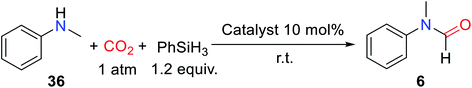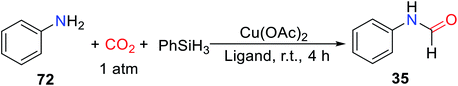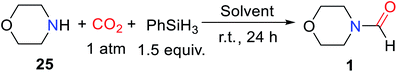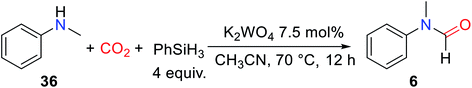Open Access Article
Open Access ArticleRecent advances in liquid hydrosilane-mediated catalytic N-formylation of amines with CO2
Zhengyi
Li
a,
Zhaozhuo
Yu
a,
Xiaoxiang
Luo
a,
Chuanhui
Li
a,
Hongguo
Wu
a,
Wenfeng
Zhao
ab,
Hu
Li
 *a and
Song
Yang
*a
*a and
Song
Yang
*a
aState Key Laboratory Breeding Base of Green Pesticide & Agricultural Bioengineering, Key Laboratory of Green Pesticide & Agricultural Bioengineering, Ministry of Education, State-Local Joint Laboratory for Comprehensive Utilization of Biomass, Center for Research & Development of Fine Chemicals, Guizhou University, Guiyang 550025, Guizhou, China. E-mail: jhzx.msm@gmail.com; hli13@gzu.edu.cn; Fax: +86-851-8829-2170; Tel: +86-851-8829-2171
bTechnical University of Denmark, Centre for Catalysis and Sustainable Chemistry, Department of Chemistry, Kemitorvet, Building 207, 2800 Kgs. Lyngby, Denmark
First published on 14th September 2020
Abstract
Carbon dioxide is an ideal raw material for the synthesis of complex organic compounds because of its rich, non-toxic, and good physical properties. It is of great significance to transform CO2 into valuable fine chemicals and develop a green sustainable cycle of carbon surplus. Based on hydrosilane as a reducing agent, this work summarizes the recent applications of reductive amidation of CO2 using different catalysts such as organocatalysts, ionic liquids (ILs), salts, transition metal complexes, and solvents. The main factors affecting the reductive amidation of CO2 and the possible reaction mechanism are discussed. Moreover, the future orientation and catalytic systems of the formylation of amines with CO2 and hydrosilane are prospected.
1 Introduction
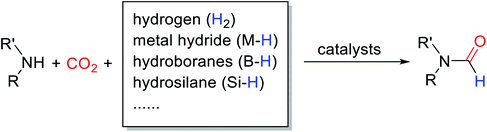
The use of fossil fuels contributes to climate change by emitting large amounts of CO2.1 But human beings have to rely on fossil fuels to get a lot of commercial chemicals.2 So far, great efforts have been made to reduce the use of fossil fuels or to find alternative sources of energy, such as the conversion of biological substrates into value-added chemicals3–10 and the preparation of biodiesel.11–15 But the environmental problems caused by CO2 need to be solved urgently. Creating a carbon balance to recycle carbon waste is necessary, which can not only reduce CO2 emissions but also achieve energy savings. Moreover, CO2 is often used as C1 building block because it is non-toxic, cheap, and readily available.16 Therefore, the conversion of CO2 into valuable chemicals has attracted the attention of many researchers. The activation of CO2 is difficult, due to its high thermodynamic stability and kinetic inertness. In recent years, many strategies have been applied to the formation of C–C, C–O, and C–N bonds. The construction of the C–N bond attracts much attention because N-formylation compounds are key chemical intermediates in the synthesis of drugs, pesticides, dyes, and fragrances.17 In addition, the in site generated formyl group can be converted into other functional groups.In general, H2 is the cleanest and most economical reducing agent. However, due to the high relay capacity of H2, the reduction of CO2 often requires harsh experimental conditions, such as high temperature, high pressure, and the use of some precious metals.18 Hydrosilane and hydroborane as more efficient and convenient reductants for reductive amidation of CO2 duo to their weaker and more polar Si–H and B–H bonds in comparison to the H–H bond in the H2.19 But metal hydride and hydroborane are generally sensitive to air and moisture, preventing other broad applications.20 Hydrosilane is a cheap, nontoxic, and easy-to-treat reducing agent, and even Si–H polar bonds with mild reducibility are more dynamically active.21 Therefore, using hydrosilane as a reducing agent, a large number of catalytic systems have been developed for the reductive amidation of CO2 under mild conditions (Table 1).
| H donor | Advantages | Disadvantages |
|---|---|---|
| Hydrogen (H2) | Clean and atom economical | Harsh reaction conditions (high reaction temperature and pressure) |
| Metal hydride (M–H) | Strong reduction ability | Sensitive to air and moisture |
| Hydroborane (B–H) | Strong reduction ability | Sensitive to air and moisture |
| Hydrosilane (Si–H) | Mild reduction potential, cheap, nontoxic, and easy-to-handle | Different hydrosilanes with a greater effect on the reaction |
In recent years, the catalytic systems for reductive amidation of CO2 using hydrosilanes as a reducing agent have been widely developed and reported. However, this method is different from the direct formylation of aryl group reported by the group of Liu.22,23 It is N-formylation requiring amines as a functional reagent, which can be roughly divided into four categories, including organocatalysts, ionic liquids (ILs) and salts, transition metal complexes, and solvents. Previous reports are mainly focused on the functional reduction of CO2 to build C–C, C–O and C–N bonds using catalytic systems of either nonmetallic or transition-metal complexes.24–27 In the present review, the emphasis was placed on the design and development of budget catalysts and catalytic systems to facilitate the reductive amidation of CO2, and attention is paid on the understanding of the reaction mechanism and process optimization. Reaction conditions, application range, and main factors affecting each catalytic system are also reviewed, in which control of the reaction conditions is necessary to selectively afford formylated products since the fractional reduction of CO2via 2-electron reduction, 4-electron reduction, and 6-electron reduction affords formamide, aminal, and methylamine, respectively.27 Besides, the problems of each catalytic system also prospect.
2 Organocatalysts for N-formylation of amines with CO2
Organic catalysts are organic compounds that do not require metal atoms to speed up chemical reactions. Because of their non-toxic properties and their stability to air and water, they are generally considered safe reagents. These characteristics eliminate concerns about the discharge of metal-containing waste streams, and allow the possibility of working under ambient conditions, which are preferred for industrial applications. In addition, many organic catalysts are readily available from renewable feedstock, offering the potential to reduce carbon emissions.2.1 N-heterocyclic carbenes (NHCs) and related compounds
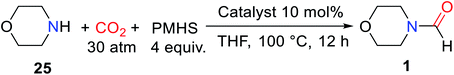
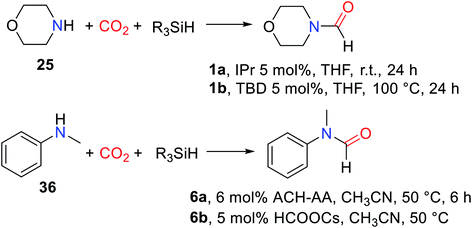
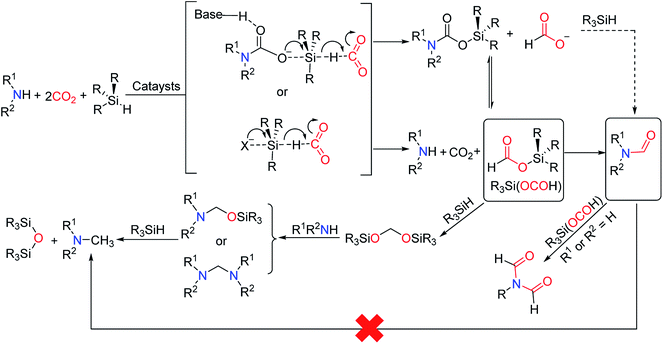
Hydrosilane was used as a reducing agent in the formylation of an amine with CO2 catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD). But the so-called diagonal transformation makes the catalytic reductive functionalization of CO2 to afford formylated products, which is limited to the basic amine.16 Therefore, Cantat and co-workers28 reported the use of unsaturated N-heterocyclic carbenes (NHCs) 1,3-bis(2,6-diisopropylphenyl)-2,3-dihydro-1H-imidazole (IPr) as a highly effective catalyst for reductive amidation of CO2 in the presence of polymethylhydrosiloxane (PMHS). In tetrahydrofuran (THF), morpholine 25 was catalyzed by 5 mol% IPr to afford N-formylmorpholine 1 at room temperature. The activity of different hydrosilanes was as follows, (EtO)3SiH (28% yield), Ph3SiH (4% yield), tetramethyldisiloxane TMDS (43% yield), and PMHS (90% yield). The PMHS is a nontoxic and stable silicon industrial chemical waste,29 and performs very good reducibility and selectivity in the reductive amidation of CO2. IPr is an effective catalyst for the formylation of amines with CO2, which is applicable to various substrates such as amine (giving 1), imine (giving N-(diphenylmethylene)formamide 3), hydrazine (giving N′,N′-diphenylformohydrazide 4), N-heterocyclic ring compounds (giving 5,6-dihydropyrimidine-1(4H)-carbaldehyde 5), and aniline derivatives (giving N-(4-butylphenyl)formamide 2, N-methyl-N-phenylformamide 6, N-(4-methoxyphenyl)-N-methylformamide 7, N-(4-butylphenyl)-N-methylformamide 8, N-mesityl-N-methylformamide 9, N-(2,6-diisopropylphenyl)-N-methylformamide 10), except that the formylation of p-nitro-N-methylaniline 11 cannot afford N-methyl-N-(4-nitrophenyl)formamide 12 (Scheme 1).28 In general, amines are used as functionalized reagents in the process of N-formylation. In 2018, an abnormal N-heterocyclic carbene (aNHC) catalyzed for N-formylation of the amide with CO2 was reported by Mandal and co-workers.30 Extended N-formylation of different substrates is of great significance in organic synthesis. Some heterogeneous catalytic systems have also been designed based on the excellent catalytic activity of NHC such as porous organic polymers,31 and poly-NHC.32 At the same time, the development of heterogeneous catalytic systems successfully realized the reuse of catalysts.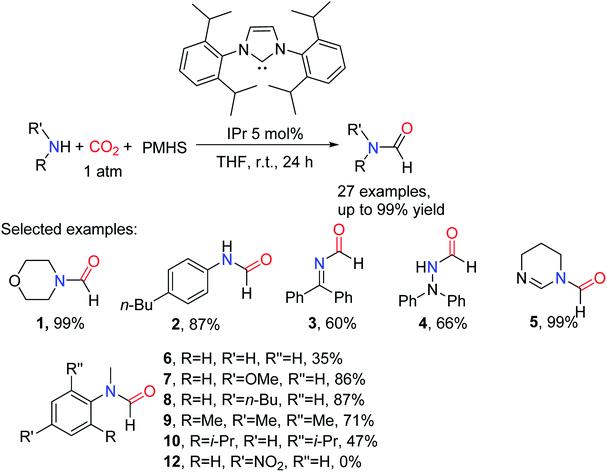 | ||
| Scheme 1 NHCs catalytic systems for the formylation of amines with CO2 and PMHS.28 | ||
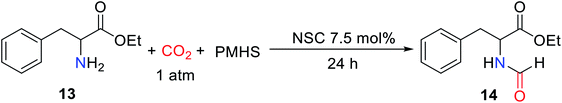
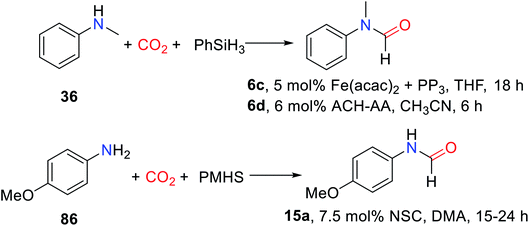
CO2 is fixed by vitamin B1 in animal tissues, which can facilitate the transformation of pyruvate into oxaloacetate. Inspired by this fact, Dyson and co-workers33 reported using non-toxic, cheap, stable, and easy to store thiazolium carbene (NSC)34 to catalyze the reductive functionalization of CO2 to afford the formylated products with PMHS as the reductant under mild conditions. It was found that at a relatively low temperature (50 °C) was more conducive to the formation of formylated products, while at 100 °C, it would lead to over-reduction to afford methylated products.33 The use of solvents in this catalytic system is critical for the control of product distribution. In a polar solvent like N,N-dimethylacetamide (DMA) and N,N-dimethylformamide (DMF), the desired formylated product ethyl formylphenylalaninate 14 was obtained with high yields (95% and 77%, respectively), while polar solvents such as acetonitrile, toluene, and THF were not active for reaction (Table 2).33 This may be due to the instability of the catalyst in these solvents, another possible reason for better performance in DMF or DMA could be its higher solubility toward CO2.
The catalytic system was suitable for aromatic amine, alicyclic amines and aliphatic amines, the formylated product N-(4-methoxyphenyl)formamide 15 (83%), N-cyclohexylformamide 16 (86%), and N-hexylformamide 17 (73%) were obtained, respectively.33 In the catalytic system, the 4-bromoaniline can also afford N-(4-bromophenyl)formamide 18 (80%). However, the application scope of secondary amines is not discussed in the catalytic system (Scheme 2).
 | ||
| Scheme 2 NSC catalytic system for the formylation of amines with CO2 and PMHS.33 | ||
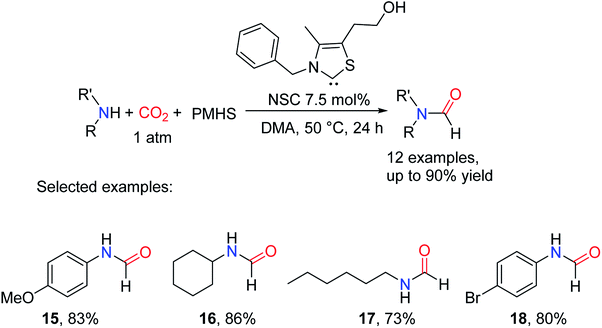
It is of great significance to apply NHCs to reductive amidation of CO2. But the drawback is that NHCs are air-sensitive and must be generated fresh before use.35 It is important to develop different methods to convert CO2 into value-added chemicals. In the metal-free catalytic systems that have been used for CO2 valorization, the activation modes of CO2 are mainly divided into three categories based on the different binding modes: one Lewis base (LB) adduct, one LB and Lewis acid (LA) adduct, and one LB and two LA adducts36 As the P atom of the phosphorus compound acts as a nucleophile, it generally reacts with CO2 to afford LB adduct rather than the phosphorus formate. In 2015, Kinjo and Chong37 provided a new method for capturing CO2. The formylation of amines with CO2 and Ph2SiH2 was finished with 72–99% yields by using 1,3,2-diazaphospholene (NHP-H) as a catalyst, an N-heterocyclic base containing a phosphorus atom in its structure. The catalytic system was widely used for aliphatic/aromatic primary and secondary amines, and alicyclic amines. The substrate with less steric hindrance can produce formylated products with high yield such as N,N-diethylformamide 19 (95% yield), and N,N-diisopropylformamide 22 (61% yield).37 Due to different reaction mechanisms involved, amines with electron-withdrawing groups still exhibit good activity such as N-(4-trifluoromethyl)formamide 24 (71% yield) (Scheme 3).
 | ||
| Scheme 3 1,3,2-Diazaphospholene-catalyzed reduction of CO2 to formamides using amines and Ph2SiH2.37 | ||
2.2 Organic superbases
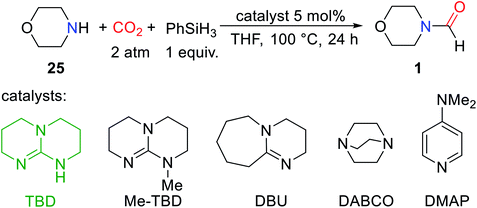
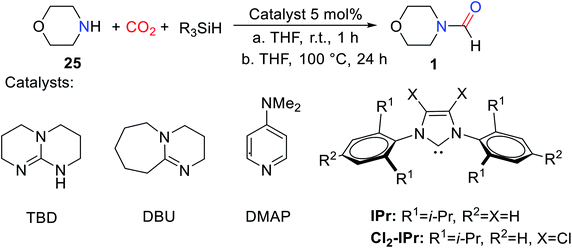
As is known to all, in the formylation reaction between amine and CO2, the use of H2 as a reducing agent requires the addition of metal catalysts. And the reaction needs to be carried out under the conditions of high temperature and high pressure.18 These harsh reaction conditions greatly limit the extensive use of H2 and the expansion of the range of directly available compounds obtained from CO2.In 2012, Cantat and coworkers16 applied organic catalysts for the first time to the formylation of amines with CO2 in the presence of hydrosilane. Replacing the commonly used CO2/H2 system with hydrosilane can not only avoid the burden of high temperature and pressure conditions but also improve the selectivity of the reaction.16 TBD, a cyclic guanidine, was found to be a more effective catalyst to embed CO2 into N–H of 25 to obtain 1 after testing the reactivity of different superbases under the same experimental conditions (Table 3).16
In the absence of any solvent, the obtained formylation product 1 is quantitative (yield > 99%) (Table 3).16 The effects of different hydrosilanes on reactivity were also explored, and PhSiH3 was found to be a more effective reductant. The study of the scope of the catalytic system found that the reaction activity of the substrate was related to its basicity and the steric hindrance around N atom of amines. Strong basicity was more conducive to the reaction. However, 19 (47% yield) and 22 (10% yield) were obtained with lower yield when the steric hindrance around N atom of amines was increased.16 At the same time, the commonly used solvent DMF (N,N-dimethylformamide 26) can be obtained. It was also found that the catalytic system showed low activity for the formylation of both aromatic amines and primary amines (Scheme 4).16
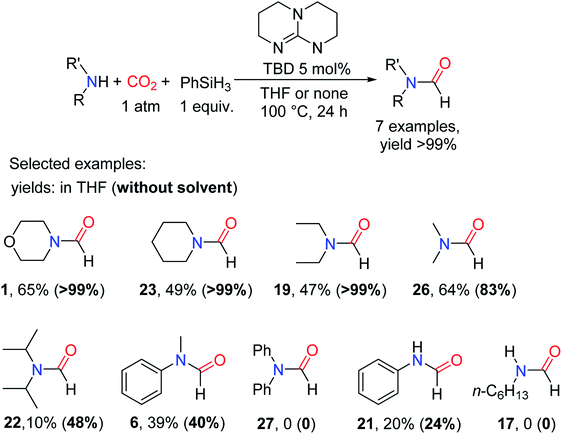 | ||
| Scheme 4 TBD catalytic reduction of CO2 to formamides using amines and PhSiH3.16 | ||
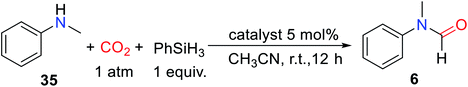
Subsequently, other superbases were reported as catalysts for the formylation of amines and CO2. In 2018, Li and Chen38 also reported that the superbase 1,8-diazabicyclo[5.4.0]undec-7-en (DBU) was used as a catalyst for reductive amidation of CO2, and the formylated product (N-methyl-N-(m-tolyl)formamide 33) yield reached 93% in CH3CN after reaction at 30 °C for 24 h. The DBU catalytic system could selectively afford formylation products by adjusting the reaction temperature.38 The catalytic system is suitable for aliphatic amines and aromatic amines, but the activity of the reaction decreases when the substrate is attached with electron-withdrawing groups such as N-(4-chlorophenyl)-N-methylformamide 32 (21% yield) (Scheme 5).38
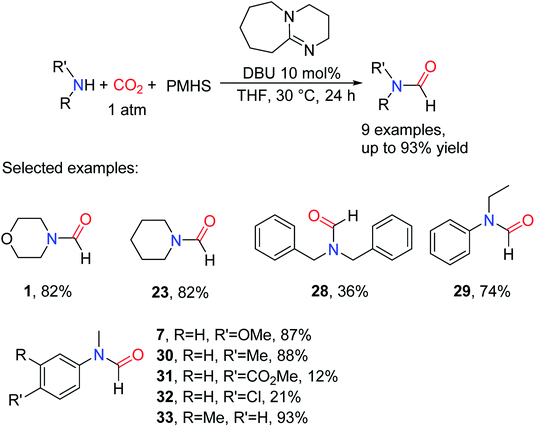 | ||
| Scheme 5 DBU catalytic systems for the formylation of amines with CO2 and PMHS.38 | ||
Cyclic carbamate can be obtained from CO2, in which the cheap and stable N,N,N,N-tetramethylguanidine (TMG) has better reactivity than the well-modified cyclic guanidine.39 Therefore, alkylated TMG, 2-butyl-1,1,3,3-tetramethylguanidine (BTMG), was applied to the formylation of amines with CO2 and proved to be an efficient catalyst.40 In MeCN, PhSiH3 used as the reducing agent, the formylated product 1 (80.5% yield) can be obtained with only 0.1 mol% catalyst (TON = 805 and TOF = 33.5 h−1). Its catalytic activity was also higher than that of complex cyclic guanidine compounds, and BTMG catalyzed reaction was completed within 50 min, which was superior to the reaction with TBD (>300 min).40 BTMG is more stable and has higher activity than TMG, since TMG itself is formylated leading to the catalyst deactivation. At the same time, the catalytic system is suitable for aliphatic/aromatic primary and secondary amines. However, when the substrate is attached with electron-withdrawing groups, it will have low reaction activity or even no reaction. Even if 11 underwent reaction for 22 h, the corresponding formylated product 12 was not obtained (Scheme 6).40
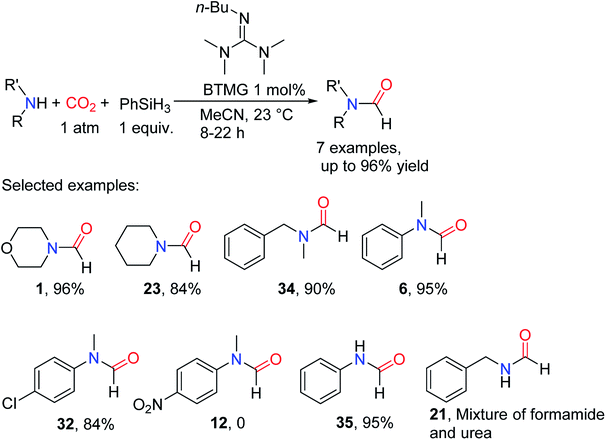 | ||
| Scheme 6 BTMG catalytic reduction of CO2 to formamides using amines and PhSiH3.40 | ||
TBD was applied to formylation of CO2 for the first time, which provided a progressive strategy for the reductive functionalization of CO2.40 By using DBU as a catalyst, the formylated products in high selectivity were obtained at a low temperature (e.g., 30 °C). With simple BTMG as a catalyst, high yields of formylated products were obtained with low catalyst load. However, the scope of application of this kind of catalysts is limited: TBD (7 examples), DBU (9 examples), BTMG (7 examples), and only some simple aliphatic and aromatic amines, especially TBD even only showed good activity to secondary amines.40 In the catalytic system, the reaction time is often longer. Therefore, more adaptable and selective catalytic systems need to be developed.
3 Ionic liquids (ILs) and salts for N-formylation of amines with CO2
3.1 Ionic liquids (ILs) and organic salts
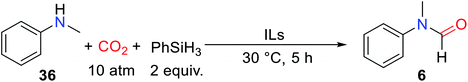
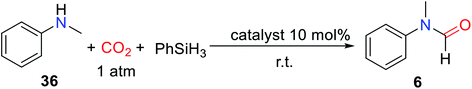
ILs are composed of organic cations and organic/inorganic anions, with adjustable performance, good thermal stability, and chemical stability. It is worth noting that the majority of ILs are non-metallic salts, which can avoid the problem of metal pollution, so it has a promising application prospect in the field of catalysis.41–46 At the same time, compared with other non-metallic catalysts, ILs are easy to separate and can be reused, and designed by the synergistic effect between anion and cation with special functions.47–50In 2015, Liu and co-workers51 reported that in the absence of solvent, ILs composed of 1-butyl-3-methylimidazolium (BMIm) and different anions were used as catalysts for the formylation of N-methylaniline 36 with CO2 to obtain formylated product 6 with different yields (Table 4).
Among the tested ILs, 1-butyl-3-methylimidazolium chloride ([BMIm]Cl), 1-butyl-3-methylimidazolium bromide ([BMIm]Br), and 1-hexyl-3-methylimidazolium chloride [HMIm]Cl exhibited high activity for catalyzing the reductive amidation of CO2.51 However, the [BMIm]Cl could catalyze the formation of formylated product 6 with 91% yield under a low catalyst load of 10 mol%. There is a huge difference in reactivity for ILs with different anions, which is attributed to the synergy between anions and cations. Although there was no change in the catalytic activity of alkylated 1-butyl-3-methylimidazolium and [HMIm]Cl, it was not enough to indicate that cations had little effect on the reaction. In the presence of PhSiH3, the [BMIm]Cl was successfully used as the catalyst for the formylation of aliphatic/aromatic primary and secondary amines.51 In addition to substrate 11, amines with electron-withdrawing groups also had good activity such as N-(4-fluorophenyl)-N-methylformamide 38 (89% yield) was obtained. However, in the process of formylation of primary amines with CO2, the diformylated products were obtained such as N-butyl-N-formylformamide 20′ and N-benzyl-N-formylformamide 21′ (Scheme 7).51
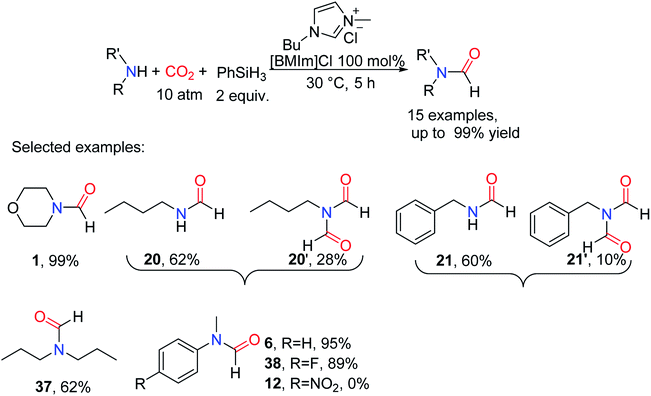 | ||
| Scheme 7 [BMIm]Cl catalytic reduction of CO2 to formamides using amines and PhSiH3.51 | ||
In 2016, Dyson et al. reported the activity of chlorine salt-containing different cations as catalysts for the formylation of amines with CO2 and PhSiH3 under the CO2 pressure of 1 atm with the catalyst load of 10 mol%.52 And the reactivity was found to follow the order of [BMIm]Cl < 1-butyl-2,3-dimethylimidazolium chloride ([BdMIm]Cl) < 1-butyl-1-methylpyrrolidinium chloride ([BMP]Cl) < tetrabutylammonium chloride (TBAC). They still showed lower activity after a longer reaction time (20 h), and the yield of formylated product 6 catalyzed by [BMIm]Cl and TBAC was 6% and 65%, respectively. However, the tetrabutylammonium fluoride (TBAF) was found to show excellent activity by testing the salts containing the same tetrabutylammonium (TBA) cation but different anions, and the formylated product 6 (99% yield) could be obtained in short reaction time (6 h).52 This result further indicates that the driving force of this type of catalyst is mainly from anion nucleophilicity: I− < Br− < Cl− ≪ F− ≈ OH−, where the more nucleophilic is more conducive to the reaction (Table 5).
The catalytic system could tolerate many unsaturated functional groups, giving methyl 4-(N-methylformamido)benzoate 31 and N-(4-formylphenyl)-N-methylformamide 41.52 The catalytic system is also applied to aliphatic amines (giving 17), aromatic amines (giving N-phenylformamide 35), imines (giving 3) and hydrazones (giving N′-(diphenylmethylene)formohydrazide 43). For aniline and its derivatives, the substrate attached with an electron-withdrawing group in the C4-position was less reactive such as N-methyl-N-(4-(trifluoromethyl)phenyl)formamide 42 (53% yield). The catalytic system can even promote 4-nitroaniline 39 being converted to the corresponding formylation product N-(4-nitrophenyl)formamide 40 (Scheme 8).52
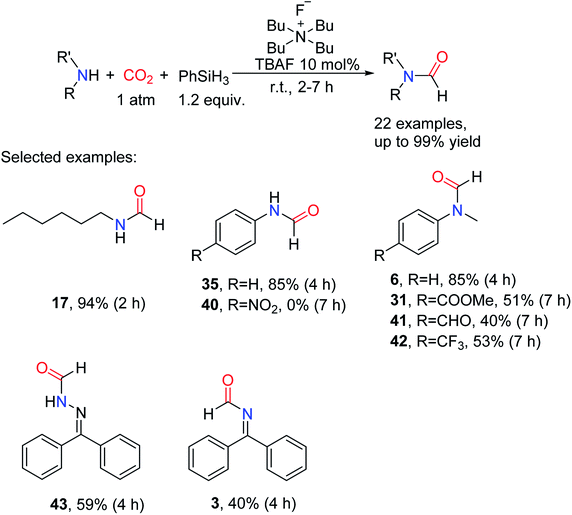 | ||
| Scheme 8 TBAF catalytic reduction of CO2 to formamides using amines and PhSiH3.52 | ||
The catalytic system was also reported and proved it is efficient for reductive amidation of CO2 under mild conditions by Liu and co-workers.53 But products at different reduction levels can be obtained by using different hydrosilanes in the work. In the reductive functionalization of CO2, (EtO)3SiH used as a reductant can afford formylated products with high selectivity. At the same time, PhSiH3 was used as a reducing agent, methylated products could be obtained. This work provides a new method to improve the selectivity of reductive amidation of CO2.
At present, the use of the natural product and bio-derived compounds as sustainable catalysts or solvents has attracted the attention of more and more researchers. Glycine betaine (GB, 1-carboxy-N,N,N-trimethylmethanaminium inner salt) is a quaternary ammonium alkaloid with an amphoteric structure that is widely found in plants.54 GB has the advantages of being biodegradable, non-toxic, and cheap. It has a broad application prospect in the catalytic field owing to its basicity.55,56 Therefore, it is of great significance to apply for the reductive amidation of CO2. So, the reaction of formylation of amines, and CO2 with Ph2SiH2 catalyzed by GB was studied by He and co-workers at a relatively low temperature of 50 °C.54 It is found that the catalytic system has a wide range of applicability. Aliphatic amines and aromatic amines with electron-withdrawing and electron-donating groups can get corresponding formylated products such as N-(4-chlorophenyl)formamide 45 (65% yield) and 15 (84% yield). And the activity of amines with the electron-withdrawing group is lower than the amines with electron-donating groups. Meanwhile, the catalytic system could tolerate some reducible functional groups, such as alkenyl (giving N-allyl-N-phenylformamide 44) and carbonyl group (giving N-(4-acetylphenyl)-N-methylformamide 46) (Scheme 9).54
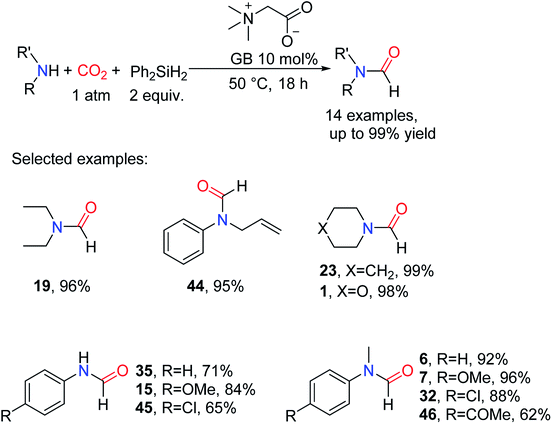 | ||
| Scheme 9 GB-catalyzed reduction of CO2 to formamides from amines using PhSiH3.54 | ||
It is worth noting that Han and Xie57 also reported the application of GB as a catalyst for the formylation of amines with CO2 and PhSiH3 in CH3CN and 3 mol% catalyst, which could afford formylated products at room temperature in good to high yields (15–92%). The catalytic system has a wide range of applicability, and can even promote substrate 39 being converted to the corresponding formylated product 40 (23% yield).57 However, substrates with high steric hindrance around N atoms are relatively inactive. The studies on the distribution of formylated products of primary amine showed that mono-formylated products were the main products (Scheme 10).
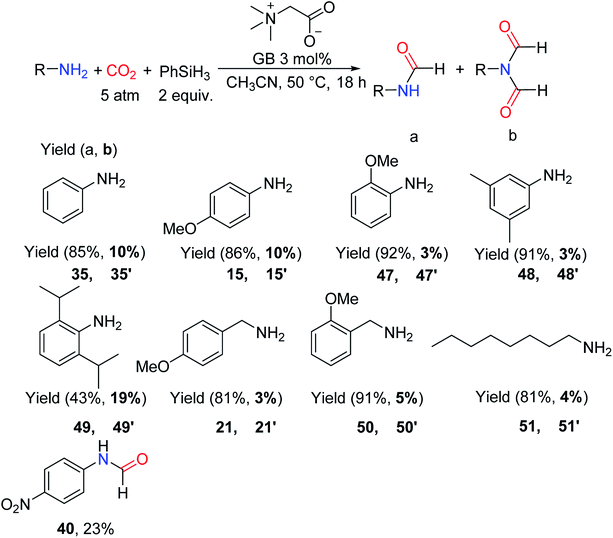 | ||
| Scheme 10 Formylation of various primary amines and 4-nitroaniline using CO2 as the carbon source.57 | ||
The successful application of GB for the formylation of amines with CO2 has proved that natural products have great potential in the reductive functionalization of CO2. Lecithin as a natural product has good biological compatibility, which is renewable, non-toxic, and widely used as food additives, biological surface-active agent, and catalyst,58–61 with a structure similar to GB (i.e., zwitterionic structure) (Fig. 1). Importantly, lecithin is more easily obtained than GB, which is widely found in soy, eggs, and rapeseed.62
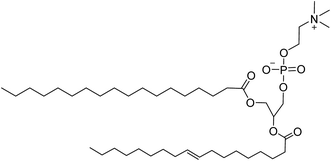 | ||
| Fig. 1 Chemical structure of lecithin.63 | ||
In 2018, Han and Hu63 successfully used lecithin as an effective catalyst for the reductive functionalization of CO2 to formylated products in the presence of an amine and PhSiH3 as a hydrogen source. In a conditioned screening experiment, 36 used as the model substrate to afford the corresponding formylated product 6 (97% yield), and PhSiH3 was demonstrated to be the more effective reducing agent. By controlling the CO2 pressure, the catalytic system can afford formylated product 6 with high selectivity (0.1 MPa, 17% yield; 0.3 MPa, 84% yield). The catalytic system is suitable for aliphatic/aromatic primary and secondary amines. Amines attached to electron-donating groups and electron-withdrawing groups can also afford corresponding formylated products with higher yields such as 7 (95% yield) and N-(4-bromophenyl)-N-methylformamide 53 (80% yield).63 Even the substrate 11 can also afford the corresponding formylated product 12 (24% yield) proving that the catalytic system has high reducibility. For primary amines, monoformylated products can also afford with high yields, indicating that the catalytic system has high selectivity (Scheme 11).
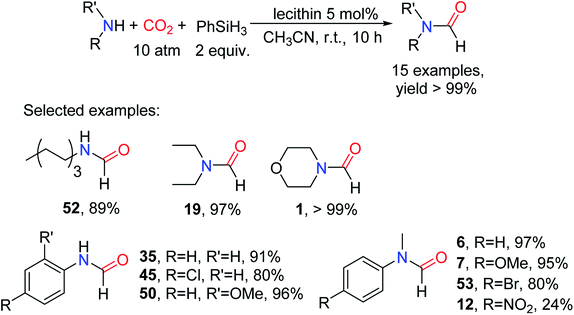 | ||
| Scheme 11 Lecithin catalytic reduction of CO2 to formamides using amines and PhSiH3.63 | ||
Choline-based ILs are completely composed of biological materials. It is a type of environmentally friendly material with low toxicity, high stability, and strong basicity.64–66 Yang and Zhao67 by screening experiments found that acetylcholine-carboxylate bio-ionic liquids (ACH-AA) composed of acetylcholine and carboxylate is an efficient catalyst for reductive amidation of CO2. It was reported that the viscosity of ILs increased with the length of the carboxylic acid carbon chain,65 thus increasing the permeability and diffusion coefficient of CO2.68 The ACH-AA had higher nucleophilicity and basicity. And it showed higher activity and selectivity for the reductive amidation of CO2. In the presence of PhSiH3, no solvent, and 30 °C reaction temperature, 6 mol% ACH-AA could catalyze pyrrolidine to afford the corresponding formylated product N-formylpyrrolidine 55 (100% yield). The catalytic system is not only suitable for secondary aliphatic amines and aromatic amines, but also can tolerate unsaturated double bonds (giving 44). However, for the larger amines, the activity was lower, e.g., for the synthesis of N-(4-methoxybenzyl)-N-phenylformamide 57 (Scheme 12).
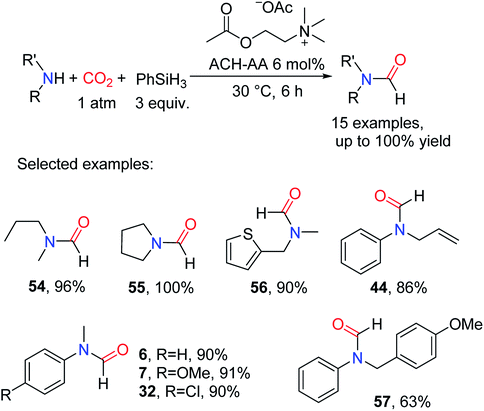 | ||
| Scheme 12 ACH-AA catalytic reduction of CO2 to formamides using amines and PhSiH3.67 | ||
[BMIm]Cl and ACH-AA are both ILs catalytic systems that could be reused many times without loss of activity. To achieve the catalyst recycles, some heterogeneous catalytic systems are also designed for reductive amidation of CO2 such as microporous polymers,69 iron-rich Gibeon meteorite,70 and zwitterionic covalent organic frameworks.71,72 Although complex preparation processes were required, it is important for the sustainable conversion of CO2. Performance of these two types of ILs and organic salts, such as TBAF catalytic systems, can be adjusted. High activity and selectivity of the catalyst can be designed by the interaction between anions and cations. It is worth noting that natural products are non-toxic, biodegradable and environment-friendly, showing great potential in the field of catalysis.
3.2 Inorganic salts
K2CO3 supported on alumina was reported as the active catalyst for the hydrosilylation of benzaldehyde.73 Cui and coworkers also reported Cs2CO3 catalytic reduction of amides, aldehydes, and ketones in the presence of hydrosilane.74,75 In 2015, Motokura and co-workers76 successfully got silyl formate by using CsF and K2CO3 to catalyze hydrosilylation of CO2. The use of low cost, readily available, and stable inorganic salt as the catalyst for the formylation of amines with CO2 was successively reported. In 2016, Lin and Fang77 used the simple and easily available inorganic salt Cs2CO3 as the catalyst for the N-formylation of amines with CO2 in the presence of PhSiH3. The formylated products were obtained in 14–99% yields at room temperature. The catalytic activity of carbonate of different alkali metals was also investigated. The presence of “cesium effect”78,79 resulted in the change of solubility or nucleophilicity of different carbonates. The results show that the reactivity of alkali-metal carbonates of different sizes is different. 15-Crown-5 ether was added to the Na2CO3-based system and it was found that the yield of formylation catalyzed by Na2CO3 increased to 21%, proving that the solubility of carbonate is an important factor affecting the catalytic activity (Table 6).77In the conditional optimization experiment with the 36 as the model substrate, PhSiH3 used as a reducing agent in CH3CN under 1 bar of CO2 at room temperature for 12 h, the formylated product 6 was obtained with 94% yield.77 In this catalytic system, the catalyst load had little influence on the reaction. Under the same reaction conditions, the yields corresponding to 5 mol%, 2.5 mol%, and 1 mol% catalyst load are 94%, 91%, and 90%, respectively. And it turns out that the solvents have a big effect on the reaction, and the desired products cannot be obtained in some solvents. By controlling the temperature of the reaction and the pressure of CO2, the formylated products can be obtained selectively. The catalytic system was found to be very resistant to many functional groups, such as aryl bromide (giving 53), allyl (giving 44), and cyano groups (N-(2-cyanoethyl)-N-phenylformamide 63).77 However, the substrate (e.g., diisopropylamine) having high steric resistance has a lower reactive activity. In general, aniline and its derivatives have a remote effect of the substituent, which results in the lower reactivity of substrates attached with electron-withdrawing groups than that of the substrates with electron-giving substituents. This method is also applicable to the non-amine nitrogenous compounds to afford corresponding formylated products like N,N′-diphenylformohydrazide 62 (Scheme 13).
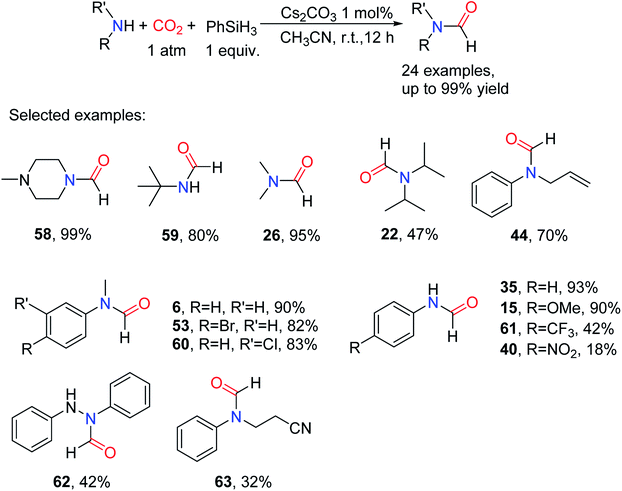 | ||
| Scheme 13 Cs2CO3 catalytic reduction of CO2 to formamides using amines and PhSiH3.77 | ||
In 2016, Bhanage and co-workers80 tested the catalytic activity of different carbonates at 100 °C and under CO2 pressure 3 atm, in the presence of PMHS. In this catalytic system, although Cs2CO3 and K2CO3 both could afford formylated product 1 (99%), the relatively cheap K2CO3 was selected as the catalyst for the N-formylation of amines with CO2 (Table 7).
The catalytic system was suitable for aliphatic/aromatic primary and secondary amines.80 And diallylamine was also formylated to afford N,N-diallylacetamide 64. However, amines attached with electron-withdrawing groups or amines with large steric hindrance showed lower activity and even could not obtain the desired formylated products such as N-methyl-N-(2-nitrophenyl)formamide 68 and N-benzyl-N-(tert-butyl)formamide 66 (Scheme 14).80 The catalytic system requires higher CO2 pressure, reductant equivalent and catalyst dosage, which may be due to the use of less reductive PMHS.
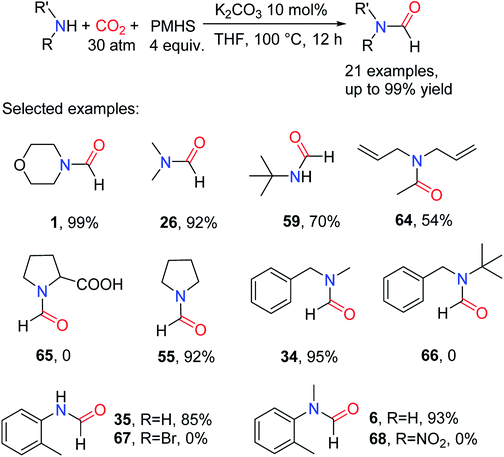 | ||
| Scheme 14 K2CO3-catalyzed reduction of CO2 to formamides using amines and PMHS.80 | ||
Since the oxygen atom of tungstate ion has high electron density.81 It is speculated that tungstate anion can activate hydrosilane to promote the reduction of CO2. In this regard, potassium tungstate has been applied for the reductive amidation of CO2 by He and co-workers.82 By adjusting the pressure of CO2 to a higher state (2 MPa), the reductive amidation of CO2 could be complete with higher selectivity. The catalytic system is suitable for most of the aromatic secondary amines and heterocyclic amines. For the substituted aniline derivatives, the activity of the substrate substituted by the electron-withdrawing groups was lower than that of the substrate substituted by the electron-donating group, such as 32 (85% yield) and 7 (92% yield).82 When the steric hindrance increases, like in the case of N,N-diphenilfamine, the formylated product was obtained in a low yield (Scheme 15).
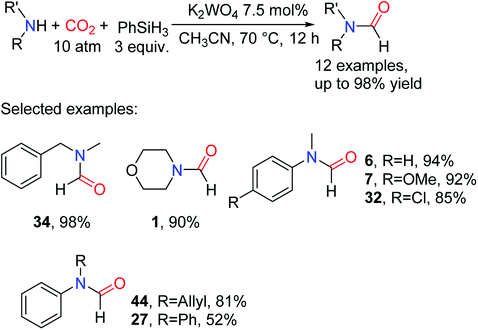 | ||
| Scheme 15 K2WO4-catalyzed reduction of CO2 to formamides using amines and PhSiH3.82 | ||
4 Transition metal complex for N-formylation of amines with CO2
As a common intermediate in the process of CO2 reduction, metallic hydrogen compounds (M–H),83,84 can promote the CO2 reduction by forming complexes with electron-donating ligands to enhance the nucleophilicity of M–H. As a new pathway to reduce CO2, different transition metal complexes (Ni, Cu, Fe, Ru, Zn, Mn) have been applied to catalyze the formylation of amines with CO2. The same metal can be combined with different ligands to develop diverse catalytic systems. And the selection of ligands developed from initially toxic diphosphophenyl to relatively green NHC and some ionic liquids. This is of great significance for the reductive functionalization of CO2.4.1 Cu-based complex
In 2012, Motokura and co-workers85 reported in the presence of PMHS, the Cu-base complex (3 equivalents of 1,2-bis(diisopropylphosphino)benzene (L1) to Cu) is an effective catalyst for the N-formylation of amines with CO2, 1 atm of CO2 pressure and reaction temperature of 80 °C in 1,4-dioxane, giving formylated products in moderate to excellent yields. The effect of different ligand structures L1–L5 (1,2-bis(diphenylphosphaneyl)benzene L2, 1,2-bis(dicyclohexylphosphaneyl)benzene L3, 1,3-bis(diphenylphosphaneyl)propane L4 (dppp), and triphenylphosphane L5 (Ph3P)) (Fig. 2) on the reaction was experimentally tested, and it was found that diphosphine (L1 or L3) with o-benzene structure was a good formylated ligand compared with the double-dentate ligand (L4) and single-dentate ligand (L5) connected with the propyl chain.85 Among these diphosphines, the activity of alkyl-functional ligands such as isopropyl and cyclohexyl was superior to that of the phenyl counterpart.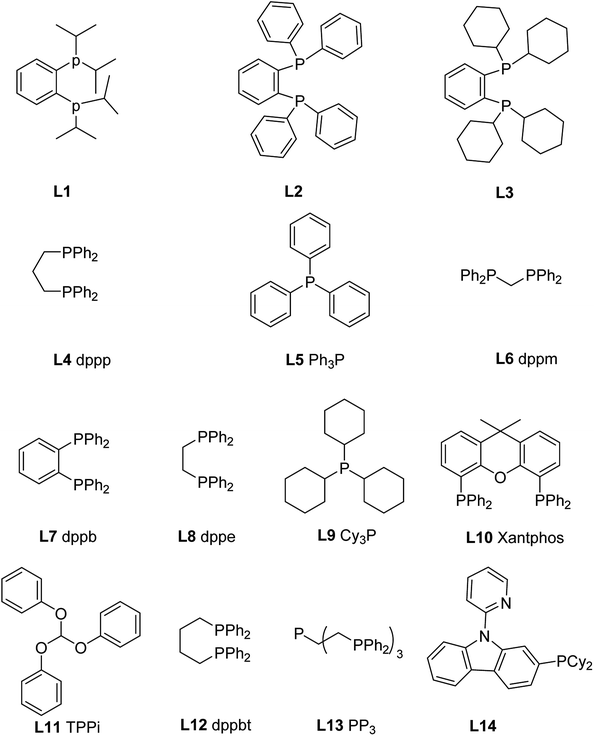 | ||
| Fig. 2 The structure of the used phosphorus ligand.85,86 | ||
The catalytic system is suitable for aliphatic secondary amines, alicyclic amine, and aromatic primary and secondary amines.85 Commonly [Me2NCO2][Me2NH2] used as the substrate to afford DMF 26 in 75% yield, which further indicates that the reaction of amines with CO2 does not affect the N-formylation. At the same time, amines with branched chains showed lower reactivity, and the reactivity decreased with the increase of the number of methyl attached to piperidine-derived such as formylated product 23 (98% yield), 2,6-dimethy-N-formylpiperidine 69 (47% yield) and 2,2,6,6-tetramethyl-N-formylpiperidine 70 (3% yield).85 This is due to the large volume of the catalyst itself, and the substrate of a large steric hindrance has a great impact on the reaction activity. Some regionally selected formylated products like N-(2,2,6,6-tetramethylpiperidin-4-yl)formamide 71 that is a precursor of the highly functional 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) derivative, were synthesized. When the substrate was aniline and its derivatives, the electron-donating group attached in the C4-position of the aromatic ring facilitated the formylation reaction.85 However, when the substrates are attached with the electron-withdrawing groups, the reaction cannot occur, e.g., for the synthesis of 40 (yield < 1%) (Scheme 16).
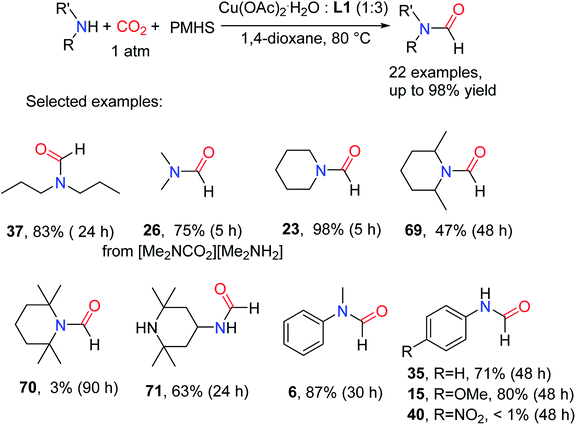 | ||
| Scheme 16 Copper–diphosphine complex catalytic reduction of CO2 to formamides using amines and PMHS.85 | ||
The complex [Ir(H)(CF3SO3)(NSiN)(coe)] (NSiN = bis(pyridine-2-yloxy)methylsilyl fac-coordinated) was used as a catalyst for the synthesis of silyl carbamates in the absence of solvents. The method was then applied to the reductive amidation of CO2, but the formylated products were obtained in very low yield.86 In 2016, Zhang and coworkers20 used bis(diphenylphosphoino)ethane (L8) (Fig. 2) as a ligand of Cu. The Cu(OAc)2-L8 as a highly effective catalyst for reductive amidation of CO2 without any solvent in the presence of PhSiH3 under room temperature and 1 atm CO2 with only 0.1 mol% catalyst loading, giving the formylated products in 91–99% yields.20 The influence of different ligands L5–L12 (L5, bis(diphenylphosphaneyl)methane L6 (dppm), 1,2-bis(diphenylphosphaneyl)benzene L7 (dppb), L8 (dppe), tricyclohexylphosphane L9 (Cy3P), 9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane L10 (xantphos), triphenoxymethane L11 (TPPi), and 1,4-bis(diphenylphosphaneyl)butane L12 (dppbt)) (Fig. 2) on the reactivity was also explored. It was found that monophosphine ligands (L5, L9, L11) are inactive for the N-formylation reaction, and bite angle (β0) of the ligand has an important effect on the activity of the catalyst. The larger bite angle is, the lower the yield of the formylated product (Table 8).20
| Ligand | Bite angle, β0 | Yield [%] |
|---|---|---|
| a Reaction conditions: aniline (1 mmol), Si–H (6 mmol), Cu(OAc)2 (1 mmol), ligand (1.2 mmol); N.R. = no reaction. | ||
| L5 | — | N.R. |
| L9 | — | N.R. |
| L11 | — | N.R. |
| L10 | 102 | 11 |
| L7 | 87 | 23 |
| L6 | 84 | 59 |
| L8 | 89 | 96 |
| L12 | 98 | 83 |
The catalytic system is suitable for aliphatic/aromatic primary and secondary amines, and can selectively give monoformylated products with high yields for primary amines.20 Indoline can be also formylated to afford N-formylindoline 74 in the catalytic system. For aniline with electron-withdrawing groups, the formylated product can be obtained by increasing reaction time. Amines with high steric hindrance required longer reaction time to obtain higher yields of formylation products, as in the case of 27 (Scheme 17).20 It is worth noting that the catalytic system showed excellent catalytic activity in the absence of solvents.
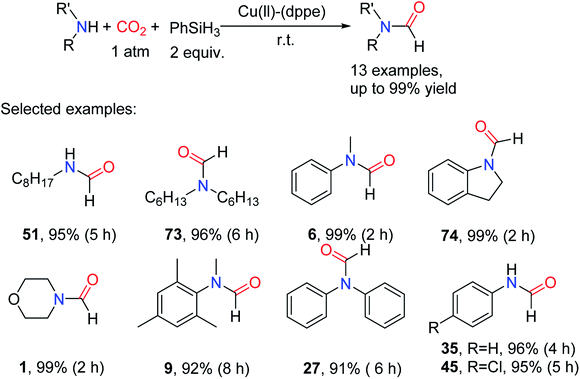 | ||
| Scheme 17 Cu(II)-(dppe)-catalyzed reduction of CO2 to formamides using amines and PhSiH3.20 | ||
4.2 Ni-based complex
In 2013, García and co-workers87 found that the intermediate (Et3SiOC(O)H) in the process of CO2 formylation could be obtained in high yields using nickel complex [(dippe)Ni(μ-H)]2 as a catalyst after only 1 h, in the presence of 10 mol% of Et3B. So this method is applied to reductive amidation of CO2 in THF at 80 °C for 1 h with Et3SiH (Scheme 18).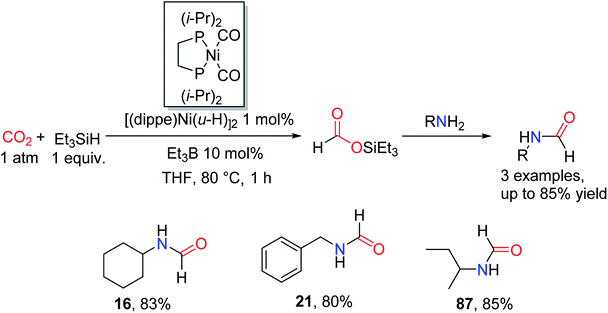 | ||
| Scheme 18 Catalytic reduction of CO2 to formamides using amines and Et3SiH over [(dippe)Ni(μ-H)]2.87 | ||
4.3 Fe-based complex
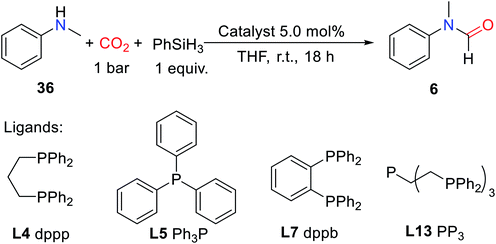
Li and coworkers reported the esters were reduced to aldehydes by iron complexes.88 In 2009, Beller et al. and Zhou et al. independently reported that carbonyl iron complex was used as a catalyst for the amide reduction to amine.89,90 In 2013, the reduction of urea to formamidine using an iron complex as catalyst was reported by Cantat and coworkers91 for the first time. Moreover, phosphorus-containing ligands proved to be effective ligands for catalytic formylation of amines with CO2.85 In 2013, Cantat and coworkers21 screened different phosphorus ligands (L4, L5, L7, and tris[2-(diphenylphosphino)ethyl]phosphine L13 (PP3); Fig. 2) to form complexes with Fe(acac)2 as catalysts for reductive amidation of CO2 in the presence of PhSiH3. In THF reaction at room temperature for 18 h, the L13 and Fe(acac)2 in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as a catalyst (5.0 mol%) could catalyze the N-formation of N-methylaniline 36 to afford 6 (yield > 95%) (Table 9).21
1 as a catalyst (5.0 mol%) could catalyze the N-formation of N-methylaniline 36 to afford 6 (yield > 95%) (Table 9).21
It was found that the catalytic system was suitable for aliphatic/aromatic primary and secondary amines. The amine containing a functional group of carbonyl group could be formylated to afford the corresponding product 46 (65% yield).21 Imine and hydrazine could also afford the corresponding formylated products 3 (8% yield) and 62 (26% yield), respectively. However, the substrate with large steric hindrance has lower activity, e.g., di-iso-propylamine being formylated to afford 22 (40% yield). For aniline and its derivatives, the substrate attached with the electron-donating group was more active than the electron-withdrawing counterpart.21 However, for primary amines, diformylated products were obtained such as N-benzyl-N-formylformamide 21′ and N-formyl-N-heptylformamide 52′ (Scheme 19), proving that the selectivity of the catalytic system was low for primary amines.21
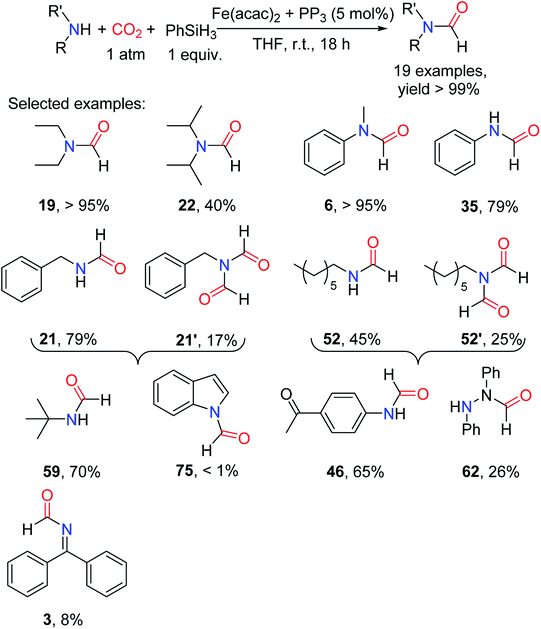 | ||
| Scheme 19 Fe-base complex catalytic reductive amidation of CO2 using amines and PhSiH3.21 | ||
4.4 Ru-based complex
To achieve industrialize reductive amidation of CO2, it is highly desirable to develop new catalysts that can promote reductive formylation at low catalyst loads and ambient temperatures. Based on the general metal catalytic reduction of CO2, the common intermediate is the metal hydrogen intermediate.83,84 Meanwhile, it is speculated that the strong electron donor ability of NHC ligands can increase the nucleophilicity of metal hydrides, thus promoting the reduction of CO2. In addition, bis(NHC) rhodium complexes as effective catalysts for hydrosilylation of ketones have also been reported.92 In 2015, Nguyen and co-workers93 reported the alkyl-bridged bis(tzNHC) (tz = 1,2,3-triazol-5-ylidene) rhodium complexes used as a catalyst for N-formylation of amines with CO2 in the presence of Ph2SiH2. The catalytic system had high catalytic activity and selectivity for aliphatic, primary and secondary amines, and aromatic amines. At the same time, the catalytic system can tolerate some reducible functional groups such as alkenyl (giving N-allyl-N-butylformamide 78), alkyne (giving N-butyl-N-(prop-2-yn-1-yl)formamide 79), and carbonyl (giving methyl N-butyl-N-formylglycinate 80). Generally, amines with large steric hindrance have relatively low activity such as 59 (60% yield). For aniline and its derivatives, the activity of substrates attached electron-withdrawing groups is lower than that of substrates attached electron-donating groups such as 18 and 15 (Scheme 20). Although reductive amidation of CO2 was achieved at lower catalyst loads (0.1 mol%) and lower temperatures (25 °C), a relatively high pressure (25 atm) was required.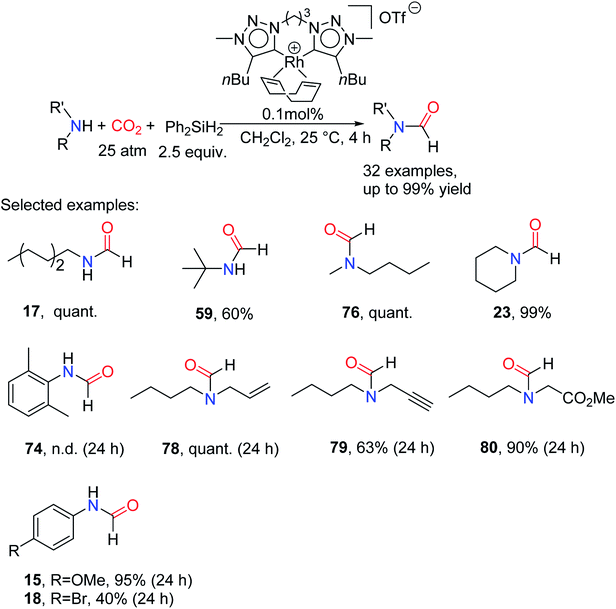 | ||
| Scheme 20 Bis(tzNHC) Rhodium complexes catalytic reduction of CO2 to formamides using amines and Ph2SiH2.93 | ||
4.5 Zn-based complex
In 2015, Yang and co-workers94 designed and synthesized a fluoro-functionalized polymeric NHC–Zn complex (F–PNHC–Zn) as a stable catalyst for the formylation of amines with CO2 and PhSiH3. N-Methylaniline was used as a substrate to study its application range, and corresponding formylation products were obtained in medium yields for amines attached with electron-withdrawing groups (Scheme 21).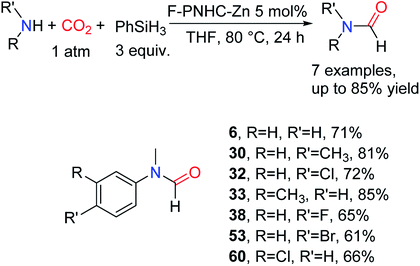 | ||
| Scheme 21 F–PNHC–Zn catalytic reduction of CO2 to formamides using amines and PhSiH3.94 | ||
The Lewis base-transition metal center (LB-TM) catalytic system could activate H2 effectively.95 In 2017, Luo and coworkers96 designed and synthesized an interesting catalyst system that integrates ILs [BMIm]Br, tetrabutylamine bromide (TBAB) and salen transition metal complex Zn(salen) into the binary catalytic system (Fig. 3), because the chiral ligands of large rings have proven to have the stronger electron-donating capacity.97,98
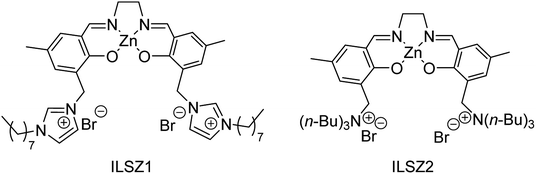 | ||
| Fig. 3 The catalysts consist of different ionic liquids and Zn(salen).96 | ||
The synergies between LB and transition metal center activate the hydrosilane and promote reductive functionalization of CO2 to formylated products in the presence of amines. Moreover, the synergistic effect between the two has experimentally proved that Zn(salen) and 1.0 mol% TBAB were used alone to induce the N-formylation of substrate 36 at 40 °C and CO2 pressure 1.5 MPa for 24 h, and the formylated product 6 was obtained in the yield of ca. 37% and 80%, respectively (Fig. 4A). However, at the temperature of 25 °C and CO2 pressure of 0.5 MPa, the single-component catalyst was basically inactive, but the two-component catalyst consisting of Zn(salen) and TBAB could afford the formylated product 6 in 99% yield after 7 h. The reaction could be finished after 3 h by increasing the temperature to 40 °C and the CO2 pressure to 1.5 MPa (Fig. 4B). It's worth noting that the catalytic system could be reused for 5 times without loss of reactivity.96
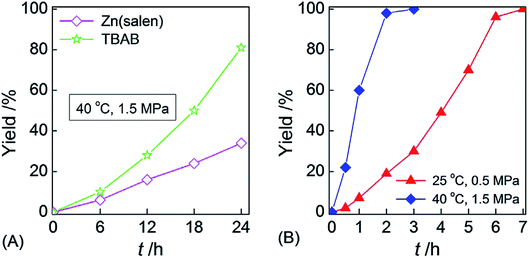 | ||
| Fig. 4 Kinetic curves from the N-formylation reaction of N-methylaniline, CO2, and PhSiH3 catalyzed by the (A) one-component Zn(salen) or (B) Zn-(salen)/TBAB bicomponent catalyst. Reaction conditions: 10 mL stainlesssteel autoclave, N-methylaniline (1.0 mmol), PhSiH3 (1.0 mmol), Zn(salen) (0.5 mol%), TBAB (1.0 mol%), CO2 pressure (0.5 MPa or 1.5 MPa), reaction temperature (25 °C or 40 °C).96 | ||
Both ILSZ1 and ILSZ2 are efficient for a wide range of amines, including aliphatic/aromatic primary and secondary amines, hydrazine (giving 62), and imines (giving 3), and can tolerate the carbonyl group (giving 46).96 However, during the formylation of primary amines, the presence of bisformylation led to a decrease in the selectivity of the reaction such as 16′, 20′, and 35′. Aniline and its derivatives could afford the formylated products in moderate to excellent yields, in addition to incomplete formylation of the substrate 11. Moreover, when the steric hindrance of substrate increased, the reaction could not take place, generally unable to give e.g., the formylated product 70 (Scheme 22).
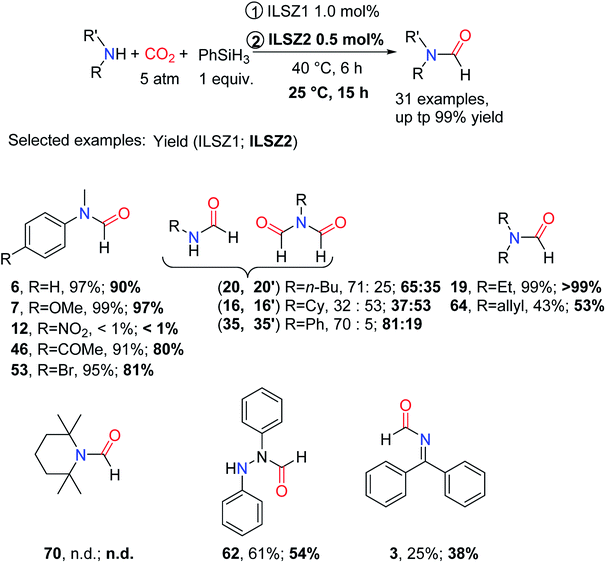 | ||
| Scheme 22 ILSZ1 and ILSZ2 catalytic reductive amidation of CO2 using amines and PhSiH3.96 | ||
4.6 Mn-based complex
In 2019, the group of Li reported the formation of Mn-based complex with ligand 2-(dicyclohexylphosphaneyl)-9-(pyridin-2-yl)-9H-carbazole (Fig. 2, L14) and Mn as the catalyst for reductive amidation of CO2.99 Interestingly, double and mono N-formylation of primary arylamines with CO2 and PhSiH3 could be obtained selectively by changing the experimental parameters (Scheme 23).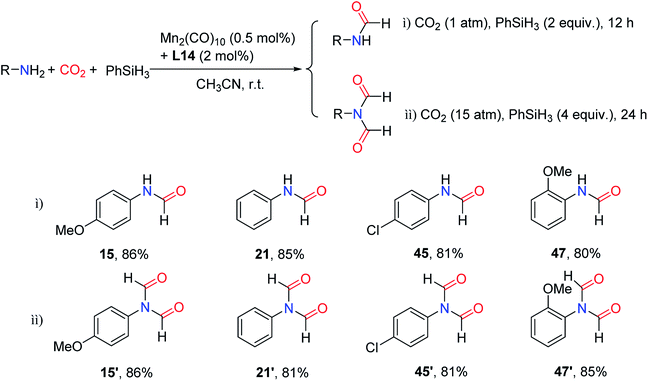 | ||
| Scheme 23 Mn-based complex catalytic reduction of CO2 to formamides using amines and PhSiH3.99 | ||
5 Solvent promoted N-formylation of amines with CO2
5.1 Dimethylsulfoxide (DMSO)
Cantat and co-workers28 reported the reaction of formylation of amine and CO2 catalyzed by NHCs, and proved that the nucleophilicity and basicity of NHCs played a key role in activating the Si–H bond of the hydrosilane to promote the reductive amidation of CO2. Considering that amines are also nucleophilic and basic, it may be possible to activate the Si–H bond in a similar way to the NHCs. But the problem is that nucleophilicity and basicity of amines are not enough to activate the Si–H bond. According to reports, solvents can regulate the nucleophilicity and alkalinity of amines.100,101 Therefore, In 2016, Lv and co-workers102 reported that without any catalyst, the effect of different solvents on the formylation of an amine with CO2 and PhSiH3 at room temperature. An experiment using 25 as a model substrate found that the formylated product 1 was obtained quantitatively in the polar aprotic solvent such as DMF and DMSO. For less polar or no polar solvents, the reaction cannot occur successfully. The effect of amine on hydrosilane was mainly influenced by the polarity of the solvent (Table 10).102It is worth noting that the simple solvent catalytic system could be widely applied for the formylation of aliphatic/aromatic primary and secondary amines and hydrazine (giving N′-phenylformohydrazide 84).102 In primary amines, monoformylated products were obtained selectively and quantitatively. In aniline and its derivatives, substrates with C4-attached electron-donating and electron-withdrawing groups could also afford corresponding formylated products 15 and N-(4-fluorophenyl)formamide 83, respectively (Scheme 24).102
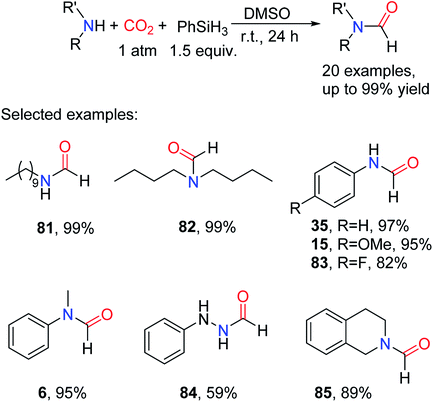 | ||
| Scheme 24 DMSO promoted the reduction of CO2 to formamides using amines and PhSiH3.102 | ||
5.2 γ-Valerolactone (GVL)
Although many efficient catalytic systems have been reported, there is no doubt that the development of a simple, efficient, non-toxic and renewable catalytic system for the formylation of amines and CO2 to produce methylamine is still very important. At present, the use of bio-derived compounds to achieve sustainable catalysis is a hot field.103–106 GVL as a bio-derived compound has the advantages of low toxicity, biodegradability, low steam, and widely existent in fruit.107,108 In 2016, Song and co-workers109 reported the use of PhSiH3 as a reducing agent in the presence of amine for the GVL-promoted the CO2 reductive functionalization to afford formylated products. The catalytic system is suitable for most aliphatic/aromatic primary and secondary amines. In the product distribution of primary amine, although there are also diformylated products N-formyl-N-phenylformamide 35' and N-formyl-N-octylformamide 51', monoformylated products 34 and N-octylformamide 51 were the main products. For aniline and its derivatives, the reaction activity could be improved by attaching the electron-donating groups in C4 of the aromatic ring. It is necessary to extend the reaction time to obtain the ideal yield or the reaction is not performed when the electron-withdrawing group is connected in C4 of the aromatic ring (Scheme 25).109 However, relatively high CO2 pressures (30 atm) were required in this simple catalytic system.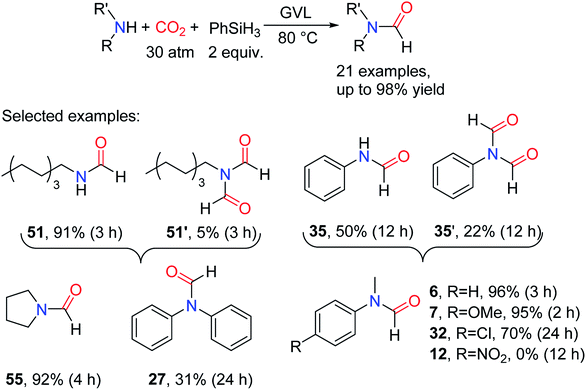 | ||
| Scheme 25 GVL-promoted the reduction of CO2 to formamides using amines and PhSiH3.109 | ||
Solvent play a vital role in the process of reductive amidation as well, in the presence of catalysts. It can effectively promote the generation and separation of carbaminate, thus increasing the nucleophilicity of carbaminate to improve the reaction activity of the catalytic systems.110
6 The influencing factors of the N-formylation of amine and CO2
6.1 Effect of the catalyst basicity
Among the reductive amidation of CO2 catalytic systems reported at present, the base catalytic system is the main one. NHCs can catalyze a wide range of amines to afford desired formylated products in good to excellent yields owing to its strong basicity. So the basicity of the catalyst has a great influence on the reaction. Cantat et al. demonstrated that the catalytic activity of the IPr was significantly higher than that of the Cl substituted IPr (Cl2–IPr), and the decreased reactivity was due to lower basicity.28 The same results were also observed in the TBD catalytic system (Table 11).16In IL and salt catalytic systems, Hao et al.51 demonstrated that the driving force of catalytic activity was mainly anion. The catalytic activity sequence of different halogen anions was I− < Br− < Cl− < F−. The higher basicity the more enhanced the catalytic activity (Table 12).
Dyson et al.111 expressed the basicity of the catalyst by the conjugate acid pKa of the catalyst and proved that the basicity of the catalyst had an important influence on the reaction. In the formylation of N-methylaniline, the pKa value of the catalytic system was between 4–7.5 and the reaction activity increased linearly. The catalytic system with pKa of 4.0 had no reactivity, while that with pKa of greater than 7.5 was almost unchanged (Fig. 5). Therefore, before designing an effective catalytic system, the basicity of the catalyst should not be neglected.
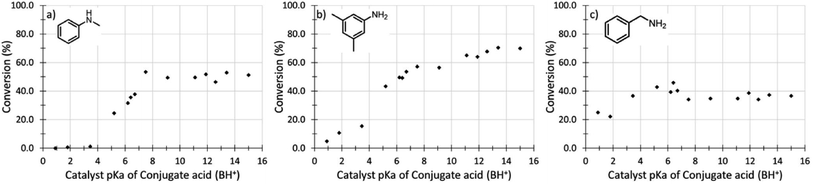 | ||
| Fig. 5 The dependency of reaction yield on the pKa of the catalyst in DMSO in the N-formylation of (a) N-methylaniline, (b) 3,5-dimethylaniline, and (c) benzylamine.111 | ||
6.2 Effect of the hydrosilane as reductant
Hydrosilane is an effective reducing agent for the formylation of the amine with CO2, so the effects of different hydrosilanes on the reaction of reverse reductive functionalization of CO2 to formylation products were briefly summarized. Hydrosilane completely replaced by alkyl or phenyl groups showed lower reduction activity (Entries 1 and 2, Table 13). Secondly, oxosilane showed moderate reducing activity. Cantat and co-workers28 reported that oxysilane PMHS used as reducing agent for reductive amidation of CO2 could afford formylated product 1 in 90% yield (Entries 6, 1a, Table 13). Meanwhile, He and co-workers112 also reported ((EtO)3SiH) as a reducing agent for the synthesis of formylated product 1 (86% yield). In addition, our research group67 and Cantat et al.16 also obtained the same results (Entries 4, 6a, Table 13). So far, PhSiH3 shows the strongest reducibility in most reported catalytic systems to promote reductive amidation of CO2. Our research group67 found that the reduction activity of Et3SiH was lower than (EtO)3SiH, indicating that the electronic effect had an important influence on the reductant. In the phenyl substituted hydrosilane, the trend order of reducing activity was PhSiH3 > Ph2SiH2 > Ph3SiH, indicating that the steric hindrance of hydrosilane was high to reduce its reduction activity.6.3 Effect of the reaction temperature and CO2 pressure
In the formylation of the amine with CO2 and hydrosilane, the reaction temperature and CO2 pressure are the important factors influencing the reaction, because the control of the two factors can selectively give formylation products. In 2014, Cantat and co-workers21 first reported that different reductive degrees of formylation products are obtained by different reaction temperatures, and the low temperature can selectively provide formylation products. Dyson and co-workers33 also obtained formylated products at a lower temperature of 50 °C, while the methylated product was obtained when the temperature rose to 100 °C. Our research group also reached this conclusion (Table 14).67 Another possible consequence of high temperatures is to affect the solubility of CO2.In 2018, He and co-workers82 reported that the formylation products were obtained by controlling CO2 pressure at a higher CO2 pressure of 2 MPa. Due to the increase of CO2 concentration at a higher pressure, the complete consumption of reducing agent hydrosilanes can stop the CO2 reduction at the formylation stage (Table 15). Therefore, low temperature and high pressure are conducive to reductive amidation of CO2.
7 Mechanism of the N-formylation of amines with CO2
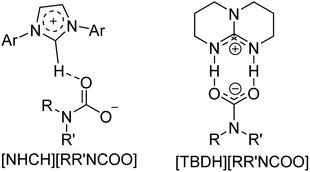
To increase the application range of CO2 and develop more efficient and sustainable catalytic systems, the mechanism of the N-formylation of amines with CO2 catalyzed by a large number of organic catalysts has been reported. Li and Zhou found that the real role of NHCs in catalyzing reductive amidation of CO2 was neither the mode of activating Si nor the mode of activating CO2. One of the most likely activation modes, the “Sn2@Si-acceptor”, was proposed.113 The reaction first forms NHC–CO2 adduct, and in the presence of amine, carbamate is generated and stabilized to produce carbamate [NHCH][RRNCOO] by catalyst NHCs or polar solvent, and then activated to reduce CO2 to generate intermediate formoxysilane and finally react with the amine to get the product. DFT calculation by Wang and Cao114 also proved the existence of this intermediate and transition state. When the organic superbase TBD was first applied to the formylation of CO2, the possible mechanism was also extensively reported. Cantat et al. also reported the generation of [TBDH] [RRNCOO],16 and the DFT calculation110 showed that the activation of hydrosilane to reduce CO2 by [TBDH] [RRNCOO] was more favorable. By comparing the actions of the two catalysts, it is found that the same is that both of them can stabilize the newly formed carbamate as a base-catalyzed reaction. The only experiment that is not consistent with this result is that the CO2 reduction rate is reduced when N,N′-diphenylthiourea as a co-catalyst.40 However, the true behavior of N,N′-diphenylthiourea in the TMG catalytic system has not been reported (Fig. 6).It is a fact that TBAF can activate the hydrosilicone in carbonyl reduction.100,101 TBAF was used as a catalyst for reductive amidation of CO2, and the possible mechanisms were also proposed (Scheme 26).52 Salt mainly attacks hydrosilanes by nucleophilic anions to make CO2 embedded in the Si–H bond of hydrosilanes, thus achieving reduction purpose. Most salts include TBAF, Cs2CO3, GB, and various carboxylates in this way.
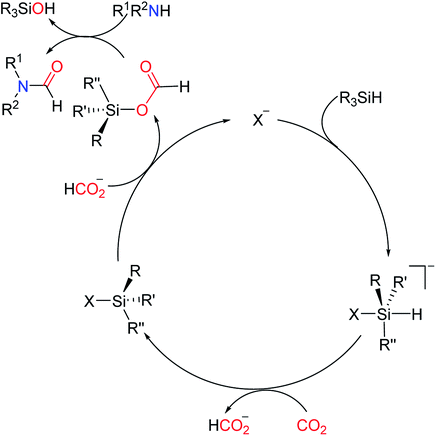 | ||
| Scheme 26 N-Formylation reaction with a TBAF catalyst.52 | ||
In 2018, Dyson and co-workers reported the mechanistic study of N-formylation with ILs [TBA][OAc] catalyst, and three possible pathways were proposed to depend on the nucleophilic of the substrate and reaction conditions (Scheme 27). Pathway 1 favors other paths (pathway 2, 3) when the substrate is non-nucleophilic amines in the presence of a catalyst, and the formoxysilane intermediate can be obtained. Pathway 2 is the process of amine-assisted formoxysilane intermediate formation. And the catalyst is used as a base to stabilize in situ generated carbaminate. Pathway 3 is more favorable when the substrates are strongly nucleophilic amines. And the silylcarbamate intermediate can be obtained. In the presence of the excess amount of hydrosilane, the silylcarbamate intermediate can directly afford formylated products. Without the excess amount of hydrosilane, silylcarbamate can afford formoxysilane intermediate. At the same time, the amine/CO2/carbamate/carbonate equilibrium is established.115 In pathway 2 and 3, a base catalyst to stabilize in situ generated carbaminate. The action of ILs catalyst is similar to that of an organic catalyst.
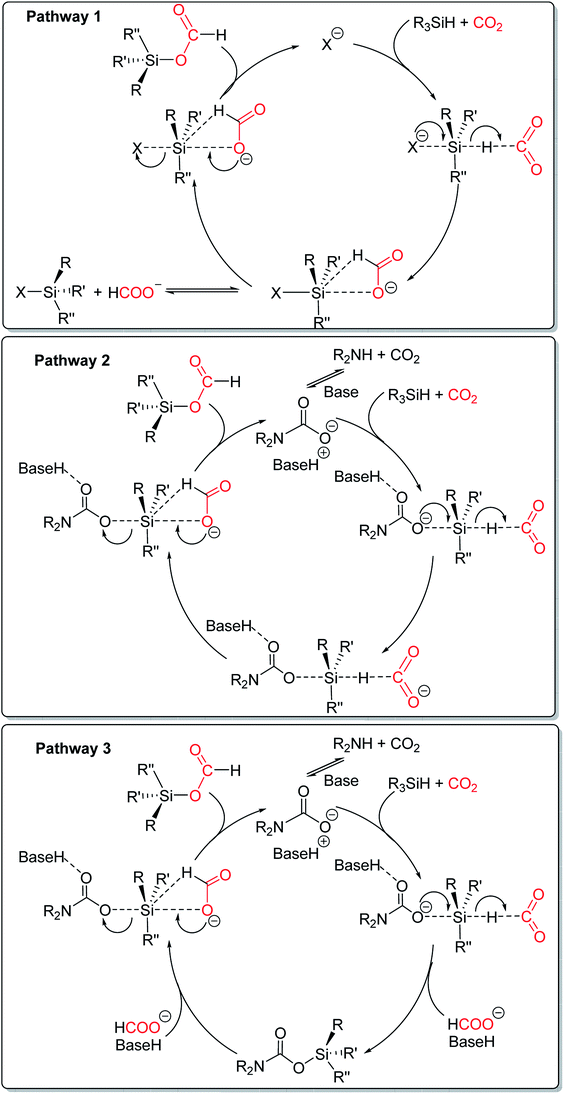 | ||
| Scheme 27 Three possible pathways for the formylation of amines with CO2.115 | ||
Generally, strong acid anions are not basic enough to support the formation of carbaminate, while can activate the hydrosilane by attacking hydrosilane directly as a nucleophile (like pathway 1). Therefore, the activation of hydrosilane by carbamate is more popular than other nucleophiles as long as a sufficiently alkaline catalyst is present in the reaction, and generally has better reactivity as a base catalyst than as a nucleophile.111 In ILs and salt catalytic systems, ion pairing can have a detrimental effect on the catalytic activity. Dyson and coworkers combined ion pairing energies with nucleophilicity and basicity data on the anion, to obtain structure–activity relationships between the salt catalyst and its catalytic activity and optimize the N-formylation reaction.111
In the study of the catalytic mechanism of inorganic salts, it was found that the anions of salts seemed to activate hydrosilanes as nucleophiles.77 However, the cations of salts did not participate in the reaction but may affect the solubility of catalysts. Based on already built amine/CO2/carbamate/carbonate equilibrium, carbaminate may also be formed in the process of inorganic salt catalytic reductive amidation of CO2.
From the distribution of products in the catalytic systems, it is found that in reductive amidation of CO2, both the monoformylation products and diformylated products are obtained. At the same time, when considering the effect of temperature on the reaction process, it can be found that methylated products are mainly obtained at high temperatures. It is indicated that side reactions such as bisformylation and N-methylation often occur in the reductive amidation of CO2. Based on the previous studies, the overall reaction mechanism is summarized in Scheme 28.
However, in the 1,3,2-diazaphospholene catalytic system, the intermediate formoxysilane is given in a different way. The catalytic system is different from the metal-free catalysis. The CO2 first forms NHPOCOH with the catalyst, which has ionic properties, rather than the NHC–CO2 adducts formed at the initial stage of the reaction as in the NHCs catalytic system. Then, in the presence of hydrosilane, the main intermediate of N-formylation formic acid derivative Ph2Si(OCHO)2 was formed. Because the Si–O bond was more stable than the P–O bond, the amine hydrolysis resulted in the formation of amides, and the possible reaction mechanism was proposed (Scheme 29).37
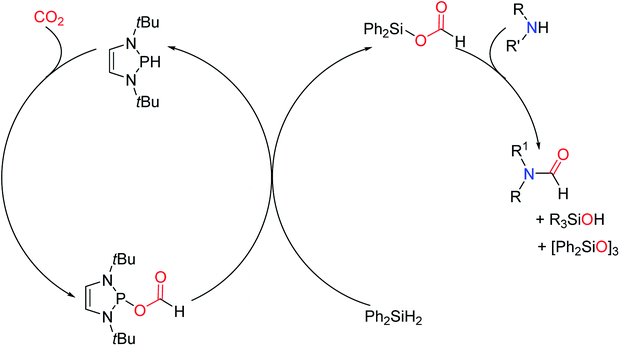 | ||
| Scheme 29 The mechanism of the 1,3,2-diazaphospholene catalytic N-formylation of amines with CO2 and Ph2SiH2.37 | ||
The catalytic mechanism of transition metal complexes has also been studied. In 2015, Nguyen and co-workers93 applied Rh-based complex to catalyze reductive amidation of CO2, and the possible reaction mechanism was proposed (Scheme 30). First, the auxiliary ligand is dissociated, and then the rhodium catalyst is oxidized and adducted with Ph2SiH2 to form a reductive silica-based rhodium intermediate. This intermediate then promotes the reduction of CO2 hydrogenation to the rhodium silyl formate. Finally, a simple addition elimination reaction is performed to obtain the desired formylation product.
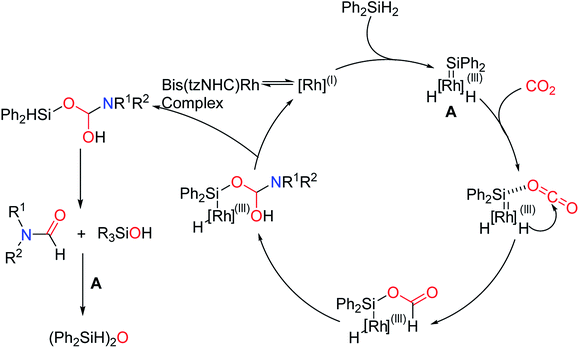 | ||
| Scheme 30 The mechanism of the Rh-based complex catalytic N-formylation of amines with CO2 and Ph2SiH2.93 | ||
Later in 2016, the mechanism of Cu-based complex catalytic N-formylation of amines was also proposed by the group of Han (Scheme 31).20 The mode of activation of hydrosilane by transition metal complexes is different from that of organic catalysts, ILs, and salt catalytic systems. At the initial stage of the reaction, M–H complex83,84 with reducing capacity is formed. The CO2 is then embedded into the activated Si–H bond to form cuprum silyl formate, and finally undergoes a simple addition elimination reaction to produce a formylated product in the presence of an amine.
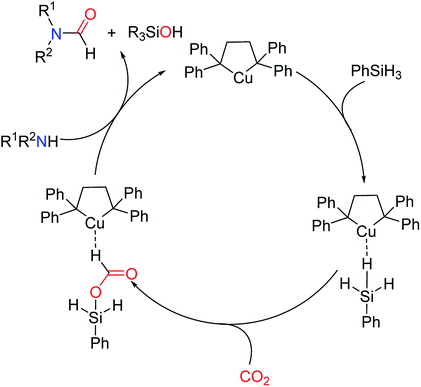 | ||
| Scheme 31 The mechanism of the Cu-based complex catalytic N-formylation of amines with CO2 and PhSiH3.20 | ||
8 Conclusions and perspectives
In this review, four catalytic systems, including organic, IL and salt, transition metal complex, and solvent catalysis have been reviewed. The biggest advantage of the organic catalytic system is that it can avoid the pollution caused by metals and can be widely applied to various amines, such as NHC, so it can be used as an alternative and relatively green method to promote the reductive functionalization of CO2. IL and salt catalytic systems are considered to be outstanding catalytic systems. Firstly, such catalytic systems can promote the reductive formylation of CO2 by using simple salts. Secondly, the performance of the catalytic system is adjustable, and the efficient catalyst can be designed according to the interaction between anions and cations in the catalyst. Finally, the reductive formylation of CO2 can be achieved by internal salt structure lecithin, GB, and biological derivative acetylcholine. Sustainable catalysis by natural products and derivatives is a hot research field at present. With these three advantages, this kind of catalytic system has great potential. As for transition metal complex catalytic systems, the use of metals and toxic ligands limits the development of such catalytic systems, leading to organic catalytic systems as an alternative to such catalytic systems. However, it is interesting to note that a two-component catalytic system with IL and metal complex can facilitate reductive formylation of CO2 at a lower load due to the synergistic effect between the two. It is proved that this kind of catalytic system also has a certain development prospect. As the solvent promoted CO2 reductive formylation is the simplest catalytic system, it is of great significance to develop environmentally friendly solvent catalyzed reductive functionalization of CO2.The applications of various catalytic systems are summarized and found that larger space steric hindrance of the substrate showed lower activity, while secondly lean electronic substrate against reaction is due to weak alkaline. In aniline and its derivatives, once the substrates attached to the electron-absorbing group are less active than those attached to the electron-giving group, especially substrates with para-nitro-bonded substrates have low or no activity in many catalytic systems. The formylation of primary amine is usually accompanied by the presence of bisformylation. The general mode of action of catalysts follows the base catalysis mode. The main influencing factors of the reaction were emphasized and it was found that low temperature and high pressure could selectively produce formylation products.
Although many excellent catalytic systems have been reported, there are still some deficiencies in these catalytic systems:
(i) For organic catalytic systems, the selectivity for primary amines is low due to the presence of diformylation. In addition, high load and long reaction time are often needed in the catalytic process. It is necessary to design catalytic systems with renewed modes for activation of hydrosilane to break these limits.
(ii) For ILs and salt catalysis systems, the design and preparation of ILs are complex. However, if simple and efficient ILs based on natural products or biological derivatives can be designed, it will be more conducive to the sustainable transformation of CO2.
(iii) Leaching of metal species the use of toxic ligands may cause serious environmental issues that should be taken into consideration for transition metal complex catalytic systems. Therefore, catalyst stability and reusability should be considered.
(iv) The extensive use of toxic solvents (e.g., DMF) in catalytic systems will also cause great damage to the environment. It is necessary to develop environmentally friendly solvents to promote the formylation of amines with CO2.
List of abbreviations
| ILs | Ionic liquids |
| NHCs | N-heterocyclic carbenes |
| NHC | N-heterocyclic carbene |
| IPr | 1,3-Bis(2,6-diisopropylphenyl)-2,3-dihydro-1H-imidazole |
| PMHS | Polymethylhydrosiloxane |
| TMDS | Tetramethyldisiloxane |
| aNHC | Abnormal N-heterocyclic carbene |
| DMA | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| THF | Tetrahydrofuran |
| DMSO | Dimethylsulfoxide |
| GVL | γ-Valerolactone |
| LA | Lewis acid |
| LB | Lewis base |
| NHP-H | 1,3,2-Diazaphospholene |
| TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene |
| Me-TBD | 1-Methyl-1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine |
| DMAP | N,N-Dimethylpyridin-4-amine |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-en |
| TMG | N,N,N,N-Tetramethylguanidine |
| BTMG | 2-Butyl-1,1,3,3-tetramethylguanidine |
| BMIm | 1-Butyl-3-methylimidazolium |
| [BMIm]Cl | 1-Butyl-3-methylimidazolium chloride |
| [BMIm]Br | 1-Butyl-3-methylimidazolium bromine |
| [HMIm]Cl | 1-Hexyl-3-methylimidazolium chloride |
| [BdMIm]Cl | 1-Butyl-2,3-dimethylimidazolium chloride |
| [BMP]Cl | 1-Butyl-1-methylpyrrolidinium chloride |
| TBA | Tetrabutylammonium |
| TBAF | Tetrabutylammonium fluoride |
| TBAC | Tetrabutylammonium chloride |
| TBAB | Tetrabutylammonium bromine |
| TBAI | Tetrabutylammonium iodide |
| GB | Glycine betaine |
| ACH-AA | Acetylcholine-carboxylate |
| L1 | 1,2-Bis-(diisopropylphosphino)-benzene |
| L2 | 1,2-Bis(diphenylphosphaneyl)benzene |
| L3 | 1,2-Bis(dicyclohexylphosphaneyl)benzene |
| L4 (dppp) | 1,3-Bis(diphenylphosphaneyl)propane |
| L5 (Ph3P) | Triphenylphosphane |
| L6 (dppm) | Bis(diphenylphosphaneyl)methane |
| L7 (dppb) | 1,2-Bis(diphenylphosphaneyl)benzene |
| L8 (dppe) | 1,2-Bis(diphenylphosphaneyl)ethane |
| L9 (Cy3P) | Tricyclohexylphosphane |
| L10 (xantphos) | (9,9-Dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane) |
| L11 (TPPi) | Triphenoxymethane |
| L12 (dppbt) | 1,4-Bis(diphenylphosphaneyl)butane |
| L13 (PP3) | Tris[2-(diphenylphosphino)ethyl]phosphine |
| L14 | 2-(Dicyclohexylphosphaneyl)-9-(pyridin-2-yl)-9H-carbazole |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl |
| LB-TM | Lewis base-transition metal center |
| [TBA][OAc] | Tetra-N-butylammonium |
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (21666008, 21908033, 21576059), Fok Ying-Tong Education Foundation (161030), Guizhou Science & Technology Foundation ([2018]1037), Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023), and Chinese State Scholarship (201906670009).References
- C. Figueres, C. Le Quéré, A. Mahindra, O. Bäte, G. Whiteman, G. Peters and D. Guan, Nature, 2018, 564, 27–30 CrossRef CAS PubMed.
- T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS.
- H. Li, T. Yang and Z. Fang, Appl. Catal., B, 2018, 227, 79–89 CrossRef CAS.
- J. He, H. Li, A. Riisager and S. Yang, ChemCatChem, 2018, 10, 430–438 CrossRef CAS.
- H. Li, W. Zhao, A. Riisager, S. Saravanamurugan, Z. Wang, Z. Fang and S. Yang, Green Chem., 2017, 19, 2101–2106 RSC.
- H. Li, X. Liu, T. Yang, W. Zhao, S. Saravanamurugan and S. Yang, ChemSusChem, 2017, 10, 1761–1770 CrossRef CAS PubMed.
- H. Li, Z. Fang, J. He and S. Yang, ChemSusChem, 2017, 10, 681–686 CrossRef CAS PubMed.
- X. Liu, H. Li, H. Pan, H. Zhang, S. Huang, K. Yang, W. Xue and S. Yang, J. Energy Chem., 2016, 25, 523–530 CrossRef.
- H. Li, Q. Zhang, P. S. Bhadury and S. Yang, Curr. Org. Chem., 2014, 18, 547–597 CrossRef CAS.
- H. Li, X. He, Q. Zhang, F. Chang, W. Xue, Y. Zhang and S. Yang, Energy Technol., 2013, 1, 151–156 CrossRef CAS.
- H. Zhang, H. Li, H. Pan, A. Wang, S. Souzanchi, C. Xu and S. Yang, Appl. Energy, 2018, 223, 416–429 CrossRef CAS.
- H. Pan, H. Li, X.-F. Liu, H. Zhang, K.-L. Yang, S. Huang and S. Yang, Fuel Process. Technol., 2016, 150, 50–57 CrossRef CAS.
- H. Li, Z. Fang and S. Yang, Chempluschem, 2016, 81, 135–142 CrossRef CAS.
- H. Zhang, Q. Zhou, F. Chang, H. Pan, X.-F. Liu, H. Li, D.-Y. Hu and S. Yang, Ind. Crops Prod., 2015, 76, 768–771 CrossRef CAS.
- F. Chang, M. A. Hanna, D. J. Zhang, H. Li, Q. Zhou, B. A. Song and S. Yang, Bioresour. Technol., 2013, 140, 435–438 CrossRef CAS.
- C. Das Neves Gomes, O. Jacquet, C. Villiers, P. Thuery, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187–190 CrossRef CAS.
- L. Schmid, A. Canonica and A. Baiker, Appl. Catal., A, 2003, 255, 23–33 CrossRef CAS.
- S. Kumar and S. L. Jain, RSC Adv., 2014, 4, 64277–64279 RSC.
- Q. Shen, X. Chen, Y. Tan, J. Chen, L. Chen and S. Tan, ACS Appl. Mater. Interfaces, 2019, 11, 38838–38848 CrossRef CAS.
- S. Zhang, Q. Mei, H. Liu, H. Liu, Z. Zhang and B. Han, RSC Adv., 2016, 6, 32370–32373 RSC.
- X. Frogneux, O. Jacquet and T. Cantat, Catal. Sci. Technol., 2014, 4, 1529–1533 RSC.
- B. Yu, Z. Yang, Y. Zhao, L. Hao, H. Zhang, X. Gao, B. Han and Z. Liu, Chemistry, 2016, 22, 1097–1102 CrossRef CAS.
- B. Yu, Y. Zhao, H. Zhang, J. Xu, L. Hao, X. Gao and Z. Liu, Chem. Commun., 2014, 50, 2330–2333 RSC.
- Y. Zhang, T. Zhang and S. Das, Green Chem., 2020, 22, 1800–1820 RSC.
- X.-F. Liu, X.-Y. Li and L.-N. He, Eur. J. Org. Chem., 2019, 2437–2447, DOI:10.1002/ejoc.201801833.
- R. A. Pramudita and K. Motokura, Green Chem., 2018, 20, 4834–4843 RSC.
- A. Tlili, E. Blondiaux, X. Frogneux and T. Cantat, Green Chem., 2015, 17, 157–168 RSC.
- O. Jacquet, C. Das Neves Gomes, M. Ephritikhine and T. Cantat, J. Am. Chem. Soc., 2012, 134, 2934–2937 CrossRef CAS PubMed.
- N. J. Lawrence, M. D. Drew and S. M. Bushell, J. Chem. Soc., Perkin Trans. 1, 1999, 3381–3391, 10.1039/a903662h.
- P. K. Hota, S. C. Sau and S. K. Mandal, ACS Catal., 2018, 8, 11999–12003 CrossRef CAS.
- H. Lv, W. Wang and F. Li, Chem.–Eur. J., 2018, 24, 16588–16594 CrossRef CAS.
- S. N. Riduan, J. Y. Ying and Y. Zhang, J. Catal., 2016, 343, 46–51 CrossRef CAS.
- S. Das, F. D. Bobbink, S. Bulut, M. Soudani and P. J. Dyson, Chem. Commun., 2016, 52, 2497–2500 RSC.
- S. E. O'Toole, C. A. Rose, S. Gundala, K. Zeitler and S. J. Connon, J. Org. Chem., 2011, 76, 347–357 CrossRef PubMed.
- F. D. Bobbink, S. Das and P. J. Dyson, Nat. Protoc., 2017, 12, 417–428 CrossRef CAS.
- I. Purushothaman, S. De and P. Parameswaran, RSC Adv., 2014, 4, 60421–60428 RSC.
- C. C. Chong and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 12116–12120 CrossRef CAS.
- G. Li, J. Chen, D.-Y. Zhu, Y. Chen and J.-B. Xia, Adv. Synth. Catal., 2018, 360, 2364–2369 CrossRef CAS.
- R. Nicholls, S. Kaufhold and B. N. Nguyen, Catal. Sci. Technol., 2014, 4, 3458–3462 RSC.
- R. L. Nicholls, J. A. McManus, C. M. Rayner, J. A. Morales-Serna, A. J. P. White and B. N. Nguyen, ACS Catal., 2018, 8, 3678–3687 CrossRef CAS.
- J. Peng and Y. Deng, New J. Chem., 2001, 25, 639–641 RSC.
- F. Shi, Y. Deng, T. SiMa, J. Peng, Y. Gu and B. Qiao, Angew. Chem., Int. Ed., 2003, 42, 3257–3260 CrossRef CAS.
- Z. Zhang, Y. Xie, W. Li, S. Hu, J. Song, T. Jiang and B. Han, Angew. Chem., Int. Ed., 2008, 47, 1127–1129 CrossRef CAS.
- W. Lu, J. Ma, J. Hu, J. Song, Z. Zhang, G. Yang and B. Han, Green Chem., 2014, 16, 221–225 RSC.
- L. Wu, Q. Liu, I. Fleischer, R. Jackstell and M. Beller, Nat. Commun., 2014, 5, 3091 CrossRef.
- Y. Zhao, B. Yu, Z. Yang, H. Zhang, L. Hao, X. Gao and Z. Liu, Angew. Chem., Int. Ed., 2014, 53, 5922–5925 CrossRef CAS PubMed.
- P. A. Futreal, C. Cochran, J. Rosenthal, Y. Miki, J. Swenson, M. Hobbs, L. M. Bennett, A. Haugen-Strano, J. Marks, J. C. Barrett, S. V. Tavtlglan, D. Shattuck-Eldens, A. Kamb, M. Skolnick and R. W. Wiseman, Hum. Mol. Genet., 1994, 3, 1359–1364 CrossRef CAS.
- M. C. Corvo, J. Sardinha, S. C. Menezes, S. Einloft, M. Seferin, J. Dupont, T. Casimiro and E. J. Cabrita, Angew. Chem., Int. Ed., 2013, 52, 13024–13027 CrossRef CAS.
- A.-L. Girard, N. Simon, M. Zanatta, S. Marmitt, P. Gonçalves and J. Dupont, Green Chem., 2014, 16, 2815–2825 RSC.
- M. Zanatta, A.-L. Girard, N. M. Simon, G. Ebeling, H. K. Stassen, P. R. Livotto, F. P. dos Santos and J. Dupont, Angew. Chem., Int. Ed., 2014, 53, 12817–12821 CrossRef CAS.
- L. Hao, Y. Zhao, B. Yu, Z. Yang, H. Zhang, B. Han, X. Gao and Z. Liu, ACS Catal., 2015, 5, 4989–4993 CrossRef CAS.
- M. Hulla, F. D. Bobbink, S. Das and P. J. Dyson, ChemCatChem, 2016, 8, 3338–3342 CrossRef CAS.
- X. F. Liu, R. Ma, C. Qiao, H. Cao and L. N. He, Chem.–Eur. J., 2016, 22, 16489–16493 CrossRef CAS.
- X. F. Liu, X. Y. Li, C. Qiao, H. C. Fu and L. N. He, Angew. Chem., Int. Ed., 2017, 56, 7425–7429 CrossRef CAS PubMed.
- F. Boissou, K. De Oliveira Vigier, B. Estrine, S. Marinkovic and F. Jérôme, ACS Sustainable Chem. Eng., 2014, 2, 2683–2689 CrossRef CAS.
- Y. Zhou, S. Hu, X. Ma, S. Liang, T. Jiang and B. Han, J. Mol. Catal. A: Chem., 2008, 284, 52–57 CrossRef CAS.
- C. Xie, J. Song, H. Wu, B. Zhou, C. Wu and B. Han, ACS Sustainable Chem. Eng., 2017, 5, 7086–7092 CrossRef CAS.
- M. H. Azizi, N. Rajabzadeh and E. Riahi, LWT--Food Sci. Technol., 2003, 36, 189–193 CrossRef CAS.
- T. T. L. Nguyen, A. Edelen, B. Neighbors and D. A. Sabatini, J. Colloid Interface Sci., 2010, 348, 498–504 CrossRef CAS PubMed.
- J. Song, B. Zhang, P. Zhang, J. Ma, J. Liu, H. Fan, T. Jiang and B. Han, Catal. Today, 2012, 183, 130–135 CrossRef CAS.
- M. J. Hülsey, H. Yang and N. Yan, ACS Sustainable Chem. Eng., 2018, 6, 5694–5707 CrossRef.
- B. F. Szuhaj, J. Am. Oil Chem. Soc., 1983, 60, 306–309 CrossRef CAS.
- Y. Hu, J. Song, C. Xie, H. Wu, Z. Wang, T. Jiang, L. Wu, Y. Wang and B. Han, ACS Sustainable Chem. Eng., 2018, 6, 11228–11234 CrossRef CAS.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2006, 8, 784–786 RSC.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155–1157 RSC.
- A. Zhu, S. Bai, L. Li, M. Wang and J. Wang, Catal. Lett., 2015, 145, 1089–1093 CrossRef CAS.
- W. Zhao, X. Chi, H. Li, J. He, J. Long, Y. Xu and S. Yang, Green Chem., 2019, 21, 567–577 RSC.
- L. C. Tomé, D. J. S. Patinha, R. Ferreira, H. Garcia, C. Silva Pereira, C. S. R. Freire, L. P. N. Rebelo and I. M. Marrucho, ChemSusChem, 2014, 7, 110–113 CrossRef.
- R. A. Molla, P. Bhanja, K. Ghosh, S. S. Islam, A. Bhaumik and S. M. Islam, ChemCatChem, 2017, 9, 1939–1946 CrossRef CAS.
- A. Gopakumar, I. Akçok, L. Lombardo, F. Le Formal, A. Magrez, K. Sivula and P. J. Dyson, ChemistrySelect, 2018, 3, 10271–10276 CrossRef CAS.
- Z.-J. Mu, X. Ding, Z.-Y. Chen and B.-H. Han, ACS Appl. Mater. Interfaces, 2018, 10, 41350–41358 CrossRef CAS PubMed.
- B. Dong, L. Wang, S. Zhao, R. Ge, X. Song, Y. Wang and Y. Gao, Chem. Commun., 2016, 52, 7082–7085 RSC.
- T. Baba, Y. Kawanami, H. Yuasa and S. Yoshida, Catal. Lett., 2003, 91, 31–34 CrossRef CAS.
- W. Xie, M. Zhao and C. Cui, Organometallics, 2013, 32, 7440–7444 CrossRef CAS.
- M. Zhao, W. Xie and C. Cui, Chem.–Eur. J., 2014, 20, 9259–9262 CrossRef CAS PubMed.
- K. Motokura, M. Naijo, S. Yamaguchi, A. Miyaji and T. Baba, Chem. Lett., 2015, 44, 1217–1219 CrossRef CAS.
- C. Fang, C. Lu, M. Liu, Y. Zhu, Y. Fu and B.-L. Lin, ACS Catal., 2016, 6, 7876–7881 CrossRef CAS.
- G. Dijkstra, W. H. Kruizinga and R. M. Kellogg, J. Org. Chem., 1987, 52, 4230–4234 CrossRef CAS.
- R. N. Salvatore, A. S. Nagle and K. W. Jung, J. Org. Chem., 2002, 67, 674–683 CrossRef CAS PubMed.
- D. Nale and B. Bhanage, Synlett, 2016, 27, 1413–1417 CrossRef CAS.
- T. Kimura, K. Kamata and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 6700–6703 CrossRef CAS.
- M.-Y. Wang, N. Wang, X.-F. Liu, C. Qiao and L.-N. He, Green Chem., 2018, 20, 1564–1570 RSC.
- S. Park, D. Bézier and M. Brookhart, J. Am. Chem. Soc., 2012, 134, 11404–11407 CrossRef CAS PubMed.
- W. Sattler and G. Parkin, J. Am. Chem. Soc., 2012, 134, 17462–17465 CrossRef CAS PubMed.
- K. Motokura, N. Takahashi, D. Kashiwame, S. Yamaguchi, A. Miyaji and T. Baba, Catal. Sci. Technol., 2013, 3, 2392 RSC.
- A. Julián, V. Polo, E. A. Jaseer, F. J. Fernández-Alvarez and L. A. Oro, ChemCatChem, 2015, 7, 3895–3902 CrossRef.
- L. González-Sebastián, M. Flores-Alamo and J. J. García, Organometallics, 2013, 32, 7186–7194 CrossRef.
- H. Li, L. C. Misal Castro, J. Zheng, T. Roisnel, V. Dorcet, J. B. Sortais and C. Darcel, Angew. Chem., Int. Ed., 2013, 52, 8045–8049 CrossRef CAS PubMed.
- Y. Sunada, H. Kawakami, T. Imaoka, Y. Motoyama and H. Nagashima, Angew. Chem., Int. Ed., 2009, 48, 9511–9514 CrossRef CAS.
- S. Zhou, K. Junge, D. Addis, S. Das and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 9507–9510 CrossRef CAS.
- J. Pouessel, O. Jacquet and T. Cantat, ChemCatChem, 2013, 5, 3552–3556 CrossRef CAS.
- S. K. U. Riederer, P. Gigler, M. P. Högerl, E. Herdtweck, B. Bechlars, W. A. Herrmann and F. E. Kühn, Organometallics, 2010, 29, 5681–5692 CrossRef CAS.
- T. V. Nguyen, W. J. Yoo and S. Kobayashi, Angew. Chem., Int. Ed., 2015, 54, 9209–9212 CrossRef CAS.
- Z.-Z. Yang, B. Yu, H. Zhang, Y. Zhao, G. Ji and Z. Liu, RSC Adv., 2015, 5, 19613–19619 RSC.
- X. Li, Y. Xue, P. Lv, H. Lin, F. Du, Y. Hu, J. Shen and H. Duan, Soft Matter, 2016, 12, 1655–1662 RSC.
- R. Luo, X. Lin, Y. Chen, W. Zhang, X. Zhou and H. Ji, ChemSusChem, 2017, 10, 1224–1232 CrossRef CAS.
- R. Luo, X. Zhou, S. Chen, Y. Li, L. Zhou and H. Ji, Green Chem., 2014, 16, 1496–1506 RSC.
- R. Luo, X. Zhou, W. Zhang, Z. Liang, J. Jiang and H. Ji, Green Chem., 2014, 16, 4179–4189 RSC.
- Z. Huang, X. Jiang, S. Zhou, P. Yang, C. X. Du and Y. Li, ChemSusChem, 2019, 12, 3054–3059 CrossRef CAS.
- D. H. Aue, H. M. Webb and M. T. Bowers, J. Am. Chem. Soc., 1976, 98, 318–329 CrossRef CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- H. Lv, Q. Xing, C. Yue, Z. Lei and F. Li, Chem. Commun., 2016, 52, 6545–6548 RSC.
- Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 CrossRef CAS.
- J. Song, B. Zhou, H. Zhou, L. Wu, Q. Meng, Z. Liu and B. Han, Angew. Chem., Int. Ed., 2015, 54, 9399–9403 CrossRef CAS PubMed.
- A. R. C. Morais, M. D. D. J. Matuchaki, J. Andreaus and R. Bogel-Lukasik, Green Chem., 2016, 18, 2985–2994 RSC.
- W. Luo, M. Sankar, A. M. Beale, Q. He, C. J. Kiely, P. C. A. Bruijnincx and B. M. Weckhuysen, Nat. Commun., 2015, 6, 6540 CrossRef CAS.
- C. Ortiz-Cervantes, M. Flores-Alamo and J. J. García, ACS Catal., 2015, 5, 1424–1431 CrossRef CAS.
- J. Song, B. Zhou, H. Liu, C. Xie, Q. Meng, Z. Zhang and B. Han, Green Chem., 2016, 18, 3956–3961 RSC.
- C. Zhang, Y. Lu, R. Zhao, W. Menberu, J. Guo and Z.-X. Wang, Chem. Commun., 2018, 54, 10870–10873 RSC.
- M. Hulla, D. Ortiz, S. Katsyuba, D. Vasilyev and P. J. Dyson, Chem.–Eur. J., 2019, 25, 11074–11079 CrossRef CAS.
- X.-F. Liu, C. Qiao, X.-Y. Li and L.-N. He, Green Chem., 2017, 19, 1726–1731 RSC.
- Q. Zhou and Y. Li, J. Am. Chem. Soc., 2015, 137, 10182–10189 CrossRef CAS PubMed.
- B. Wang and Z. Cao, RSC Adv., 2013, 3, 14007 RSC.
- M. Hulla, G. Laurenczy and P. J. Dyson, ACS Catal., 2018, 8, 10619–10630 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |