 Open Access Article
Open Access ArticleInfluence of nanoparticle-based nano-nutrients on the growth performance and physiological parameters in tilapia (Oreochromis niloticus)
M. Z. H. Khan *a,
M. M. M. Hossainb,
M. Khanb,
M. S. Alib,
S. Aktarc,
M. Moniruzzamanc and
Mala Khanc
*a,
M. M. M. Hossainb,
M. Khanb,
M. S. Alib,
S. Aktarc,
M. Moniruzzamanc and
Mala Khanc
aDept. of Chemical Engineering, Jashore University of Science and Technology, Jashore 7408, Bangladesh. E-mail: zaved.khan@just.edu.bd
bDept. of Fisheries and Marine Bioscience, Jashore University of Science and Technology, Jashore 7408, Bangladesh
cDRiCM Institute, Bangladesh Council for Scientific and Industrial Research, Dhaka 1000, Bangladesh
First published on 13th August 2020
Abstract
The present study was conducted to evaluate the effects of dietary nano-nutrients on the growth, physiological and amino acid responses in tilapia fish. Vitamins were incorporated with chemically synthesized nanoparticles (Fe, Zn, Cu and Se) to form a nano-nutrient complex (NNC). Powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM) analyses were performed to confirm the structure and morphology of the as-prepared nutrients. A commercial basal diet without the addition of any NNC was used as a control and compared with the other two diets formulated with different levels of NNC. In a 60 day feeding trial, the fish fed with a diet of NNC60 showed significant differences in final weight and length compared with the basal diet. Furthermore, a high value of nutrient content was observed in the muscles of fish fed with nano diets. In addition, protein, total fat, vitamin C, and essential amino acid levels were significantly higher in the NNC60-treated fish compared with the other groups. The present study suggests that the addition of NNC to a commercial diet has the potential to enhance the growth performance and biochemical parameters in tilapia fish.
1. Introduction
In the last few decades, commercial aquaculture has contributed more than half of the fish globally consumed.1 According to the Food and Agriculture Organization of the United Nations (FAO), global aquaculture production in 2016 was 110.2 million tonnes, among which the total fish production was 80.0 million tonnes with the total farm-gate value estimated at USD 243.5 billion.2 Adaptation of modern fisheries and the use of technology has become of primary importance to support this high productivity. Stringent control over the feed is an important requirement to maintain water quality and to reduce fish stress.3 Therefore, the most challenging and essential aspect is to supply proper nutrition for intensive aquaculture.4 However, fish farmers from developing countries often choose conventional cheaper feeds, rather than nutritionally rich but expensive feeds. The indiscriminate use of commercial feed has resulted in overexploitation, which reduces fish production and diversity significantly.5 Major problems that may result from low-quality feeds are poor appetite, slow growth, high feed conversion ratio, and low survival. These usually develop as a result of problems in the quality of raw materials, feed formulation, processing technology, storage, and feed manage-merit.Nanotechnology has been growing explosively worldwide and become a ubiquitous tool for solving various aquaculture problems, including fish nutrition,6 water quality management, and disease treatment. During the past few years, various engineered nanoparticles [NPs] have been reported for micronutrient delivery, such as FeNPs,6 ZnNPs,4 SeNPs,7–9 AgNPs,10 MnNPs,11 CuNPs12 etc. Iron (Fe) is an essential micronutrient for the functioning of tissues and organs of animals, including fish. It plays a vital role in the immune system, the lipid oxidation reaction and in hematological parameters.13–15 On the other hand, zinc (Zn) is an indispensable element for stabilizing cellular membranes and plays an important role in preventing peroxidation.16 In addition, copper (Cu) is a cofactor for numerous oxidation-reduction enzyme systems. It plays an important role in the physiological and biological response of aquatic animals and has functions within the central nervous system.17 However, due to a lack of proper understanding of NP-based nutrient delivery systems, extensive study is needed.
Vitamins and minerals are also essential micronutrients and important for maintaining normal catalytic processes and body functions. Many metabolic disorders and infections/diseases in fish and other animals can be caused by a deficiency of these nutrients.18 For the healthy growth of fish, several researchers have reported the significant role of vitamin E.1
The present study was conducted to evaluate the comparative efficacy of hybrid nanoparticle-based nano-nutrients on tilapia growth performance and physiological parameters. The present study was designed to synthesize and characterization different elemental nanoparticles and their incorporation with essential vitamins. It was also aimed at determining the synergistic effect of the prepared nano-nutrients on the meat composition and amino acid parameters of the studied fish.
2. Materials and methods
2.1 Preparation of the hybrid nano-nutrient diet
All of the chemicals and reagents were purchased of analytical grade either from Sigma Aldrich, China, or Merck, Germany. Deionized water was used for all purposes unless otherwise stated. At first, Fe nanoparticles (FeNPs) were synthesized as described previously.19 Zn nanoparticles (ZnNPs) were prepared through the precipitation method as described by Kumar et al.20 In addition, the simple one-step reduction method for the synthesis of Cu nanoparticles (CuNPs) was explained in our previous report,17 whereas nano Se was prepared according to the method described by Khan et al.18 The formulation and approximate composition of the nano-nutrient diet are shown in Table 1.| Ingredients | Actual amount |
|---|---|
| a Vitamin premix: thiamine hydrochloride, 10 mg kg−1; riboflavin, 20 mg kg−1; calcium pantothenate, 40 mg kg−1; nicotinic acid, 50 mg kg−1; folic acid, 5 mg kg−1; inositol, 400 mg kg−1; choline chloride, 2000 mg kg−1; biotin, 1 mg kg−1; vitamin B12, 0.05 mg kg−1; vitamin A, 3000 IU; vitamin E, 200 IU; and vitamin C, 200 mg kg−1. | |
| FeNPs | 35% |
| ZnNPs | 30% |
| SeNPs | 25% |
| CuNPs | 9% |
| Vitamin premixa | 1% |
2.2 Characterization of the synthesized nanoparticles
To study the surface morphology and size of the nanoparticles, scanning electron microscopy (SEM) was performed using a Hitachi S-3000H model. On the other hand, powder X-ray diffraction (PXRD, Bruker D8 Advance, Germany) with Cu Kα radiation was used for phase identification of the nanocomposite.2.3 Fish trial
Tilapia (average size of ∼16 g) were purchased from a local Hatchery, Jashore. After immediate oxygenated transportation, they were kept in ambient laboratory conditions for one week in a concrete tank with aerated groundwater (pH: 7.2, DO: 4.5 mg L−1, conductivity: 425 μS cm−1, temp: 26.2 °C). The fish were fed with basal diet pellets once daily at a ratio of 3% body weight. The locally bought commercial basal diet formulation and approximate composition are shown in Table 2.| Ingredients | Approximate composition | |
|---|---|---|
| Fish meal | Protein | >33% |
| Corn | Lipid | >5% |
| Rice bran | Fiber | 8% |
| Soybean meal | Moisture | <12% |
| Animal protein | ||
| Minerals | ||
| Additives | ||
Then, the fish were distributed into six tanks at 10 per group with similar body weight. Finally, experimental diets were allocated as follows:
(i) COM: commercial basal diet (control group)
(ii) NNC30: 30 mg kg−1 nano-nutrient complex mixed with basal diet
(iii) NNC60 group: 60 mg kg−1 nano-nutrient complex mixed with basal diet.
During the experiment, the uneaten feed was removed on a weekly basis by siphoning and the tank of 500 L capacity was cleaned with 50% of the water being changed. Untreated groundwater was used in the fish tank throughout the experiment. The experiment was continued for 60 days.
2.4 Sampling and analytical method
During the 60 day rearing period, each fish was weighed from the first day (initial weight) on a weekly basis and the final weight was recorded (final weight). The specific growth rate (SGR), the feed conversion ratio (FCR) and the survival rate were calculated according to the method described by Mahdi et al.,1 which is as follows:| SGR (% per day) = 100 × ln(final weight) − ln(initial weight)/days |
| FCR (g g−1) = (total feed casting − total fish residue)/(total fish final weight − total fish initial weight + total fish mortality weight) |
The ash (muffle furnace at 550 °C for 24 h) and moisture (oven-dry at 105 °C for 24 h) were determined using standard methods. To measure the mineral content in the fish meat, whole-body samples of the fish were oven-dried (70 °C) followed by sun-drying (3 days). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) instruments (Model Trilogy-7) were used to analyse different elements. The standard sample preparation method was followed for individual element analysis. All experiments were carried out in triplicate with a randomized block design.
The composition of fatty acids was determined by GC analysis with a Supelco™ SP-2560 capillary column. The Kjeldahl method was used to measure the protein content, whereas high-performance liquid chromatography (HPLC) used for the detection of ascorbic acid. The composition of essential amino acids was determined following Ishida et al.21 At first, 6 N HCl was used to hydrolyse the muscle protein at 110 °C. Later, the solution was neutralized with 6 N NaOH, and finally, an amino acid analyser (Hitachi L-8900, USA) instrument was used to determine the composition of the sixteen essential amino acids.
3. Results and discussion
3.1 Characterization of nano-nutrients
The PXRD patterns of the as-prepared different nanoparticles are shown in Fig. 1. The peaks presented in Fig. 1a at the positions of 35.1, 54.8, and 62.3 confirm the presence of FeNPs.22 Fig. 1b represents the PXRD of ZnNPs, where the clear peak positions observed at 31.78, 34.5, 36.7, 47.4, and 56.2 prove the presence of Zn.23 The availability of SeNPs was confirmed by the peaks at 24.3, 30.9, 51.3 and 62.7 as shown in Fig. 1c.24 Meanwhile, the peaks at 43.1, 51.34 and 74.09 (Fig. 1d) suggest the presence of copper nanoparticles.25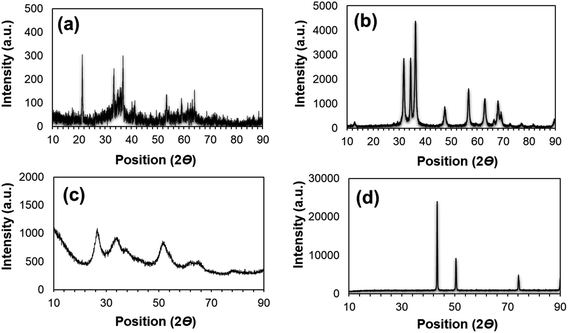 | ||
| Fig. 1 Wide-angle PXRD patterns of the as-prepared nanoparticles: (a) FeNPs, (b) ZnNPs, (c) SeNPs, and (d) CuNPs. | ||
The formation, surface morphology, and particle size distribution of the as-prepared different nanoparticles were evaluated by SEM microscopy. Fig. 2a–d represent the SEM images of FeNPs, CuNPs, SeNPs, and ZnNPs, respectively. The average particle size was observed to be about 11 nm and 15 nm for the FeNPs and CuNPs. Meanwhile, the particle sizes of the SeNPs and ZnNPs were 70 and 105 nm, respectively. A homogeneous distribution was noticed for all of the synthesized nanoparticles.
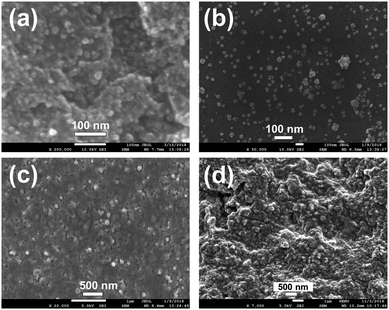 | ||
| Fig. 2 Morphologies of the samples. SEM images of the (a) FeNPs, (b) CuNPs, (c) SeNPs, and (d) ZnNPs. | ||
3.2 Water quality and growth performance study
We used normal tap water for fish cultivation. The initial pH of the water was approximately 7.2. The pH, conductivity, and DO of the water were calculated once a week. There were no adverse effects of nano-nutrient feeding on water quality. Table 3 represents the water quality of the fish tank after one week of each experiment. It can be observed that the pH and conductivity of the water decreased after introducing nano-nutrients into the water, which is favorable for fish farming. The reason for such changes was not understood. In another study, Mansour et al. also observed higher water pH with commercial feed meals used for lampam fish.26 However, pH 6–9 is suitable for tilapia culture for optimum growth performance and survival rate as reported in the literature.27 Meanwhile, the DO and water temperature values were almost unchanged.| pH | DO (mg L−1) | Conductivity (μS cm−1) | Temp (°C) | |
|---|---|---|---|---|
| COM | 8.2 | 6.7 | 535 | 25.4 |
| NNC30 | 7.7 | 6.4 | 527 | 26.1 |
| NNC60 | 8.0 | 6.9 | 505 | 25.8 |
The effects of different diets on the growth performance and survival of the fish are presented in Table 4. The fish fed with the NNC60 diet displayed the highest growth performance. Meanwhile, the survival rate was the same for all of the experimental groups after the 60 day experiment. No significant difference was observed in the initial weight of the fish across all groups. However, NNC60 treatment gave a 33% higher final weight than feeding with a commercial basal diet. In addition, feeding with NNC60 produced better SGR and FCR values. There is no significant difference observed between the NNC30 and NNC60 diets. Several researchers reported the growth of many fish species with dietary SeNPs.7,8 The results are in agreement with those of Zhou et al., who demonstrated that SeNPs supplemented in the basal diet could improve the growth performance of crucian carp.7 Moreover, Behera et al. have recently demonstrated that FeNPs supplemented in the basal diet could improve the final weight of treated fish.6 In another study, Muralisankar and his co-workers observed improved performance in the survival, growth and activities of fresh water prawn fish treated with a ZnNP supplementary diet.4
| COM | NNC30 | NNC60 | |
|---|---|---|---|
| a Values are the mean ± SE of three replicate groups. Mean values with different superscript letters are significantly different from each other (the significance level is defined as P < 0.05). | |||
| Initial weight (g) | 16.2 ± 0.45a | 15.9 ± 0.53a | 16.3 ± 0.58a |
| Final weight (g) | 44.9 ± 0.64a | 56.1 ± 0.55b | 61.0 ± 0.38a |
| Length (cm) | 12.6 ± 0.89a | 13.4 ± 0.12ab | 14.1 ± 0.22bb |
| SGR (% per day) | 1.19 ± 0.02a | 1.38 ± 0.01a | 1.68 ± 0.02a |
| FCR (g g−1) | 2.34 ± 0.12a | 2.11 ± 0.18a | 3.19 ± 0.08b |
| Survival (%) | 100 | 100 | 100 |
3.3 Meat quality
To study the nutritional value of the studied fish after various treatments, protein, fat, mineral, vitamin, and amino acid contents were measured. The protein content of the fish treated with nano-nutrients was higher than that of the basal diet. This result suggests that nano-nutrient treated fish represent an adequate source of protein. The fat content of the fish treated with the commercial basal diet and NNC60 was 2.46 and 1.50 respectively. However, both the fat and trans-fat contents of the fish treated with nano were lower than those treated with a basal diet. The content of Vit. C for the fish treated with nano-nutrients was slightly higher. The normal ash content of the fish treated with all types of diet showed similar ranges from 2.2 ± 0.2%.The effect of different feeding on the tilapia muscle composition was analyzed. The contents of elements Fe, Zn, and Cu in the muscle of tilapia at the end of the feeding trial are shown in Table 5. The fish treated with nano-nutrients showed increased contents of Fe, Zn, and Cu compared to those treated with a commercial diet.16 Several research studies have reported improved fish weight with iron-supplements. It is clear from Table 5 that the iron content in the fish markedly changed with nano-nutrient treatment. Fe and Zn have a potent stimulatory effect on bone formation and mineralization.4 However, extensive studies are needed to determine the optimal inclusion levels of nano-nutrients and trace elements.
It is well known that amino acids play an important role as building blocks of proteins, in nutrient transport and as intermediates in the metabolic pathways of animals. Dietary amino acids contained in high-quality proteins are necessary for key body functions of humans. From Table 5, it can be observed that the nano-composite mixed diet NNC60 shows higher values of cysteine, leucine, methionine, and lysine essential amino acids. Leucine is responsible for stimulating muscle protein synthesis, whereas methionine is used for treating depression in fish. On the other hand, cysteine plays an important role in removing toxic peroxide from the fish body. Lysine is another important amino acid for fish growth performance and preventing cold sores.21 The enrichment of essential amino acids after treatment with NNC would enhance the incorporation of nanomaterials compared with the basal diet. Further studies are being conducted to elucidate potentially negative effects and also perform a toxicity study of the nano-nutrients for other fish (Table 6).
| Amino acids (g/100 g protein) | COM | CN30 | CN60 |
|---|---|---|---|
| Thr | nd | 0.2 | nd |
| Ser | 1.7 | nd | nd |
| Glu | 1.7 | 1.9 | nd |
| Gly | nd | 0.3 | 0.4 |
| Cys | 2.0 | 3.2 | 3.7 |
| Val | 1.2 | 1.7 | 1.9 |
| Met | 1.1 | 1.2 | 1.4 |
| IIe | 0.6 | 0.6 | 0.9 |
| Leu | 1.7 | 1.5 | 2.2 |
| Tyr | 0.3 | 0.4 | 0.5 |
| Phe | 0.1 | 0.2 | 0.1 |
| Lys | 1.2 | 1.0 | 1.4 |
| NH3 | 0.1 | 0.9 | 1.1 |
| His | 0.3 | 0.4 | 0.4 |
| Arg | 0.4 | 0.6 | 0.8 |
4. Conclusion
The inclusion of nano-nutrients and vitamins with the commercial basal diet for tilapia promoted maximum growth, and increased the protein, fat, and amino acid content. The incorporation of various important minerals showed strong synergistic interaction with the basal diet. The proposed NNC60 supplemented diet significantly improves fish meat quality and can be considered as an interesting source of minerals in future aquaculture. This research demonstrated that nano-nutrient supplements in fish diets could improve the growth rate and induce the physiological parameters of tilapia fish. Moreover, nano Fe, Zn, and Cu appeared to increase the muscle Fe, Zn, and Cu content. However, extensive studies are needed to determine the optimal inclusion levels of nano-nutrients and trace elements.Conflicts of interest
The authors declare that there is no conflict of interest.Ethical statement
All experiments were performed in compliance with the relevant laws or guidelines of Jashore University of Science and Technology. All experiments followed the institutional guidelines. All fish experiment procedures were performed in accordance with the Guidelines and approved by the Animal Ethics Committee of Jashore University of Science and Technology.Acknowledgements
This research work was done with financial support from Jashore University of Science and Technology, Jashore, Bangladesh under the scheme ‘‘Special Research Grant’’ for 2019–2020.References
- M. Naderi, S. Keyvanshokooh, A. P. Salati and A. Ghaedi, Aquaculture, 2017, 474, 40–47 CrossRef CAS.
- Fish Species|GLOBEFISH – Information and Analysis on World Fish Trade|Food and Agriculture Organization of the United Nations Market Reports|GLOBEFISH|Food and Agriculture Organization of the United Nations, http://www.fao.org/in-action/globefish/market-reports/fish-products/en/, accessed 10 July 2020 Search PubMed.
- Y. Wang and J. Li, Nanotoxicology, 2011, 5, 425–431 CrossRef CAS PubMed.
- T. Muralisankar, P. S. Bhavan and S. Radhakrishnan, et al., Biol. Trace Elem. Res., 2014, 160, 56–66 CrossRef CAS PubMed.
- S. M. Hixson, J. Aquacult. Res. Dev., 2014, 5(3) DOI:10.4172/2155-9546.1000234.
- T. Behera, P. Swain, P. V Rangacharulu and M. Samanta, Appl. Nanosci., 2014, 4, 687–694 CrossRef CAS.
- X. Zhou, Y. Wang, Q. Gu and W. Li, Aquaculture, 2009, 291, 78–81 CrossRef CAS.
- S. Ashouri, S. Keyvanshokooh, A. Parviz, S. Ali and H. Pasha-zanoosi, Aquaculture, 2015, 446, 25–29 CrossRef CAS.
- H. Li, J. Zhang, T. Wang, W. Luo, Q. Zhou and G. Jiang, Aquat. Toxicol., 2008, 89, 251–256 CrossRef CAS PubMed.
- A. K. Jha and K.J. Prasad, Chin. Adv. Mater. Soc., 2014, 2(3), 179–185 CrossRef CAS.
- M. S. Izquierdo, W. Ghrab, J. Roo, K. Hamre, C. M. Hernández-Cruz, G. Bernardini, G. Terova and R. Saleh, Aquacult. Res., 2016, 1, 1–16 Search PubMed.
- T. Muralisankar, P. Saravana Bhavan, S. Radhakrishnan, C. Seenivasan and V. Srinivasan, J. Trace Elem. Med. Biol., 2016, 34, 39–49 CrossRef CAS PubMed.
- W. R. Beisel, Am. J. Clin. Nutr., 1982, 35, 417–468 CrossRef CAS PubMed.
- S. Singha, K. Das and N. Jha, Ann. Aquac. Res., 2017, 4, 1–12 Search PubMed.
- F. Andersen, B. Lygren, A. Maage and R. Waagbø, Aquaculture, 1998, 161, 437–451 CrossRef CAS.
- A. Awad, A. W. Zaglool, S. A. A. Ahmed and S. R. Khalil, Fish Shellfish Immunol., 2019, 93, 336–343 CrossRef CAS PubMed.
- M. Z. H. Khan, M. A. Rahman, P. Yasmin, F. K. Tareq, N. Yuta, T. Komeda and R. A. Jahan, J. Nanomater., 2017, 2017, 5702838 Search PubMed.
- K. Ullah, A. Zuberi, S. Nazir, I. Ullah and Z. Jamil, Aquac. Rep., 2017, 5, 70–75 CrossRef.
- H. Ping, K. Lu, Y. Jun, Y. Fan, W. Kuaishe, D. Jinjing, Y. Zhanlin, C. Weicheng and L. Dongxin, Rare Met. Mater. Eng., 2016, 45, 3112–3114 CrossRef.
- S. S. Kumar, P. Venkateswarlu, V. R. Rao and G. N. Rao, Int. Nano Lett., 2013, 3, 30 CrossRef.
- Y. Ishida, T. Fujita and K. Asai, J. Chromatogr., 1981, 204, 143–148 CrossRef CAS PubMed.
- D. Chen, Q. Tang, X. Li, X. Zhou, J. Zang, W. Xue, J. Xiang and C. Guo, Int. J. Nanomed., 2012, 7, 4973–4982 CrossRef CAS PubMed.
- M. J. Akhtar, M. Ahamed, S. Kumar, M. M. Khan, J. Ahmad and S. A. Alrokayan, Int. J. Nanomed., 2012, 7, 845–857 CAS.
- C. P. Shah, K. K. Singh, M. Kumar and P. N. Bajaj, Mater. Res. Bull., 2010, 45, 56–62 CrossRef CAS.
- A. Sharma, J. Hickman, N. Gazit, E. Rabkin and Y. Mishin, Nat. Commun., 2018, 9, 4102 CrossRef CAS PubMed.
- O. Mansour, M. Idris, N. M. Noor, M. S. B. Ruslan and S. K. Das, Effects of organic and commercial feed meals on water quality and growth of Barbonymus schwanenfeldii juvenile, 2017, vol. 10 Search PubMed.
- M. K. Mustapha and S. D. Atolagbe, J. Basic Appl. Zool., 2018, 79, 46 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |
