Open Access Article
Open Access ArticlePerformance of the dye-sensitized quasi-solid state solar cell with combined anthocyanin-ruthenium photosensitizer†
Eka Cahya Prima a,
Harbi Setyo Nugrohob,
Nugrahabc,
Gema Refanterob,
Camelia Panataranid and
Brian Yuliarto
a,
Harbi Setyo Nugrohob,
Nugrahabc,
Gema Refanterob,
Camelia Panataranid and
Brian Yuliarto *bc
*bc
aDepartment of Science Education, Faculty of Mathematics and Science Education, Universitas Pendidikan Indonesia, Bandung, Indonesia
bDepartment of Engineering Physics, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung, Indonesia. E-mail: brian@tf.itb.ac.id
cNational Research Center of Nanotechnology (NRCN), Institut Teknologi Bandung, Bandung, Indonesia
dDepartment of Physics, Faculty of Mathematics and Natural Science, Universitas Padjadjaran, Bandung, Indonesia
First published on 7th October 2020
Abstract
This work contributes to combining 12.2 mM purified anthocyanin of cyanidin-3-glucoside extracted from Indonesian black rice as the natural pigment with a ruthenium photosensitizer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
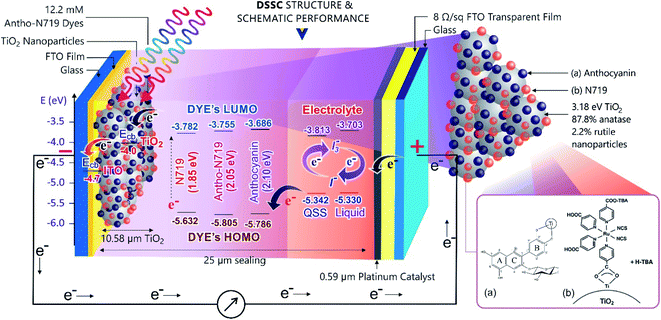
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in dye-sensitized solar cells (DSSCs) in liquid and quasi solid-state electrolytes. The findings essentially highlight the spectroscopic and electron transfer mechanism for the future trend of D–π–A natural pigment modification. The complete pigment comparison, dye absorbance, dye adsorption onto the semiconductor, dye electronic properties, electron excitation, and regeneration were investigated using spectroscopic methods. Cells employ TiO2 mesoporous nanoparticles (19.18 nm grain size, 50.99 m2 g−1 surface area, 87.8% anatase 12.2% rutile, 10.58 μm thickness, 3.18 eV band gap) sensitized by anthocyanin-N719 photosensitizer (12.2 mM) with the I−/I3− electrolyte (0.1 M lithium iodide/0.05 M iodine/0.6 M 1-buty-3-methylimidazolium iodide/0.5 M 4-tert-butylpyridine/polyethylene oxide Mw = 1 × 106) – Pt film. As a result, the quasi-solid state with combined anthocyanin-ruthenium dye-sensitized solar cell (3.51%) is achieved and reported for the first time. The work also achieved the highest efficiency of the anthocyanin dye-sensitized quasi-solid state solar cells of 2.65%. The insight on how the combined anthocyanin-N719 and the quasi-solid state electrolytes exhibit better performances will be further discussed.
1) in dye-sensitized solar cells (DSSCs) in liquid and quasi solid-state electrolytes. The findings essentially highlight the spectroscopic and electron transfer mechanism for the future trend of D–π–A natural pigment modification. The complete pigment comparison, dye absorbance, dye adsorption onto the semiconductor, dye electronic properties, electron excitation, and regeneration were investigated using spectroscopic methods. Cells employ TiO2 mesoporous nanoparticles (19.18 nm grain size, 50.99 m2 g−1 surface area, 87.8% anatase 12.2% rutile, 10.58 μm thickness, 3.18 eV band gap) sensitized by anthocyanin-N719 photosensitizer (12.2 mM) with the I−/I3− electrolyte (0.1 M lithium iodide/0.05 M iodine/0.6 M 1-buty-3-methylimidazolium iodide/0.5 M 4-tert-butylpyridine/polyethylene oxide Mw = 1 × 106) – Pt film. As a result, the quasi-solid state with combined anthocyanin-ruthenium dye-sensitized solar cell (3.51%) is achieved and reported for the first time. The work also achieved the highest efficiency of the anthocyanin dye-sensitized quasi-solid state solar cells of 2.65%. The insight on how the combined anthocyanin-N719 and the quasi-solid state electrolytes exhibit better performances will be further discussed.
1. Introduction
Dye-sensitized solar cells (DSSCs) have attracted considerable attention for the future generation of solar cells with a wide area of application such as window glass, car windshields, mobile chargers, or flexible and transparent solar cells due to their abundant, non-toxic, low-cost, and renewable sources.1,2 The natural dyes in DSSCs are obtained from organic sources such as anthocyanin, chlorophyll, betalain, and carotenoid.3 The dye absorber materials are bonded to the TiO2 porous material in order to broaden the semiconductor film absorbance. The electron is injected from the dye absorber materials into the n-type semiconductor during the illumination, and then a hole is left on the surface-absorbed dye ion.4 Due to the wide band gap and large surface area, TiO2 nanoparticles are used as photoelectrodes in this solar cell system.5The optimization of DSSC has become an attractive research interest for the further development of renewable energy due to abundant, low-cost, and renewable sources. One of key aims of DSSC development is to enhance the electron transferability. In comparison to the standard organometallic pigments based on the ruthenium (N719) material complexes, the electron transferability of the natural pigment remains lower even though the natural pigment has higher absorbance than that of N719.6 The molar extinction coefficient of anthocyanin dye is 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 at 550 nm, whereas that of the N719 photosensitizer is 14
900 M−1 cm−1 at 550 nm, whereas that of the N719 photosensitizer is 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 515 nm.7–9 Therefore, different methods are applied in this work so as to enhance the performance of natural photosensitizers such as dye combination,1,10–13 dye purification,14–18 and electrolyte layer modification.19–21
000 M−1 cm−1 at 515 nm.7–9 Therefore, different methods are applied in this work so as to enhance the performance of natural photosensitizers such as dye combination,1,10–13 dye purification,14–18 and electrolyte layer modification.19–21
The development of natural photosensitizers based on anthocyanin has become an attractive topic due to the hydroxyl group-containing pigment, which facilitates proper dye attachment.22,23 Kimura and co-workers explored the use of five single anthocyanin derivatives as purified dye photosensitizers.22 The best device with an efficiency of 1.42% in MeOH solvent was obtained using Pg3G as the dye photosensitizer. Another work by a different research group revealed that long-hydroxyl and carbonyl-chain bearing anthocyanin generates maximum electron transfer rate (κεT) and results in the highest efficiency of 1.87% for anthocyanin with a concentration of 1 g dye per 10 mL water.24 The use of pomegranate dye has been reported by Ghann et al., which successfully narrowed the band gap, and as a result, the power conversion efficiency of 2.0% was obtained.11 The use of four synthetic environment-friendly flavylium salts as anthocyanin-like sensitizers has been reported by Calogero et al.25 In our previous work, we investigated the pigments of single and combined cyanidin-3-O-glucoside and peonidin-3-O-glucoside with a concentration of 2.0 mM and pH 2.0.1 Experimental and theoretical investigations were done to broaden the understanding of how the combination of these pigments contributes to the electronic structure and the photochemical performance of the DSSC.
Natural DSSC photosensitizer studies have shown a novel trend for improving the natural pigment performance by modifying the D–π–A structure.26,27 Regardless of the contamination by toxic materials of the ruthenium pigment and the non-toxic materials of the natural pigment, the low natural performance needs to be improved due to the lack of absorbance, adsorption, and electron transfer ability compared to the standard organometallic pigments based on ruthenium complex materials. Although the previously established N719/TiO2 cell has achieved an efficiency 11.1%,28 we are concerned about benchmark natural pigments with N719. By studying and comparing these pigments, we believe that the findings will contribute to the development of novel modified natural photosensitizer pigments for future natural dye-sensitized solar cells. However, there is no complete head-to-head comparison between the natural sensitizer and N719 pigment, although this information is very fundamental for developing the natural pigment as a low-cost material with high efficiency. Interestingly, in comparison with N719, the molar extinction coefficient of anthocyanin dye is 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 at 550 nm, whereas that of the N719 photosensitizer is 14
900 M−1 cm−1 at 550 nm, whereas that of the N719 photosensitizer is 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 515 nm.16,18,29 Luo et al. reported the co-sensitized dithiafulvenyl-phenothiazine (DTF-PTZ)-based organic dyes with N719 for improving the performance of the DSSC.7 The DTF-PTZ and N719 co-sensitized DSSC showed significant improvement by about 3%. A different work by Liu et al. reported the combined PbS-N719 dye-sensitized material to improve the performance of the DSSC.30 This combination has elevated the open-circuit voltage (VOC) and increased the device efficiency from 5.95% to 6.35%. To the best of our knowledge, we have reported the combined anthocyanin-N719 photosensitizer in quasi-solid state dye-sensitized solar cells for the first time.
000 M−1 cm−1 at 515 nm.16,18,29 Luo et al. reported the co-sensitized dithiafulvenyl-phenothiazine (DTF-PTZ)-based organic dyes with N719 for improving the performance of the DSSC.7 The DTF-PTZ and N719 co-sensitized DSSC showed significant improvement by about 3%. A different work by Liu et al. reported the combined PbS-N719 dye-sensitized material to improve the performance of the DSSC.30 This combination has elevated the open-circuit voltage (VOC) and increased the device efficiency from 5.95% to 6.35%. To the best of our knowledge, we have reported the combined anthocyanin-N719 photosensitizer in quasi-solid state dye-sensitized solar cells for the first time.
Lately, the discussion of a liquid versus quasi-solid state electrolyte for DSSC has attracted research interest due to its unique properties.20,31 Quasi-solid state electrolytes have the features of solids such as definite shape and volume. Nevertheless, they also have liquid properties such as high ionic conductivity and better interfacial contact with the mesoporous photoanode. Unfortunately, DSSC based on quasi-solid electrolyte tend to generate lower efficiency compared to liquid electrolyte-based DSSC due to poorer ion diffusion and sub-optimal interfacial contact with the mesoporous layer.32 Although the use of liquid electrolytes usually shows better ionic conductivity, the treatment also leads to several problems concerning solar cell sealing and manufacturing such as electrolyte leakage, volatilization, desorption of the adsorbed photosensitizers, and corrosion of the counter electrode, which contribute to instability during long-term usage.31 For these reasons, the utilization of quasi-solid state electrolytes has interested researchers to optimize the performance of DSSC.
Regarding the quasi solid-state electrolyte for natural pigment-based DSSC assembly, the problem of quasi solid-state electrolyte penetration through the dye-adsorbed TiO2 has been treated by providing the preheated quasi solid-state electrolyte before insertion into the cell sandwich. The semi-liquid phase of the electrolyte facilitates the electrolyte to penetrate through the semiconductor before being dry as a gel. In this work, the quasi-solid state electrolyte has been compared to the liquid electrolyte with the same I−/I3− concentration ratio. 0.6 M 1-buty-3-methylimidazolium iodide (BMII) and 0.5 M 4-tert-butylpyridine (TBP) have been employed as the stabilizing additives for the LiI/I2-based electrolyte in acetonitrile solution. The quasi-solid state electrolyte has been prepared based on the polyethylene oxide polymer (Mw = 1 × 106).
The characterization was performed by the spectroscopic methods in order to consistently compare the performance of the anthocyanin-N719 pigment, including the absorbance, adsorption, electronic properties, energy efficiency, and charge transfer properties. Fourier transform infrared (FTIR) analysis was performed to analyze the molecular vibration of the dye photosensitizer. UV-Vis spectroscopy was performed to investigate the dye's absorbance. Cyclic voltammetry was performed to analyze the redox potentials. Also, the oxidation potential, the reduction potential, the HOMO–LUMO orbitals, and the gap energy were included in the potential redox analysis.39 The I–V characteristics were studied to analyze the low current density (JSC), the open-circuit voltage (VOC), the fill factor (FF), and the power conversion efficiency (η). Moreover, the electron injection investigation was also performed using several electrochemical impedance spectroscopy (EIS) parameters such as the diffusion resistance (Rd), recombination resistance (Rr), diffusion lifetime (τd), recombination lifetime (τn), diffusion rate (Dn), chemical capacitance (Cμelec), and conductivity (σn).
2. Materials and methods
2.1 Preparation of the photosensitizers
Indonesian black rice (Oryza sativa L.) pigment was extracted and purified to obtain cyanidin-3-O-glucoside anthocyanin photosensitizers. The black rice grains were firstly dried and ground to prepare black rice powder. Unpurified anthocyanin extract was prepared by adding 200 g black rice powder into a solution and then stirring it at 800 rpm at the temperature of 50 °C for 2.5 hours. The solution was previously prepared by stirring together 400 mL methanol and 5 mL hydrochloric acid in 95 mL distilled water for 5 minutes under room temperature. The mixture was then macerated for three weeks at room temperature in dark conditions. The mixture was then filtered using a vacuum pump through 110 mm Whatman's filter paper after previously centrifuging it at 4000 rpm for 30 minutes. The extract was then poured into 50 mL aluminum-coated glass and placed in a refrigerator at 5 °C to store the pigment. Then, the extract was evaporated at 40 °C for two days in a darkroom and vacuum conditions to obtain 4.5 g of the unpurified anthocyanin powder. The anthocyanin concentration was measured using eqn (1). Moreover, it was then adjusted by diluting a similar solvent.18,30| Anthocyanin concentration (mg L−1) = A × MW × DF × 103/ε × l | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 relative to the cyanidin-3-glucoside, l is the 1 cm light-path length, and A is the absorbance given by eqn (2).
900 M−1 cm−1 relative to the cyanidin-3-glucoside, l is the 1 cm light-path length, and A is the absorbance given by eqn (2).| A = (Amax − A700)pH 1 − (Amax − A700)pH 4.5 | (2) |
The dye's pH was adjusted by diluting the 0.025 M potassium chloride buffer solution to result in pH 1.0 and 0.4 M sodium acetate to result in pH 4.5. The particular buffer solution was diluted by 225 mL H2O, and pH was adjusted to 1.0 and 4.5 by adding hydrochloric acid dropwise. Next, the purification of the unpurified anthocyanin powder was done to separate cyanidin-3-glucoside and peonidin-3-glucoside using the standardized method previously reported by Park et al. and Ordaz-Galindo et al.18,33 The method was carried out by dissolving the unpurified anthocyanin powder in 15 mL n-hexane and then pouring it into an Amberlite XAD-7 Aldrich® calibrated chromatography column with the solvent reservoir. The polar constituents were removed by eluting the column with 15 mL HCl 0.3% and then the solution was sequentially eluted using 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 30
80, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, and 40
70, and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 vol% methanol
60 vol% methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water containing HCl 0.3%. Precoated RP-18 F254s thin-layer chromatography (TLC) plate (0.25 mm layer thickness) was then used to test the isolated anthocyanin extract with 10
water containing HCl 0.3%. Precoated RP-18 F254s thin-layer chromatography (TLC) plate (0.25 mm layer thickness) was then used to test the isolated anthocyanin extract with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol% methanol
90 vol% methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water containing HCL 0.3% to analyze the XAD-7 Aldrich®-calibrated chromatography column fractions.34,35 The detailed analysis of anthocyanin concentration can be found in the ESI in Fig. S3–S5.† The purification provided 3.06 g cyanidin-3-glucoside and 0.27 g peonidin-3-glucoside. In this work, cyanidin-3-glucoside has been subsequently named as anthocyanin. Finally, the combined anthocyanin-N719 dye-photosensitizer was prepared by mixing purified anthocyanin and N719 in the stoichiometric ratio of 1
water containing HCL 0.3% to analyze the XAD-7 Aldrich®-calibrated chromatography column fractions.34,35 The detailed analysis of anthocyanin concentration can be found in the ESI in Fig. S3–S5.† The purification provided 3.06 g cyanidin-3-glucoside and 0.27 g peonidin-3-glucoside. In this work, cyanidin-3-glucoside has been subsequently named as anthocyanin. Finally, the combined anthocyanin-N719 dye-photosensitizer was prepared by mixing purified anthocyanin and N719 in the stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a solution containing 19 mL H2O, 80 mL MeOH, and 1 mL HCl, resulting in 12.2 mM pigment concentration with pH 1.0 in all the cases.
1 in a solution containing 19 mL H2O, 80 mL MeOH, and 1 mL HCl, resulting in 12.2 mM pigment concentration with pH 1.0 in all the cases.
2.2 Preparation of liquid and quasi solid-state electrolytes
The liquid-state electrolyte was prepared by mixing 0.1 M lithium iodide (LiI) and 0.05 M iodine (I2) in 100 mL acetonitrile. The solution was then stirred at 300 rpm for 5 minutes until a well-mixed solution was formed. Next, 0.6 M 1-buty-3-methylimidazolium iodide (BMII) and 0.5 M 4-tert-butylpyridine (TBP) were added and stirred overnight. The electrolyte was then poured into a dark bottle and placed at room temperature. Afterwards, the electrolyte solution was ready to use as a liquid-state electrolyte mediator for DSSC containing I−/I3−. Finally, the quasi-solid electrolyte was prepared by mixing 10 mL electrolyte solution and 2 g polyethylene oxide (Mw = 1 × 106). This mixture was then stirred at room temperature for 24 hours until it became a quasi-solid state. The quasi-solid state electrolyte was maintained at 60 °C before being attached to the dye-adsorbed TiO2.2.3 Preparation of the Surlyn spacer
The Surlyn polymer was cut to have an area of 2 × 2 cm2 with 25 μm thickness in order to prepare the sealant material. The inside spacer hole was also cut to have an area of 0.7 × 0.7 cm2 so as to make the film frame. The cell was assembled after uncovering the thicker plastic from the sealant material and then the two sealants were clipped to each other. Lastly, the spacer was cleaned using isopropyl alcohol.2.4 Preparation of TiO2 nanoparticle photoelectrode and Pt-Counter electrode
The TiO2 nanoparticle film was prepared using the sol–gel method. First, the solvent solution was prepared by stirring 30 mL acetic acid and 30 mL water at 600 rpm for 5 minutes. 5 g titanium dioxide P25 and 1.5 g polyethylene glycol (PEG, molecular weight = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) as a polymer was added into the solution and stirred at 600 rpm for 3 hours. This material was used to prevent agglomeration. A few drops of the Triton X-100 surfactant were added to the solution. Moreover, few drops of hydrochloric acid were also added to the paste so as to adjust the pH to 1.0. This treatment has a vital role in improving the number of anatase phases and the stability of TiO2 nanoparticles, following the previous work by Chae, et al.36 This evidence was supported by Tryba et al., who found that under acidic conditions, TiO2 nanoparticles possessed the most crystallized anatase phase in comparison to the others, although this treatment did not contribute to the enlargement of the surface area of the particles.37
000) as a polymer was added into the solution and stirred at 600 rpm for 3 hours. This material was used to prevent agglomeration. A few drops of the Triton X-100 surfactant were added to the solution. Moreover, few drops of hydrochloric acid were also added to the paste so as to adjust the pH to 1.0. This treatment has a vital role in improving the number of anatase phases and the stability of TiO2 nanoparticles, following the previous work by Chae, et al.36 This evidence was supported by Tryba et al., who found that under acidic conditions, TiO2 nanoparticles possessed the most crystallized anatase phase in comparison to the others, although this treatment did not contribute to the enlargement of the surface area of the particles.37
Furthermore, the solution was heated and kept at 80 °C with 600 rpm stirring. Sonication treatment was applied for 3 hours to obtain the uniform TiO2 paste for further TiO2 film preparation. The TiO2 paste was then spin-coated onto fluorine-doped tin oxide (FTO) glass at 4000 rpm for 30 seconds. The FTO glass has an area of 2 × 2.5 cm2 with a frame size of 0.5 × 0.5 cm2 and a sheet resistance of 8 Ω per sq. The TiO2 film photoelectrode with a film area of 0.25 cm2 was fabricated using the spin-coating process, as seen in the ESI in Fig. S7.† The spin-coating process was repeated twice to obtain the desired thickness of about 10.58 μm. The film was then heated using a three-stage temperature gradient from 125 °C for 15 minutes, 325 °C for 15 minutes, and 450 °C for 30 minutes with the heating rate of 2 °C per minute.
The dye photosensitizers were then deposited onto the TiO2 film photoelectrode by soaking the TiO2 film photoelectrode in the dye solution for four days. In this work, we prepared three different dye solutions with the same concentration of 12.2 mM and volume of 10 mL, namely, anthocyanin, anthocyanin-N719 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and N719; hence, we present three different variations of the dye absorber. After the soaking process, the loose pigments were then removed from the TiO2 film photoelectrode using isopropyl alcohol. To evaluate the dye adsorption, we analyzed the pigment concentration before and after dye adsorption. All the film glasses were immersed in the pigments. Regardless of the small amount of pigment adsorption on FTO when we removed the films from the dyes, we assumed that the pigment was predominantly adsorbed onto the TiO2 film. The dye before and after adsorption was diluted 50 times with the same solvent so as to investigate it using UV-Vis spectroscopy, as reported in Fig. 7. The counter electrode film was then prepared by cleaning it using acetone, 2-propanol, and water, followed by drying at 60 °C. The Pt layer was sputtered as the counter electrode film under a pressure and applied current of 10 Pa and 20 mA, respectively; the film was calcined at 300 °C for 30 minutes.
1), and N719; hence, we present three different variations of the dye absorber. After the soaking process, the loose pigments were then removed from the TiO2 film photoelectrode using isopropyl alcohol. To evaluate the dye adsorption, we analyzed the pigment concentration before and after dye adsorption. All the film glasses were immersed in the pigments. Regardless of the small amount of pigment adsorption on FTO when we removed the films from the dyes, we assumed that the pigment was predominantly adsorbed onto the TiO2 film. The dye before and after adsorption was diluted 50 times with the same solvent so as to investigate it using UV-Vis spectroscopy, as reported in Fig. 7. The counter electrode film was then prepared by cleaning it using acetone, 2-propanol, and water, followed by drying at 60 °C. The Pt layer was sputtered as the counter electrode film under a pressure and applied current of 10 Pa and 20 mA, respectively; the film was calcined at 300 °C for 30 minutes.
2.5 DSSC assembling
The devices were then completed by sandwiching the dye-absorbed TiO2 film and the Pt-sputtered film with the insertion of the I−/I3− electrolyte and sealing with the prepared Surlyn frame. Besides, two different electrolyte states, namely, the liquid state and the quasi-solid state, were prepared in this work. We present six different samples with the variation of the dyes and the electrolyte state: (a) anthocyanin with the liquid-state electrolyte, (b) anthocyanin with the quasi-solid state electrolyte, (c) anthocyanin-N719 with the liquid-state electrolyte, (d) anthocyanin-N719 with the quasi-solid state electrolyte, (e) N719 with the liquid-state electrolyte, and (f) N719 with the quasi-solid state electrolyte. Each sample was inserted by the 25 μm Surlyn polymer, as described before, clipped on the four sides of the sandwiched film, and then put under a 350 nm UV lamp for 30 seconds. Subsequently, the samples were put on a hotplate and then dried at 100 °C for 60 seconds. Finally, the samples were cooled under room temperature and were then ready to conduct the characterization and measurements.2.6 Characterization and measurements
Spectroscopic studies were used for the investigation in this work. A FT/IR Jasco-6100, Japan, equipped with a potassium bromide (KBr) plate with scanning range from 400 to 4500 cm−1, was used for the spectroscopic analysis, the binding vibrations of the organic molecule in solution. A UV-Vis spectrophotometer from Hewlett Packard 8453 Agilent Technologies and a Jasco V-670 Spectrophotometer were used to check the dye's absorbance. A Hitachi S-4800 FE-SEM was used to study the morphology and the thickness of the photoanode coatings. The NMR spectra were recorded on a JEOL JNM-ECP500 (1H: 500 MHz). The anthocyanin NMR analysis referred to the previous finding by Fernandes et al., Lee, and Park et al.33,38,40 The eDAQ potentiostat is equipped with an e-coder 401, software Echem Ver. 2.1, and the scan rate of 50 mV s−1, the range of −1.8 V to +1.8 mV, and the positive initial direction was used for cyclic voltammetric measurements. The three electrodes used were a platinum counter electrode, a glassy carbon electrode, and an Ag/AgCl reference in 3 M NaCl solution as the reference electrode. The electrolyte used was 0.1 M tetrabutylammonium tetrafluoroborate (n-C4H9)4NPF6 in acetonitrile as the supporting electrolyte. The empirical Bredas equation was estimated to determine the HOMO position based on the onset curve of voltammetry.41 The dye's highest occupied molecular orbital (HOMO) is given by eqn (3).| EHOMO = −e(Eonsetox + 4.4) | (3) |
The Eonsetox data is plotted from the oxidation potential voltammetry measured by the eDAQ potentiostat. The dyes' direct energy gaps were calculated from the UV-Vis spectral data using Tauc's Relation, as estimated in eqn (4).42
| αhv ≈ (hv − Eg)n | (4) |
The dyes' direct band gaps (Eg) were obtained from the α extrapolation to 0 when n = 2. On the other hand, Eg is the dye's band gap, α is the dye's absorbance, and hv is the photon energy. The absorbance data was be used to obtain the band gap and light-harvesting efficiency (LHE) of the absorber layer of each sample. The band gap was obtained using the Tauc plot method and LHE was obtained using eqn (5).43
| LHE(λ) = (1 − 10−∞) × 100% | (5) |
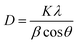 | (6) |
3. Results and discussion
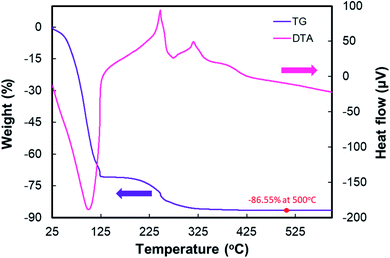
Fig. 1 describes the Thermo Gravimetric-Differential Thermal Analysis (TG-DTA) results for TiO2 P25. From the TGA curve, when the sample was heated up to 125 °C, there was a dramatic weight loss of about 70.8% due to the hydroxyl (–OH) group solvent evaporation from acetic acid and water.51 The second region between 125–325 °C was attributed to organic decomposition. As the temperature was increased up to 420 °C, the weight loss was due to residual polymer oxidation and decomposition of chlorine (obtained from hydrochloric acid) bonded to Ti–OH.51 The TGA curve also shows that the residual polymer was removed entirely at calcination temperatures above 450 °C. As the temperature increased up to 500 °C, the weight loss was about 86.55%.The DTA curve clearly presents two peaks, an endothermic peak in the region of 100–250 °C, which is assigned to solvent evaporation, and a broad exothermic peak at about 325 °C–420 °C, which is assigned to the decomposition of the chlorine, the polymer, and the phase transformation from amorphous to TiO2 anatase. The phase transformation starts from 325 °C.51 As shown in the full TG-DTA curve, the data from the TGA curve directly corresponds to the DTA record because the endothermic or the exothermic processes are always accompanied by weight loss.
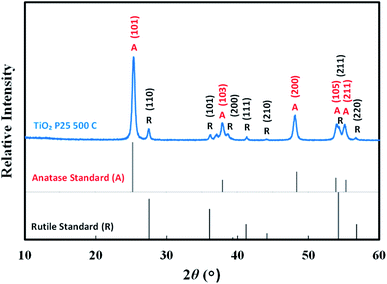
Fig. 2 depicts the X-ray diffraction pattern of TiO2 nanoparticles compared to the standard anatase and rutile peaks from JCPDS 21-1272 and 21-1276, respectively. The graph indicates that TiO2 nanoparticles result in the structure of mixed anatase and rutile. The photoelectrode contains 87.8% anatase, as confirmed by the diffraction peaks at (101), (103), (200), (105), and (211), which respectively appear at the 2θ of 25.30°, 37.78°, 47.98°, 51.58°, and 55.02°, respectively. The semiconductor also contains 12.2% rutile phase, as confirmed by the diffraction peaks at (110), (101), (200), (111), (210), (211), and (220), which respectively appear at 2θ of 27.51°, 36.04°, 39.31°, 41.19°, 44.14°, 54.23°, and 56.78°. According to the grain size calculation corresponding to all the planes using Scherrer's equation on the plane of (101), the average particle size is approximately 19.185 nm. A smaller crystallite size of the particles will facilitate a higher number of dye molecules attaching the TiO2 film. Moreover, the band gap analysis of TiO2 shows that the film has a 3.18 eV optical TiO2 band gap.
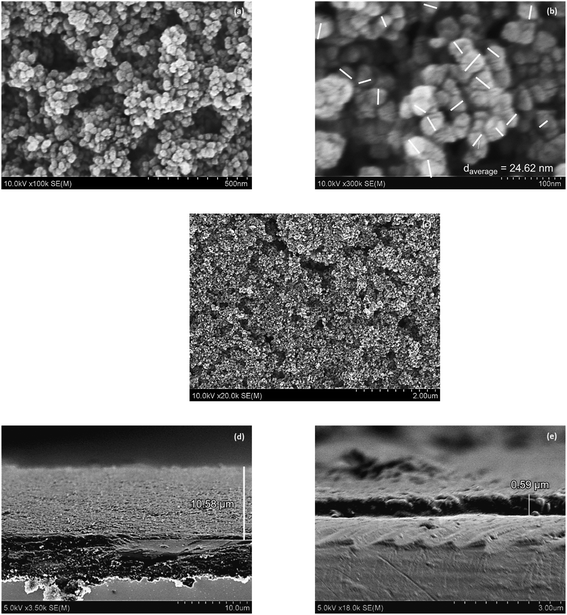
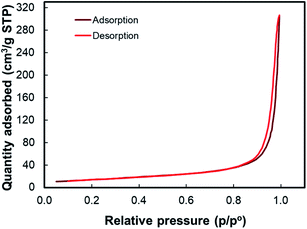
Fig. 3 shows the scanning electron microscopy (SEM) images of TiO2 nanoparticles. According to Fig. 3(a), it can be seen that the film thickness influences the number of pigment attachments in contrast to the electron transports. Therefore, the TiO2 photoelectrode has been adjusted to the film thickness of 10.58 μm according to the previously optimized result.6 Fig. 3(e) also displays that the Pt film has a thickness of 0.59 μm. The TiO2 images in Fig. 3(a–c) show that the TiO2 nanoparticles have a unique mesoporous morphology. The mesoporous morphology facilitates the dyes to adsorb as well as redox couple (I−/I3−) penetration into the film. The average grain size obtained from the SEM image is about 24.62 nm. The surface analysis of TiO2 nanoparticles conducted using the Brunauer–Emmett–Teller (BET) method demonstrates the graph of the isothermal linear plot of the adsorption–desorption of N2 gas surrounding the TiO2 nanoparticles and the pore size distribution curves, as seen in Fig. 4. The sample demonstrated the isotherm of type IV (BDDT classification) with the H3 hysteresis loop type in the relative pressure range of 0.86–1.0. The result shows the existence of a mesoporous structure. The average surface area of the TiO2 nanoparticles is 50.9931 m2 g−1.
The Fourier transform infrared (FTIR) spectra of the pigments and photoelectrodes from anthocyanin, N719, combined anthocyanin-N719, and TiO2 nanoparticles are shown in Fig. 5. The FTIR spectra of the combined anthocyanin-N719 dye were compared to the infrared spectra of anthocyanin and the N719 standards.29,52–55 Three vibrational peaks at anthocyanin also appear in the combined anthocyanin-N719. First, the peak presented at 588 cm−1 is affected by the stretching vibration of the C–O–C aliphatic ether group from glucose. Second, the peak shown at 1075.1 cm−1 is assigned to the in-plane bending vibration of C–H and stretching vibration of C–O. Third, the absorption peak at 1639.2 cm−1 is assigned to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O aromatic group.
O aromatic group.
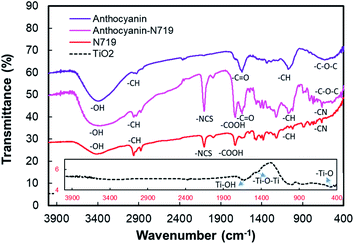 | ||
| Fig. 5 Fourier transform infrared spectra of anthocyanin, anthocyanin-N719, N719, and TiO2 nanoparticles. | ||
Moreover, four vibration peaks at N719 also appear in the combined anthocyanin-N719. First, the peak presented at 663.4 cm−1 is affected by the out-of-plane bending vibration of C–H and C–N. Second, the peak shown in the range 670–829 cm−1 is assigned to the third-order stretching vibration of C–H and C–N. Third, the absorption peak at 1720.2 cm−1 is assigned to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH groups. Fourth, the peak shown at 2101.1 cm−1 is affected by the stretching vibration of the NCS group. The two vibrational peaks also display all the pigments. The vibrational peaks in the range of 2872–2975 cm−1 are due to the stretching vibration of the alkane group C–H. The group exists in all the pigments. Finally, the vibrational peak at the range 2975–3600 cm−1 is assigned to the stretching vibration of O–H. The group plays an essential role in binding the TiO2 semiconductors. The vibrational analysis of TiO2 shows that the first vibration peak at 460.7 cm−1 is due to the stretching vibration of –Ti–O. The vibration peaks in the range from 983.5 through 1000.9 cm−1 indicate the stretching vibration of Ti–O–Ti. Finally, the vibration peak at 1637.3 cm−1 is assigned to the stretching vibration of Ti–OH. Detailed information regarding the vibrational analysis is described in Tables S1 and S2 in the ESI.†
O and COOH groups. Fourth, the peak shown at 2101.1 cm−1 is affected by the stretching vibration of the NCS group. The two vibrational peaks also display all the pigments. The vibrational peaks in the range of 2872–2975 cm−1 are due to the stretching vibration of the alkane group C–H. The group exists in all the pigments. Finally, the vibrational peak at the range 2975–3600 cm−1 is assigned to the stretching vibration of O–H. The group plays an essential role in binding the TiO2 semiconductors. The vibrational analysis of TiO2 shows that the first vibration peak at 460.7 cm−1 is due to the stretching vibration of –Ti–O. The vibration peaks in the range from 983.5 through 1000.9 cm−1 indicate the stretching vibration of Ti–O–Ti. Finally, the vibration peak at 1637.3 cm−1 is assigned to the stretching vibration of Ti–OH. Detailed information regarding the vibrational analysis is described in Tables S1 and S2 in the ESI.†
The hydroxyl group is present as the anchoring group, as seen in 2975–3600 cm−1 from the vibrational analysis in the anthocyanin as well as the N719 molecules, which facilitates the molecules binding the semiconductor surface. Fig. 6 shows that cyanidin-3-O-glucoside possesses eight hydroxyl groups, while N719 contains two carboxylic acid and two carboxylate groups. Thus, it can anchor onto the TiO2 surface by the close overlap of the ligand π* orbitals and the 3D orbitals of TiO2. The carboxylic group tends to strengthen the binding by creating a combination of bidentate-bridging and H-bonding involving a donating group from the N719 (and/or Ti–OH) units and an acceptor from the Ti–OH (and/or N719) groups,56 while the hydroxyl group of anthocyanin results in the monodentate, bridge bidentate, or chelate mode since the first studies reported on anthocyanin DSSCs, while chelate catechol binding has been indicated as the primary and most efficient mode for anthocyanin adsorption on TiO2.7,8
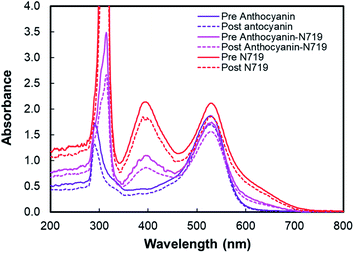
Table 1 tabulates the dye adsorption analysis of anthocyanin, combined anthocyanin-N719, and N719 from UV-Vis analysis following the previous works.57–61 ‘Pre’ means pigment absorbance before TiO2 adsorption, while ‘Post’ means pigment absorbance after TiO2 adsorption. From the information based on the dye-adsorption on TiO2 for anthocyanin, combined anthocyanin-N719, and N719, it was found that the dyes were adsorbed on TiO2 by 5.657, 6.325, and 7.355 nmol cm−2, respectively. The combination of anthocyanin-N719 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) also makes the dye adsorption 11.81% stronger than anthocyanin binding. It can be analyzed that 0.89 nmol N719 and 0.69 nmol anthocyanin dyes were adsorbed on the 0.25 cm2 TiO2 nanoparticle film compared to the individual N719 and anthocyanin pigments, which resulted in 1.84 and 1.41 nmol, respectively. These findings were confirmed by the dye-adsorbed TiO2 absorbance, as seen in Fig. 8. The finding implies that the dye's anchoring group plays a fundamental role in improving the natural pigment's efficiency by strengthening the pigment attachment. The anthocyanin pigment with the carboxylic anchoring group was exciting for further investigation.
1) also makes the dye adsorption 11.81% stronger than anthocyanin binding. It can be analyzed that 0.89 nmol N719 and 0.69 nmol anthocyanin dyes were adsorbed on the 0.25 cm2 TiO2 nanoparticle film compared to the individual N719 and anthocyanin pigments, which resulted in 1.84 and 1.41 nmol, respectively. These findings were confirmed by the dye-adsorbed TiO2 absorbance, as seen in Fig. 8. The finding implies that the dye's anchoring group plays a fundamental role in improving the natural pigment's efficiency by strengthening the pigment attachment. The anthocyanin pigment with the carboxylic anchoring group was exciting for further investigation.
| Dye | Peak1 | Peak2 | Peak3 | ΔAbsλ 530 nm (a.u.) | Dye-adsorbed TiO2 (nmol cm−2) | |||
|---|---|---|---|---|---|---|---|---|
| λmax (nm) | Abs (a.u.) | λmax (nm) | Abs (a.u.) | λmax (nm) | Abs (a.u.) | |||
| Pre anthocyanin | 290 | 1.727 | — | — | 528 | 1.868 | 0.173 | 5.657 |
| Post anthocyanin | 289 | 1.328 | — | — | 529 | 1.695 | ||
| Pre anthocyanin-N719 | 314 | 3.486 | 396 | 1.098 | 530 | 1.744 | 0.181 | 6.325 |
| Post anthocyanin-N719 | 314 | 2.666 | 396 | 0.862 | 529 | 1.563 | ||
| Pre N719 | 314 | 7.000 | 393 | 2.139 | 530 | 2.116 | 0.255 | 7.355 |
| Post N719 | 314 | 7.000 | 395 | 1.792 | 530 | 1.861 | ||
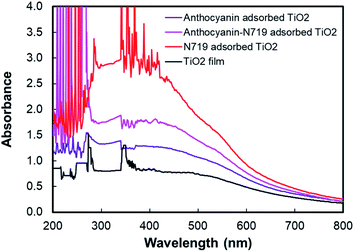 | ||
| Fig. 8 UV-Vis spectra of anthocyanin-adsorbed TiO2, combined anthocyanin-N719 adsorbed TiO2, and N719 adsorbed TiO2. | ||
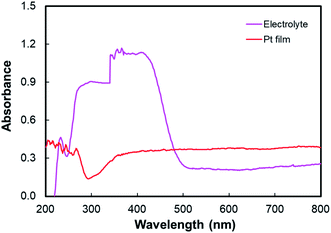
Fig. 9 depicts the light-harvesting efficiencies of the pigments. The introduction of N719 into anthocyanin successfully increased the light-harvesting efficiency by 11.2%, as seen in Table 2. Moreover, N719 has 24.5 times better efficiency compared to the anthocyanin dye. The pigment combination successfully reduced the pigment's tail band gap by 0.28 eV. The narrower band gap facilitates better electron transfer. The evidence correlates the dye attachment ability with the presence of many carboxylic groups. It is believed that the bidentate mode of binding is the most effective way of facilitating charge transfers. The middle peak at 395 nm in the combined anthocyanin-N719 pigment appears due to the existence of N719 dye in the combined pigment. The peaks at 395 and 530 nm appear because the low-energy metal-to-ligand charge-transfer transition band exists in N719. During the anthocyanin-N719 combination, the change in the energy of the lowest unoccupied molecular orbital (LUMO) of the N719 ligand causes the π–π* and dπ–π* transitions to occur at higher and lower energies, respectively.57 As a result, in Table 1, the first peak of anthocyanin has a bathochromic shift of about 24 nm from 290 nm to 314 nm. In the same case, the second peak of anthocyanin has a bathochromic shift of about 1 nm from 528 nm to 529 nm. Moreover, the primary absorption spectra of anthocyanin structure due to the benzoyl (ring A) and cinnamoyl (ring B) systems are identified as Band I (289–300 nm) and Band II (261–266 nm), respectively. Band I shifted due to the presence of a glucose group, showing the absorption spectra in the range of 507–530 nm.1 We also tested the absorbance spectra of the liquid I−/I3− electrolyte mediator as well as the Pt Film, as seen in Fig. 10. Although this material does not play a significant role in generating electron–hole separation, the result has confirmed that Pt does not significantly absorb the light spectrum within all the UV-Vis range. Moreover, the electrolyte mediator tends to absorb the light spectrum in the range of 220–530 nm. This absorbance range results in the overlap band of I−/I3−, as seen in the usual mechanism of DSSC acting as a redox mediator to regenerate the oxidized dyes. We also summarized the DSSC mechanism based on our investigation in Fig. 6.
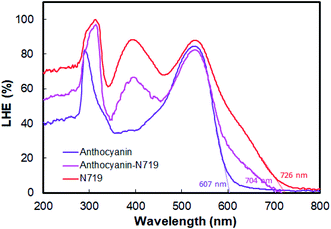 | ||
| Fig. 9 Light-harvesting efficiency of anthocyanin, combined anthocyanin-N719, and N719 photosensitizers. | ||
| Dye | LHE200–500 nmmean (%) | λtail (nm) | Etail band gap (eV) | ΔLHEDye-Antho (%) |
|---|---|---|---|---|
| Anthocyanin | 54.5 | 607 | 2.04 | — |
| Anthocyanin-N719 | 65.7 | 704 | 1.76 | 11.2 |
| N719 | 79.0 | 726 | 1.70 | 24.5 |
Fig. 11, 12, and Table 3 show the direct band gaps plotted from the Tauc's plots. The direct band gap corresponds to the vertical electron transition during the excitation.14 Nguyen showed that a direct band gap transition would dominantly lead to the electron injection process.58 Therefore, the direct band gap was then investigated to examine the character of the dye's vertical transition during photoexcitation. The electronic structure of the photosensitizers focused on the investigation of anthocyanin, as well as combined anthocyanin-N719 before and after the pigment adsorption. As shown in Fig. 11, anthocyanin, combined anthocyanin-N719, and N719 show a precise fit, resulting in a direct band gap of 2.10, 2.05, and 1.85 eV, respectively. Interestingly, the band gaps decrease since the pigments have been attached to the semiconductor; the direct band gap of anthocyanin, combined anthocyanin-N719, and N719 after adsorption are 1.76, 1.69, and 1.63 eV, respectively, as depicted in Fig. 12. The finding indicates that TiO2 contributes to the broadening of light absorption, which is consistent with the evidence from Fig. 8. The ruthenium pigment adsorptions contribute to extending the natural pigment photosensitization. Consequently, the pigment lowered the tail band gaps. To find out the exact position of molecular orbital transition, we investigated the HOMO–LUMO level via the cyclic voltammetric method, as seen in Fig. 13 and Table 5. The details of the HOMO–LUMO transition have been investigated in our previous work.1 Although the other level of HOMO–LUMO also contributes to the electronic transitions, the previous work has resulted in the highest electron transition probability of about 49.9%. Table 4 tabulates the HOMO–LUMO level and the gap for the photosensitizer. The LUMO position lowered with the contamination of N719 (LUMON719 < LUMOAnthocyanin-N719 < LUMOAnthocyanin). In contrast, the HOMO position decreased with the contamination of anthocyanin (HOMOAnthocyanin < HOMOAnthocyanin-N719 < HOMON719). This finding was confirmed by the previous bathochromic shift of the first peak of anthocyanin in Table 1 and Fig. 7. The pigment shows a bathochromic shift of about 24 nm from 290 nm to 314 nm when N719 was introduced into anthocyanin in the combined pigment. In the same case, the second peak of anthocyanin shows a bathochromic shift of about 1 nm from 528 nm to 529 nm. The summary of the HOMO–LUMO levels is illustrated in Fig. 6. It is observed that these findings affect the performance and electron transfer properties of DSSC. The closer the LUMO level to the conduction band of TiO2, the easier the transfer of the excited electron to the semiconductor. On the other hand, the closer the HOMO position towards the I− potential, the easier the regeneration of the oxidized dye by the electrons. According to this condition, it is proven explained why N719 has the best performance among the investigated pigments. Interestingly, N719 contamination in anthocyanin results in the lowering of the LUMO level and the raising of the HOMO level. Apart from broadening the pigment's absorption, these levels play a crucial role in facilitating electron transfer. Moreover, the time of electron diffusion and dye regeneration is expected to be the key to improving the stability of natural DSSC in the future.
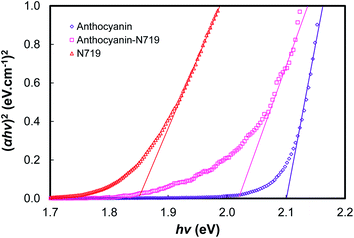 | ||
| Fig. 11 The plot of (αhv)2 versus hv for the direct transition of anthocyanin, combined anthocyanin-N719, and N719. | ||
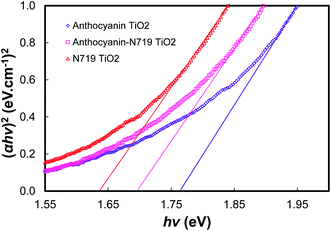 | ||
| Fig. 12 The plot of (αhv)2 versus hv for the direct transition of anthocyanin adsorbed TiO2, combined anthocyanin-N719 adsorbed TiO2, and N719 adsorbed TiO2. | ||
| Dye | EBand Gap (eV) | Dye–TiO2 | EBand Gap (eV) |
|---|---|---|---|
| Anthocyanin | 2.10 | Anthocyanin–TiO2 | 1.76 |
| Anthocyanin-N719 | 2.05 | Anthocyanin N719–TiO2 | 1.69 |
| N719 | 1.85 | N719–TiO2 | 1.63 |
| Dye | Eox (V) | EHOMO (eV) | Ered (V) | ELUMO (eV) | EBand Gap (eV) |
|---|---|---|---|---|---|
| Anthocyanin | −1.386 | −5.786 | −0.714 | −3.686 | 2.10 |
| Anthocyanin-N719 | −1.405 | −5.805 | −0.645 | −3.755 | 2.05 |
| N719 | −1.232 | −5.632 | −0.618 | −3.782 | 1.85 |
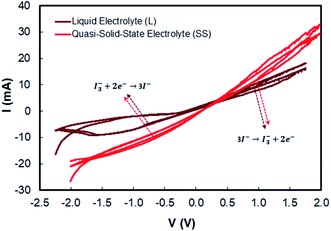
| Eox (V vs. Ag/AgCl) | Eox (V) | Ered (V vs. Ag/AgCl) | Ered (V) | ΔEred–ox (V) | Ipc (mA) | Ipa (mA) | n = Ipc/Ipa | |
|---|---|---|---|---|---|---|---|---|
| Liquid electrolyte (L) | 0.930 | −5.330 | −0.697 | −3.703 | −1.627 | 8.87 | 5.06 | 1.753 |
| Quasi-solid state electrolyte (SS) | 0.942 | −5.342 | −0.587 | −3.813 | −1.529 | 11.33 | 8.81 | 1.286 |
Based on the intermediate results of optical band gap, LUMO, and HUMO analysis, it is clear that the combination does not significantly exert a good synergetic effect; it seems that the number of individually attached dyes are directly transferred from the electrons to the TiO2 nanoparticles.
The previous work implied that the novel electrolyte must be introduced into the natural pigment so that the problem of the time for the regeneration of the oxidized dye can be boosted by providing a more stable electrolyte. In this work, we introduce the quasi-solid state electrolyte, which is to be applied to the combined anthocyanin-N719 dye. However, there is a previous exciting discussion regarding the conductivity of quasi-solid state pigments that lowers the cell performance. We believe that a better assembling process also contributes to the attachment of the electrolyte penetrating the semiconductor mesoporous firmly. The better stability of the quasi-solid state electrolyte is also the reason for researchers to fabricate solar cells with more stability.31,62–67 In this case, by comparing two electrolytes with a similar I− composition, then considering the dye regeneration by I− with the mechanism given eqn (4), we can analyze that the quasi-solid state electrolyte presented better dye regeneration due to the 0.012 V lower oxidation potential compared to the liquid electrolyte, resulting in a sharper peak current Ipc by about 2.46 mA than that of the liquid electrolyte. As a result, all the dyes have higher open potential voltages by about 0.056, 0.006, and 0.004 V for anthocyanin, combined anthocyanin-N719, and N719, respectively, as tabulated in Table 6. The previous investigation regarding the stability of the dye has been presented by Suyitno.68 The better electrolyte ability for cycling leads to the reduction and oxidation reaction (Ipc/Ipa of the quasi-solid state electrolyte < Ipc/Ipa of liquid electrolyte), resulting in better electrolyte conductivity by about 19.6% based on the analysis from Tables 5, 7, and Fig. 13. This condition is an essential key for stabilizing the dye. The faster electron excitation from the excited dye must be compensated by faster dye regeneration by I−. Therefore, the condition successfully increased the efficiency of anthocyanin, combined anthocyanin-N719, and N719 based DSSC performance by 0.7, 0.22, and 1.06%, respectively.
| Dye | Electrolyte | JSC (mA cm−2) | VOC (V) | Jmax (mA cm−2) | Vmax (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a This work employed TiO2 (nanoparticles, 19.18 nm, 50.99 m2 g−1 surface area, 87.8% anatase 12.2% rutile, 10.58 μm thickness, 3.18 eV band gap) – combined purified anthocyanin-N719 (12.2 mM, pH 1.0) – I−/I3− with liquid electrolyte (containing lithium iodide (0.1 M)/Iodine (0.050 M) in acetonitrile and 0.6 M 1-buty-3-methylimidazolium iodide (BMII) and 0.5 M 4-tert-butylpyridine (TBP), + polyethylene oxide (MW = 1 × 106) for quasi-solid state phase) – Pt and 25 μm sealing thickness.b TiO2 (nanoparticles, 13 nm, 120 m2 g−1 surface area, 100% anatase, 7.3 μm thickness, 3.2 eV band gap) – unpurified pigment with chlorophyll b copigment (1.92 mM, pH 7.0) – I−/I3− with liquid electrolyte (containing tetrabutylammonium iodide (0.5 M)/iodine (0.05 M) in acetonitrile and 70 mM tert-butylpyridine, 0.1 M lithium iodide, and 35 mM pyridine) – Pt and 50 μm sealing thickness.c TiO2 (nanoparticles, 20.47 nm, 52 m2 g−1 surface area, mixed 70% anatase 30% rutile, 11.06 μm thickness, 3.2 eV band gap) – double anthocyanin pigments (2.00 mM, pH 2.0) – I−/I3− with liquid electrolyte (containing lithium iodide (0.1 M)/iodine (0.05 M) in acetonitrile and 0.6 M 1-buty-3-methylimidazolium iodide (BMII) and 0.5 M 4-tert-butylpyridine (TBP)) – Pt and 25 μm sealing thickness.d TiO2 (nanoparticles, 13 nm, 120 m2 g−1 surface area, 100% anatase, 7.3 μm thickness, 3.2 eV band gap) – unpurified pigment with chlorophyll b copigment (1.92 mM, pH 7.0) – I−/I3− with liquid electrolyte (containing tetrabutylammonium iodide (0.5 M)/iodine (0.05 M) in acetonitrile and 70 mM tert-butylpyridine, 0.1 M lithium iodide, and 35 mM pyridine) – Pt and 50 μm sealing thickness. | ||||||||
| Anthocyanin (L) | Liquid | 5.69 | 0.558 | 4.52 | 0.431 | 61.3 | 1.95 | — |
| Anthocyanin (SS) | Quasi-solid state | 6.46 | 0.614 | 5.46 | 0.486 | 66.7 | 2.65 | — |
| Anthocyanin-N719 (L) | Liquid | 8.25 | 0.615 | 5.45 | 0.468 | 64.9 | 3.29 | — |
| Anthocyanin-N719 (SS) | Quasi-solid state | 12.66 | 0.621 | 7.47 | 0.470 | 44.6 | 3.51 | — |
| N719 (L) | Liquid | 16.01 | 0.647 | 13.86 | 0.465 | 62.2 | 6.45 | — |
| N719 (SS) | Quasi-solid state | 20.78 | 0.651 | 15.71 | 0.478 | 55.5 | 7.51 | — |
| Hesperidinc | Liquid | 3.23 | 0.480 | 2.16 | 0.320 | 45.0 | 0.71 | 2 |
| C3G + P3Gb | Liquid | 5.46 | 0.523 | 4.69 | 0.449 | 74.0 | 2.08 | 1 |
| Unpurified black riced | Liquid | 5.44 | 0.550 | 4.67 | 0.473 | 74.0 | 2.17 | 4 |
| Dye | Rd (Ω) | Rr (Ω) | Rpt (Ω) | τd (μs) | τn (ms) | Ln (μm) | Dn 10−3 (cm2 s−1) | Cμelec (μF) | σn 10−4 (S m−1) | η (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthocyanin L | 2.53 | 96.18 | 6.97 | 33.30 | 13.55 | 65.23 | 33.58 | 140.86 | 16.73 | 1.95 | — |
| Anthocyanin S | 2.10 | 81.18 | 6.50 | 31.00 | 13.27 | 65.78 | 36.12 | 163.45 | 20.15 | 2.65 | — |
| Anthocyanin-N719 L | 1.78 | 64.49 | 4.81 | 28.10 | 1.71 | 63.68 | 39.82 | 264.49 | 23.78 | 3.29 | — |
| Anthocyanin-N719 S | 1.58 | 58.53 | 4.41 | 26.50 | 1.67 | 64.39 | 42.27 | 285.43 | 26.78 | 3.51 | — |
| N719 L | 1.00 | 43.40 | 2.70 | 23.40 | 1.05 | 69.70 | 47.74 | 242.84 | 42.32 | 6.45 | — |
| N719 S | 0.90 | 38.41 | 2.46 | 21.00 | 1.03 | 69.12 | 53.22 | 267.48 | 47.02 | 7.51 | — |
| Hesperidin | 13.60 | 265.00 | — | 220.00 | 4.29 | 32.20 | 2.56 | 15.20 | 7.14 | 0.71 | 2 |
| C3G + P3G | 0.90 | 73.32 | 25.07 | 25.07 | 1.03 | — | 21.30 | 14.09 | 10.8 | 2.08 | 1 |
| Unpurified black rice | 21.90 | 281.60 | — | 70.92 | 3.63 | 28.6 | 16.96 | 14.32 | — | 2.17 | 4 |
Fig. 14 shows the DSSC sensitized by anthocyanin, combined anthocyanin-N719, and N719 with liquid and quasi solid-state electrolytes. The photovoltaic parameters, including short current density (JSC), open-circuit voltage (VOC), fill factor (FF), and power conversion efficiency (η), are tabulated in Table 6. The investigated anthocyanin and N719-based DSSC were compared to the combined anthocyanin-N719 DSSC. The result shows that the N719 dye-sensitized solid-state solar cell with 7.51% efficiency is superior to the other dyes. The photocurrent density, open-circuit voltage, fill factor, and overall cell efficiency of the N719 dye were observed to be 20.78 mA cm−2, 0.651 V, 0.55, and 7.51%, respectively. All the quasi-solid state dye-sensitized solar cells resulted in better performance than the liquid-based dye-sensitized solar cells by about 0.70, 0.22, and 1.06% for anthocyanin, anthocyanin-N719, and N719, respectively. The lower oxidation potential of the quasi-solid state electrolyte by about 0.012 V contributes to the broader open-circuit voltages of anthocyanin, anthocyanin-N719, and N719 by about 0.056, 0.006, and 0.004 V, respectively. The combination of lower LUMO gaps of anthocyanin-N719 and N719 of about 0.069 and 0.096 eV, respectively, towards anthocyanin's LUMO with a broader dye absorbance of anthocyanin-N719 and N719 of 11.2 and 24.5%, respectively, contribute to higher short current densities of anthocyanin-N719 and N719 by about 2.56–6.20 and 10.32–14.32 mA cm−2, respectively, for liquid and quasi solid-state DSSCs. Although the tendency of changes in the fill factor due to the change in the total internal resistance of DSSC has not been found, the stronger pigment adsorptions of anthocyanin-N719 and N719 by about 0.668 and 1.698 nmol cm−2 towards anthocyanin on the TiO2 semiconductor due to the presence of hydroxyl, carboxyl, and carbonyl groups is also an essential factor facilitating faster electron transfer.
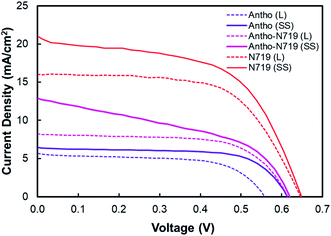 | ||
| Fig. 14 I–V curve of DSSC sensitized by anthocyanin, combined anthocyanin-N719, and N719 with liquid and quasi solid-state electrolytes. | ||
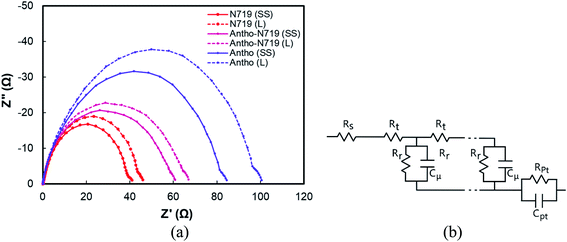
Fig. 15(a) shows the Nyquist plot of DSSC sensitized by anthocyanin, combined anthocyanin-N719, and N719 with liquid and quasi solid-state electrolytes. At the same time, Fig. 15(b) illustrates that the equivalent circuit elements showing the diffusion-recombination transmission line under reflecting boundary conditions fit overlays a highly idealized photoanode schematic at various potentials in the dark. Based on the EIS investigation, this evidence has been shown in Table 7 for the analysis of diffusion time. Anthocyanin-N719 and N719 are 4.5–5.2 μs and 9.9–10 μs faster than anthocyanin DSSC for both liquid and quasi-solid state electrolytes, respectively. The lower oxidation potential of the quasi-solid state electrolyte shortened the regeneration time by 0.28, 0.04, and 0.02 ms for anthocyanin, combined anthocyanin-N719, and N719 pigments. The table also presents that the faster the electron transfer, the higher the conductivity of the pigment; thus, the greater the cell performance. The lower the electrolyte oxidation potential towards HOMO, the wider the open-circuit voltage of cells. These findings are also supported by the tendency of electrolyte chemical capacitance enhancements.
To strengthen our findings towards our previously published works,1,2,12,14,60,69 we strengthen our findings as to the state of the art of the engineering of black rice-based anthocyanin pigments. To the best of our knowledge, this work has achieved the highest efficiency of anthocyanin dye-sensitized quasi-solid state solar cells of 2.65%. The analysis of the absorbance, the adsorption, the HOMO–LUMO level, and the electron transfer play essential roles in determining and comparing anthocyanin with the standardized N719 pigment. There is no previous work reporting the details of the head-to-head performance of natural and synthetic pigments, considering the above-mentioned parameters. The dye combination of anthocyanin-N719 successfully improved the performance of both liquid and quasi-solid state anthocyanin dye-sensitized solar cells by about 1.34 and 0.86%, respectively. Regardless of the DSSC structures, which have been investigated previously, compared to the previously investigated anthocyanin-based dye, the result presents superior performance among them. This improvement is significantly affected by the enhancement in the short circuit photocurrent density due to the dye's higher concentration and faster-oxidized dye regeneration. Other dye considerations such as pH, dye attachment, HOMO–LUMO, co-sensitization, purification, and the chemical structure of D–π–A also contribute to the final performance of the DSSC. As tabulated in Table 7, the condition of the EIS tests, such as applying forward bias or placing the cell under irradiation, will significantly affect the diffusion resistance, recombination resistance, and the ratio between them. The table shows that this work has obtained a better diffusion rate with a range of (33.58–53.22) × 10−3 cm2 s−1 as well as better conductivity with a range of (16.73–47.02) × 10−4 S m−1. The condition leads to verifying the better performance of the cells.
4. Conclusions
The natural pigment was modified to improve the cell performance by modifying the D–π–A structure. Interestingly, in comparison with N719, the molar extinction coefficient of anthocyanin dye is 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 at 550 nm, whereas that of the N719 photosensitizer is 14
900 M−1 cm−1 at 550 nm, whereas that of the N719 photosensitizer is 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 515 nm. By investigating and comparing these pigments, we believe that the finding will contribute to the development of novel modified natural photosensitizer pigments for future natural dye-sensitized solar cells. Moreover, the quasi-solid state electrolyte introduced in this work also presents a better performance of the photosensitizer compared to the liquid-based electrolyte.
000 M−1 cm−1 at 515 nm. By investigating and comparing these pigments, we believe that the finding will contribute to the development of novel modified natural photosensitizer pigments for future natural dye-sensitized solar cells. Moreover, the quasi-solid state electrolyte introduced in this work also presents a better performance of the photosensitizer compared to the liquid-based electrolyte.
The combination of anthocyanin-N719 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) improved the dye adsorption by making it 11.81% stronger than anthocyanin binding. The pigment combination successfully enhanced the absorbance of anthocyanin adsorbed on TiO2 by 38.0%. At the same time, the pigment combination decreased the N719 adsorbed TiO2 by about 32.4%. Interestingly, the N719 contamination in the anthocyanin results in the lowering of the LUMO level and the raising of the HOMO level. The combination of lower LUMO gaps of anthocyanin-N719 and N719 of 0.069 and 0.096 eV, respectively, towards anthocyanin's LUMO with a broader dye absorbance of anthocyanin-N719 and N719 by about 11.2 and 24.5%, respectively. As a consequence, the cell shows a better diffusion rate with the range 33.58–53.22 × 10−3 cm2 s−1 as well as better conductivity with the range of 16.73–47.02 × 10−4 S m−1, leading to the verification of the better performance of the cells. A quasi-solid state electrolyte with the combined anthocyanin-ruthenium dye-sensitized solar cell achieved 3.51%. The work also obtained the highest anthocyanin dye-sensitized quasi-solid state solar cell efficiency of 2.65%.
1) improved the dye adsorption by making it 11.81% stronger than anthocyanin binding. The pigment combination successfully enhanced the absorbance of anthocyanin adsorbed on TiO2 by 38.0%. At the same time, the pigment combination decreased the N719 adsorbed TiO2 by about 32.4%. Interestingly, the N719 contamination in the anthocyanin results in the lowering of the LUMO level and the raising of the HOMO level. The combination of lower LUMO gaps of anthocyanin-N719 and N719 of 0.069 and 0.096 eV, respectively, towards anthocyanin's LUMO with a broader dye absorbance of anthocyanin-N719 and N719 by about 11.2 and 24.5%, respectively. As a consequence, the cell shows a better diffusion rate with the range 33.58–53.22 × 10−3 cm2 s−1 as well as better conductivity with the range of 16.73–47.02 × 10−4 S m−1, leading to the verification of the better performance of the cells. A quasi-solid state electrolyte with the combined anthocyanin-ruthenium dye-sensitized solar cell achieved 3.51%. The work also obtained the highest anthocyanin dye-sensitized quasi-solid state solar cell efficiency of 2.65%.
In terms of the quasi-solid state electrolyte investigation, it has presented better dye regeneration due to the 0.012 V lower oxidation potential compared to that of the liquid electrolyte, resulting in a stronger peak current Ipc by about 2.46 mA than that of the liquid electrolyte. As a result, all the dyes have higher open potential voltages by about 0.056, 0.006, and 0.004 V for anthocyanin, combined anthocyanin-N719, and N719, respectively. The better the electrolyte ability for cycling the reduction and oxidation reaction (Ipc/Ipa of the quasi-solid state electrolyte < Ipc/Ipa of the liquid electrolyte), the better the electrolyte conductivity, which is about 19.6% better. The faster electron excitation from the excited dye must be compensated by faster dye regeneration by I−. Therefore, the condition successfully increased the efficiency of anthocyanin, combined anthocyanin-N719, and N719-based DSSC by about 0.7, 0.22, and 1.06%, respectively.
The finding implies that the dye's anchoring group plays a fundamental role in improving the natural pigment efficiency by strengthening the pigment attachment. The anthocyanin pigment with carboxylic anchoring groups is exciting for further investigation.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Program Penelitian Kolaborasi Indonesia, 2020 (Grant Number 924 /UN40.D/PT/2020). Moreover, this work was also partially supported by Indonesia Ministry of Education and Culture, and Indonesia Ministry of Research and Technology under grant scheme of World Class University Program managed by Institut Teknologi Bandung.References
- E. C. Prima, A. Nuruddin, B. Yuliarto, G. Kawamura and A. Matsuda, New J. Chem., 2018, 42, 11616–11628 RSC.
- E. C. Prima, N. N. Hidayat, B. Yuliarto, Suyatman and H. K. Dipojono, Spectrochim. Acta Mol. Biomol. Spectrosc., 2017, 171, 112–125 CrossRef CAS.
- G. Calogero, A. Bartolotta, G. Di Marco, A. Di Carlo and F. Bonaccorso, Chem. Soc. Rev., 2015, 44, 3244–3294 RSC.
- Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2009, 2903–2934 CrossRef.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- Z.-S. Wang, H. Kawauchi, T. Kashima and H. Arakawa, Coord. Chem. Rev., 2004, 248, 1381–1389 CrossRef CAS.
- J. Luo, Z. Wan, C. Jia, Y. Wang, X. Wu and X. Yao, Electrochim. Acta, 2016, 211, 364–374 CrossRef CAS.
- P. Sharma, I. Sharma and S. C. Katyal, J. Appl. Phys., 2009, 105, 053509 CrossRef.
- S. A. Sapp, C. M. Elliott, C. Contado, S. Caramori and C. A. Bignozzi, J. Am. Chem. Soc., 2002, 124, 11215–11222 CrossRef CAS.
- F. Kabir, M. M. H. Bhuiyan, M. S. Manir, M. S. Rahaman, M. A. Khan and T. Ikegami, Results Phys., 2019, 14, 102474 CrossRef.
- W. Ghann, H. Kang, T. Sheikh, S. Yadav, T. Chavez-Gil, F. Nesbitt and J. Uddin, Sci. Rep., 2017, 7, 41470 CrossRef CAS.
- E. C. Prima, B. Yuliarto, V. Suendo and Suyatman, J. Mater. Sci.: Mater. Electron., 2014, 25, 4603–4611 CrossRef CAS.
- S. Nakade, Y. Saito, W. Kubo, T. Kitamura, Y. Wada and S. Yanagida, J. Phys. Chem. B, 2003, 107, 8607–8611 CrossRef CAS.
- E. C. Prima, M. Qibtiya, B. Yuliarto, Suyatman and H. K. Dipojono, Ionics, 2016, 22, 1687–1697 CrossRef CAS.
- N. Lahmer, N. Belboukhari, A. Cheriti and K. Sekkoum, Der Pharma Chem., 2015, 7, 1–4 CAS.
- C.-Y. Chien and B.-D. Hsu, Sol. Energy, 2013, 98, 203–211 CrossRef CAS.
- N. Anuar, A. F. Mohd Adnan, N. Saat, N. Aziz and R. Mat Taha, Sci. World J., 2013, 2013, 10 Search PubMed.
- A. Ordaz-Galindo, P. Wesche-Ebeling, R. E. Wrolstad, L. Rodriguez-Saona and A. Argaiz-Jamet, Food Chem., 1999, 65, 201–2016 CrossRef CAS.
- N. T. R. N. Kumara, P. Ekanayake, A. Lim, L. Y. C. Liew, M. Iskandar, L. C. Ming and G. K. R. Senadeera, J. Alloys Compd., 2013, 581, 186–191 CrossRef CAS.
- Y. Duan, Q. Tang, Y. Chen, Z. Zhao, Y. Lv, M. Hou, P. Yang, B. He and L. Yu, J. Mater. Chem. A, 2015, 3, 5368–5374 RSC.
- Y. Yang, C.-h. Zhou, S. Xu, H. Hu, B.-l. Chen, J. Zhang, S.-j. Wu, W. Liu and X.-z. Zhao, J. Power Sources, 2008, 185, 1492–1498 CrossRef CAS.
- Y. Kimura, T. Maeda, S. Iuchi, N. Koga, Y. Murata, A. Wakamiya and K. Yoshida, J. Photochem. Photobiol., A, 2017, 335, 230–238 CrossRef CAS.
- K. Kalyanasundaram and M. Grätzel, Coord. Chem. Rev., 1998, 177, 347–414 CrossRef CAS.
- S. Akın, S. Açıkgöz, M. Gülen, C. Akyürek and S. Sönmezoğlu, RSC Adv., 2016, 6, 85125–85134 RSC.
- G. Calogero, I. Citro, G. Di Marco, S. Caramori, L. Casarin, C. A. Bignozzi, J. Avó, A. Jorge Parola and F. Pina, Photochem. Photobiol. Sci., 2017, 16, 1400–1414 RSC.
- M. A. M. Al-Alwani, H. Abu Hasan, N. Kaid Nasser Al-Shorgani and A. B. S. A. Al-Mashaan, Mater. Chem. Phys., 2020, 240, 122204 CrossRef CAS.
- J. M. Cole, G. Pepe, O. K. Al Bahri and C. B. Cooper, Chem. Rev., 2019, 119, 7279–7327 CrossRef CAS.
- Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS.
- A. Versari, R. Boulton and J. Thorngate, in Red Wine Color, American Chemical Society, 2004, vol. 886, ch. 5, pp. 53–67 Search PubMed.
- Y. Liu and J. Wang, Thin Solid Films, 2010, 518, e54–e56 CrossRef.
- A. A. Mohamad, J. Power Sources, 2016, 329, 57–71 CrossRef CAS.
- J. H. Yang, C. W. Bark, K. H. Kim and H. W. Choi, Materials, 2014, 7, 3522–3532 CrossRef CAS.
- Y. S. Park, S. J. Kim and H. I. Chang, Korean J. Microbiol. Biotechnol., 2008, 36, 55–60 CAS.
- G. R. Takeoka, L. T. Dao, H. Tamura and L. A. Harden, J. Agric. Food Chem., 2005, 53, 4932–4937 CrossRef CAS.
- S. N. Ryu, S. J. Han, S. Z. Park and H. Y. Kim, Korean J. Crop. Sci., 2000, 45, 257–260 Search PubMed.
- S. Y. Chae, M. K. Park, S. K. Lee, T. Y. Kim, S. K. Kim and W. I. Lee, Chem. Mater., 2003, 15, 3326–3331 CrossRef CAS.
- B. Tryba, M. Tygielska, C. Colbeau-Justin, E. Kusiak-Nejman, J. Kapica-Kozar, R. Wróbel, G. Żołnierkiewicz and N. Guskos, Mater. Res. Bull., 2016, 84, 152–161 CrossRef CAS.
- J. H. Lee, Food Sci. Biotechnol., 2010, 19, 391–397 CrossRef CAS.
- A. G. Al-Sehemi, A. Irfan and A. M. Fouda, Spectrochim. Acta, Part A, 2013, 111, 223–229 CrossRef CAS.
- A. Fernandes, G. Ivanova, N. F. Brás, N. Mateus, M. J. Ramos, M. Rangel and V. de Freitas, Carbohydr. Polym., 2014, 102, 269–277 CrossRef CAS.
- J. L. Bredas, R. Silbey, D. S. Boudreaux and R. R. Chance, J. Am. Chem. Soc., 1983, 105, 6555–6559 CrossRef CAS.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- Q. Wang, J.-E. Moser and M. Grätzel, J. Phys. Chem. B, 2005, 109, 14945–14953 CrossRef CAS.
- J. Bisquert, J. Phys. Chem. B, 2004, 108, 2323–2332 CrossRef CAS.
- J. Bisquert, G. Garcia-Belmonte and Á. Pitarch, ChemPhysChem, 2003, 4, 287–292 CrossRef CAS.
- J. Bisquert, Phys. Chem. Chem. Phys., 2003, 5, 5360–5364 RSC.
- J. Bisquert and V. S. Vikhrenko, Electrochim. Acta, 2002, 47, 3977–3988 CrossRef CAS.
- J. Bisquert, J. Phys. Chem. B, 2002, 106, 325–333 CrossRef CAS.
- J. Bisquert and A. Compte, J. Electroanal. Chem., 2001, 499, 112–120 CrossRef CAS.
- B. Yuliarto, W. Septina, K. Fuadi, F. Fanani, L. Muliani and Nugraha, Adv. Mater. Sci. Eng., 2010, 2010, 6 Search PubMed.
- L. K. Singh, T. Karlo and A. Pandey, Spectrochim. Acta, Part A, 2014, 118, 938–943 CrossRef CAS.
- K. E. Lee, M. A. Gomez, S. Elouatik, G. B. Shan and G. P. Demopoulos, J. Electrochem. Soc., 2011, 158, H708–H714 CrossRef CAS.
- A. Jantasee, K. Thumanu, N. Muangsan, W. Leeanansaksiri and D. Maensiri, Food Anal. Methods, 2014, 7, 389–399 CrossRef.
- A. Soriano, P. M. Pérez-Juan, A. Vicario, J. M. González and M. S. Pérez-Coello, Food Chem., 2007, 104, 1295–1303 CrossRef CAS.
- K. E. Lee, M. A. Gomez, S. Elouatik and G. P. Demopoulos, Langmuir, 2010, 26, 9575–9583 CrossRef CAS.
- P. Wen, Y. Han and W. Zhao, Int. J. Photoenergy, 2012, 2012, 7 Search PubMed.
- V.-H. Nguyen, H.-Y. Chan and J. C. S. Wu, J. Chem. Sci., 2013, 125, 859–867 CrossRef CAS.
- E. C. Prima, B. Yuliarto, Suyatman and H. K. Dipojono, Mater. Sci. Forum, 2017, 889, 178–183 Search PubMed.
- M. A. Qibtiya, E. C. Prima, B. Yuliarto and Suyatman, Mater. Sci. Forum, 2016, 864, 154–158 Search PubMed.
- E. C. Prima, B. Yuliarto, Suyatman and H. K. Dipojono, J. Phys.: Conf. Ser., 2016, 739, 012031 CrossRef.
- M. Suzuka, N. Hayashi, T. Sekiguchi, K. Sumioka, M. Takata, N. Hayo, H. Ikeda, K. Oyaizu and H. Nishide, Sci. Rep., 2016, 6, 28022 CrossRef CAS.
- L. Tao, Z. Huo, Y. Ding, Y. Li, S. Dai, L. Wang, J. Zhu, X. Pan, B. Zhang, J. Yao, M. K. Nazeeruddin and M. Grätzel, J. Mater. Chem. A, 2015, 3, 2344–2352 RSC.
- T. S. Senthil, N. Muthukumarasamy, D. Velauthapillai, S. Agilan, M. Thambidurai and R. Balasundaraprabhu, Renewable Energy, 2011, 36, 2484–2488 CrossRef CAS.
- H. Yang, M. Huang, J. Wu, Z. Lan, S. Hao and J. Lin, Mater. Chem. Phys., 2008, 110, 38–42 CrossRef CAS.
- J. Wu, Z. Lan, D. Wang, S. Hao, J. Lin, Y. Huang, S. Yin and T. Sato, Electrochim. Acta, 2006, 51, 4243–4249 CrossRef CAS.
- E. Stathatos, P. Lianos, S. M. Zakeeruddin, P. Liska and M. Grätzel, Chem. Mater., 2003, 15, 1825–1829 CrossRef CAS.
- S. Suyitno, T. J. Saputra, A. Supriyanto and Z. Arifin, Spectrochim. Acta Mol. Biomol. Spectrosc., 2015, 148, 99–104 CrossRef CAS.
- E. Cahya Prima, B. Yuliarto and H. Kresno Dipojono, Adv. Mater. Res., 2015, 1112, 317–320 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06550a |
| This journal is © The Royal Society of Chemistry 2020 |