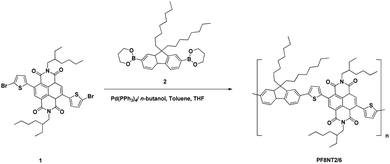
Open Access Article
Open Access ArticleNear-infrared polyfluorene encapsulated in poly(ε-caprolactone) nanoparticles with remarkable large Stokes shift†
Jaruwan Joothamongkona,
Udom Asawapiroma,
Raweewan Thiramanas a,
Kulachart Jangpatarapongsa
a,
Kulachart Jangpatarapongsa b and
Duangporn Polpanich
b and
Duangporn Polpanich *a
*a
aNational Nanotechnology Center, National Science and Technology Development Agency (NSTDA), 111 Thailand Science Park, Phahonyothin Road, Khlong Nueng, Khlong Luang, Pathum Thani 12120, Thailand. E-mail: duangporn@nanotec.or.th
bCenter for Innovation Development and Technology Transfer, Faculty of Medical Technology, Mahidol University, Bangkok-Noi, Bangkok 10700, Thailand
First published on 9th September 2020
Abstract
Near-infrared (NIR) fluorescent dyes have attracted increasing attention as fluorescent probes in biomedical applications due to their low biological autofluorescence as well as high tissue penetration depth. However, their being hydrophobic in nature limits their clinical use as they are prone to aggregate in the physiological environment. Herein, we have designed and synthesized a novel polymeric NIR fluorescent dye and then encapsulated it into a poly(ε-caprolactone) (PCL) matrix by way of an emulsion–diffusion technique. The effect of the structure of the surfactant on the nanoparticle properties is investigated. Results show that polymeric surfactant, Kolliphor® P188, allows the formation of a high fluorescence intensity of the nanoparticles with the highest level homogeneity and stability. The synthesized nanoparticles show significant advantages in terms of a remarkable large stokes shift (276 nm) in the aqueous solution and excellent biocompatibility. The fabrication process is not limited to encapsulation of polymeric fluorescent dye. The synthesized NIR polymeric nanoparticles would be potentially applicable for biomedical applications.
1. Introduction
Near-infrared (NIR) fluorescent dyes hold great promise as imaging and therapeutic agents in biomedical applications. Due to the high sensitivity of fluorescence and minimization of unspecific background signal, this type of fluorescent dye is becoming increasingly more employed, especially in early detection and in vivo monitoring of disease-related pathological changes.1–5 In this, the excitation of dye using NIR light offers the advantages of extremely low absorption and autofluorescence from organic tissue in the NIR spectrum window (650–900 nm), which is favorable in terms of providing an increase in penetration depth of the excitation light.3–6 An ideal NIR fluorophore must exhibit a large Stokes shift for minimum interference between absorption and emission spectral, high molar absorption coefficient and quantum yield for intense fluorescence and good photostability in buffers or biological conditions.7 Chemists continue to design and synthesize a variety of novel NIR dyes, including organic fluorophores such as cyanines, rhodamine analogs, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPYs), squaraines, phthalocyanines, porphyrin derivatives, conjugated polymer and inorganic dyes (quantum dots).7–11 Although the quantum dots principally present interesting alternatives, cytotoxicity and stability concerns, as well as difficulties in the reproducibility of their preparation, need to be resolved.2,11 Compared to inorganic dyes, organic NIR fluorophores display an improvement in photophysical properties and have availability for large-scale chemical synthesis.7,9 However, only a few of them are readily available owing to poor water solubility, which probably brings about dye accumulation in normal organs causing systemic toxicity.Developments of encapsulation of NIR fluorophores into polymeric liposomes or nanoparticles have been extensively studied. This can direct the protection of the dyes from degradation and improve fluorescent signal (a high quantity of dye molecules per nanoparticle), biocompatibility and especially stability in a physiological condition.1,2,12–14 Behnke et al. reported that the encapsulation of hydrophobic cyanine Itrybe dye into amino-modified polystyrene (PS) nanoparticles using a one-step staining procedure yielded a bright labeling probe for in vitro and in vivo sensitive tumor detection.2,15 The presence of an amino group on the surface of particles provides an active site for antibody functionalization to improve tumor targeting efficiency.1,2 Moreover, polymeric nanoparticles loaded with NIR dyes and anticancer agents brought about the integration of imaging and therapeutic functions to achieve the ultimate goal of simultaneous diagnosis and treatment.7
Polyfluorene conjugated polymer and its derivatives, has gained considerable attention due to its excellent thermal and chemical stability, high photostability, and good photoluminescence yield.16,17 However, it displays a short-wavelength photoluminescence which is not preferable for in vivo biomedical applications. Various low-band gap monomer units have been incorporated into the polyfluorene backbone to increase emission wavelengths to become long-wavelengths or even to the near-infrared region.16,18,19 Meng et al. prepared conjugated copolymers comprising alternating fluorene and BODIPY units in the main chain.16 The existence of BODIPY dyes gave rise to significant red shifts in the UV-vis absorption and emission spectral maxima. Later, Piyakulawat et al. alternately copolymerized bis-[2,2′-(5,5′-dibromo)-thienyl]-2,6-naphthalene-1,4,5,8-tetracarboxylic diimide and fluorene monomers through Suzuki type cross-coupling reactions. This led to the formation of alternated copolymers of poly{5,5′-[bis(2,2′-thiophene)-2,6-naphthalene-1,4,5,8-tetracarboxylic-N,N′-dihexyldiimide]-alt-[2,7-(9,9′-dioctyl)fluorene]} (PNATF) and poly{5,5’-[bis(2,2′-thiophene)-2,6-naphthalene-1,4,5,8-tetracarboxylic-N,N′-ditolyldiimide]-alt-[2,7-(9,9′-dioctyl)fluorene]} (PNTTOF).19 The extension of π-conjugation throughout the main chain allowed for the tunable emission spectral maxima of the modified copolymer to the NIR wavelength region (684–770 nm).
In this study, we intended to synthesize the NIR PNATF conjugated copolymer encapsulated in a biocompatible polymer. To enhance encapsulation efficiency in polymer material by increasing hydrophobicity of the dye, molecular structure of the NIR PNATF conjugated copolymer was modified by replacing the hexyl group of naphthalene-diimide-based monomers with the ethylhexyl group to achieve poly{[2,7-(9,9′-dioctyl)fluorene]-alt-[5,5′-bis(2,2′-thiophene)-2,6-naphthalene-1,4,5,8-tetracarboxylic-N,N′-diethylhexyldiimide]} (PF8NT2/6). After being well-characterized using NMR, the modified dye was encapsulated into a poly(ε-caprolactone) (PCL) matrix by way of an emulsion-diffusion technique. Influence of surfactant structure on hydrodynamic diameter, zeta potential, and fluorescent properties of the PF8NT2/6 encapsulated PCL (PCL-PF8NT2/6) nanoparticles was systematically assessed. The adjusted amount of PF8NT2/6 in the encapsulation process was also studied to tune encapsulation efficiency (EE). Finally, as a proof-of-concept for its potential for in vivo applications, the cytotoxicity of the prepared particles was tested using fibroblasts.
2. Materials and methods
2.1. Materials
Bis-[2,2′-(5,5′-dibromo)-thienyl]-2,6-naphthalene-1,4,5,8-tetracarboxylic-N,N′-di(2-ethylhexyl)imide was synthesized in close analogy to method described in the literature.19 9,9-Dioctylfluorene-2,7-bis(trimethylborate), 97% and tetrakis(triphenylphosphine)palladium(0), 99% (Pd(PPh3)4) were purchased from Aldrich and Acros organics, respectively. PCL (molecular weight 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), Kolliphor® P 188 (molecular weight 7680–9510 g mol−1), Tween® 80 (molecular weight 1310 g mol−1) and poly(vinyl alcohol) (PVA) (99+% hydrolyzed, molecular weight 89
000 g mol−1), Kolliphor® P 188 (molecular weight 7680–9510 g mol−1), Tween® 80 (molecular weight 1310 g mol−1) and poly(vinyl alcohol) (PVA) (99+% hydrolyzed, molecular weight 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–98
000–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were purchased from Sigma-Aldrich (Analytical Grade). Organic solvent including chloroform, toluene, tetrahydrofuran (THF), n-butanol, methanol and acetone purchased from Carlo-Erba (Analytical Grade) was used as received. Deionized (DI) water purified by PureLab Ultra water system (ELGA, UK) was used throughout the work.
000 g mol−1) were purchased from Sigma-Aldrich (Analytical Grade). Organic solvent including chloroform, toluene, tetrahydrofuran (THF), n-butanol, methanol and acetone purchased from Carlo-Erba (Analytical Grade) was used as received. Deionized (DI) water purified by PureLab Ultra water system (ELGA, UK) was used throughout the work.
2.2. Synthesis of PF8NT2/6 NIR dye
The PF8NT2/6 NIR dye was synthesized through a Suzuki-type coupling reaction using Pd(PPh3)4 as the catalyst (Scheme 1). Bis-[2,2′-(5,5′-dibromo)-thienyl]-2,6-naphthalene-1,4,5,8-tetra carboxylic-N,N′-di(2-ethylhexyl)imide (1) (503.8 mg, 0.62 mmol), 9,9-dioctylfluorene-2,7-bis(trimethylborate) (2) (346.2 mg, 0.62 mmol) and Pd(PPh3)4 (25.7 mg, 0.02 mmol) were dissolved in a degassed mixture of aqueous Na2CO3 (2.5 mL; 20% w/v), n-butanol (2.5 mL), toluene (20 mL) and THF (20 mL). After stirring under reflux for 3 days and cooling to room temperature, aqueous HCl (2 N, 20 mL) was poured into the mixture. Extraction with chloroform was done before allowing phase separation. The organic phase containing PF8NT2/6 NIR dye was isolated and then washed with saturated aqueous EDTA and subsequently NaHCO3 solution. It was then harvested, dried over anhydrous Na2SO4, and evaporated solvent off. The dye was solvated in chloroform and precipitated into a mixture of methanol/aqueous HCl (2 N) (10/1, vol%), filtered, and extracted in a Soxhlet apparatus with methanol, acetone, and hexane, respectively to remove impurities and oligomers. The obtained PF8NT2/6 was dissolved in chloroform, concentrated and precipitated in methanol/aqueous HCl (2 N) (10/1, vol%) before drying under vacuum. Yield: 0.272 g (42%).1H NMR (500 MHz, CDCl3): δ 8.85 (2H), 7.63–7.75 (6H), 7.48 (2H), 7.42 (2H), 4.12 (4H), 2.05 (4H), 1.96 (2H), 1.56 (4H), 1.29–1.41 (12H), 1.10 (24H), 0.87–0.96 (12H), 0.76–0.81 (6H) (Fig. S1†). 13C NMR (500 MHz, CDCl3): δ 162.7, 151.9, 148.4, 140.7, 139.7, 139.6, 136.6, 132.9, 130.2, 127.6, 125.4, 125.1, 123.5, 122.7, 120.3, 120.1, 55.4, 44.6, 40.6, 37.8, 31.8, 30.6, 30.1, 29.3, 28.6, 23.9, 23.1, 22.6, 14.1, 10.6 (Fig. S2†). Mn = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 586. Mw = 36
586. Mw = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 358. Mw/Mn = 1.96.
358. Mw/Mn = 1.96.
2.3. Preparation and characterizations of PCL-PF8NT2/6 nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 30 min) and the supernatant was collected to measure the amount of free PF8NT2/6 (non-encapsulated PF8NT2/6) using UV-Vis-NIR spectrometer (Agilent Technologies, Cary 5000, USA) at 610 nm. All measurements were performed in triplicate. The EE (%) was then calculated as the following equation:
000 rpm, 30 min) and the supernatant was collected to measure the amount of free PF8NT2/6 (non-encapsulated PF8NT2/6) using UV-Vis-NIR spectrometer (Agilent Technologies, Cary 5000, USA) at 610 nm. All measurements were performed in triplicate. The EE (%) was then calculated as the following equation:
 | (1) |
Morphology of the PCL-PF8NT2/6 nanoparticles was investigated using Transmission Electron Microscopy (TEM, JEM-2100, JEOL, Japan) operated at an accelerating voltage of 200 kV. Size distribution of the nanoparticles was analysed from TEM imaging (n = 50) using image processing program (ImageJ). Elemental analysis was done using an Energy Dispersive X-ray Spectrometer (EDS) detector equipped with TEM. For specimen preparation, a few drops of the diluted sample were deposited on a carbon coated copper grid without staining and air dried overnight. Composition of the nanoparticles was investigated through thermogravimetric analysis (TGATGA/DSC 3+, Mettler Toledo, Switzerland). The samples were heated from 25 to 700 °C using a heating rate of 10 °C min−1 under nitrogen atmosphere. NIR fluorescence spectra of the prepared nanoparticles were recorded using NIR spectroscopy (QuantaMaster™ 500, Photon Technologies International, USA) at excitation wavelength (λex) of 610 nm and emission spectra recorded in range of 700–1200 nm. All measurements were performed in 1 cm quartz cell with the excitation and emission slit of 10 nm at room temperature. Flow cytometer (FACScanto II, BD) was also applied for determination of homogeneity of fluorescence emission of the PCL-PF8NT2/6 nanoparticles.
The PCL-PF8NT2/6 nanoparticles (0.1 g) were separately diluted using the completed medium (1 mL), i.e. RPMI-1640 supplemented with HEPES (25 mM), D-glucose (1.8 mg mL−1), glutamine (2 mM), gentamicin (40 mg mL−1) and 10% heat-inactivated fetal calf serum (FCS) to attain a given concentration. L-929 cells were seeded at the density of 100 cells per well in 24-well plates and incubated for 24 h (37 °C, 5% CO2). After incubation for 6 days, the number of colonies on each well was counted under the optical microscope and % inhibition of cell growth was evaluated. Culture medium was used instead of the encapsulated nanoparticles in the case of negative control. Statistical significance was evaluated using one-way analysis of variance (ANOVA) (Minitab). Results with p-values < 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Preparation of PCL-PF8NT2/6 nanoparticles
With the aim to apply the synthesized PCL-PF8NT2/6 nanoparticles in biomedical applications, it is expected that the monodisperse nanoparticles possess high stability and cell compatibility. Herein the encapsulation of hydrophobic PF8NT2/6 NIR dye into a polymer matrix was carried out by way of an emulsion–diffusion technique as this offers monodispersed particles with high encapsulation efficiency of active compounds (generally >70%) from preformed polymer. This process exhibits a high batch-to-batch reproducible manner and ease of scale-up.22,23 Generally, this method uses ethyl acetate (water solubility 8.3 g/100 mL at 20 °C, dielectric constant 6.0) as an organic solvent to dissolve polymers and the active molecules.24,25 However, in our study, very hydrophobic PF8NT2/6 dye cannot be completely dissolved in ethyl acetate. Chloroform (water solubility 0.809 g/100 mL at 20 °C, dielectric constant 4.8) was used instead to dissolve the NIR dye and PCL yielded a blue transparent mixture.26 Firstly, the partially water-soluble chloroform was saturated with DI water ensuring an initial thermodynamic equilibrium of both liquids which can avoid mass-transfer during the process.27 The reaction began after the addition of surfactant dissolved in chloroform-saturated water into water-saturated chloroform containing a mixture of PCL and NIR dye under ultrasonic treatment. After adding water in the dilution step, chloroform continuously flowed away from initial homogeneous droplets of PCL-PF8NT2/6-chloroform stabilized with non-ionic surfactant to the aqueous-rich phase. This gave rise to polymer aggregation and consequently formed solid PCL-PF8NT2/6 nanoparticles.27,28 Moinard-Chécot suggested that the advantage of eliminating organic solvent using the diffusion process (water addition) is the fact that it is milder than the direct evaporation as the nanodispersion does not directly resist mechanical stress inside the aqueous suspension during the evaporation.25It is noteworthy that a surfactant is a critical preparative variable to obtain the stable nanoparticles and can affect the formation mechanism, as well as surface characteristics of the prepared nanoparticles.29 In this study, various biocompatible non-ionic surfactants, including Tween® 80 (small molecule surfactant), PVA and Kolliphor® P188 (polymeric surfactant) with different hydrophilic–lipophilic balance (HLB) values, were employed in the preparation process. Surfactants with high HLB values correspond to the high hydrophilic/lipophobic characteristics of the molecule.30 Hydrodynamic diameter, PDI and zeta potential of the encapsulated nanoparticles prepared using those surfactants at the same concentration of PCL (10 mg) and PF8NT2/6 NIR dye (1 mg) are summarized in Table 1.
| Sample | Surfactant | HLB | Hydrodynamic diametera (nm) | PDIa | Zeta potentiala (mV) |
|---|---|---|---|---|---|
| a Each value represents the mean ± SD (n = 3). | |||||
| PCL-PF8NT2/6-1 | Tween® 80 | 15 | 21.2 ± 1.3 | 0.65 ± 0.02 | −5.52 ± 0.44 |
| PCL-PF8NT2/6-2 | PVA | 18 | 282.7 ± 4.3 | 0.51 ± 0.04 | −1.55 ± 0.06 |
| PCL-PF8NT2/6-3 | Kolliphor® P188 | 29 | 285.7 ± 4.9 | 0.25 ± 0.01 | −6.65 ± 0.44 |
As shown in Table 1, when using polymeric surfactants, PVA or Kolliphor® P188, the prepared PCL-PF8NT2/6-2 and PCL-PF8NT2/6-3 exhibited a drastically higher hydrodynamic diameter of 282.7 and 285.7 nm compared to that using Tween® 80 (PCL-PF8NT2/6-1). This might be attributed to the small molecular size of Tween® 80 which can lower surface tension at the oil–water interface compared to that of polymeric surfactant (PVA and Kolliphor® P188) at the same concentration. This led to reduction of particle sizes during the emulsification step which encourages solvent diffusion to the aqueous phase during dilution and evaporation steps resulted in small size nanoparticles.31 However, upon short-term storage, precipitation of PCL-PF8NT2/6-1 in aqueous medium can be observed.32 This indicates a low efficiency of Tween® 80 at a given concentration for stabilization of the prepared nanoparticles. Based on emulsion–diffusion technique, it has been believed that a stable nanoparticle would generate if the sufficient surfactant remains on the interface area and adequately protects the globule from coalescence or aggregation during dilution step.29 In the case of Kolliphor® P188, poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG-b-PPG-b-PEG), this block copolymer acts as steric stabilizer in a manner where the hydrophobic PPG chain aligns on the surface of the nanoparticles and the other two hydrophilic PEG chains extend into the aqueous phase. This structure promotes steric repulsion to prevent droplet aggregation during PCL-PF8NT2/6-3 formation.33,34 In addition, the high HLB value of Kolliphor® P188 indicates the strong tendency to migrate at oil/water interface and then stabilized the prepared nanoparticles, as well as lowering the exchange rate of entrapped PF8NT2/6 dye to the surrounding aqueous solution.32 Although PVA and Kolliphor® P188 are non-ionic polymeric surfactants, higher HLB values of Kolliphor® P188 can stabilize smaller, more uniform and homogeneous emulsion droplets compared to PVA. This led to the low PDI value of PCL-PF8NT2/6-3. In addition, larger PDI values of the PCL-PF8NT2/6-2 may attribute to high extent of hydrolysis of PVA (99+% hydrolyzed), inducing strong intermolecular and intramolecular hydrogen bonding occurrence in PVA chain. This results in a low efficiency of PVA to prevent the globule coalescence by steric stabilization during solvent diffusion process.25,33,35 These results suggest that the structure of the surfactant is a factor that significantly influences hydrodynamic diameter and PDI of the prepared PCL-PF8NT2/6 nanoparticles.
According to Table 1, it can be seen that the zeta potential of all PCL-PF8NT2/6 nanoparticles exhibited slightly negative values. This relates to dissociation of hydrophilic –COOH end group of PCL. During the dilution step, the migration of chloroform in the PCL matrix to the chloroform-saturated aqueous phase possibly encourages the re-arrangement of PCL by moving the hydrophobic part of PCL chain close to the oil and water interface.29,36 In addition, using TGA technique, two steps thermal degradation of PCL-PF8NT2/6-2 and PCL-PF8NT2/6-3 (320–440 °C and 460–600 °C) compared to neat PCL and PF8NT2/6 were observed (Fig. S4†).37 The data implied the presence of PF8NT2/6 encapsulated in PCL matrix.
Based on intrinsic characteristics of PF8NT2/6, the fluorescence intensity of the prepared PCL-PF8NT2/6 nanoparticles was investigated in comparison to free PF8NT2/6 dye at λex of 610 nm, as presented in Fig. 1.
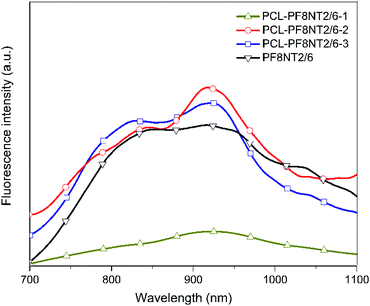 | ||
| Fig. 1 Fluorescence spectra of free PF8NT2/6 NIR dye (0.05 mg mL−1 in chloroform) and PCL-PF8NT2/6 nanoparticles (0.05 mg mL−1 in DI water) prepared using different surfactants (λex = 610 nm). | ||
As can be seen in Fig. 1, the prepared PCL-PF8NT2/6-2 and -3 exhibited two obvious maximum emission wavelengths (λmax) (at 841 and 918 nm for PCL-PF8NT2/6-2 and 840 and 921 nm for PCL-PF8NT2/6-3), which resembled the λmax of free PF8NT2/6 NIR dye at 843 and 922 nm. Significant lower fluorescence intensity of PCL-PF8NT2/6-1 might be originated from the low amount of the dye encapsulated in the PCL matrix due to the low efficiency of Tween® 80 to stabilize the nanoparticles as mentioned above. It is worth noting that λmax of PCL-PF8NT2/6-2 and -3 nanoparticles at approximately 840 nm is in the NIR optical window (between 700–900 nm in wavelength), enabling deeper tissue penetration with a higher signal-to-background ratio.38 Ding et al. noted that the emission wavelength of far-red/near-infrared fluorescent conjugated polymer (CP) nanoparticles is 600–800 nm (λmax = 698 nm) and can be utilized as a fluorescent probe for bio-imaging applications.39 The red-shift of emission spectra of CP nanoparticles is attributed to the increased conjugation system.40 The quantum yield of PCL-PF8NT2/6-1, 2 and 3 nanoparticles measured using indocyanine green (ICG) dye dissolved in DMSO as a reference was found to be 0.9, 1.6 and 3.3%, respectively. Although the fluorescent intensity of PCL-PF8NT2/6-2 and -3 nanoparticles was quite similar, PDI and stability of PCL-PF8NT2/6-2 measured from the DLS was lower than that of PCL-PF8NT2/6-3. Therefore, Kolliphor® P188 block copolymer was found to be a suitable surfactant for preparing the PCL-PF8NT2/6 nanoparticles in this system.
3.2. Influence of PF8NT2/6 concentration on optical and colloidal characteristics of PCL-PF8NT2/6 nanoparticles
Dye concentration is an important factor yielding high fluorescent intensity, colloidal stability and EE of the nanoparticles. In this study, initial concentration of the PF8NT2/6 was varied from 0.25 to 2.0 mg while keeping other parameters including concentrations of PCL and surfactant, reaction temperature and time constant.Optical characteristics of the PCL-PF8NT2/6-3 nanoparticles with various amount of dye were investigated using UV-vis absorption and NIR photoluminescence. Fig. 2a shows the absorption spectra of the aqueous suspension of the encapsulated nanoparticles as a function of dye concentration. All PCL-PF8NT2/6-3 nanoparticles exhibit the two maximum absorption peaks at wavelengths of 400 and 645 nm. The absorbance increases with increasing dye concentration which relates to color intensity of the prepared nanoparticles, as displayed in Fig. 2b. The absorption peak at 400 nm is attributed to π–π* transition of the conjugated backbone of the PF8NT2/6 dye, while the broad absorption peak at 550–700 nm is resulted from charge transfer between fluorene and naphthalene units of dye molecule.41 It can be noticed that the fluorescence signal from the PCL-PF8NT2/6-3 nanoparticles is detectable upon excitation at 610 nm, indicating that most excited electrons return to the ground state via radiative decay.42 Fig. 3 shows the near IR photoluminescence spectra of the prepared nanoparticles suspended in DI water. As can be seen in Fig. 2a and 3, the PCL-PF8NT2/6-3 nanoparticles present the absorption peak at 645 nm and the emission maximum peak at 921 nm with an obvious large Stokes shift of 276 nm. This value is remarkably larger than the previously reported NIR conjugated dye nanoparticles.39,43 Slight red-shift of absorption and the emission peaks can be observed when the concentration of PF8NT2/6 was increased. These results are in line with the previous research reported by Wu et al., in which the shift of spectra is probably due to the aggregation of dye which is attributed to the dye molecules getting close to each other inside the nanoparticle.44 Additionally, the encapsulated nanoparticles exhibit the increasing fluorescence intensity of two emission peaks (837 and 919 nm) when the dye concentration increased, as shown in Fig. 3. These results reveal that there is an increasing number of dye molecules in the PCL matrix.
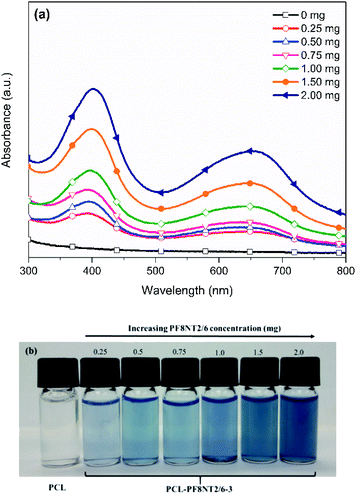 | ||
| Fig. 2 (a) UV-vis absorption spectra and (b) photographs of the PCL-PF8NT2/6-3 nanoparticles at different dye concentrations. | ||
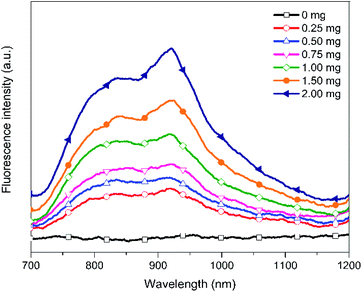 | ||
| Fig. 3 Fluorescence intensity of the PCL-PF8NT2/6-3 nanoparticles in aqueous media at different dye concentrations. | ||
Hydrodynamic diameter, PDI and zeta potential of the synthesized PCL-PF8NT2/6-3 at various dye concentrations are displayed in Table 2.
| Amount of dye (mg) | Hydrodynamic diametera (nm) | PDI | Zeta potentiala (mV) | EE (%) |
|---|---|---|---|---|
| a Each value represents the mean ± SD (n = 3). | ||||
| 0.0 | 179.1 ± 6.0 | 0.49 ± 0.03 | −4.77 ± 0.39 | — |
| 0.25 | 265.7 ± 12.6 | 0.41 ± 0.02 | −8.83 ± 0.30 | 12.5 ± 6.4 |
| 0.5 | 261.6 ± 2.1 | 0.25 ± 0.01 | −7.75 ± 0.51 | 64.5 ± 4.0 |
| 0.75 | 259.6 ± 1.7 | 0.25 ± 0.03 | −8.62 ± 0.42 | 67.7 ± 6.5 |
| 1.0 | 285.7 ± 4.9 | 0.25 ± 0.01 | −6.65 ± 0.44 | 70.4 ± 2.4 |
| 1.5 | 285.0 ± 6.7 | 0.26 ± 0.01 | −8.38 ± 0.67 | 75.9 ± 6.3 |
| 2.0 | 297.7 ± 5.6 | 0.41 ± 0.02 | −7.59 ± 0.28 | 82.8 ± 3.5 |
As shown in Table 2, the hydrodynamic diameter of PCL-PF8NT2/6-3 nanoparticles tends to increase with increasing NIR dye concentration. Bare PCL nanoparticles exhibit the smallest diameter and highest PDI, indicating its inhomogeneous size distribution.
However, after loading PF8NT2/6 dye, the PDI of the nanoparticles decreases, especially when using PF8NT2/6 concentration in the range of 0.5 to 1.5 mg. Increasing dye concentration higher than 1.5 mg caused the formation of large aggregates of the excess dye in aqueous phase which can be seen via the naked eye. Considering the absolute zeta potential of the PCL-PF8NT2/6-3 nanoparticles, an increase in the absolute value is observed compared to that of the bare PCL nanoparticles. However, the value does not significantly differ as the dye concentration increased. These nanoparticles are stabilized owing to the steric repulsion of the hydrophilic part of Kolliphor® P188 extending into aqueous phase.45 The near neutral surface charges of these synthesized PCL-PF8NT2/6-3 nanoparticles would be advantageous for in vivo use as the large positive or negative charges can lead to rapid blood clearance.46
Based on eqn (1), EE of the PCL-PF8NT2/6-3 nanoparticles prepared using various concentrations of the dye are summarized in Table 2. Our results indicate that EE increased up to 82.8% with increasing the dye concentration from 0.25 to 2.0 mg. This result agrees well with the photographs of the PCL-PF8NT2/6-3 shown in Fig. 2b. Since the structure of the PF8NT2/6 molecule is quite hydrophobic, its interaction with PCL chain and consequential encapsulation is preferable. Although the use of 2.0 mg NIR dye showed the highest EE of 82.8%, its PDI value indicated low homogeneity of nanoparticles. Monodisperse particles are an important requirement to understand the physical property (size and shape) of nanoparticles and to minimize the variability in biological application.47 Therefore, an optimal concentration of PF8NT2/6 NIR dye giving high fluorescence intensity, EE, as well as low PDI value, is 1.5 mg. Fluorescence intensity histogram obtained from flow cytometer in Fig. 4b clearly demonstrates homogeneity of fluorescence emission of PCL-PF8NT2/6-3 nanoparticles (1.5 mg of PF8NT2/6) compared with that of bare PCL in Fig. 4a at the same particle concentration. The histogram shifts more to the right-hand side compared to that of PCL nanoparticles (Fig. 4a), suggesting a strong fluorescence signal of these PCL-PF8NT2/6-3 nanoparticles, which could provide a sufficient fluorescence signal for cell labelling.
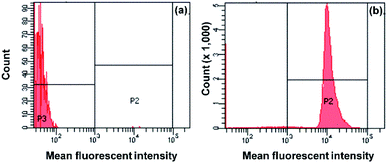 | ||
| Fig. 4 Fluorescence intensity histogram determined by flow cytometer of (a) bare PCL nanoparticles and (b) PCL-PF8NT2/6-3 nanoparticles at 1.5 mg of NIR dye. | ||
Morphology and elemental composition of the PCL-PF8NT2/6-3 nanoparticles (1.5 mg of NIR dye) was investigated using TEM equipped with EDS. Results are displayed in Fig. 5.
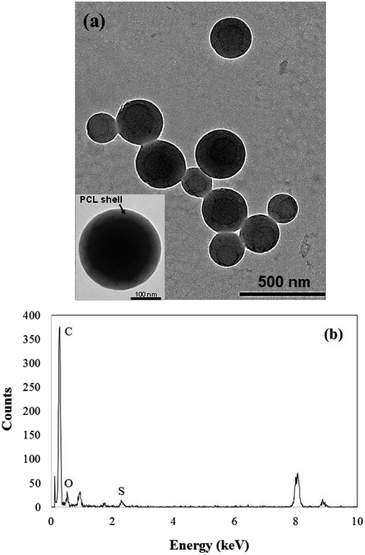 | ||
| Fig. 5 (a) TEM and high-magnification TEM micrographs (inset) and (b) EDS spectrum of PCL-PF8NT2/6-3 nanoparticles (1.5 mg of NIR dye). | ||
As can be seen in Fig. 5a, the prepared PCL-PF8NT2/6-3 nanoparticles (1.5 mg of PF8NT2/6) are spherical in shape with mean diameter ranged from 110 to 350 nm. Size distribution analysed from TEM image demonstrates a uniform particle size distribution of the nanoparticles (Fig. S6†) which corresponds to its low PDI value, as shown in Table 2. Theoretically, the dried size observed by TEM is smaller than that measured by DLS involving the shrinkage of hydrophilic part (PEO chain) of Kolliphor® P188 in dried state. In addition, there is a distinct difference in the contrast of dye (dark core) and PCL (light shell) having thickness approximately 36–39 nm, indicating that most PF8NT2/6 were preferentially located inside the nanoparticle as previously reported.24,48 Results obtained from EDS in Fig. 5b confirm the presence of sulphur at 2.307 keV which is an element composed only in PF8NT2/6 compared to that of PCL nanoparticles (Fig. S7†).
3.3. In vitro cytotoxicity of the PCL-PF8NT2/6-3 nanoparticles
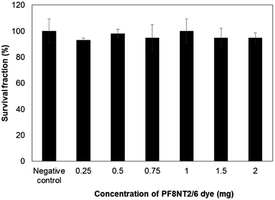
With the aim to apply this nanoparticle in biomedical applications, the cytotoxic effect of the PCL-PF8NT2/6-3 nanoparticles was evaluated. Proliferation capacity of a single cell in the presence of the PCL-PF8NT2/6-3 nanoparticles prepared at various dye concentrations was studied based on colony-forming efficiency assay using L-929 fibroblast cell line. In RPMI culture media composed of protein and salt components, the PCL-PF8NT2/6-3 nanoparticles demonstrate good stability as their hydrodynamic diameter is close to that in original synthesis media (Fig. S8†). Changes in cell viability after incubation with the samples result in a reduction of the number of colonies formed compared to a negative control. The percentages of survival fraction as a function of NIR dye concentration are depicted in Fig. 6.As can be seen in Fig. 6, after 6 days' exposure to the PCL-PF8NT2/6-3, the percentage of survival fraction of L-929 cells was higher than 90% irrespective of concentration of PF8NT2/6 dye. The values do not significantly differ from negative control (P < 0.05). Slight negative charges of these nanoparticles may not drastically affect cell viability as it has been reported that positively charged particles likely interact with negatively charged portions of cell membrane inducing toxicity response via endocytosis.49 An almost neutral charge of the synthesized PCL-PF8NT2/6-3 might hinder them from getting internalization through the cell membrane. In addition, Kolliphor® P188 is defined as a FDA approved surfactant and suitable for human use. The zeta potential of the PCL-PF8NT2/6-3 at various NIR dye concentrations is quite similar, so that survival fraction was not remarkably different. It has been previously described that dynamic layer of protein corona occurred from interaction between cell culture media components and nanoparticles diminished the toxic response to the cells.50 A similar contribution was possibly observed in this study. Additionally, the adsorbed proteins might increase the colloidal stability of the nanoparticles by taking part in their stabilization via steric stabilization. These results highlighted that the synthesized nanoparticles are not toxic to L-929 cells at this investigated concentration.
4. Conclusions
We have successfully synthesized the NIR fluorescent dye (PF8NT2/6) encapsulated into PCL matrix via emulsion–diffusion technique. Using Kolliphor® P188 as a surfactant, the prepared PCL-PF8NT2/6-3 nanoparticles exhibited a spherical morphology and uniform size distribution. Increasing dye concentration gave rise in absorbance and fluorescence intensity of the nanoparticles. Remarkably, a large Stokes shift of 276 nm in aqueous medium was obtained. These results reveal the PCL-PF8NT2/6-3 nanoparticles with great potential to diminish background interference for bio-imaging applications. Finally, the results from the colony-forming technique demonstrated low toxicity of the nanoparticles to the cells irrespective of PF8NT2/6 concentration.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by National Nanotechnology Center, National Science and Technology Development Agency (NSTDA) (P1450519). K. J. is grateful to Mahidol University for research support. The Authors thank the Center for Research and Innovation, Faculty of Medical Technology, Mahidol University for facility support.References
- M. Kolitz-Domb, I. Grinberg, E. Corem-Salkmon and S. Margel, Engineering of near infrared fluorescent proteinoid-poly(L-lactic acid) particles for in vivo colon cancer detection, J. Nanobiotechnol., 2014, 12, 30–42 CrossRef.
- T. Behnke, L. E. Mathejczyk, R. Brehm, C. Würth, F. R. Gomes, C. Dullin, J. Napp, F. Alves and U. Resch-Genger, Target-specific nanoparticles containing a broad band emissive NIR dye for the sensitive detection and characterization of tumor development, Biomaterials, 2013, 34, 160–170 CrossRef CAS.
- Z. Yang, S. Zheng, W. J. Harrison, J. Harder, X. Wen, J. G. Gelovani, A. Qiao and C. Li, Long-circulating near-infrared fluorescence core-cross-linked polymeric micelles: synthesis, characterization, and dual nuclear/optical imaging, Biomacromolecules, 2007, 8, 3422–3428 CrossRef CAS.
- S. M. Yoon, S. J. Myung, I. W. Kim, E. J. Do, B. D. Ye, J. H. Ryu, K. Park, K. Kim, I. C. Kwon, M. J. Kim, D. H. Moon, D. H. Yang, K. J. Kim, J. S. Byeon, S. K. Yang and J. H. Kim, Application of Near-infrared fluorescence imaging using a polymeric nanoparticle-based probe for the diagnosis and therapeutic monitoring of colon cancer, Dig. Dis. Sci., 2011, 56, 3005–3013 CrossRef CAS.
- A. Topete, M. Alatorre-Meda, P. Iglesias, E. M. Villar-Alvarez, S. Barbosa, J. A. Costoya, P. Taboada and V. Mosquera, Fluorescent drug-loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells, ACS Nano, 2014, 8, 2725–2738 CrossRef CAS.
- A. Yuan, J. Wu, X. Tang, L. Zhao, F. Xu and Y. Hu, Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies, J. Pharm. Sci., 2013, 102, 6–28 CrossRef CAS.
- S. Luo, E. Zhang, Y. Su, T. Cheng and C. Shi, A review of NIR dyes in cancer targeting and imaging, Biomaterials, 2011, 32, 7127–7138 CrossRef CAS.
- X. Yi, F. Wang, W. Qin, X. Yang and J. Yuan, Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field, Int. J. Nanomed., 2014, 9, 1347–1365 CrossRef.
- M. Laine, N. A. Barbosa, A. Kochel, B. Osiecka, G. Szewczyk, T. Sarna, P. Ziółkowski, R. Wieczorek and A. Filarowski, Synthesis, structural, spectroscopic, computational and cytotoxic studies of BODIPY dyes, Sens. Actuators, B, 2017, 238, 548–555 CrossRef CAS.
- L. Hu, Z. Yan and H. Xu, Advances in synthesis and application of near-infrared absorbing squaraine dyes, RSC Adv., 2013, 3, 7667–7676 RSC.
- J. O. Escobedo, O. Rusin, S. Lim and R. M. Strongin, NIR dyes for bioimaging applications, Curr. Opin. Chem. Biol., 2010, 14, 64–70 CrossRef CAS.
- J. Zhang, Z. Zhang, B. Yu, C. Wang, W. Wu and X. Jiang, Synthesis and biological properties of porphyrin-containing polymeric micelles with different sizes, ACS Appl. Mater. Interfaces, 2016, 8, 5794–5803 CrossRef CAS.
- K. Hoffmann, T. Behnke, D. Drescher, J. Kneipp and U. Resch-Genger, Near-infrared-emitting nanoparticles for lifetime-based multiplexed analysis and imaging of living cells, ACS Nano, 2013, 7, 6674–6684 CrossRef CAS.
- T. Behnke, C. Würth, K. Hoffmann, M. Hübner, U. Panne and U. Resch-Genger, Encapsulation of hydrophobic dyes in polystyrene micro- and nanoparticles via swelling procedures, J. Fluoresc., 2011, 21, 937–944 CrossRef CAS.
- J. Napp, T. Behnke, L. Fischer, C. Würth, M. Wottawa, D. M. Katschinski, F. Alves, U. Resch-Genger and M. Schäferling, Targeted luminescent near-infrared polymer-nanoprobes for in vivo imaging of tumor hypoxia, Anal. Chem., 2011, 83, 9039–9046 CrossRef CAS.
- G. Meng, S. Velayudham, A. Smith, R. Luck and H. Liu, Color tuning of polyfluorene emission with BODIPY monomers, Macromolecules, 2009, 42, 1995–2001 CrossRef CAS.
- D. Neher, Polyfluorene homopolymers: conjugated liquid-crystalline polymers for bright blue emission and polarized electroluminescence, Macromol. Rapid Commun., 2001, 22, 1365–1385 CrossRef CAS.
- U. Asawapirom, R. Güntner, M. Forster, T. Farrell and U. Scherf, Dialkylfluorene-oligothiophene and dialkylfluorene-dithienylvinylene alternating copolymers, Synthesis, 2002, 9, 1136–1142 Search PubMed.
- P. Piyakulawat, A. Keawprajak, A. Chindaduang, M. Hanusch and U. Asawapirom, Synthesis and preliminary characterization of novel naphthalene bisimide based copolymers, Synth. Met., 2009, 159, 467–472 CrossRef CAS.
- J. Hu, J. Guo, Z. Xie, D. Shan, E. Gerhard, G. Qian and J. Yang, Fluorescence imaging enabled poly(lactide-co-glycolide), Acta Biomater., 2016, 29, 307–319 CrossRef CAS.
- International Organization for Standardization, Biological evaluation of medical devices - Part 5: Tests for In Vitro Cytotoxicity, International Organization for Standardization, Geneva, Switzerland, 2009, ISO 10993-5:2009(en) Search PubMed.
- B. V. N. Nagavarma, K. S. Y. Hemant, A. Ayaz, L. S. Vasuha and H. G. Shivakumar, Different techniques for preparation of polymeric nanoparticles-a review, Asian J. Pharm. Clin. Res., 2012, 5, 16–23 CAS.
- C. E. Mora-Huertas, H. Fessi and A. Elaissari, Polymer-based nanocapsules for drug delivery, Int. J. Pharm., 2010, 385, 113–142 CrossRef CAS.
- M. Choi, A. Soottitantawat, O. Nuchuchua, S. Min and U. Ruktanonchai, Physical and light oxidative properties of eugenol encapsulated by molecular inclusion and emulsion–diffusion method, Food Res. Int., 2009, 42, 148–156 CrossRef CAS.
- D. Moinard-Chécot, Y. Chevalier, S. Briançon, L. Beney and H. Fessi, Mechanism of nanocapsules formation by the emulsion–diffusion process, J. Colloid Interface Sci., 2008, 317, 458–468 CrossRef.
- S. Lee, E. Yoo, H. Ghim and S. Lee, Alginate nanohydrogels prepared by emulsification-diffusion method, Macromol. Res., 2009, 17, 168–173 CrossRef CAS.
- S. Guinebretière, S. Briancon, H. Fessi, V. S. Teodorescu and M. G. Blanchin, Nanocapsules of biodegradable polymers: preparation and characterization by direct high resolution electron microscopy, Mater. Sci. Eng., C, 2002, 21, 137–142 CrossRef.
- M. Hassou, F. Couenne, Y. Gorrec and M. Tayakout, Modeling and simulation of polymeric nanocapsule formation by emulsion diffusion method, AIChE J., 2009, 55, 2094–2105 CrossRef CAS.
- C. E. Mora-Huertas, F. Couenne, H. Fessi and A. Elaissari, Electrokinetic properties of poly-ε-caprolactone-based nanoparticles prepared by nanoprecipitation and emulsification-diffusion methods: a comparative study, J. Nanopart. Res., 2010, 14, 876–891 CrossRef.
- R. W. Egan, M. A. Jones and A. L. Lehninger, Hydrophile-lipophile balance and critical micelle concentration as key factors influencing surfactant disruption of mitochondrial membranes, J. Biol. Chem., 1976, 251, 4442–4447 CAS.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Formation and stability of nano-emulsions, Adv. Colloid Interface Sci., 2004, 108, 303–318 CrossRef.
- N. Garti and D. J. McClements, Encapsulation Technologies and Delivery Systems for Food Ingredients and nutraceuticals, Woodhead Publishing Limited, Cambridge, 2012 Search PubMed.
- N. Sharma, P. Madan and S. Lin, Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: a co-surfactant study, Asian J. Pharm. Sci., 2016, 11, 404–416 CrossRef.
- T. Tadros, Polymeric surfactants in disperse systems, Adv. Colloid Interface Sci., 2009, 147, 281–299 CrossRef.
- H. Murakami, Y. Kawashima, T. Niwa, T. Hino, H. Takeuchi and M. Kobayashi, Influence of the degrees of hydrolyzation and polymerization of poly(vinylalcohol) on the preparation and properties of poly(DL-lactide-co-glycolide) nanoparticle, Int. J. Pharm., 1997, 149, 43–49 CrossRef CAS.
- U. Bazylinska, A. Pucek, M. Sowa, E. Matczak-Jon and K. A. Wilk, Engineering of phosphatidylcholine-based solid lipid nanocarriers for flavonoids delivery, Colloids Surf., 2014, 460, 483–493 CrossRef CAS.
- M. R. Tavares, L. R. Menezes, J. C. D. Filho, L. M. Cabral and M. I. B. Taveres, Surface-coated polycaprolactone nanoparticles with pharmaceutical application: Structural and molecular mobility evaluation by TD-NMR, Polym. Test., 2017, 60, 39–48 CrossRef CAS.
- J. Mérian, J. Gravier, F. Navarro and I. Texier, Fluorescent nanoprobes dedicated to in vivo imaging: from preclinical validations to clinical translation, Molecules, 2012, 17, 5564–5591 CrossRef.
- D. Ding, J. Liu, G. Feng, K. Li, Y. Hu and B. Liu, Bright far-red/near-infrared conjugated polymer nanoparticles for in vivo bioimaging, Small, 2013, 9, 3093–3102 CrossRef CAS.
- H. Liu, P. Wu, S. Kuo, C. Chen, E. Chang, C. Wu and Y. Chan, Quinoxaline-based polymer dots with ultrabright red to near-infrared fluorescence for in vivo biological imaging, J. Am. Chem. Soc., 2015, 137, 10420–10429 CrossRef CAS.
- J. Geng, C. Sun, J. Liu, L. Liao, Y. Yuan, N. Thakor, J. Wang and B. Liu, Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy, Small, 2015, 11, 1603–1610 CrossRef CAS.
- C. L. Amiot, S. Xu, S. Liang, L. Pan and J. X. Zhao, Near-infrared fluorescent materials for sensing of biological targets, Sensors, 2008, 8, 3082–3105 CrossRef CAS.
- K. Li, D. Ding, D. Huo, K. Pu, N. N. Phuong Thao, Y. Hu, Z. Li and B. Liu, Conjugated polymer based nanoparticles as dual-modal probes for targeted in vivo fluorescence and magnetic resonance imaging, Adv. Funct. Mater., 2012, 22, 3107–3115 CrossRef CAS.
- W. Wu, C. Chen, Y. Tian, S. Jang, Y. Hong, Y. Liu, R. Hu, B. Tang, Y. Lee, C. Chen, W. Chen and A. K.-Y. Jen, Enhancement of aggregation-induced emission in dye-encapsulating polymeric micelles for bioimaging, Adv. Funct. Mater., 2010, 20, 1413–1423 CrossRef CAS.
- Y. Zhang, L. Tang, L. Sun, J. Bao, C. Song, L. Huang, K. Liu, Y. Tian, G. Tian, Z. Li, H. Sun and L. Mei, A novel paclitaxel-loaded poly(ε-caprolactone)/poloxamer 188 blend nanoparticle overcoming multidrug resistance for cancer treatment, Acta Biomater., 2010, 6, 2045–2052 CrossRef CAS.
- N. Ramalingam, G. Natesan, B. Dhandayuthapani, P. Perumal, J. Balasundaram and S. Natesan, Design and characterization of ofloxacin niosomes, Pak. J. Pharm. Sci., 2013, 26, 1089–1096 CAS.
- J. D. Robertson, L. Rizzello, M. Avila-Olias, J. Gaitzsch, C. Contini, M. S. Magoń, S. A. Renshaw and G. Battaglia, Purification of nanoparticles by size and shape, Sci. Rep., 2016, 6, 27494–27502 CrossRef CAS.
- K. Lia and B. Liu, Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC.
- S. Nandhakumar, M. D. Dhanaraju, V. D. Sundar and B. Heera, Influence of surface charge on the in vitro protein adsorption and cell cytotoxicity of paclitaxel loaded poly(ε-caprolactone) nanoparticles, Bull. Fac. Pharm., 2017, 55, 249–258 Search PubMed.
- I. Lynch and K. A. Dawson, Protein-nanoparticle interactions, Nano Today, 2008, 3, 40–47 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05809b |
| This journal is © The Royal Society of Chemistry 2020 |