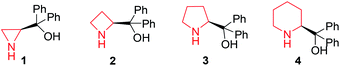Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Azaheterocyclic diphenylmethanol chiral solvating agents for the NMR chiral discrimination of alpha-substituted carboxylic acids†
Gao-Wei Li ab,
Xiao-Juan Wang
ab,
Xiao-Juan Wang *a,
Dan-Dan Cuia,
Yu-Fei Zhanga,
Rong-Yao Xua,
Shuai-Hua Shia,
Lan-Tao Liu*a,
Min-Can Wang
*a,
Dan-Dan Cuia,
Yu-Fei Zhanga,
Rong-Yao Xua,
Shuai-Hua Shia,
Lan-Tao Liu*a,
Min-Can Wang b,
Hong-Min Liu
b,
Hong-Min Liu b and
Xin-Xiang Lei
b and
Xin-Xiang Lei *c
*c
aCollege of Chemistry and Chemical Engineering and Henan Engineering Laboratory of Green Synthesis for Pharmaceuticals, Shangqiu Normal University, Shangqiu, 476000, P. R. China. E-mail: juanzi409e@126.com; liult05@iccas.ac.cn
bSchool of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, 450001, P. R. China
cSchool of Pharmaceutical Sciences, South-Central University for Nationalities, Wuhan, 430074, P. R. China. E-mail: xxlei@mail.scuec.edu.cn
First published on 18th September 2020
Abstract
A series of small-membered heterocycle probes, so-called azaheterocycle-containing diphenylmethanol chiral solvating agents (CSAs), have been developed for NMR enantiodiscrimination. These chiral sensors were readily synthesized were inexpensive and efficiently used for the chiral analysis of alpha-substituted carboxylic acids. The sensing method was operationally simple and the processing was straightforward. Notably, we propose (S)-aziridinyl diphenylmethanol as a promising CSA, which has excellent chiral discriminating properties and offers multiple detectable possibilities pertaining to the 1H NMR signals of diagnostic split protons (including 25 examples, up to 0.194 ppm, 77.6 Hz). Its ability to detect the molecular recognition of fluorinated carboxylic acids were further investigated, with a good level of discrimination via the 19F NMR spectroscopic analysis. In addition, an accurate enantiomeric excess (ee) analysis of the p-methoxyl-mandelic acid with different optical compositions have been calculated based on the integration of well-separated proton signals.
The detection and identification of chiral compounds have attracted considerable scientific attention due to their indispensable role in technological applications including pharmacology, biology, and modern chemistry for the last several decades.1 So far, spectroscopic and chromatographic approaches are generally utilized for the enantioselective molecular recognition or quantitative analysis including enantio-discrimination, measurement of enantiomeric excess (ee) and assignment of absolute configuration.2 Among them, the nuclear magnetic resonance (NMR) spectroscopy is one of the most convenient, versatile, and routinely available analytical techniques because it provides structural information in a multidimensional manner. For these NMR-based analytical methods, the use of diastereomers prepared from chiral solvating agents (CSAs) and guest compounds has become an efficient and operationally simple analytical approach for chiral discrimination.3 Compared with chiral derivatization agents (CDAs), CSAs form two in situ-formed diastereomeric complexes with analytes via non-covalent interactions, which can be tested immediately after mixing, avoiding chiral derivatization in advance. Over the last few decades, a number of CSAs have been developed, such as coordinative unsaturated chiral lanthanide or transition metal complexes,4 low molecular-weight synthetic Brønsted acids/bases,5 and supramolecular receptors,6 to generate anisochronous chemical shifts. Furthermore, some of them are obtained via synthesis and some from nature. It has been reported that enantiopure compounds/complexes are good NMR CSAs for the determination of the enantiomeric purity of different chiral substrates including alcohols or 1,2-diols, cyanohydrins, and hydroxy acids or amino acid derivatives.7 Although several CSAs are commercially available, the current methods possess several drawbacks such as line-broadening, narrow substrate scope, poor solubility, and poor resolution. Thus, the development of excellent CSAs with wide applications of the 1H NMR chiral analysis is still highly desirable.
Small-membered azaheterocycle derivatives have gained a lot of interest among organic and medicinal chemists for the synthesis of biological active nitrogen-containing compounds. Now, 2-substituted aziridines or azetidines have been already used as synthons for the synthesis of a wide range of compounds in stereoselective transformations, such as highly stereoselective addition or reduction, regioselective ring expansion reaction, and nucleophilic ring-opening polymerization.8 Their broad utility has made these aziridine, azetidine, and even pyrrolidine-based more specifically optically pure amino alcohols, a class of useful and attractive chiral ligands/catalysts in a number of catalytic asymmetric transformations, including the addition of transition metal-catalyzed and organocatalytic asymmetric processes.9 Among various N-heterocyclic amino alcohols, such as N-trityl aziridine carbinols,10 Ferrocenyl-substituted aziridinylalcohols,11 N-phenylethyl aziridine carbinols,12 N-substituted azetidino alcohols,13 and azetidine-2-carboxamides,14 diphenylprolinols15 have received considerable attention and have been successfully applied to catalyze asymmetric reactions. Nevertheless, the literature concerning the guests of chiral analysis using a small-membered N-heterocyclic moiety and the study on enantiodiscrimination in isotropic solutions by NMR techniques are still not abundant. A few representative ones are the following: Rachwalski et al. explored chiral aziridine-mediated imines to evaluate their action as CSAs towards mandelic derivatives,16 and proline-derived diphenylprolinol had been successfully developed as an effective CSA for the analysis of chiral carboxylic acids.17 Therefore, the development of structurally simple efficient receptors for the enantioselective recognition of carboxylic acids is still highly desirable. Taking this fact into account and basing on our experience in the field of the synthesis of a different class of CSAs for the determination of enantiomer ratio, and the application of enantiodiscrimination.18 The suitably designed chiral heterocyclic amino alcohol derivatives might be a good candidate because of the existence of multiple H-bonding and the rigid ring of this candidate compounds. Thus, we specifically focus on the further applications of these 2-(diphenylhydroxy) azaheterocycles as chemical probes for the discrimination of carboxylic acids and the measurement of their ee 1H NMR spectroscopy.
The synthesis of chiral diphenylmethanol-containing N-heterocyclic amino alcohols, in general, was accomplished from commercially available methyl 1-aza-heterocycle-2-carboxylate in a two-step synthesis (the general synthetic procedures are illustrated in ESI Scheme S1†). Four molecular structures of aza-heterocycle-containing diphenylmethanols 1–4 are shown in Fig. 1; in principle, these compounds are suitable candidates for H bonding receptors and they are often used by chemists as enantioselective catalysts in various reactions, and our finding that they are useful CSAs extend their utility.
We conducted the initial exploratory study using racemic analytes in chloroform-d as the most common solvent to determine their abilities to split the signals in the NMR analysis, and explored the binding properties of chiral aza-heterocycle-containing diphenylmethanols 1–4. The (S)-pyrrolidinyl diphenylmethanol 3 as CSA has been proved in our previous study,17 we had found that the suitable stoichiometries of the host–guest complex, the stoichiometric amounts of racemic mandelic acid and (S)-pyrrolidinyl diphenylmethanol 3 molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was finally utilized to carry the application for the resolution of discrimination and measurement of carboxylic acids. Thus, equimolar amounts of all of the chiral aza-heterocycle-containing diphenylmethanols, relative to (±)-3,5-difluoro mandelic acid (MA), were found to be sufficient to discriminate between the corresponding enantiomers. The comparing results of these experiments are shown in Table 1, and their 1H NMR spectra show that the single peak of α-H has particularly significant splitting. The nonequivalent chemical shift difference (ΔΔδ) of α-H in racemic 3,5-difluoro-MA was calculated to be 0.194 ppm for (S)-aziridinyl diphenylmethanol 1, 0.046 ppm for (S)-azetidinyl diphenylmethanol 2, 0.050 ppm for (S)-pyrrolidinyl diphenylmethanol 3 and 0.033 ppm for (S)-piperidinyl diphenylmethanol 4 (Table 1, entries 1–4). Compared with its higher homologues, such as pyrrolidine and piperidine, aziridine and azetidine were seen as more suitable for application as effective NMR discriminating agents for mandelic acids (ESI Fig. S11–S14†). Interestingly, the signal splitting was particularly significant with (S)-aziridinyl diphenylmethanol 1, achieving a value of 77.6 Hz (at 400 Hz) for methine proton (CαH) signals. The preliminary results evidenced that CSA 1 can act as a more efficient chiral solvating agent towards MA because of its rigid structure of the aziridine ring. Enantiopure aziridinyl diphenylmethanol, i.e., ent-1 has also explored its efficacy in binding with racemic 3,5-difluoro-MA, a similar degree of splitting was obtained in the separated peaks (0.184 ppm, Table 1, entry 5).
1 was finally utilized to carry the application for the resolution of discrimination and measurement of carboxylic acids. Thus, equimolar amounts of all of the chiral aza-heterocycle-containing diphenylmethanols, relative to (±)-3,5-difluoro mandelic acid (MA), were found to be sufficient to discriminate between the corresponding enantiomers. The comparing results of these experiments are shown in Table 1, and their 1H NMR spectra show that the single peak of α-H has particularly significant splitting. The nonequivalent chemical shift difference (ΔΔδ) of α-H in racemic 3,5-difluoro-MA was calculated to be 0.194 ppm for (S)-aziridinyl diphenylmethanol 1, 0.046 ppm for (S)-azetidinyl diphenylmethanol 2, 0.050 ppm for (S)-pyrrolidinyl diphenylmethanol 3 and 0.033 ppm for (S)-piperidinyl diphenylmethanol 4 (Table 1, entries 1–4). Compared with its higher homologues, such as pyrrolidine and piperidine, aziridine and azetidine were seen as more suitable for application as effective NMR discriminating agents for mandelic acids (ESI Fig. S11–S14†). Interestingly, the signal splitting was particularly significant with (S)-aziridinyl diphenylmethanol 1, achieving a value of 77.6 Hz (at 400 Hz) for methine proton (CαH) signals. The preliminary results evidenced that CSA 1 can act as a more efficient chiral solvating agent towards MA because of its rigid structure of the aziridine ring. Enantiopure aziridinyl diphenylmethanol, i.e., ent-1 has also explored its efficacy in binding with racemic 3,5-difluoro-MA, a similar degree of splitting was obtained in the separated peaks (0.184 ppm, Table 1, entry 5).
| Entry | Aza-heterocycle-containing diphenylmethanols | Probe signal α-H ΔΔδa (ppm) |
|---|---|---|
| a ΔΔδ = chemical shift non-equivalences for α-H signals. | ||
| 1 | (S)-Aziridinyl diphenylmethanol 1 | 0.194 |
| 2 | (S)-Azetidinyl diphenylmethanol 2 | 0.046 |
| 3 | (S)-Pyrrolidinyl diphenylmethanol 3 | 0.044 |
| 4 | (S)-Piperidinyl diphenylmethanol 4 | 0.034 |
| 5 | (R)-Aziridinyl diphenylmethanol ent-1 | 0.184 |
Having the most active (S)-aziridinyl diphenylmethanol 1 in hand, to define the binding interactions, NMR titration experiments were performed. The effect of concentration on the chemical shift differences (ΔΔδ) was examined by the gradual addition of CSA 1 to a solution of (rac)-4-MeO-MA (Fig. 2). Baseline resolution was achieved over a considerably wide concentration ratio of [(S)-aziridinyl diphenylmethanol 1]/[(rac)-4-MeO-MA] (ca. 0.25–4.0), with an upfield chemical shift (first upfield and then reach equilibrium). The largest ΔΔδ values of the methane proton were observed when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of host and guest were used, and it is worth pointing out that the split separation signals of the methoxy group of (rac)-4-MeO-MA are also observed (see ESI Fig. S16†).
1 mixtures of host and guest were used, and it is worth pointing out that the split separation signals of the methoxy group of (rac)-4-MeO-MA are also observed (see ESI Fig. S16†).
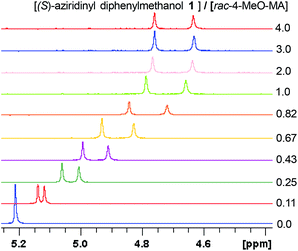 | ||
| Fig. 2 Part of the 1H NMR spectra of (rac)-4-MeO-MA in the presence of different molar amounts of (S)-aziridinyl diphenylmethanol 1. | ||
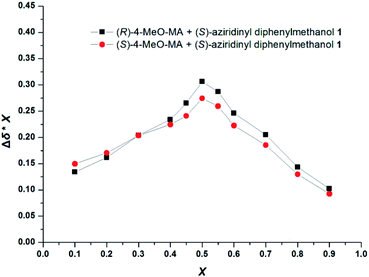
The stoichiometries of the CSA and guests were further established by the 1H NMR analysis using Job plots. Fig. 3 plots the chemical shift non-equivalencies (Δδ) of 4-MeO-MA enantiomers observed for the CSA 1 as a function of molar ratio. In most cases, the enantiomeric separation increases sharply as the molar ratio increases from 0 to about 0.5 and then steadily declines. The measured Δδ values represent the shift in the NMR signal of the α-H proton of (R)-4-MeO-MA in the presence of CSA. The X represents the molar fraction of the guest in the mixture. The Job plots of X × Δδ versus the molar fraction (X) of (R)- or (S)-4-MeO-MA in the mixture were obtained at 10 mM in CDCl3, which showed a maximum at X = 0.5, and the best baseline resolution was both achieved with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CSA 1/4-MeO-MA, as shown in Fig. 3. This further indicated that CSA 1 and the acid bind in a 1
1 ratio of CSA 1/4-MeO-MA, as shown in Fig. 3. This further indicated that CSA 1 and the acid bind in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex under these conditions.
1 complex under these conditions.
After obtaining the most active (S)-aziridinyl diphenylmethanol 1 and using the optimal conditions, in the next step of studies, the influence of various electron-donating and electron-accepting substituents in the racemic alpha-substituted carboxylic acids on the chiral recognition was examined and evaluated. All the experiments were carried out by adding a certain amount of solution of racemic carboxylic acid in CDCl3 (10 mM) to a solution of (S)-aziridinyl diphenylmethanol 1 in CDCl3 (10 mM). Immediately after each addition, the 1H NMR spectrum was acquired in a 400 MHz spectrometer at room temperature. As shown in Table 2, all racemic analytes can be well-resolved (ΔΔδ values up to 0.194 ppm, 77.6 Hz). To our delight, the ΔΔδ values of the α-H signals were large enough to give very good baseline resolution for most tested aromatic carboxylic acids containing α-OH (Table 2, entries 1–18). Notably, fluorine-substituted aromatic carboxylic acids (Table 2, entries 4–7, 9 and 11) almost showed more baseline resolution and had larger ΔΔδ values than other diverse substituents such as chlorine and bromine, irrespective of their electronic nature or positions, the role of fluorine in the aromatic MA systems for the strongly enhance the binding ability of the aziridinyl diphenylmethanol (S)-CSA 1, which results in a better splitting of the NMR signal of the corresponding guests.
| Entry | Carboxylic acids | ΔΔδb | Spectra |
|---|---|---|---|
a All samples were prepared by mixing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of carboxylic acids (10 mM in CDCl3) with (S)-CSA 1 in NMR tubes, and the spectra is recorded at 25 °C on a 400 MHz spectrometer.b ΔΔδ values in ppm and Hz for α-H are shown.c Chemical shift non-equivalences of the α-methoxy group.d Chemical shift non-equivalences of the α-methyl group.e Use of 2 equiv. of (S)-CSA 1 for the dicarboxylic acid. 1 of carboxylic acids (10 mM in CDCl3) with (S)-CSA 1 in NMR tubes, and the spectra is recorded at 25 °C on a 400 MHz spectrometer.b ΔΔδ values in ppm and Hz for α-H are shown.c Chemical shift non-equivalences of the α-methoxy group.d Chemical shift non-equivalences of the α-methyl group.e Use of 2 equiv. of (S)-CSA 1 for the dicarboxylic acid. |
|||
| 1 | 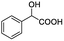 |
0.134, 53.6 | 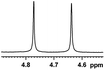 |
| 2 | 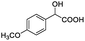 |
0.137, 54.8 | 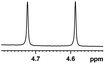 |
| 3 | 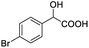 |
0.100, 40.0 | 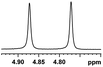 |
| 4 | 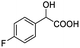 |
0.148, 59.2 | 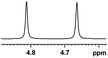 |
| 5 | 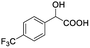 |
0.138, 55.2 | 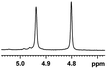 |
| 6 | 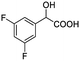 |
0.194, 77.6 | 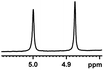 |
| 7 | 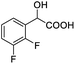 |
0.125, 50.0 | 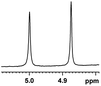 |
| 8 | 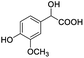 |
0.121, 48.4 | 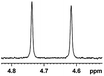 |
| 9 | 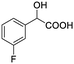 |
0.134, 53.6 | 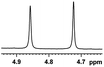 |
| 10 | 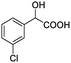 |
0.118, 47.2 | 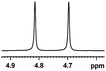 |
| 11 | 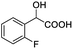 |
0.109, 43.6 | 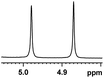 |
| 12 | 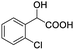 |
0.050, 20.0 | 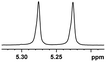 |
| 13 | 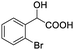 |
0.053, 21.2 | 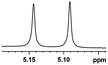 |
| 14 | 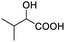 |
0.111, 44.4 | 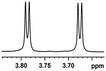 |
| 15 | 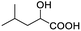 |
0.084, 33.6 | 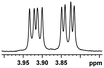 |
| 16 | 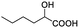 |
0.068, 27.2 | 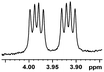 |
| 17 | 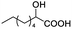 |
0.058, 23.2 | 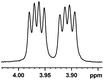 |
| 18 | 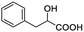 |
0.079, 31.6 | 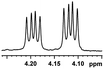 |
| 19 | 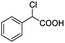 |
0.150, 60.0 | 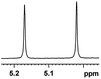 |
| 20 | 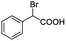 |
0.101, 40.4 | 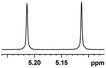 |
| 21 | 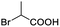 |
0.012, 4.8 | 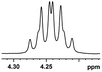 |
| 22 | 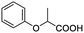 |
0.104, 41.6 | 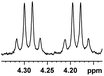 |
| 0.188, 75.2d | 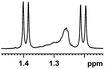 |
||
| 23 |  |
0.039, 15.6 | 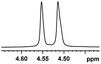 |
| 0.052, 20.8c | 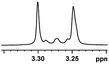 |
||
| 24 | 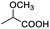 |
0.041, 16.4c | 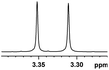 |
| 0.049, 19.6d | 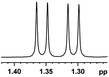 |
||
| 25 | 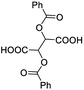 |
0.029, 11.6e | 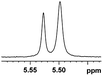 |
In addition, we further evaluated the chiral discriminating abilities of (S)-aziridinyl diphenylcarbinol 1 for aliphatic 2-hydroxymonocarboxylic acids as guests, and these 2-hydroxycarboxylic acids are known for their relationship with particular human illnesses, especially but not only with inherited metabolic diseases.19 2-hydroxy-3-methylbutyric acid (2-H-3-MBA), 2-hydroxyisocaproic acid (2-HICA), and 2-hydroxyoctanoic acid (2-HOA) are related to certain types of organic acidurias. For example, an increased amount of 2-HICA has been found in patients with the Zellweger syndrome, a congenital disorder.20 The inspection of results presented in the fragments of 1H NMR spectra (0.111 ppm, 44.4 Hz; 0.084 ppm, 33.6 Hz; 0.058 ppm, 23.2 Hz; 0.058 ppm, 23.2 Hz, and 0.079 ppm, 31.6 Hz, Table 2, entries 14–18) indicates that the robust (S)-aziridinyl diphenylmethanol 1 as CSA can give a good baseline resolution.
Moreover, clearly resolved signals from α-H of 2-halogenocarboxylic acids are observed with almost baseline separation, and their partial spectra are shown in Table 2 (entries 19–20 (0.150 ppm, 60.0 Hz and 0.101 ppm, 40.4 Hz)). Interestingly, similar fluorine substituted effect on the ability of discrimination was observed in the aforementioned racemic mandelic derivatives. 2-Bromo propionic acid displayed poor enantiodiscriminating ability (0.012 ppm, 4.8 Hz, Table 2, entry 21).
Besides, when the free α-hydroxyl group was replaced by the methoxy group, the chiral discrimination was also observed for OMe and methine proton (CαH) signals (Table 2, entries 22–24), while the dicarboxylic acid derivative was tested, 2 equiv. of (S)-CSA 1 was used and the ΔΔδ value decreased slightly (Table 2, entry 25).
The fluorine element has polar and steric properties as a substituent, and the effects that fluorinated groups can have on the physical and chemical properties of molecules have increased the number of new methods for incorporating fluorine into target compounds. Since the 19F NMR frequency and sensitivity are similar to the proton, it is convenient to transition from the proton to fluorine NMR experiments. Then, we recorded the 19F–{1H} NMR spectra of fluorinated carboxylic acids. The results are listed in Table 3 (entries 1–8). It can be seen that the fluorine signal separation is visible for fluorinated MAs (entries 1–6). As for fluorinated substrates, obtaining a distinguishable NMR split signal highly depends on the distance; therefore, 4-CF3-substituted MA showed the lowest fluorine NMR chemical shift with 0.015 ppm, while the α-fluorine phenylacetic acid gave the well-split F signal with 0.859 ppm chemical shift difference value (entry 7 vs. 8).
| Entry | Carboxylic acids | ΔΔδ | Spectra |
|---|---|---|---|
| a The proton-decoupled 19F NMR spectra was obtained from o-substituted fluorine.b The proton-decoupled 19F NMR spectra was obtained from m-substituted fluorine. | |||
| 1 | 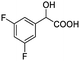 |
0.076, 28.6 | 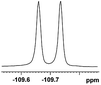 |
| 2 | 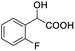 |
0.057, 21.5 | 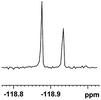 |
| 3 | 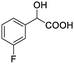 |
0.054, 20.0 | 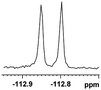 |
| 4 | 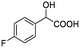 |
0.256, 96.2 | 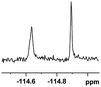 |
| 5 | 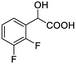 |
0.056, 21.3a | 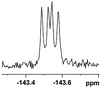 |
| 0.054, 20.4b | 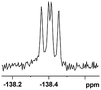 |
||
| 6 | 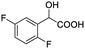 |
0.068, 25.9a | 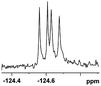 |
| 0.080, 30.2b | 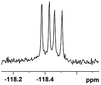 |
||
| 7 | 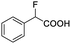 |
0.859, 323.3 | 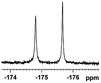 |
| 8 | 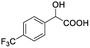 |
0.015, 5.6 | 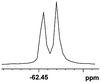 |
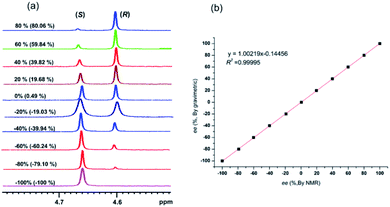
Now that (S)-aziridinyl diphenylcarbinol 1 had been established to demonstrate efficiently chiral discriminating abilities towards α-hydroxyl acids via 1H NMR spectroscopy, to further demonstrate the practical utility of (S)-aziridinyl diphenylcarbinol 1 as a CSA for enantiomeric determination. It allowed determining the enantiomeric composition of multiple nonracemic 4-MeO-MA samples by the simple integration of the split signals, and samples containing (R)-4-MeO-MA with 80, 60, 40, 20, 0, −20, −40, −60, −80 and −100% were prepared in the presence of (S)-aziridinyl diphenylcarbinol 1, and their 1H NMR spectra were measured. Enantiomeric excesses of all samples were calculated based on the integration of the 1H NMR signals of the α-H signal protons of the enantiomers of (R)- and (S)-4-MeO-MA. The overlaid partial 1H NMR spectra and linear correlation between the theoretical (X) and observed ee% (Y) are shown in Fig. 4. The linear relationship between the NMR-determined values and those gravimetrically determined values is excellent with R2 = 0.99995. The average absolute error in the ee measurement plotted in Fig. 4 is within 1%.
Conclusions
In summary, we have demonstrated a general and efficient chiral solvation of carboxylic acids with a chiral aza-semi crown amino alcohol, (S)-aziridinyl diphenylcarbinol 1. Efficient peak separation of racemic analytes was achieved after the addition of small amounts of the CSA in CDCl3 at room temperature (25 examples), the fluorinated carboxylic acids were further investigated, with a good level of discrimination by the 19F NMR spectroscopic analysis. Moreover, quantitative analysis was performed according to the integration of the 1H NMR spectra, and an excellent linear relationship was observed between the experimental and observed values of ee. The easy accessibility of the CSA (1–4) from commercially available methyl 1-aza-heterocycle-2-carboxylate should make this methodology very attractive for practical applications as CSAs for alpha-substituted carboxylic acids. The mode of recognition was based on effective hydrogen bond formation. It has shown the possibility that this class of CSAs to be used for determining the optical purity of unknown samples as well as other supramolecular and macrocyclic host-based applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was financially co-supported by the National Natural Science Foundation of China (21804086, U1504207). We are also grateful for the financial support from the Science and Technology Program of Henan Province (192102310143).Notes and references
- For recent reviews on chiral analysis, see: (a) L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840 CrossRef CAS; (b) Z. Chen, Q. Wang, X. Wu, Z. Li and Y. B. Jiang, Chem. Soc. Rev., 2015, 44, 4249 RSC; (c) J. Shen and Y. Okamoto, Chem. Rev., 2016, 116, 1094 CrossRef CAS.
- For select reviews on analytical methods, see: (a) E. L. Izake, J. Pharm. Sci., 2007, 96, 1659 CrossRef CAS; (b) Y. Okamoto and T. Ikai, Chem. Soc. Rev., 2008, 37, 2593 RSC; (c) L. Pu, Chem. Rev., 2004, 104, 1687 CrossRef CAS; (d) H. H. Jo, C. Y. Lin and E. V. Anslyn, Acc. Chem. Res., 2014, 47, 2212 CrossRef CAS.
- (a) J. M. Seco, E. Quinoa and R. Riguera, Chem. Rev., 2004, 104, 17 CrossRef CAS; (b) T. J. Wenzel and C. D. Chisholm, Prog. NMR Spectrosc., 2011, 59, 1 CrossRef CAS; (c) Z. Xu, C. Liu, S. Zhao, S. Chen and Y. Zhao, Chem. Rev., 2019, 119, 195 CrossRef CAS; (d) T. J. Wenzel, Discrimination of Chiral Compounds Using NMR Spectroscopy, John Wiley & Sons, Inc., 2nd edn, 2018, pp. 441–481 CrossRef; (e) F. Balzano, G. Uccello-Barretta, and F. Aiello, Chiral Analysis by NMR Spectroscopy: Chiral Solvating Agents, Elsevier B.V., 2018, pp. 367–427 Search PubMed.
- For select recent examples, see: (a) Y. Zhao and T. M. Swager, J. Am. Chem. Soc., 2015, 137, 3221 CrossRef CAS; (b) M. S. Seo and H. Kim, J. Am. Chem. Soc., 2015, 137, 14190 CrossRef CAS; (c) L. P. Li and B. H. Ye, Inorg. Chem., 2017, 56, 10717 CrossRef CAS; (d) S. I. Aizawa, M. Okano and T. Kidani, Chirality, 2017, 29, 273 CrossRef CAS; (e) S. Jang and H. Kim, iScience, 2019, 19, 425 CrossRef CAS; (f) Z. Sun, Z. Chen, Y. Wang, X. Zhang, J. Xu, G. Bian and L. Song, Org. Lett., 2020, 22, 589 CrossRef CAS; (g) S. Jang and H. Kim, Org. Lett., 2020, 22, 4185 CrossRef CAS.
- For select recent examples, see: (a) A. Couffin, O. T. d. Boullay, M. Vedrenne, C. Navarro, B. Martin-Vaca and D. Bourissou, Chem. Commun., 2014, 50, 5997 RSC; (b) N. Jain, M. B. Mandal and A. V. Bedekar, Tetrahedron, 2014, 70, 4343 CrossRef CAS; (c) G. Bian, H. Fan, H. Huang, S. Yang, H. Zong, L. Song and G. Yang, Org. Lett., 2015, 17, 1369 CrossRef CAS; (d) E. Monteagudo, P. March, A. Alvarez-Larena and A. Virgili, ChemistrySelect, 2017, 2, 7362 CrossRef CAS; (e) A. Khanvilkar and A. Bedekar, Chem. Commun., 2018, 54, 11037 RSC; (f) P. Cuřínová, P. Hájek, K. Janků and R. Holakovský, Chirality, 2019, 31, 410 CrossRef; (g) D. Prasad, S. Mogurampelly and S. R. Chaudhari, RSC Adv., 2020, 10, 2303 RSC; (h) F. Balzano and G. Uccello-Barretta, RSC Adv., 2020, 10, 4869 RSC; (i) A. Recchimurzo, C. Micheletti, G. Uccello-Barretta and F. Balzano, J. Org. Chem., 2020, 85, 5342 CrossRef CAS.
- For select recent examples, see: (a) S. Y. Wang, Le. Li, Y. Xiao and Y. Wang, Trends Anal. Chem., 2019, 121, 115691 CrossRef CAS; (b) L. Feng, G. Gao, H. Zhao, L. Zheng, Y. Wang, P. Stavropoulos, L. Ai and J. Zhang, J. Org. Chem., 2018, 83, 13874 CrossRef CAS; (c) T. Ema, T. Yamasaki, S. Watanabe, M. Hiyoshi and K. Takaishi, J. Org. Chem., 2018, 83, 10762 CrossRef CAS; (d) Y. C. Rodriguez, T. M. Duarte, Z. Szakonyi, E. Forró, F. Fülöp and T. J. Wenzel, Chirality, 2015, 27, 708 CrossRef CAS; (e) A. Tyszka-Gumkowska, G. Pikus and J. Jurczak, Molecules, 2019, 24, 2635 CrossRef.
- For select examples, see: (a) I. Pal, R. Chaudhari and N. R. Suryaprakash, Magn. Reson. Chem., 2015, 53, 142 CrossRef CAS; (b) T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591 CrossRef CAS; (c) L. S. Moon, R. S. Jolly, Y. Kasetti and P. V. Bharatam, Chem. Commun., 2009, 1067 RSC; (d) L. S. Moon, M. Pal, Y. Kasetti, P. V. Bharatam and R. S. Jolly, J. Org. Chem., 2010, 75(16), 5487 CrossRef CAS; (e) F. Freire, E. Quinoa and R. Riguera, Chem. Commun., 2008, 4147 RSC; (f) S. E. Gunal, S. T. Tuncel and I. Dogan, Tetrahedron, 2020, 76, 131141 CrossRef; (g) S. K. Mishra and N. Suryaprakash, Tetrahedron: Asymmetry, 2017, 28, 1220 CrossRef CAS; (h) G. Gao, C. Lv, G. Wang, L. Feng, P. Stavropoulos, G. Gao and L. Ai, Tetrahedron Lett., 2015, 56, 6742 CrossRef CAS; (i) D. Kumari, P. Bandyopadhyay and N. Suryaprakash, J. Org. Chem., 2013, 78, 2373 CrossRef CAS.
- (a) D. Tanner, Angew. Chem., Int. Ed., 1994, 33, 599 CrossRef; (b) L. Degennaro, P. Trinchera and R. Luisi, Chem. Rev., 2014, 114, 7881 CrossRef CAS; (c) S. Stankovic, M. D'hooghe, S. Catak, H. Eum, M. Waroquire, V. V. Speybroeck, N. D. Kimpe and H. J. Ha, Chem. Soc. Rev., 2012, 41, 643 RSC; (d) G. Callebaut, T. Meiresonne, N. De Kimpe and S. Mangelinckx, Chem. Rev., 2014, 114, 7954 CrossRef CAS; (e) H. J. Ha, J. H. Jung and W. K. Lee, Asian J. Org. Chem., 2014, 3, 1020 CrossRef CAS; (f) T. Gleede, L. Reisman, E. Rieger, P. C. Mbarushimana, P. A. Rupar and F. R. Wurm, Polym. Chem., 2019, 10, 3257 RSC.
- (a) A. M. Pieczonka, M. Rachwalski and S. Lesniak, Curr. Org. Chem., 2014, 18, 3045 Search PubMed; (b) A. Brandi, S. Cicchi and F. M. Cordero, Chem. Rev., 2008, 108, 3988 CrossRef CAS; (c) F. Couty, G. Evano and D. Prim, Mini-Rev. Org. Chem., 2004, 1, 133 CrossRef CAS; (d) S. Meninno and A. Lattanzi, Chem. Commun., 2013, 49, 3821 RSC; (e) S. Meninno, C. Volpe and A. Lattanzi, ChemCatChem, 2019, 11, 3716 CrossRef CAS.
- (a) J. G. H. Willems, F. J. Dommerholt, J. B. Hammink, A. M. Vaarhorst, L. Thijs and B. Zwanenburg, Tetrahedron Lett., 1995, 36, 603 CrossRef CAS; (b) P. G. Andersson, D. Guijarro and D. Tanner, J. Org. Chem., 1997, 62, 7364 CrossRef CAS; (c) C. F. Lawrence, S. K. Nayak, L. Thijs and B. Zwanenburg, Synlett, 1999, 1571 CrossRef CAS; (d) P. C. B. Page, S. M. Allin, S. J. Maddocks and M. R. J. Elsegood, J. Chem. Soc., Perkin Trans. 1, 2002, 1, 2827 RSC; (e) U. Shadakshari and S. K. Nayak, Tetrahedron, 2001, 57, 8185 CrossRef CAS; (f) M. C. Wang, Z. K. Liu, S. Li, X. Ding, Y. Li and M. S. Tang, Tetrahedron: Asymmetry, 2010, 21, 486 CrossRef CAS; (g) S. Jarzyński, M. Rachwalski, A. M. Pieczonka, Z. Wujkowska and S. Leśniak, Tetrahedron: Asymmetry, 2015, 26, 924 CrossRef; (h) S. Jarzyński, S. Leśniak, A. M. Pieczonka and M. Rachwalski, Tetrahedron: Asymmetry, 2015, 26, 35 CrossRef.
- (a) Ö. Dogan, H. Koyuncu, P. Garner, A. Bulut, W. J. Youngs and M. Panzner, Org. Lett., 2006, 8, 4687 CrossRef; (b) A. Bulut, A. Aslan and Ö. Dogan, J. Org. Chem., 2008, 73, 7373 CrossRef CAS; (c) A. Isleyen and Ö. Dogan, Tetrahedron: Asymmetry, 2007, 18, 679 CrossRef CAS; (d) A. Bulut, A. Aslan, E. C. Izgu and Ö. Dogan, Tetrahedron: Asymmetry, 2007, 18, 1013 CrossRef CAS.
- (a) M. C. Wang, Y. H. Wang, G. W. Li, P. P. Sun, J. X. Tian and H. J. Lu, Tetrahedron: Asymmetry, 2011, 22, 761 CrossRef CAS; (b) X. Song, Y. Z. Hua, J. G. Shi, P. P. Sun, M. C. Wang and J. B. Chang, J. Org. Chem., 2014, 79, 6087 CrossRef CAS.
- (a) M. C. Wang, Q. J. Zhang, W. X. Zhao, X. D. Wang, X. Ding, T. T. Jing and M. P. Song, J. Org. Chem., 2008, 73, 168 CrossRef CAS; (b) J. K. Niu, M. C. Wang, L. J. Lu, G. L. Ding, H. J. Lu, Q. T. Chen and M. P. Song, Tetrahedron: Asymmetry, 2009, 20, 2616 CrossRef CAS.
- X. Song, A. X. Liu, S. S. Liu, W. C. Gao, M. C. Wang and J. B. Chang, Tetrahedron, 2014, 70, 1464 CrossRef CAS.
- (a) Y. Takemoto, Y. Baba, I. Noguchi and C. Iwata, Tetrahedron Lett., 1996, 37, 3345 CrossRef CAS; (b) Y. Takemoto, Y. Baba, A. Honda, S. Nakao, I. Noguchi, C. Iwata, T. Tanaka and T. Ibuka, Tetrahedron, 1998, 54, 15567 CrossRef CAS; (c) M. Lombardo, M. Chiarucci and C. Trombini, Chem.–Eur. J., 2008, 14, 11288 CrossRef CAS; (d) J. W. Xie, L. Yue, D. Xue, X. L. Ma, Y. C. Chen, Y. Wu, J. Zhu and J. G. Deng, Chem. Commun., 2006, 1563 RSC; (e) S. Wang, X. Li, H. Liu, L. Xu, J. Zhuang, J. Li, H. Li and W. Wang, J. Am. Chem. Soc., 2015, 137, 2303 CrossRef CAS; (f) X. Tang, X. Huang, T. Cao, Y. Han, X. Jiang, W. Lin, Y. Tang, J. Zhang, Q. Yu, C. Fu and S. Ma, Org. Chem. Front., 2015, 2, 688 RSC.
- A. M. Pieczonka, S. Leniak and M. Rachwalski, Tetrahedron, 2018, 74, 1571 CrossRef CAS.
- G. W. Li, J. M. Cao, W. Zong, X. X. Lei and R. X. Tan, Org. Chem. Front., 2016, 3, 96 RSC.
- (a) X. X. Lei, L. Liu, X. J. Chen, X. C. Yu, L. S. Ding and A. J. Zhang, Org. Lett., 2010, 12, 2540 CrossRef CAS; (b) L. Liu, M. D. Ye, X. G. Hu, X. C. Yu, L. X. Zhang and X. X. Lei, Tetrahedron: Asymmetry, 2011, 22, 1667 CrossRef CAS; (c) L. Bai, P. Chen, J. Xiang, J. Sun and X. X. Lei, Org. Biomol. Chem., 2019, 17, 1466 RSC; (d) G. W. Li, M. S. Ma, G. F. Wang, X. J. Wang and X. X. Lei, Front. Chem., 2020, 8, 336 CrossRef CAS.
- (a) G. R. Villani, G. Gallo, E. Scolamiero, F. Salvatore and M. Ruoppolo, Clin. Exp. Med., 2017, 17, 305 CrossRef CAS; (b) O. A. Mamer and M. L. Reimer, J. Biol. Chem., 1992, 267, 22141 CAS.
- A. Muth, J. Jung, S. Bilke, A. Scharrer, A. Mosandl, A. C. Sewell and H. Böhles, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2003, 792, 269 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional supporting data, figures, and experimental details. See DOI: 10.1039/d0ra06312f |
| This journal is © The Royal Society of Chemistry 2020 |