Open Access Article
Open Access ArticlePreparation of novel morning glory structure γ-MnO2/carbon nanofiber composite materials with the electrospinning method and their high electrochemical performance
Ziwei Lan†
a,
Lulin Luo†a,
Jiaye Yea,
Qingyue Luoa and
Lei Zhao *ab
*ab
aDepartment of Physical Science and Technology, Lingnan Normal University, Zhanjiang, 524048, China. E-mail: leizhaolingnan@163.com
bKey Laboratory of Environmentally Friendly Functional Materials and Devices, Lingnan Normal University, Zhanjiang, 524048, China
First published on 5th October 2020
Abstract
A novel γ-MnO2/carbon nanofiber composite electrode material with a morning glory structure was prepared for the first time by electrospinning a mixture solution of Mn(CH3COO)2 salt and polyacrylonitrile and subsequently treating it with NH3 atmosphere. The resultant materials were applied in supercapacitors, exhibiting a high voltage of 2 V and high energy density of 15.7 W h kg−1 at the current density of 0.5 A g−1 in 1 M Na2SO4 solution. In this study, precisely controlling the concentration and time of the reaction resulted in the novel morning glory structure. And the morning glory structure at the surface of the carbon nanofibers increased the specific surface area and shortened the diffusion path for charge transport, increasing the Na+/H+ ion intercalation capacity, accounting for the high voltage and energy density of the present supercapacitors. These results demonstrated that this new-type of carbon nanofiber with morning glory structure electrode material is potentially superior in obtaining a high voltage for electrode materials and supercapacitors. We hope that these novel materials can expand to different applications in the energy or catalytic field.
Introduction
Supercapacitors, a new energy storage system, are energy storage devices between batteries with faradaic redox and dielectric capacitors and have been arousing more intensive interest for the purpose of protecting the environment.1,2 Usually, supercapacitors can be divided into two categories according to their energy storage mechanism.3 One is electrical double-layer capacitors (EDLCs), which involve a non-faradaic redox reaction of pure electrostatic charge accumulation at the electrode–electrolyte interface. The other is pseudocapacitors due to fast and reversible surface redox reactions, which include a metal oxide and conductive polymer involving faradaic redox reactions in the charge/discharge process.4 In these two categories of supercapacitors, EDLCs use traditional carbonaceous materials (the specific capacitance and energy density are 100–300 F g−1 and 5–7 W h kg−1, respectively) as electrode materials, resulting in low energy density, which limits their application.5 In addition, because of their high electrochemical activity and ability to directly store energy through rapid and reversible redox reactions on the electrode surface, pseudocapacitors with transition metal oxides as an electrode material have been more extensively studied than EDLCs. However, compared to the faradaic process of EDLCs, the non-faradaic process of pseudocapacitors is slow, resulting in low power density and a lack of long-term stability during cycling.6,7Although the energy density of EDLCs using traditional carbon materials as electrode materials is relatively low, carbon materials have always been sought after by researchers due to their high surface area, good electrical conductivity and high electrochemical stability.8 Recently, it has been reported that researchers have conducted a large number of experimental studies to further realize the application of EDLCs in portable electronic devices, electric vehicles and flexible energy storage systems. Zhou et al. obtained MXene-functionalized and cross-linked reduced graphene oxide (rGO) platelets through Ti–O–C covalent bonding, which achieved the bonding of heterogeneous plates between MXene and rGO. As a result, a supercapacitor with super-tough MXene-functionalized graphene sheets realized the dual combination of energy and power density.9 The interlayer spacing and pore structure of carbon materials are important factors that determine the electrochemical performance of EDLCs. Li et al. designed a graphene electrode with high pore utilization rate by tuning the layer spacing of the graphene laminate film. Consequently, the supercapacitor fabricated using this electrode can store capacitance performance compactly and deliver a stack volumetric energy density of 88.1 W h L−1 in an ionic liquid electrolyte.9 For the purpose of understanding the energy conversion process of supercapacitors, Krishnamoorthy et al. used piezoelectrochemical spectroscopy to probe the energy conversion process of an electrochemical self-charging supercapacitor power cell driven by piezoelectricity. This demonstrated that the self-charging supercapacitor made of siloxene sheets had a self-charging capacity of up to 207 mV under various levels of compressive force.10 Therefore, tuning the structure of carbon materials and designing high voltage carbon electrodes are the optimum choice for customizing high performance EDLC electrodes.
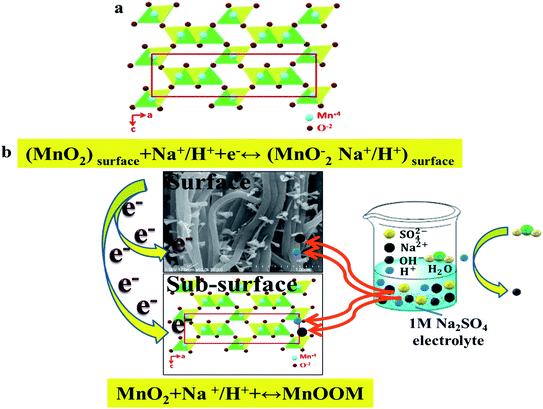
Among all kinds of pseudocapacitor materials, such as MnO2, RuO2, Co3O4 and NiO etc., MnO2 is widely used in supercapacitors because of its excellent theoretical advantages, environmental friendliness, abundant natural storage and simple preparation process.11 However, MnO2 materials have poor electrical conductivity, small specific surface area, and easy expansion and shedding during the redox reaction, which has hindered their widespread practical application.12 Therefore, for practical applications in the future, such as in large energy storage systems, hybrid vehicles, medical equipment, etc.,13 it is imperative to develop supercapacitors with higher working voltages and higher energy. In this way, it is possible to achieve a high cycle life without sacrificing power density under fast charging/discharging to meet the urgent energy needs of people in the present day. According to eqn (1):14,15
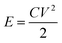 | (1) |
In order to achieve the purpose of high operating voltage and energy density, it is very necessary to combine nanostructured MnO2 with a highly conductive carbon material to prepare a composite electrode.16 Through compositing carbon materials, such as activated carbon,17 carbon nanofibers,18–20 carbon nanotubes6,16,21,22 and graphene,23,24 etc., it is possible to not only shorten the transport path of electrons and reduce the contact resistance, but also provide a larger surface area and increase the number of electroactive sites of electrochemical reaction. Among these carbon materials, carbon nanofibers have attracted much attention due to their unique one-dimensional framework, controllable specific surface area and high conductivity.25 Simultaneously, the conductivity of electrons can also be further improved by adopting the method of ion doping in the MnO2-based electrode. Doping atoms (such as Co-doping,25–27 Ni-doping,27 N-doping,28–30 Fe-doping,27,31 Ce-doping,32 and Cr-doping,33 etc.) can generate new energy levels and adjust the binding energy of electrons,25 thereby achieving the purpose of improving the pseudocapacitance and electrocatalytic performance of MnO2. Recently, many references have combined carbon materials with MnO2 and doped them with atoms to actualize the effective unification of the EDLC performance and pseudocapacitance. R. Nasser et al. synthesized cobalt-doped MnO2 nanowires modified with graphene oxide by hydrothermal and ultrasonic methods. The analysis results showed that the electrode had a nanocomposite phase structure, and the MnO2 nanowires had good dispersion on graphene oxide sheets.26 Li et al. prepared a MnO2/N-APC composite by growing MnO2 nanosheets on N-doped porous carbon, and the composite exhibited a specific capacitance of 330 F g−1 at the current density of 1 A g−1.28 Li et al. synthesized MnO2 nanosheets doped with transition metals (Fe, Co, Ni, and V) (MONSs) on the internal surface of macroporous carbon (MC), in which the supercapacitor assembled from Ni-doped MONSs had a high specific capacitance of 445 F g−1 at the current density of 1 A g−1.27 Wang et al. prepared a Ce-doped MnO2/rGO composite material by a hydrothermal method. The Ce–MnO2/rGO composite material was convenient for the quick transport and migration of electrolyte ions during the charge/discharge process, and exhibited good rate capability.32 Lu et al. synthesized a MnO2/N-doped ultra-microporous carbon nanosphere (MnO2/N-UCN) composite material by a simple and efficient strategy, which had regular ultramicropores, high surface area, nitrogen heteroatoms, and a high content of MnO2.29 Le et al. prepared a 3D N-doped graphene/MnO2 composite material (N-G/MnO2) using naturally abundant diatomite as a biotemplate, which was used as a bifunctional material for a supercapacitor and oxygen reduction reaction catalyst. Accordingly, the N-G/MnO2 electrode exhibited a high specific capacitance of 411.5 F g−1 at 0.5 A g−1 in 1.0 M Na2SO4 aqueous electrolyte and achieved a working voltage of up to 1.8 V and 46.1 W h kg−1 high energy density.30 However, there is no characterization study and mechanism analysis of MnO2/CNF-based supercapacitors using electrospinning technology to prepare biomass carbon material morning glories as conductive skeletons, especially concerning the charging and discharging mechanism of the electrodes.
Herein, for the first time, electrospinning of polyacrylonitrile and Mn(CH3COO)2 salts was performed to produce carbon and γ-MnO2 composite materials with a novel morning glory structure, which were used as an electrode material for supercapacitors. The obtained morning glory structure enlarges the cross-section of the carbon nanofibers, and N-doping during the heat treatment of NH3 improved the conductivity, thus expanding the working potential compared with the conventional carbon nanofibers. In addition, the morning glory structure provided high wettability, conductivity and specific surface area, so as to ameliorate the ion transfer process between the γ-MnO2/NCNF composites and H+/Na+ ions in neutral electrolyte, thus obtaining a high working voltage. The γ-MnO2/NCNFs-30 composite electrode exhibited a maximum energy density of 15.6 W h kg−1 at the current density of 0.5 A g−1 in a two-electrode system and the working range in 1 M Na2SO4 solution reached 2 V. Furthermore, the electrochemical performance of the electrode materials based on MnO2 found in this study and other reported works are compared in Table 1, which will provide guidance for the development of high-voltage aqueous supercapacitors and allow profound and lasting development of large-scale energy storage devices in the future.
| Material | Synthesis | Electrolyte | Specific capacitance/F g−1 | Working voltage/V | Stability (cycles) | Ref. |
|---|---|---|---|---|---|---|
| MnO2 | Hydrothermal | 1 M Na2SO4 | 197.3 (1 A g−1) | 1.0 | 94.6% (1000) | 11 |
| MnO2/AC | Wet impregnation | 1 M Na2SO4 | 291 (5 mV s−1) | 2.0 | 70% (500) | 17 |
| CNFs/MnO2 | Electrospinning | 1 M Na2SO4 | 428 (1 A g−1) | 0.8 | 98.8% (1500) | 20 |
| CNTs/MnO2 | Hydrothermal | 1 M Na2SO4 | 405.15 (100 mV s−1) | 0.8 | 81% (1000) | 22 |
| rGO/MnO2 | Hydrothermal | 1 M Na2SO4 | 270 (0.5 A g−1) | 1.0 | 90.12% (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
24 |
| Ni-MONSs/MC | Redox reaction | 1 M Na2SO4 | 445 (1 A g−1) | 1.8 | 93% (1000) | 27 |
| Ce–MnO2/rGO | Hydrothermal | 1 M Na2SO4 | 130.44 (1 A g−1) | 1.0 | 85% (1000) | 32 |
| MnO2/N-UCNs | Redox reaction | 1 M Na2SO4 | 401 (1 A g−1) | 1.0 | 86.3% (1000) | 29 |
| N-G/MnO2 | Chemical vapor deposition | 1 M Na2SO4 | 411.5 (0.5 A g−1) | 1.8 | 88.3% (4000) | 30 |
| γ-MnO2/NCNFs | Electrospinning | 1 M Na2SO4 | 125 (0.5 A g−1) | 2.0 | 92.1% (1000) | This work |
Experimental details
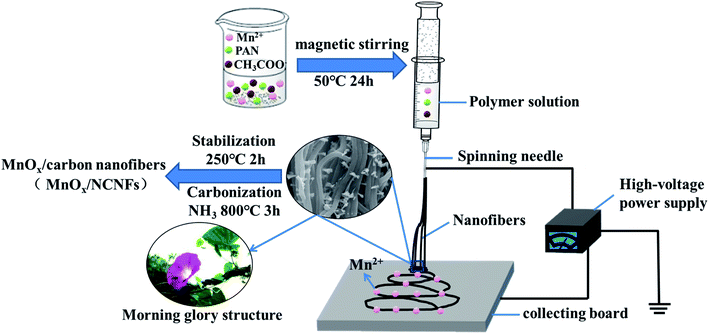
Polyacrylonitrile (PAN), Mn(CH3COO)2 salt and N,N-dimethylformamide (DMF) solvent were used for electrospinning. DMF solution was prepared by stirring 3 wt% PAN polymer and 0.9 wt% Mn(CH3COO)2 (30 wt% compared with PAN) in a magnetic field at 50 °C for 24 hours, to obtain a homogeneously-distributed solution.The parameters were as follows: the PAN nanofibers were prepared by a conventional electrospinning setup (DW-P503-2ACCD, Dongwen High Voltage Power Supply Company, China), which consists of a high voltage power supply (0–50 kV) and an ordinary hypodermic syringe with a stainless steel needle. During electrospinning the positive terminal (copper wire) of the power supply was connected with the syringe needle and the ground electrode was connected with the collecting substrates. The distance between the needle nozzle and collecting substrates was set at about 15 cm and the electrospinning voltage was 25 kV. Graphitic papers were used to collect the electrospun nanofibers, and no syringe pump was used during electrospinning. The NCNFs were prepared by stabilization and carbonization on the electrospun PAN nanofibers. Stabilization was carried out in air at 250 °C for 2 hours with a heating rate of 5 °C min−1. Carbonization was carried out in an NH3 atmosphere with a flow rate of 60 mL min−1 for 3 hours at 800 °C with a heating rate of 5 °C min−1. Finally, the samples with different amounts of Mn(CH3COO)2 salt (30 wt%, 60 wt%, and 150 wt% compared with PAN) after NH3 atmosphere treatment were denoted as MnOx/NCNFs-30, MnOx/NCNFs-60 and MnOx/NCNFs-150.
The structures were characterized by scanning electron microscopy (SEM, Hitachi S-4800), X-ray diffraction (XRD, Rigaku D/max. 2500/PC) and Raman spectroscopy (RM-1000 by Renishaw). A CHI760D (Shanghai Chenhua Instrument Co., Ltd., China) was used for the electrochemical performance test. In the three-electrode electrochemical test, a platinum wire electrode (CHI115) and an Ag/AgCl electrode (CHI111, saturated) were used as the counter electrode and reference electrode, respectively. The mass of electrode material was 1.6 mg and the electrolyte was 1 M Na2SO4 solution. The cyclic voltammetry (CV) curves of the three-electrode system were obtained at room temperature in the range of −1 to 1 V at the scan rate of 10–100 mV s−1 in 1 M Na2SO4 electrolyte. Electrochemical impedance spectroscopy (EIS) measurements were recorded from 10 kHz to 10 MHz with an AC amplitude of 5 mV. Galvanostatic charge/discharge (GCD) measurements were performed at a current density of 0.5, 1, 2, 5 and 10 A g−1.
The specific capacitance can be calculated using eqn (2):34
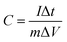 | (2) |
Results and discussion
As seen from Fig. 1, the prepared polymer solution was injected into the propulsion device of the electrospinning equipment. Under the action of high DC voltage, a high voltage electric field was formed between the spinning needle and the collecting board, so that the solution was ejected from the spinning needle to generate carbon nanofibers. The generated carbon nanofibers were adsorbed on the collecting board, while Mn2+ was regularly attached to the carbon nanofibers. After the prepared carbon nanofibers were subjected to high temperature treatment in an NH3 atmosphere, NH3 was decomposed into three active substances (namely H atoms, NH, and NH2). During the carbonization process, these substances would diffuse to all parts of the internal space of the carbon nanofibers.35 Then, by combining with their surface atomic bonds, the carbon nanofibers were prevented from agglomerating together to form a large channel with a morning glory structure, so as to stabilize the structure. Meanwhile, Mn2+ was oxidized to Mn4+ to form MnO2, and the metal oxide was used as a catalyst to promote the further growth of carbon nanofibers along the radial direction. As mentioned above, the reaction of NH3 with the active substances in the carbon nanofibers would produce carbon-containing active substances during the gas phase reaction, and the diameter of the carbon nanofibers was reduced under the corrosion of NH3.35 Moreover, under the action of the MnO2 catalyst, these carbon-containing active substances in the form of a carbon source induce the further growth of carbon nanofibers in the radial direction during the chemical phase deposition, thereby promoting the generation of MnOx/NCNFs.The carbonization of fibers with different concentrations at 800 °C is shown in Fig. 2. The γ-MnO2/NCNFs-30 (Fig. 2a–d) shows a morning glory structure, which is more stable and uniform. Correspondingly, MnOx/NCNFs-60 (Fig. 2e) and MnOx/NCNFs-150 (Fig. 2f) show a columnar structure, which is not uniform. It can be seen that during the carbonization process, only a fiber shape with too many nodes appeared. The structure of the MnOx/NCNF composites is a morning glory structure with an average opening diameter of 70 nm and a length of 170 nm growing along the vertical fiber direction. These results can be interpreted as follows: (1) the etching reaction between NH3 and carbon leads to a reduction in the diameter of NH3 during the carbonization of nanofibers. (2) At a high temperature of 800 °C, NH3 decomposes to generate hydrogen atoms or H-containing active species, and immediately reacts with carbon to generate carbon-containing active species or molecules, and thus the diameter of the resulting fibers dwindles. (3) Manganese, as the core catalyst, augments the effective length of H atoms on the surface of the carbon nanofibers in the vertical direction.
 | ||
| Fig. 2 (a) An image of a morning glory, SEM images of γ-MnO2/NCNFs-30 at (b) high magnification, (c) medium magnification and (d) low magnification, (e) MnOx/NCNFs-60 and (f) MnOx/NCNFs-150. | ||
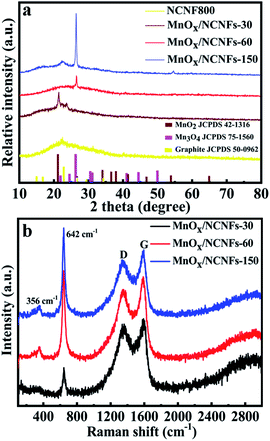
The crystallinity and phase composition of the different MnOx/NCNFs composites were further investigated by XRD as shown in Fig. 3a. All of the observed diffraction peaks can be indexed as graphite, MnO2 and Mn3O4. The 2θ values of the carbon peaks are 26.4°, 44.4°, and 54.5°, corresponding to the (002), (101) and (004) planes of graphite (JCPDS 50-0962).36 The peaks of MnOx/NCNFs-30 at 2θ values of 21.9°, 35.2°, 38.4° and 36.8° correspond to the (101), (301), (210), and (111) planes of γ-MnO2 (JCPDS 42-1316), respectively. Therefore, the prepared MnOx/NCNFs-30 sample is γ-MnO2/NCNFs-30.37,38 However, it can be seen from Fig. 3a that the prepared MnOx/NCNFs-60 and MnOx/NCNFs-150 samples are indexed as Mn3O4 (JCPDS 75-0962). The Raman spectra of the MnOx/NCNF composites are shown in Fig. 3b. Two prominent peaks at 1345 and 1588 cm−1 are observed, corresponding to the D and G peaks of the MnOx/NCNF composites, because N-doping introduces a large amount of topological defects. The G band is caused by doping, defects, stains and other factors, and our results are homogeneous with those of N-doped nanofibers, resulting in the downshift of the G band. Furthermore, the peak value in the range of 500–700 cm−1 is deemed to be characteristic of γ-MnO2, and its specific position is in the range of 630–660 cm−1.37 What we can see from Fig. 3b is that the most intense peak is located at 645 cm−1, corresponding to the γ-MnO2 donating the symmetric Mn–O stretching vibration of the MnO6 group.39 That of the γ-MnO2/NCNFs-30 is at 642 cm−1, nearly the same as the value above.37 The small peaks at 356 cm−1 are assigned to the bending modes of Mn–O and the peak at 269 cm−1 is a property of γ-MnO2. The results of the Raman bands for the γ-MnO2 are in good agreement with the XRD results.
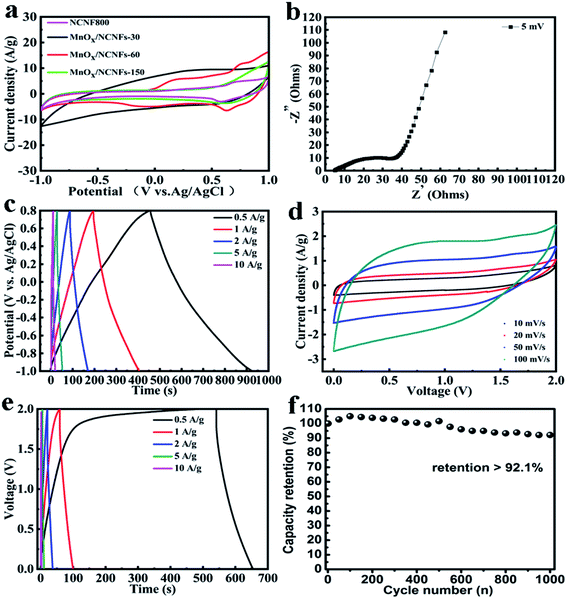
In the three-electrode system, the CV curves of γ-MnO2/NCNFs-30 are shown at a stable working voltage of 2 V with the scan velocity of 10–100 mV s−1 in Fig. 4a. Contrasting with MnOx/NCNFs-60 and γ-MnOx/NCNFs-150, γ-MnO2/NCNFs-30 shows the largest current density in the same potential range. This is because, as compared with the pure carbon nanofiber treated by NH3 atmosphere, the addition of metal oxides increases the specific capacitance. Furthermore, the addition of manganese salts makes it a catalyst for the growth of carbon materials at 800 °C, which increases the radial growth of the carbon materials and the metallic tendency. As seen from Fig. 2 and 3, γ-MnO2/NCNFs-30 shows more avenue structures than the other two samples and a γ-MnO2 structure by contrasting the XRD patterns, which can vastly facilitate the ion charge storage in MnO2.40,41 Two mechanisms are based on the surface process and the intercalation/extraction of protons or cations.42,43 One process is the adsorption/desorption of electrolyte protons or cations, which is often anticipated on the surface of amorphous MnO2.
| (MnO2)suface + M+ + e− ↔ (MnO−2M+)surface |
The other process is expected to be in crystalline MnO2.
| MnO2 + M+ ↔ MnOOM |
M+ is Na+ or H+ in Na2SO4 electrolyte.
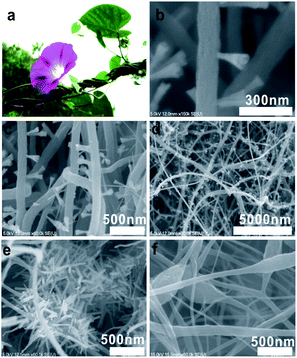
The CV curves of γ-MnO2/NCNFs-30 in the two-electrode system are shown in Fig. 4d, which exhibits a high working voltage. It can be seen that the potential window of the symmetrical supercapacitor assembled with the γ-MnO2/NCNFs-30 is 0–2 V, and it can work stably at a scanning rate of 0–100 mV s−1 at 2 V. Furthermore, the CV curve still maintains the rectangular feature at a high scan rate (100 mV s−1), which demonstrates an outstanding rate capability. Two processes enhance the water decomposition potential, which makes the potential of the γ-MnO2/NCNFs-30 larger than 1.23 V in 1 M Na2SO4 electrolyte. XRD and Raman show that the electrospun MnO2 is the amorphous structure of γ-MnO2 as shown in Fig. 5a, which is the irregular alternating growth structure of the pyrolusite (1 × 1) tunnels and ramsdellite (1 × 2) tunnels.44,45 The structure is incomplete, poorly ordered and defective, but it can compensate for the lack of charge caused by the cation vacancies and low-valence manganese cations through connecting with oxide anions.44 This study considered that when the potential was lower than the decomposition of water, the nascent hydrogen was absorbed to the surface, resulting in a large specific surface area of the MnO2 morning glory structure, which immediately interacted into crystalline MnO2. At fast scan rates, some ions do not have enough time to absorb onto the MnO2 surface due to the polarization of the hydrated Na+ ions, and consequently the adsorption on the electrode surface was incomplete. The greatly enhanced ion intercalation capacity is ascribed to the relatively large surface area, ultra-stable γ-MnO2/carbon nanofiber structure and shorter diffusion path for mass and charge transport.46 Finally, these two reasons above can be fully proved from the redox mechanism diagram of γ-MnO2/NCNFs-30 in Fig. 5b. As seen from Fig. 5b, the schematic diagram of the redox mechanism of γ-MnO2/NCNFs-30 can be divided into two parts. In the left part, electrons come in from an external circuit and reduce high-valence ions (Mn4+) to low-valence ions (Mn3+) on the surface and sub-surface of the carbon nanofibers, which is a process of rapid reduction of manganese ions.45,47 In the right part, one process is that the H+ generated by the decomposition of water and the H+ in the Na2SO4 electrolyte are inserted into the structure of the γ-MnO2 and react with O2− immediately. The other is that Na+ in the electrolyte is also inserted into the γ-MnO2 structure. Eventually, under the action of kinetics, H+ and Na+ quickly and uniformly diffuse into the γ-MnO2 structure. These results finally indicate that this series of processes enhances the intercalation capacity of Na+/H+ ions due to the relatively large surface area, ultra-stable γ-MnO2/carbon nanofiber structure and shorter diffusion path for charge transport, thereby increasing the working voltage.
 | ||
| Fig. 5 (a) Crystal structures of γ-MnO2. (b) Schematic of the γ-MnO2/NCNFs-30 redox reaction mechanism. | ||
From Fig. 4b, the Nyquist plot includes three parts. The high frequency intercept of the semicircle with the real axis represents the internal resistance (Rs),48 which is 5 ohms. A semicircle in the high-to-medium frequency region is the charge-transfer resistance (Rct).49,50 It can be seen that Rct (49.7 ohms) is equal to the diameter of the semicircle. The low Rct of γ-MnO2/NCNFs-30 is due to the morning glory structure, which has a high specific surface area, so that it facilitates the electrolyte cation insertion/extraction process into the nanofiber. In the low frequency region, the Nyquist plot shows a nearly 45° slope relating closely to the middle frequency region (the Warburg impedance),33 which corresponds to the honeycomb porous nature of the MnO2 fibers.
The capacitance at the current density of 0.5 A g−1 is 125 F g−1, which is shown in Fig. 4c. The variation of the charge/discharge curve with increasing current density not only exhibits symmetry, but also maintains a good specific capacitance. And the initial voltage drop is not obvious with the current density increasing from 0.5 to 10 A g−1, which is attributed to the morning glory structure of the γ-MnO2/NCNFs-30. The two-electrode system was tested with the total mass of 870 μg (two γ-MnO2/NCNFs-30 electrodes). It can be calculated that the energy density is 15.6 W h kg−1 at the current density of 0.5 A g−1 in Fig. 4e. In order to meet the demand of providing high energy density for long cycles at high charge/discharge rates, an excellent electrochemical energy storage device is required. Therefore, the cycling life was investigated over 1000 cycles as shown in Fig. 4f, which showed a stable capacitance retention that remained at 92.1%.
Conclusions
In summary, a new type of γ-MnO2/carbon nanofiber composite with a morning glory structure was obtained for the first time. It was prepared by electrospinning PAN and Mn(CH3COO)2 salts, simultaneously, treated by 800 °C thermal treatment under an NH3 atmosphere. These new-type carbon nanofibers with a morning glory structure not only exhibit an increased specific surface area, but also have an enhanced working potential. The high working voltage of 2 V can be attributed to the following three points. (1) Radial growth can improve the conductivity while improving the wetting performance, and then undergo NH3 treatment to generate activated functional groups, thereby increasing the forward voltage and expanding the potential window. (2) The material is mainly carbon, because carbon itself is a negative potential material, so the introduction of functional groups can expand the positive potential and increase the working voltage. (3) The large specific surface area of the morning glory structure, ultra-stable γ-MnO2/carbon nanofiber radial structure and shorter diffusion path for charge transport can enhance the Na+/H+ ion intercalation capacity effectively, so as to increase the working voltage. The γ-MnO2/NCNFs-30 exhibits an energy density of 15.7 W h kg−1 at the current density of 0.5 A g−1 and a working voltage of 2 V in 1 M Na2SO4 electrolyte. The novel structure in this paper not only enriches the family of manganese/carbon composites but also improves the working voltage in the application of supercapacitors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Science and Technology Program of Zhanjiang government (Grant No. 2019B01002).Notes and references
- J. Yan, S. Li, B. Lan, Y. Wu and P. S. Lee, Adv. Funct. Mater., 2020, 30, 1902564 CrossRef CAS.
- C. Young, T. Park, J. W. Yi, J. Kim, M. S. A. Hossain, Y. V. Kaneti and Y. Yamauchi, ChemSusChem, 2018, 11, 3546–3558 CrossRef CAS.
- Q. Zheng, A. Kvit, Z. Cai, Z. Ma and S. Gong, J. Mater. Chem. A, 2017, 5, 12528–12541 RSC.
- A. Xie, F. Tao, C. Jiang, W. Sun, Y. Li, L. Hu, X. Du, S. Luo and C. Yao, J. Electroanal. Chem., 2017, 789, 29–37 CrossRef CAS.
- B. Chang, L. Wang, W. Shi, Y. Chai, S. Zhang and B. Yang, Sustainable Energy Fuels, 2020, 4, 2527–2540 RSC.
- L. Li, Z. A. Hu, N. An, Y. Y. Yang, Z. M. Li and H. Y. Wu, J. Phys. Chem. C, 2014, 118, 22865–22872 CrossRef CAS.
- K. Ghosh, C. Y. Yue, M. M. Sk, R. K. Jena and S. Bi, Sustainable Energy Fuels, 2018, 2, 280–293 RSC.
- S. Korkmaz and İ. A. Kariper, J. Energy Storage, 2020, 27, 101038 CrossRef.
- T. Zhou, C. Wu, Y. Wang, A. P. Tomsia, M. Li, E. Saiz, S. Fang, R. H. Baughman, L. Jiang and Q. Cheng, Nat. Commun., 2020, 11, 2077 CrossRef CAS.
- K. Krishnamoorthy, P. Pazhamalai, V. K. Mariappan, S. S. Nardekar, S. Sahoo and S.-J. Kim, Nat. Commun., 2020, 11, 2351 CrossRef CAS.
- S. Zhao, T. Liu, D. Hou, W. Zeng, B. Miao, S. Hussain, X. Peng and M. S. Javed, Appl. Surf. Sci., 2015, 356, 259–265 CrossRef CAS.
- J. Ju, H. Zhao, W. Kang, N. Tian, N. Deng and B. Cheng, Electrochim. Acta, 2017, 258, 116–123 CrossRef CAS.
- Z. Wu, L. Li, J. Yan and X. Zhang, Adv. Sci., 2017, 4, 1600382 CrossRef.
- Z. Fan, J. Yan, W. Tong, L. Zhi, G. Ning, T. Li and W. Fei, Adv. Funct. Mater., 2011, 21, 2366–2375 CrossRef CAS.
- N. Swain, A. Mitra, B. Saravanakumar, S. K. Balasingam, S. Mohanty, S. K. Nayak and A. Ramadoss, Electrochim. Acta, 2020, 342, 136041 CrossRef CAS.
- H. Wu, M. La, J. Li, Y. Han, Y. Feng, Q. Peng and C. Hao, Compos. Interfaces, 2019, 26, 659–677 CrossRef CAS.
- D. Deng, B. Kim, M. Gopiraman and I. S. Kim, RSC Adv., 2015, 5, 81492–81498 RSC.
- J. H. Jeong, Y. A. Kim and B.-H. Kim, Carbon, 2020, 164, 296–304 CrossRef CAS.
- J.-G. Wang, Y. Yang, Z.-H. Huang and F. Kang, J. Power Sources, 2013, 224, 86–92 CrossRef CAS.
- H. Zhao, W. Han, W. Lan, J. Zhou, Z. Zhang, W. Fu and E. Xie, Electrochim. Acta, 2016, 222, 1931–1939 CrossRef CAS.
- S. Ramesh, H. S. Kim, Y. Haldorai, Y.-K. Han and J.-H. Kim, Mater. Lett., 2017, 196, 132–136 CrossRef CAS.
- W. Li, M. Chang, K. Chuang, Y. Li, J. Luo and H. Cheng, J. Electrochem. Soc., 2019, 166, A2194–A2198 CrossRef CAS.
- X. Meng, L. Lu and C. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 16474–16481 CrossRef CAS.
- X. Wang, L. Chen, S. Zhang, X. Chen, Y. Li, J. Liu, F. Lu and Y. Tang, Colloids Surf., A, 2019, 573, 57–66 CrossRef CAS.
- X. Yang, W. Peng, K. Fu, L. Mao, J. Jin, S. Yang and G. Li, Electrochim. Acta, 2020, 340, 135989 CrossRef CAS.
- R. Nasser, G.-F. Zhang and J.-M. Song, Electrochim. Acta, 2020, 345, 136198 CrossRef CAS.
- J. Li, Y. Ren, S. Wang, Z. Ren and J. Yu, Appl. Mater. Today, 2016, 3, 63–72 CrossRef.
- D. Li, J. Lin, Y. Lu, Y. Huang and C. Tang, J. Alloys Compd., 2019, 815, 152344 CrossRef.
- W.-J. Lu, S.-Z. Huang, L. Miao, M.-X. Liu, D.-Z. Zhu, L.-C. Li, H. Duan, Z.-J. Xu and L.-H. Gan, Chin. Chem. Lett., 2017, 28, 1324–1329 CrossRef CAS.
- Q. J. Le, M. Huang, T. Wang, X. Y. Liu, L. Sun, X. L. Guo, D. B. Jiang, J. Wang, F. Dong and Y. X. Zhang, J. Colloid Interface Sci., 2019, 544, 155–163 CrossRef CAS.
- Z. Wang, F. Wang, Y. Li, J. Hu and M. Xu, Nanoscale, 2016, 8, 7309–7317 RSC.
- Y. Wang, Y. Zhan, X. Yan and Z. Ma, Russ. J. Electrochem., 2018, 54, 283–291 CrossRef CAS.
- S. Zhao, T. Liu, Z. Yu, Z. Wen, T. Li, S. Hussain, D. Hou and X. Peng, J. Mater. Sci.: Mater. Electron., 2016, 27, 3265–3270 CrossRef CAS.
- L. Zhang, X. Peng, J. Wan, Q. Cui, X. Chu, H. Suo, C. Zhao and D. He, Nano, 2020, 15, 2050031 CrossRef CAS.
- L. Zhao, Y. Qiu, J. Yu, X. Deng, C. Dai and X. Bai, Nanoscale, 2013, 5, 4902 RSC.
- A. Gomez-Martin, A. Gutierrez-Pardo, J. Martinez-Fernandez and J. Ramirez-Rico, Fuel Process. Technol., 2020, 199, 106279 CrossRef CAS.
- C. Julien, M. Massot, S. Rangan, M. Lemal and D. Guyomard, J. Raman Spectrosc., 2002, 33, 223–228 CrossRef CAS.
- Y. Chabre and J. Pannetier, Prog. Solid State Chem., 1995, 23, 1–130 CrossRef CAS.
- L. Balan, C. M. Ghimbeu, L. Vidal and C. Vixguterl, Green Chem., 2013, 15, 2191–2199 RSC.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2004, 16, 3184–3190 CrossRef CAS.
- L. Zhao, J. Yu, W. Li, S. Wang, C. Dai, J. Wu, X. Bai and C. Zhi, Nano Energy, 2014, 4, 39–48 CrossRef CAS.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2002, 14, 3946–3952 CrossRef CAS.
- M. I. Said, A. H. Rageh and F. A. Abdel-aal, RSC Adv., 2018, 8, 18698–18713 RSC.
- J. Arnott, R. Williams, A. Pandolfo and S. Donne, J. Power Sources, 2007, 165, 581–590 CrossRef CAS.
- X. Duan, J. Yang, H. Gao, J. Ma, L. Jiao and W. Zheng, CrystEngComm, 2012, 14, 4196–4204 RSC.
- Z. Hong and C. Zhenhai, J. Electrochem. Soc., 1989, 136, 2771 CrossRef.
- S. Hassan, M. Suzuki and A. A. El-Moneim, Am. J. Mater. Sci., 2012, 2, 11–14 CrossRef.
- Z.-H. Huang, Y. Song, D.-Y. Feng, Z. Sun, X. Sun and X.-X. Liu, ACS Nano, 2018, 12, 3557–3567 CrossRef CAS.
- J. Kavil, P. M. Anjana, P. Periyat and R. B. Rakhi, Sustainable Energy Fuels, 2018, 2, 2244–2251 RSC.
Footnote |
| † Equal contribution in this paper. |
| This journal is © The Royal Society of Chemistry 2020 |