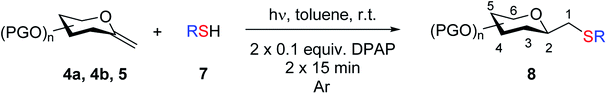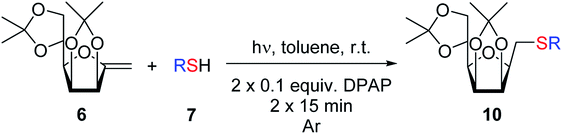Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and photoinitiated thiol–ene reactions of exo-mannals – a new route to C-β-D-mannosyl derivatives†
János Józsefac,
Nóra Debreczenibcd,
Dániel Eszenyib,
Anikó Borbás b,
László Juhász
b,
László Juhász *a and
László Somsák
*a and
László Somsák *a
*a
aDepartment of Organic Chemistry, University of Debrecen, PO Box 400, H-4002 Debrecen, Hungary. E-mail: juhasz.laszlo@science.unideb.hu; somsak.laszlo@science.unideb.hu; Tel: +36-52-512900/22464, +36-52-512900/22348
bDepartment of Pharmaceutical Chemistry, University of Debrecen, PO Box 400, H-4002 Debrecen, Hungary
cUniversity of Debrecen, Doctoral School of Chemistry, PO Box 400, H-4002 Debrecen, Hungary
dHAS-UD Molecular Recognition and Interaction Research Group, University of Debrecen, Egyetem tér 1, Debrecen 4032, Hungary
First published on 22nd September 2020
Abstract
Syntheses of acyl protected exo-mannal derivatives were developed starting from O-peracylated mannopyranoses via the corresponding anhydro-aldose tosylhydrazones under modified Bamford–Stevens conditions. The synthesis of analogous O-peralkylated (benzylated and isopropylenated) derivatives was carried out from pyranoid and furanoid mannonolactones using methylene transfer reagents. Photoinitiated thiol–ene additions of these exo-mannals resulted in the corresponding C-(mannopyranosyl/mannofuranosyl)methyl sulfides in medium to good yields with exclusive regio- and β(D) stereoselectivities.
1. Introduction
D-Mannose occurs in microbes, plants, animals in free form, but more often as a component of glycans or glycoproteins. Several articles were published in recent years about the metabolic study and biological function of mannose and mannose containing glycoconjugates demonstrating that the therapeutic applications of these derivatives receive increasing attention.1–4 Mannose can be used as a drug in the case of specific bacterial infections, but it can be lethal or teratogenic, too.5–7 The mannose binding lectins (MBL) of the human cells play a central role in innate immunity by the interaction with surface sugars of a wide series of microorganisms, but this specific interaction can also be used for selective delivery of anti-cancer drugs, using glycosylated (mannosylated) bioconjugates.8–10The O-glycosidic bond in natural glycosides is characterized with low hydrolytic and/or enzymatic stability but by replacing the glycosidic oxygen with other atoms (C, N, S)11 or groups (S–S, S–Se, SO2–N, and N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N),12 more stable molecules can be synthesized with similar biological activity. These molecules are the glycomimetics,11 which are frequently used as leads of drug discovery.
O)–N),12 more stable molecules can be synthesized with similar biological activity. These molecules are the glycomimetics,11 which are frequently used as leads of drug discovery.
Several routes have been published in the literature for the synthesis of C-glycosyl derivatives but the yield and stereoselectivity of these reactions are highly dependent on the circumstances, protecting groups and configuration of the starting compounds.13–15 C-Mannosyl derivatives received special attention as summarized in a recent review.16
Photoinitiated thiol–ene additions, also called thio-click reactions are widely used in synthetic organic chemistry and material science for the synthesis of sulfur containing compounds.17–20 In carbohydrate chemistry, the sugar moiety can be used both as a thiol or an alkene components, and such transformations show excellent regio- and stereoselectivities.21 The hydrothiolation of exo-glycals allows the synthesis of novel, glycosylmethyl sulfide (Gly–CH2–SR) type mimetics with very high or exclusive β-selectivity.22–25
Based on the above experiences we set out to study the thiol–ene additions with various exo-mannals with the expectation that the β-stereoselectivity observed with other exo-glycal configurations will be maintained here as well. This synthetic route can provide mimetics of O-β-D-mannosyl derivatives whose syntheses are otherwise very challenging tasks.26
2. Results
2.1. Synthesis of exo-mannal derivatives
Several methods are known in the literature for the synthesis of exo-glycal derivatives possessing different protecting groups.27,28 In the case of base stable ether or acetal type protection, they can be synthesized from the corresponding aldonolactones, using well-known olefination methods with the Petasis and the Tebbe reagents29–31 or Julia/modified Julia olefinations32,33 under strongly basic conditions. We have developed a simple method for the preparation of exo-glycals with ester type protection of hydroxyl groups via anhydro-aldose tosylhydrazones starting from glycosyl cyanides.34,35By using the latter method, exo-mannals 4a and 4b were synthesized in multistep reactions from commercially available 1,2,3,4,6-penta-O-acetyl- (1a) and -benzoyl-D-mannopyranose (1b), as summarized in Scheme 1. First, O-peracylated mannopyranoses 1a or 1b were reacted36 with TMSCN in the presence of BF3·OEt2 to give mannosyl cyanides 2a37 and 2b in 35 and 64% yields, respectively, as single anomers. Their NMR study clearly showed the 4C1 conformation of the ring and the α(D) anomeric configuration which can be explained by the anchimeric effect of the 2-O-acyl substituent and also corresponds to the anomeric effect of the CN substituent forcing this group to occupy an axial position.38–40
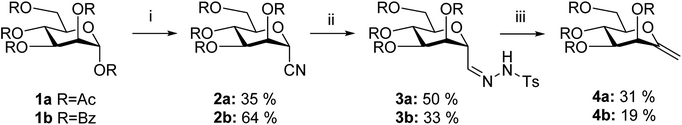 | ||
| Scheme 1 (i) 3.2 equiv. TMSCN, 2 equiv. BF3·OEt2 in CH3NO2, 40 °C; (ii) 1.3 equiv. TsNHNH2, 8.4 equiv. NaH2PO2, RANEY®-Ni in pyridine–AcOH–H2O; (iii) 5 equiv. K3PO4 in dry dioxane, reflux. | ||
Subsequently cyanides 2 were transformed into the tosylhydrazones 3 under reductive conditions in the presence of tosylhydrazine, which on deprotonation by K3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 (instead of the less easily handled NaH34) and heating to reflux temperature gave exo-mannals 4. The pyranoid ring of tosylhydrazones 3 and exo-mannals 4 had a 5C2 conformation according to the 3J coupling constants between H-2, H-3, H-4, H-5 and H-6 (Table 1).
41 (instead of the less easily handled NaH34) and heating to reflux temperature gave exo-mannals 4. The pyranoid ring of tosylhydrazones 3 and exo-mannals 4 had a 5C2 conformation according to the 3J coupling constants between H-2, H-3, H-4, H-5 and H-6 (Table 1).
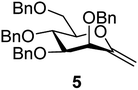
The benzylated pyranoid exo-mannal 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 and the isopropylenated furanoid exo-mannal 6
31 and the isopropylenated furanoid exo-mannal 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32,42 were synthesized by literature procedures.
32,42 were synthesized by literature procedures.
2.2. Thiol–ene additions of exo-mannal derivatives
The addition of thiols was carried out in toluene at room temperature (unless otherwise indicated) in the presence of 2,2-dimethoxy-2-phenylacetophenone (DPAP, 0.1 equiv.) as the photoinitiator with irradiation at λmax 365 nm for 15 min. The progress of the reaction was monitored by TLC after this reaction period and irradiation and addition of DPAP were repeated if necessary (in most cases two irradiation cycles had to be applied for total consumption of exo-mannals 4–6). The thiols were used in a 5-fold excess for 7a and 7b and in slightly more than equimolar amounts (1.1 equiv.) for 7c–f.The results of the addition of thiols 7 to exo-mannals 4 and 5 are summarized in Table 2. The reactions were carried out under an Ar atmosphere (except in the case of thiol 7d) to give the expected products 8 in high yields. On addition of thiol 7d to both exo-mannals 4a and 4b under air, beside the glycosylmethyl sulfides 8ad and 8bd, respectively, as the major products, sulfoxides 9ad and 9bd were also isolated as minor components. The structure of these side products was identified by NMR and MS measurements, and their formation could be eliminated by using an inert atmosphere.
| Exo-Glycal | Thiols | Yieldb (%) of 8 | Structure of adducts 8 |
|---|---|---|---|
a Total conversion of 4a, b was detected after two irradiations of 15 min.b Isolated yields after purification by column chromatography.c The reaction was performed at −78 °C. At room temperature, no conversion of the exo-glycal was detected.d The reaction was performed under air. Formation of corresponding sulfoxide 9 in low yield was also observed: 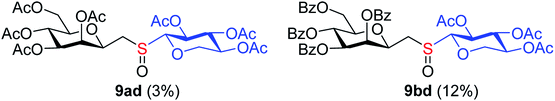 |
|||
 |
7a | 79c | 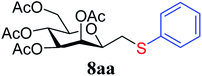 |
| 7b | 69 | 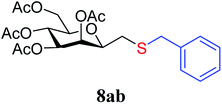 |
|
| 7c | 78 | 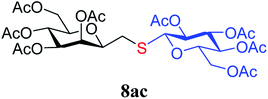 |
|
| 7d | 71d | 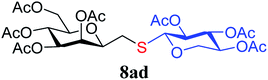 |
|
| 7e | 79 | 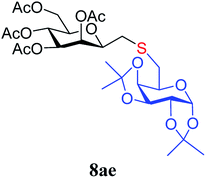 |
|
 |
7a | 47c | 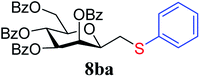 |
| 7b | 51 | 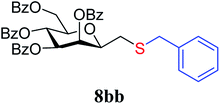 |
|
| 7d | 68d | 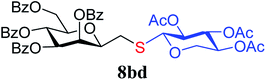 |
|
| 7e | 73 | 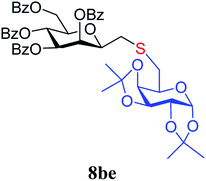 |
|
 |
Low conversion and decomposition of exo-mannal 5 were observed with 7a, b, d | ||
In the case of benzenethiol 7a no transformation was detected at room temperature, but at −78 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43,44 the sulfides 8aa and 8ba were isolated by column chromatography in 79 and 47% yields, respectively.
43,44 the sulfides 8aa and 8ba were isolated by column chromatography in 79 and 47% yields, respectively.
There are only a few examples in the literature for thiol–ene addition to benzylated carbohydrate derivatives, due to the low stability of this protecting group under radical conditions.23,45 This experience was corroborated with exo-mannal 5, whose reactions proceeded only with low conversion and decomposition of the starting benzylated glycal (Table 2).
The addition of thiols 7 to the furanoid exo-mannal 6 was carried out under the same conditions as described above (under argon atmosphere and at room temperature), and the results are summarized in Table 3. After the second irradiation total conversion of starting compound was observed, and the desired glycosylmethyl sulfides 10 were isolated in moderate to good yields (53–82%). In the case of benzenethiol 7a the reaction at room temperature gave 10a in 53% yield, while at low temperature the yield raised to 70%.
The structure of the products 8 and 10 was identified by assigning each signal and connectivity in their 1H NMR spectra by using COSY experiments (selected data are collected in Table 4).
| H-1A | H-1B | H-2 | H-3 | H-4 | H-5b | H-6 | ||
|---|---|---|---|---|---|---|---|---|
| a The NMR experiments were performed at 400 MHz in CDCl3.b In the case of compounds 8 the signals H-5 were split into triplet. | ||||||||
 |
δ | 3.16 | 2.92 | 3.68 | 5.55 | 5.02 | 5.19 | 3.61 |
| J | 13.9, 6.8 | 13.9, 7.0 | 7.0, 6.8, 1.0 | 3.4, 1.0 | 10.0, 3.4 | 10.0 | 10.0, 5.6, 2.3 | |
 |
δ | 2.64 | 2.40 | 3.60–3.56 | 5.41 | 4.97 | 5.19 | 3.60–3.56 |
| J | 14.0, 7.3 | 14.0, 6.2 | m | 3.2 | 10.0, 3.4 | 10.0 | m | |
 |
δ | 3.25 | 3.05 | 4.01 | 6.03 | 5.59 | 6.02 | 4.08 |
| J | 14.1, 7.1 | 14.1, 6.5 | 7.1, 6.5, 0.9 | 3.2, 0.9 | 10.0, 3.2 | 10.0 | 10.0, 4.6, 2.7 | |
 |
δ | 2.73 | 2.51 | 3.95 | 5.89 | 5.56 | 6.01 | 4.07 |
| J | 14.4, 7.8 | 14.4, 5.5 | 7.8, 5.5, 0.8 | 3.2, 0.8 | 10.0, 3.2 | 10.0 | 10.0, 4.6, 2.5 | |
 |
δ | 3.24 | 3.22 | 3.67 | 4.79–4.73 | 3.49 | 4.38 | |
| J | 13.5, 6.1 | 13.5, 7.7 | 7.7, 6.1, 2.9 | m | 7.5, 2.9 | 7.5, 6.0, 4.7 | ||
 |
||||||||
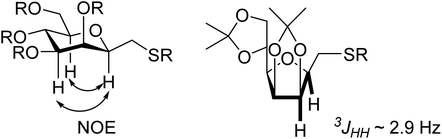
The vicinal coupling constant between H-2, H-3, H-4, H-5 and H-6 indicated the 5C2 conformation of the sugar ring of 8. The β(D)-configuration at C-2 of 8 could not be determined from the coupling constants between H-2 and H-3 but it was easily assigned from the observed NOE-s between H-2, H-4 and H-6, which also confirm the 5C2 ring conformation of the products. In the case of furanoid derivatives the 3JH2,H3 values of ∼2.9 Hz clearly indicated the β(D)-configuration at C-2 of 10.
The exclusive regio- and stereoselectivity of these reactions can be explained by the following mechanistic considerations. The regiochemistry of the additions is determined by the different stability of the radicals that may form upon addition of the thiyl radicals to the exocyclic double bonds. The resonance stabilized C-glycosyl radical provides a reaction pathway with a significantly lower activation barrier than the glycosylmethyl radical (Fig. 1A). In the 5C2 conformation of mannopyranosyl radicals46 there are stabilizing overlaps between the 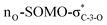 orbitals due to the homo-anomeric effect. Similar considerations refer to the furanosyl radicals. The abstraction of the hydrogen by these radicals are clearly more favourable from the α-side, since both the steric shielding by the O-3-substituent and the preservation of the above stabilizing overlaps act in synergy (Fig. 1B). These effects lead to a more favorable transition state (TS) with lower energy, thus determining the exclusively observed β configuration of C-2.
orbitals due to the homo-anomeric effect. Similar considerations refer to the furanosyl radicals. The abstraction of the hydrogen by these radicals are clearly more favourable from the α-side, since both the steric shielding by the O-3-substituent and the preservation of the above stabilizing overlaps act in synergy (Fig. 1B). These effects lead to a more favorable transition state (TS) with lower energy, thus determining the exclusively observed β configuration of C-2.
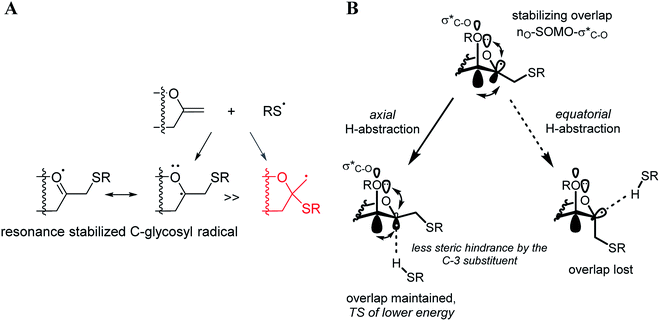 | ||
| Fig. 1 Mechanistic considerations. (A) Relative stabilities of adduct radical; (B) stabilizing overlaps in mannopyranosyl radicals and transition states of H-abstraction. | ||
3. Conclusion
A synthesis of O-peracylated exo-mannals was developed from mannopyranosyl cyanides and the respective anhydro-aldose tosylhydrazones. Photoinitianed thiol–ene additions of these O-peracylated and also O-peracetalated exo-mannals of both pyranoid and furanoid structures were studied to result in C-mannosylmethyl sulfide type compounds with the R-S-CH2 appendage in (pseudo)equatorial position as the only products. This study demonstrated that the radical-mediated thiol–ene reactions of exo-mannals with a wide range of thiols took place with exclusive regio- and stereoselectivities, thereby providing a new way for the construction of novel types of glycomimetic compounds of high biological relevance.4. Materials and methods
4.1. General methods
Melting points were measured on a Kofler hot-stage and are uncorrected. Optical rotations were measured at r.t. with a Jasco P-2000 polarimeter. TLC was performed on Kieselgel 60 F254 (Merck) with detection by immersing into 5% ethanolic sulfuric acid soln. followed by heating. Column chromatography was performed on Silica gel 60 (Merck 0.063–0.200 mm). Organic solutions were dried over MgSO4 and concentrated under diminished pressure. The 1H (400 MHz) and 13C NMR (100.28 MHz) NMR spectra were recorded with a Bruker DRX-400 spectrometer. Chemical shifts are referenced to Me4Si (0.00 ppm for 1H) and to the residual solvent signals (CDCl3: 77.16 ppm for 13C). The coupling constant values (J) are given in Hz. Mass spectra were recorded with MicroTOF-Q type Qq-TOF MS (Bruker Daltonik, Bremen, Germany) instruments. The photoinitiated reactions were carried out by irradiation with a Hg-lamp (maximum emission at 365 nm) in a borosilicate vessel. The benzylated pyranoid exo-mannal (5) and isopropylenated furanoid exo-mannal (6) were synthesized by literature procedures.31,32,42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). When the starting material disappeared, the solvent was evaporated, and the residue was dissolved in Et2O (50 mL). This was washed with saturated NaHCO3 solution (2 × 20 mL) and brine (1 × 20 mL), dried, then concentrated and purified by column chromatography.
1). When the starting material disappeared, the solvent was evaporated, and the residue was dissolved in Et2O (50 mL). This was washed with saturated NaHCO3 solution (2 × 20 mL) and brine (1 × 20 mL), dried, then concentrated and purified by column chromatography.
4.1.1.1. 2,3,4,6-Tetra-O-acetyl-α-D-mannopyranosyl cyanide (2a). Prepared from 1a (5 g, 12.8 mmol) according to Method A to give 2a by column chromatography (eluent: hexane–acetone = 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow syrup (1.6 g, 35%). Rf = 0.39 (hexane–EtOAc = 1
1) as a yellow syrup (1.6 g, 35%). Rf = 0.39 (hexane–EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +11.4 (c = 0.244, CHCl3); lit37 +27.8 (c 3.32, CHCl3).
1). [α]D = +11.4 (c = 0.244, CHCl3); lit37 +27.8 (c 3.32, CHCl3).1H-NMR of 2a (400 MHz, CDCl3) δ: 5.44 (dd, 1H, J = 3.1, 2.3 Hz, H-2), 5.38–5.28 (m, 2H, H-3, H-4), 4.92 (d, 1H, J = 2.3 Hz, H-1), 4.34 (dd, 1H, J = 12.6, 5.3 Hz, H-6A), 4.17 (dd, 1H, J = 12.6, 2.0 Hz, H-6B), 4.08 (ddd, 1H, J = 9.4, 5.3, 2.0 Hz, H-5), 2.19, 2.11, 2.08, 2.03 (4 × s, 4 × 3H, OAc); data correspond to lit37 values. 13C-NMR of 2a (100 MHz, CDCl3) δ: 170.5, 169.7, 169.6, 169.6 (CO), 113.5 (CN), 74.3, 69.0, 68.8, 65.7, 65.1 (C-1, C-2, C-3, C-4, C-5), 61.8 (C-6), 20.8, 20.7, 20.7, 20.6 (OAc). ESI-MS positive mode (m/z): calcd for C15H19NNaO9+ [M + Na]+ = 380.0952. Found: [M + Na]+ = 380.0970.
4.1.1.2. 2,3,4,6-Tetra-O-benzoyl-α-D-mannopyranosyl cyanide (2b). Prepared from 1b (5 g, 7.1 mmol) according to Method A to give 2b by column chromatography (eluent: hexane–acetone = 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow syrup (2.2 g, 53%). Rf = 0.31 (hexane–EtOAc = 2
1) as a yellow syrup (2.2 g, 53%). Rf = 0.31 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −32.9 (c = 0.254, CHCl3).
1). [α]D = −32.9 (c = 0.254, CHCl3).1H-NMR of 2b (400 MHz, CDCl3) δ: 8.10 (dd, 2H, J = 8.1, 1.3 Hz, aromatic), 8.02 (dd, 2H, J = 8.2, 1.1 Hz, aromatic), 7.98 (dd, 2H, J = 8.2, 1.0 Hz, aromatic), 7.85 (dd, 2H, J = 8.3, 1.1 Hz, aromatic), 7.63–7.26 (m, 12H, aromatic), 6.18 (pseudo t, 1H, J = 9.8 Hz, H-4), 5.94–5.89 (m, 2H, H-2, H-3), 5.21 (d, 1H, J = 2.0 Hz, H-1), 4.77 (dd, 1H, J = 10.6, 3.2 Hz, H-6A), 4.56–4.51 (m, 2H, H-5, H-6B). 13C-NMR of 2b (100 MHz, CDCl3) δ: 165.4, 165.2 (CO), 134.1–128.6 (aromatic), 113.8 (CN), 74.6, 70.1, 69.8, 65.9, 65.7 (C-1, C-2, C-3, C-4, C-5), 62.1 (C-6). ESI-MS positive mode (m/z): calcd for C35H27NNaO9+ [M + Na]+ = 628.1578. Found: [M + Na]+ = 628.1573.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the insoluble materials were filtered off with suction and washed with CH2Cl2 (10 mL). The organic layer of the filtrate was separated, washed sequentially with H2O (5 mL), 10% aqueous solution of HCl (2 × 5 mL), cold, saturated NaHCO3 solution (2 × 5 mL) and H2O (5 mL), and then dried over anhydrous MgSO4. The solution was concentrated under reduced pressure, and traces of pyridine were removed by repeated co-evaporations of toluene. The residue was purified by column chromatography.
1) the insoluble materials were filtered off with suction and washed with CH2Cl2 (10 mL). The organic layer of the filtrate was separated, washed sequentially with H2O (5 mL), 10% aqueous solution of HCl (2 × 5 mL), cold, saturated NaHCO3 solution (2 × 5 mL) and H2O (5 mL), and then dried over anhydrous MgSO4. The solution was concentrated under reduced pressure, and traces of pyridine were removed by repeated co-evaporations of toluene. The residue was purified by column chromatography.
4.1.2.1. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-D-glycero-D-talo-heptose tosylhydrazone (3a). Prepared from 2a (1.6 g, 4.5 mmol) according to Method B to give 3a by column chromatography (eluent: hexane–EtOAc = 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (1.2 g, 50%). Rf = 0.36 (hexane–EtOAc = 1
1) as a yellow amorphous solid (1.2 g, 50%). Rf = 0.36 (hexane–EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −37.7 (c = 0.200, CHCl3).
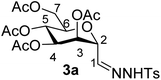
1). [α]D = −37.7 (c = 0.200, CHCl3).1H-NMR of 3a (400 MHz, CDCl3) δ: 8.63 (s, 1H, NH), 7.92 (d, 2H, J = 8.4 Hz, aromatic), 7.34 (d, 2H, J = 8.4 Hz, aromatic), 7.17 (d, 1H, J = 3.2 Hz, H-1), 5.57 (dd, 1H, J = 3.5, 2.6 Hz, H-3), 5.30 (dd, 1H, J = 9.2, 3.5 Hz, H-4), 5.23 (pseudo t, 1H, J = 9.2 Hz, H-5), 4.56 (dd, 1H, J = 3.2, 2.6 Hz, H-2), 4.24 (dd, 1H, J = 12.3, 5.3 Hz, H-7A), 4.05 (dd, 1H, J = 12.3, 2.6 Hz, H-7B), 3.66 (ddd, 1H, J = 9.2, 5.3, 2.6 Hz, H-6), 2.42 (CH3), 2.12, 2.09, 2.05, 2.03 (4 × s, 4 × 3H, OAc). 13C-NMR of 3a (100 MHz, CDCl3) δ: 170.9, 170.3, 169.8, 169.8 (CO), 144.1 (C-1), 144.6–128.2 (aromatic), 74.7, 72.1, 69.3, 68.2, 66.5 (C-2, C-3, C-4, C-5, C-6), 62.3 (C-7), 21.7 (CH3), 20.9, 20.8, 20.8, 20.7 (OAc). ESI-MS positive mode (m/z): calcd for C22H28N2NaO11S+ [M + Na]+ = 551.1306. Found: [M + Na]+ = 551.1316.
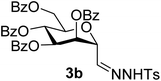
4.1.2.2. 3,4,5,7-Tetra-O-benzoyl-2,6-anhydro-D-glycero-D-talo-heptose tosylhydrazone (3b). Prepared from 2b (1.7 g, 2.8 mmol) according to Method B to give 3b by column chromatography (eluent: hexane–EtOAc = 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (0.7 g, 33%). Rf = 0.44 (hexane–EtOAc = 1
1) as a yellow amorphous solid (0.7 g, 33%). Rf = 0.44 (hexane–EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +3.7 (c = 0.198, CHCl3).
1). [α]D = +3.7 (c = 0.198, CHCl3).1H-NMR of 3b (400 MHz, CDCl3) δ: 8.90 (s, 1H, NH), 8.10–7.26 (m, 25H, aromatic, H-1), 6.09 (pseudo t, 1H, J = 9.8 Hz, H-5), 6.04 (dd, 1H, J = 3.1, 1.9 Hz, H-3), 5.88 (dd, 1H, J = 9.8, 3.1 Hz, H-4), 4.84 (dd, 1H, J = 2.7, 1.9 Hz, H-2), 4.65 (dd, 1H, J = 12.2, 2.2 Hz, H-7A), 4.40 (dd, 1H, J = 12.2, 4.1 Hz, H-7B), 4.12 (ddd, 1H, J = 9.8, 4.1, 2.2 Hz, H-6), 2.41 (CH3). 13C-NMR of 3b (100 MHz, CDCl3) δ: 166.3, 165.6, 165.4 (CO), 144.3 (C-1), 144.8–128.5 (aromatic), 75.0, 72.4, 70.5, 69.5, 67.3 (C-2, C-3, C-4, C-5, C-6), 62.8 (C-7), 21.7 (CH3). ESI-MS positive mode (m/z): calcd for C42H36N2NaO11S+ [M + Na]+ = 799.1932. Found: [M + Na]+ = 799.1923.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the mixture was cooled and the insoluble material filtered off. The solvent was removed under reduced pressure, and the residue was purified by column chromatography.
1), the mixture was cooled and the insoluble material filtered off. The solvent was removed under reduced pressure, and the residue was purified by column chromatography.
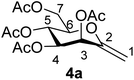
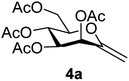
4.1.3.1. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-1-deoxy-D-manno-hept-1-enitol (4a). Prepared from 3a (1.0 g, 1.9 mmol) according to Method C to give 4a by column chromatography (eluent: hexane–EtOAc = 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil (0.2 g, 31%). Rf = 0.41 (hexane–EtOAc = 1
1) as a yellow oil (0.2 g, 31%). Rf = 0.41 (hexane–EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +34.7 (c = 0.126, CHCl3).
1). [α]D = +34.7 (c = 0.126, CHCl3).1H-NMR of 4a (400 MHz, CDCl3) δ: 5.71 (d, 1H, J = 3.5 Hz, H-3), 5.43 (pseudo t, 1H, J = 9.5 Hz, H-5), 5.10 (dd, 1H, J = 9.5, 3.5 Hz, H-4), 4.88 (d, 1H, J = 1.4 Hz, H-1A), 4.73 (d, 1H, J = 1.4 Hz, H-1B), 4.31 (dd, 1H, J = 12.4, 5.2 Hz, H-7A), 4.21 (dd, 1H, J = 12.4, 2.6 Hz, H-7B), 3.82 (ddd, 1H, J = 9.5, 5.2, 2.6 Hz, H-6), 2.14, 2.12, 2.07, 2.03 (4 × s, 4 × 3H, OAc). 13C-NMR of 4a (100 MHz, CDCl3) δ: 170.7, 170.0, 169.9, 169.6 (CO), 152.5 (C-2), 102.0 (C-1), 77.1, 71.1, 69.0, 65.6 (C-3, C-4, C-5, C-6), 62.4 (C-7), 21.1, 20.8, 20.7, 20.7 (OAc). ESI-MS positive mode (m/z): calcd for C15H20NaO9+ [M + Na]+ = 367.1000. Found: [M + Na]+ = 367.0996.
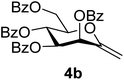
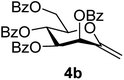
4.1.3.2. 2,6-Anhydro-3,4,5,7-tetra-O-benzoyl-1-deoxy-D-manno-hept-1-enitol (4b). Prepared from 3b (0.2 g, 0.26 mmol) according to Method C to give 4b by column chromatography (eluent: hexane–EtOAc = 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil (64 mg, 42%). Rf = 0.43 (hexane–EtOAc = 2
1) as a yellow oil (64 mg, 42%). Rf = 0.43 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +19.0 (c = 0.260, CHCl3).
1). [α]D = +19.0 (c = 0.260, CHCl3).1H-NMR of 4b (400 MHz, CDCl3) δ: 8.13–7.85 (m, 8H, aromatic), 7.60–7.26 (m, 13H, aromatic, H-1), 6.28 (pseudo t, 1H, J = 9.7 Hz, H-5), 6.17 (d, 1H, J = 3.4 Hz, H-3), 5.68 (dd, 1H, J = 9.7, 3.4 Hz, H-4), 5.03 (d, 1H, J = 1.3 Hz, H-1A), 4.94 (d, 1H, J = 1.3 Hz, H-1B), 4.78 (dd, 1H, J = 12.3, 2.7 Hz, H-7A), 4.55 (dd, 1H, J = 12.3, 4.1 Hz, H-7B), 4.29 (ddd, 1H, J = 9.7, 4.1, 2.7 Hz, H-6). 13C-NMR of 4b (100 MHz, CDCl3) δ: 165.7, 165.6, 165.4, 165.4 (CO), 152.6 (C-2), 133.7–128.5 (aromatic), 102.8 (C-1), 77.3, 72.2, 70.1, 66.4 (C-3, C-4, C-5, C-6), 62.8 (C-7). ESI-MS positive mode (m/z): calcd for C35H28NaO9+ [M + Na]+ = 615.1626. Found: [M + Na]+ = 615.1626.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) indicated incomplete transformation of the starting material another 0.1 equiv. of DPAP was added and irradiation was continued for 15 min. When the reaction was complete, the solvent was removed, then the residue was purified by column chromatography.
1) indicated incomplete transformation of the starting material another 0.1 equiv. of DPAP was added and irradiation was continued for 15 min. When the reaction was complete, the solvent was removed, then the residue was purified by column chromatography.
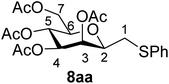
4.1.4.1. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-1-S-phenyl-1-thio-D-glycero-D-galacto-heptitol (8aa). Prepared from 4a (35 mg, 0.11 mmol) and 7a (56 μL, 0.55 mmol) using DPPA (3 × 2.8 mg, 0.011 mmol) in toluene (1.1 mL) at −78 °C according to Method D to give 8aa by column chromatography (eluent: hexane–acetone = 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (31 mg, 62%). Rf = 0.41 (hexane–EtOAc = 2
1) as a yellow amorphous solid (31 mg, 62%). Rf = 0.41 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +91.9 (c = 0.102, CHCl3).
1). [α]D = +91.9 (c = 0.102, CHCl3).1H-NMR of 8aa (400 MHz, CDCl3) δ: 7.39–7.22 (m, 5H, aromatic), 5.55 (dd, 1H, J = 3.4, 1.0 Hz, H-3), 5.23 (pseudo t, 1H, J = 10.0 Hz, H-5), 5.02 (dd, 1H, J = 10.0, 3.4 Hz, H-4), 4.27 (dd, 1H, J = 12.3, 5.6 Hz, H-7A), 4.09 (dd, 1H, J = 12.3, 2.3 Hz, H-7B), 3.68 (ddd, 1H, J = 7.0, 6.8, 1.0 Hz, H-2), 3.61 (ddd, 1H, J = 10.0, 5.6, 2.3 Hz, H-6), 3.16 (dd, 1H, J = 13.9, 6.8 Hz, H-1A), 2.92 (dd, 1H, J = 13.9, 7.0 Hz, H-1B), 2.14, 2.10, 2.03, 1.98 (4 × s, 4 × 3H, OAc). 13C-NMR of 8aa (100 MHz, CDCl3) δ: 170.9, 170.3, 170.3, 169.8 (CO), 135.0–127.1 (aromatic), 76.5, 76.1, 72.4, 68.6, 66.1 (C-2, C-3, C-4, C-5, C-6), 62.8 (C-7), 34.3 (C-1), 20.9, 20.8, 20.7 (OAc). ESI-MS positive mode (m/z): calcd for C21H26NaO9S+ [M + Na]+ = 477.1190. Found: [M + Na]+ = 477.1189.
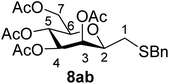
4.1.4.2. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-1-S-benzyl-1-thio-D-glycero-D-galacto-heptitol (8ab). Prepared from 4a (100 mg, 0.29 mmol) and 7b (170 μL, 1.45 mmol) using DPPA (3 × 7.4 mg, 0.029 mmol) in toluene (2.9 mL) according to Method D to give 8ab by column chromatography (eluent: hexane–acetone = 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (94 mg, 69%). Rf = 0.26 (hexane–EtOAc = 2
1) as a yellow amorphous solid (94 mg, 69%). Rf = 0.26 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +60.5 (c = 0.152, CHCl3).
1). [α]D = +60.5 (c = 0.152, CHCl3).1H-NMR of 8ab (400 MHz, CDCl3) δ: 7.32–7.30 (m, 5H, aromatic), 5.41 (d, 1H, J = 3.2 Hz, H-3), 5.19 (pseudo t, 1H, J = 10.0 Hz, H-5), 4.97 (dd, 1H, J = 10.0, 3.4 Hz, H-4), 4.24 (dd, 1H, J = 12.3, 5.8 Hz, H-7A), 4.12 (dd, 1H, J = 12.3, 2.3 Hz, H-7B), 3.79 (d, 1H, J = 13.4 Hz, CH2Ph), 3.72 (d, 1H, J = 13.4 Hz, CH2Ph), 3.60–3.56 (m, 1H, H-2, H-6), 2.64 (dd, 1H, J = 14.0, 7.3 Hz, H-1A), 2.40 (dd, 1H, J = 14.0, 6.2 Hz, H-1B), 2.09, 2.09, 2.04, 1.97 (4 × s, 4 × 3H, OAc). 13C-NMR of 8ab (100 MHz, CDCl3) δ: 170.7, 170.3, 170.2, 169.8 (CO), 138.1–127.3 (aromatic), 77.7, 76.4, 72.3, 68.9, 66.1 (C-2, C-3, C-4, C-5, C-6), 62.9 (C-7), 37.1 (CH2Ph), 31.0 (C-1), 20.9, 20.8, 20.7, 20.6 (OAc). ESI-MS positive mode (m/z): calcd for C22H28NaO9S+ [M + Na]+ = 491.1346. Found: [M + Na]+ = 491.1346.
4.1.4.3. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-1-S-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)-1-thio-D-glycero-D-galacto-heptitol (8ac). Prepared from 4a (61 mg, 0.18 mmol) and 7c (72 mg, 0.20 mmol) using DPPA (3 × 4.6 mg, 0.018 mmol) in toluene (1.8 mL) according to Method D to give 8ac by column chromatography (eluent: hexane–acetone = 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (82 mg, 50%). Rf = 0.32 (hexane–EtOAc = 2
1) as a yellow amorphous solid (82 mg, 50%). Rf = 0.32 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −25.10 (c = 0.1195, CHCl3).
1). [α]D = −25.10 (c = 0.1195, CHCl3).1H-NMR of 8ac (400 MHz, CDCl3) δ: 5.51 (dd, 1H, J = 3.4, 1.0 Hz, H-3), 5.26–5.18 (m, 2H, H-5, H-3′), 5.12–5.05 (m, 2H, H-4, H-4′), 5.00 (pseudo t, 1H, J = 9.9 Hz, H-2′), 4.49 (d, 1H, J = 10.1 Hz, H-1′), 4.30–4.19 (m, 3H, H-7A, 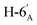 ,
, 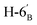 ), 4.12 (dd, 1H, J = 12.3, 2.4 Hz, H-7B), 3.87 (ddd, 1H, J = 7.2, 6.8, 1.0 Hz, H-2), 3.74–3.67 (m, 2H, H-6, H-5′), 2.87 (dd, 1H, J = 13.9, 6.8 Hz, H-1A), 2.69 (dd, 1H, J = 13.9, 7.2 Hz, H-1B), 2.17, 2.12, 2.11, 2.06, 2.04, 2.03, 2.01, 1.98 (8 × s, 8 × 3H, OAc). 13C-NMR of 8ac (100 MHz, CDCl3) δ: 170.8, 170.7, 170.2, 170.1, 169.8, 169.5, 169.4 (CO), 83.3 (C-1′), 76.9, 76.4, 76.2, 73.8, 72.3, 69.9, 68.6, 68.2, 66.1 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 62.8 (C-7), 61.9 (C-6′), 29.6 (C-1), 20.9, 20.8, 20.7, 20.6 (OAc). Anal. calcd for C27H40O14S (620.21): C 52.24, H 6.5, S 5.16; measured C 53.07, H 6.60, S 5.03.
), 4.12 (dd, 1H, J = 12.3, 2.4 Hz, H-7B), 3.87 (ddd, 1H, J = 7.2, 6.8, 1.0 Hz, H-2), 3.74–3.67 (m, 2H, H-6, H-5′), 2.87 (dd, 1H, J = 13.9, 6.8 Hz, H-1A), 2.69 (dd, 1H, J = 13.9, 7.2 Hz, H-1B), 2.17, 2.12, 2.11, 2.06, 2.04, 2.03, 2.01, 1.98 (8 × s, 8 × 3H, OAc). 13C-NMR of 8ac (100 MHz, CDCl3) δ: 170.8, 170.7, 170.2, 170.1, 169.8, 169.5, 169.4 (CO), 83.3 (C-1′), 76.9, 76.4, 76.2, 73.8, 72.3, 69.9, 68.6, 68.2, 66.1 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 62.8 (C-7), 61.9 (C-6′), 29.6 (C-1), 20.9, 20.8, 20.7, 20.6 (OAc). Anal. calcd for C27H40O14S (620.21): C 52.24, H 6.5, S 5.16; measured C 53.07, H 6.60, S 5.03.
4.1.4.4. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-1-S-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)-1-thio-D-glycero-D-galacto-heptitol (8ad). Prepared from 4a (150 mg, 0.44 mmol) and 7d (150 mg, 0.48 mmol) using DPPA (3 × 11.3 mg, 0.044 mmol) in toluene (4.4 mL) according to Method D to give 8ad by column chromatography (eluent: hexane–acetone = 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (200 mg, 71%). Rf = 0.23 (hexane–EtOAc = 1
1) as a yellow amorphous solid (200 mg, 71%). Rf = 0.23 (hexane–EtOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −22.4 (c = 0.216, CHCl3).
1). [α]D = −22.4 (c = 0.216, CHCl3).1H-NMR of 8ad (400 MHz, CDCl3) δ: 5.50 (dd, 1H, J = 3.2, 0.9 Hz, H-3), 5.22 (pseudo t, 1H, J = 10.0 Hz, H-5), 5.15 (pseudo t, 1H, J = 8.3 Hz, H-3′), 5.04 (dd, 1H, J = 10.0, 3.3 Hz, H-4), 4.96–4.91 (m, 2H, H-2′, H-4′), 4.51 (d, 1H, J = 8.4 Hz, H-1′), 4.28–4.19 (m, 2H, 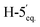 , H-7A), 4.12 (dd, 1H, J = 12.3, 2.2 Hz, H-7B), 3.79 (ddd, 1H, J = 7.2, 6.6, 0.9 Hz, H-2), 3.66 (ddd, 1H, J = 10.0, 5.4, 2.3 Hz, H-6), 3.41 (dd, 1H, J = 11.7, 9.0 Hz,
, H-7A), 4.12 (dd, 1H, J = 12.3, 2.2 Hz, H-7B), 3.79 (ddd, 1H, J = 7.2, 6.6, 0.9 Hz, H-2), 3.66 (ddd, 1H, J = 10.0, 5.4, 2.3 Hz, H-6), 3.41 (dd, 1H, J = 11.7, 9.0 Hz, 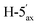 ), 2.88 (dd, 1H, J = 13.8, 6.6 Hz, H-1A), 2.69 (dd, 1H, J = 13.8, 7.2 Hz, H-1B), 2.18, 2.10, 2.08, 2.05, 2.05, 2.04, 1.98, (7 × s, 7 × 3H, OAc). 13C-NMR of 8ad (100 MHz, CDCl3) δ: 170.8, 170.3, 170.2, 169.9, 169.8, 169.7, 169.6 (CO), 83.4, 77.0, 76.5, 72.4, 71.9, 69.9, 68.5, 66.0, (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′), 65.5 (C-7), 62.8 (C-5′), 29.6 (C-1), 20.9, 20.8, 20.7 (OAc). ESI-MS positive mode (m/z): calcd for C26H36NaO16S+ [M + Na]+ = 659.1616. Found: [M + Na]+ = 659.1615.
), 2.88 (dd, 1H, J = 13.8, 6.6 Hz, H-1A), 2.69 (dd, 1H, J = 13.8, 7.2 Hz, H-1B), 2.18, 2.10, 2.08, 2.05, 2.05, 2.04, 1.98, (7 × s, 7 × 3H, OAc). 13C-NMR of 8ad (100 MHz, CDCl3) δ: 170.8, 170.3, 170.2, 169.9, 169.8, 169.7, 169.6 (CO), 83.4, 77.0, 76.5, 72.4, 71.9, 69.9, 68.5, 66.0, (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′), 65.5 (C-7), 62.8 (C-5′), 29.6 (C-1), 20.9, 20.8, 20.7 (OAc). ESI-MS positive mode (m/z): calcd for C26H36NaO16S+ [M + Na]+ = 659.1616. Found: [M + Na]+ = 659.1615.
4.1.4.5. 3,4,5,7-Tetra-O-acetyl-2,6-anhydro-1-S-(1,2:3,4-di-O-isopropylidene-β-D-galactopyranos-6-yl)-1-thio-D-glycero-D-galacto-heptitol (8ae). Prepared from 4a (60 mg, 0.17 mmol) and 7e (52 mg, 0.19 mmol) using DPPA (3 × 4.4 mg, 0.017 mmol) in toluene (1.7 mL) according to Method D to give 8ae by column chromatography (eluent: hexane–acetone = 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (102 mg, 83%). Rf = 0.28 (hexane–EtOAc = 2
1) as a yellow amorphous solid (102 mg, 83%). Rf = 0.28 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −30.92 (c = 0.084, CHCl3).
1). [α]D = −30.92 (c = 0.084, CHCl3).1H-NMR of 8ae (400 MHz, CDCl3) δ: 5.54–5.52 (m, 2H, H-3, H-1′), 5.20 (pseudo t, 1H, J = 9.9 Hz, H-5), 5.10 (dd, 1H, J = 10.0, 3.4 Hz, H-4), 4.61 (dd, 1H, J = 7.8, 2.4 Hz, H-3′), 4.31 (dd, 1H, J = 5.1, 2.4 Hz, H-2′), 4.26–4.22 (m, 2H, H-7A, H-4′), 4.12 (dd, 1H, J = 12.3, 2.4 Hz, H-7B), 3.90 (dd, 1H, J = 7.0, 6.7, 0.8 Hz, H-2), 3.86 (ddd, 1H, J = 7.4, 6.2, 1.7 Hz, H-5′), 3.68 (ddd, 1H, J = 9.9, 5.9, 2.4 Hz, H-6), 2.82–2.76 (m, 3H, H-1A, 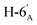 ,
, 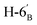 ), 2.62 (dd, 1H, J = 14.1, 6.7 Hz, H-1B), 2.15, 2.10, 2.05, 1.98 (4 × s, 4 × 3H, CH3), 1.56, 1.45, 1.34, 1.26, (4 × s, 4 × 3H, OAc). 13C-NMR of 8ae (100 MHz, CDCl3) δ: 170.8, 170.3, 170.1, 169.8 (CO), 109.4, 108.8 (Cacetal), 96.6 (C-1′), 77.7, 76.4, 72.3, 72.0, 71.0, 70.5, 69.0, 68.3, 66.4 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 63.0 (C-7), 33.3 (C-1), 29.8 (C-6′), 26.1, 26.0, 25.0, 24.5 (CH3), 20.9, 20.8, 20.7 (OAc). Anal. calcd for C27H40O14S (620.21): C 52.24, H 6.50, S 5.16; measured C 52.58, H 6.62, S 5.08.
), 2.62 (dd, 1H, J = 14.1, 6.7 Hz, H-1B), 2.15, 2.10, 2.05, 1.98 (4 × s, 4 × 3H, CH3), 1.56, 1.45, 1.34, 1.26, (4 × s, 4 × 3H, OAc). 13C-NMR of 8ae (100 MHz, CDCl3) δ: 170.8, 170.3, 170.1, 169.8 (CO), 109.4, 108.8 (Cacetal), 96.6 (C-1′), 77.7, 76.4, 72.3, 72.0, 71.0, 70.5, 69.0, 68.3, 66.4 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 63.0 (C-7), 33.3 (C-1), 29.8 (C-6′), 26.1, 26.0, 25.0, 24.5 (CH3), 20.9, 20.8, 20.7 (OAc). Anal. calcd for C27H40O14S (620.21): C 52.24, H 6.50, S 5.16; measured C 52.58, H 6.62, S 5.08.
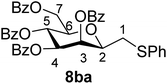
4.1.4.6. 2,6-Anhydro-3,4,5,7-tetra-O-benzoyl-1-S-phenyl-1-thio-D-glycero-D-galacto-heptitol (8ba). Prepared from 4b (110 mg, 0.19 mmol) and 7a (195 μL, 1.9 mmol) using DPPA (3 × 5.0 mg, 0.019 mmol) in toluene (1.9 mL) at −78 °C according to Method D to give 8ba by column chromatography (eluent: hexane–acetone = 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (60 mg, 47%). Rf = 0.33 (hexane–EtOAc = 2
1) as a yellow amorphous solid (60 mg, 47%). Rf = 0.33 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +24.3 (c = 0.180, CHCl3).
1). [α]D = +24.3 (c = 0.180, CHCl3).1H-NMR of 8ba (400 MHz, CDCl3) δ: 8.12 (dd, 2H, J = 8.3, 1.3 Hz, aromatic), 8.02 (dd, 2H, J = 8.3, 1.2 Hz, aromatic), 7.89 (dd, 2H, J = 8.4, 1.3 Hz, aromatic), 7.79 (dd, 2H, J = 8.4, 1.3 Hz, aromatic), 7.63–7.18 (m, 17H, aromatic), 6.03 (dd, 1H, J = 3.2, 0.9 Hz, H-3), 6.02 (pseudo t, 1H, J = 10.0 Hz, H-5), 5.59 (dd, 1H, J = 10.0, 3.2 Hz, H-4), 4.71 (dd, 1H, J = 12.2, 2.7 Hz, H-7A), 4.46 (dd, 1H, J = 12.2, 4.6 Hz, H-7B), 4.08 (ddd, 1H, J = 10.0, 4.6, 2.7 Hz, H-6), 4.01 (ddd, 1H, J = 7.1, 6.5, 0.9 Hz, H-2), 3.25 (dd, 1H, J = 14.1, 7.1 Hz, H-1A), 3.05 (dd, 1H, J = 14.1, 6.5 Hz, H-1B). 13C-NMR of 8ba (100 MHz, CDCl3) δ: 166.3, 165.9, 165.6, 165.5 (CO), 133.6–127.0 (aromatic), 76.6, 76.5, 73.4, 69.7, 67.1 (C-2, C-3, C-4, C-5, C-6), 63.3 (C-7), 34.6 (C-1). ESI-MS positive mode (m/z): calcd for C41H34NaO9S+ [M + Na]+ = 725.1816. Found: [M + Na]+ = 725.1818.
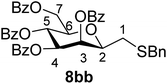
4.1.4.7. 2,6-Anhydro-3,4,5,7-tetra-O-benzoyl-1-S-benzyl-1-thio-D-glycero-D-galacto-heptitol (8bb). Prepared from 4b (110 mg, 0.19 mmol) and 7b (110 μL, 0.95 mmol) using DPPA (3 × 4.9 mg, 0.019 mmol) in toluene (1.9 mL) according to Method D to give 8bb by column chromatography (eluent: hexane–acetone = 8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (70 mg, 51%). Rf = 0.43 (hexane–EtOAc = 2
1) as a yellow amorphous solid (70 mg, 51%). Rf = 0.43 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +25.2 (c = 0.110, CHCl3).
1). [α]D = +25.2 (c = 0.110, CHCl3).1H-NMR of 8bb (400 MHz, CDCl3) δ: 8.14 (dd, 2H, J = 8.2, 1.5 Hz, aromatic), 7.97 (dd, 2H, J = 8.4, 1.4 Hz, aromatic), 7.92 (dd, 2H, J = 8.4, 1.3 Hz, aromatic), 7.79 (dd, 2H, J = 8.3, 1.5 Hz, aromatic), 7.62–7.13 (m, 19H, aromatic), 6.01 (pseudo t, 1H, J = 10.0 Hz, H-5), 5.89 (dd, 1H, J = 3.2, 0.8 Hz, H-3), 5.56 (dd, 1H, J = 10.0, 3.2 Hz, H-4), 4.78 (dd, 1H, J = 12.2, 2.5 Hz, H-7A), 4.46 (dd, 1H, J = 12.2, 4.6 Hz, H-7B), 4.07 (ddd, 1H, J = 10.0, 4.6, 2.5 Hz, H-6), 3.95 (ddd, 1H, J = 7.8, 5.5, 0.8 Hz, H-2), 3.86 (d, 1H, J = 13.4 Hz, CH2Ph), 3.70 (d, 1H, J = 13.4 Hz, CH2Ph), 2.73 (dd, 1H, J = 14.4, 7.8 Hz, H-1A), 2.51 (dd, 1H, J = 14.4, 5.5 Hz, H-1B). 13C-NMR of 8bb (100 MHz, CDCl3) δ: 166.3, 165.8, 165.6, 165.0 (CO), 138.1–127.2 (aromatic), 78.7, 76.6, 73.4, 70.1, 66.9 (C-2, C-3, C-4, C-5, C-6), 63.2 (C-7), 37.2 (CH2Ph), 31.1 (C-1). ESI-MS positive mode (m/z): calcd for C42H36NaO9S+ [M + Na]+ = 739.1972. Found: [M + Na]+ = 739.1973.
4.1.4.8. 2,6-Anhydro-3,4,5,7-tetra-O-benzoyl-1-S-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)-1-thio-D-glycero-D-galacto-heptitol (8bd). Prepared from 4b (70 mg, 0.12 mmol) and 7d (40 mg, 0.13 mmol) using DPPA (3 × 3.1 mg, 0.012 mmol) in toluene (1.2 mL) according to Method D to give 8bd by column chromatography (eluent: hexane–acetone = 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (60 mg, 68%). Rf = 0.33 (hexane–EtOAc = 2
1) as a yellow amorphous solid (60 mg, 68%). Rf = 0.33 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +0.5 (c = 0.162, CHCl3).
1). [α]D = +0.5 (c = 0.162, CHCl3).1H-NMR of 8bd (400 MHz, CDCl3) δ: 8.13 (dd, 2H, J = 8.1, 1.1 Hz, aromatic), 8.02 (dd, 2H, J = 8.2, 1.2 Hz, aromatic), 7.91 (dd, 2H, J = 8.3, 1.4 Hz, aromatic), 7.79 (dd, 2H, J = 8.5, 1.3 Hz, aromatic), 7.62–7.23 (m, 12H, aromatic), 6.04 (pseudo t, 1H, J = 10.1 Hz, H-5), 6.00 (dd, 1H, J = 3.1, 0.9 Hz, H-3), 5.62 (dd, 1H, J = 10.1, 3.1 Hz, H-4), 5.14 (pseudo t, 1H, J = 8.3 Hz, H-2′), 4.94 (pseudo t, 1H, J = 8.2 Hz, H-3′), 4.92–4.87 (m, 1H, H-4′), 4.75 (dd, 1H, J = 12.2, 2.5 Hz, H-7A), 4.55 (d, 1H, J = 8.3 Hz, H-1′), 4.45 (dd, 1H, J = 12.2, 4.2 Hz, H-7B), 4.16–4.10 (m, 3H, H-2, H-6, 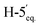 ), 3.38 (dd, 1H, J = 11.7, 8.9 Hz,
), 3.38 (dd, 1H, J = 11.7, 8.9 Hz, 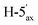 ), 2.99 (dd, 1H, J = 14.0, 6.6 Hz, H-1A), 2.84 (dd, 1H, J = 14.0, 7.1 Hz, H-1B), 2.04, 2.03, 2.02 (3 × s, 3 × 3H, OAc). 13C-NMR of 8bd (100 MHz, CDCl3) δ: 170.1, 170.0, 169.8, 169.6, 166.3, 165.5, 165.4 (CO), 133.5–128.4 (aromatic), 83.7, 77.6, 76.5, 73.4, 72.0, 70.1, 69.5, 68.6, 66.9, (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′), 65.4 (C-7), 63.1 (C-5′), 30.4 (C-1), 20.8 (OAc). ESI-MS positive mode (m/z): calcd for C46H44NaO16S+ [M + Na]+ = 907.2242. Found: [M + Na]+ = 907.2238.
), 2.99 (dd, 1H, J = 14.0, 6.6 Hz, H-1A), 2.84 (dd, 1H, J = 14.0, 7.1 Hz, H-1B), 2.04, 2.03, 2.02 (3 × s, 3 × 3H, OAc). 13C-NMR of 8bd (100 MHz, CDCl3) δ: 170.1, 170.0, 169.8, 169.6, 166.3, 165.5, 165.4 (CO), 133.5–128.4 (aromatic), 83.7, 77.6, 76.5, 73.4, 72.0, 70.1, 69.5, 68.6, 66.9, (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′), 65.4 (C-7), 63.1 (C-5′), 30.4 (C-1), 20.8 (OAc). ESI-MS positive mode (m/z): calcd for C46H44NaO16S+ [M + Na]+ = 907.2242. Found: [M + Na]+ = 907.2238.
4.1.4.9. 2,6-Anhydro-3,4,5,7-tetra-O-benzoyl-1-S-(2,3,4-tri-O-acetyl-β-D-xylopyranosyl)-1-thio-D-glycero-D-galacto-heptitol-S-oxide (9bd). Prepared from 4b (70 mg, 0.12 mmol) and 7d (40 mg, 0.13 mmol) using DPPA (3 × 3.1 mg, 0.012 mmol) in toluene (1.2 mL) according to Method D to give 9bd by column chromatography (eluent: hexane–acetone = 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (11 mg, 12%). Rf = 0.21 (hexane–acetone = 1
1) as a yellow amorphous solid (11 mg, 12%). Rf = 0.21 (hexane–acetone = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).1H-NMR of 9bd (400 MHz, CDCl3) δ: 8.13 (dd, 2H, J = 8.2, 1.1 Hz, aromatic), 8.05 (dd, 2H, J = 8.2, 1.2 Hz, aromatic), 7.92 (dd, 2H, J = 8.2, 1.3 Hz, aromatic), 7.78 (dd, 2H, J = 8.4, 1.3 Hz, aromatic), 7.65–7.22 (m, 12H, aromatic), 6.08 (pseudo t, 1H, J = 10.0 Hz, H-5), 5.87 (dd, 1H, J = 3.2, 0.9 Hz, H-3), 5.66 (dd, 1H, J = 10.1, 3.2 Hz, H-4), 5.27 (pseudo t, 1H, J = 8.2 Hz, H-2′), 5.21 (pseudo t, 1H, J = 8.2 Hz, H-3′), 4.92 (ddd, 1H, J = 9.0, 8.2, 5.1 Hz, H-4′), 4.74 (dd, 1H, J = 12.2, 2.5 Hz, H-7A), 4.55 (ddd, 1H, J = 10.7, 2.3, 0.9 Hz, H-2), 4.47 (dd, 1H, J = 12.2, 4.5 Hz, H-7B), 4.41 (d, 1H, J = 8.3 Hz, H-1′), 4.23–4.18 (m, 2H, H-6, 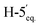 ), 3.47 (dd, 1H, J = 11.6, 9.0 Hz,
), 3.47 (dd, 1H, J = 11.6, 9.0 Hz, 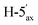 ), 3.17 (dd, 1H, J = 13.0, 10.7 Hz, H-1A), 3.04 (dd, 1H, J = 13.0, 2.3 Hz, H-1B), 2.03, 2.02, 1.96 (3 × s, 3 × 3H, OAc). ESI-MS positive mode (m/z): calcd for C46H44KO17S+ [M + K]+ = 939.1936. Found: [M + K]+ = 939.1934.
), 3.17 (dd, 1H, J = 13.0, 10.7 Hz, H-1A), 3.04 (dd, 1H, J = 13.0, 2.3 Hz, H-1B), 2.03, 2.02, 1.96 (3 × s, 3 × 3H, OAc). ESI-MS positive mode (m/z): calcd for C46H44KO17S+ [M + K]+ = 939.1936. Found: [M + K]+ = 939.1934.
4.1.4.10. 2,6-Anhydro-3,4,5,7-tetra-O-benzoyl-1-S-(1,2:3,4-di-O-isopropylidene-β-D-galactopyranos-6-yl)-1-thio-D-glycero-D-galacto-heptitol (8be). Prepared from 4b (50 mg, 0.08 mmol) and 7e (24 mg, 0.088 mmol) using DPPA (3 × 2.1 mg, 0.008 mmol) in toluene (0.8 mL) according to Method D to give 8be by column chromatography (eluent: hexane–acetone = 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (51 mg, 73%). Rf = 0.35 (hexane–EtOAc = 2
1) as a yellow amorphous solid (51 mg, 73%). Rf = 0.35 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −22.3 (c = 0.160, CHCl3).
1). [α]D = −22.3 (c = 0.160, CHCl3).1H-NMR of 8be (400 MHz, CDCl3) δ: 8.11 (dd, 2H, J = 8.4, 1.4 Hz, aromatic), 8.04 (dd, 2H, J = 8.1, 1.0 Hz, aromatic), 7.92 (dd, 2H, J = 8.0, 0.9 Hz, aromatic), 7.78 (dd, 2H, J = 8.0, 0.9 Hz, aromatic), 7.62–7.22 (m, 12H, aromatic), 6.03 (dd, 1H, J = 3.2, 0.7 Hz, H-3), 6.01 (pseudo t, 1H, J = 10.0 Hz, H-5), 5.68 (dd, 1H, J = 10.0, 3.2 Hz, H-4), 5.52 (d, 1H, J = 5.0 Hz, H-1′), 4.73 (dd, 1H, J = 12.2, 2.7 Hz, H-7A), 4.58 (dd, 1H, J = 7.9, 2.4 Hz, H-3′), 4.46 (dd, 1H, J = 12.2, 4.6 Hz, H-7B), 4.28 (dd, 1H, J = 5.0, 2.4 Hz, H-2′), 4.24 (dd, 1H, J = 7.9, 1.8 Hz, H-4′), 4.23 (m, 1H, H-2), 4.15 (ddd, 1H, J = 10.0, 4.6, 2.7 Hz, H-6), 3.86 (ddd, 1H, J = 7.6, 5.8, 1.8 Hz, H-5′), 2.91 (dd, 1H, J = 14.1, 7.0 Hz, H-1A), 2-86-2.74 (m, 3H, H-1B, 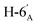 ,
, 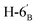 ), 1.46, 1.41, 1.29, 1.27 (4 × s, 4 × 3H, CH3). 13C-NMR of 8be (100 MHz, CDCl3) δ: 166.3, 165.7, 165.6 (CO), 133.5–128.4 (aromatic), 109.4, 108.7 (Cacetal), 96.7 (C-1′), 78.2, 76.5, 73.3, 72.0, 71.0, 70.6, 70.0, 68.3, 67.3 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 63.5 (C-7), 33.4 (C-1), 33.0 (C-6′), 26.2, 26.1, 25.0, 24.5 (CH3). ESI-MS positive mode (m/z): calcd for C47H48NaO14S+ [M + Na]+ = 891.2657. Found: [M + Na]+ = 891.2659.
), 1.46, 1.41, 1.29, 1.27 (4 × s, 4 × 3H, CH3). 13C-NMR of 8be (100 MHz, CDCl3) δ: 166.3, 165.7, 165.6 (CO), 133.5–128.4 (aromatic), 109.4, 108.7 (Cacetal), 96.7 (C-1′), 78.2, 76.5, 73.3, 72.0, 71.0, 70.6, 70.0, 68.3, 67.3 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 63.5 (C-7), 33.4 (C-1), 33.0 (C-6′), 26.2, 26.1, 25.0, 24.5 (CH3). ESI-MS positive mode (m/z): calcd for C47H48NaO14S+ [M + Na]+ = 891.2657. Found: [M + Na]+ = 891.2659.
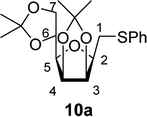
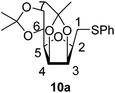
4.1.4.11. 2,5-Anhydro-3,4:6,7-di-O-isopropylidene-1-S-phenyl-1-thio-D-glycero-D-galacto-heptitol (10a). Prepared from 6 (60 mg, 0.23 mmol) and 7a (230 μL, 2.3 mmol) using DPPA (4 × 5.9 mg, 0.023 mmol) in toluene (2.3 mL) at room temperature according to Method D to give 10a by column chromatography (eluent: hexane–acetone = 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (45 mg; 53%).
1) as a yellow amorphous solid (45 mg; 53%).Prepared from 6 (56 mg, 0.22 mmol) and 7a (220 μL, 2.2 mmol) using DPPA (2 × 5.6 mg, 0.022 mmol) in toluene (2.2 mL) at −78 °C according to Method D to give 10a by column chromatography (eluent: hexane–acetone = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow amorphous solid (56 mg; 69%). Rf = 0.44 (hexane–EtOAc = 2
1) as a yellow amorphous solid (56 mg; 69%). Rf = 0.44 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = +39.9 (c = 0.172, CHCl3).
1). [α]D = +39.9 (c = 0.172, CHCl3).
1H-NMR of 10a (400 MHz, CDCl3) δ: 7.39 (dd, 2H, J = 8.3, 1.2 Hz, aromatic), 7.30–7.26 (m, 2H, aromatic), 7.21–7.17 (m, 1H, aromatic), 4.76–4.72 (m, 2H, H-3, H-4), 4.38 (ddd, 1H, J = 7.5, 6.0, 4.7 Hz, H-6), 4.07 (dd, 1H, J = 8.7, 6.0 Hz, H-7A), 4.03 (dd, 1H, J = 8.7, 4.7 Hz, H-7B), 3.67 (ddd, 1H, J = 7.7, 6.1, 2.9 Hz, H-2), 3.49 (dd, 1H, J = 7.5, 2.9 Hz, H-5), 3.24 (dd, 1H, J = 13.5, 6.1 Hz, H-1A), 3.22 (dd, 1H, J = 13.5, 7.7 Hz, H-1B), 1.49, 1.43, 1.37, 1.34 (4 × s, 4 × 3H, CH3). 13C-NMR of 10a (100 MHz, CDCl3) δ: 136.0–126.3 (aromatic), 112.7, 109.2 (Cacetal), 81.8, 80.8, 80.6, 80.6, 73.2 (C-2, C-3, C-4, C-5, C-6), 66.9 (C-7), 31.4 (C-1), 27.1, 25.9, 25.3, 24.7 (CH3). ESI-MS positive mode (m/z): calcd for C19H27O5S+ [M + H]+ = 367.0987. Found: [M + H]+ = 367.1012.
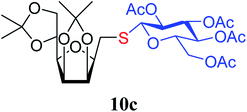
4.1.4.12. 2,5-Anhydro-3,4:6,7-di-O-isopropylidene-1-S-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)-1-thio-D-glycero-D-galacto-heptitol (10c). Prepared from 6 (79 mg, 0.31 mmol) and 7c (135 mg, 0.37 mmol) using DPPA (3 × 7.9 mg, 0.031 mmol) in toluene (3.1 mL) according to Method D to give 10c by column chromatography (eluent: hexane–acetone = 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a colorless syrup (168 mg; 88%). Rf = 0.28 (hexane–EtOAc = 2
1) as a colorless syrup (168 mg; 88%). Rf = 0.28 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −6.4 (c = 0.200, CHCl3).
1). [α]D = −6.4 (c = 0.200, CHCl3).1H NMR of 10c (400 MHz, CDCl3) δ: 5.21 (pseudo t, 1H, J = 9.3 Hz, H-3′), 5.08 (pseudo t, 1H, J = 9.7 Hz, H-4′), 5.07 (pseudo t, 1H, J = 9.6 Hz, H-2′), 4.79–4.73 (m, 2H, H-3, H-4), 4.61 (d, 1H, J = 10.1 Hz, H-1′), 4.38 (dt, 1H, J = 6.1, 5.0 Hz, H-6), 4.24 (dd, 1H, J = 12.4, 5.0 Hz, 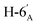 ), 4.14 (dd, 1H, J = 12.4, 2.2 Hz,
), 4.14 (dd, 1H, J = 12.4, 2.2 Hz, 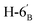 ), 4.12–4.00 (m, 2H, H-7A, H-7B), 3.77 (ddd, 1H, J = 8.5, 5.2, 2.8 Hz, H-2), 3.70 (ddd, 1H, J = 10.1, 5.0, 2.2 Hz, H-5′), 3.55 (dd, 1H, J = 7.2, 2.8 Hz, H-5), 3.07 (dd, 1H, J = 13.8, 8.5 Hz, H-1A), 2.85 (dd, 1H, J = 13.8, 5.2 Hz, H-1B), 2.09 2.06, 2.03, 2.01 (4 × s, 4 × 3H, OAc), 1.47, 1.44, 1.37, 1.36 (4 × s, 4 × 3H, CH3). 13C NMR of 10c (100 MHz, CDCl3) δ:170.7, 170.4, 169.6, 169.5 (CO), 112.7, 109.1 (Cacetal), 84.2, 82.1, 81.8, 80.8, 80.7, 76.0, 74.0, 73.2, 70.2, 68.4 (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′, C-5′), 66.9 (C-7), 62.2 (C-6′), 28.4 (C-1), 27.0, 25.9, 25.3, 24.9 (OAc), 20.9, 20.8, 20.7, 20.7 (CH3). Elemental analysis: calcd for C27H40O14S (620.663): C: 52.25; H: 6.50; S: 5.17. Found: C: 50.28; H: 6.66; S: 5.12.
), 4.12–4.00 (m, 2H, H-7A, H-7B), 3.77 (ddd, 1H, J = 8.5, 5.2, 2.8 Hz, H-2), 3.70 (ddd, 1H, J = 10.1, 5.0, 2.2 Hz, H-5′), 3.55 (dd, 1H, J = 7.2, 2.8 Hz, H-5), 3.07 (dd, 1H, J = 13.8, 8.5 Hz, H-1A), 2.85 (dd, 1H, J = 13.8, 5.2 Hz, H-1B), 2.09 2.06, 2.03, 2.01 (4 × s, 4 × 3H, OAc), 1.47, 1.44, 1.37, 1.36 (4 × s, 4 × 3H, CH3). 13C NMR of 10c (100 MHz, CDCl3) δ:170.7, 170.4, 169.6, 169.5 (CO), 112.7, 109.1 (Cacetal), 84.2, 82.1, 81.8, 80.8, 80.7, 76.0, 74.0, 73.2, 70.2, 68.4 (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′, C-5′), 66.9 (C-7), 62.2 (C-6′), 28.4 (C-1), 27.0, 25.9, 25.3, 24.9 (OAc), 20.9, 20.8, 20.7, 20.7 (CH3). Elemental analysis: calcd for C27H40O14S (620.663): C: 52.25; H: 6.50; S: 5.17. Found: C: 50.28; H: 6.66; S: 5.12.
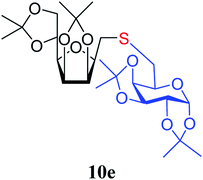
4.1.4.13. 2,5-Anhydro-3,4:6,7-di-O-isopropylidene-1-S-(1,2:3,4-di-O-isopropylidene-β-D-galactopyranose-6-yl)-1-thio-D-glycero-D-galacto-heptitol (10e). Prepared from 6 (111 mg, 0.40 mmol) and 7e (100 mg, 0.36 mmol) using DPPA (3 × 10.3 mg, 0.040 mmol) in toluene (4.0 mL) according to Method D to give 10e by column chromatography (eluent: hexane–acetone = 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a colorless syrup (154 mg; 81%). Rf = 0.36 (hexane–EtOAc = 2
1) as a colorless syrup (154 mg; 81%). Rf = 0.36 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −28.7 (c = 0.300, CHCl3).
1). [α]D = −28.7 (c = 0.300, CHCl3).1H NMR of 10e (400 MHz, CDCl3) δ: 5.53 (d, 1H, J = 5.0 Hz, H-1′), 4.77–4.70 (m, 2H, H-3, H-4), 4.62 (dd, 1H, J = 7.9, 2.2 Hz, H-3′), 4.35–4.28 (m, 2H, H-2′, H-4′), 4.38 (dd, 1H, J = 12.1, 6.1 Hz, H-6), 4.12–4.01 (m, 2H, H-7A, H-7B), 3.87 (pseudo t, 1H, J = 6.8 Hz, H-2), 3.71 (td, 1H, J = 6.8, 2.8 Hz, H-5′), 3.51 (dd, 1H, J = 7.6, 2.9 Hz, H-5), 2.95–2.78 (m, 4H, H-1A, H-1B, 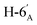 ,
, 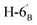 ), 1.53, 1.45, 1.44, 1.37, 1.35, 1.33 (8 × s, 8 × 3H, CH3). 13C NMR of 10e (100 MHz, CDCl3) δ: 112.5, 109.3, 109.1, 108.6 (Cacetal), 96.7 (C-1′), 82.1, 81.9, 80.9, 80.6, 73.1, 71.7, 71.0, 70.6, 67.6 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 67.0 (C-7′), 32.8 (C-6′) 30.4 (C-1), 27.0, 26.2, 26.1, 25.8, 25.3, 25.0, 24.7, 24.5 (CH3). Elemental analysis: calcd for C25H40O10S (532.65): C: 56.37; H: 7.57; S: 6.02. Found: C: 58.31; H: 7.75; S: 5.94.
), 1.53, 1.45, 1.44, 1.37, 1.35, 1.33 (8 × s, 8 × 3H, CH3). 13C NMR of 10e (100 MHz, CDCl3) δ: 112.5, 109.3, 109.1, 108.6 (Cacetal), 96.7 (C-1′), 82.1, 81.9, 80.9, 80.6, 73.1, 71.7, 71.0, 70.6, 67.6 (C-2, C-3, C-4, C-5, C-6, C-2′, C-3′, C-4′, C-5′), 67.0 (C-7′), 32.8 (C-6′) 30.4 (C-1), 27.0, 26.2, 26.1, 25.8, 25.3, 25.0, 24.7, 24.5 (CH3). Elemental analysis: calcd for C25H40O10S (532.65): C: 56.37; H: 7.57; S: 6.02. Found: C: 58.31; H: 7.75; S: 5.94.
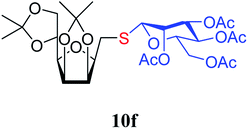
4.1.4.14. 2,5-Anhydro-3,4:6,7-di-O-isopropylidene-1-S-(2,3,4,6-tetra-O-acetyl-β-D-mannopyranosyl)-1-thio-D-glycero-D-galacto-heptitol (10f). Prepared from 6 (123 mg, 0.48 mmol) and 7f (210 mg, 0.58 mmol) using DPPA (3 × 12.3 mg, 0.048 mmol) in toluene (4.8 mL) according to Method D to give 10f by column chromatography (eluent: hexane–acetone = 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a colorless syrup (168 mg; 88%). Rf = 0.34 (hexane–EtOAc = 2
1) as a colorless syrup (168 mg; 88%). Rf = 0.34 (hexane–EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). [α]D = −15.8 (c = 0.300, CHCl3).
1). [α]D = −15.8 (c = 0.300, CHCl3).1H NMR of 10f (400 MHz, CDCl3) δ: 5.53 (d, 1H, J = 2.5 Hz, H-3), 5.25 (pseudo t, 1H, J = 10.0 Hz, H-4′), 5.04 (dd, 1H, J = 10.1, 3.4 Hz, H-2′), 4.92 (s, 1H, H-1′), 4.80–4.74 (m, 2H, H-3, H-4), 4.38 (dd, 1H, J = 11.7, 6.2 Hz, H-6), 4.26 (dd, 1H, J = 12.3, 5.9 Hz, 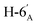 ), 4.15 (dd, 1H, J = 12.3, 1.9 Hz,
), 4.15 (dd, 1H, J = 12.3, 1.9 Hz, 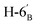 ), 4.10–3.98 (m, 2H, H-7A, H-7B), 3.78–3.71 (m, 1H, H-2), 3.70–3.64 (m, 1H, H-5′), 3.55 (dd, 1H, J = 6.9, 2.2 Hz, H-5), 3.04 (dd, 1H, J = 13.6, 9.1 Hz, H-1A), 2.94 (dd, 1H, J = 13.6, 5.1 Hz, H-1B), 2.19, 2.08, 2.05, 1.98 (4 × s, 4 × 3H, OAc), 1.47, 1.44, 1.37, 1.35 (4 × s, 4 × 3H, CH3). 13C NMR of 10f (100 MHz, CDCl3) δ: 170.7, 170.3, 170.2, 169.7 (CO), 112.7, 109.1 (Cacetal), 83.6, 81.9, 80.7, 80.5, 76.7, 73.2, 72.0, 70.4, 65.9 (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′, C-5′), 66.8 (C-7), 63.0 (C-6′), 29.4 (C-1), 27.0, 25.8, 25.3, 24.8 (OAc), 20.9, 20.8, 20.8, 20.7 (CH3). Elemental analysis: calcd for C27H40O14S (620.66): C: 52.25; H: 6.50; S: 5.17. Found: C: 52.06; H: 6.26; S: 5.21.
), 4.10–3.98 (m, 2H, H-7A, H-7B), 3.78–3.71 (m, 1H, H-2), 3.70–3.64 (m, 1H, H-5′), 3.55 (dd, 1H, J = 6.9, 2.2 Hz, H-5), 3.04 (dd, 1H, J = 13.6, 9.1 Hz, H-1A), 2.94 (dd, 1H, J = 13.6, 5.1 Hz, H-1B), 2.19, 2.08, 2.05, 1.98 (4 × s, 4 × 3H, OAc), 1.47, 1.44, 1.37, 1.35 (4 × s, 4 × 3H, CH3). 13C NMR of 10f (100 MHz, CDCl3) δ: 170.7, 170.3, 170.2, 169.7 (CO), 112.7, 109.1 (Cacetal), 83.6, 81.9, 80.7, 80.5, 76.7, 73.2, 72.0, 70.4, 65.9 (C-2, C-3, C-4, C-5, C-6, C-1′, C-2′, C-3′, C-4′, C-5′), 66.8 (C-7), 63.0 (C-6′), 29.4 (C-1), 27.0, 25.8, 25.3, 24.8 (OAc), 20.9, 20.8, 20.8, 20.7 (CH3). Elemental analysis: calcd for C27H40O14S (620.66): C: 52.25; H: 6.50; S: 5.17. Found: C: 52.06; H: 6.26; S: 5.21.
Funding
This research was funded by the National Research, Development and Innovation Office of Hungary (Grant K109450, K132870 and FK128766), and by the EU co-financed by the European Regional Development Fund under the projects GINOP-2.3.2-15-2016-00008 and GINOP-2.3.3-15-2016-00004.Author contributions
JJ: synthesis and structure elucidation of compounds (1a, b – 4a, b; every adduct except 8ac, 8ae, 10c, 10a, 10f) wrote the manuscript; ND: synthesis and structure elucidation of compounds (8ac, 8ae); ED: synthesis and structure elucidation of compounds (10c, 10e 10f); AB: planned and controlled the experiments, structure elucidations (8ac, 8ae, 10c, 10a, 10f) and reviewed manuscript; LJ: planned and controlled the experiments, structure elucidations, wrote the manuscript; SL: conceived the research and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.References
- V. Sharma, M. Ichikawa and H. H. Freeze, Biochem. Biophys. Res. Commun., 2014, 453, 220–228 CrossRef CAS.
- M. Loibl and S. Strahl, Biochim. Biophys. Acta, 2013, 1833, 2438–2446 CrossRef CAS.
- Y. Ihara, Y. Inai and M. Ikezaki, Trends Glycosci. Glycotechnol., 2011, 23, 1–13 CrossRef CAS.
- I. Loke, D. Kolarich, N. H. Packer and M. Thaysen-Andersen, Mol. Aspects Med., 2016, 51, 31–55 CrossRef CAS.
- B. Kranjčec, D. Papeš and S. Altarac, World J. Urol., 2014, 32, 79–84 CrossRef.
- V. Sharma, J. Nayak, C. DeRossi, A. Charbono, M. Ichikawa, B. G. Ng, E. Grajales-Esquivel, A. Srivastava, L. Wang, P. He, D. A. Scott, J. Russell, E. Contreras, C. M. Guess, S. Krajewski, K. D. Rio-Tsonis and H. H. Freeze, FASEB J., 2014, 28, 1854–1869 CrossRef.
- N. Freinkel, N. J. Lewis, S. Akazawa, S. I. Roth and L. Gorman, N. Engl. J. Med., 1984, 310, 223–230 CrossRef CAS.
- M. Scorza, R. Liguori, A. Elce, F. Salvatore and G. Castaldo, Clin. Chim. Acta, 2015, 451, 78–81 CrossRef CAS.
- D. L. Worthley, P. G. Bardy and C. G. Mullighan, Int. Med. J, 2005, 35, 548–555 CrossRef CAS.
- L. Cai, Z. Gu, J. Zhong, D. Wen, G. Chen, L. He, J. Wu and Z. Gu, Drug Discovery Today, 2018, 23, 1126–1138 CrossRef CAS.
- P. Bach, M. Bols, A. Damsgard, S. U. Hansen, L. Lohse, N. Asano, H. Hashimoto, Y. Kobayashi, P. Hadwiger, A. E. Stütz, T. M. Wrodnigg, J.-M. Beau, B. Vauzeilles, T. Skrydstrup and H. P. Wessel, in Glycoscience: Chemistry and Chemical Biology, ed. B. O. Fraser-Reid, K. Tatsuta and J. Thiem, Springer, Berlin, Heidelberg, 2001, vol. III, pp. 2533–2752 Search PubMed.
- V. Kelemen and A. Borbás, in Recent Trends in Carbohydrate Chemistry, ed. A. P. Rauter, B. E. Christensen, L. Somsák, P. Kosma and R. Adamo, Elsevier, 2020, pp. 161–215, DOI:10.1016/B978-0-12-817467-8.00005-0.
- K. Kitamura, Y. Ando, T. Matsumoto and K. Suzuki, Chem. Rev., 2018, 118, 1495–1598 CrossRef CAS.
- Y. Yang and B. Yu, Chem. Rev., 2017, 117, 12281–12356 CrossRef CAS.
- É. Bokor, S. Kun, D. Goyard, M. Tóth, J.-P. Praly, S. Vidal and L. Somsák, Chem. Rev., 2017, 117, 1687–1764 CrossRef.
- M. Choumane, A. Banchet, N. Probst, S. Gérard, K. Plé and A. Haudrechy, C. R. Chim., 2011, 14, 235–273 CrossRef CAS.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS.
- A. B. Lowe, Polym. Chem., 2014, 5, 4820–4870 RSC.
- F. Denes, M. Pichowicz, G. Povie and P. Renaud, Chem. Rev., 2014, 114, 2587–2693 CrossRef CAS.
- A. K. Sinha and D. Equbal, Asian J. Org. Chem., 2019, 8, 32–47 CrossRef CAS.
- A. Borbás, Chem.–Eur. J., 2020, 26, 6090–6101 CrossRef.
- J. Gervay, T. M. Flaherty and D. Holmes, Tetrahedron, 1997, 53, 16355–16364 CrossRef CAS.
- L. Lázár, M. Csávás, Á. Hadházi, M. Herczeg, M. Tóth, L. Somsák, T. Barna, P. Herczegh and A. Borbás, Org. Biomol. Chem., 2013, 11, 5339–5350 RSC.
- J. József, L. Juhász, T. Z. Illyés, M. Csávás, A. Borbás and L. Somsák, Carbohydr. Res., 2015, 413, 63–69 CrossRef.
- J. József, L. Juhász and L. Somsák, New J. Chem., 2019, 43, 5670–5686 RSC.
- H. Liao, J. Ma, H. Yao and X.-W. Liu, Org. Biomol. Chem., 2018, 16, 1791–1806 RSC.
- C. Taillefumier and Y. Chapleur, Chem. Rev., 2004, 104, 263–292 CrossRef CAS.
- C. J. M. Frédéric and S. P. Vincent, Tetrahedron, 2018, 74, 6512–6519 CrossRef.
- T. V. RajanBabu and G. S. Reddy, J. Org. Chem., 1986, 51, 5458–5461 CrossRef CAS.
- A. M. Gomez, A. Pedregosa, S. Valverde and J. C. Lopez, Tetrahedron Lett., 2003, 44, 6111–6116 CrossRef CAS.
- D. Waschke, J. Thimm and J. Thiem, Org. Lett., 2011, 13, 3628–3631 CrossRef CAS.
- D. Gueyrard, R. Haddoub, A. Salem, N. Bacar and P. Goekjian, Synlett, 2005, 36, 520–522 CrossRef.
- B. Bourdon, M. Corbet, P. Fontaine, P. G. Goekjian and D. Gueyrard, Tetrahedron Lett., 2008, 49, 747–749 CrossRef CAS.
- M. Tóth and L. Somsák, J. Chem. Soc., Perkin Trans. 1, 2001, 942–943 RSC.
- M. Tóth, S. Kun, L. Somsák and D. Goyard, in Carbohydrate Chemistry: Proven Synthetic Methods, ed. P. Kovac, CRC Press, Boca Raton, 2012, vol. 1, pp. 367–375 Search PubMed.
- R. W. Myers and Y. C. Lee, Carbohydr. Res., 1986, 154, 145–163 CrossRef CAS.
- G. D. Kini, C. R. Petrie, W. J. Hennen, N. K. Dalley, B. E. Wilson and R. K. Robins, Carbohydr. Res., 1987, 159, 81–94 CrossRef CAS.
- L. Somsák and M. Szabó, J. Carbohydr. Chem., 1990, 9, 755–759 CrossRef.
- M. Rotella, M. Bezpalko, N. Piro, N. Lazzara, S. Kassel, D. Zubris and R. Giuliano, in Stereochemistry and Global Connectivity: The Legacy of Ernest L. Eliel Volume 1, American Chemical Society, 2017, ch. 8, vol. 1257, pp. 155–170 Search PubMed.
- M. Rotella, M. Giovine, W. Dougherty, W. Boyko, S. Kassel and R. Giuliano, Carbohydr. Res., 2016, 425, 40–42 CrossRef CAS.
- T. Kaszás, M. Tóth, S. Kun and L. Somsák, RSC Adv., 2017, 7, 10454–10462 RSC.
- R. Csuk and B. I. Glänzer, Tetrahedron, 1991, 47, 1655–1664 CrossRef CAS.
- D. Eszenyi, V. Kelemen, F. Balogh, M. Bege, M. Csávás, P. Herczegh and A. Borbás, Chem.–Eur. J., 2018, 24, 4532–4536 CrossRef CAS.
- V. Kelemen, M. Bege, D. Eszenyi, N. Debreczeni, A. Bényei, T. Stürzer, P. Herczegh and A. Borbás, Chem.–Eur. J., 2019, 25, 14555–14571 CrossRef CAS.
- M. Fiore, A. Marra and A. Dondoni, J. Org. Chem., 2009, 74, 4422–4425 CrossRef CAS.
- J. Dupuis, B. Giese, D. Rüegge, H. Fischer, H.-G. Korth and R. Sustmann, Angew. Chem., Int. Ed., 1984, 23, 896–898 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07115c |
| This journal is © The Royal Society of Chemistry 2020 |