Open Access Article
Open Access ArticleAmino group dependent sensing properties of metal–organic frameworks: selective turn-on fluorescence detection of lysine and arginine†
Jing Dongab,
Xiu-Du Zhanga,
Xia-Fei Xiea,
Fan Guoa and
Wei-Yin Sun *a
*a
aCoordination Chemistry Institute, State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing National Laboratory of Microstructures, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210023, China. E-mail: sunwy@nju.edu.cn
bNanjing Tech University, Nanjing 211816, China
First published on 9th October 2020
Abstract
Recently, metal–organic frameworks (MOFs) have been extensively investigated as fluorescence chemsensors due to their tunable porosity, framework structure and photoluminescence properties. In this paper, a well-known Zr(IV)-based MOF, UiO-66-NH2 was demonstrated to have capability for detection of L-lysine (Lys) and L-arginine (Arg) selectively from common essential amino acids in aqueous media via a fluorescence turn-on mechanism. Further investigation reveals its high sensitivity and strong anti-interference properties. Moreover, the possible mechanism for sensing Lys and Arg was explored by FT-IR and 1H-NMR, and the results indicate that the enhancement of the fluorescence could be ascribed to the adsorption of Lys/Arg and the hydrogen bonding interactions between Lys/Arg and the amino group of UiO-66-NH2. The difference of the sensing capacity and sensitivity between UiO-66 and UiO-66-NH2 revealed that the amino group plays an essential role in the sensing performance. This work presents a unique example of the functional group dependent sensing properties of MOFs.
Introduction
As important biological molecules, amino acids (AAs) are an indispensable constituent of living systems, which are the basic substances that make up proteins as well as enzymes and exist widely in food, blood and tissues.1 AAs are considered to have an inseparable relationship with the metabolism of proteins and peptides in a series of physiological processes.2 Detection of definite AAs in food and organisms is particularly important in nutritional analysis and disease diagnosis.3 Among the amino acid family, lysine (Lys) is one of the essential AAs for human beings and mammals.4 Lys has a great influence on the Krebs–Henseleit cycle and polyamine synthesis, which is related to the metabolic function of human beings and animals.5 What is more, Lys cannot be synthesized by itself in the human body and must be supplemented from food. As a result, the content of Lys is usually proposed as an evaluation criterion to judge the nutritional value of food, and hence the detection of Lys has a positive nutritional significance.6 On the other hand, as another important natural amino acid, arginine (Arg) has been recognized as an essential amino acid for young/adult mammals and birds. The increase of Arg metabolism can lead to the abnormality of human related system.7 Meanwhile, Arg has been demonstrated to be a biomarker of specific metabolic diseases, such as sulfite oxidase deficiency8 and has also been considered to be closely related to the pathogenesis of a number of diseases involving cardiovascular and renal.9 As a result, the detection of Arg is very helpful for drug analysis and medical diagnosis, which has great significance for further clinical diagnosis and treatment.8,10 Therefore, it is necessary to develop simple, sensitive and selective probe to distinguish Lys/Arg from other AAs. However, one of the biggest problem and challenge of biosensors for the detection of AAs is the stability and biocompatibility of the sensing materials in aqueous media.11Metal–organic frameworks (MOFs) are porous organic–inorganic hybrid materials formed by metal ions or metal clusters and organic bridging ligands through coordination interactions.12 In the past several decades, they have been intensively investigated and successfully applied in varied areas including energy storage,13 heterogeneous catalysis,14 gas adsorption and separation,15,16 drug delivery17 and so on due to their structural diversity, controllability, high porosity and large surface area.18–20 In particular, the investigation employing MOFs as fluorescent materials to detect a variety of substances, for example, the hazardous gas molecules,21 volatile organic chemicals (VOCs),22 explosives23 etc., has attracted great attention. On the one hand, the fluorescent properties of MOFs depend on the nature of the metal ions/clusters and organic ligands in the construction of MOFs and the regulation of the fluorescence behavior of MOFs could be realized through the rational selection and design.24,25 On the other hand, the pores and/or channels of MOFs could adsorb appropriate guest molecules and the interactions between the guest molecules and the metal nodes or organic ligands of MOFs may influence the fluorescence behavior to some extent.26 In addition, quite a lot of MOFs are easy to be modified with different functional groups and thus high selectivity can be achieved by modification of specific functional groups.27 Therefore, MOFs have been admired by researchers and gained popularity in the development of fluorescence sensing materials.
As a kind of Zr(IV)-based MOFs, the series of MOFs developed by Lillerud and coworkers28–31 including UiO-66, UiO-67 and UiO-68 (UiO = University of Oslo) have become one of the most famous and studied MOFs due to the high porosity, surface area, excellent stability and easy modification. In this paper, we studied the fluorescence properties of amino group-functionalized UiO-66, namely UiO-66-NH2, and found that Lys and Arg could enhance its fluorescence emission intensity significantly in aqueous suspension, which realized the purpose of detecting Lys and Arg in aqueous media. Further experiments not only demonstrated its high sensing selectivity in various AAs, but also revealed that the emission intensity of UiO-66-NH2 in aqueous suspension is in proportional to the concentration of Lys and Arg, which made it possible for the quantitatively detection of Lys and Arg. In addition, UiO-66-NH2 also exhibits strong anti-interference in the detection of Lys and Arg. According to the FT-IR and 1H-NMR spectral measurements, the fluorescence turn-on mechanism may be ascribed to the adsorption of Lys/Arg into the pores and the hydrogen bonding interactions between Lys/Arg and the amino groups in the framework of UiO-66-NH2. Furthermore, the results of the sensing capacity and sensitivity of UiO-66 compared with that of UiO-66-NH2 illustrate that the amino groups of UiO-66-NH2 play an essential role in the sensing performance.
Experimental
Materials and methods
UiO-66-NH2 and UiO-66 were synthesized following previously reported procedure with slight modification.28,32 All the chemicals and solvents were purchased commercially and used without further purification. FT-IR spectra were recorded in the range of 400–4000 cm−1 using a Bruker Vecotr22 FT-IR spectrophotometer with KBr pellets. Powder X-ray diffraction (PXRD) measurements were conducted using a Bruker D8 Advance X-ray diffractometer equipped with a Cu-Kα radiation (λ = 1.5418 Å) source, in which the X-ray tube was operated at 40 kV and 40 mA. Fluorescence spectra were measured on a PerkinElmer LS-55 fluorescence spectrometer. 1H NMR spectra were recorded on a Bruker-DRX 500 MHz instrument. UV-Vis spectral measurements were conducted at room temperature on a Shimadzu UV3600 spectrophotometer.Fluorescence and sensing experiments
The solid-state fluorescence property of UiO-66-NH2 was examined at room temperature. In order to explore the sensing capacity for AAs, the as-synthesized samples of UiO-66-NH2 were fully ground and then dispersed in deionized water or the aqueous solutions of different AAs (0.1 M) with ultrasonic treatment to prepare the steady suspension (0.5 mg mL−1). The suspension was stirred at a constant rate during fluorescence measurements for homogeneity and the fluorescence emission spectra in the range of 370–680 nm were recorded at room temperature. Each experiment was repeated three times to obtain reliable data.Quantitative titration and anti-interference experiments
In order to investigate the relationship between the emission enhancement ratio and the concentration of Lys/Arg, quantitative titration experiments were conducted by gradual addition of the Lys (0.1 M) or Arg (0.02 M) solution into the suspension of UiO-66-NH2 (0.5 mg mL−1) in deionized water and the fluorescent emission spectra were recorded every five minutes after the addition of Lys/Arg. Moreover, the anti-interference experiments were carried out by the addition in sequence of the solutions of other AAs and Lys/Arg (0.1 M) into the suspension of UiO-66-NH2 (0.5 mg mL−1) in water. All the experiments were repeated three times to achieve reliable data.Results and discussion
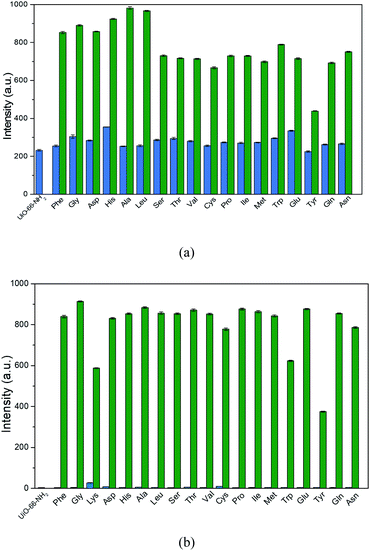
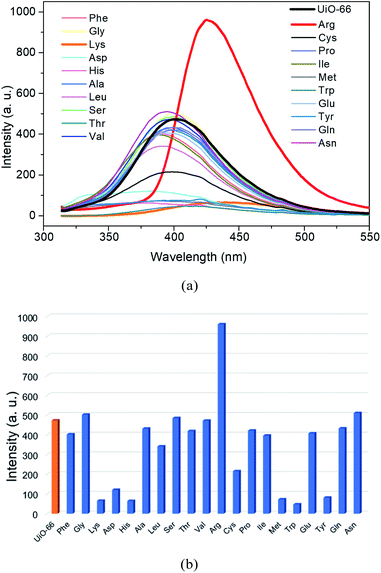
The bulk-phase purity of the as-synthesized UiO-66-NH2 was confirmed by using powder X-ray diffraction (PXRD) measurements. As depicted in Fig. S1 (ESI†), the peak positions of the obtained crystalline sample UiO-66-NH2 matched well with the simulated ones from the single crystal X-ray diffraction data,33 manifesting the phase purity of the synthesized samples. Then the solid-state fluorescence properties of the synthesized UiO-66-NH2 were examined at room temperature. It can be observed in Fig. S2 (ESI†) that UiO-66-NH2 exhibits characteristic emission band with maxima at 456 nm under the excitation wavelength of 375 nm. Furthermore, in order to investigate the sensing ability of UiO-66-NH2 towards AAs in aqueous media, the as-synthesized samples of UiO-66-NH2 were fully ground and dispersed in deionized water containing different AAs including L-phenylalanine (Phe), glycine (Gly), L-lysine (Lys), L-aspartic acid (Asp), L-histidine (His), L-alanine (Ala), L-leucine (Leu), L-serine (Ser), L-threonine (Thr), L-valine (Val), L-arginine (Arg), L-cysteine (Cys), L-proline (Pro), L-isoleucine (Ile), L-methionine (Met), L-tryptophan (Trp), L-glutamic acid (Glu), L-tyrosine (Tyr), L-glutamine (Gln) and L-asparagine (Asn) with concentration of 0.1 M to prepare the stable suspension (0.5 mg mL−1) by ultrasonication, respectively. Then, the fluorescence emission spectra of these suspension were recorded in the range of 370–680 nm excited at 350 nm. Compared to the emission of the aqueous suspension of UiO-66-NH2 without any AAs, increase in the emission intensities were observed with some shift in the maximum wavelength for all the suspensions with addition of AAs (Fig. 1a and S3, ESI†). As clearly shown in Fig. 1b, it is worth noting that only a slight emission increment could be monitored for the suspensions of most of the AAs but Lys and Arg could greatly enhance the emission intensity of UiO-66-NH2. Particularly, the presence of Arg could result in a greater than 181-fold increase in the emission intensity, which is much more significant than that of Lys (about 60 times). UV-Vis spectra of the suspension of UiO-66-NH2 with various amino acids were measured and they show absorption between 250–400 nm (Fig. S4, ESI†).The great fluorescence increment behavior indicates that UiO-66-NH2 is possible to be employed as a fluorescent sensor for detection of Lys and Arg. In order to explore the detection sensitivity of UiO-66-NH2 towards Lys and Arg, quantitative fluorescence titration experiments upon the gradual addition of the aqueous solution of Lys (0.1 M) and Arg (0.02 M) to the aqueous suspension of UiO-66-NH2 were carried out, respectively. As depicted in Fig. 2a and c, the emission intensities of the suspension of UiO-66-NH2 increase continuously with the addition of Lys and Arg. The plots of the emission enhancement ratio I/I0 vs. the concentration of Lys and Arg revealed a linear relationship between them in the concentration range of 0–3.475 and 0–0.645 mM with the correlation coefficients (R2) of 0.99976 (Fig. 2b) and 0.98633 (Fig. 2d) for Lys and Arg, respectively, where I0 and I are the emission intensities of the aqueous suspension before and after the addition of the aqueous solution of Lys/Arg. Thus, the formula of I/I0 = K[A] + 1 is used to fit the linear curves, where K is the slope, [A] is the concentration of analyte. The slope (K) of the linear curves were estimated to be 1.81 × 103 M−1 for Lys and 8.03 × 103 M−1 for Arg (Fig. 2b and d), which is higher or comparable to some reported materials (Tables S1 and S2, ESI†). According to the standard equation 3δ/K for the calculation of the limit of detection (LOD), where δ is the standard deviation for replicating detections of blank suspensions, the LOD is calculated to be 60.22 and 21.50 μM for Lys and Arg, respectively. The results manifested that UiO-66-NH2 has high sensing sensitivity towards Arg than Lys.
In addition to the AAs, the sensing ability of UiO-66-NH2 towards some biogenic amines was also checked. As shown in Fig. S5 (ESI†), the addition of putrescine, cadaverine and spermidine had little influence on the fluorescence of UiO-66-NH2, while the presence of spermine could strengthen the fluorescence of UiO-66-NH2. Such phenomenon indicated that UiO-66-NH2 may be able to detect spermine as well.
In practical application, there are usually different AAs existing simultaneously in the real sample. Therefore, it is of great significance to be capable to identify the target analyte in the presence of other competing species. In order to verify its anti-interference, competing experiments were conducted by gradual addition of the aqueous solution of other AAs followed by Lys/Arg. As shown in Fig. 3a, it was found that the addition of the aqueous solutions of other AAs (1 mL, 0.1 M) only cause slight fluorescence emission increment. However, significant increase in the emission intensities could be observed upon the addition of aqueous solution of Lys (1 mL, 0.1 M). Similar phenomenon was also observed for Arg, furthermore, the emission intensity of the aqueous suspension of UiO-66-NH2 could still be enhanced obviously in the presence of Lys (Fig. 3b). Such phenomenon implied that UiO-66-NH2 is capable to detect Lys and Arg in the presence of interfering AAs.
The effects of anions on the sensing performance of UiO-66-NH2 towards Lys and Arg were investigated. As exhibited in Fig. S6 (ESI†), the presence of sodium salt of acetate, benzoate, salicylate, Cl−, Br− had almost no influence on the sensing capability of UiO-66-NH2 towards Lys and Arg, while the addition of ATPs could induce the loss of sensing capacity. As for phosphate and pyrophosphate, the addition of them could also cause the sharp increase in the emission intensities of the suspension of UiO-66-NH2 as reported previously34 and the following addition of Lys/Arg could not induce further enhancement of the emission intensities. Such phenomenon indicated that the presence of ATPs, phosphate and pyrophosphate could affect the sensing performance of UiO-66-NH2 towards Lys and Arg.
The reusability of UiO-66-NH2 for sensing Lys and Arg has been checked. After centrifuging the suspension and washing with deionized water several times, the sample of the treated UiO-66-NH2 was prepared into the suspension again and the fluorescence return to the weak emission. The sensing experiments with the recovered samples revealed that the sensing capacity towards Lys without obvious changes, however, the sensing performance for Arg weakened (Fig. S7, ESI†), implying that UiO-66-NH2 could be reused to detect Lys but not Arg.
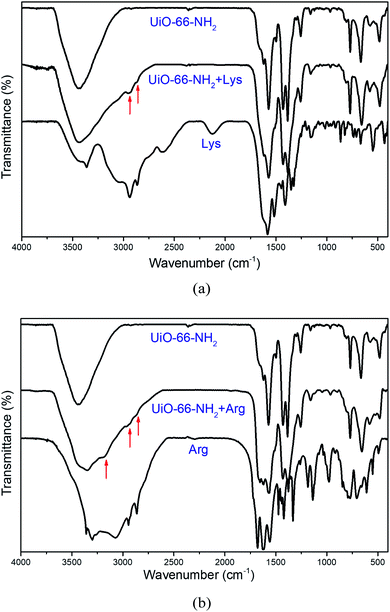
In addition, we also conducted experiments to further understand the insight for the detection of Lys and Arg by UiO-66-NH2. Firstly, the stability of UiO-66-NH2 in aqueous solution of Lys and Arg were examined. As shown in Fig. 4, the correspondence between the PXRD patterns of the UiO-66-NH2 before and after soaking in aqueous solution of Lys and Arg demonstrates the stability of the framework in the presence of Lys and Arg, suggesting that UiO-66-NH2 could retain the framework structure, and accordingly the turn-on fluorescence caused by the collapse of the framework can be excluded. Afterwards, we speculated that the fluorescence enhancement may be originated by the adsorption of Lys or Arg into the pores of UiO-66-NH2. Therefore, 1H-NMR and FT-IR spectral measurements were carried out to examine this assumption. Prior to the 1H NMR experiments, the samples of the untreated and Lys/Arg-treated UiO-66-NH2 were digested in HF/DMSO-d6. The 1H-NMR spectra of Lys-treated UiO-66-NH2 shows the appearance of new peaks at 1.28–1.48, 1.55, 1.70–1.85, 2.78, 3.80 ppm belonging to Lys (Fig. 5a and Table S3, ESI†), and also the new peaks at 1.38–1.69, 1.71–1.88, 3.09, 3.83 ppm belonging to Arg were observed in the 1H-NMR spectra of Arg-treated UiO-66-NH2 (Fig. 5b and Table S3, ESI†), which confirmed the adsorption of Lys and Arg into the pores of UiO-66-NH2. The calculated molecular sizes of Lys and Arg are smaller than the pore size of UiO-66-NH2 (Fig. S8, ESI†), which allow the adsorption. In addition, after incubation with the aqueous solution of Lys/Arg, the FT-IR spectra revealed the appearance of C–H stretching vibrations around 2952 and 2962 cm−1 for Lys and Arg respectively, and N–H stretching vibrations around 3178 cm−1 for Arg (Fig. 6a and b). Meanwhile, compared to the original FT-IR spectrum of UiO-66-NH2, the shift of the peaks at 1621, 1499, 1256 cm−1 (Fig. 6a and b) could be observed, which not only demonstrated the adsorption of Lys/Arg into the pores of UiO-66-NH2, but also implied the existence of hydrogen bonding interactions between Lys/Arg and UiO-66-NH2.6,35 It has been reported that the weakened emission of UiO-66-NH2, compared with the organic ligand due to the ligand-to-metal charge transfer (LMCT), could be recovered by addition of phosphate through the interactions between the analyte and UiO-66-NH2.34 Furthermore, its sensing capacity towards Lys and Arg under different pH was examined as well and the results revealed that the sensing performance was weakened significantly at pH = 2 (Fig. S9, ESI†), which also suggested that the hydrogen bonding interactions between Lys/Arg and UiO-66-NH2 play an important role in the detection of Lys/Arg. Compared to the other AAs, there are amino and guanidyl groups in the side chains of Lys and Arg, respectively, which may be responsible for the interactions of Lys/Arg with UiO-66-NH2.
 | ||
| Fig. 5 1H-NMR spectra of Lys (a) and Arg (b) in DCl/DMSO-d6, UiO-66-NH2 digested in HF/DMSO-d6 before and after the immersion in the solution of Lys (a) and Arg (b). | ||
To further ensure the interactions between Lys/Arg and amino group in the UiO-66-NH2 playing important role in detection of the analyte, we use the corresponding MOF without amino group functionalization, namely UiO-66, to recognize the AAs under the same conditions. As illustrated in Fig. 7a and b, the addition of the selected AAs except Arg could not induce fluorescence enhancements of the aqueous suspension of UiO-66, in contrast, Lys, Asp, His, Met, Trp and Tyr lead to the obvious fluorescence quenching. In addition, although the addition of Arg was still capable to cause the increment of the emission intensity of UiO-66, the emission enhancement amplitude in the case of UiO-66 was far lower than that of UiO-66-NH2, which distinctly confirmed the essential role of amino group in UiO-66-NH2 for the fluorescent turn-on sensing of Lys/Arg. These phenomena indicate that the introduction of amino functional group may be the critical factor to realize the sensing capacity and high sensitivity of UiO-66-NH2 towards Lys and Arg.
Conclusions
In summary, we demonstrate the sensing capability of a well-used MOF, UiO-66-NH2 towards Lys and Arg in aqueous media via the fluorescence turn-on effect. Quantitative titration experiments revealed that it can detect Lys and Arg selectively in various amino acids and the emission enhancement had linear correlation with the concentration of Lys/Arg, which indicated the realization of quantitative determination of Lys/Arg. Moreover, the selective and sensitive sensing Lys/Arg could be ascribed to the adsorption of Lys/Arg and the hydrogen bonding interactions between Lys/Arg and the amino group in UiO-66-NH2. Comparing the sensing capacity and sensitivity of UiO-66 with that of UiO-66-NH2, it could be concluded that the amino functional group play an essential role in the sensing performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Basic Research Program of China (grant no. 2017YFA0303504) and the Fundamental Research Funds for the Central Universities (grant no. 14380232) for financial support of this work. This work was also supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- C. P. Han and H. B. Li, Small, 2008, 4, 1344–1350 CrossRef CAS.
- J. Wang, H. B. Liu, Z. F. Tong and C. S. Ha, Coord. Chem. Rev., 2015, 303, 139–184 CrossRef CAS.
- Y. W. Zhao, Y. Wang and X. M. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 20991–20999 CrossRef CAS.
- X. R. Wang, Z. Huang, J. Du, X. Z. Wang, N. Gu, X. Tian, Y. Li, Y. Y. Liu, J. Z. Huo and B. Ding, Inorg. Chem., 2018, 57, 12885–12899 CrossRef CAS.
- Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- Y. H. Liu, M. J. Huangfu, P. Y. Wu, M. Jiang, X. L. Zhao, L. L. Liang, L. H. Xie, J. G. Bai and J. Wang, Dalton Trans., 2019, 48, 13834–13840 RSC.
- X. Z. Wang, X. R. Wang, Y. Y. Liu, J. Z. Huo, Y. Li, Q. Wang, K. Liu and B. Ding, Ultrason. Sonochem., 2019, 59, 104734 CrossRef CAS.
- R. Cui, Y. Wan, G. Ji and Z. Liu, Analyst, 2019, 144, 5875–5881 RSC.
- W. D. Pu, H. W. Zhao, C. Z. Huang, L. P. Wu and D. Xu, Anal. Chim. Acta, 2013, 764, 78–83 CrossRef CAS.
- R. S. Bhosale, G. V. Shitre, R. Kumar, D. O. Biradar, S. V. Bhosale, R. Narayan and S. V. Bhosale, Sens. Actuators, B, 2017, 241, 1270–1275 CrossRef CAS.
- J. Dong, D. Zhao, Y. Lu and W. Y. Sun, J. Mater. Chem. A, 2019, 7, 22744–22767 RSC.
- S. R. Batten, N. R. Champness, X. M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Ohrstrom, M. O'Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CAS.
- J. S. Chavez, K. L. Harrison and D. F. Sava Gallis, RSC Adv., 2017, 7, 24312–24320 RSC.
- F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS.
- N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127–1129 CrossRef CAS.
- J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS.
- K. M. L. Taylor-Pashow, J. Della Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- M. Eddaoudi, H. L. Li and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391–1397 CrossRef CAS.
- B. L. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Science, 2001, 291, 1021–1023 CrossRef CAS.
- E. Haghighi and S. Zeinali, Microporous Mesoporous Mater., 2020, 300, 110065 CrossRef CAS.
- M. Zhang, G. Feng, Z. Song, Y. P. Zhou, H. Y. Chao, D. Yuan, T. T. Y. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 7241–7244 CrossRef CAS.
- H. Xu, F. Liu, Y. Cui, B. Chen and G. Qian, Chem. Commun., 2011, 47, 3153–3155 RSC.
- W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev., 2017, 46, 3242–3285 RSC.
- Y. Zhang, S. Yuan, G. Day, X. Wang, X. Yang and H. C. Zhou, Coord. Chem. Rev., 2018, 354, 28–45 CrossRef CAS.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS.
- A. Buragohain and S. Biswas, CrystEngComm, 2016, 18, 4374–4381 RSC.
- M. Kandiah, S. Usseglio, S. Svelle, U. Olsbye, K. P. Lillerud and M. Tilset, J. Mater. Chem., 2010, 20, 9848–9851 RSC.
- S. Oien-Odegaard, B. Bouchevreau, K. Hylland, L. P. Wu, R. Blom, C. Grande, U. Olsbye, M. Tilset and K. P. Lillerud, Inorg. Chem., 2016, 55, 1986–1991 CrossRef.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- S. Chavan, J. G. Vitillo, D. Gianolio, O. Zavorotynska, B. Civalleri, S. Jakobsen, M. H. Nilsen, L. Valenzano, C. Lamberti, K. P. Lillerud and S. Bordiga, Phys. Chem. Chem. Phys., 2012, 14, 1614–1626 RSC.
- H. Chevreau, W. Liang, G. J. Kearley, S. G. Duyker, D. M. D'Alessandro and V. K. Peterson, J. Phys. Chem. C, 2015, 119, 6980–6987 CrossRef CAS.
- C. A. Trickett, K. J. Gagnon, S. Lee, F. Gandara, H. B. Burgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 11162–11167 CrossRef CAS.
- J. Yang, Y. Dai, X. Zhu, Z. Wang, Y. Li, Q. Zhuang, J. Shi and J. Gu, J. Mater. Chem. A, 2015, 3, 7445–7452 RSC.
- H. Zhu, J. Huang, Q. Zhou, Z. Lv, C. Li and G. Hu, J. Lumin., 2019, 208, 67–74 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD, UV-Vis and additional emission spectra, comparison for lysine and arginine sensing. See DOI: 10.1039/d0ra06879a |
| This journal is © The Royal Society of Chemistry 2020 |