Open Access Article
Open Access ArticleBio-elastomers based on polyocimene synthesized via coordination polymerization using neodymium-based catalytic systems
Luis Valencia *a,
Francisco Javier Enríquez-Medranob,
Héctor Ricardo López Gonzálezb,
Rishab Handac,
Hened Saade Caballerob,
Ricardo Mendoza Carrizalesb,
José Luis Olivares-Romerod and
Ramón Enrique Díaz de León Gómez*b
*a,
Francisco Javier Enríquez-Medranob,
Héctor Ricardo López Gonzálezb,
Rishab Handac,
Hened Saade Caballerob,
Ricardo Mendoza Carrizalesb,
José Luis Olivares-Romerod and
Ramón Enrique Díaz de León Gómez*b
aMaterials Technology and Chemistry, Alfa Laval Tumba AB, SE-14782 Tumba, Sweden. E-mail: luisalexandro.valencialopez@alfalaval.com
bResearch Center for Applied Chemistry, Blvd. Enrique Reyna 140, San José de los Cerritos, 25294, Saltillo, Coahuila, Mexico. E-mail: ramon.diazdeleon@ciqa.edu.mx
cExperimental Physics, Saarland University, 66123, Saarbrücken, Germany
dInstitute of Ecology A.C., Red de Estudios Moleculares Avanzados, Clúster Científico y Tecnológico BioMimic®, Campus III, Carretera Antigua a Coatepec N. 351, 91070, Xalapa, Veracruz, Mexico
First published on 5th October 2020
Abstract
Towards the development of eco-friendly alternatives of elastomeric materials, which can replace petroleum-based materials, it is crucial to explore different monomers and catalytic systems in order to find the best possible combinations for specific applications. Herein, we report the synthesis of polyocimene via coordination polymerization using two different neodymium-based catalysts (NdV3 and Nd(Oi-Pr)3), activated by alkylaluminums/organoboron compounds. By varying the type of co-catalyst species, halide donors, and reaction parameters, we have demonstrated the possibility to obtain polymers with a controlled microstructure and tunable properties, in terms of molecular weight characteristics and kinetics. Our results provide important insights towards the search for the optimum catalytic system to produce bio-elastomers.
Introduction
The pressing need to abate the current environmental issues and to reduce the consumption of fossil resources impels the search for alternative bio-based monomers that can (at least partially) replace synthetic rubbers based on butadiene or isoprene monomers, which are obtained from steam cracking processes. A prominent alternative for this challenge is the use of bio-based terpenes, which are natural hydrocarbons present in renewable resources like plant resins and essential oils. Most terpenes contain a hemiterpene moiety in their chemical structure, and therefore are polymerizable to form elastomers. Some of the most abundant terpenes, such as β-pinene or limonene, have readily been reported as monomers for radical,1–4 or cationic5–9 polymerizations. Nevertheless, the resultant polymers are in most cases of low molecular weight due to steric hindrance effects caused by the voluminous monomeric structures, as well as due to the phenomenon of degradative chain-transfer reaction that occurs in radical polymerization of some terpenes. Therefore, there is a current need to investigate alternative initiating or catalytic systems to achieve polymers with the desired characteristics.Rare-earth metal-based catalysts have been reported for their efficiency to polymerize 1,3-dienes with high catalytic activities, yielding polymers with good control in both their stereoregularity and molecular weight characteristics.10–14 The potential of such catalytic systems has lately been extended to the polymerization of unconventional bio-terpenes, such as β-myrcene,15,16 β-farnesene,17,18 and (E)-α-ocimene,18,19 obtained from renewable resources. Although the aforesaid monomers produce bio-elastomers with low carbon footprint and acceptable performance, they fall short when compared to conventional petroleum-based materials in terms of cost-efficiency and performance. Thus, it becomes essential to explore different bio-based terpenes as well as different catalytic systems. In this work we emphasize ocimene, being our monomer of interest, and address the ways to polymerize it via neodymium (Nd) based catalysts.
Ocimene is a diene monomer containing three double bonds in the backbone chain and can be polymerized to potentially manufacture materials with rubbery features. Despite several reports of rare-earth metal-based catalysts for the stereoregulated polymerization of terpenes,20,21 a well-defined approach to successfully polymerize ocimene is still scarce. Some previous works are for instance Peng et al., who reported the regio- and stereo-chemical control in the polymerization of ocimene using half-sandwich catalytic systems based on scandium, lutetium, yttrium, and dysprosium, in which the synthesis of syndiotactic cis-1,4-polyocimene (POc) and isotactic trans-1,2-polyocimene was presented depending on the central metal of the complex used, as well as the cyclopentadienyl ligand employed.21 Moreover, Naddeo et al. reported the use of titanium (Ti)-based complexes, activated with methylaluminoxane (MAO), to polymerize ocimene at different temperatures, from 0 to 90 °C. The catalytic systems exhibited a temperature-dependent stereoselectivity, yielding preferably trans-1,4-polyocimenes (∼70%) at the higher temperatures, while at the lowest temperatures, isotactic 1,2-polyocimenes (>99%) were reported.22
Recently, our research group has investigated the polymerization of 1,3-butadiene and β-myrcene using Nd-based catalytic systems.18,23–25 However, to the best of our knowledge, no reports have been filed for ocimene polymerization using Nd-based catalytic systems. Therefore, in this study we evaluate two different catalysts: neodymium isopropoxide (Nd(Oi-Pr)3) and neodymium versatate (NdV3) (see Fig. 1) to polymerize α-ocimene, using different alkylaluminums and/or organoboron compounds as activators. Moreover, the influence of different reactions conditions, such as molar ratios or temperatures, is also addressed.
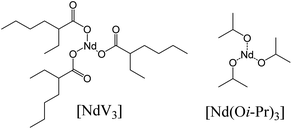 | ||
| Fig. 1 Chemical structure of the neodymium-based catalysts used in this study: (left) neodymium versatate [NdV3] and (right) neodymium isopropoxide [Nd(Oi-Pr)3]. | ||
Experimental part
Materials
All manipulations were carried out in an Mbraun glove box or under an inert atmosphere using a dual vacuum-argon line and standard Schlenk techniques. α-Ocimene monomer (purity ≥ 90%, as it contains other isomers) was acquired from Aldrich and distilled before use. The catalysts Nd(Oi-Pr)3 and NdV3 were obtained from Strem Chemicals and Rhodia respectively. The co-catalysts diisobutylaluminum hydride (Al(i-Bu)2H or DIBAH) and triisobutylaluminum (Al(i-Bu)3 or TIBA) were both acquired from Sigma-Aldrich in the form of 1.0 M solutions in hexane. The halide donors diethylaluminum chloride (AlEt2Cl or DEAC), dimethylaluminum chloride (AlMe2Cl or DMAC), diisobutylaluminum chloride (Ali-Bu2Cl or DIBAC), and ethylaluminum sesquichloride (Al2Et3Cl3 or EASC) were acquired from Aldrich; 1.0 M solutions in hexanes for DEAC and DMAC and 97% pure reagents for DIBAC and EASC. The organoboron compounds trityl tetrakis(pentafluorophenyl)borate ([CPh3][B(C6F5)4] or B1), N,N-dimethylanilinium tetrakis(pentafluorophenyl)borate ([HNMe2Ph][B(C6F5)4] or B2) and tris(pentafluorophenyl)borane (B(C6F5)3 or B3) were supplied by Strem Chemicals. All catalysts, co-catalysts, and activators were used as received. Cyclohexane was used as solvent in polymerization reactions and it was twice distilled from sodium under argon atmosphere prior use.Polymerization procedure
The polymerizations were carried out under argon atmosphere in a 1 L stainless steel Parr reactor equipped with a turbine-type mechanical stirrer. Heating and cooling were regulated by a PID controller coupled to an electrical-heating element and the internal tubing (for cold water flow).Two different catalysts: Nd(Oi-Pr)3 and NdV3 were used to polymerize ocimene (Fig. 1).
A typical ocimene polymerization procedure using the NdV3 as catalyst is described as follows: the catalytic system was first prepared in a glass vial, adding the components in the following order: (i) cyclohexane (5 mL), (ii) DIBAH, (iii) NdV3 and (iv) halide donor (DEAC, DIBAC, EASC or DMAC). The catalytic system was then aged at 30 °C for 30 minutes before use. The ocimene monomer (12 mL) and cyclohexane (180 mL) were added into the reactor and the temperature was stabilized to 50 °C and stirred at 100 rpm. The aged catalytic system was then fed to the reactor by means of a syringe to initiate the polymerization reaction. Several samples of the reaction mixture were evaluated gravimetrically for the %-conversion. The polymerization reaction was deactivated by adding acidified methanol. The resulting POc (dissolved in cyclohexane) was stabilized by adding Irganox 1076, precipitated in methanol, and dried under vacuum at 25 °C, until constant weight.
The polymerization procedure using Nd(Oi-Pr)3 as a catalyst was as follows: the catalytic system was prepared by mixing first Nd(Oi-Pr)3 in toluene + DIBAH and aged at room temperature for 1 hour. Ocimene and cyclohexane were added into the reactor at 50 °C and stirred at 100 rpm. The organoboron compound solution (in toluene) was then injected to the reactor, followed by the catalytic system, initiating the polymerization reaction. Deactivation and purification steps were identical to those previously described for NdV3.
Characterization
The molecular weight characteristics of the resultant polymers were estimated by Size Exclusion Chromatography (SEC) using a PLGel mixed column in a PL-GPC 50 from Agilent, equipped with a refractive index detector. The calibration curve was carried out using polystyrene standards and tetrahydrofuran (HPLC grade from Aldrich) was used as an eluent at a flow rate of 1 mL min−1.The stereoregularity of the synthesized polymers was investigated by 1H Nuclear Magnetic Resonance (NMR) spectroscopy, using a Bruker-400 MHz spectrometer. CDCl3 was used as a solvent and the analyses were performed at room temperature.
Differential Scanning Calorimetry (DSC) thermograms were obtained using a TA Instruments DSC 2920. The analyses were carried out under nitrogen atmosphere and at a heating rate of 5 °C min−1.
Results and discussions
With the aim of providing insights towards the synthesis of stereoregular bio-elastomers, conceived by coordination polymerization, we carried out a series of experiments related to the synthesis of POc. The reaction was catalysed by two different systems, however sharing a common class of ternary neodymium-based catalysts (see Fig. 2), illustrated as (1) NdV3 activated by alkylaluminums, and (2) Nd(Oi-Pr)3 activated by organoboron compounds. To avoid complications, the results are addressed separately in the following sections of the article.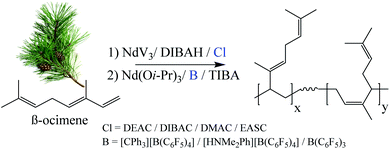 | ||
| Fig. 2 Conceptual schematic illustration of the synthesis of POc via the two different ternary neodymium-based catalytic systems. | ||
(1) Ocimene polymerization using NdV3/DIBAH/Cl as catalytic system
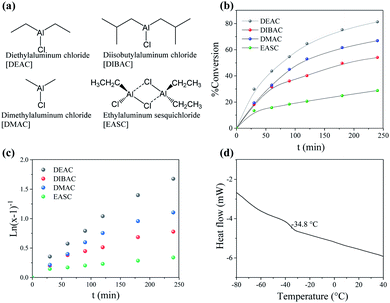
Neodymium versatate catalyst (the name comes from versatic acid, a mixture of α,α-disubstituted C10 carboxylic acids, whereas versatetes are the salts of such carboxylic acids),26 in combination with DIBAH, an alkylaluminum acting as co-catalyst, was used for the polymerization of ocimene. Note that alkylaluminums act as Lewis acids, abstracting alkyl groups from the Nd precursor to create free-coordination sites. Such abstraction can also be accomplished by other Lewis acids, such as borates (discussed later).To optimize the ocimene polymerization process, different halide donors were tested (DEAC, DIBAC, DMAC, or EASC, Fig. 3a) under the impression that halide donors are essential to achieve high catalytic activities and stereocontrol as shown by neodymium based catalysts in the polymerization of dienes.27 The halide atoms are transferred from the donor to the catalyst complex, coordinating the Nd atom, inducing 4f orbitals, and thus enhancing the cis coordination of dienes. Moreover, the use of halide donors prevents the anti–syn isomerization by favouring the coordination back-biting of the penultimate double bond of the polydienes, promoting stereocontrol.26,28 Among halide donors, chlorine-containing compounds are preferred (like the ones here used), especially in large scale-production. The most significant results of our attempt to synthesize POc using the forenamed halide donors are summarized in Table 1.
| Run | Halide donor | Yield (%) | Ab | Mwc (kDa) | Đc | 1,4d (%) | Tge (°C) | kappf (10−3) |
|---|---|---|---|---|---|---|---|---|
| a Isothermal polymerizations were performed in 180 mL of cyclohexane for 240 min. [Oc]/[Nd] = 600, DIBAH was used as co-catalyst. [Nd]/[Al]/[Cl] = 1/35/2. The reaction temperature was 50 °C in all cases.b Catalytic activity (kgPOc molNd−1 h−1) calculated after 60 min of reaction.c Dispersity index (Mw/Mn) determined by SEC.d cis + trans determined by 1H NMR.e Determined by DSC.f Apparent first-order rate constant (L mol−1 min−1) calculated considering the kinetic law d[M]/dt = ka[Nd][M], where kapp = k[Nd], and from the plots ln(1 − x)−1 = f(t), where x is the conversion. | ||||||||
| POc-1 | DEAC | 85.5 | 35.9 | 210 | 5.1 | 79 | −38.6 | 11.53 |
| POc-2 | DIBAC | 54.1 | 25.7 | 192 | 4.3 | 71 | −34.8 | 8.69 |
| POc-3 | DMAC | 66.9 | 26.9 | 161 | 5.1 | 70 | −38.7 | 5.18 |
| POc-4 | EASC | 28.8 | 13.0 | 151 | 9.4 | 52 | −40.3 | 2.00 |
The ocimene polymerization was greatly influenced by the type of halide donor employed in the reaction. Fig. 3b shows the kinetics of the polymerization reactions by tracking the time–conversion relations. Clearly, the evolution of conversion is significantly influenced by the halide donor, since the highest yield was achieved using DEAC (85.5% after 60 min), followed by DMAC (67%), DIBAC (54%), and EASC (29%). This trend was further used to extrapolate the catalytic activities, ranging from 13–36 kgPOc molNd−1 h−1 (see Table 1). The %-conversion could be furthermore be described as a first-order kinetics relation with respect to the monomer (see Fig. 3c), and the calculated apparent kinetic constant (ka) values are shown in Table 1.
The molecular weight characteristics of the POc's were also influenced by the halide donor, as the molar mass varied between 150–210 kDa, at which DEAC > DIBAC > DMAC > EASC. Note that the dispersity (Đ) ranged between 4–5 for DEAC, DMAC, and DIBAC as halide donor, whereas EASC led to a much higher Đ of 9.4. Among other factors, the Đ in the molecular weight distributions (MWD) strongly depends on the chain transfer reaction sites (for instance, chain transfer to aluminium, β-elimination, and transference to monomer) that are responsible for deactivating the catalyst.26 Moreover, this suggests the generation of variable catalytic active sites, with unique kinetic behaviours, governing the overall polymerization kinetics. Such behaviour was further analysed by deconvoluting the MWD using Gaussian functions (Fig. 4), where bimodal distributions were obtained when using DEAC, DMAC, or EASC as halide donor, whilst DIBAC resulted in a monomodal distribution, additionally corroborating to the lowest Đ value (Table 1).
 | ||
| Fig. 4 Deconvoluted molecular weight distributions of POc's synthesized using NdV3/DIBAH catalytic system with the different halide donors. | ||
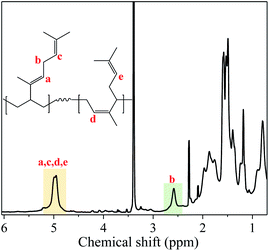
The microstructure, here referring to the 1,4-content (cis + trans), was examined by 1H NMR (see Fig. 5), following the previous reports in the literature.22 It can be observed that the content of 1,4-units was slightly influenced by the type of halide donor, where EASC showed the highest 3,4-content (Table 1) and showing also a slightly lower glass transition temperature (Tg). A representative DSC plot for the synthesized POc (POc-2) is shown in Fig. 3d. No presence of a melting peak was observed, strongly indicating the amorphous nature of the synthesized materials, corroborating previous reports for POc.22 Further polymerization reactions were carried out, varying the Nd/Al/Cl molar ratios, keeping DEAC as halide donor, as it gave the highest catalytic activity. The results are summarized in Table 2. Increasing the amount of alkylaluminum co-catalyst led to a dramatic increase in dispersity, presumably as higher co-catalyst concentration would promote chain transfer reactions to aluminium and therefore broader MWD. Apparently, a non-monotonic increase in the catalytic activity was observed by incrementing the amount of co-catalyst, whereas 1/35 seems to be an optimal ratio leading to 35.9 kgPOc molNd−1 h−1 with a yield of 85.5%. On the other hand, the catalytic activity as a function of halide donor content also appears to show a non-monotonic behaviour, apparently finding the optimum value at Nd/Cl molar ratio of 3, see Table 2.
| Run | Nd/Al/Cl | Yield (%) | Ab | Mw (kDa) | Đc |
|---|---|---|---|---|---|
| a Isothermal polymerizations were performed in 180 mL of cyclohexane for 240 min. [Oc]/[Nd] = 600. The reaction temperature was 50 °C in all cases. DIBAH was used as co-catalyst. DEAC was used as halide donor in all cases. n.d. = not determined.b Catalytic activity (kgPOc molNd−1 h−1) calculated after 60 min of reaction.c Dispersity index (Mw/Mn) determined by SEC. | |||||
| POc-5 | 1/15/2 | 18.4 | 9.1 | 232 | 5.7 |
| POc-1 | 1/35/2 | 85.5 | 35.9 | 210 | 5.1 |
| POc-6 | 1/45/2 | 74.4 | 26.7 | 169 | 8.3 |
| POc-7 | 1/35/3 | 86.7 | 49.5 | 128 | 4.5 |
| POc-8 | 1/35/5 | 81.3 | 38 | 122 | 8.1 |
(2) Ocimene polymerization using Nd(Oi-Pr)3/B/TIBA as catalytic system
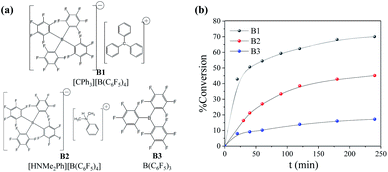
A neodymium trialkoxide-based ternary catalytic system was also studied for the polymerization of α-ocimene, comprising Nd(Oi-Pr)3, activated by both TIBA and organoboron compounds, which have repeatedly proved their capacity of producing highly active catalytic systems in Ziegler–Natta polymerizations. Both components, together with neodymium isopropoxide, would then form the catalytic system which is responsible for the coordination and insertion of the monomer, carrying out the polymerization. In this process, there is firstly an exchange of species between the Nd(Oi-Pr)3 and the alkylaluminum which form heterometallic species with isopropyl/isopropoxy groups and subsequently form heterometallic cationic complexes with the organoboron compound.29 TIBA was the chosen alkylaluminum to be part of the catalytic system, due to its effective function in the polymerization of other terpenes, such as myrcene.25Two different types of organoboron activators were used, being of ionic and non-ionic nature. The non-ionic, such as B3, abstracts an alkyl group of the catalysts, leading to the formation of an anionic boron compound and an activated catalytic species which is then coordinated by a monomer unit. On the other hand, organoboron of ionic nature, such as B1 and B2, are constituted of an ion-pair configuration where the cationic species (typically anilinium or carbonium) activates the catalyst species, while the borate anionic species prevent the interaction among the abstracted alkyl group and the metal centre. The chemical structure of the organoboron compounds used in this study is shown in Fig. 6a, as well as the rate of conversion in the α-ocimene polymerization (Fig. 6b). The general features of the resultant POc's are shown in Table 3.
| Run | Bb | Yield (%) | Ac | Mw (kDa) | Đd | 1,4e (%) | kappf (10−3) |
|---|---|---|---|---|---|---|---|
| a Isothermal polymerizations were performed in 180 mL of cyclohexane for 240 min. [Oc]/[Nd] = 650, [Nd] = 0.5 mM. [Nd]/[Al]/[B] = 1/35/1. Reaction temperature = 65 °C.b B1 = [CPh3][B(C6F5)4]; B2 = [HNMe2Ph][B(C6F5)4]; B3 = B(C6F5)3.c Catalytic activity (kgPOc molNd−1 h−1) calculated after 60 min of reaction.d Dispersity index (Mw/Mn) determined by SEC.e cis + trans determined by 1H NMR.f Apparent first-order rate constant (L mol−1 min−1) calculated considering the kinetic law d[M]/dt = ka[Nd][M], where kapp = k[Nd] and from the plots ln(1 − x)−1 = f(t), where x is the conversion. | |||||||
| POc-9 | B1 | 70.0 | 47.7 | 89 | 2.0 | 51 | 7.69 |
| POc-10 | B2 | 45.1 | 23.7 | 31 | 2.6 | 67 | 4.42 |
| POc-11 | B3 | 17.2 | 8.9 | 72 | 3.3 | 55 | 1.34 |
As it can be observed in Fig. 6b and Table 3, the polymerization behaviour was strongly influenced by the type of organoboron compound. B2 appear to lead to the highest 1,4-units content suggesting a better microstructure control. B1, on the other hand, leads to the highest catalytic activity, achieving 70% of yield after 240 min, proving to be 101% and 434% more active than when using B2 or B3 respectively, attributed to the different capacity to reduce the Nd metal nucleus, yielding the active cationic complex.
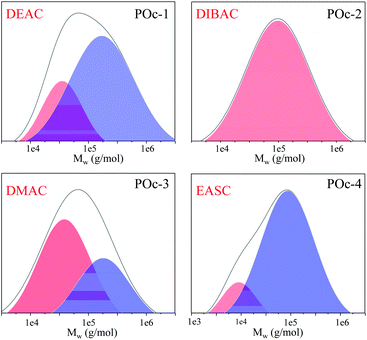
Moreover, B1 also led to the highest molecular weight polymers with dispersities of 2.0. Considering this, Nd(Oi-Pr)3/B1/TIBA was selected as catalytic system to explore different reactions parameters and find the optimum conditions. The results are shown in Fig. 7 and Table 4.
 | ||
| Fig. 7 Influence of (a) reaction temperature, (b) B/Nd ratio and (c) TIBA/Nd molar ratio, over the rate of conversion in the ocimene polymerization. | ||
| Run | Tb (°C) | Nd/Al/B | Yield (%) | Ac | 1,4d (%) | Mw (kDa) | Đe |
|---|---|---|---|---|---|---|---|
| a Isothermal polymerizations were performed in 180 mL of cyclohexane for 240 min. [Oc]/[Nd] = 650, [Nd] = 0.5 mM.b Reaction temperature.c Catalytic activity (kgPoc molNd−1 h−1) calculated after 60 min of reaction.d cis + trans determined by 1H NMR.e Dispersity index (Mw/Mn) determined by SEC. | |||||||
| POc-12 | 50 | 1/35/1 | 56.1 | 42.9 | 45 | 45 | 2.0 |
| POc-13 | 80 | 1/35/1 | 63.6 | 51.8 | 50 | 32 | 1.6 |
| POc-14 | 65 | 1/35/1.2 | 70.0 | 57.9 | 47 | 35 | 1.7 |
| POc-15 | 65 | 1/35/0.8 | 60.4 | 52.9 | 59 | 60 | 2.1 |
| POc-16 | 65 | 1/35/0.6 | 57.1 | 37.1 | 51 | 45 | 2.3 |
| POc-17 | 65 | 1/15/1 | 50.2 | 33.1 | 46 | 121 | 1.9 |
| POc-18 | 65 | 1/20/1 | 62.9 | 46.8 | 45 | 96 | 1.5 |
Three different reaction temperatures were evaluated in the polymerization of ocimene, namely 50, 65, and 80 °C (Fig. 7a). During the first 90 min of reaction, the rate of polymerization increased gradually as a function of temperature, in such a way that the catalytic activity at 80 °C was 8 and 17% higher than at 65 and 50 °C, respectively. Nevertheless, after 240 min of reaction, the polymerization at 65 °C led to the highest yield (70%), as shown in Table 4. Suggesting the eventual deactivation of the catalytic system at high temperatures. Considering this, the selected reaction temperature was 65 °C for further studies. Four different B/Nd molar ratios (0.6, 0.8, 1.0, and 1.2) were tested for the polymerization of ocimene using Nd(Oi-Pr)3/B1/TIBA as catalytic system, as well as three different TIBA/Nd (15, 20, and 35) ratios. The results can be observed in Fig. 7b and c and are summarized in Table 4.
As expected, the rate of polymerization (Fig. 5b), as well as the catalytic activity, is promoted by greater content of organoboron co-catalysts. Similar behaviour is observed by increasing the amount of TIBA (Fig. 7c). As previously mentioned, the variation in co-catalyst concentration (both organoboron and TIBA) can promote (i) different amounts of heterometallic cationic complexes to carry out the polymerization, and (ii) the formation of complexes with variable alkyl groups. In this context, the increment of catalytic activity with a higher concentration of co-catalyst is expectable considering the higher amount of catalytic species. The increase in alkylaluminum species was accompanied by a decrease in molecular weight of the synthesized polymers. However, the low dispersity values (Table 4) do not suggest chain transfer reactions be predominant in this system.
Conclusions
In this work, we provide important insights towards the synthesis of polyocimene, which is a prominent alternative to produce bio-based elastomers and partially replace polybutadiene as building block. For this purpose, two different neodymium-based catalysts (NdV3 and Nd(Oi-Pr)3) systems were explored, activated by alkylaluminums/organoboron compounds. Most catalytic systems exhibited a high activity and 1,4 microstructure (cis + trans) content around 60%. The interplay of reactive species, as well as reaction parameters, allowed the obtention of polymers with variable features, in terms of molecular weight characteristics, Tg, and microstructure. For NdV3, DEAC was the best alternative as halide donor, and the optimum Nd/Al/Cl ratios appeared to be 1/35/3. When using Nd(Oi-Pr)3 as the catalyst, on the other hand, the organoboron compound [CPh3][B(C6F5)4] was the best co-catalyst alternative, used in combination with TIBA. The highest yield-% was observed at 65 °C, and the catalytic activity incremented as a function of co-catalyst concentration without significantly altering the dispersity, therefore neglecting the generation of chain-transfer reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of the Mexican National Council of Science and Technology (CONACyT) through the Basic Science Project 258278. Also, the authors thank José Díaz Elizondo, Guadalupe Mendez, and Judith Cabello for their technical support in the characterization of the samples.References
- A. Singh and M. Kamal, J. Appl. Polym. Sci., 2012, 125, 1456–1459 CrossRef CAS.
- S. Sharma and A. K. Srivastava, Eur. Polym. J., 2004, 40, 2235–2240 CrossRef CAS.
- S. Sharma and A. K. Srivastava, J. Appl. Polym. Sci., 2007, 106, 21–23 CrossRef.
- S. Sharma and A. K. Srivastava, Polym.-Plast. Technol. Eng., 2007, 42, 37–41 Search PubMed.
- J. A. A. M. Castro and R. P. F. Guine, J. Appl. Polym. Sci., 2001, 82, 2558–2565 CrossRef.
- F. Martinez, J. Appl. Polym. Sci., 1984, 22, 673–677 CAS.
- J. Lu, M. Kamigaito, M. Sawamoto, T. Higashimura and Y.-X. Deng, J. Appl. Polym. Sci., 1996, 61, 1011–1016 CrossRef CAS.
- J. Lu, M. Kamigaito and M. Sawamoto, Macromolecules, 1997, 3, 22–26 CrossRef.
- A. H. H. Derdar, M. Belbachir, F. Hennaoui and M. Akeb, Polym. Sci., 2018, 60, 555–562 Search PubMed.
- D. K. Jenkins, Polymer, 1985, 26, 147–151 CrossRef CAS.
- B. Liu, G. Sun, S. Li, D. Liu and D. Cui, Organometallics, 2015, 34, 4063–4068 CrossRef CAS.
- Q. Zhang, X. Ni and Z. Shen, Polym. Int., 2002, 212, 208–212 CrossRef.
- C. O. Hollfelder, L. N. Jende, D. Diether, T. Zelger and R. Stauder, Catalysts, 2018, 8, 1–21 Search PubMed.
- N. Martins, F. Bonnet and M. Visseaux, Polymer, 2014, 55, 5013–5016 CrossRef CAS.
- S. Loughmari, A. Hafid, A. Bouazza, A. El Bouadili, P. Zinck and M. Visseaux, J. Appl. Polym. Sci., 2012, 2898–2905 CAS.
- C. Ligated, B. Liu, B. Han, C. Zhang, S. Li, G. Sun and D. Cui, Chin. J. Polym. Sci., 2015, 33, 792–796 CrossRef.
- E. Laur and A. Welle, Catalysts, 2017, 7, 1–12 CrossRef.
- R. D. De León, R. López, L. Valencia, R. Mendoza, J. Cabello and J. Enríquez, Key Eng. Mater., 2018, 779, 115–121 Search PubMed.
- F. Yang and X. Li, Polym. Chem., 2017, 55, 2271–2280 CrossRef CAS.
- B. Liu, L. Li, G. Sun, D. Liu and D. Cui, Chem. Commun., 2014, 51, 1039–1041 RSC.
- D. Peng, G. Du, P. Zhang, B. Yao and X. Li, Macromol. Rapid Commun., 2016, 37, 987–992 CrossRef CAS.
- M. Naddeo and A. Buonerba, Polymer, 2017, 131, 151–159 CrossRef CAS.
- D. Leon and Y. A. De Santiago-rodriguez, Macromol. Symp., 2013, 325, 125–131 Search PubMed.
- F. J. Enríquez-Medrano, L. A. V. López, Y. A. de Santiago-Rodríguez, F. S. Corral, H. S. Caballero, M. L. L. Quintanilla and R. D. de León-Gómez, J. Polym. Eng., 2015, 35, 1–7 Search PubMed.
- F. J. Enr, R. M. Carrizales, K. R. Acosta and H. R. Lopez, Can. J. Chem. Eng., 2016, 94, 823–832 CrossRef.
- O. Nuyken and W. O. Lars Friebe, Adv. Polym. Sci., 2006, 206, 1–154 CrossRef.
- N. G. Marina, Y. B. Monakov, S. R. Rafikov and K. K. Gadeleva, Polym. Sci., 1984, 26, 1251–1268 Search PubMed.
- G. Kwag, P. Kim, S. Han and H. Choi, Polymer, 2005, 46, 3782–3788 CrossRef CAS.
- Y. Taniguchi, W. M. Dong, T. Katsumata, M. Shiotsuki and T. Masuda, Polym. Bull., 2005, 54, 173–178 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |