Open Access Article
Open Access ArticleScreening of multiclass pesticide residues in maca and Moringa oleifera by a modified QuEChERS sample preparation procedure and UPLC-ESI-MS/MS analysis
Yanqin Zhu *abc,
Ping Duab,
Jun Yangab,
Qinhong Yind and
Yaling Yangc
*abc,
Ping Duab,
Jun Yangab,
Qinhong Yind and
Yaling Yangc
aResearch Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming 650093, China. E-mail: zyq23788@126.com; Fax: +86-87165113971; Tel: +86-87165113971
bAnalysis and Test Center of Yunnan Province, Kunming 650093, China
cFaculty of Life Science and Technology, Kunming University of Science and Technology, Kunming 650500, China
dFaculty of Narcotics Control, Yunnan Police College, Kunming 650223, China
First published on 7th October 2020
Abstract
In the present study, a modified QuEChERS (quick, easy, cheap, effective, rugged, and safe) method was proposed for the simultaneous analysis of 75 pesticides in maca and Moringa oleifera with ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS). The developed method was validated in accordance with linearity, linear range, limit of detection, limit of quantification, accuracy, precision, and matrix effect. Each analyte had good linearity (R2 > 0.99) in the corresponding concentration range. The method LOD and LOQ values of all the analytes ranged from 0.01 μg kg−1 to 303.35 μg kg−1 and 0.03 μg kg−1 to 1011.15 μg kg−1, respectively. The recoveries (n = 6) of the analyzed pesticides were in the range of 75.92–113.43%. The RSDs of precision were between 0.60% and 7.36%. All matrix effect values ranged from 81.79% to 118.71% and 80.36% to 119.64% in maca and Moringa oleifera, respectively. The analysis of 103 samples showed the presence of isofenphos-methyl in some of them. The method had a good application prospect and could be used as a general approach for the quantitative determination of pesticide residues in food.
1. Introduction
Maca can enhance physical strength, improve fertility, adjust endocrine, enhance immunity, and so on.1–4 In 2011, China approved maca as a new resource food. At present, China has become the largest maca producing country in the world, with maca being widely cultivated in Lijiang and Diqing of Yunnan province.5 Moringa oleifera can enhance immunity, promote digestion, and improve human intestinal health with rich nutrients and medicinal ingredients, so it is the umbrella of human health. Moringa oleifera, the Chinese Ganoderma lucidum and American ginseng were known as the “world's three treasures”, and Moringa oleifera was even named as the “miracle tree” by scientists.6,7 In November 2012, China's ministry of health approved Moringa oleifera leaves as a new resource food. It is widely cultivated in the Yunnan province of China.8,9 Maca and Moringa oleifera can be used as both food and medicine with rich nutrients and secondary metabolites. With the growth of the socio-economic level, people's health consciousness constantly improve, get more attention to food safety, residents also have higher requirement for the quality of food.Pesticide residues in food are one of the main factors that endanger food safety and human health.10–12 In order to ensure food safety, many countries and regions in the world have established strict limits for pesticide residues in food, and a lot of researches have been conducted on the detection technology and methods. In recent years, techniques such as gas chromatography (GC), high-performance liquid chromatography (HPLC), GC-tandem mass spectrometry (GC-MS/MS), and LC-tandem mass spectrometry (LC-MS/MS) have been widely used in the detection of pesticide residues and the risk assessment in food.13–18 However, the application of these technologies needs to be based on reliable sample pretreatment methods. In view of this, Anastassiades et al.19 of the United States agricultural department in 2003 researched a new sample preparation method, namely the QuEChERS (quick, easy, cheap, effective, rugged, and safe) method. This method was mainly applied to the rapid extraction and purification of pesticide residues in food.20–23 This technique can solve the problems of long pretreatment time, large amount of toxic solvent, qualitative and quantitative interference of coexisting substances in traditional analytical methods. However, because the physical and chemical properties of different analytes differ greatly, and different substrates have different effects on the same analyte, it is difficult to achieve the optimal recovery of all the analytes at the same time. In addition, since the main advantage of the pre-treatment method of QuEChERS is simple and fast, the matrix cannot be completely purified, and hence it is easy to produce a matrix effect affecting the accuracy and reliability of the test results when it is combined with various sophisticated high-end detection instruments for trace or ultra-trace analysis.24,25 No current method can probe all pesticides from all matrices using a single approach. The multiclass and multi-residue analysis of pesticides is a constantly evolving process. The emergence of new pesticides, instruments and techniques has brought both opportunities and challenges.26 QuEChERS is not just a method or a pair of official methods (AOAC2007.01 and EN 15662), but it is also a flexible approach for chemical sample preparation that is being used in multiple applications.27 In order to improve the recovery rate and detection efficiency as much as possible, researchers have made a variety of improvements to the QuEChERS method.28–30 Therefore, the QuEChERS method is optimized according to the differences in the properties of different samples and components to be tested, so as to improve the recovery rate, reduce the interference of impurities, and increase the types of components to be extracted at the same time.
A general method for a large-scale multi-residue pesticide analysis should be established. The pesticide residue analysis is an important quality assessment that solves consumer concerns about food safety and accords with national and international food safety regulations. With the establishment of the food safety standards agency, the scope of pesticide residue monitoring has been expanded dramatically over the past decade. As a result, a multi-residue analysis method should contain as many chemicals as possible and be applicable to multiple substrates.
There are four types of QuEChERS, namely the original QuEChERS method,19,31 AOAC QuEChERS method,32,33 European (EN) official QuEChERS method,34 and modifications of the standard QuEChERS technique.29,30 Food matrices in pesticide analysis using QuEChERS are as follows: fruits and vegetables,35 cereals,36 and animal products.37 This method has the advantages of dynamic, simple, rapid, fewer analysis steps, and fewer errors. In addition, it is cheap and environmentally friendly due to the use of small amounts of organic solvents. However, few studies have combined the QuEChERS technique with other pre-concentration methods regarding maca and Moringa oleifera. In order to improve the extraction efficiency and selectivity, the coupling of QuEChERS is the focus of future research.
In this study, a modified QuEChERS-UPLC-ESI-MS/MS method was developed for multiclass pesticide analysis in maca and Moringa oleifera. In the proposed method, the N-EVAP-based extraction method has been added with an optimized concentrated volume that can provide a high enrichment factor for the determination of trace amounts of pesticides in the samples. Furthermore, after using the QuEChERS technology, a certain proportion of matrix interference components is still present in the samples, and these interfering components may get coextracted and adversely affect the chromatographic analysis. Therefore, a pretreatment C18 column was added to the UPLC-MS/MS column to make the sample clean enough and greatly improve the cleanliness of the samples. The increase in the concentration of the measured components in the clean samples can reduce the matrix effect and improve the sensitivity, accuracy, and selectivity of the target compounds.
2. Materials and methods
2.1 Chemicals and reagents
The certified reference standards of all the test compounds were of >99% purity and purchased from A ChemTek, Inc. (Worcester, MA, USA). Deionized water was obtained from a Milli-Q filtration system (Millipore Filter Cor., Bedford, MA, USA). LC/MS grade acetonitrile and formic acid were obtained from Fisher (Thermo Fisher Scientific Inc., Waltham, MA, USA). Other chemicals and solvents were of analytical grade and purchased from Zhiyuan Chemical Factory (Tianjin, China). A dispersive solid-phase extraction purification tube (anhydrous magnesium sulfate (MgSO4): 1500 mg, N-primary secondary amine (PSA): 500 mg, C18: 500 mg, silicone: 500 mg, graphitized carbon black (GCB): 150 mg) was purchased from Agela Technologies (Bona Agela Technology Co. Ltd, Tianjin, China).2.2 Apparatus
A grinding machine (200 g capacity, model DFT-200, Wenling Linda Machinery Co. Ltd, Zhejiang, China), a vortex mixer (XW-90, Instrument Factory of Shanghai Medical University, Shanghai, China), an oscillator (SHA-C, Jintan Kexi Instrument Co. Ltd, Jiangsu, China), a centrifuge (TGL-16C, Shanghai Precision Instrument Co. Ltd, Shanghai, China), and N-EVAP(ND200-1, Hangzhou Ruicheng Instrument Co. Ltd, Zhejiang, China) were used. The residue analysis was performed using an LC-MS/MS [Acquity I-class UPLC connected to ESI TQD (Waters Corp., Milford, MA, USA) mass spectrometer].2.3 Selection of pesticides
The paper studied 75 multiclass pesticides with different biological activities such as fungicides, acaricides, insecticides, herbicides, and plant growth regulators. The analytes were selected considering the MRL database of the National Health Commission in china.2.4 Preparation of standard solutions
The stock solutions of certified reference standards of about 100 μg mL−1 were prepared in acetonitrile. The intermediate standard mixtures of 1 μg mL−1 and working standard solutions of 0.1 μg mL−1 were prepared by diluting the standard stock solutions with acetonitrile. The stock, intermediate, and working standard solutions were stored at −20 °C before use. All the solutions were filtered through a 0.22 μm membrane filter before injection.2.5 Liquid chromatographic conditions
In this study, the chromatographic analysis was performed on an Acquity I-class UPLC system (Waters Corp., Milford, MA, USA) coupled with an autosampler, two pumps, a controller, and a degasser. The separation of pesticides was carried out using an ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) kept at 40 °C, and the autosampler was maintained at 10 °C. The mobile phase consisted of acetonitrile (A) and 0.1% formic acid aqueous solution (B) and a chromatographic gradient program of 5% (A) for 0.0–1.0 min, 5–60% (A) for 1.0–4.0 min, 60–100% (A) for 4.0–10.0 min, 100% (A) for 10.0–12.0 min, 100–5% (A) for 12.0–12.2 min, and 5% (A) for 12.2–16.0 min was used. The flow rate of the mobile phase was set at 0.2 mL min−1, and the injection volume was 1 μL.2.6 MS/MS conditions
A Xevo tandem quadruple detector (TQD) mass spectrometer was operated using an electrospray ionization (ESI) source (Waters Corp., Milford, MA, USA) with positive and negative modes (ESI+ and ESI−). After the direct infusion of reference standards, the multiple reaction monitoring (MRM) conditions were optimized for each pesticide. Other MS conditions were as follows: capillary voltage, 3 kV; cone voltage, 32 V; desolvating temperature, 350 °C; source temperature, 130 °C; source desolvating gas flow, 1000 L h−1; and cone gas flow, 50 L h−1. Each pesticide had two different products. The MS/MS parameters (the type of pesticide, retention time, precursor/product, cone voltage, and collision energy) for the determination of pesticide residues in the MRM ESI+ and ESI− modes are presented in Table 1.| Pesticide | Type of pesticide | Retention time (min) | Precursor (m/z) | Product (m/z) | Cone (V) | Collision energy (eV) | Remark |
|---|---|---|---|---|---|---|---|
| Chlorfluazuron | Insect growth regulator | 9.12 | 539.8 | 158.0 | 42 | 20 | Quantifier |
| 382.9 | 42 | 20 | Qualifier | ||||
| Methamidophos | Insecticide | 1.44 | 142.0 | 93.9 | 28 | 13 | Quantifier |
| 124.9 | 28 | 13 | Qualifier | ||||
| Carbaryl | Insecticide | 5.39 | 202.0 | 117.0 | 28 | 28 | Quantifier |
| 145.0 | 28 | 22 | Qualifier | ||||
| Dichlorvos | Insecticide, acaricide | 5.08 | 221.0 | 79.0 | 34 | 34 | Quantifier |
| 109.0 | 34 | 22 | Qualifier | ||||
| Parathion-methyl | Insecticide | 6.41 | 263.9 | 79.0 | 38 | 36 | Quantifier |
| 109.0 | 38 | 22 | Qualifier | ||||
| Phorate sulfoxide | Insecticide | 5.35 | 277.0 | 96.9 | 24 | 32 | Quantifier |
| 143.0 | 24 | 20 | Qualifier | ||||
| Pendimethalin | Herbicide | 9.01 | 282.2 | 194.1 | 21 | 17 | Quantifier |
| 212.2 | 21 | 10 | Qualifier | ||||
| Parathion | Insecticide | 7.35 | 291.9 | 110.0 | 36 | 33 | Quantifier |
| 236.0 | 36 | 14 | Qualifier | ||||
| Phorate sulfone | Insecticide | 5.96 | 293.0 | 96.9 | 24 | 30 | Quantifier |
| 115.0 | 24 | 24 | Qualifier | ||||
| Diflubenzuron | Insecticide | 6.93 | 311.1 | 227.0 | 32 | 8 | Quantifier |
| 269.0 | 32 | 8 | Qualifier | ||||
| Chlorpyrifos | Insecticide, acaricide | 8.99 | 349.9 | 97.0 | 36 | 32 | Quantifier |
| 198.0 | 36 | 20 | Qualifier | ||||
| Fenpropathrin | Insecticide, acaricide | 8.99 | 350.1 | 97.0 | 24 | 34 | Quantifier |
| 125.0 | 24 | 14 | Qualifier | ||||
| Chlorantraniliprole | Insecticide | 5.79 | 484.0 | 286.0 | 18 | 12 | Quantifier |
| 453.0 | 18 | 17 | Qualifier | ||||
| Tau-fluvalinate | Insecticide, acaricide | 10.20 | 503.0 | 181.1 | 24 | 30 | Quantifier |
| 208.1 | 24 | 12 | Qualifier | ||||
| Abamectin (B1a) | Insecticide, acaricide, bactericide | 9.69 | 890.6 | 305.2 | 24 | 25 | Quantifier |
| 567.4 | 24 | 11 | Qualifier | ||||
| Methomyl | Insecticide | 3.78 | 163.0 | 88.0 | 26 | 10 | Quantifier |
| 106.0 | 26 | 10 | Qualifier | ||||
| Carbendazim | Bactericide | 3.72 | 192.0 | 105.0 | 24 | 41 | Quantifier |
| 160.0 | 24 | 16 | Qualifier | ||||
| Pyrimethanil | Bactericide | 6.10 | 200.0 | 82.0 | 51 | 24 | Quantifier |
| 107.0 | 51 | 24 | Qualifier | ||||
| Carbofuran | Insecticide | 5.28 | 222.1 | 123.0 | 34 | 16 | Quantifier |
| 165.1 | 34 | 16 | Qualifier | ||||
| Acetamiprid | Insecticide | 4.42 | 223.0 | 56.1 | 34 | 15 | Quantifier |
| 126.0 | 34 | 20 | Qualifier | ||||
| Imidacloprid | Insecticide | 4.31 | 256.1 | 175.1 | 34 | 20 | Quantifier |
| 209.1 | 34 | 15 | Qualifier | ||||
| Chlorobenzuron | Insecticide | 6.94 | 310.1 | 43.1 | 42 | 24 | Quantifier |
| 70.2 | 42 | 18 | Qualifier | ||||
| Iprodione | Bactericide | 6.76 | 330.0 | 245.0 | 35 | 15 | Quantifier |
| 288.1 | 35 | 15 | Qualifier | ||||
| Isofenphos-methyl | Insecticide | 11.18 | 332.4 | 58.1 | 44 | 30 | Quantifier |
| 91.0 | 44 | 28 | Qualifier | ||||
| Tebufenozide | Insecticide | 7.03 | 353.1 | 133.0 | 19 | 20 | Quantifier |
| 297.1 | 19 | 8 | Qualifier | ||||
| Pyridaben | Acaricide | 9.76 | 365.1 | 147.1 | 28 | 24 | Quantifier |
| 309.1 | 28 | 12 | Qualifier | ||||
| Prochloraz | Bactericide | 6.94 | 376.1 | 266.0 | 27 | 17 | Quantifier |
| 308.0 | 27 | 12 | Qualifier | ||||
| Dimethomorph | Bactericide | 5.88 | 388.1 | 165.0 | 41 | 30 | Quantifier |
| 300.9 | 41 | 20 | Qualifier | ||||
| Emamectin benzoate | Insecticide, acaricide | 7.49 | 886.6 | 126.0 | 45 | 38 | Quantifier |
| 158.0 | 45 | 37 | Qualifier | ||||
| Propamocarb | Bactericide | 3.26 | 189.1 | 102.0 | 31 | 17 | Quantifier |
| 144.0 | 31 | 12 | Qualifier | ||||
| Aldicarb sulfoxide | Insecticide | 3.19 | 207.0 | 89.0 | 22 | 14 | Quantifier |
| 132.0 | 22 | 10 | Qualifier | ||||
| Aldicarb | Insecticide | 4.85 | 213.1 | 89.1 | 30 | 16 | Quantifier |
| 116.1 | 30 | 11 | Qualifier | ||||
| Omethoate | Insecticide | 3.13 | 214.1 | 125.1 | 26 | 22 | Quantifier |
| 183.1 | 26 | 11 | Qualifier | ||||
| Aldicarb sulfone | Insecticide | 3.63 | 223.0 | 86.0 | 31 | 14 | Quantifier |
| 148.0 | 31 | 10 | Qualifier | ||||
| Carbofuran-3-hydroxy | Insecticide | 4.23 | 238.0 | 163.0 | 34 | 16 | Quantifier |
| 181.0 | 34 | 10 | Qualifier | ||||
| Forchlorfenuron | Plant growth regulator | 5.33 | 248.1 | 93.0 | 36 | 35 | Quantifier |
| 129.0 | 36 | 15 | Qualifier | ||||
| Fenitrothion | Insecticide | 6.77 | 278.0 | 79.1 | 38 | 34 | Quantifier |
| 109.1 | 38 | 20 | Qualifier | ||||
| Thiamethoxam | Insecticide | 3.96 | 292.0 | 132.0 | 28 | 22 | Quantifier |
| 211.2 | 28 | 12 | Qualifier | ||||
| Triadimefon | Bactericide | 6.39 | 294.1 | 69.3 | 31 | 20 | Quantifier |
| 197.2 | 31 | 15 | Qualifier | ||||
| Phoxim | Insecticide | 7.80 | 299.0 | 129.0 | 22 | 13 | Quantifier |
| 153.0 | 22 | 7 | Qualifier | ||||
| Phosmet | Insecticide | 6.19 | 318.0 | 77.0 | 28 | 46 | Quantifier |
| 160.0 | 28 | 22 | Qualifier | ||||
| Azoxystrobin | Bactericide | 6.22 | 404.0 | 329.0 | 28 | 30 | Quantifier |
| 372.0 | 28 | 15 | Qualifier | ||||
| Fipronil sulphone | Insecticide | 5.77 | 453.0 | 112.0 | 60 | 54 | Quantifier |
| 194.0 | 60 | 32 | Qualifier | ||||
| Mevinphos | Insecticide | 4.52 | 225.1 | 127.1 | 24 | 15 | Quantifier |
| 193.1 | 24 | 8 | Qualifier | ||||
| Phorate | Insecticide, acaricide | 7.94 | 261.0 | 75.0 | 17 | 12 | Quantifier |
| 97.0 | 17 | 32 | Qualifier | ||||
| Fenthion | Insecticide | 7.43 | 279.1 | 169.1 | 36 | 16 | Quantifier |
| 247.1 | 36 | 13 | Qualifier | ||||
| Phosphamidon | Insecticide | 4.76 | 300.1 | 127.1 | 28 | 25 | Quantifier |
| 174.1 | 28 | 14 | Qualifier | ||||
| Tolclofos-methyl | Bactericide | 4.74 | 302.1 | 127.5 | 43 | 20 | Quantifier |
| 176.5 | 43 | 13 | Qualifier | ||||
| Fenamiphos | Insecticide | 6.26 | 304.1 | 202.1 | 36 | 36 | Quantifier |
| 217.1 | 36 | 24 | Qualifier | ||||
| Triazophos | Insecticide | 6.75 | 314.1 | 118.9 | 31 | 35 | Quantifier |
| 161.9 | 31 | 18 | Qualifier | ||||
| Isazofos | Insecticide | 6.75 | 314.1 | 97.0 | 34 | 30 | Quantifier |
| 162.2 | 34 | 20 | Qualifier | ||||
| Dichlofenthion | Insecticide | 7.74 | 316.2 | 46.2 | 20 | 12 | Quantifier |
| 74.2 | 20 | 30 | Qualifier | ||||
| Phenthoate | Insecticide | 7.48 | 321.0 | 135.0 | 18 | 20 | Quantifier |
| 163.0 | 18 | 12 | Qualifier | ||||
| Malathion | Insecticide | 6.73 | 331.0 | 99.0 | 20 | 24 | Quantifier |
| 127.0 | 20 | 12 | Qualifier | ||||
| Phosalone | Insecticide, acaricide | 7.83 | 367.9 | 110.9 | 22 | 42 | Quantifier |
| 181.9 | 22 | 14 | Qualifier | ||||
| Profenofos | Insecticide | 8.23 | 372.9 | 127.9 | 36 | 40 | Quantifier |
| 302.6 | 36 | 20 | Qualifier | ||||
| Difenoconazole | Bactericide | 7.28 | 406.0 | 111.1 | 46 | 60 | Quantifier |
| 251.1 | 46 | 25 | Qualifier | ||||
| Dimethoate | Insecticide, acaricide | 4.41 | 230.1 | 125.0 | 24 | 20 | Quantifier |
| 199.0 | 24 | 10 | Qualifier | ||||
| Methacrifos | Insecticide, acaricide | 6.16 | 241.1 | 125.0 | 20 | 20 | Quantifier |
| 209.1 | 20 | 8 | Qualifier | ||||
| Ethoprophos | Insecticide | 6.58 | 243.2 | 97.0 | 32 | 31 | Quantifier |
| 131.0 | 32 | 20 | Qualifier | ||||
| Fonofos | Insecticide | 7.81 | 247.1 | 109.0 | 24 | 20 | Quantifier |
| 137.0 | 24 | 10 | Qualifier | ||||
| Cadusafos | Insecticide | 7.72 | 271.1 | 131.0 | 28 | 22 | Quantifier |
| 159.0 | 28 | 16 | Qualifier | ||||
| Fosthiazate | Insecticide | 5.43 | 284.0 | 104.0 | 28 | 22 | Quantifier |
| 228.0 | 28 | 10 | Qualifier | ||||
| Etrimfos | Insecticide | 7.61 | 293.1 | 125.0 | 38 | 26 | Quantifier |
| 265.1 | 38 | 16 | Qualifier | ||||
| Quinalphos | Insecticide | 7.28 | 299.0 | 96.9 | 24 | 30 | Quantifier |
| 162.9 | 24 | 24 | Qualifier | ||||
| Methidathion | Insecticide | 6.14 | 303.0 | 85.1 | 18 | 20 | Quantifier |
| 145.0 | 18 | 10 | Qualifier | ||||
| Diazinon | Insecticide | 7.75 | 305.1 | 96.9 | 31 | 35 | Quantifier |
| 169.0 | 31 | 22 | Qualifier | ||||
| Pirimiphos-methyl | Insecticide, acaricide | 8.14 | 306.1 | 108.1 | 36 | 32 | Quantifier |
| 164.1 | 36 | 22 | Qualifier | ||||
| Fensulfothion | Insecticide | 5.54 | 309.0 | 157.1 | 36 | 25 | Quantifier |
| 173.1 | 36 | 22 | Qualifier | ||||
| Azinphos-methyl | Insecticide, acaricide | 6.18 | 318.0 | 160.0 | 20 | 8 | Quantifier |
| 261.0 | 20 | 8 | Qualifier | ||||
| EPN | Insecticide | 8.03 | 324.0 | 157.0 | 31 | 25 | Quantifier |
| 296.0 | 31 | 14 | Qualifier | ||||
| Isofenphos | Insecticide | 8.14 | 346.1 | 217.0 | 16 | 22 | Quantifier |
| 245.1 | 16 | 12 | Qualifier | ||||
| Ethion | Insecticide, acaricide | 9.00 | 385.0 | 142.9 | 30 | 25 | Quantifier |
| 199.0 | 30 | 10 | Qualifier | ||||
| Fipronil desulfinyl | Insecticide | 7.33 | 386.9 | 282.0 | 35 | 30 | Quantifier |
| 351.0 | 35 | 15 | Qualifier | ||||
| Fipronil sulphide | Insecticide | 7.64 | 418.9 | 262.0 | 35 | 25 | Quantifier |
| 383.0 | 35 | 10 | Qualifier |
2.7 Sample preparation
One hundred and three samples were collected from February to March 2018 in Yunnan province for the quantification of multiclass pesticide residues. Each sample was smashed and passed through a 100 mesh sieve. Five grams of each sample powder was accurately weighed in 100 mL polystyrene centrifuge tubes, followed by the addition of 25 mL of 1% glacial acetic acid solution, which was then dispersed by vortexing and placed for 30 min. Subsequently, 25 mL of acetonitrile was added, and the mixture was agitated vigorously for 1 min and 5 min using a vortex mixer and an oscillator, respectively. Then, 12.5 g of the salt mixture of anhydrous magnesium sulphate (MgSO4) and anhydrous sodium acetate (NaAc) (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
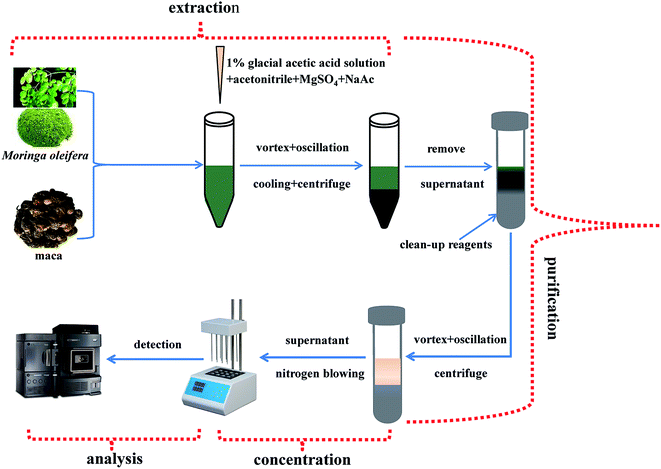
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) was added to the tube and oscillated forcibly for 3 min, followed by placing the tube in cold water for 10 min to cool the sample. The sample was then centrifuged for 5 min at 4000 rpm, and 15 mL of the supernatant was added into a dispersive solid-phase extraction purification tube (anhydrous magnesium sulfate (MgSO4): 1500 mg, N-primary secondary amine (PSA): 500 mg, C18: 500 mg, silicone: 500 mg, graphitized carbon black (GCB): 150 mg). The tube was vortexed fully and oscillated tempestuously for 5 min, and then centrifuged for 5 min at 4000 rpm. Subsequently, 8 mL of the supernatant was dried to approximately 0.6 mL under nitrogen gas at 40 °C. Then, the concentrate was diluted to 1 mL with acetonitrile and vortexed adequately. Lastly, the solution was filtered through a 0.22 μm membrane filter before the UPLC-ESI-MS/MS (LC-MS/MS using a pretreatment C18 column) analysis. The flowchart of the sample preparation is shown in Fig. 1.
1 w/w) was added to the tube and oscillated forcibly for 3 min, followed by placing the tube in cold water for 10 min to cool the sample. The sample was then centrifuged for 5 min at 4000 rpm, and 15 mL of the supernatant was added into a dispersive solid-phase extraction purification tube (anhydrous magnesium sulfate (MgSO4): 1500 mg, N-primary secondary amine (PSA): 500 mg, C18: 500 mg, silicone: 500 mg, graphitized carbon black (GCB): 150 mg). The tube was vortexed fully and oscillated tempestuously for 5 min, and then centrifuged for 5 min at 4000 rpm. Subsequently, 8 mL of the supernatant was dried to approximately 0.6 mL under nitrogen gas at 40 °C. Then, the concentrate was diluted to 1 mL with acetonitrile and vortexed adequately. Lastly, the solution was filtered through a 0.22 μm membrane filter before the UPLC-ESI-MS/MS (LC-MS/MS using a pretreatment C18 column) analysis. The flowchart of the sample preparation is shown in Fig. 1.
2.8 Method validation
The validation of the method was performed in accordance with the following parameters: linearity, linear range, limit of detection, limit of quantification, accuracy, precision, and matrix effect. Linearity was established on the basis of the quantitative ion peak area (Y-axis) versus the corresponding concentration (X-axis, μg mL−1) for the injection of standard solutions of appropriate concentrations in the linear range 1–500 ng mL−1. The limits of detection (LOD) and quantification (LOQ) were defined as the lowest concentration with signal-to-noise ratios (S/N) of 3 and 10, respectively. To identify a compound, the qualifier ion must have an S/N ratio higher than 3, and the S/N quantifier was many times higher than the qualifier. Indeed, the quantifier yielded an S/N ratio higher than 10, thereby permitting the quantification of the residue. Accuracy was expressed in terms of recovery, and the spiking experiments were carried out in duplicate to estimate the recovery of the target analytes. Precision was usually evaluated in terms of the relative standard deviation (RSD). The matrix effect might affect the exact content of the target compound in a complex sample matrix. In the experiment, sample 1 was a sample spiked to a concentration of 0.1 μg mL−1 with a negative sample, and sample 2 was a standard solution of 0.1 μg mL−1. The matrix effect was calculated using the equation given below, where “A” represents the area of the corresponding sample. Commonly, the matrix effect < 0.8 indicates significant matrix suppression, while the matrix effect > 1.2 represents the matrix enhancement.38,393. Results and discussion
3.1 Optimization of UPLC and MS conditions
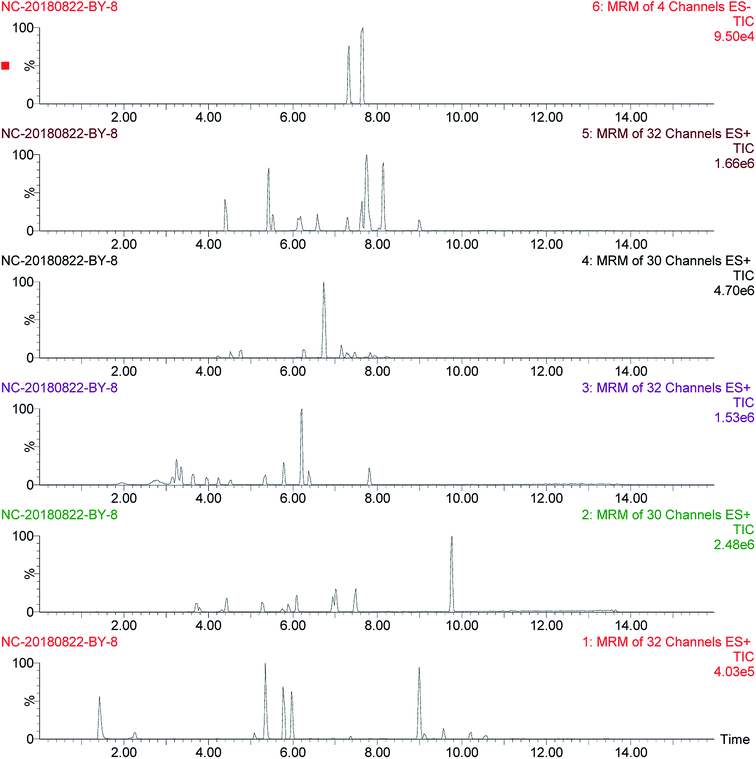
The mobile phase, flow rate, and chromatographic column for UPLC were chosen to sufficiently acquire the optimal signal response of each target analyte. Different proportions of the mobile phase composition (methanol/water or acetonitrile/water) could not obtain better improvement in the peak shape. However, the mobile phase consisting of acetonitrile and 0.1% formic acid aqueous solution with gradient elution could get good symmetric peaks and preferable analyte ionization, with the elution condition being optimized, as described in the section “Liquid chromatographic conditions”. Different flow rates (0.1 mL min−1, 0.2 mL min−1, and 0.3 mL min−1) were performed, and the best separation was achieved when the flow rate was 0.2 mL min−1. Agilent Eclipse Plus (50 × 2.1 mm, 1.8 μm), ACQUITY UPLC BEH C18 (50 × 2.1 mm, 1.7 μm), and ACQUITY UPLC BEH C18 (100 × 2.1 mm, 1.7 μm) were used for the separation, and it was observed that better chromatographic peaks were obtained by using the ACQUITY UPLC BEH C18 (100 × 2.1 mm, 1.7 μm) column. In a representative sample, the total ion chromatogram (TIC) of target analytes is shown in Fig. 2.Each standard solution at a concentration of 50 ng mL−1 was injected directly into the mass spectrometer. In order to acquire maximum signal strength for the quantifier and qualifier of each analyte, an electrospray ionization source with positive and negative modes (ESI+ and ESI−) was selected. After fragmentation by collision gas, two characteristic peaks of fragment ions with strong responses were found. Therefore, the largest response product was regarded as the quantifier, and the other one was viewed as the qualifier. The multiple reaction monitoring (MRM) conditions of pesticide residues via tandem quadruple detector-mass spectrometry (TQD-MS) are summarized in Table 1.
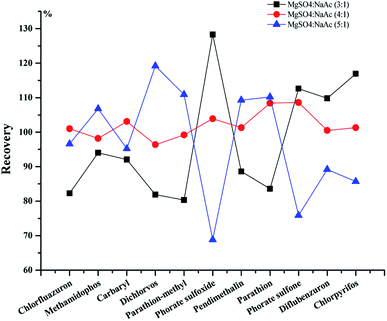
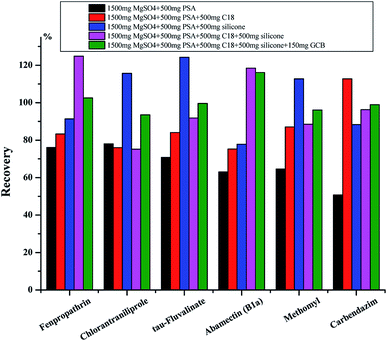
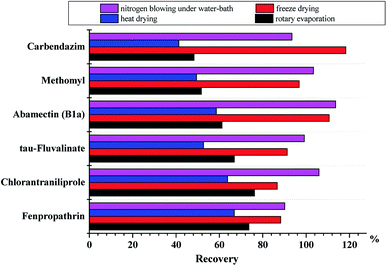
3.2 Optimization of sample pretreatment
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w).
1 w/w).
3.3 Method validation
In this paper, the standard curves for each pesticide acquired good linearity in the range of 1–500 ng mL−1 with the correlation coefficient (R2) > 0.990. The LOD and LOQ values of all the analytes ranged from 0.01 μg kg−1 to 303.35 μg kg−1 and 0.03 μg kg−1 to 1011.15 μg kg−1, respectively, thereby manifesting the high sensitivity of the proposed method. Furthermore, the recoveries (n = 6) of the analyzed pesticides were in the range of 75.92–113.43%, which demonstrated that the developed method had excellent accuracy for the determination of the target analytes. The precision based on RSD from six replicate samples was evaluated, and the RSDs were between 0.60% and 7.36%, revealing the good repeatability of the developed UPLC-ESI-MS/MS method. Simultaneously, the matrix effect was considered to be one of the most important and common problems in the pesticide analysis, which had adverse effects on the quantitative analysis. The matrix effect was usually caused by the insufficient removal of fatty acids, phospholipids, pigments, and sugars from the extracting solution. All the matrix effect values ranged from 81.79% to 118.71% and 80.36% to 119.64% in maca and Moringa oleifera, respectively, which showed that the quantitative detection of the sample was not affected by the matrix. The regression equations, linear ranges, R2, LODs, LOQs, recoveries, RSDs, and matrix effects of 75 compounds are listed in Table 2. Meanwhile, the proposed method was compared with the published literature about the determination of pesticides in floristics (Table 3).| Compounds | Linear equation | Linear range (ng mL−1) | R2 | LOD (μg kg−1) | LOQ (μg kg−1) | Recovery (%) | RSDprecision (n = 6) (%) | Matrix effect (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Maca | Moringa oleifera | ||||||||
| Chlorfluazuron | y = 4403.98x + 127.899 | 1–100 | 0.9967 | 0.73 | 2.42 | 95.32 | 5.71 | 96.61 | 101.23 |
| Methamidophos | y = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388.7x + 4201.16 388.7x + 4201.16 |
5–500 | 0.9982 | 4.42 | 14.72 | 94.11 | 3.52 | 99.22 | 106.81 |
| Carbaryl | y = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001.3x − 248.311 001.3x − 248.311 |
1–100 | 0.9948 | 0.52 | 1.71 | 103.10 | 4.83 | 95.19 | 92.10 |
| Dichlorvos | y = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 032.7x − 255.536 032.7x − 255.536 |
10–500 | 0.9977 | 76.84 | 256.15 | 96.42 | 4.29 | 81.88 | 119.18 |
| Parathion-methyl | y = 1622.44x − 100.796 | 1–200 | 0.9999 | 3.14 | 10.45 | 99.91 | 7.17 | 99.16 | 100.86 |
| Phorate sulfoxide | y = 143![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529x − 2705.6 529x − 2705.6 |
2–500 | 0.9946 | 13.61 | 45.36 | 103.89 | 2.92 | 81.79 | 99.12 |
| Pendimethalin | y = 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799.7x − 413.757 799.7x − 413.757 |
1–100 | 0.9984 | 0.07 | 0.22 | 98.84 | 2.73 | 97.88 | 88.63 |
| Parathion | y = 4189.73x − 83.6592 | 1–200 | 0.9955 | 1.14 | 3.81 | 97.86 | 4.77 | 110.16 | 83.56 |
| Phorate sulfone | y = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 992.1x + 195.135 992.1x + 195.135 |
1–200 | 0.9905 | 0.08 | 0.27 | 75.92 | 2.57 | 91.08 | 80.36 |
| Diflubenzuron | y = 1981.39x − 105.615 | 2–200 | 0.9982 | 3.65 | 12.18 | 89.22 | 5.60 | 100.47 | 109.79 |
| Chlorpyrifos | y = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095x − 120.144 095x − 120.144 |
1–100 | 0.9964 | 0.22 | 0.73 | 101.25 | 4.19 | 116.92 | 97.46 |
| Fenpropathrin | y = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 394.6x − 107.92 394.6x − 107.92 |
1–100 | 0.9957 | 0.14 | 0.46 | 110.16 | 3.43 | 104.87 | 93.28 |
| Chlorantraniliprole | y = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 379.3x − 796.367 379.3x − 796.367 |
1–100 | 0.9968 | 0.07 | 0.22 | 95.74 | 3.63 | 103.23 | 99.51 |
| Tau-fluvalinate | y = 7740.1x + 69.9103 | 1–200 | 0.9963 | 0.44 | 1.47 | 94.14 | 4.27 | 99.59 | 104.28 |
| Abamectin (B1a) | y = 156.624x − 11.4867 | 10–500 | 0.9976 | 35.84 | 119.48 | 107.78 | 7.34 | 99.14 | 93.06 |
| Methomyl | y = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844.9x − 63.5845 844.9x − 63.5845 |
20–500 | 0.9937 | 303.35 | 1011.15 | 97.09 | 3.98 | 96.10 | 99.42 |
| Carbendazim | y = 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 884x − 1543.79 884x − 1543.79 |
10–500 | 0.9986 | 26.52 | 88.42 | 88.33 | 2.50 | 98.36 | 90.75 |
| Pyrimethanil | y = 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330.1x + 63.1455 330.1x + 63.1455 |
1–100 | 0.9938 | 0.05 | 0.18 | 94.44 | 3.03 | 118.23 | 110.11 |
| Carbofuran | y = 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 373x − 1772.69 373x − 1772.69 |
1–100 | 0.9975 | 0.05 | 0.17 | 93.26 | 3.05 | 98.02 | 105.37 |
| Acetamiprid | y = 185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492x − 9477.32 492x − 9477.32 |
1–100 | 0.9963 | 0.04 | 0.13 | 94.02 | 1.22 | 107.48 | 105.89 |
| Imidacloprid | y = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718.9x − 201.094 718.9x − 201.094 |
2–200 | 0.9984 | 0.68 | 2.27 | 81.39 | 4.08 | 99.25 | 105.13 |
| Chlorobenzuron | y = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 129.6x + 437.562 129.6x + 437.562 |
1–100 | 0.9973 | 0.05 | 0.18 | 87.67 | 2.62 | 93.20 | 119.08 |
| Iprodione | y = 3156.04x − 240.147 | 2–200 | 0.9985 | 3.27 | 10.89 | 90.82 | 6.29 | 100.63 | 109.36 |
| Isofenphos-methyl | y = 6207.22x − 125.747 | 2–200 | 0.9998 | 0.81 | 2.69 | 103.49 | 5.53 | 103.04 | 97.74 |
| Tebufenozide | y = 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816x + 468.153 816x + 468.153 |
1–100 | 0.9918 | 0.04 | 0.13 | 96.56 | 4.34 | 108.78 | 107.32 |
| Pyridaben | y = 459![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 203x + 216.086 203x + 216.086 |
1–100 | 0.9965 | 0.01 | 0.03 | 104.22 | 1.73 | 96.16 | 105.41 |
| Prochloraz | y = 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 479.1x − 922.982 479.1x − 922.982 |
1–100 | 0.9943 | 0.07 | 0.22 | 77.14 | 3.20 | 115.02 | 101.26 |
| Dimethomorph | y = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433.9x − 621.198 433.9x − 621.198 |
1–100 | 0.9912 | 0.07 | 0.23 | 89.17 | 2.79 | 89.11 | 105.43 |
| Emamectin benzoate | y = 283![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 956x − 1525.82 956x − 1525.82 |
1–100 | 0.9992 | 0.02 | 0.06 | 95.71 | 1.76 | 111.73 | 96.24 |
| Propamocarb | y = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570.4x + 1634.29 570.4x + 1634.29 |
1–100 | 0.9908 | 0.02 | 0.07 | 93.14 | 1.67 | 97.71 | 109.32 |
| Aldicarb-sulfoxide | y = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 944.6x − 152.452 944.6x − 152.452 |
1–200 | 0.9956 | 0.25 | 0.83 | 107.52 | 5.44 | 96.74 | 95.92 |
| Aldicarb | y = 1650.99x − 52.811 | 2–500 | 0.9978 | 3.48 | 11.59 | 90.91 | 7.24 | 101.51 | 107.62 |
| Omethoate | y = 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447x − 1454.94 447x − 1454.94 |
1–100 | 0.9978 | 0.09 | 0.30 | 92.62 | 7.36 | 100.78 | 106.59 |
| Aldicarbsulfone | y = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481.2x + 726.102 481.2x + 726.102 |
1–100 | 0.9994 | 0.08 | 0.26 | 95.82 | 4.00 | 102.89 | 101.56 |
| Carbofuran-3-hydroxy | y = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453.9x − 289.78 453.9x − 289.78 |
1–200 | 0.9972 | 0.22 | 0.73 | 85.13 | 4.44 | 113.04 | 85.13 |
| Forchlorfenuron | y = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444.9x − 1291.53 444.9x − 1291.53 |
1–200 | 0.9936 | 0.16 | 0.53 | 95.11 | 3.05 | 83.62 | 102.53 |
| Fenitrothion | y = 3535.14x − 39.6721 | 5–500 | 0.9992 | 2.18 | 7.25 | 86.36 | 5.75 | 97.41 | 114.84 |
| Thiamethoxam | y = 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 061.2x − 716.041 061.2x − 716.041 |
1–100 | 0.9975 | 0.10 | 0.32 | 94.67 | 3.93 | 101.25 | 81.63 |
| Triadimefon | y = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365.3x − 1039.06 365.3x − 1039.06 |
1–100 | 0.9950 | 0.05 | 0.18 | 86.68 | 3.63 | 96.62 | 108.32 |
| Phoxim | y = 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111.5x − 135.064 111.5x − 135.064 |
1–100 | 0.9989 | 0.10 | 0.32 | 85.80 | 2.87 | 100.51 | 91.23 |
| Phosmet | y = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610.6x − 549.165 610.6x − 549.165 |
1–200 | 0.9982 | 0.18 | 0.59 | 95.16 | 4.64 | 102.83 | 107.92 |
| Azoxystrobin | y = 378![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 351x − 2332.66 351x − 2332.66 |
1–100 | 0.9956 | 0.01 | 0.04 | 101.354 | 2.26 | 111.45 | 109.94 |
| Fipronil-sulfone | y = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332.4x − 511.308 332.4x − 511.308 |
10–500 | 0.9975 | 44.17 | 147.24 | 94.09 | 2.72 | 118.71 | 84.13 |
| Mevinphos | y = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838.5x − 775.089 838.5x − 775.089 |
1–200 | 0.9969 | 0.11 | 0.36 | 102.91 | 5.23 | 102.54 | 93.62 |
| Phorate | y = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294.9x − 1152.52 294.9x − 1152.52 |
1–100 | 0.9976 | 0.07 | 0.23 | 92.89 | 2.09 | 95.22 | 115.23 |
| Fenthion | y = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 726.6x − 243.559 726.6x − 243.559 |
1–200 | 0.9976 | 0.28 | 0.93 | 95.90 | 3.96 | 93.74 | 107.81 |
| Phosphamidon | y = 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158x − 2071.14 158x − 2071.14 |
1–100 | 0.9976 | 0.05 | 0.16 | 88.23 | 1.32 | 100.43 | 101.14 |
| Tolclofos-methyl | y = 4072.56x − 89.1861 | 2–200 | 0.9942 | 1.23 | 4.11 | 90.40 | 4.19 | 112.03 | 93.52 |
| Fenamiphos | y = 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909x − 1479.86 909x − 1479.86 |
1–100 | 0.9987 | 0.03 | 0.09 | 96.68 | 2.17 | 101.78 | 107.28 |
| Triazophos | y = 379![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606x − 950.747 606x − 950.747 |
1–100 | 0.9984 | 0.01 | 0.04 | 94.21 | 1.93 | 102.34 | 105.78 |
| Isazofos | y = 686![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684x − 3209.08 684x − 3209.08 |
1–200 | 0.9993 | 6.43 | 21.44 | 96.29 | 0.60 | 103.13 | 101.02 |
| Dichlofenthion | y = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725.3x + 483.144 725.3x + 483.144 |
1–500 | 0.9906 | 250.41 | 834.71 | 108.2 | 4.72 | 99.62 | 119.64 |
| Phenthoate | y = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548x + 145.255 548x + 145.255 |
1–100 | 0.9963 | 0.09 | 0.31 | 97.14 | 2.45 | 98.21 | 86.78 |
| Malathion | y = 367![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 467x − 2199.49 467x − 2199.49 |
1–100 | 0.9992 | 0.01 | 0.05 | 92.18 | 2.28 | 103.62 | 110.23 |
| Phosalone | y = 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828x − 138.642 828x − 138.642 |
1–100 | 0.9992 | 0.05 | 0.16 | 82.38 | 1.66 | 110.04 | 108.52 |
| Profenofos | y = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307.6x − 42.0899 307.6x − 42.0899 |
1–200 | 0.9962 | 0.23 | 0.76 | 87.8 | 4.23 | 105.67 | 99.43 |
| Difenoconazole | y = 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220x − 635.362 220x − 635.362 |
1–100 | 0.9990 | 0.05 | 0.15 | 85.39 | 1.78 | 101.31 | 104.04 |
| Dimethoate | y = 234![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 934x − 2342.4 934x − 2342.4 |
1–100 | 0.9985 | 0.03 | 0.11 | 84.46 | 2.31 | 103.78 | 109.56 |
| Methacrifos | y = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 311.1x − 138.44 311.1x − 138.44 |
1–200 | 0.9984 | 0.23 | 0.77 | 95.51 | 3.58 | 101.62 | 111.89 |
| Ethoprophos | y = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643.6x − 947.642 643.6x − 947.642 |
5–500 | 0.9991 | 34.26 | 114.19 | 96.47 | 2.43 | 101.85 | 97.64 |
| Fonofos | y = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 849.2x − 686.454 849.2x − 686.454 |
1–200 | 0.9987 | 0.43 | 100.40 | 100.70 | 3.70 | 100.42 | 105.51 |
| Cadusafos | y = 176![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382x − 868.372 382x − 868.372 |
5–500 | 0.9934 | 9.82 | 32.73 | 97.42 | 1.60 | 102.62 | 87.05 |
| Fosthiazate | y = 286![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765x − 2455.26 765x − 2455.26 |
1–100 | 0.9953 | 0.05 | 0.17 | 83.91 | 2.39 | 99.60 | 96.02 |
| Etrimfos | y = 158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015x − 1371.18 015x − 1371.18 |
1–100 | 0.9988 | 0.03 | 0.10 | 92.89 | 2.79 | 96.81 | 118.33 |
| Quinalphos | y = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587.5x − 319.706 587.5x − 319.706 |
2–200 | 0.9985 | 1.29 | 4.29 | 102.67 | 4.32 | 110.14 | 89.32 |
| Methidathion | y = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 479.6x − 309.929 479.6x − 309.929 |
1–100 | 0.9973 | 0.09 | 0.31 | 98.42 | 1.81 | 97.21 | 88.89 |
| Diazinon | y = 296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816x − 751.324 816x − 751.324 |
1–100 | 0.9993 | 0.02 | 0.06 | 91.56 | 1.69 | 97.42 | 93.56 |
| Pirimiphos-methyl | y = 283![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855x − 85.2483 855x − 85.2483 |
1–100 | 0.9983 | 0.02 | 0.05 | 113.43 | 1.74 | 107.04 | 82.11 |
| Fensulfothion | y = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725.6x − 360.82 725.6x − 360.82 |
1–200 | 0.9984 | 0.15 | 0.52 | 80.40 | 4.52 | 98.23 | 117.31 |
| Azinphos-methyl | y = 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309x − 753.843 309x − 753.843 |
1–100 | 0.9957 | 0.08 | 0.25 | 104.90 | 2.98 | 112.65 | 97.14 |
| EPN | y = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.7x − 55.1401 800.7x − 55.1401 |
1–200 | 0.9997 | 0.32 | 1.07 | 96.54 | 3.82 | 100.33 | 106.22 |
| Isofenphos | y = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093.2x − 109.971 093.2x − 109.971 |
1–200 | 0.9965 | 0.52 | 1.72 | 76.12 | 3.23 | 101.02 | 96.63 |
| Ethion | y = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 378.8x − 664.326 378.8x − 664.326 |
1–100 | 0.9948 | 0.07 | 0.23 | 81.27 | 2.39 | 99.82 | 93.41 |
| Fipronil desulfinyl | y = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500.5x − 79.4482 500.5x − 79.4482 |
1–200 | 0.9985 | 0.21 | 0.70 | 98.65 | 3.47 | 98.56 | 109.32 |
| Fipronil sulphide | y = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 024.9x − 25.3713 024.9x − 25.3713 |
1–200 | 0.9979 | 0.17 | 0.58 | 96.43 | 3.84 | 103.53 | 93.67 |
| Floristics | Analytes | Chromatographic methods | Extraction technique | Analyte time | Recovery | LOQ | References |
|---|---|---|---|---|---|---|---|
| Tea | 10 pesticides | HPLC-MS/MS | QuEChERS | 15 min | 72–116% | 1.7–9.0 μg kg−1 | 48 |
| Pepper | 3 pesticides | UPLC-MS/MS | QuEChERS | 15 min | 104.91% | 2–10 μg kg−1 | 49 |
| Green tea | 102 pesticides | HPLC-MS/MS | Modified QuEChERS | 42 min | 62–125% | 0.1–50 μg kg−1 | 50 |
| Maca and Moringa oleifera | 75 pesticides | QuEChERS-UPLC-ESI-MS/MS | Modified QuEChERS | 16 min | 75.92–113.43% | 0.03–1011.15 μg kg−1 | This method |
| Tomato | 3 pesticides | UPLC-MS/MS | QuEChERS | 15 min | 60–140% | — | 51 |
| Strawberry | 16 pesticides | UPLC-MS/MS | Modified QuEChERS | 3.5 min | 81.8–117.2% | 0.3–2.8 μg kg−1 | 52 |
| Cinnamon bark | 60 pesticides | UPLC-MS/MS | d-SPE and QuEChERS | 16 min | 71–118% | 0.5–50 μg kg−1 | 53 |
| Pecan nuts | 47 pesticides | UPLC-MS/MS | Modified QuEChERS | 30 min | 70–120% | 5–10 μg kg−1 | 54 |
| Oat | 60 pesticides | UPLC-MS/MS | QuEChERS | 25 min | 70–120% | 5–10 μg kg−1 | 55 |
| Strawberry | 203 pesticides | UPLC-MS/MS | QuEChERS | 27 min | 70–120% | 2–10 μg kg−1 | 56 |
| Cucumber and grapefruit | 233 pesticides | HPLC-MS/MS | QuEChERS | — | 77.87–104.15% | 0.42–39.35 μg kg−1 | 57 |
| Zizania latifolia | 25 pesticides | UPLC-MS/MS | QuEChERS | 10 min | 72–118% | 0.5–3.3 μg kg−1 | 58 |
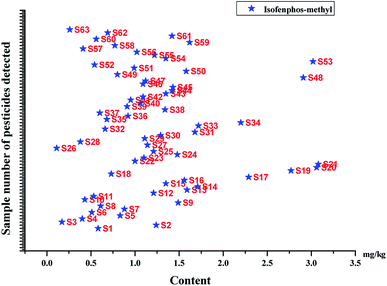
3.4 Analysis of real samples
The developed modified QuEChERS-UPLC-ESI-MS/MS method was used to analyze the contents of 75 pesticide residues in 103 samples (Table 4 and Fig. 7). Among 103 samples (63 dried fruits of maca and 40 Moringa oleifera leaves), isofenphos-methyl was detected in all maca samples with the concentrations in the range of 0.11–3.08 mg kg−1. However, other pesticides were not detected. As a result, 40 Moringa oleifera leaves were found as negative samples. Isofenphos-methyl is a kind of soil insecticide, which has a strong contact action and causes gastric toxicity to pests. Meanwhile, it is a broad-spectrum insecticide with a long residual period, mainly used for preventing and treating grubs, mole crickets, wireworms, and other underground pests coming from wheat, peanuts, soybeans, corns, sweet potatoes, beets, apples, and other crops but is only used in seed mixing or soil treatment; however, it is prohibited from spraying on the fruit tree leaves. For maca, the edible part was mainly the root of maca that was used as the research object in this paper. In order to prevent and control pests, the planting farmers may add isofenphos-methyl to seed dressing or soil treatment, as a result, isofenphos-methyl remains in the root of maca. Regarding isofenphos-methyl, no MRLs had been reported in the dried maca and Moringa oleifera samples by the Codex Alimentarius.59| Sample name | No. | Sample source | Date of collection | Pesticides detected | Content (mg kg−1) | Codex Alimentarius MRLs (mg kg−1) |
|---|---|---|---|---|---|---|
| a n.e.: not established by the Codex Alimentarius. | ||||||
| Maca | S1 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.58 ± 0.05 | n.e. |
| S2 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.24 ± 0.11 | n.e. | |
| S3 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.17 ± 0.02 | n.e. | |
| S4 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.40 ± 0.03 | n.e. | |
| S5 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.83 ± 0.06 | n.e. | |
| S6 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.51 ± 0.05 | n.e. | |
| S7 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.88 ± 0.06 | n.e. | |
| S8 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.61 ± 0.05 | n.e. | |
| S9 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.49 ± 0.13 | n.e. | |
| S10 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.43 ± 0.03 | n.e. | |
| S11 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.53 ± 0.05 | n.e. | |
| S12 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.21 ± 0.10 | n.e. | |
| S13 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.59 ± 0.14 | n.e. | |
| S14 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.71 ± 0.15 | n.e. | |
| S15 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.35 ± 0.12 | n.e. | |
| S16 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.56 ± 0.14 | n.e. | |
| S17 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 2.29 ± 0.19 | n.e. | |
| S18 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.73 ± 0.06 | n.e. | |
| S19 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 2.77 ± 0.23 | n.e. | |
| S20 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 3.06 ± 0.26 | n.e. | |
| S21 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 3.08 ± 0.26 | n.e. | |
| S22 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.00 ± 0.07 | n.e. | |
| S23 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.10 ± 0.08 | n.e. | |
| S24 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.48 ± 0.13 | n.e. | |
| S25 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.21 ± 0.10 | n.e. | |
| S26 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.11 ± 0.01 | n.e. | |
| S27 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.14 ± 0.10 | n.e. | |
| S28 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.38 ± 0.03 | n.e. | |
| S29 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.11 ± 0.08 | n.e. | |
| S30 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.29 ± 0.11 | n.e. | |
| S31 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.68 ± 0.14 | n.e. | |
| S32 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.66 ± 0.05 | n.e. | |
| S33 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.72 ± 0.15 | n.e. | |
| S34 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 2.20 ± 0.19 | n.e. | |
| S35 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.68 ± 0.06 | n.e. | |
| S36 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.92 ± 0.07 | n.e. | |
| S37 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.60 ± 0.05 | n.e. | |
| S38 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.34 ± 0.12 | n.e. | |
| S39 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.91 ± 0.07 | n.e. | |
| S40 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.06 ± 0.08 | n.e. | |
| S41 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.95 ± 0.07 | n.e. | |
| S42 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.09 ± 0.08 | n.e. | |
| S43 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.35 ± 0.12 | n.e. | |
| S44 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.42 ± 0.13 | n.e. | |
| S45 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.43 ± 0.13 | n.e. | |
| S46 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.09 ± 0.08 | n.e. | |
| S47 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.12 ± 0.08 | n.e. | |
| S48 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 2.91 ± 0.25 | n.e. | |
| S49 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.80 ± 0.06 | n.e. | |
| S50 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.58 ± 0.14 | n.e. | |
| S51 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.99 ± 0.07 | n.e. | |
| S52 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.54 ± 0.05 | n.e. | |
| S53 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 3.02 ± 0.26 | n.e. | |
| S54 | Yongsheng (Lijiang) | Mar-18 | Isofenphos-methyl | 1.35 ± 0.12 | n.e. | |
| S55 | Yongsheng (Lijiang) | Mar-18 | Isofenphos-methyl | 1.22 ± 0.11 | n.e. | |
| S56 | Yongsheng (Lijiang) | Mar-18 | Isofenphos-methyl | 1.02 ± 0.09 | n.e. | |
| S57 | Litang (Ganzi) | Mar-18 | Isofenphos-methyl | 0.41 ± 0.03 | n.e. | |
| S58 | Litang (Ganzi) | Mar-18 | Isofenphos-methyl | 0.77 ± 0.06 | n.e. | |
| S59 | Xianggelila (Yunnan) | Mar-18 | Isofenphos-methyl | 1.62 ± 0.14 | n.e. | |
| S60 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.56 ± 0.05 | n.e. | |
| S61 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 1.42 ± 0.12 | n.e. | |
| S62 | Yulong (Lijiang) | Mar-18 | Isofenphos-methyl | 0.69 ± 0.05 | n.e. | |
| S63 | Luquan (Kunming) | Mar-18 | Isofenphos-methyl | 0.26 ± 0.02 | n.e. | |
4. Conclusions
A sensitive and effective modified QuEChERS coupled with the UPLC-ESI-MS/MS method was successfully applied for the determination of 75 multiclass pesticides in maca and Moringa oleifera samples. The feasibility of the method was evaluated in the light of linearity, linear range, LOD, LOQ, accuracy, precision, and matrix effect. The validation studies manifested that the proposed method had good linearity, accuracy, and precision. The developed method was used to analyze the practical samples, and the analyses of 63 maca samples from Yunnan in China demonstrated the presence of isofenphos-methyl, but the MRL was not set by the Codex Alimentarius. Consequently, the risk and exposure levels of isofenphos-methyl in the dried fruit of maca should be further investigated and explored. Meanwhile, this method showed a good application prospect and could be used as a general method for the quantitative determination of pesticide residues in food.Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
The paper was supported by the Analysis Test Fund of Kunming University of Science and Technology (No. 2020T20070029) and Natural Science Fund of Yunnan Province (No. 2017ZF004).References
- A. F. Cicero, E. Bandieri and R. Arletti, J. Ethnopharmacol., 2001, 75, 225–229 CrossRef CAS.
- G. F. Gonzales, C. Gonzales and C. Gonzales-Castaneda, Forsch. Komplementmed., 2009, 16, 373–380 CrossRef.
- K. J. Lee, K. Dabrowski, J. Rinchard and C. Gomez, Aquacult. Res., 2004, 35, 215–223 CrossRef.
- B. Feret, Formulary, 2005, 40, 227–230 CAS.
- M. Stone, A. Ibarra, M. Roller, A. Zangara and E. Stevenson, J. Ethnopharmacol., 2009, 126, 574–576 CrossRef.
- B. Padayachee and H. Baijnath, J. Med. Plants Res., 2012, 6, 5831–5839 Search PubMed.
- P. Siddhuraju and K. Becker, J. Agric. Food Chem., 2003, 51, 2144–2155 CrossRef CAS.
- J. M. Das, Curr. Sci., 1965, 34, 374–375 CAS.
- N. Ramiah and G. A. Nair, J. Inst. Chem., 1977, 49, 163–165 Search PubMed.
- J. R. Ingelfinger, N. Engl. J. Med., 2008, 359, 2745–2748 CrossRef CAS.
- P. A. Murphy, S. Hendrich, C. Landgren and C. M. Bryant, J. Food Sci., 2006, 71, R51–R65 CrossRef CAS.
- P. H. Abelson, Science, 1993, 259, 1235 CrossRef CAS.
- W. J. Kong, R. W. Wei, A. F. Logrieco, J. H. Wei, J. Wen, X. H. Xiao and M. H. Yang, Food Chem., 2014, 146, 320–326 CrossRef CAS.
- J. S. Aulakh, A. K. Malik, V. Kaur and P. S. Kopplin, Crit. Rev. Anal. Chem., 2005, 35, 71–85 CrossRef CAS.
- R. Raina, Chapter 5-Chemical analysis of pesticides using GC/MS, GC/MS/MS, and LC/MS/MS. In Pesticides-strategies for pesticides analysis; Stoytcheva, M.; Academic Press: In Tech, Vienna, Austria, 2011; pp. 105–130 Search PubMed.
- K. F. Nielsen and U. Thrane, Mycotoxin Res., 2000, 16, 252–256 CrossRef.
- M. A. Cámara, A. Barba, S. Cermeño, G. Martínez and J. Oliva, J. Environ. Sci. Health, Part B, 2017, 52, 1–7 CrossRef.
- M. A. Cámara, S. Cermeño, G. Martínez and J. Oliva, Food Chem., 2020, 325, 126936 CrossRef.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck, J. AOAC Int., 2003, 86, 412–431 CrossRef CAS.
- M. M. Rahman, H. S. Lee, A. M. Abd El-Aty, Md. H. Kabir, H. S. Chung, J. H. Park, M. R. Kim, J. Kim, H. C. Shin, S. S. Shin and J. H. Shim, Food Chem., 2018, 263, 59–66 CrossRef CAS.
- S. Valverde, A. M. Ares, J. L. Bernal, M. J. Nozal and J. Bernal, Microchem. J., 2018, 142, 70–77 CrossRef CAS.
- L. Han, J. Matarrita, Y. Sapozhnikova and S. J. Lehotay, J. Chromatogr. A, 2016, 1449, 17–29 CrossRef CAS.
- B. Y. Durak, D. S. Chormey, M. Firat and S. Bakirdere, Food Chem., 2020, 305, 125487 CrossRef CAS.
- R. M. Gonzalez-Rodriguez, R. Rial-Otero, B. Cancho-Grande and J. Simal-Gándara, J. Chromatogr. A, 2008, 1196–1197, 100–109 CrossRef CAS.
- T. D. Nguyen, J. E. Yu, D. M. Lee and G. H. Lee, Food Chem., 2008, 110, 207–213 CrossRef CAS.
- S. J. Lehotay, Multiclass, Multiresidue Analysis of Pesticides, Strategies for, in Encyclopedia of Analytical Chemistry, 2006, DOI:10.1002/9780470027318.a1715.
- S. J. Lehotay, QuEChERS Sample Preparation Approach for Mass Spectrometric Analysis of Pesticide Residues in Foods, in Mass Spectrometry in Food Safety: Methods in Molecular Biology (Methods and Protocols), ed. J. Zweigenbaum, 2011, DOI:10.1007/978-1-61779-136-9_4.
- Y. L. Wu, L. W. Chen, Y. P. Xian, X. C. Hou, M. Liang, H. Dong and J. F. Chen, Food Chem., 2019, 298, 125048 CrossRef CAS.
- I. R. Ghoniem, E. R. Attallah and M. M. Abo-Aly, Int. J. Environ. Anal. Chem., 2017, 97, 301–312 CrossRef CAS.
- A. Steinborn, L. Alder, M. Spitzke, D. Dork and M. Anastassiades, J. Agric. Food Chem., 2017, 65, 1296–1305 CrossRef CAS.
- A. Suganthi, K. Bhuvaneswari and M. Ramya, Food Chem., 2018, 241, 275–280 CrossRef CAS.
- J. A. Ferreira, J. M. S. Ferreira, V. Talamini, J. de Fátima Facco, T. M. Rizzetti, O. D. Prestes, M. B. Adaime, R. Zanella and C. B. G. Bottoli, Food Chem., 2016, 213, 616–624 CrossRef CAS.
- O. Golge, A. Koluman and B. Kabak, Food Anal. Methods, 2018, 11, 1122–1148 CrossRef.
- W. J. Zheng, J. A. Park, A. M. Abd El-Aty, S. K. Kim, S. H. Cho, J. M. Choi, H. Yi, S. M. Cho, A. Ramadan, J. H. Jeong, J. H. Shim and H. C. Shin, J. Chromatogr. B: Biomed. Sci. Appl., 2018, 1072, 60–69 CrossRef CAS.
- M. Sajid, C. Basheer and M. Mansha, J. Chromatogr. A, 2016, 1475, 110–115 CrossRef CAS.
- G. F. Xia, X. Y. Fang, Y. R. Wang and X. Y. Yang, Anal. Lett., 2017, 50, 787–796 CrossRef CAS.
- Q. Z. Guo, S. Zhao, J. Zhang, K. L. Qi, Z. X. Du and B. Shao, Food Addit. Contam., Part A, 2018, 35, 1543–1552 CrossRef CAS.
- H. Dong, X. F. Zeng and W. D. Bai, Food Chem., 2018, 258, 206–213 CrossRef CAS.
- A. G. Frenich, R. Romero-González, M. L. Gómez-Pérez and J. L. M. Vidal, J. Chromatogr. A, 2011, 1218, 4349–4356 CrossRef CAS.
- X. C. Wang, B. Shu, S. Li, Z. G. Yang and B. Qiu, Talanta, 2017, 162, 90–97 CrossRef CAS.
- P. A. S. Tette, F. A. da Silva Oliveira, E. N. C. Pereira, G. Silva, M. B. de Abreu Glória and C. Fernandes, Food Chem., 2016, 211, 130–139 CrossRef CAS.
- A. Z. Zhang, Q. L. Wang, L. L. Cao, Y. Li, H. Shen, J. Shen, S. F. Zhang and Z. Y. Man, Chin. J. Chromatogr., 2016, 34, 158–164 CrossRef CAS.
- S. J. Lehotay, K. Mastovska and A. R. Lightfield, J. AOAC Int., 2005, 88, 615–629 CrossRef CAS.
- M. S. Abbas, A. S. Soliman, H. A. El-Gammal, M. E. Amer and E. R. Attallah, Int. J. Environ. Anal. Chem., 2017, 97, 1003–1023 CrossRef CAS.
- Y. J. Lee, M. M. Rahman, A. M. Abd El-Aty, J. H. Choi, H. S. Chung, S. W. Kim, A. M. Abdel-Aty, H. C. Shin and J. H. Shim, Food Chem., 2016, 210, 442–450 CrossRef CAS.
- G. Bernardi, M. Kemmerich, L. C. Ribeiro, M. B. Adaime, R. Zanella and O. D. Prestes, Talanta, 2016, 161, 40–47 CrossRef CAS.
- M. Anastassiades, E. Scherbaum, B. Tasdelen and D. Stajnbaher, Recent developments in QuEChERS methodology for pesticide multiresidue analysis, in Pesticide chemistry: Crop protection, public health, environmental safety, H. Ohkawa, H. Miyagawa and P. W. Lee, Academic Press, Wiley-VCH, Weinheim, Germany, 2007, pp. 439–458 Search PubMed.
- C. C. Yu, D. Y. Hao, Q. Chu, T. Wang, S. N. Liu, T. Lan, F. H. Wang and C. P. Pan, Food Chem., 2020, 321, 126657 CrossRef CAS.
- B. Polat and O. Tiryaki, J. Environ. Sci. Health, Part B, 2020, 55, 1–10 CrossRef CAS.
- Y. S. Huang, T. Shi, X. Luo, H. L. Xiong, F. F. Min, Y. Chen, S. P. Nie and M. Y. Xie, Food Chem., 2019, 275, 255–264 CrossRef CAS.
- B. Polat and O. Tiryaki, J. Environ. Sci. Health, Part B, 2019, 54, 112–117 CrossRef CAS.
- L. Song, Z. Z. Zhong, Y. T. Han, Q. L. Zheng, Y. H. Qin, Q. Wu, X. P. He and C. P. Pan, Ecotoxicol. Environ. Saf., 2020, 188, 109842 CrossRef CAS.
- Z. H. Zhang, M. F. Dong, X. H. Hao, L. J. Han, S. Y. Song and W. Yao, Food Chem., 2019, 276, 140–146 CrossRef CAS.
- P. E. P. Barci, L. S. Alves, A. A. S. Avellar, L. R. Cendon, P. J. Santos, F. M. Stringhini, O. D. Prestes and R. Zanella, Food Anal. Methods, 2020, 13, 793–801 CrossRef.
- E. M. C. Matos, L. C. Ribeiro, O. D. Prestes, J. A. G. Silva, B. S. Farias, L. A. A. Pinto and R. Zanella, Food Anal. Methods, 2019, 12, 2835–2844 CrossRef.
- N. E. Song, M. Yoo and T. G. Nam, CyTA--J. Food, 2019, 17, 976–987 CrossRef CAS.
- F. Hepsağ, Appl. Ecol. Environ. Sci., 2019, 17, 6887–6916 Search PubMed.
- F. Xu, J. Y. Yu, Q. S. Wang, Y. Fu, H. Zhang and Y. L. Wu, Sci. Rep., 2019, 9, 10031 CrossRef.
- Food and Agriculture Organization of the United Nations, Pesticides Database, Codex Alimentarius, 2020, http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/en/, accessed on 3 June 2020 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |