y. Li, Q. D. Zhao, L. B. Zeng, M. M. Zhang, Z. F. Yin, T. T. Lian, M. O. Tad and S. M. Liu, Dalton Trans., 2018, 47, 12769–12782 RSC.
- Y. P. Zhu, J. Yin, E. Abou-Hamad, X. Liu, W. Chen, T. Yao, O. F. Mohammed and H. N. Alshareef, Adv. Mater., 2020, 32, e1906368 CrossRef.
- C. V. Reddy, K. R. Reddy, V. V. N. Harish, J. Shim, M. V. Shankar, N. P. Shetti and T. M. Aminabhavi, Int. J. Hydrogen Energy, 2020, 45, 7656–7679 CrossRef CAS.
- J. Wang, X. Liu, C. Li, M. Yuan, B. Zhang, J. Zhu and Y. Ma, J. Photochem. Photobiol., A, 2020, 401, 112795 CrossRef CAS.
- L. Zeng, X. Li, S. Chen, J. Wen, W. Huang and A. Chen, J. Mater. Chem. A, 2020, 8, 7339–7349 RSC.
- S. Zhang, M. Du, Z. Xing, Z. Li, K. Pan and W. Zhou, Appl. Catal., B, 2020, 262, 118202 CrossRef CAS.
- J. Ye, J. Liu, Z. Huang, S. Wu, X. Dai, L. Zhang and L. Cui, Chemosphere, 2019, 227, 505–513 CrossRef CAS.
- Z. H. Yang, J. Cao, Y. P. Chen, X. Li, W. P. Xiong, Y. Y. Zhou, C. Y. Zhou, R. Xu and Y. R. Zhang, Microporous Mesoporous Mater., 2019, 277, 277–285 CrossRef CAS.
- M. A. Nasalevich, M. van der Veen, F. Kapteijn and J. Gascon, CrystEngComm, 2014, 16, 4919–4926 RSC.
- J. Liu and Y. Pan, Metal-Organic Frameworks for Biomedical Applications, 2020, pp. 45–68, DOI:10.1016/b978-0-12-816984-1.00004-4.
- A. Beziau, S. A. Baudron, A. Fluck and M. W. Hosseini, Inorg. Chem., 2013, 52, 14439–14448 CrossRef CAS.
- W. S. El-Yazeed, Y. G. El-Reash, L. A. Elatwy and A. I. Ahmed, RSC Adv., 2020, 10, 9693–9703 RSC.
- M. Y. Masoomi, A. Morsali, A. Dhakshinamoorthy and H. Garcia, Angew. Chem., 2019, 58, 15188–15205 CrossRef CAS.
- M. R. D. Khaki, M. S. Shafeeyan, A. A. A. Raman and W. Daud, J. Environ. Manage., 2017, 198, 78–94 CrossRef CAS.
- D. Ge, G. Qu, X. Li, K. Geng, X. Cao and H. Gu, New J. Chem., 2016, 40, 5531–5536 RSC.
- W. Zhang, Y. Shi, C. Li, Q. Zhao and X. Li, Catal. Lett., 2016, 146, 1956–1964 CrossRef CAS.
- T. A. Vu, G. H. Le, C. D. Dao, L. Q. Dang, K. T. Nguyen, P. T. Dang, H. T. K. Tran, Q. T. Duong, T. V. Nguyen and G. D. Lee, RSC Adv., 2014, 4, 41185–41194 RSC.
- M. Perfecto-Irigaray, J. Albo, G. Beobide, O. Castillo, A. Irabien and S. Pérez-Yáñez, RSC Adv., 2018, 8, 21092–21099 RSC.
- S. Wang, F. Meng, X. Sun, M. Bao, J. Ren, S. Yu, Z. Zhang, J. Ke and L. Zeng, Appl. Surf. Sci., 2020, 528, 147053 CrossRef CAS.
- L. Wang, S. Duan, M. Fang, J. Liu, J. He, J. Li and J. Lei, RSC Adv., 2016, 6, 71250–71261 RSC.
- S. A. Park, H. J. Lee, Y. J. Cho, S. Choi and M. Oh, Chem. – Eur. J., 2014, 20, 5559–5564 CrossRef CAS.
- A. Samui, A. R. Chowdhuri, T. K. Mahto and S. K. Sahu, RSC Adv., 2016, 6, 66385–66393 RSC.
- Z. D. Lei, Y. C. Xue, W. Q. Chen, L. Li, W. H. Qiu, Y. Zhang and L. Tang, Small, 2018, 14, e1802045 CrossRef.
- H. Y. Zhang, L. Wang, C. F. Guo, J. Q. Ning, Y. J. Zhong and Y. Hu, ChemNanoMat, 2020, 6, 1325–1331 CrossRef CAS.
- H. Liu, K. Tian, J. Ning, Y. Zhong, Z. Zhang and Y. Hu, ACS Catal., 2019, 9, 1211–1219 CrossRef CAS.
- H. Zhang, C. Guo, J. Ren, J. Ning, Y. Zhong, Z. Zhang and Y. Hu, Chem. Commun., 2019, 55, 14050–14053 RSC.
- L. Li, C. Guo, J. Shen, J. Ning, Y. Zhong and Y. Hu, Chem. Eng. J., 2020, 400, 125925 CrossRef CAS.
- H. Liu, L. Li, C. Guo, J. Ning, Y. Zhong and Y. Hu, Chem. Eng. J., 2020, 385, 123929 CrossRef CAS.
- X. Li, Y. Pi, L. Wu, Q. Xia, J. Wu, Z. Li and J. Xiao, Appl. Catal., B, 2017, 202, 653–663 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07487j |
| This journal is © The Royal Society of Chemistry 2020 |