Open Access Article
Open Access ArticleEffect of soybean milk fermented with Lactobacillus plantarum HFY01 isolated from yak yogurt on weight loss and lipid reduction in mice with obesity induced by a high-fat diet
Chong
Li†
abc,
Huilin
Liu†
d,
Jiao
Yang
ae,
Jianfei
Mu
abc,
Ranran
Wang
ae and
Xin
Zhao
 *abc
*abc
aChongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing 400067, China. E-mail: zhaoxin@cque.edu.cn; Tel: +86-23-6265-3650
bChongqing Engineering Research Center of Functional Food, Chongqing University of Education, Chongqing 400067, China
cChongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing 400067, China
dDepartment of Clinical Nutrition, Chongqing University Three Gorges Hospital, Chongqing 500101, China
eCollege of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China
First published on 15th September 2020
Abstract
Soybean milk fermented with Lactobacillus plantarum HFY01 (LP-HFY01) was used for weight and lipid reduction in mice with obesity induced by a high-fat diet. We evaluated the gastrointestinal tolerance in vitro, organ index, body fat rate, pathological changes, serum index, mRNA expression and changes of isoflavones in soybean milk. Results indicated that LP-HFY01 exhibited good tolerance to pH 3.0 artificial gastric juice (69.87 ± 0.04%) and 0.3% bile salt (15.94 ± 0.3%). LP-HFY01-fermented soybean milk reduced the body fat rate and liver index of obese mice (p < 0.05). Organ sections showed that LP-HFY01-fermented soybean milk improved fatty degeneration and liver cell damage caused by a high-fat diet. LP-HFY01-fermented soybean milk inhibited increases in low-density lipoprotein cholesterol (LDL-c), triglyceride (TG), alkaline phosphatase (AKP), and glutamic oxaloacetic transaminase (GOT) and the decrease in high-density lipoprotein cholesterol (HDL-c) in the serum of obese mice, and inhibited CCAAT/enhancer-binding protein-α (C/EBP-α) and peroxisome proliferator-activated receptor-γ (PPAR-γ) mRNA expression, as well as activated cuprozinc-superoxide dismutase (SOD1) and lipoprotein lipase (LPL) mRNA expression in the liver and epididymal fat of obese mice (p < 0.05). Daidzin, glycitin, daidzein, glycitein, genistein, and genistin contents in soybean milk were determined before and after fermentation by high-performance liquid chromatography (HPLC); the daidzin and genistin contents in the fermented soybean milk decreased, whereas the daidzein and genistein contents increased significantly. Therefore, the LP-HFY01-fermented soybean milk strongly inhibits obesity induced by a high-fat diet, and shows good potential for utilization.
1 Introduction
Obesity indicates that the long-term energy intake of the body is higher than energy consumption and disrupts the original balance of energy intake. Excessive energy is converted into fat for storage, which leads to the excessive accumulation of fat in the body, becoming overweight (BMI between 25–30 kg m−2), and resulting in abnormal human physiological functions or other diseases. Simple obesity refers to the “non-disease” form of obesity induced by higher energy intake than energy expenditure; it comprises about 95% of obesity cases. Obesity is mainly related to lifestyle (i.e., fast-paced and irregular), the westernization of diets (excessive intake of saturated fatty acids, animal protein and simple carbohydrates), and the lack of physical exercise.1,2Excess energy in the body is converted into triglycerides (TGs) and accumulates in subcutaneous adipocytes or visceral organs through oil droplets, causing liver diseases (e.g., nonalcoholic fatty liver disease or NAFLD) and cardiovascular diseases (e.g., hypertension, hyperlipidemia, coronary heart disease, and diabetes).3 The state of fat cells can be assessed by evaluating the quantity of oil droplets in each adipocyte or the size of fat cells. The World Health Organization listed obesity as a chronic disease in 1996. The data published by the International Agency for Research on Cancer (IARC) in 2002 also indicated that cancer such as esophageal cancer, colon cancer, and breast cancer are related to obesity.4,5 High levels of fatty acid and cholesterol caused by obesity can damage the immune system, reduce the phagocytic function of macrophages, decrease the anti-infective ability of the body, and cause the fatty degeneration of the posterior pituitary gland. Such an effect on the pituitary gland can reduce the secretion of growth and sex hormones, affecting the sexual maturity and growth and the development of the human body.6
People often reduce their energy intake by eating less high-fat and high-sugar foods and increase their physical activity to enhance their energy expenditure and thereby induce the body to achieve a negative energy balance to ultimately realize the effect of weight loss. However, both reduce energy intake and strengthen physical activity need long-term persistence, once the persistence fails, it can disrupt the negative energy balance in the body, preventing weight loss.7 Therefore, the use of different weight-loss drugs (orlistat, lorcaserin, phenylbutylamine, topiramate sustained-release capsules, etc.) has gradually been considered. However, the long-term use of weight-loss drugs can lead to diarrhea, nausea, insomnia, vomiting, and other adverse effects, as well as severe liver injury, acute kidney injury, headache, and other diseases.8,9 Alternatively, the potential risk for infection and the high cost discourage people from taking bariatric surgery (reducing the size of the stomach). To effectively lose weight and prevent diseases without toxic and side effects, natural products, such as tea polyphenols,10 Chinese herbal medicine,11 and probiotic fermented milk12 have been studied. Reports have indicated that probiotics or their fermented milk can reduce serum cholesterol and improve lipid metabolism.13 Therefore, research interest has been directed toward supplementation with probiotic fermentation products to alleviating and treating obesity.14
Soybean milk is an important plant protein resource for humans because of the high quality of plant protein and trace calcium. After fermentation, ingredients become diverse and the effect of weight loss and lipid reduction is improved.15 Probiotics refer to live bacteria preparations and their metabolites, which can improve the intestinal microecological balance and health of the host.16Lactobacillus plantarum can produce proteases, organic acids, and other substances that can decompose protein, dietary fiber, oligosaccharides, and other macromolecular substances in soybean into small molecules during fermentation of L. plantarum, and realize pre-digestion. Therefore, compared with unfermented soybean milk, L. plantarum-fermented soybean milk can increase the nutritional value of soybean products and degrade small molecular proteins, increase total amino acid content and small peptide content, promote the dual regulation of intestinal peristalsis, diarrhea, and constipation,17 and influences the decrease of blood lipid, improvement of non-alcoholic fatty liver, and reduction of cardiovascular and cerebrovascular diseases.18
In the current study, we selected L. plantarum KFY01 (LP-KFY01), a “probiotic” strain from yak yogurt. We used LP-KFY01 fermented soybean milk as an intervention for obesity induced by a high-fat diet in mice. We also explored the weight loss and lipid-lowering mechanisms of LP-HFY01-fermented soybean milk at the biochemical, molecular and gene levels.
2 Materials and methods
2.1 Strains and reagents
L. plantarum HFY01 (LP-HFY01) was isolated from the homemade traditional fermented yak yogurt collected from local herdsmen in Hongyuan County (Abe Tibetan and Qiang Autonomous Prefecture, Sichuan, China) and then deposited at China General Microbiological Culture Collection Center (CGMCC, Beijing, China) with the preservation number 16629. Local soybeans were obtained from Chongqing, China. Commercial Lactobacillus bulgaricus (LB) was provided by Beijing Beina Chuanglian Biotechnology Research Institute. L-Carnitine was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). The high-fat diet used in this study was customized by Chongqing ENSIWEIER Biotechnology Co., Ltd. (Chongqing, China).2.2 Preparation of soybean milk fermented with LP-HFY01
A single colony of LP-HFY01 was cultured in an MRS (BD, Franklin Lakes, NJ, USA) liquid culture tube (37 °C, 20 h) until the initial stages of stabilization. The LP-HFY01 strain was inoculated into an MRS liquid culture medium in accordance with the 2% inoculation amount. The strain was then cultured and activated for 2 generations (in a constant temperature water bath shaker at 37 °C). The MRS liquid culture medium containing bacteria after activation for two generations was removed and centrifuged at 4000 rpm for 10 min. The upper culture medium was poured out, and 0.9% normal saline of the same volume was added to prepare a bacterial suspension. The commercial L. bulgaricus treatment was also prepared using the aforementioned procedure.As much as 55.0 g of dry soybeans were soaked in 110.0 mL of water for 12 h; 110.0 mL of water was added to beat into soymilk. The soymilk was filtered into a conical flask by using double gauze, and the conical flask was placed in an autoclave, which was sterilized at 121 °C for 15 min. The sterilized soymilk was cooled on a superclean working table and then subpacked into 10.0 mL of sterilized centrifuge tubes. Each centrifuge tube was filled with 5.0 mL of soymilk and inoculated with 50.0 μL of activated bacterial stock solution. The centrifuge tube was sealed and placed in a 37 °C constant temperature incubator for fermentation for 12 h and then used for gavage the next day.
2.3 Evaluation of gastrointestinal tolerance in vitro
The pH value of the 100 mL artificial simulated gastric juice (0.35 g pepsin and 0.2 g sodium chloride) was adjusted to 3.0 with hydrochloric acid (1.0 mol L−1), filtered and disinfected (0.45 μm filter), centrifuged (4000 rpm, 10 min), washed, and resuspended with an equal amount of normal saline. Subsequently, 1.0 mL of bacterial solution was mixed with 9.0 mL of artificial gastric juice and then cultured in a shaking tube (37 °C, 150 rpm) for 3 h to count the colonies (0 and 3 h). Up to 100 μL of the bacterial solution was inoculated into 5 mL of MRS-THIO (0.3% (w/v) bile salt; 0.2% sodium thioglycolate); the medium without the inoculated bacterial solution was used as a control. After the incubation at 37 °C for 24 h, the absorbance (600 nm) was measured, and the survival rate was calculated.192.4 Experimental animal grouping and establishment of the obesity model
A total of 60 C57BL/6 mice were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). The mice were fed under the following conditions: humidity, 50% ± 20%; temperature, (22 °C ± 4 °C); duration, 1 week. They were then randomly divided into 6 groups of 10 mice each (normal group, model group, L-carnitine group, soymilk group, Lactobacillus plantarum HFY01-fermented soymilk group and Lactobacillus bulgaricus-fermented soymilk group), with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 male-to-female ratio. The daily diet and oral components of the experimental animals are listed in Tables 1 and 2. During the experiment, mice were given ad libitum access to food and water, and their bedding was changed 2 times per week. New high-fat feed was given daily to prevent the production of an odor (via fat oxidation) that might affect the normal intake of the mice. The experiment continued for 12 weeks. This study was conducted in accordance with the Declaration of Helsinki, and the research scheme was approved by the ethics committee of Chongqing Functional Food Collaborative Innovation Center, Chongqing University of Education, China (201903001b).
50 male-to-female ratio. The daily diet and oral components of the experimental animals are listed in Tables 1 and 2. During the experiment, mice were given ad libitum access to food and water, and their bedding was changed 2 times per week. New high-fat feed was given daily to prevent the production of an odor (via fat oxidation) that might affect the normal intake of the mice. The experiment continued for 12 weeks. This study was conducted in accordance with the Declaration of Helsinki, and the research scheme was approved by the ethics committee of Chongqing Functional Food Collaborative Innovation Center, Chongqing University of Education, China (201903001b).
| Product | Ingredient | Mass/% |
|---|---|---|
| Normal diet | Wheat flour | 25 |
| Oatmeal | 25 | |
| Corn flour | 25 | |
| Soybean flour | 10 | |
| Fish meal | 8 | |
| Hog bone powder | 4 | |
| Yeast powder | 2 | |
| Refined salt | 1 | |
| High-fat diet | High-fat diet was composed of 90% normal diet, 8.2% lard, 1.5% cholesterol and 0.3% bile salt | |
| a N: normal group; M: model group; L: L-carnitine group (medication group); LP: Lactobacillus plantarum HFY01-fermented soymilk group; LB: Lactobacillus bulgaricus-fermented soymilk group. S: soymilk group (unfermented soymilk group). | ||||||
|---|---|---|---|---|---|---|
| Groups | N | M | L | LP | LB | S |
| Daily diet | Normal diet | High-fat diet | ||||
| Daily oral components | Physiological saline | Physiological saline | L-Carnitine (200 mg kg−1) | LP-HFY01-fermented soymilk | LB-fermented soymilk | Soymilk |
| Oral dose | 0.1 mL/10 g | |||||
2.5 Collection of blood and tissue samples from mice
After feeding for 12 weeks, the mice were fasted but were free to drink water for 24 h. Blood was collected from the living eyeball, which was centrifuged at 4 °C for 15 min at 4000 rpm after they were allowed to clot in a refrigerator at 4 °C for 0.5 h. The upper serum was collected and stored in a cryogenic refrigerator at −80 °C. After cervical dislocation, the mice were dissected. The liver and epididymal fat were collected, washed, and weighed. Part of the fresh liver and epididymal fat was stored in a 10% neutral formalin solution for pathological determination. The remaining liver and epididymal fat were labeled and stored in a cryogenic refrigerator at −80 °C.2.6 Evaluation of organ index and body fat rate
Blood was collected from the living eyeball of each mouse. The liver and epididymal fat were dissected and obtained, washed with sterile water, dried using filter paper, and weighed to determine the organ index and body fat rate of the mice.2.7 Histological analyses
After the liver and epididymal fat tissues were fixed with 10% neutral formalin solution and dehydrated with 95% (v/v) ethanol, xylene was used to replace the ethanol in organs and tissues. Tissue sections were paraffin-embedded, cooled, and stained with hematoxylin and eosin. Pathological changes in the liver and fat were observed from low- to high-power magnification under an electron microscope, and photographs were taken. Image-Pro Plus 6.0 (Media Cybernetics, America) was used to analyze the significance of different pictures.2.8 Serum index determination
The serum samples of the mice were taken out of the ultralow-temperature refrigerator set at −80 °C, and the contents of LDL, HDL, TG, alkaline phosphatase (AKP), and GOT were determined using an enzyme labeling instrument, following the instructions provided with the kit (Nanjing Jiancheng Bioengineering Institute).2.9 Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) analysis
We studied the mRNA expression of CCAAT/enhancer-binding protein (C/EBP-α), cuprozinc-superoxide dismutase (SOD1), peroxisome proliferator-activated receptor (PPAR-γ), and lipoprotein lipase (LPL) (primer sequences in Table 3) in the liver and epididymal fat of the soybean milk fermented with LP-HFY01 to examine the mechanism underlying its weight loss and lipid-lowering effects. Initially, total RNA in the liver and epididymal fat of the mice was extracted using the TRIzol method. The concentration and purity of mRNA were then measured and adjusted to 1 μg mL−1, and the mRNA was reverse-transcripted to cDNA (Thermo Fisher Scientific). The effect of LP-HFY01-fermented soybean milk on the mRNA expression of related genes in the liver and epididymal fat of the mice was detected by RT-qPCR. The reaction conditions were as follows: for pre-denaturation, 95 °C for 30 s; for PCR reaction, 95 °C for 5 s and 60 °C for 30 s; 40 cycles. β-Actin was used as an internal reference gene, and 3 parallel experiments were conducted for each sample. Finally, the average of the 3 parallel experiments was calculated, and the mRNA expression of the target gene was calculated using the 2−ΔΔCT method.20| Primer name | Forward primer (3′ to 5′) | Reverse primer (3′ to 5′) |
|---|---|---|
| PPAR | CCTCAGGGTACCACTACGGAGT | GCCGAATAGTTC GCC GAA |
| SOD1 | AACCAGTTGTGTTGTCAGGAC | CCATGTTTTCTTAGAGTGAGG |
| LPL | AGGGCTCTGCCTGAGTTGTA | AGAAATCTCGAAGGCCTGGT |
| C/EBP-α | TGGAGAACAGCAACGAGTAC | GCAGTTGCCCATGGCCTTGAC |
| β-Actin | GGCATCACACTTTCTACAACG | GGCAGGAACATTAAAGGTTTC |
2.10 HPLC analysis
Daidzin, glycitin, daidzein, glycitein, genistein, and genistin were supplied by Beijing Putian Tongchuang Biotechnology Co., Ltd. (Beijing, China). Six kinds of soybean isoflavone standards were prepared using 70% methanol to obtain a standard solution with a concentration of 100 μg mL−1. The 6 standards were mixed at 40 μg mL−1. LP-HFY01-fermented soybean milk, LB-fermented soybean milk, and unfermented soybean milk were pre-frozen at −80 °C, and then lyophilized using a freeze-drying machine. The lyophilized products were dissolved again by adding 5 mL of 80% ethanol as the extraction solution. The ultrasonic power was set at 100 W at room temperature for 1 h. The supernatant was extracted by centrifugation (4 °C, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min). The extract was again placed in the freeze-drying machine and then sealed and stored at −80 °C. The 0.01 g fermented soybean milk extract was weighed accurately and dissolved in 5 mL of 70% methanol solution to prepare 2 mg mL−1 of the fermented soybean milk extract. After being passed through an organic filter measuring 0.22 μm, it was stored at a low temperature for subsequent use. The UltiMate 3000 HPLC system (Thermo Fisher Scientific) was used to analyze fermented soybean milk samples. The chromatographic column was an Accucore C18 column (2.6 μm, 4.6 × 150 mm; Thermo FisherScientific); the mobile phase A was acetonitrile, and the mobile phase B was water containing 0.5% acetic acid. The conditions were as follows: flow rate, 0.5 mL min; column temperature, 30 °C; detection wavelength, 260 nm; injection volume, 10 μL. The gradient elution conditions were as follows: 15% A (isocratic) pre-balance for 10 min; 15–35% A (linear gradient) at 0–28 min; 35–45% A (linear gradient) at 28–45 min; 45–90% A (linear gradient) at 45–47 min; and 90–15% A (linear gradient) at 47–60 min.
000 rpm, 10 min). The extract was again placed in the freeze-drying machine and then sealed and stored at −80 °C. The 0.01 g fermented soybean milk extract was weighed accurately and dissolved in 5 mL of 70% methanol solution to prepare 2 mg mL−1 of the fermented soybean milk extract. After being passed through an organic filter measuring 0.22 μm, it was stored at a low temperature for subsequent use. The UltiMate 3000 HPLC system (Thermo Fisher Scientific) was used to analyze fermented soybean milk samples. The chromatographic column was an Accucore C18 column (2.6 μm, 4.6 × 150 mm; Thermo FisherScientific); the mobile phase A was acetonitrile, and the mobile phase B was water containing 0.5% acetic acid. The conditions were as follows: flow rate, 0.5 mL min; column temperature, 30 °C; detection wavelength, 260 nm; injection volume, 10 μL. The gradient elution conditions were as follows: 15% A (isocratic) pre-balance for 10 min; 15–35% A (linear gradient) at 0–28 min; 35–45% A (linear gradient) at 28–45 min; 45–90% A (linear gradient) at 45–47 min; and 90–15% A (linear gradient) at 47–60 min.
2.11 Statistical analysis
The experimental data were statistically analyzed by one-way ANOVA with Duncan multivariate test (P < 0.05, significant) in SPSS 19.0. All experiments were conducted 3 times, and the values were expressed as mean ± SD.3 Results
3.1 Tolerance of artificial gastric juice and bile salt
The poor tolerance of probiotics to harsh environments such as gastric juice and high bile salts can affect their physiological activity in the body. We evaluated the survival rate and growth efficiency of LP-HFY01 in pH 3.0 artificial gastric juice and 0.3% bile salt (Table 4) and found that they were well tolerated by both.| Strain | Survival rate in pH 3.0 artificial gastric juice (%) | Growth efficiency in 0.3% bile salt (%) |
|---|---|---|
| a Survival rate (%) = (Na/Nb) × 100, where Nb is the number of live cells at 0 h, Na is the number of live cells at 3 h, and the unit is CFU mL−1. Bile tolerance rate (%) = (A1/A0) × 100, where A1 is OD containing 0.3% (w/v) bile salt, and A0 is OD without 0.3% (w/v) bile salt. | ||
| LP-HFY01 | 69.87 ± 0.04 | 15.94 ± 0.3 |
3.2 Analysis of organ index and body fat rate
As shown Table 5, the liver weight and liver index are lowest in the normal group among the six groups because of the constant intake of common feed; compared with the normal group, the model group has significantly higher liver weight and liver index because of the long-term intake of a high-fat feed; this result indicates that the experiment is successful. Compared with the model group, other intervention groups show controlled decreases in liver weight and liver index to varying degrees. The LP-HFY01-fermented soybean milk group has lower liver weight and liver index, compared with the LB-fermented soybean milk group and unfermented soybean milk group. Moreover, compared with the normal group, the model group shows significantly higher fat weight and body fat rate, indicating the successful establishment of the obesity model. This result also indicates that a high-fat diet can cause increase the epididymal fat in mice, leading to obesity. Compared with the model group, the LP-HFY01-fermented soybean milk group exhibit lower fat weight and body fat ratio, similarly, the LB-fermented soybean milk group has lower fat weight and body fat ratio, but the differences are not apparent. These results show that soybean milk fermented with LP-HFY01 can significantly reduce the liver index and body fat ratio in mice and exerts an effect most similar to that of L-carnitine.| Mouse groups | Body weight | Liver | Epididymal fat | ||
|---|---|---|---|---|---|
| Weight | Ratio (%) | Weight | Ratio (%) | ||
| a The calculation formula is as follows: organ index (%) = organ weight/body weight × 100; body fat rate (%) = fat mass/mouse weight × 100. a–e Mean values with different letters in the same bar graph are significantly different (p < 0.05) as determined using Duncan's multiple range test. | |||||
| Normal | 26.785 | 0.937 ± 0.83e | 3.5e | 0.374 ± 0.050d | 1.4d |
| Model | 30.842 | 1.2 ± 0.31a | 3.9a | 0.863 ± 0.062a | 2.8a |
| L-Carnitine | 27.71 | 0.997 ± 0.041d | 3.6d | 0.443 ± 0.061c | 1.6c |
| LP-HFY01-fermented soymilk | 28.387 | 1.051 ± 0.023c | 3.7c | 0.596 ± 0.029b | 2.1b |
| LB-fermented soymilk | 28.421 | 1.079 ± 0.091b | 3.8b | 0.767 ± 0.021ab | 2.7ab |
| Soymilk | 28.894 | 1.097 ± 0.135b | 3.8b | 0.809 ± 0.032a | 2.8a |
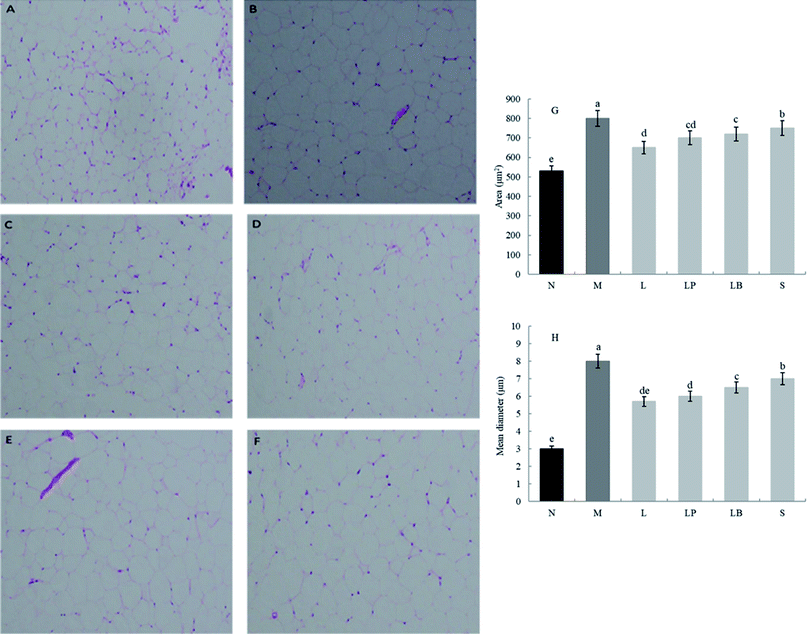
3.3 Histological analysis of epididymal fat and liver
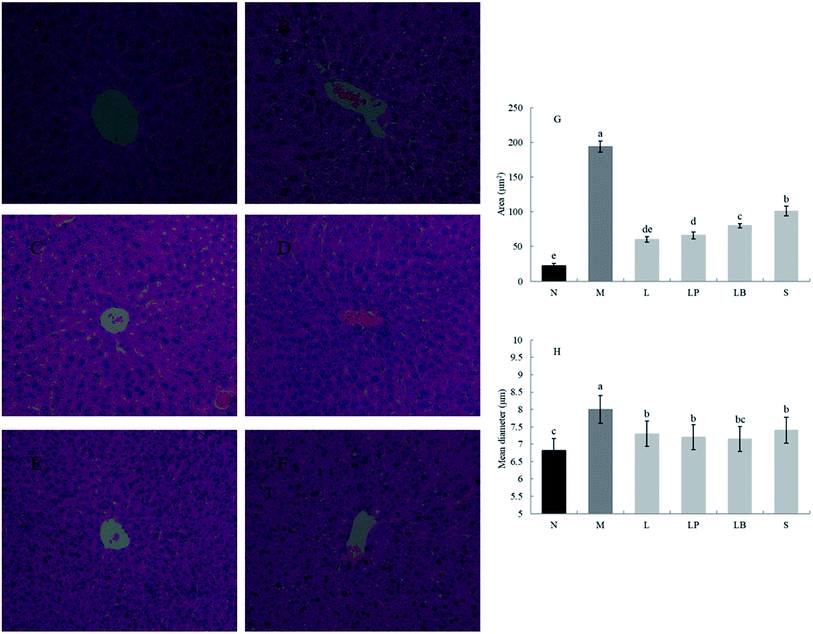
As shown in Fig. 1A–F, compared with the normal group, the model group has a significantly higher volume of adipose tissue cells. The other groups have significantly different sizes of adipose tissue cells, indicating that a high-fat diet can increase the volume of adipose cells, and gastric perfusion intervention can effectively change the size of adipose tissue cells. Compared with the model group, the L-carnitine group has a significantly lower cell volume, close to that of the normal group. L-Carnitine reduces the fat cell volume, according to existing experimental research;21 the fat cell volume of the LP-HFY01-fermented soymilk group is significantly reduced; the fat cell volumes of the unfermented soymilk group and LB-fermented soymilk group are reduced to a certain extent but Fig. 1G and H showed that the area and mean diameter of adipocytes in mice fed with high-fat diet were larger than those in other groups, but the area and mean diameter of adipocytes were decreased after different treatment groups (p < 0.05). The results show soybean milk fermented with LP-HFY01 can effectively improve the oxidative decomposition of fat tissue and inhibit the increase in fat cell volume.As shown in Fig. 2A–F, the liver in the normal group shows no pathological; no fat cavity is observed, the structure of the liver cells is complete and clear, and the arrangement is orderly. In the model group, many and small fat droplets are present in the liver; some large fat droplets are regular and round, indicating that the dynamic balance of energy is disrupted by the long-term intake of a high-fat diet in mice. Thus, preventing the complete decomposition of triglycerides taken in and storage of fat in the liver results in fatty liver. After gavage intervention, the number of fat cavities in the liver of the L-carnitine group was significantly reduced to a level close to that of the normal group, indicating that the drug treatment exerted a better therapeutic effect than model group. In the LP-HFY01-fermented group and LB-fermented group, the fatty lesions in the liver were alleviated. More fat droplets were observed in the unfermented soybean milk group. This finding suggests that compared with the unfermented soybean milk, LP-HFY01 and LB can improve the accumulation of fat more effectively. The reason could be that LP-HFY01 and LB can improve the composition of soymilk, promote the metabolism of fat, and reduce the accumulation of fat. The area of fat cavities in the liver was analyzed by Image Pro-Plus 6.0 software (Fig. 2G and H), and similar results were obtained (p < 0.05), but the mean diameter of fat cavities in different groups (except the model group) did not change significantly.
3.4 Detection of indexes in serum
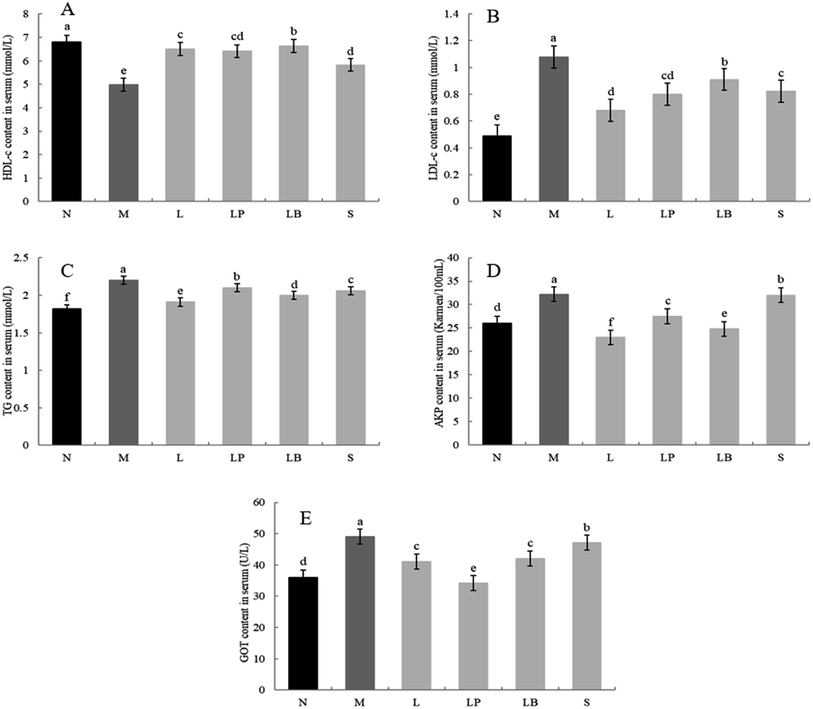
As shown in Fig. 3A, the HDL-c content is significantly higher in the normal group than in the other groups; moreover, it is significantly lower in the model group than in the other groups. Other gastric perfusion intervention groups can effectively improve the decrease in HDL-c caused by a high-fat diet. Compared with the LB-fermented soymilk group, the LP-HFY01-fermented soymilk group shows a significantly inhibited decrease in serum HDL-c, and the effect of LP-HFY01-fermented soymilk is similar to that of the L-carnitine.As shown in Fig. 3B, compared with the normal group, the model group has a significantly higher LDL content owing to the long-term intake of a high-fat diet. Compared with that in the model group, the LDL content in the L-carnitine group, LP-HFY01-fermented group, LB-fermented group and soymilk group are lower. The LP-HFY01-fermented soymilk can more effectively control the increase in LDL content in serum, compared with the unfermented soybean milk and LB-fermented soymilk. This finding suggests that fermentation with LP-HFY01 can inhibit the increase in LDL content in serum.
As shown in Fig. 3C, in contrast to the normal group, the model group exhibits TG accumulation in the liver, significantly increasing the TG content in the liver. This result suggests the successful establishment of the obesity model. When the LP-HFY01-fermented soymilk, L-carnitine, LB-fermented soymilk, and unfermented soymilk were administered via gavage, the blood damage caused by the high-fat diet was controlled to varying degrees. The L-carnitine group exhibited the largest increase in TG content in the serum. The effects on the unfermented soymilk group, LP-HFY01-fermented group, and LB-fermented group were evident but not as much as that on the L-carnitine group.
As shown in Fig. 3D, the serum AKP content is significantly higher in the model group than in the normal group, indicating that the long-term intake of a high-fat diet can cause liver damage in the model group. The AKP content is lower in the L-carnitine and LB groups than in the model group, even lower than that in the normal group. The increase in AKP content is more greatly inhibited in the LP-HFY01-fermented soymilk group than in the unfermented soymilk group. This result suggests that LP-HFY01-fermented soymilk can effectively reduce liver damage caused by the long-term intake of a high-fat diet.
As shown in Fig. 3E, the GOT enzyme activity in the model group is significantly higher than that in the normal group, indicating that long-term feeding of a high-fat feed can induce an increase in GOT enzyme activity in mouse serum. Compared with other treatment groups, the GOT activity in the LP-HFY01-fermented group decreased more significantly than that in the L-carnitine and LB-fermented groups; GOT content was close to the GOT content in the normal group. The results show that LP-HFY01-fermented soybean milk can inhibit liver damage, and the increase in GOT enzyme activity caused by long-term intake of a high-fat diet.
3.5 Expression of related genes in the liver and epididymal fat of mice
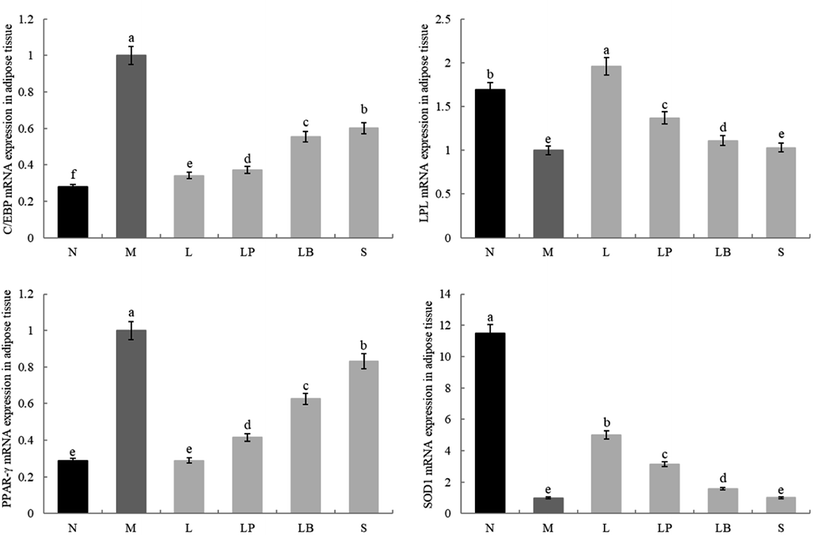
As shown in Fig. 4 and 5, the C/EBP-α and PPAR-γ gene expression in the liver and adipose tissue is significantly higher in the model group than in the normal group. Compared with the model group, the L-carnitine group showed reductions in PPAR-γ and C/EBP-α gene expression levels in the liver and adipose tissues of the intervention group to varying degrees and most significant reductions in the PPAR-γ gene expression levels in the liver and adipose tissues of L-carnitine group, close to the normal group. Compared with the LB-fermented and unfermented groups, the LP-HFY01-fermented group exhibited the largest decreases in the PPAR-γ gene expression in the liver and adipose tissues of the LP-HFY01-fermented group, indicating that fermentation with LP-HFY01 was more effective in regulating PPAR-γ gene expression. Fermentation with LP-HFY01 significantly inhibited the expression of the C/EBP-α gene in the liver and adipose tissues, and its effect was similar to that of L-carnitine. The LB-fermented soymilk and unfermented soymilk were weaker than the other treatments. This finding suggests that the LP-HFY01-fermented soymilk can effectively inhibit the increase in C/EBP-α gene expression caused by a high-fat diet.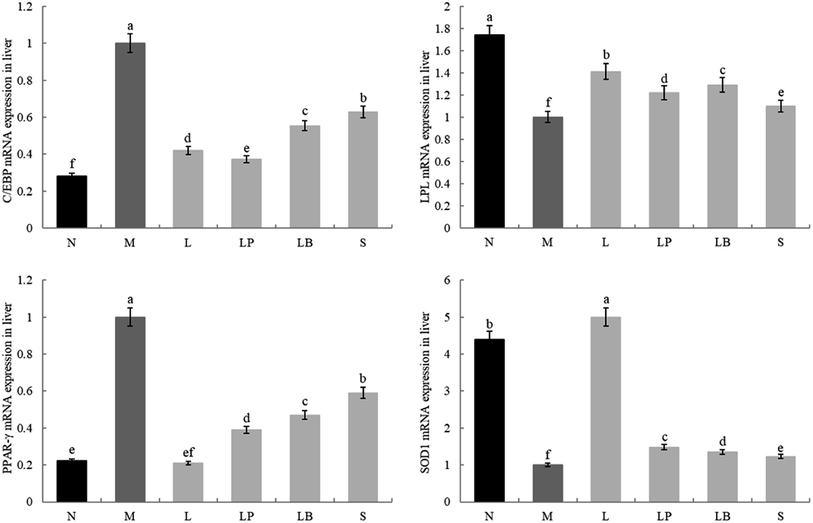 | ||
| Fig. 4 Gene expression in liver tissue. a–e Mean values with different letters in the same bar graph indicate significant differences (p < 0.05) as determined using Duncan's multiple range test. | ||
The LPL and SOD1 gene expression levels in the liver and adipose tissues in the model group were significantly lower than those in the normal group; compared with that in the model group, the LPL gene expression in the fat and liver tissues in the LP-HFY01- and LB-fermented groups increased to varying degrees; however, the increase in fat was weaker in the LB-fermented group than in the LP-HFY01-fermented group. The SOD1 gene expression in the liver and adipose tissues of the intervention groups, particularly the L-carnitine group, increased to varying degrees; SOD1 expression in the liver and adipose tissues was more strongly alleviated in the LP-HFY01-fermented group than in the unfermented and LB-fermented groups. This result suggests that LPL and SOD1 gene expression is more effectively regulated by LP-HFY01-fermented soybean milk.
3.6 HPLC analysis of the fermented and unfermented soybean milk
Fig. 6 presents a comparison of chromatograms, with the standard chromatogram as the reference (Fig. 6A). We identified 6 soybean isoflavone components from soybean milk, LB-fermented soybean milk, and LP-HFY01-fermented soybean milk: daidzin (peak 1, 5.590 min), glycitin (peak 2, 5.987 min), genistin (peak 3, 9.227 min), daidzein (peak 4, 16.940 min), glycitein (peak 5, 18.147 min), and genistein (peak 6, 24.193 min).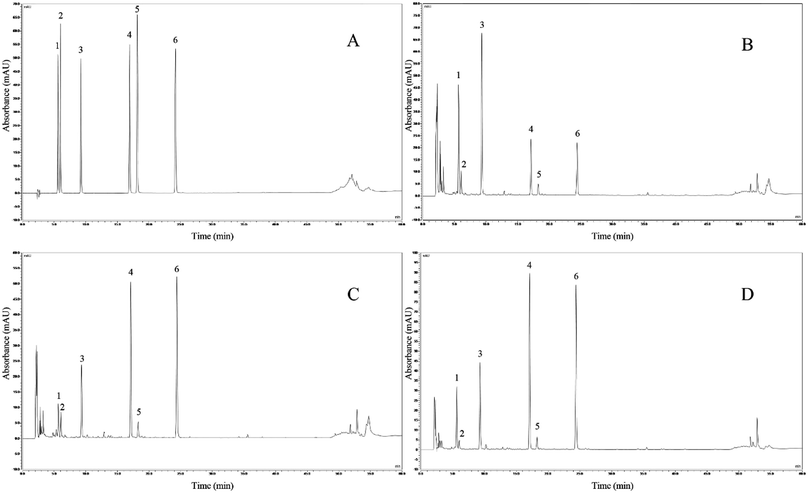 | ||
| Fig. 6 HPLC assay. (A) Mixed standard chromatograms; (B) soybean milk chromatograms; (C) LB-fermented soybean milk chromatograms; (D) LP-HFY01-fermented soybean milk chromatograms. | ||
Compared with soybean milk, the LP-HFY01- and LB-fermented soybean milk showed lower daidzin and genistin contents but higher daidzein and genistein contents. Changes in specific component content are listed in Table 6. These changes may be attributable to the fermentation characteristics of the probiotic LP and LB strains. The composition of the six soybean isoflavones is shown in Fig. 7.
| Samples | Daidzin | Glycitin | Genistin | Daidzein | Glycitein | Genistein |
|---|---|---|---|---|---|---|
| Soybean milk | 0.0376 | 0.0077 | 0.0603 | 0.0192 | 0.0057 | 0.0187 |
| LB-fermented soybean milk | 0.0023 | 0.0018 | 0.0053 | 0.0390 | 0.0014 | 0.0423 |
| LP-HFY01-fermented soybean milk | 0.0209 | 0.0026 | 0.0365 | 0.0707 | 0.0068 | 0.0678 |
 | ||
| Fig. 7 Chemical structures of the 6 isoflavones. (1) Daidzin; (2) glycitin; (3) genistin; (4) daidzein; (5) glycitein; (6) genistein. | ||
4 Discussion
A high-fat diet can induce TGs in animals to form fat and accumulate in adipocytes, leading to the swelling of adipocytes and an increase in weight, ultimately resulting in obesity. Obesity can not only enhance oxidative stress but also lead to chronic inflammation, which can easily induce the occurrence of metabolic syndrome, and its derivative complications such as diabetes, cardiovascular disease, and NAFLD are abnormalities of organs.22–24 As the source of various complications, obesity needs to be prevented and controlled to improve weight, reduce inflammation, and lower the risk for cancer. The literature shows that exogenous probiotics can improve obesity, cardiovascular disease, fat accumulation, abnormal glucose, and lipid metabolism, mainly by inhibiting the increase in serum total cholesterol, triglyceride, and other related blood lipid levels. L. plantarum is recognized as a safe microorganism, and numerous experiments have proved that it can not only degrade cholesterol in vitro but also regulate blood lipid in vivo.12,13,25When the normal human body feeds on large quantities of a high-fat diet, TGs accumulating in the liver cells cannot be completely decomposed and transported. Consequently; they form a round fat drop in the cells to create a fat cavity, damaging the normal structure of the liver cells, which then results in NAFLD.26 This condition is highly associated with obesity. Fat accumulation develops into chronic inflammation of fatty liver, although it can be reversed and recovered by fat metabolism; however, if diets and lifestyle habits are not modified, fatty liver inflammation may worsen and develop into liver fibrosis, cirrhosis, or liver failure.27 Studies have shown that L-carnitine treatment can effectively reduce the fat accumulation and fat cavities, as well as reduce the TG content in the liver.28 The organ index can reflect the health status of the mice, and the body fat rate can directly reflect fat content in mice, that is, the degree of obesity.29 The increase in serum LDL-c is the main risk factor for atherosclerotic cardiovascular and cerebrovascular diseases. The Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program (NECP) in 2001 recognized LDL-c as the cornerstone of blood lipid control and indicated that a decrease in LDL-c can reduce the risk for cardiovascular and cerebrovascular disease in the future.30 The HDL-c level is negatively correlated with the occurrence of coronary heart disease > HDL-c mainly acts as an anti-atherosclerosis by promoting reverse cholesterol transport, as well as anti-oxidant, anti-inflammatory, anti-thrombotic, and other mechanisms.31 AKP and GOT can reflect the degree of liver injury. AKP needs to pass through the liver and then be excreted by bile. Liver damage can cause abnormalities in the excretion process, which can induce high AKP levels; GOT is mainly distributed in the myocardium and then in the liver, skeletal muscles, and kidney tissues. When liver cells are damaged, the cell membrane permeability increases, and GOT in the cytoplasm is released into the blood; subsequently, its serum concentration rises, suggesting damage to the liver parenchyma.32–35 The results of the current study confirm that the high-fat diet-induced model group exhibits apparent obesity, visceral fat accumulation, fat cell enlargement, and significant increases in serum TG and LDL-c contents; meanwhile HDL-c content decreased significantly, indicating that the model was established successfully. In the high-fat diet model group, the epididymal adipocytes increased significantly, accumulation of fat droplets in the liver tissue, and significant increases in AKP and GOT, indicating that a high-fat diet led to significant liver damage in mice.36 In the experiment, unfermented soybean milk can reduce the TG, LDL-c, AKP, and GOT levels while increasing the HDL-c level to improve dyslipidemia, obesity, and liver injury in the model group. L. plantarum is proved to exert significant weight loss and lipid-lowering effects. The synergistic effect of the combined use of both is considerably greater than that of the single use of unfermented soybean milk, and the lipid-lowering effect is significantly enhanced. Meanwhile, the overall effect of the LP-HFY01-fermented soybean milk on weight loss is stronger than that of the LB-fermented soybean milk, which is closer to that of L-carnitine. This lipid-lowering effect, which could be attributable to the probiotic intervention, improved the intestinal flora structure, changed the intestinal permeability, and inhibited the absorption of a high-fat diet.37
Adipose tissue is a complex endocrine system, which secretes active factors and participates in the regulation of the neuro-endocrine–immune network; the abnormal differentiation of adipocytes can cause fat accumulation and leads to endocrine dysfunction of adipocytes, resulting in metabolic diseases.38 Two transcription factors closely related to adipocyte differentiation are the PPAR-γ and C/EBP, the expression of which is closely related to the development of obesity. Peroxisome proliferator-activated receptors are ligand-activated receptors in the nuclear hormone receptor family with 3 subtypes. PPAR-γ is the most adipose tissue-specific and plays an important role in adipocyte differentiation. It can regulate fat metabolism, inflammation, immunity, and cell differentiation. The C/EBP family consists of 3 members. C/EBP-α plays a key role in adipocyte differentiation. It highly expressed in the final stages of adipocyte differentiation. It is associated with PPAR-γ and can activate the expression of fat-specific genes to synthesize, extract, and store long-chain fatty acids, as well as to stop the proliferation of cells, showing a state of complete differentiation.39,40 The current study explored the two main genes that affect adipocyte differentiation. The results show that both LP-HFY01-fermented soybean milk and LB-fermented soybean milk tend to inhibit the growth of adipocytes, reduce the accumulation of intracellular lipids, and inhibit the expression of the genes PPAR-γ and C/EBP-α, which are related to adipocyte differentiation, at the mRNA level; however, the effect of the LP-HFY01-fermented soybean milk is better. LPL is an enzyme that catalyzes the hydrolysis of triglycerides linked to proteins, which are mainly derived from fat cells, muscle cells, and other parenchyma cells. Inactive monomer often changes into active dimer by glycosylation, which act on adipose tissue and striated muscle capillary lumen. They also hydrolyze triglycerides carried by chylous particles and very-low-density lipoprotein to produce fatty acids and monoacylglycerides, which are converted into low-molecular-weight fatty acids for energy supply of tissue oxidation or storage.41 Soybean milk fermented with L. plantarum HFY01 can significantly increase the LPL activity in epididymal adipose tissue and liver tissue, promote the transformation of triglycerides, alleviate the pathological changes in liver tissue. In addition, obesity can enhance the damage caused by oxidative stress. In the experiment, the SOD activity in the fat and liver tissue of the mice fed with a high-fat diet was significantly decreased, which led to adverse effects similar to damage to cell membrane integrity. After the intervention of LP-HFY01-fermented soybean milk, SOD activity in the liver and fat of the mice fed with a high-fat diet was significantly increased, and oxidative damage was improved to a certain extent.
Probiotics can be colonized in the host intestinal tract and reproductive system, which can produce definite health effects, improving the microecological balance of the host and providing benefits. L. plantarum, a kind of lactic acid bacteria, produces organic acid, bacteriocin, hydrogen peroxide, diacetyl, and other natural antibacterial substances after metabolism. These substances can maintain the balance of the intestinal flora, prevent the colonization of pathogenic bacteria and adhesion of toxins, improve the immunity of the organism, promote nutrient absorption and other functions, improve intestinal permeability, enhance immunity, reduce cholesterol level, reduce oxidative stress, relieve lactose intolerance, and inhibit the formation of tumor cells.42–46 The probiotic properties of Lactobacillus-fermented products consumed for an extended period can generally reduce cholesterol content in vivo, according to a study conducted among the Masai tribe of Africa by Mann and Spoerry in the 1970s.47 Yak yogurt is a naturally fermented dairy product in the Tibetan area. Owing to its special natural environment, specifically the large difference between milk fermented with microorganisms and ordinary fermented yogurt, yak yogurt has special flavor and quality; in addition, it contains rich nutritional components, which can inhibit oxidation, reduce cholesterol, and regulate immune response.48 Therefore, we studied yak yogurt in Aba Tibetan and Qiang Autonomous Prefecture of Sichuan Province. We isolated and identified the probiotics in yak yogurt, and labeled one of them as L. plantarum HFY01 (LP-HFY01). The experiment showed that LP-HFY01 exhibits good tolerance to gastric acid and bile salt in vitro and further studied the potential probiotic characteristics of LP-HFY01-fermented soybean milk.
A nutrient-rich plant protein resource, soybean is rich in protein, vitamins, unsaturated fatty acids, soybean isoflavones, and trace elements. The long-term use of soybean or soybean processing can prevent or alleviate cardiovascular disease, fatty liver disease and other diseases. Isoflavones and other bioactive components in common soybean exist in a combined form, which hinders its effective utilization by the body. However, the macromolecular substances in LP-HFY01-fermented soybean milk are hydrolyzed into small molecular substances, and vitamins and other nutrients are increased, facilitating its digestion and absorption by the body; the soybean milk fermented with LP-HFY01 can also remove the original beany smell and add soft acid taste and aroma, enhance the characteristics that render it edible, and produce organic acids that can effectively inhibit the reproduction of spoilage bacteria.49–51 Soybean isoflavones are also known as phytoestrogens for the similarity of their structure to animal estrogens. They affect hormone secretion, metabolic biological activity, protein synthesis, growth factor activity, and cancer chemoprevention.52 Ten kinds of soybean isoflavones have been identified, and these are mainly divided, based on structure, into free aglycones and combined glycosides. Daidzein, genistein, and glycitin belong to the former and exhibit high biological activity and high utilization efficiency. Meanwhile, daidzin and genistin belong to the latter group, characterized by a large molecular weight, hydrophilicity, and difficulty of absorption by the small intestine; however, intestinal microorganisms can degrade them into active and easily absorbed aglycones, although they are poorly hydrolyzed. The adverse situation can be improved by ingesting probiotics.53,54 In this experiment, after the soybean milk was fermented with LP-HFY01, the glycosidic daidzin and genistin (which were not effectively utilized by the body) were hydrolyzed, and aglycone daidzein and genistein (which were not easily absorbed by the body) increased. The fermentation of oral soybean milk with by LP-HFY01 not only provided rich nutrition for the body; it also facilitated β-glucosidase in the hydrolysis of the isoflavone glycoside structure, allowing its direct absorption by the small intestine. Consequently, the biological function of isoflavones was promoted, and their health care function was greatly enhanced. In addition, the changes in the intestinal flora composition are related to diet-induced obesity, insulin resistance, and diabetes. The intervention and regulation of host intestinal flora can selectively increase the number of intestinal probiotics in mice, adjust the balance of the intestinal flora, alleviate obesity and diabetes induced by a high-fat diet, as well as prevent and treat the effects of a metabolic disorder.55 Nutritional components in the unfermented soybean milk had high molecular weight and low digestibility. After being fermented with LP-HFY01 or LB, the digestibility of soybean milk improved and was efficiently used in the intestinal tract. Ingestion of probiotics or their fermented products could modify the colonization of relevant microorganisms in the intestinal tract, form a biological barrier with other anaerobic bacteria, inhibit the colonization of Enterobacter, Enterococcus, and other pathogenic bacteria, and adjust the intestinal microbial balance. It could also ferment in the colon to produce many short-chain fatty acids and regulate the metabolism of blood lipids.56
5 Conclusions
The experimental study showed that soybean milk fermented with L. plantarum HFY01 markedly alleviates high-fat induced obesity in mice. LP-HFY01-fermented soybean milk can reduce the body fat rate and liver index of obese mice, significantly inhibit the increase in serum TG, AKP, GOT, and HDL caused by a high-fat diet, decrease LDL, regulate lipid metabolism disorders and improve liver injury. LP-HFY01-fermented soybean milk can improve the decrease in SOD1 and LPL mRNA expression in the liver and adipose tissue of mice with obesity induced by a high-fat diet and inhibit the increase in the mRNA expression of C/EBP-α and PPAR-γ. It can also increase energy consumption, reduce oil formation, lower the weight gain caused by fat tissue, and influence weight loss, and promote the reproduction of intestinal probiotics. With a probiotic effect closest to that of L-carnitine, LP-HFY01 shows good potential for development and utilization.Conflicts of interest
No conflicts of interest in this article.Acknowledgements
This research was funded by Children's Research Institute of National Center for Schooling Development Programme and Chongqing University of Education (CSDP19FS01103) and the Science and Technology Research Program of Chongqing Municipal Education Commission (KJZD-K201901601), China.References
- G. A. Bray and J. Delany, Obes. Res., 1995, 3, 419–423 CrossRef PubMed.
- M. Labib, J. Clin. Pathol., 2003, 56, 17–25 CrossRef CAS PubMed.
- D. Y. Park, Y. T. Ahn, C. S. Huh, S. M. Jeon and M. S. Choi, J. Med. Food, 2011, 14, 670–675 CrossRef CAS.
- L. Yang and G. A. Colditz, JAMA Intern. Med., 2015, 175, 1412–1413 CrossRef.
- Y. Wang, J. Mi, X. Y. Shan, Q. J. Wang and K. Y. Ge, Int. J. Obes., 2007, 31, 177–188 CrossRef CAS PubMed.
- A. R. Saltiel and J. M. Olefsky, J. Clin. Invest., 2017, 127, 1–4 CrossRef.
- A. Urdampilleta, P. Gonzalez-Muniesa, M. P. Portillo and J. A. Martinez, J. Physiol. Biochem., 2012, 68, 289–304 CrossRef CAS PubMed.
- A. Astrup, Lancet, 2010, 376, 567–568 CrossRef PubMed.
- R. A. Adan, Trends Neurosci., 2013, 36, 133–140 CrossRef CAS.
- Y. Zhao and X. Zhang, J. Sci. Food Agric., 2020, 100, 897–903 CrossRef CAS PubMed.
- G. B. Lenon, K. X. Li, Y. H. Chang, A. W. Yang, C. Da Costa, C. G. Li, M. Cohen, N. Mann and C. C. Xue, J. Evidence-Based Complementary Altern. Med., 2012, 2012, 435702–435713 Search PubMed.
- Y. Y. Eiichiro Naito and K. Satoru, Biosci. Microbiota, Food Health, 2017, 37, 9–18 CrossRef PubMed.
- L. Santiago-López, A. Hernández-Mendoza, H. S. Garcia, V. Mata-Haro, B. Vallejo-Cordoba and A. F. González-Córdova, Int. J. Dairy Technol., 2015, 68, 153–165 CrossRef.
- M. J. Saad, A. Santos and P. O. Prada, Physiology, 2016, 31, 283–293 CrossRef CAS PubMed.
- M. Kushida, R. Okouchi, Y. Iwagaki, M. Asano, M. X. Du, K. Yamamoto and T. Tsuduki, Mol. Nutr. Food Res., 2018, 62, 1–34 CrossRef.
- G. La Fata, P. Weber and M. H. Mohajeri, Probiotics Antimicrob. Proteins, 2018, 10, 11–21 CrossRef CAS PubMed.
- Q. Guo, J. Z. Goldenberg, C. Humphrey, R. El Dib and B. C. Johnston, Cochrane Database Syst. Rev., 2019, 4, 4827–4929 Search PubMed.
- R. Yadav, S. H. Khan, S. B. Mada, S. Meena, R. Kapila and S. Kapila, Probiotics Antimicrob. Proteins, 2019, 11, 509–518 CrossRef CAS.
- R. Yi, F. Tan, W. Liao, Q. Wang, J. Mu, X. Zhou, Z. Yang and X. Zhao, Microorganisms, 2019, 7, 530–550 CrossRef CAS PubMed.
- C. Li, F. Tan, J. Yang, Y. Yang, Y. Gou, S. Li and X. Zhao, Antioxidants, 2019, 8, 381–397 CrossRef CAS PubMed.
- S. K. Panchal, H. Poudyal, L. C. Ward, J. Waanders and L. Brown, Food Funct., 2015, 6, 2496–2506 RSC.
- G. Marchesini, M. Brizi, G. Bianchi, S. Tomassetti and E. Bugianesi, Diabetes, 2001, 50, 1844–1850 CrossRef CAS.
- S. Furukawa, T. Fujita, M. Shimabukuro, M. Iwaki, Y. Yamada, Y. Nakajima, O. Nakayama, M. Makishima, M. Matsuda and I. Shimomura, J. Clin. Invest., 2004, 114, 1752–1761 CrossRef CAS.
- L. M. Martins, A. R. S. Oliveira, K. J. C. Cruz, F. L. Torres-Leal and DdN. Marreiro, Braz. J. Pharm. Sci., 2014, 50, 677–692 CrossRef.
- T. D. Nguyen, J. H. Kang and M. S. Lee, Int. J. Food Microbiol., 2007, 113, 358–361 CrossRef CAS PubMed.
- V. T. Samuel and G. I. Shulman, Cell Metab., 2018, 27, 22–41 CrossRef CAS.
- N. Peixoto-Silva, E. G. Moura, J. C. Carvalho, J. L. Nobre, F. T. Quitete, C. R. Pinheiro, A. P. Santos-Silva, E. de Oliveira and P. C. Lisboa, Clin. Exp. Pharmacol. Physiol., 2017, 44, 488–499 CrossRef CAS PubMed.
- R. Z. Hamza, R. A. Al-Eisa, A. E. Mehana and N. S. El-Shenawy, J. Basic Clin. Physiol. Pharmacol., 2019, 30, 219–232 CrossRef CAS.
- T. Saida, W. Fukushima, S. Ohfuji, K. Kondo, I. Matsunaga and Y. Hirota, J. Gastroenterol. Hepatol., 2014, 29, 128–136 CrossRef PubMed.
- J. G. Robinson, B. S. Nedergaard, W. J. Rogers, J. Fialkow, J. M. Neutel, D. Ramstad, R. Somaratne, J. C. Legg, P. Nelson and R. Scott, JAMA, J. Am. Med. Assoc., 2014, 311, 1870–1882 CrossRef.
- C. Chen and J. L. Dai, Lipids Health Dis., 2018, 17, 130–139 CrossRef PubMed.
- J. S. Greenberger, S. A. Aaronson, D. S. Rosenthal and W. C. Moloney, Nature, 1975, 257, 143–144 CrossRef CAS.
- J. Macias, J. Mira, I. Gilabert, K. Neukam, C. Roldan, M. Viloria, A. Moro and J. A. Pineda, HIV Med., 2011, 12, 14–21 CrossRef CAS PubMed.
- S. C. Chen, S. P. Tsai, J. Y. Jhao, W. K. Jiang, C. K. Tsao and L. Y. Chang, Sci. Rep., 2017, 7, 4649 CrossRef.
- S. Zareei, M. M. A. Boojar and M. Amanlou, Life Sci., 2017, 178, 49–55 CrossRef CAS.
- B. A. Ouzidane and M. E. Hajjam, Int. J. Sports Med., 1991, 12, 413–418 CrossRef PubMed.
- J. Balanzó, C. Guarner, E. G. Schiffrin, B. Mirelis, M. Chiva, G. Soriano and I. Rochat, J. Hepatol., 2002, 37, 456–462 CrossRef.
- Q. A. Wang, A. Song, W. Chen, P. C. Schwalie, F. Zhang, L. Vishvanath, L. Jiang, R. Ye, M. Shao and C. Tao, Cell Metab., 2018, 28, 282–288 CrossRef CAS PubMed.
- P. Fan, B. Abderrahman, T. S. Chai, S. Yerrum and V. C. Jordan, Mol. Cancer Ther., 2018, 17, 2732–2745 CrossRef CAS PubMed.
- J. Lekstrom-Himes and K. G. Xanthopoulos, J. Biol. Chem., 1998, 273, 28545–28548 CrossRef CAS.
- J. R. Mead, S. A. Irvine and D. P. Ramji, J. Mol. Med., 2002, 80, 753–769 CrossRef CAS PubMed.
- M. L. L. A. Niku-Paavola and T. Mattila-Sandholm, J. Appl. Microbiol., 2010, 86, 29–35 CrossRef PubMed.
- R. Paolillo, C. Romano Carratelli, S. Sorrentino, N. Mazzola and A. Rizzo, Int. Immunopharmacol., 2009, 9, 1265–1271 CrossRef CAS.
- S. C. V. Michael and L. A. Dieleman, Inflamm. Bowel Dis., 2002, 8, 71–80 CrossRef PubMed.
- C. Li, S. P. Nie, K. X. Zhu, Q. Ding, T. Xiong and M. Y. Xie, Food Funct., 2014, 5, 3216–3223 RSC.
- O. Palocz, E. Paszti-Gere, P. Galfi and O. Farkas, PLoS One, 2016, 11, 1–16 CrossRef.
- G. V. Mann, Am. J. Clin. Nutr., 1974, 27, 464–499 CrossRef CAS PubMed.
- T. Hong, Z. Xiao-ping and Y. Yi-lan, Food Sci., 2010, 31, 152–156 Search PubMed.
- A. D. G. C. Gaddi and G. Noseda, Arch. Dis. Child., 1987, 62, 274–278 CrossRef CAS PubMed.
- S. Inoguchi, Y. Ohashi, A. Narai-Kanayama, K. Aso, T. Nakagaki and T. Fujisawa, Int. J. Food Sci. Nutr., 2012, 63, 402–410 CrossRef CAS.
- A. Umeno, M. Horie, K. Murotomi, Y. Nakajima and Y. Yoshida, Molecules, 2016, 21, 708–723 CrossRef.
- M. Messina and B. Lane, Future Lipidol., 2007, 2, 55–74 CrossRef CAS.
- Y. Zhu, Y. Chen, P. Huray and X. Dong, Microw. Opt. Technol. Lett., 2002, 35, 10–14 CrossRef CAS.
- C. Hu, W. T. Wong, R. Wu and W. F. Lai, Crit. Rev. Food Sci. Nutr., 2020, 60, 2098–2112 CrossRef CAS PubMed.
- A. Yazigi, B. Gaborit, J. P. Nogueira, M. E. Butiler and F. Andreelli, Presse Med., 2008, 37, 1427–1430 CrossRef PubMed.
- G. Y. Mei, C. M. Carey, S. Tosh and M. Kostrzynska, Can. J. Microbiol., 2011, 57, 857–865 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |