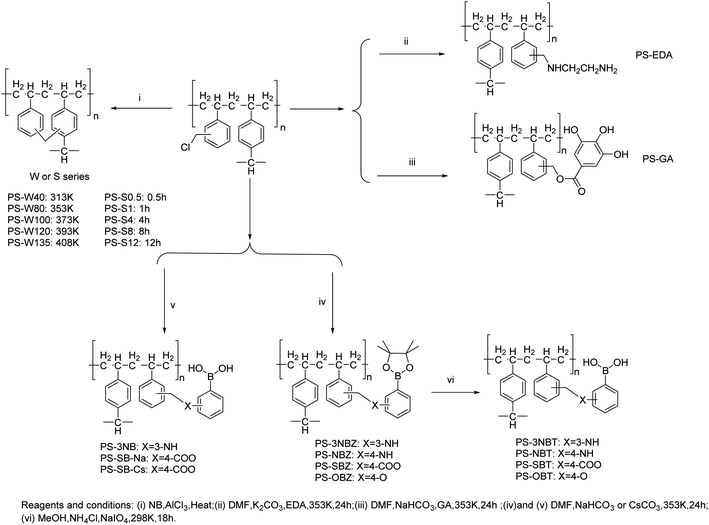
Open Access Article
Open Access ArticleThe design and synthesis of high efficiency adsorption materials for 1,3-propanediol: physical and chemical structure regulation†
Kexin Zheng‡
ab,
Long Jiang‡b,
Shitao Yua,
Mo Xianb,
Zhanqian Songac,
Shiwei Liu*a and
Chao Xu *b
*b
aChemical Engineering and Technology, Qingdao University of Science & Technology, Qingdao 266042, China. E-mail: liushiweiqust@126.com; Tel: +86-0532-8402-2782
bKey Laboratory of Biobased Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China. E-mail: xuchao@qibebt.ac.cn; Tel: +86-0532-5878-2981
cNational Engineering & Technology Research Center of Forest Chemical Industry, Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry, Nanjing 210042, China
First published on 15th October 2020
Abstract
In this study, a series of polystyrene-divinylbenzene resins with precise physical structure regulation and chemical modification were successfully synthesized. The regulation of Friedel–Crafts reaction conditions resulted in several physical resins with various BET surface areas and pore structures, while the adsorption of 1,3-propanediol revealed that the molecular size and other physical properties exhibited a moderate contribution to the adsorption of hydrophilic compounds. The adsorption processes between 1,3-propanediol and nitrogen, oxygen and boron functional group modified resins were further explored, and boronic acid modified resins named PS-3NB and PS-SBT exhibited higher adsorption capacities than commercial resin CHA-111. The adsorption capacity of PS-3NB and PS-SBT reached 17.54 mg g−1 and 17.23 mg g−1, respectively, which were 37% and 35% higher than that of commercial resin CHA-111. Furthermore, the adsorption mechanism demonstrated that the content of boronic acid, solution pH and adsorbate hydrophobicity were the primary adsorption driving forces. Herein, we provided a method to modify polystyrene-divinylbenzene materials with boronic acid to selectively adsorb hydrophilic polyols via the specific affinity between boronic acid and diol molecule.
1 Introduction
Polyols including glycerol, 1,3-propanediol and 1,2-butanediol are important raw materials and intermediates in pharmaceuticals, chemicals, food and other fields.1 Currently, the environmentally friendly fermentative polyols have been widely applied, and the separation and purification of them has been an important research field in chemistry.2–4 However, the high boiling point and strong hydrophilicity restrict the development of related separation technologies.In recent years, distillation and membrane separation are commonly used to separate polyols from fermentation broth or aqueous media. The traditional distillation method5 has been used to separate 1,2-propanediol, 1,3-propanediol, etc., while the key problem is the relatively high boiling point resulting in high energy consumption and high cost. Membrane separation method6,7 can be used to separate and purify polyols from biological fermentation broth and biodiesel, but the membrane service life, effective membrane retention capacity and membrane fouling limit the practical application. As an efficient and green separation method, adsorption has attracted more and more attention with the advantages of low cost, low pollution and reproducibility.
Currently, the commonly utilized adsorbents are activated carbon,8,9 zeolite,10–12 silica gel13,14 and adsorption resin.15,16 Adsorption resin has attracted extensive attention due to their high specific surface area, adjustable pore structure and stable mechanical strength.17 In the early 1970s, Davankov first reported the synthesis of hyper-crosslinked polystyrenes resin.18 Hyper-crosslinked resin as a physical regulated resin owns high specific surface area and rich pore diameter structure, which has been widely used in the adsorption and separation of phenols,19,20 anilines,21–23 dyes24 and other substances. Besides, the introduction of chemical functional groups on resin skeleton can improve the surface properties of resins, exhibiting good adsorption and separation performance for phenols,25–27 amines28–30 and other substances. However, there are few reports on the selective adsorption of polyols by adjusting the physical or chemical structure of resin. Therefore, it is necessary to study the effects and mechanisms of physical structure regulation and chemical modification on the adsorption and separation of polyols.
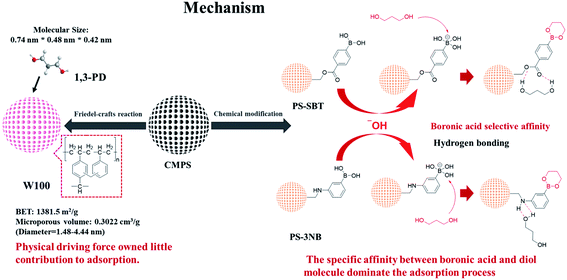
In our previous study,16 we proved that it is feasible to introduce boronic acid functional group into chloromethyl sites of CMPS to realize polyol adsorption. However, the in-depth studies on the influence of physical structure regulation and chemical functional modification to the adsorption of polyol and the rational utilization of chloromethyl sites remained to be explored. In this study, a series of physical structure regulated and nitrogen, oxygen, boron functional groups modified resins were synthesized with chloromethyl polystyrene-divinylbenzene as precursor. 1,3-Propanediol was taken as the research object, and the influences of adsorption capacity and pore structure, specific surface area, functional group, pH, temperature and other factors were deeply explored.
2 Experimental
2.1 Materials
Chloromethyl styrene divinylbenzene (CMPS), with cross-linking degree of 7% and chlorine content of 18.9%, was purchased from Zhengzhou Diligent Technology Co. Ltd. Commercial resin CHA-111 was obtained from Zhengzhou High-Tech Technology Co. Ltd. Gallic acid (GA), ethylenediamine (EDA), cesium carbonate (CsCO3), sodium periodate (NaIO4), 4-carboxyphenylboronic acid (SB), 4-carboxylphenylboronic acid pinacol ester (SBZ), 4-aminophenylboronic acid pinacol ester (NBZ), 3-aminophenylboronic acid pinacol ester (3NBZ), 3-aminophenylboronic acid (3NB) and 4-hydroxyphenylboronic acid pinacol ester (OBZ) in AR grade were purchased from Aladdin Chemical Reagent Co. Ltd. Nitrobenzene (NB), anhydrous aluminum chloride, N,N-dimethylformamide (DMF), potassium carbonate (K2CO3), sodium bicarbonate (NaHCO3), ammonium chloride (NH4Cl), sodium hydroxide, hydrochloric acid, methanol (MeOH), ethanol and 1,3-propanediol (1,3-PD) were purchased from Sinopharm Chemical Reagent Co. Ltd. All reagents were AR grade, without further purified.2.2 Synthesis of resins
Synthesis of boronic acid modified resins: a series of boronic acid modified resins were synthesized by the method shown in ESI Section 1.2.† PS-SB and PS-SBZ modified resins were prepared by nucleophilic substitution reaction of CMPS with boronic acid under the action of strong base NaHCO3 or CsCO3. The deprotection scheme of PS-SBZ boronic acid pinacol ester modified resins were employed NaIO4 to remove the protection group to obtain PS-SBT resins. Detailed synthesis conditions were shown in Table 1.
| Resin | Precursor resin | Functional group | Reaction conditions |
|---|---|---|---|
| PS-EDA | CMPS | EDA | DMF/K2CO3/353 K/24 h |
| PS-GA | CMPS | GA | DMF/NaHCO3/353 K/24 h |
| PS-SB-Na | CMPS | SB | DMF/NaHCO3/353 K/24 h |
| PS-SB-Cs | CMPS | SB | DMF/CsCO3/353 K/24 h |
| PS-3NB | CMPS | 3NB | DMF/CsCO3/353 K/24 h |
| PS-3NBZ | CMPS | 3NBZ | DMF/CsCO3/353 K/24 h |
| PS-NBZ | CMPS | NBZ | DMF/CsCO3/353 K/24 h |
| PS-OBZ | CMPS | OBZ | DMF/CsCO3/353 K/24 h |
| PS-SBZ | CMPS | SBZ | DMF/NaHCO3/353 K/24 h |
| PS-3NBT | PS-3NBZ | 3NBT | NaIO4/MeOH/NH4Cl/298 K/18 h |
| PS-NBT | PS-NBZ | NBT | NaIO4/MeOH/NH4Cl/298 K/18 h |
| PS-OBT | PS-OBZ | OBT | NaIO4/MeOH/NH4Cl/298 K/18 h |
| PS-SBT | PS-SBZ | SBT | NaIO4/MeOH/NH4Cl/298 K/18 h |
2.3 Adsorption experiments
| Qe = V(C0 − Ce)/M | (1) |
| Qe = CeKLQm/(1 + KLCe) | (2) |
| Qe = KFCe1/n | (3) |
The residual chlorine content of resin was determined by Volhard method.46 The specific determination method as follows: accurately weighed 0.2 g resin into the crucible and added 1 g NaOH and 1 g KNO3 to stir evenly, then covered the surface with a layer of KNO3, put the crucible in muff furnace and decomposed it under 873 K for 4 h. After the muff furnace being cooled to room temperature, the sample was taken out, dissolved in pure water and then added phenolphthalein indicator. Added concentrated nitric acid into the system to adjust pH to acidity and 2 mL nitrobenzene to protect chloride ion. After adding an excess of 0.1 M silver nitrate standard solution and alum iron as an indicator, titrate with potassium thiocyanate standard solution and the chlorine content was calculated. The boronic acid content of boronic acid modified resins were determined by titration method (ESI Section 2†).
3 Results and discussion
3.1 Physical structure regulated resins
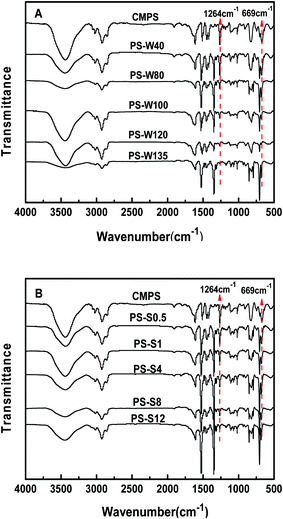 | ||
| Fig. 1 IR spectra (wavenumbers from 500–4000 cm−1) of PS-W and PS-S series physical resins (A and B). | ||
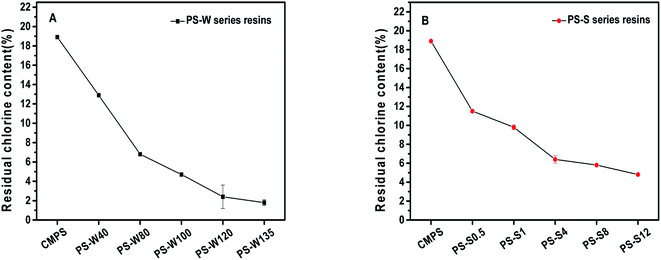
In order to further study the residual chlorine content of PS-W and PS-S series resins, Volhard method was employed for quantitative determination (Fig. 2A and B). There were 18.9%, 12.9% and 1.8% residual chlorine in the precursor resin CMPS, PS-W40 and PS-W135, respectively, and the residual chlorine curve of PS-W series resins decreased rapidly with the increase of temperature and then decreased slowly. Meanwhile, the residual chlorine content decreased from 11.5% to 4.8% as the reaction time increased from 0.5 h to 12 h, exhibiting an inverse relation with Friedel–Craft reaction time. It presented the increase of reaction temperature and time leaded to the rapid consumption of chloromethyl. Subsequently, the reaction was slowed down due to the increase of steric hindrance and the decrease of chloromethyl site, which was consistent with the FT-IR results. Hence, the PS-W and PS-S series physical structure regulated resins were successful synthesized.
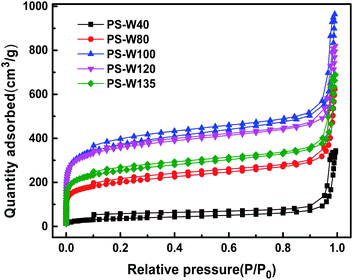
The specific surface area and pore structure of PS-W and PS-S series resins are related to the adsorption capacity. High specific surface area and appropriate pore size are conducive to the adsorption of small molecule. As the N2 physical adsorption–desorption shown in Fig. 3, the physical structure regulated resins owned obvious specific surface area advantage and abundant pore structure (Table 2). Both PS-W and PS-S series resins showed a sharp increase in nitrogen adsorption capacity when the relative pressure was low (P/P0 < 0.05), indicating that there were quite a large proportion of micropores in the resin. At the same time, the adsorption branch and desorption branch on the nitrogen adsorption–desorption curve had obvious hysteresis loop, indicating the existence of resin mesopores. When the relative pressure was high (P/P0 > 0.95), the nitrogen adsorption capacity also showed a sharp increase, indicating that some macropores still existed in the resin26 (PS-S series N2 adsorption–desorption attached figure and pore diameter distribution figure were shown in Fig. S1†). The specific surface area of the resin increases with the increase in micropores and mesopores, which provides a powerful physical force for the adsorption of 1,3-propanediol.
| Pore diameter (nm) | 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | Total | |
|---|---|---|---|---|---|---|---|
| BET (m2 g−1) | CHA-111 | 443.8 | 360.4 | 58.2 | 23.7 | 11.4 | 1414.4 |
| PS-W100 | 494.2 | 300.9 | 34.4 | 9.3 | 3.7 | 1381.5 | |
| Volume (cm3 g−1) | CHA-111 | 0.157 | 0.254 | 0.069 | 0.042 | 0.027 | 1.32 |
| PS-W100 | 0.180 | 0.204 | 0.038 | 0.016 | 0.009 | 0.86 | |
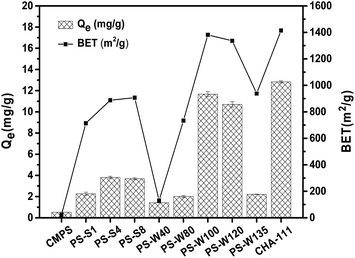 | ||
| Fig. 4 Adsorption capacities for 1,3-propanediol and BET surface area of CMPS and physical resins (initial concentration 2 g L−1, 30 °C, 16 h). | ||
For small molecular organics such as p-aminobenzoic acid, micropores were more suitable for solute–solute interaction, Huang et al.24 believed that the best match between the pore size of the resin and the molecular volume of the adsorbate was 2–6 times. According to the molecular size of 1,3-propanediol (0.74 × 0.48 × 0.42 nm, optimized simulation by software Gaussian 09W and Multiwfn), the optimal pore size of resin was 1.48–4.44 nm. Although the pore sizes of commercial resin CHA-111 and PS-W100 distributed at 1.48–4.44 nm have a high proportion of optimal adsorption pore diameter (Table 2), the considerable match between the pore size and molecule size failed to capture 1,3-propanediol. Therefore, the adsorption of 1,3-propanediol on the resin modified by different functional groups were further studied.
3.2 Chemical functional group modified resins
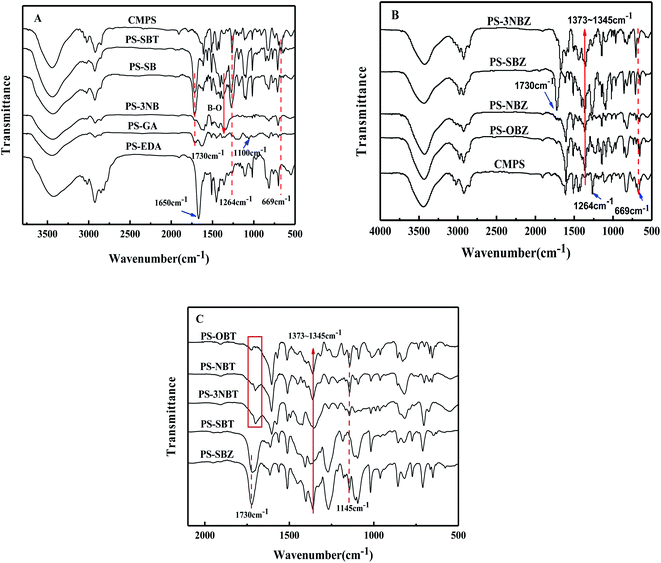
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appearing on PS-GA at 1730 cm−1, and 1100 cm−1 was the stretching vibration peak of C–O and bending vibration peak in the O–H plane, which preliminarily indicated that gallic acid has been successfully introduced.33 In the PS-EDA spectrum of aminated resin, the stretching vibration peak of N–H bond appeared at 1650 cm−1,34 indicating the success of amino modification. The characteristic peak of B–O bond appeared at 1373–1345 cm−1 of phenylboronic acid modified resins,16 and the ester C
O appearing on PS-GA at 1730 cm−1, and 1100 cm−1 was the stretching vibration peak of C–O and bending vibration peak in the O–H plane, which preliminarily indicated that gallic acid has been successfully introduced.33 In the PS-EDA spectrum of aminated resin, the stretching vibration peak of N–H bond appeared at 1650 cm−1,34 indicating the success of amino modification. The characteristic peak of B–O bond appeared at 1373–1345 cm−1 of phenylboronic acid modified resins,16 and the ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak appeared at 1730 cm−1 of 4-carboxylphenylboronic acid modified resins (PS-SB and PS-SBT), indicating that the modification was successful. In the PS-3NB spectra of 3-aminophenylboronic acid modified resin, the characteristic peak of N–H bond stretching vibration appeared at 1650 cm−1, and the characteristic peak of B–O appeared at 1373–1345 cm−1, indicating the successful modification of amination reaction. The boronic acid pinacol ester modified resins were characterized (Fig. 5B) and the C–Cl vibration peak strength of the four modified resins was greatly reduced. Besides, B–O bond characteristic peak appeared at 1373–1345 cm−1, indicating the successful introduction of B–O bond of the four resins. Compared with the 4-carboxyphenylboronic acid modified resins (PS-SBZ and PS-SBT), the C–O–C characteristic peak at 1145 cm−1 had obviously decrease (Fig. 5C), indicating the usefulness of the deprotection scheme.35 By infrared comparison of the other three modified resins, it was declared that the characteristic peak strength of C–O–C was not weakened, and unknown new peak appeared around 1700 cm−1 with the deprotection reaction, indicating that the three monomer modified resins' removal protection schemes had no obvious effect.
O stretching vibration peak appeared at 1730 cm−1 of 4-carboxylphenylboronic acid modified resins (PS-SB and PS-SBT), indicating that the modification was successful. In the PS-3NB spectra of 3-aminophenylboronic acid modified resin, the characteristic peak of N–H bond stretching vibration appeared at 1650 cm−1, and the characteristic peak of B–O appeared at 1373–1345 cm−1, indicating the successful modification of amination reaction. The boronic acid pinacol ester modified resins were characterized (Fig. 5B) and the C–Cl vibration peak strength of the four modified resins was greatly reduced. Besides, B–O bond characteristic peak appeared at 1373–1345 cm−1, indicating the successful introduction of B–O bond of the four resins. Compared with the 4-carboxyphenylboronic acid modified resins (PS-SBZ and PS-SBT), the C–O–C characteristic peak at 1145 cm−1 had obviously decrease (Fig. 5C), indicating the usefulness of the deprotection scheme.35 By infrared comparison of the other three modified resins, it was declared that the characteristic peak strength of C–O–C was not weakened, and unknown new peak appeared around 1700 cm−1 with the deprotection reaction, indicating that the three monomer modified resins' removal protection schemes had no obvious effect.
3.3 Influence factors of adsorption
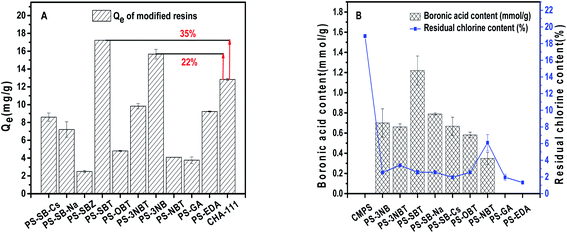
In order to study the influencing factors in the adsorption process of boronic acid modified resins, we further explored the effects of adsorbent loading boronic acid content, hydroxide ion concentration in aqueous media, temperature and other factors on adsorption.At the same time, the residual chloromethyl of modified resin was quantitatively calculated by Volhard method, and it was demonstrated that the chloromethyl content of boronic acid modified resins were 2–6%, and that of gallic acid PS-GA and amination resin PS-EDA were less than 2%. It indicates that the functional groups cannot react all chloromethyl sites in resin, which is mainly due to the large steric hindrance of the resin itself. The steric hindrance increases with the further introduction of functional monomer, which limits the reaction degree.
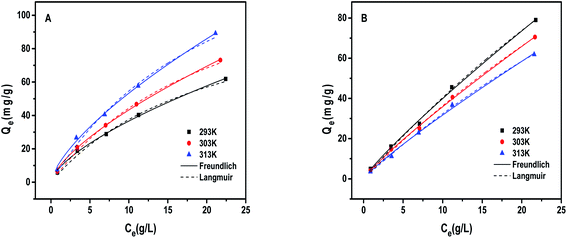
As shown in Table 3, the Freundlich isothermal model is more suitable than Langmuir model for the adsorption performance of 1,3-propanediol by PS-SBT resin. Its correlation coefficient (R2) is higher than that of Langmuir. The maximum adsorption capacity of PS-SBT at three temperatures is 117.698 mg g−1 at 293 K, 149.817 mg g−1 at 303 K and 184.620 mg g−1 at 313 K, respectively.
| Resin | Temperature (K) | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (L g−1) | R2 | KF (mg1−n) | n | R2 | ||
| PS-SBT | 293 | 117.698 | 0.047 | 0.994 | 8.085 | 1.524 | 0.998 |
| 303 | 149.817 | 0.042 | 0.995 | 8.978 | 1.464 | 0.998 | |
| 313 | 184.620 | 0.042 | 0.995 | 10.925 | 1.452 | 0.997 | |
| CHA-111 | 293 | 420.402 | 0.011 | 0.997 | 5.238 | 1.134 | 0.997 |
| 303 | 363.973 | 0.011 | 0.998 | 4.775 | 1.140 | 0.999 | |
| 313 | 305.315 | 0.012 | 0.998 | 4.148 | 1.132 | 0.996 | |
Adsorption enthalpy (ΔH, kJ mol−1), adsorption free energy (ΔG, kJ mol−1) and adsorption entropy (ΔS, J (mol K)−1) are also discussed, and the transformation relationship among them can be described by the following equation:24
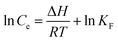 | (4) |
| ΔG = −nRT | (5) |
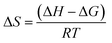 | (6) |
The ΔG, ΔH and ΔS of 1,3-propanediol adsorbed onto PS-SBT are listed in Table. S1.† It is seen that ΔG is negative, which implies the favorable and spontaneous adsorption process. The positive ΔH indicates that the adsorption is an endothermic process,41 which is consistent with the isothermal adsorption model. The ΔH of 1,3-propanediol adsorbed onto boronic acid modified resin PS-SBT is calculated to need 0.5065 kJ mol−1 with the initial concentration of 1,3-propanediol from 1 g L−1 to 24 g L−1. The positive ΔS reveals that more ordered structure of 1,3-propanediol is formed on the PS-SBT surface.
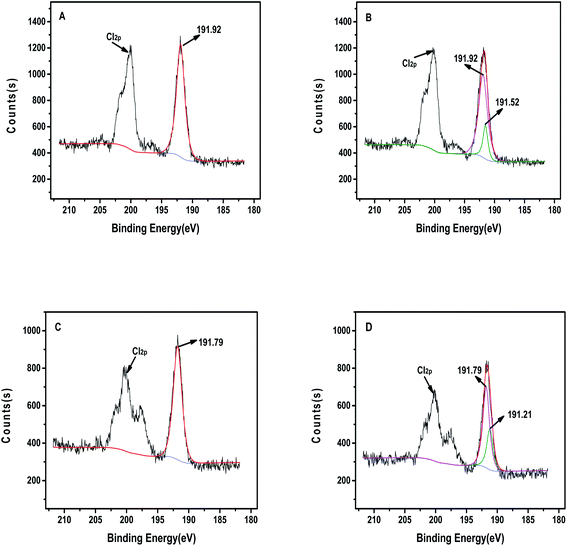
We performed XPS characterization analysis to clearly show the difference in binding energy before and after adsorption of boronic acid resin. Fig. 10A and B showed B1s peak at about 191.92 eV before adsorption in PS-SBT resin, and a new peak appeared at 191.52 eV after adsorption, which may be caused by specific binding of boronic acid and diol structure. At the same time, the binding energy of boron in PS-3NB also changed before and after adsorption in Fig. 10C and D, and two peaks appeared in boron after adsorption, which were located at 191.21 and 191.79 eV, respectively. This indicated that the boron functional group participated in the adsorption process, and the electron cloud density around boron changed after adsorption, thus confirming the specific binding of boronic acid and diol structure. And after PS-3NB resin adsorbed 1,3-propanediol, the binding energy of nitrogen element in Fig. S4† appeared two peaks at 399.57 and 399.51 eV, and a new peak appeared at 399.51 eV after adsorption, which may be caused by hydrogen bonding generated.
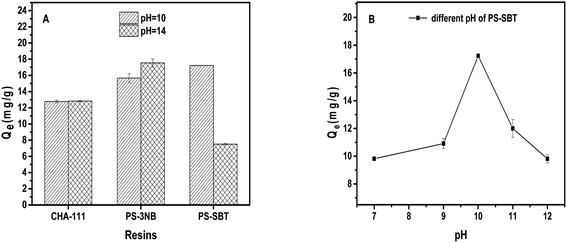
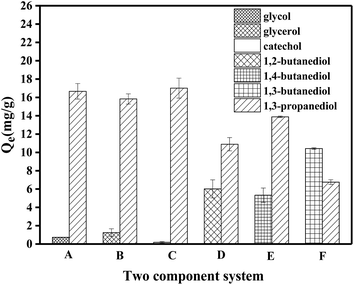
The adsorption processes of PS-SBT, PS-3NB to 1,3-propanediol are pH-dependent process. In aqueous media, boronic acid exists as a balance of the neutral trigonal state and the anionic tetrahedral state which could generate boronate ester with 1,3-pronanediol, respectively.42,43 Meanwhile, the neutral state boronate ester tends to hydrolyze reversibly to boronic acid. In contrary, the anionic tetrahedral state boronic acid could remain stable boronate ester (Fig. 11). Consequently, the introduction of hydroxyl ion could promote the reaction balance to shift to the stable anionic boronate ester and benefits the specific adsorption process. Besides, the optimal pH is related to the pKa of the polyol and boronic acid.44,45 According to the adsorption behaviors of PS-SBT and PS-3NB, the optimal pH was 10 and 14, respectively. Furthermore, the FT-IR before and after adsorption of PS-SBT modified resin revealed that the characteristic peak of B–O bond at 1373–1345 cm−1 existed and vibration band of C–O–C at 1145 cm−1 generated after adsorption in the solution pH of 10 (Fig. S5†).
4 Conclusions
In this paper, a series of physical structure regulation and chemical functional group modification resins were designed and synthesized on basis of chloromethyl polystyrene divinylbenzene resin, and the adsorption process of 1,3-propanediol was systematically studied. Although the physical regulation resins were regulated with accurately specific surface area and pore structure through temperature and time factors, the adsorption affinity of 1,3-propanediol based on molecular size and other physical interactions was poor. The chemical modified resins with nitrogen and oxygen functional groups modification exhibited certain adsorption capacity for 1,3-propandiol which were only 71.9% and 29.3% of commercial resin CHA-111. The boronic acid modified resins exhibited effectively 1,3-propanediol adsorption affinity. The highest adsorption capacity between PS-SBT and 1,3-propanediol reached to 17.23 mg g−1, which is 35% higher than that of commercial resin CHA-111. The further study demonstrated that the boronic acid content was positively correlated with the adsorption capacity, and the boronic acid content of PS-SBT was up to 1.220 mmol g−1. The effect of boronic acid modified resin on the adsorption of pH in aqueous medium was also discussed. It was indicated that the adsorption capacity of PS-3NB to 1,3-propanediol was 37% higher than CHA-111 when pH was 14, and the excellent resin structure of PS-SBT might be destroyed. It illustrated that stability of resin and affinity with adsorbates are different in different pH media and laid the material foundation for the selective separation of polyols compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the generous support provided by Taishan Scholars Project of Shandong (No. ts201712076), Qingdao Original Innovation Program Basic Research for Application Project (18-2-2-47-jch).References
- R. Mishra, S. R. Jain and A. Kumar, Biotechnol. Adv., 2008, 26(4), 293–303 CrossRef CAS.
- C. Cui, Z. Zhang and B. Chen, Bioresour. Technol., 2017, 245(Pt A), 477–482 CrossRef CAS.
- K. Prochaska, J. Antczak, M. Regel-Rosocka and M. Szczygiełda, Sep. Purif. Technol., 2018, 192, 360–368 CrossRef CAS.
- Z. Li, L. Yan, J. Zhou, X. Wang, Y. Sun and Z.-L. Xiu, Sep. Purif. Technol., 2019, 209, 246–253 CrossRef CAS.
- Z. Wang, Z. Wu and T. Tan, Biotechnol. Bioprocess Eng., 2013, 18(4), 697–702 CrossRef CAS.
- M. C. S. Gomes, N. C. Pereira and S. T. D. Barros, J. Membr. Sci., 2010, 352(1–2), 271–276 CrossRef CAS.
- J. Saleh, A. Y. Tremblay and M. A. Dubé, Fuel, 2010, 89(9), 2260–2266 CrossRef CAS.
- M. Naushad, A. A. Alqadami, Z. A. AlOthman, I. H. Alsohaimi, M. S. Algamdi and A. M. Aldawsari, J. Mol. Liq., 2019, 293, 111442 CrossRef CAS.
- P. Ma, S. Wang, T. Wang, J. Wu, X. Xing and X. Zhang, Environ. Sci. Pollut. Res. Int., 2019, 26(29), 30119–30129 CrossRef CAS.
- M. Guisnet and J. P. Gilson, Zeolites for Cleaner Technologies Catalytic Science Series, Imperial College Press, London, 2002 Search PubMed.
- A. Brito, M. E. Borges and N. Otero, Energy Fuels, 2007, 21, 3280–3283 CrossRef CAS.
- L. Yang, L. Kong, H. Shi, M. Gu, S. Qin, Z. Shen, W. Zhang and Y. Zhang, J. Fuel Chem. Technol., 2018, 45(1), 48–54 Search PubMed.
- Y. Wang, N. Liu, H. Li and J. Liu, Inorg. Chem. Ind., 2019, 51(8), 60–63 Search PubMed.
- D. Liu, Y. He, C. Zhang and L. Zhang, Funct. Mater., 2011, 5(42), 721–723 Search PubMed.
- L. Cheng, Y. Zhang, Y. Li, L. Li and S. Sun, Journal of Jilin University of Chemical Technology, 2018, 35(7), 48–52 Search PubMed.
- Y.-X. Jia, W. Sun, C. Yin, C. Xu, S. Yu and M. Xian, J. Chem. Technol. Biotechnol., 2019, 94(4), 1259–1268 CrossRef CAS.
- C. Long, Z. Lu, A. Li, W. Liu, Z. Jiang, J. Chen and Q. Zhang, Sep. Purif. Technol., 2005, 44(2), 115–120 CrossRef.
- V. A. Davankov, S. V. Rogozhin and M. P. Tsyurupa, US Pat., 3729457, 1971.
- R. Xing, F. Liu, Z. Fei, J. Chen, Q. Zhang and A. Li, Ion Exch. Adsorpt., 2006, 22(4), 330–338 CAS.
- Z. Fu, H. Li, L. Yang, H. Yuan, Z. Jiao, L. Chen, J. Huang and Y.-N. Liu, Chem. Eng. J., 2015, 273, 240–246 CrossRef CAS.
- C.-G. Oh, J.-H. Ahn and S.-K. Ihm, React. Funct. Polym., 2003, 57(2–3), 103–111 CrossRef CAS.
- G. Xiao and L. Long, Appl. Surf. Sci., 2012, 258(17), 6465–6471 CrossRef CAS.
- W. Yu, C. Xu, C. Yin, S. Yu, W. Sun, C. Xie and M. Xian, Water Sci. Technol., 2018, 78(10), 2096–2103 CrossRef CAS.
- J.-H. Huang, K.-L. Huang, S.-Q. Liu, A. T. Wang and C. Yan, Colloids Surf., A, 2008, 330(1), 55–61 CrossRef CAS.
- Y. Ma, Q. Zhou, A. Li, C. Shuang, Q. Shi and M. Zhang, J. Hazard. Mater., 2014, 266, 84–93 CrossRef CAS.
- X. Wang, X. Yuan, S. Han, H. Zha, X. Sun, J. Huang and Y.-N. Liu, Chem. Eng. J., 2013, 233, 124–131 CrossRef CAS.
- X. Wang, R. Deng, X. Jin and J. Huang, Chem. Eng. J., 2012, 191, 195–201 CrossRef CAS.
- C. He, K. Huang and J. Huang, J. Colloid Interface Sci., 2010, 342(2), 462–466 CrossRef CAS.
- W. Yu, W. Sun, C. Xu, C. Wang, Y. Jia, X. Qin, C. Xie, S. Yu and M. Xian, J. Taiwan Inst. Chem. Eng., 2018, 89, 105–112 CrossRef CAS.
- X. Wang, J. Huang and K. Huang, Chem. Eng. J., 2010, 162(1), 158–163 CrossRef CAS.
- D. Štefanec and P. Krajnc, React. Funct. Polym., 2005, 65(1–2), 37–45 CrossRef.
- A. Li, Q. Zhang, G. Zhang, J. Chen, Z. Fei and F. Liu, Chemosphere, 2002, 47, 981–989 CrossRef CAS.
- S. K. Alamsetti and G. Sekar, Chem. Commun., 2010, 46(38), 7235–7237 CAS.
- C. Yin, C. Xu, Y. X. Jia, W. Z. Sun, C. H. Wang, G. Z. Zhou and M. Xian, J. Taiwan Inst. Chem. Eng., 2019, 95, 383–392 CrossRef CAS.
- S. P. A. Hinkes and C. D. P. Klein, Org. Lett., 2019, 21(9), 3048–3052 CrossRef CAS.
- T. Nichele, C. Favero and A. Monteiro, Catal. Commun., 2009, 10(5), 693–696 CrossRef CAS.
- T. E. Pennington, C. Kardiman and C. A. Hutton, Tetrahedron Lett., 2004, 45(35), 6657–6660 CrossRef CAS.
- P. Leszczyński, T. Hofman and A. Sporzyński, J. Solution Chem., 2020, 49(6), 814–824 CrossRef.
- B. Liu, J. Liu, D. Huang, J. Wei and D. Di, Colloids Surf., A, 2020, 595, 124674 CrossRef CAS.
- X. Qiu, N. Li, X. Ma, S. Yang, Q. Xu, H. Li and J. Lu, J. Environ. Chem. Eng., 2014, 2(1), 745–751 CrossRef CAS.
- S. Chowdhury, R. Mishra, P. Saha and P. Kushwaha, Desalination, 2011, 265(1–3), 159–168 CrossRef CAS.
- B. L. A. William and S. S. Brent, Chem. Rev., 2016, 116(3), 1375–1397 CrossRef.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 57, 5291–5300 CrossRef.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60(49), 11205–11209 CrossRef CAS.
- Q. Meng, J. Yu, L. Yang, Y. Li, M. Xian and H. Liu, Sep. Purif. Technol., 2020, 117728 Search PubMed.
- L. Carlitz, Duke Math. J., 1973, 40(4), 893–901, DOI:10.1215/S0012-7094-73-04083-0.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06167k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |