Open Access Article
Open Access ArticlePreparation and facile addition reactions of iminium salts derived from amino ketene silyl acetal and amino silyl enol ether†
Makoto Shimizu *ab,
Shingo Hatab,
Koichi Kondob,
Kazuhiro Murakamib,
Isao Mizota
*ab,
Shingo Hatab,
Koichi Kondob,
Kazuhiro Murakamib,
Isao Mizota b and
Yusong Zhua
b and
Yusong Zhua
aSchool of Energy Science and Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu Province, China
bDepartment of Chemistry for Materials, Graduate School of Engineering, Mie University, Tsu, Mie 514-8507, Japan. E-mail: mshimizu@chem.mie-u.ac.jp
First published on 24th July 2020
Abstract
While iminium salts generated by the oxidation of amino ketene silyl acetals show intriguing reactivities to give useful γ-oxo-α-amino esters via reactions with silyl enol ethers in good yields, new iminium salts are also prepared by the oxidation of amino silyl enol ethers. They undergo facile addition reaction with various nucleophiles to give α-amino ketone derivatives in good yields.
1 Introduction
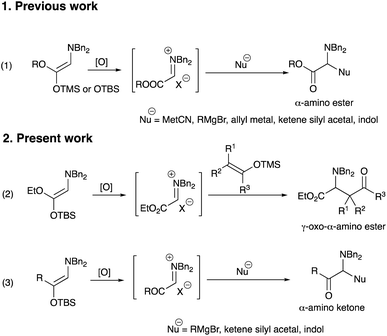
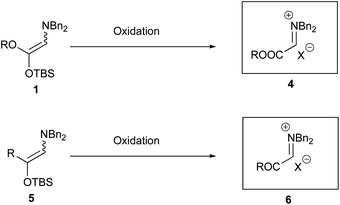
Iminium salts are usually very reactive species and used widely in organic synthesis. Several methods are known to generate these useful species. Among them the synthesis of natural products, such as alkaloids, was reported using cyclization mediated by iminium salts as a crucial step.1 Representative examples using iminium species involve the addition of organometallic reagents for the synthesis of β-amino acids,2a,b β-amino ketones, 1,3-amino alcohols,2c and so on.2d−j The synthesis of α-monosubstituted α-amino acids was reported by the nucleophilic addition of boronates to the iminium ions, which were prepared by the condensation of primary or secondary amines with ethyl glyoxylate in situ.3 Although the use of iminium salts is a promising procedure, it is not trivial to generate these species in a chemo- and regioselective manner under mild reaction conditions.We have been interested in the generation of iminium salts and have already disclosed several intriguing features.4 In these studies, we focused on the generation of iminium species by oxidizing a readily accessible and stable enol derivative with oxidants and found that the alkoxycarbonyl iminium salts from amino ketene silyl acetals were reasonably stable5 and highly reactive to give addition products with metal cyanides, Grignard reagents, allyl metals,4b ketene silyl acetals,4g and indoles4c (eqn (1), Scheme 1). However, we have not fully studied reactions with silyl enol ethers, since an initial examination using the silyl enol ether derived from cyclopentanone met with a disappointing result where only a trace amount of the addition product was obtained. We now reexamined the reaction with other silyl enol ethers and found that an acceptable range of product yields was obtained under carefully controlled conditions. We did not study generation and reaction of the iminium salts derived from amino silyl enol ethers, either, although these iminium salts are highly attractive in terms of reactivity and chemoselectivity on an addition reaction with nucleophiles. This paper describes addition reactions of the alkoxycarbonyl iminium salts with silyl enol ethers to produce γ-oxo-α-amino esters (eqn (2)). Reactions of the iminium salts derived from amino silyl enol ethers with various nucleophiles are also presented to give addition products (eqn (3)).
2 Results and discussion
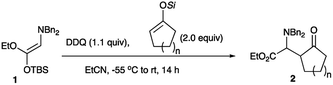
The starting amino ketene silyl acetal 1 was readily prepared according to the reported procedures in good overall yield.4b,f First, silyl enol ethers derived from cyclic ketones were subjected to the addition reaction, and Table 1 summarizes the results.Although an initial examination was carried out with the TMS enol ether derived from cyclopentanone, only a small amount of the desired addition product was obtained (entry 1). Switching the ring size from 5 to 6, 7, and 8 resulted in the formation of the desired products in 73, 71, and 68% yields, respectively (entries 2, 5, and 6). We next examined the effect of the silyl substituent on the diastereoselectivity.6 However, the use of bulky substituents such as TBS and TIPS did not improve the diastereoselectivity (entries 3 and 4). Reactions of acyclic silyl enol ethers were next examined. Table 2 summarizes the results.
| Entry | R | Si | Z/E | Solv. | Lewis acid | Yield (%)a | syn/anti |
|---|---|---|---|---|---|---|---|
| a Isolated yield.b Abbrebiation, DMS: dimethylsilyl. | |||||||
| 1 | Cy | TMS | 85/15 | EtCN | — | 3a: 32 | 52/48 |
| 2 | Cy | TMS | 85/15 | DMF | — | 3a: 67 | 77/23 |
| 3 | Cy | TMS | 85/15 | DME | BF3·Et2O | 3a: 22 | 76/24 |
| 4 | Cy | TBS | 85/15 | DMF | — | 3a: 62 | 78/22 |
| 5 | Cy | TBS | 85/15 | DME | BF3·Et2O | 3a: 25 | 65/35 |
| 6 | Ph | TMS | 90/10 | DME | BF3·Et2O | 3b: 57 | 57/43 |
| 7 | Ph | TMS | 90/10 | DME | Et2AlCl | 3b: 30 | 66/34 |
| 8 | Ph | TMS | 90/10 | DMF | — | 3b: 78 | 51/49 |
| 9 | Ph | TBS | 90/10 | DME | — | 3b: 8 | 60/40 |
| 10 | Ph | TBS | 90/10 | DME | BF3·Et2O | 3b: 38 | 58/42 |
| 11 | Ph | TBS | 90/10 | DME | Et2AlCl | 3b: 31 | 72/28 |
| 12 | Ph | TBS | 90/10 | DMF | — | 3b: 64 | 56/44 |
| 13 | Ph | TIPS | 85/15 | DME | — | 3b: 13 | 92/8 |
| 14 | Ph | TIPS | 85/15 | DME | BF3·Et2O | 3b: 60 | 86/14 |
| 15 | Ph | TIPS | 85/15 | DME | Et2AlCl | 3b: 30 | 88/12 |
| 16 | Ph | TIPS | 85/15 | DMF | — | 3b: 79 | 60/40 |
| 17 | Ph | DMSb | 88/12 | DME | — | 3b: 57 | 73/27 |
| 18 | Ph | DMS | 88/12 | DME | BF3·Et2O | 3b: 40 | 51/49 |
| 19 | Ph | DMS | 88/12 | DMF | — | 3b: 62 | 59/41 |
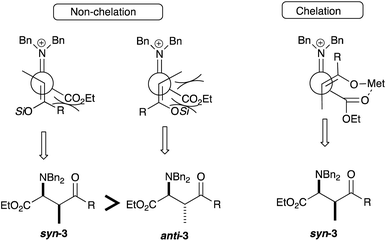
In terms of the product yields using the silyl enol ether derived from 1-cyclohexylpropan-1-one, the reaction in EtCN did not give a satisfactory result (entry 1), whereas the use of DMF in the absence of a Lewis acid gave better results (entries 2 and 4). The effects of a Lewis acid are not prominent on the diastereoselctivity, giving the addition products with moderate selectivities (entries 2 to 5).7 Regarding the silyl enol ether derived from propiophenone, both TMS and TIPS derivatives in DMF in the absence of a Lewis acid gave high yields of the product (entries 8 and 16). In general, the TIPS derivative recorded good syn-selectivities (entries 13 to 15). Among them the reaction in the presence of a Lewis acid (BF3·Et2O or Et2AlCl) gave the syn-adduct with good selectivities (entries 14 and 15), whereas the presence of a Lewis acid destroyed selectivities in certain cases (entries 6, 10, and 18). For the explanation of the syn-selectivity, Scheme 2 shows possible transition state models.
In the absence of a Lewis acid or in the presence of BF3·Et2O (monodentate Lewis acid), the non-chelation model would explain the preferred formation of the syn-isomer 3 due to the less steric congestion. In the cases where the reaction was carried out in the presence of Et2AlCl (bidentate Lewis acid), the chelation model would also support the formation of the syn-isomer 3. On the basis of the above transition state models, the formation of the syn-isomer 3 would be preferred regardless of the presence of Lewis acids.
During investigations into the reactivity of iminium salts 4 derived from amino ketene silyl acetals, new iminium salts from amino silyl enol ethers intrigued us (Scheme 3). However, no reliable reports are available for the generation of the iminium salts of type 6. We therefore investigated the generation of this type of iminium salt using a method similar to those from amino ketene silyl acetals.
The amino silyl enol ether 5 was readily prepared as follows in good yield (Scheme 4).
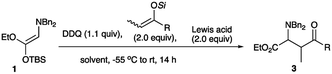
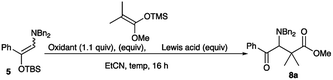
First, the formation of the iminium salt followed by addition reactions using the amino silyl enol ether 5 was examined to find the best reaction conditions involving the oxidation reagent and a Lewis acid. Table 3 summarizes the results. Iodosylbenzene did not effect the oxidation, while the use of BPO,4a NCS, and DBDMH gave the desired product in low yields (entries 1 to 4). DDQ4b,8 which was used for the oxidation of the amino ketene silyl acetal 1 effected the reaction to give the addition product 8a in 24% yield (entry 5). Among the oxidation reagents examined here the use of NBS recorded the best result (entry 6).4f We next examined the use of a Lewis acid for the present transformation. Et2AlCl was not effective, while an increased amount of the addition product was obtained in the presence of TiCl4 (entries 7 and 8). A better result was obtained when the reaction was carried out with NBS in the presence of BF3·Et2O, and the addition product was obtained in 74% yield (entry 9). The amounts of ketene silyl acetal and BF3·Et2O were further examined. The use of the reduced amounts of the ketene silyl acetal (1.5 equiv.) and BF3·Et2O (1.2 equiv.) gave a slightly better result (entry 10). Regarding the reaction temperature, the reaction at a higher temperature gave a reduced amount of the desired product (entry 13). The best result was obtained when the reaction was carried out with the ketene silyl acetal (1.5 equiv.) and BF3·Et2O (2.0 equiv.) at rt, and the addition product 8a was obtained in 80% yield (entry 15). Under the optimized conditions, various ketene silyl acetals were subjected to this addition reaction, and Table 4 summarizes the results.
| Entry | Oxidant | Nu (equiv.) | Lewis acid (equiv.) | Temp | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | PhIO | 2.0 | — | rt | 0 |
| 2 | BPO | 2.0 | — | rt | 7 |
| 3 | NCS | 2.0 | — | rt | 2 |
| 4 | DBDMH | 2.0 | — | rt | 10 |
| 5 | DDQ | 2.0 | — | rt | 24 |
| 6 | NBS | 2.0 | — | rt | 27 |
| 7 | NBS | 2.0 | Et2AlCl (2.0) | rt | 10 |
| 8 | NBS | 2.0 | TiCl4 (2.0) | rt | 48 |
| 9 | NBS | 2.0 | BF3·Et2O (2.0) | rt | 74 |
| 10 | NBS | 1.5 | BF3·Et2O (1.2) | rt | 76 |
| 11 | NBS | 1.5 | BF3·Et2O (1.5) | rt | 69 |
| 12 | NBS | 1.2 | BF3·Et2O (2.0) | rt | 53 |
| 13 | NBS | 1.5 | BF3·Et2O (2.0) | 50 °C | 40 |
| 14 | NBS | 1.5 | BF3·Et2O (2.0) | 0 °C to rt | 77 |
| 15 | NBS | 1.5 | BF3·Et2O (2.0) | rt | 80 |
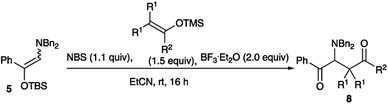
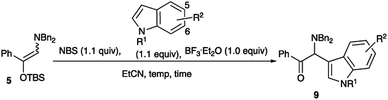
As shown in Table 4, tetra-substituted ketene silyl acetals underwent addition reaction readily to give the adducts in good yields. Regarding the ester alkoxy part, methyl and ethyl esters were obtained in good yields (entries 1 and 2), whereas a bulky tbutyl derivative recorded a decreased yield of the product (entry 4). Di-substituted thioester also participated in the present addition to give the adduct in moderate yield (entry 7). We next examined the use of indole derivatives as nucleophiles, since indole skeletons are often found in many biologically active compounds such as tryptophan, indomethacin, and dragmacidin derivatives, and several methodologies have been reported to functionalize indoles.9 Table 5 summarizes the results.
N-TIPS indole was subjected to the present reaction conditions to give a moderate yield of the addition product 9a (entry 1). The best result was obtained when the reaction was conducted at 0 °C to rt for 5 h (entry 2). An electron-withdrawing group, 5-nitro substituent decreased the product yield considerably (entry 5), whereas 5-MeO, 5-Br, and 6-Br groups did not affect the addition reaction to give the adducts in good yields (entries 6 to 8). However, N–H free and N-Ts derivatives were not suitable for the present reaction, presumably due to the decreased electron-density at the 3-position of the indole skeleton (entries 9 and 10). We next examined the use of Grignard reagents as nucleophiles.
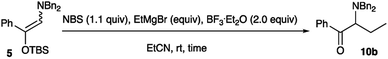
Treatment of the amino silyl enol ether 5 with NBS followed by ethylmagnesium bromide in the presence of BF3·Et2O actually gave the ethylated product 10b. Table 6 summarizes the optimization of reaction conditions.
As can be seen form Table 6, the best conditions were found when the amino silyl enol ether 5 was treated with NBS (1.1 equiv.) and ethylmagnesium bromide (2.0 equiv.) in the presence of BF3·Et2O (2.0 equiv.) in EtCN at rt for 30 min, and the ethylation product 10b was obtained in 74% yield (entry 4). Under the optimized conditions, a variety of Grignard reagents were subjected to the alkylation, and Table 7 summarizes the results.
| Entry | R | Product | Yielda (%) | Yieldb (%) |
|---|---|---|---|---|
| a RMgBr (2.0 equiv.) was used.b RMgBr (2.5 equiv.) was used.c BnMgCl was used. | ||||
| 1 | Me | 10a | 46 | 64 |
| 2 | Et | 10b | 74 | — |
| 3 | nPr | 10c | 71 | 62 |
| 4 | iPr | 10d | 63 | 63 |
| 5 | cPr | 10e | 27 | 35 |
| 6 | Cy | 10f | 58 | 60 |
| 7 | Bnc | 10g | 30 | 43 |
| 8 | Ph | 10h | 0 | 0 |
| 9 | 4-MeC6H4 | 10i | 46 | 38 |
| 10 | 2-Thienyl | 10j | 64 | 45 |
| 11 | Ethynyl | 10k | 0 | 0 |
Methyl, ethyl, npropyl, iso-propyl, cyclohexyl, and 2-thiehyl Grignard reagents recorded good product yields (entries 1–4, 6, and 10), whereas cylopropyl, benzyl, and 4-tolyl derivatives gave the addition products in moderate yields (entries 5, 6, and 9). However, phenyl and ethylnylmagnesium bromides did not give the desired products but gave complex mixtures (entries 8 and 11). Compared with the results obtained from the reactions of the iminium salts generated by the oxidation of amino ketene silyl acetals with Grignard reagents,4b this α-amino ketone-derived iminium salt appears to be only a little bit less reactive than ester-derived ones. Thus, the present iminium salt derived from α-amino ketone shows good reactivity as an electrophile to give a variety of addition products. The following Scheme 5 shows possible reaction pathways.
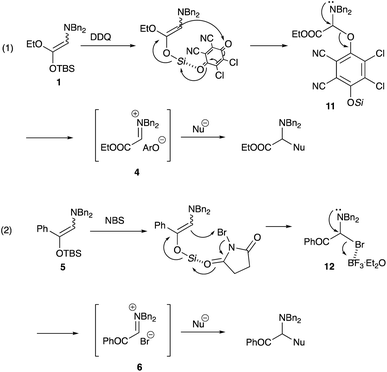
Regarding the formation of the iminium salt 4 derived from α-amino ester, DDQ oxidizes the amino ketene silyl acetal 1 to form the N,O-acetal 11, which collapses to form the iminium salt 4. This iminium salt 4 is responsible for the formation of the addition products with silyl enol ethers. In the case of α-amino ketone, NBS reacts with the amino silyl enol ether 5 to form the bromide 12. An activation with BF3·Et2O promotes the elimination of a Br ion to form the iminium salt 6, which reacts with a variety of nucleophiles.
3 Conclusions
In conclusion, the iminium salt prepared from amino ketene silyl acetal showed good reactivity and reacted with silyl enol ethers to give γ-oxo-α-amino esters in good yields. Regarding the diastereoseletivity of the reactions with the silyl enol ethers derived from ethyl ketones, a good syn-selectivitiy was observed. In particular, in the case with the TIPS enol ether derived from propiophenone, the presence of Lewis acids improved the syn-selectivity. The formation of the iminium salt derived from 2-aminoacetophenone was readily carried out via the oxidation of its TBS enol ether with NBS. The presence of BF3·Et2O facilitated the reaction to give addition products in good yields. These results indicate that the iminium salts possessing an electron-withdrawing groups next to the iminium carbon are readily prepared via the oxidation of their trialkylsilyl enolates with certain oxidants. These iminium species are reasonably reactive and act as good acceptors of nucleophiles to give addition products with ketene silyl acetals, indoles, and Grignard reagents in good yields.4 Experimental
General aspects
Infrared spectra were determined on a JASCO FT/IR-460 plus spectrometer. 1H NMR and 13C NMR spectra were recorded with a JEOL ECX-400P, or a JEOL A-500 spectrometer using tetramethylsilane as an internal standard. Mass spectra were recorded on a JEOL MS-700D spectrometer. Propionitrile (EtCN) was distilled from phosphorus pentaoxide and then from calcium hydride, and stored over molecular sieves 4 Å. Dimethyl formamide (DMF) was distilled from calcium hydride and stored over molecular sieves 4 Å. Dimethoxyethane (DME) was distilled from calcium hydride and then cupper(I) chloride, and stored over sodium. 1-Ethoxy-2-dibenzylamino-1-tbutyldimethylsiloxyethylene 13b was synthesized by the reported procedure. Purification of products was performed by column chromatography on silica gel (Kanto Silica Gel 60N) and/or preparative TLC on silica gel (Merck Kiesel Gel GF254 or Wako Gel B-5F).Synthesis of ethyl 2-(dibenzylamino)-2-(2-oxocyclohexyl)acetate 2b (general procedure for the addition reaction with cyclic silyl enol ethers)
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was introduced a solution of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (37.4 mg, 0.165 mmol) in EtCN (1.0 mL). The solution was cooled to −55 °C, and to it were added successively solutions of 1-ethoxy-2-dibenzylamino-1-tbutyldimethylsiloxyethylene 1 (59.6 mg, 0.150 mmol) in EtCN (1 mL) and 1-cyclohexenyloxytrimethylsilane (25.0 mg, 0.150 mmol) in EtCN (1 mL). The reaction mixture was allowed to warm to room temperature with stirring for 14 h. The reaction was quenched with 10% aq. Na2CO3, and the whole mixture was extracted with ethyl acetate (10 mL × 3). The combined extracts were washed with brine (15 mL), dried (Na2SO4), and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nhexane = 6
nhexane = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound (syn-2b (27.2 mg, 48%) and anti-2b (14.0 mg, 25%)).
1) to give the title compound (syn-2b (27.2 mg, 48%) and anti-2b (14.0 mg, 25%)).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.02–1.61 (m, 6H including triplet at 1.40 ppm, J = 7.3 Hz, 3H), 1.86–2.05 (m, 2H), 2.26–2.35 (m, 2H), 2.59–2.63 (m, 1H), 2.96–2.98 (m, 1H), 3.37 (d, J = 13.7 Hz, 2H), 3.39 (d, J = 10.7 Hz, 1H), 3.86 (d, J = 13.7 Hz, 2H), 4.29 (dq, J = 7.3, 10.9 Hz, 1H), 4.30 (dq, J = 7.3, 10.9 Hz, 1H), 7.22–7.37 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.8, 25.2, 28.1, 31.1, 42.5 50.0, 54.8, 60.3, 60.5, 127.2, 128.4, 129.1, 139.2, 170.9, 212.2; IR (neat) 1712, 1637, 1453, 1176, 1150, 1030, 799, 700, 621, 496, 470, 438, 420 cm−1; HRMS (EI) calcd for C24H29NO3 (M)+ 379.2147, found 379.2164.
1); 1H NMR (400 MHz, CDCl3) δ 1.02–1.61 (m, 6H including triplet at 1.40 ppm, J = 7.3 Hz, 3H), 1.86–2.05 (m, 2H), 2.26–2.35 (m, 2H), 2.59–2.63 (m, 1H), 2.96–2.98 (m, 1H), 3.37 (d, J = 13.7 Hz, 2H), 3.39 (d, J = 10.7 Hz, 1H), 3.86 (d, J = 13.7 Hz, 2H), 4.29 (dq, J = 7.3, 10.9 Hz, 1H), 4.30 (dq, J = 7.3, 10.9 Hz, 1H), 7.22–7.37 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.8, 25.2, 28.1, 31.1, 42.5 50.0, 54.8, 60.3, 60.5, 127.2, 128.4, 129.1, 139.2, 170.9, 212.2; IR (neat) 1712, 1637, 1453, 1176, 1150, 1030, 799, 700, 621, 496, 470, 438, 420 cm−1; HRMS (EI) calcd for C24H29NO3 (M)+ 379.2147, found 379.2164.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 1.35–1.81 (m, 10H including triplet at 1.56 ppm, J = 7.3 Hz), 2.02–2.05 (m, 1H), 3.04–3.08 (m, 1H), 3.32 (d, J = 13.1 Hz, 2H), 3.88 (d, J = 11.0 Hz, 1H), 3.96 (d, J = 13.1 Hz, 2H), 4.27 (dq, J = 7.3, 10.7 Hz, 1H), 4.35 (dq, J = 7.3, 10.7 Hz, 1H), 7.22–7.33 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.8, 20.7, 28.0, 38.0, 49.5, 53.6, 54.5, 60.6, 61.2, 127.3, 128.5, 129.0, 139.2, 170.5, 219.0; IR (neat) 2860, 1710, 1638, 1494, 1451, 1373, 1335, 1307, 1233, 1175, 1135, 1027, 969, 794, 744, 699, 501 cm−1; HRMS (EI) calcd for C24H29NO3 (M)+ 379.2147, found 379.2148.
1); 1H NMR (500 MHz, CDCl3) δ 1.35–1.81 (m, 10H including triplet at 1.56 ppm, J = 7.3 Hz), 2.02–2.05 (m, 1H), 3.04–3.08 (m, 1H), 3.32 (d, J = 13.1 Hz, 2H), 3.88 (d, J = 11.0 Hz, 1H), 3.96 (d, J = 13.1 Hz, 2H), 4.27 (dq, J = 7.3, 10.7 Hz, 1H), 4.35 (dq, J = 7.3, 10.7 Hz, 1H), 7.22–7.33 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.8, 20.7, 28.0, 38.0, 49.5, 53.6, 54.5, 60.6, 61.2, 127.3, 128.5, 129.0, 139.2, 170.5, 219.0; IR (neat) 2860, 1710, 1638, 1494, 1451, 1373, 1335, 1307, 1233, 1175, 1135, 1027, 969, 794, 744, 699, 501 cm−1; HRMS (EI) calcd for C24H29NO3 (M)+ 379.2147, found 379.2148.Ethyl 2-(dibenzylamino)-2-(2-oxocyclopentyl)acetate 2a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 1.41 (t, J = 7.0 Hz, 3H), 1.59–1.67 (m, 1H), 1.75–1.83 (m, 1H), 1.92–2.07 (m, 2H), 2.24–2.30 (m, 1H), 2.35–2.41 (m, 1H), 2.90–2.96 (m, 1H), 3.19 (d, J = 11.0 Hz, 1H), 3.36 (d, J = 13.7 Hz, 2H), 3.92 (d, J = 13.7 Hz, 2H), 4.28–4.41 (m, 2H), 7.23–7.39 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.6, 20.5, 27.8, 37.9, 49.4 54.3, 60.4, 61.0, 127.2, 128.3, 128.8, 139.0, 170.3, 218.9; IR (neat) 3029, 2931, 1731, 1494, 1452, 1371, 1311, 1030, 966, 743, 698, 598 cm−1; HRMS (EI) calcd for C23H27NO3(M)+ 365.1991, found 365.2003.
1); 1H NMR (500 MHz, CDCl3) δ 1.41 (t, J = 7.0 Hz, 3H), 1.59–1.67 (m, 1H), 1.75–1.83 (m, 1H), 1.92–2.07 (m, 2H), 2.24–2.30 (m, 1H), 2.35–2.41 (m, 1H), 2.90–2.96 (m, 1H), 3.19 (d, J = 11.0 Hz, 1H), 3.36 (d, J = 13.7 Hz, 2H), 3.92 (d, J = 13.7 Hz, 2H), 4.28–4.41 (m, 2H), 7.23–7.39 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.6, 20.5, 27.8, 37.9, 49.4 54.3, 60.4, 61.0, 127.2, 128.3, 128.8, 139.0, 170.3, 218.9; IR (neat) 3029, 2931, 1731, 1494, 1452, 1371, 1311, 1030, 966, 743, 698, 598 cm−1; HRMS (EI) calcd for C23H27NO3(M)+ 365.1991, found 365.2003.Ethyl 2-(dibenzylamino)-2-(2-oxocycloheptyl)acetate 2c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.85–0.99 (m, 1H), 1.09–1.18 (m, 1H), 1.21–1.40 (m, 5H including triplet at 1.38 ppm, J = 7.3 Hz, 3H), 1.60–1.94 (m, 4H), 2.23–2.32 (m, 1H), 2.53–2.60 (m, 1H), 3.08–3.15 (m, 1H), 3.45 (d, J = 13.8 Hz, 2H), 3.48 (d, J = 11.0 Hz, 1H), 3.85 (d, J = 13.8 Hz, 2H), 4.16–4.35 (m, 2H), 7.21–7.34 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.8, 23.3, 27.6, 28.7, 29.3, 43.6, 50.4, 55.6, 60.4, 61.9, 127.2, 128.4, 129.3, 139.2, 171.7, 215.0; IR (neat) 3029, 2929, 2853, 1453, 1375,1183, 1134, 1024, 938, 750, 700 cm−1; HRMS (EI) calcd for C25H31NO3 (M)+ 393.2304, found 393.2327.
1); 1H NMR (400 MHz, CDCl3) δ 0.85–0.99 (m, 1H), 1.09–1.18 (m, 1H), 1.21–1.40 (m, 5H including triplet at 1.38 ppm, J = 7.3 Hz, 3H), 1.60–1.94 (m, 4H), 2.23–2.32 (m, 1H), 2.53–2.60 (m, 1H), 3.08–3.15 (m, 1H), 3.45 (d, J = 13.8 Hz, 2H), 3.48 (d, J = 11.0 Hz, 1H), 3.85 (d, J = 13.8 Hz, 2H), 4.16–4.35 (m, 2H), 7.21–7.34 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.8, 23.3, 27.6, 28.7, 29.3, 43.6, 50.4, 55.6, 60.4, 61.9, 127.2, 128.4, 129.3, 139.2, 171.7, 215.0; IR (neat) 3029, 2929, 2853, 1453, 1375,1183, 1134, 1024, 938, 750, 700 cm−1; HRMS (EI) calcd for C25H31NO3 (M)+ 393.2304, found 393.2327.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 1.03–1.40 (m, 6H including a triplet at 1.38 ppm, J = 7.0 Hz, 3H), 1.56–1.79 (m, 5H), 2.02–2.15 (m, 2H), 3.13–3.21 (m, 1H), 3.28 (d, J = 13.3 Hz, 2H), 3.60 (d, J = 11.4 Hz, 1H), 3.96 (d, J = 13.3 Hz, 2H), 4.21–4.37 (m, 2H), 7.18–7.32 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.8, 25.7, 26.8, 28.0, 29.6, 41.2, 52.3, 54.8, 60.5, 63.6, 127.2, 128.3, 129.4, 139.1, 169.9, 212.7; IR (neat) 3027, 2933, 2855, 1722, 1494, 1451, 1370, 1326, 1236, 1170, 1025, 750 cm−1; HRMS (EI) calcd for C25H31NO3 (M)+ 393.2304, found 393.2324.
1); 1H NMR (500 MHz, CDCl3) δ 1.03–1.40 (m, 6H including a triplet at 1.38 ppm, J = 7.0 Hz, 3H), 1.56–1.79 (m, 5H), 2.02–2.15 (m, 2H), 3.13–3.21 (m, 1H), 3.28 (d, J = 13.3 Hz, 2H), 3.60 (d, J = 11.4 Hz, 1H), 3.96 (d, J = 13.3 Hz, 2H), 4.21–4.37 (m, 2H), 7.18–7.32 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.8, 25.7, 26.8, 28.0, 29.6, 41.2, 52.3, 54.8, 60.5, 63.6, 127.2, 128.3, 129.4, 139.1, 169.9, 212.7; IR (neat) 3027, 2933, 2855, 1722, 1494, 1451, 1370, 1326, 1236, 1170, 1025, 750 cm−1; HRMS (EI) calcd for C25H31NO3 (M)+ 393.2304, found 393.2324.Ethyl 2-(dibenzylamino)-2-(2-oxocyclooctyl)acetate 2d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.92–0.95 (m, 1H), 1.08–1.13 (m, 1H), 1.17–1.26 (m, 1H), 1.37–1.57 (m, 9H including triplet at 1.38 ppm, J = 7.0 Hz, 3H), 1.69–1.73 (m, 1H), 2.00–2.04 (m, 1H), 2.14 (d,d,d, J = 2.8 Hz, 7.6 Hz, 15.1 Hz, 1H), 3.19 (d,t, J = 3.7 Hz, 10.6 Hz, 1H), 3.46 (d, J = 13.4 Hz, 2H), 3.58 (d, J = 10.6 Hz, 1H), 3.85 (d, J = 13.4 Hz, 2H), 4.17–4.32 (m, 2H), 7.23–7.34 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.7, 22.8, 24.4, 25.1, 28.5, 31.8 43.9, 48.0, 55.6, 60.4, 63.8, 127.2, 128.3, 129.3, 139.0, 171.8, 219.2; IR (neat) 2931, 2854, 1719, 1696, 1647,1454, 1027, 748, 698 cm−1; HRMS (EI) calcd for C26H33NO3 (M)+ 407.2460, found 407.2457.
1); 1H NMR (400 MHz, CDCl3) δ 0.92–0.95 (m, 1H), 1.08–1.13 (m, 1H), 1.17–1.26 (m, 1H), 1.37–1.57 (m, 9H including triplet at 1.38 ppm, J = 7.0 Hz, 3H), 1.69–1.73 (m, 1H), 2.00–2.04 (m, 1H), 2.14 (d,d,d, J = 2.8 Hz, 7.6 Hz, 15.1 Hz, 1H), 3.19 (d,t, J = 3.7 Hz, 10.6 Hz, 1H), 3.46 (d, J = 13.4 Hz, 2H), 3.58 (d, J = 10.6 Hz, 1H), 3.85 (d, J = 13.4 Hz, 2H), 4.17–4.32 (m, 2H), 7.23–7.34 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.7, 22.8, 24.4, 25.1, 28.5, 31.8 43.9, 48.0, 55.6, 60.4, 63.8, 127.2, 128.3, 129.3, 139.0, 171.8, 219.2; IR (neat) 2931, 2854, 1719, 1696, 1647,1454, 1027, 748, 698 cm−1; HRMS (EI) calcd for C26H33NO3 (M)+ 407.2460, found 407.2457.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 1.26–1.85 (m, 13H including a triplet at 1.38 ppm, J = 7.0 Hz, 3H), 2.04–2.19 (m, 2H), 3.25 (d, J = 13.7 Hz, 2H), 3.27 (d, J = 10.8 Hz, 1H), 3.56 (d, J = 11.6 Hz, 1H), 3.98 (d, J = 13.7 Hz, 2H), 4.21–4.37 (m, 2H), 7.22–7.34 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.7, 24.6, 25.8, 26.1, 26.7, 28.9, 41.1, 50.4, 54.8, 60.3, 63.8, 127.1, 128.2, 129.2, 138.7, 170.0, 216.0; IR (neat) 2930, 1727, 1702, 1494, 1453, 1154, 1135, 1028, 749, 699 cm−1; HRMS (EI) calcd for C26H33NO3 (M)+ 407.2460, found 407.2460.
1); 1H NMR (500 MHz, CDCl3) δ 1.26–1.85 (m, 13H including a triplet at 1.38 ppm, J = 7.0 Hz, 3H), 2.04–2.19 (m, 2H), 3.25 (d, J = 13.7 Hz, 2H), 3.27 (d, J = 10.8 Hz, 1H), 3.56 (d, J = 11.6 Hz, 1H), 3.98 (d, J = 13.7 Hz, 2H), 4.21–4.37 (m, 2H), 7.22–7.34 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 14.7, 24.6, 25.8, 26.1, 26.7, 28.9, 41.1, 50.4, 54.8, 60.3, 63.8, 127.1, 128.2, 129.2, 138.7, 170.0, 216.0; IR (neat) 2930, 1727, 1702, 1494, 1453, 1154, 1135, 1028, 749, 699 cm−1; HRMS (EI) calcd for C26H33NO3 (M)+ 407.2460, found 407.2460.Synthesis of ethyl 4-cyclohexyl-2-(dibenzylamino)-3-methyl-4-oxobutanoate 3a (general procedure for the addition reaction with acyclic silyl enol ethers)
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was introduced a solution of DDQ (37.4 mg, 0.165 mmol) in EtCN (1.0 mL). The solution was cooled to −55 °C, and to it were added successively solutions of 1-ethoxy-2-dibenzylamino-1-tbutyldimethylsiloxyethylene 1 (59.6 mg, 0.150 mmol) in EtCN (1 mL) and (Z)-((1-cyclohexylprop-1-en-1-yl)oxy)trimethylsilane (63.6 mg, 0.150 mmol) in EtCN (1 mL). The reaction mixture was allowed to warm to room temperature with stirring for 14 h. The reaction was quenched with 10% aq. Na2CO3, and the whole mixture was extracted with ethyl acetate (10 mL × 3). The combined extracts were washed with brine (15 mL), dried (Na2SO4), and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nhexane = 5
nhexane = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound (syn-3a (32.5 mg, 52%) and anti-3a (9.7 mg, 15%)).10
1) to give the title compound (syn-3a (32.5 mg, 52%) and anti-3a (9.7 mg, 15%)).10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.86 (d, J = 6.9 Hz, 3H), 0.95–1.37 (m, 9H including a triplet at 1.29 ppm, J = 7.1 Hz, 3H), 1.61–1.82 (m, 4H), 2.14–2.22 (m, 1H), 3.22–3.31 (m, 3H including a doublet at 3.29 ppm, J = 14.2 Hz, 3H), 3.59 (d, J = 11.0 Hz, 1H), 3.95 (d, J = 14.2 Hz, 2H), 4.11–4.26 (m, 2H), 7.15–7.26 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.5, 15.0, 25.2, 25.8, 25.9, 28.0, 28.7, 43.7, 51.0, 55.2, 60.2, 65.0, 127.1, 128.2, 128.8, 138.5, 170.4, 213.9; IR (neat) 3027, 2933, 2852, 1728, 1711, 1495, 1450, 1375, 1027, 994, 732, 698 cm−1; HRMS (EI) calcd for C27H35NO3 (M)+ 421.2617, found 421.2623.
1); 1H NMR (400 MHz, CDCl3) δ 0.86 (d, J = 6.9 Hz, 3H), 0.95–1.37 (m, 9H including a triplet at 1.29 ppm, J = 7.1 Hz, 3H), 1.61–1.82 (m, 4H), 2.14–2.22 (m, 1H), 3.22–3.31 (m, 3H including a doublet at 3.29 ppm, J = 14.2 Hz, 3H), 3.59 (d, J = 11.0 Hz, 1H), 3.95 (d, J = 14.2 Hz, 2H), 4.11–4.26 (m, 2H), 7.15–7.26 (m, 10H); 13C NMR (100 MHz, CDCl3) δ 14.5, 15.0, 25.2, 25.8, 25.9, 28.0, 28.7, 43.7, 51.0, 55.2, 60.2, 65.0, 127.1, 128.2, 128.8, 138.5, 170.4, 213.9; IR (neat) 3027, 2933, 2852, 1728, 1711, 1495, 1450, 1375, 1027, 994, 732, 698 cm−1; HRMS (EI) calcd for C27H35NO3 (M)+ 421.2617, found 421.2623.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 0.97 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 7.3 Hz, 3H), 1.37 (t, J = 7.1 Hz, 3H), 2.40–2.58 (m, 2H), 3.01–3.09 (m, 1H), 3.45 (d, J = 13.3 Hz, 2H), 3.53 (d, J = 11.0 Hz, 1H), 3.85 (d, J = 13.3 Hz, 2H), 4.14–4.32 (m, 2H), 7.22–7.36 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 17.6, 14.6, 14.8, 34.9, 45.1, 55.2, 60.4, 62.7, 127.2, 128.3, 129.2, 138.9, 171.9, 214.0; IR (neat) 2931, 2851, 1725, 1495, 1452, 1368, 1027, 914, 698 cm−1; HRMS (EI) calcd for C27H35NO3 (M)+ 421.2617, found 421.2619.
1); 1H NMR (500 MHz, CDCl3) δ 0.97 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 7.3 Hz, 3H), 1.37 (t, J = 7.1 Hz, 3H), 2.40–2.58 (m, 2H), 3.01–3.09 (m, 1H), 3.45 (d, J = 13.3 Hz, 2H), 3.53 (d, J = 11.0 Hz, 1H), 3.85 (d, J = 13.3 Hz, 2H), 4.14–4.32 (m, 2H), 7.22–7.36 (m, 10H); 13C NMR (126 MHz, CDCl3) δ 17.6, 14.6, 14.8, 34.9, 45.1, 55.2, 60.4, 62.7, 127.2, 128.3, 129.2, 138.9, 171.9, 214.0; IR (neat) 2931, 2851, 1725, 1495, 1452, 1368, 1027, 914, 698 cm−1; HRMS (EI) calcd for C27H35NO3 (M)+ 421.2617, found 421.2619.Ethyl 2-(dibenzylamino)-3-methyl-4-oxo-4-phenylbutanoate 3b 10
A mixture of syn- and anti-isomers (35.4 mg, 57%, syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti = 57
anti = 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43, ratio determined by 1H NMR). Yellow oil; Rf = 0.52 (nhexane
43, ratio determined by 1H NMR). Yellow oil; Rf = 0.52 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (neat) 3061, 2979, 1724, 1683, 1495, 1027, 748, 698 cm−1; HRMS (EI) calcd for C27H29NO3 (M)+ 415.2147, found 415.2136.
1); IR (neat) 3061, 2979, 1724, 1683, 1495, 1027, 748, 698 cm−1; HRMS (EI) calcd for C27H29NO3 (M)+ 415.2147, found 415.2136.
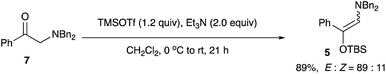
Synthesis of (E)-N,N-dibenzyl-2-[(tert-butyldimethylsilyl)oxy]-2-phenylethen-1-amine 5
Under an argon atmosphere, to a solution of 2-(dibenzylamino)-1-phenylethanone 7 (4.00 g, 12.7 mmol) in CH2Cl2 (19.0 mL) were successively added triethylamine (3.54 mL, 25.4 mmol) and TBSOTf (3.50 mL, 15.2 mmol) at 0 °C. The reaction mixture was allowed to warm to ambient temperature with stirring for 21 h. It was quenched with sat. aq. NaHCO3 (20 mL). The layers were separated, and the aqueous layer was extracted with CH2Cl2 (30 mL × 3). The combined extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt
AcOEt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et3N = 20
Et3N = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1) to give the title amino silyl enol ether 5 (89%, 4.84 g, E
1.1) to give the title amino silyl enol ether 5 (89%, 4.84 g, E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 89
Z = 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11).
11).
Yield 89% (4.84 g); yellow solid; mp = 55–56 °C; Rf = 0.75 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δ 0.15 (s, 6H), 1.11 (s, 9H), 4.33 (s, 4H), 5.82 (s, 1H), 7.32–7.45 (m, 15H); 13C NMR (100 MHz, CDCl3): δ −3.9, 18.3, 26.0, 55.8, 123.4, 124.5, 125.5, 126.9, 127.9, 128.2, 128.7, 133.1, 139.0, 140.4; IR (neat): 3059, 3031, 2926, 2854, 1649, 1453, 1349, 1257, 1162, 1058, 1024, 923, 749, 703 cm−1. HRMS (EI) calcd for C28H35NOSi (M)+ 429.2488, found 429.2500.
1); 1H NMR (400 MHz, CDCl3): δ 0.15 (s, 6H), 1.11 (s, 9H), 4.33 (s, 4H), 5.82 (s, 1H), 7.32–7.45 (m, 15H); 13C NMR (100 MHz, CDCl3): δ −3.9, 18.3, 26.0, 55.8, 123.4, 124.5, 125.5, 126.9, 127.9, 128.2, 128.7, 133.1, 139.0, 140.4; IR (neat): 3059, 3031, 2926, 2854, 1649, 1453, 1349, 1257, 1162, 1058, 1024, 923, 749, 703 cm−1. HRMS (EI) calcd for C28H35NOSi (M)+ 429.2488, found 429.2500.
Synthesis of methyl 3-(dibenzylamino)-2,2-dimethyl-4-oxo-4-phenylbutanoate 8a (general procedure for the reaction of the amino silyl enol ether with ketene silyl acetals)
Under an argon atmosphere, to N-bromosuccinimide (19.6 mg, 0.11 mmol) were successively added a solution of the amino silyl enol ether 5 (42.9 mg, 0.10 mmol) in EtCN (1.0 mL), BF3·Et2O (0.025 mL, 0.20 mmol), and a solution of [(1-methoxy-2-methylprop-1-en-1-yl)oxy]trimethylsilane (26.1 mg, 0.10 mmol) in EtCN (1.0 mL) at room temperature. The reaction mixture was stirred for 16 h. It was quenched with sat. aq. NaHCO3 (10 mL). The layers were separated, and the aqueous layer was extracted with ethyl acetate (30 mL × 3). The combined organic extracts were washed with brine (5 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel (developed twice with nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt = 6
AcOEt = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give methyl 3-(dibenzylamino)-2,2-dimethyl-4-oxo-4-phenylbutanoate 8a (80%, 33.4 mg).
1) to give methyl 3-(dibenzylamino)-2,2-dimethyl-4-oxo-4-phenylbutanoate 8a (80%, 33.4 mg).
Yield 80% (33.4 mg); white solid; mp = 73−74 °C; Rf = 0.48 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.11 (s, 3H), 1.44 (s, 3H), 3.26 (d, J = 14.4 Hz, 2H), 3.49 (s, 3H), 4.01 (d, J = 14.4 Hz, 2H), 4.68 (s, 1H), 7.23–7.37 (m, 10H), 7.52–7.57 (m, 2H), 7.60–7.65 (m, 1H), 7.87–7.90 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 21.6, 25.6, 46.6, 51.8, 56.9, 64.4, 127.2, 128.2, 128.3, 128.7, 129.0, 132.8, 139.4, 141.2, 177.7, 202.1; IR (neat) 2949, 1729, 1678, 1451, 1268, 1145, 970, 745, 700 cm−1; HRMS (EI) calcd for C26H26NO2 (M-CH3O)+ 384.1958, found 384.1961.
1); 1H NMR (400 MHz, CDCl3) δ 1.11 (s, 3H), 1.44 (s, 3H), 3.26 (d, J = 14.4 Hz, 2H), 3.49 (s, 3H), 4.01 (d, J = 14.4 Hz, 2H), 4.68 (s, 1H), 7.23–7.37 (m, 10H), 7.52–7.57 (m, 2H), 7.60–7.65 (m, 1H), 7.87–7.90 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 21.6, 25.6, 46.6, 51.8, 56.9, 64.4, 127.2, 128.2, 128.3, 128.7, 129.0, 132.8, 139.4, 141.2, 177.7, 202.1; IR (neat) 2949, 1729, 1678, 1451, 1268, 1145, 970, 745, 700 cm−1; HRMS (EI) calcd for C26H26NO2 (M-CH3O)+ 384.1958, found 384.1961.
Ethyl 3-(dibenzylamino)-2,2-dimethyl-4-oxo-4-phenylbutanoate 8b
Yield 82% (35.1 mg); white solid; mp = 65−67 °C; Rf = 0.41 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.06 (t, J = 7.3 Hz, 3H), 1.08 (s, 3H), 1.46 (s, 3H), 3.26 (d, J = 14.2 Hz, 2H), 3.90 (dq, J = 7.3, 10.5 Hz, 1H), 3.97–4.05 (m, 3H, including doublet at 4.01 ppm J = 14.2 Hz, 2H), 4.67 (s, 1H), 7.22–7.35 (m, 10H), 7.52–7.56 (m, 2H), 7.59–7.64 (m, 1H), 7.88–7.90 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.9, 21.3, 25.9, 46.5, 56.8, 60.5, 64.4, 127.2, 128.2, 128.4, 128.6, 129.0, 132.8, 139.4, 141.3, 177.3, 202.2; IR (neat): 3061, 3028, 2980, 2842, 1735, 1675, 1455, 1274, 1152, 968, 748, 699 cm−1; HRMS (EI) calcd for C26H26NO2 (M–C2H5O)+ 384.1958, found 384.1957.
1); 1H NMR (400 MHz, CDCl3) δ 1.06 (t, J = 7.3 Hz, 3H), 1.08 (s, 3H), 1.46 (s, 3H), 3.26 (d, J = 14.2 Hz, 2H), 3.90 (dq, J = 7.3, 10.5 Hz, 1H), 3.97–4.05 (m, 3H, including doublet at 4.01 ppm J = 14.2 Hz, 2H), 4.67 (s, 1H), 7.22–7.35 (m, 10H), 7.52–7.56 (m, 2H), 7.59–7.64 (m, 1H), 7.88–7.90 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.9, 21.3, 25.9, 46.5, 56.8, 60.5, 64.4, 127.2, 128.2, 128.4, 128.6, 129.0, 132.8, 139.4, 141.3, 177.3, 202.2; IR (neat): 3061, 3028, 2980, 2842, 1735, 1675, 1455, 1274, 1152, 968, 748, 699 cm−1; HRMS (EI) calcd for C26H26NO2 (M–C2H5O)+ 384.1958, found 384.1957.
Isopropyl 3-(dibenzylamino)-2,2-dimethyl-4-oxo-4-phenylbutanoate 8c
Yield 73% (32.6 mg); white solid mp = 112−113 °C; Rf = 0.55 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.01 (d, J = 6.4 Hz, 3H), 1.02 (s, 3H), 1.08 (d, J = 6.4 Hz, 3H), 1.49 (s, 3H), 3.26 (d, J = 14.2 Hz, 2H), 4.00 (d, J = 14.2 Hz, 2H), 4.64 (s, 1H), 4.84 (sept, J = 6.4 Hz, 1H), 7.22–7.36 (m, 10H), 7.52–7.56 (m, 2H), 7.60–7.64 (m, 1H), 7.88–7.91 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.7, 21.3, 21.6, 26.5, 46.4, 56.8, 64.5, 67.8, 127.2, 128.2, 128.4, 128.6, 129.0, 132.7, 139.4, 141.4, 176.9, 202.3; IR (neat): 3063, 3030, 2981, 2855, 1711, 1676, 1455, 1281, 1165, 1107, 970, 749, 694 cm−1; HRMS (EI): calcd for C26H26NO2 (M-C3H7O)+ 384.1958, found 384.1957.
1); 1H NMR (400 MHz, CDCl3) δ 1.01 (d, J = 6.4 Hz, 3H), 1.02 (s, 3H), 1.08 (d, J = 6.4 Hz, 3H), 1.49 (s, 3H), 3.26 (d, J = 14.2 Hz, 2H), 4.00 (d, J = 14.2 Hz, 2H), 4.64 (s, 1H), 4.84 (sept, J = 6.4 Hz, 1H), 7.22–7.36 (m, 10H), 7.52–7.56 (m, 2H), 7.60–7.64 (m, 1H), 7.88–7.91 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.7, 21.3, 21.6, 26.5, 46.4, 56.8, 64.5, 67.8, 127.2, 128.2, 128.4, 128.6, 129.0, 132.7, 139.4, 141.4, 176.9, 202.3; IR (neat): 3063, 3030, 2981, 2855, 1711, 1676, 1455, 1281, 1165, 1107, 970, 749, 694 cm−1; HRMS (EI): calcd for C26H26NO2 (M-C3H7O)+ 384.1958, found 384.1957.
tert-Butyl 3-(dibenzylamino)-2,2-dimethyl-4-oxo-4-phenylbutanoate 8d
Yield 57% (26.3 mg); white solid; mp = 115−117 °C; Rf = 0.58 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.98 (s, 3H), 1.27 (s, 9H), 1.47 (s, 3H), 3.28 (d, J = 14.2 Hz, 2H), 3.98 (d, J = 14.2 Hz, 2H), 4.62 (s, 1H), 7.22–7.37 (m, 10H), 7.51–7.56 (m, 2H), 7.59–7.63 (m, 1H), 7.88–7.90 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.6, 26.8, 27.8, 46.9, 56.7, 64.6, 80.5, 127.2, 128.2, 128.5, 128.6, 129.0, 132.6, 139.4, 141.5, 176.6, 202.4; IR (neat): 2976, 1678, 1456, 1247, 1146, 968, 848, 698 cm−1; HRMS (EI) calcd for C30H35NO3 (M)+ 457.2617, found 457.2607.
1); 1H NMR (400 MHz, CDCl3) δ 0.98 (s, 3H), 1.27 (s, 9H), 1.47 (s, 3H), 3.28 (d, J = 14.2 Hz, 2H), 3.98 (d, J = 14.2 Hz, 2H), 4.62 (s, 1H), 7.22–7.37 (m, 10H), 7.51–7.56 (m, 2H), 7.59–7.63 (m, 1H), 7.88–7.90 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.6, 26.8, 27.8, 46.9, 56.7, 64.6, 80.5, 127.2, 128.2, 128.5, 128.6, 129.0, 132.6, 139.4, 141.5, 176.6, 202.4; IR (neat): 2976, 1678, 1456, 1247, 1146, 968, 848, 698 cm−1; HRMS (EI) calcd for C30H35NO3 (M)+ 457.2617, found 457.2607.
Methyl 3-(dibenzylamino)-2,2-dimethoxy-4-oxo-4-phenylbutanoate 8e
Yield 73% (32.6 mg); colorless oil; Rf = 0.38 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 3.17 (s, 3H), 3.27 (s, 3H), 3.65 (d, J = 13.7 Hz, 2H), 3.71 (s, 3H), 4.26 (d, J = 13.7 Hz, 2H), 4.92 (s, 1H), 7.17–7.28 (m, 10H), 7.39–7.43 (m, 2H), 7.53–7.57 (m, 1H), 7.76–7.79 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 51.0, 51.2, 52.5, 55.5, 63.4, 103.8, 127.0, 128.0, 128.2, 128.8, 129.4, 132.9, 139.1, 139.6, 168.4, 200.9; IR (neat) 2950, 2821, 1757, 1681, 1547, 1214, 1073, 749, 696 cm−1; HRMS (EI) calcd for C27H29NO5 (M)+ 447.2046, found 447.2047.
1); 1H NMR (400 MHz, CDCl3) δ 3.17 (s, 3H), 3.27 (s, 3H), 3.65 (d, J = 13.7 Hz, 2H), 3.71 (s, 3H), 4.26 (d, J = 13.7 Hz, 2H), 4.92 (s, 1H), 7.17–7.28 (m, 10H), 7.39–7.43 (m, 2H), 7.53–7.57 (m, 1H), 7.76–7.79 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 51.0, 51.2, 52.5, 55.5, 63.4, 103.8, 127.0, 128.0, 128.2, 128.8, 129.4, 132.9, 139.1, 139.6, 168.4, 200.9; IR (neat) 2950, 2821, 1757, 1681, 1547, 1214, 1073, 749, 696 cm−1; HRMS (EI) calcd for C27H29NO5 (M)+ 447.2046, found 447.2047.
Ethyl 3-(dibenzylamino)-2,2-diethoxy-4-oxo-4-phenylbutanoate 2–8f
Yield 70% (34.1 mg); white solid mp = 84−85 °C; Rf = 0.46 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); δ 1H NMR (400 MHz, CDCl3): δ 1.06 (t, J = 6.9 Hz, 6H), 1.20 (t, J = 7.3 Hz, 3H), 3.30–3.40 (m, 2H), 3.48 (dq, J = 6.9, 9.1 Hz, 1H), 3.61 (dq, J = 6.9, 9.1 Hz, 1H), 3.72 (d, J = 13.7 Hz, 2H), 4.05 (dq, J = 7.3, 10.5 Hz, 1H), 4.23–4.32 (m, 3H, including doublet at 4.27 ppm J = 13.7 Hz, 2H), 4.89 (s, 1H), 7.20–7.27 (m, 10H), 7.35–7.39 (m, 2H), 7.49–7.53 (m, 1H), 7.72–7.74 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.9, 14.8, 15.1, 55.5, 58.9, 59.2, 61.5, 64.7, 103.0, 126.8, 127.8, 127.9, 128.8, 129.5, 132.4, 139.5, 139.9, 168.2, 201.5; IR (neat) 2979, 1750, 1682, 1559, 1452, 1249, 1122, 1062, 748, 696 cm−1; HRMS (EI) calcd for C28H30NO4 (M–C2H5O)+ 444.2169, found 444.2170.
1); δ 1H NMR (400 MHz, CDCl3): δ 1.06 (t, J = 6.9 Hz, 6H), 1.20 (t, J = 7.3 Hz, 3H), 3.30–3.40 (m, 2H), 3.48 (dq, J = 6.9, 9.1 Hz, 1H), 3.61 (dq, J = 6.9, 9.1 Hz, 1H), 3.72 (d, J = 13.7 Hz, 2H), 4.05 (dq, J = 7.3, 10.5 Hz, 1H), 4.23–4.32 (m, 3H, including doublet at 4.27 ppm J = 13.7 Hz, 2H), 4.89 (s, 1H), 7.20–7.27 (m, 10H), 7.35–7.39 (m, 2H), 7.49–7.53 (m, 1H), 7.72–7.74 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.9, 14.8, 15.1, 55.5, 58.9, 59.2, 61.5, 64.7, 103.0, 126.8, 127.8, 127.9, 128.8, 129.5, 132.4, 139.5, 139.9, 168.2, 201.5; IR (neat) 2979, 1750, 1682, 1559, 1452, 1249, 1122, 1062, 748, 696 cm−1; HRMS (EI) calcd for C28H30NO4 (M–C2H5O)+ 444.2169, found 444.2170.
S-tert-Butyl 3-(dibenzylamino)-4-oxo-4-phenylbutanethioate 8g
Yield 63% (30.0 mg); colorless oil; Rf = 0.64 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.45 (s, 9H), 2.97 (dd, J = 3.6, 15.5 Hz, 1H), 3.30 (dd, J = 8.7, 15.5 Hz, 1H), 3.46 (d, J = 13.3 Hz, 2H), 3.66 (d, J = 13.3 Hz, 2H), 4.83 (dd, J = 3.6, 8.7 Hz, 1H), 7.10–7.13 (m, 4H), 7.23–7.32 (m, 8H), 7.47–7.50 (m, 1H), 7.51–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 29.8, 38.3, 48.2, 54.5, 59.0, 127.3, 128.1, 128.2, 129.0, 129.3, 132.7, 136.4, 138.5, 198.6, 198.9; IR (neat) 3061, 3028, 2980, 2842, 1735, 1675, 1455, 1274, 1152, 968, 748, 699 cm−1; HRMS (EI) calcd for C28H31NO2S (M)+ 445.2076, found 445.2077.
1); 1H NMR (400 MHz, CDCl3) δ 1.45 (s, 9H), 2.97 (dd, J = 3.6, 15.5 Hz, 1H), 3.30 (dd, J = 8.7, 15.5 Hz, 1H), 3.46 (d, J = 13.3 Hz, 2H), 3.66 (d, J = 13.3 Hz, 2H), 4.83 (dd, J = 3.6, 8.7 Hz, 1H), 7.10–7.13 (m, 4H), 7.23–7.32 (m, 8H), 7.47–7.50 (m, 1H), 7.51–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 29.8, 38.3, 48.2, 54.5, 59.0, 127.3, 128.1, 128.2, 129.0, 129.3, 132.7, 136.4, 138.5, 198.6, 198.9; IR (neat) 3061, 3028, 2980, 2842, 1735, 1675, 1455, 1274, 1152, 968, 748, 699 cm−1; HRMS (EI) calcd for C28H31NO2S (M)+ 445.2076, found 445.2077.
Synthesis of 2-(dibenzylamino)-1-phenyl-2-[1-(triisopropylsilyl)-1H-indol-3-yl]ethenone 9a (general procedure for the reaction of the amino silyl enol ether with indoles)
Under an argon atmosphere, to a solution of N-bromosuccinimide (39.2 mg, 0.22 mmol) in EtCN (1.0 mL) were successively added a solution of the amino silyl enol ether 5 (85.9 mg, 0.20 mmol) in EtCN (1.0 mL), BF3·Et2O (0.025 mL, 0.20 mmol), and a solution of 1-(triisopropylsilyl)-1H-indole (43.1 mg, 0.22 mmol) in EtCN at 0 °C. The reaction mixture was allowed to warm to ambient temperature with stirring for 5 h. It was quenched with sat. aq. NaHCO3 (10 mL). The layers were separated, and the aqueous layer was extracted with ethyl acetate (30 mL × 3). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel (developed twice with nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt = 6
AcOEt = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 2-(dibenzylamino)-1-phenyl-2-[1-(triisopropylsilyl)-1H-indol-3-yl]ethanone 9a (77%, 90.7 mg).
1) to give 2-(dibenzylamino)-1-phenyl-2-[1-(triisopropylsilyl)-1H-indol-3-yl]ethanone 9a (77%, 90.7 mg).
Yield 77% (90.7 mg); yellow oil; Rf = 0.61 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.95 (d, J = 7.3 Hz, 9H), 0.99 (d, J = 7.3 Hz, 9H), 1.55 (sept, J = 7.3 Hz, 3H), 3.94 (d, J = 13.7 Hz, 2H), 4.02 (d, J = 13.7 Hz, 2H), 5.78 (s, 1H), 6.91 (s, 1H), 7.16–7.30 (m, 14H), 7.34–7.37 (m, 1H), 7.44–7.46 (m, 1H), 7.65–7.68 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 12.6, 17.8, 17.9, 54.8, 59.8, 113.0, 113.9, 119.5, 119.9, 121.9, 126.7, 128.0, 128.1, 128.2, 129.1, 130.6, 132.5, 137.0, 140.6, 141.4, 201.7; IR (neat) 3060, 3026, 2948, 2868, 1685, 1494, 1449, 1165, 1144, 1074, 967, 882, 746, 696, 664 cm−1; HRMS (EI) calcd for C39H46N2OSi (M)+ 586.3380, found 586.3379.
1); 1H NMR (400 MHz, CDCl3) δ 0.95 (d, J = 7.3 Hz, 9H), 0.99 (d, J = 7.3 Hz, 9H), 1.55 (sept, J = 7.3 Hz, 3H), 3.94 (d, J = 13.7 Hz, 2H), 4.02 (d, J = 13.7 Hz, 2H), 5.78 (s, 1H), 6.91 (s, 1H), 7.16–7.30 (m, 14H), 7.34–7.37 (m, 1H), 7.44–7.46 (m, 1H), 7.65–7.68 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 12.6, 17.8, 17.9, 54.8, 59.8, 113.0, 113.9, 119.5, 119.9, 121.9, 126.7, 128.0, 128.1, 128.2, 129.1, 130.6, 132.5, 137.0, 140.6, 141.4, 201.7; IR (neat) 3060, 3026, 2948, 2868, 1685, 1494, 1449, 1165, 1144, 1074, 967, 882, 746, 696, 664 cm−1; HRMS (EI) calcd for C39H46N2OSi (M)+ 586.3380, found 586.3379.
2-(Dibenzylamino)-2-[5-nitro-1-(triisopropylsilyl)-1H-indol-3-yl]-1-phenylethan-1-one 9b
Yield 13% (15.9 mg); yellow oil; Rf = 0.43 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.92–1.00 (m, 18H), 1.49–1.60 (m, 3H), 3.96 (dd, J = 13.8, 29.1 Hz, 4H), 5.80 (s, 1H), 7.04–8.65 (m, 19H); 13C NMR (100 MHz, CDCl3) δ 12.5, 17.7, 17.8, 54.8, 59.0, 113.7, 115.5, 116.8, 117.6, 127.1, 128.1, 128.4, 128.5, 129.1, 130.3, 133.0, 135.2, 136.9, 140.0, 141.9, 144.5, 201.2; IR (neat) 3064, 3025, 2945, 2866, 1683, 1508, 1447, 1338, 1264, 1206, 1136, 970, 808, 688, 659 cm−1; HRMS (EI) calcd for C30H39N3O3Si (M)+ 631.3230, found 631.3239.
1); 1H NMR (400 MHz, CDCl3) δ 0.92–1.00 (m, 18H), 1.49–1.60 (m, 3H), 3.96 (dd, J = 13.8, 29.1 Hz, 4H), 5.80 (s, 1H), 7.04–8.65 (m, 19H); 13C NMR (100 MHz, CDCl3) δ 12.5, 17.7, 17.8, 54.8, 59.0, 113.7, 115.5, 116.8, 117.6, 127.1, 128.1, 128.4, 128.5, 129.1, 130.3, 133.0, 135.2, 136.9, 140.0, 141.9, 144.5, 201.2; IR (neat) 3064, 3025, 2945, 2866, 1683, 1508, 1447, 1338, 1264, 1206, 1136, 970, 808, 688, 659 cm−1; HRMS (EI) calcd for C30H39N3O3Si (M)+ 631.3230, found 631.3239.
2-(Dibenzylamino)-2-[5-methoxy-1-(triisopropylsilyl)-1H-indol-3-yl]-1-phenylethanone 9c
Yield 67% (82.7 mg); yellow oil; Rf = 0.55 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.92 (d, J = 7.6 Hz, 9H), 0.96 (d, J = 7.6 Hz, 9H), 1.50 (sept, J = 7.6 Hz, 3H), 3.89 (s, 3H), 3.99 (s, 4H), 5.73 (s, 1H), 6.80–6.83 (m, 2H), 7.05–7.07 (m, 1H), 7.18–7.33 (m, 13H), 7.38–7.40 (m, 1H), 7.68–7.70 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 12.5, 17.8, 17.9, 54.7, 55.7, 60.0, 99.9, 100.9, 112.3, 113.1, 114.7, 126.8, 128.1, 128.3, 129.3, 131.1, 132.5, 133.0, 136.2, 137.1, 140.9, 154.2, 202.0; IR (neat) 3061, 3027, 2950, 2867, 1674, 1618, 1485, 1447, 1216, 1038, 1019, 884, 794, 744, 693, 660, 583 cm−1; HRMS (EI) calcd for C40H48N2O2Si (M)+ 616.3485, found 616.3482.
1); 1H NMR (400 MHz, CDCl3) δ 0.92 (d, J = 7.6 Hz, 9H), 0.96 (d, J = 7.6 Hz, 9H), 1.50 (sept, J = 7.6 Hz, 3H), 3.89 (s, 3H), 3.99 (s, 4H), 5.73 (s, 1H), 6.80–6.83 (m, 2H), 7.05–7.07 (m, 1H), 7.18–7.33 (m, 13H), 7.38–7.40 (m, 1H), 7.68–7.70 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 12.5, 17.8, 17.9, 54.7, 55.7, 60.0, 99.9, 100.9, 112.3, 113.1, 114.7, 126.8, 128.1, 128.3, 129.3, 131.1, 132.5, 133.0, 136.2, 137.1, 140.9, 154.2, 202.0; IR (neat) 3061, 3027, 2950, 2867, 1674, 1618, 1485, 1447, 1216, 1038, 1019, 884, 794, 744, 693, 660, 583 cm−1; HRMS (EI) calcd for C40H48N2O2Si (M)+ 616.3485, found 616.3482.
2-[5-Bromo-1-(triisopropylsilyl)-1H-indol-3-yl]-2-(dibenzylamino)-1-phenylethan-1-one 9d
Yield 75% (100.0 mg); yellow oil; Rf = 0.63 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.94–0.99 (m, 18H), 1.53 (q, J = 7.6 Hz, 3H), 3.97 (dd, J = 13.6, 32.2 Hz, 4H), 5.71 (s, 1H), 6.92 (s, 1H), 7.15–7.48 (m, 16H), 7.68–7.79 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 12.6, 17.9, 18.0, 54.8, 59.5, 113.0, 113.5, 115.4, 122.4, 124.9, 127.1, 128.2, 128.3, 128.5, 129.3, 132.6, 132.8, 133.6, 137.0, 140.1, 140.5, 201.5; IR (neat) 3061, 3026, 2947, 2867, 1688, 1597, 1495, 1445, 1199, 1159, 1135, 967, 881, 795, 750, 718, 701, 689, 653, 567 cm−1; HRMS (EI) calcd for C30H39N2OSiBr (M)+ 664.2485, found 664.2452.
1); 1H NMR (400 MHz, CDCl3) δ 0.94–0.99 (m, 18H), 1.53 (q, J = 7.6 Hz, 3H), 3.97 (dd, J = 13.6, 32.2 Hz, 4H), 5.71 (s, 1H), 6.92 (s, 1H), 7.15–7.48 (m, 16H), 7.68–7.79 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 12.6, 17.9, 18.0, 54.8, 59.5, 113.0, 113.5, 115.4, 122.4, 124.9, 127.1, 128.2, 128.3, 128.5, 129.3, 132.6, 132.8, 133.6, 137.0, 140.1, 140.5, 201.5; IR (neat) 3061, 3026, 2947, 2867, 1688, 1597, 1495, 1445, 1199, 1159, 1135, 967, 881, 795, 750, 718, 701, 689, 653, 567 cm−1; HRMS (EI) calcd for C30H39N2OSiBr (M)+ 664.2485, found 664.2452.
2-[6-Bromo-1-(triisopropylsilyl)-1H-indol-3-yl]-2-(dibenzylamino)-1-phenylethan-1-one 9e
Yield 73% (97.0 mg); yellow oil; Rf = 0.62 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.88–1.09 (m, 18H), 1.47–1.59 (m, 3H), 3.96 (dd, J = 13.7, 34.8 Hz, 4H), 5.73 (s, 1H), 6.84–7.68 (m, 18H); 13C NMR (100 MHz, CDCl3) δ 3047, 2948, 2868, 1686, 1599, 1456, 1151, 1073, 967, 883, 814, 750, 695, 599; IR (neat) 3047, 2948, 2868, 1686, 1599, 1456, 1151, 1073, 967, 883, 814, 750, 695, 599 cm−1; HRMS (EI) calcd for C30H39N2OSiBr (M)+ 664.2485, found 664.2488.
1); 1H NMR (400 MHz, CDCl3) δ 0.88–1.09 (m, 18H), 1.47–1.59 (m, 3H), 3.96 (dd, J = 13.7, 34.8 Hz, 4H), 5.73 (s, 1H), 6.84–7.68 (m, 18H); 13C NMR (100 MHz, CDCl3) δ 3047, 2948, 2868, 1686, 1599, 1456, 1151, 1073, 967, 883, 814, 750, 695, 599; IR (neat) 3047, 2948, 2868, 1686, 1599, 1456, 1151, 1073, 967, 883, 814, 750, 695, 599 cm−1; HRMS (EI) calcd for C30H39N2OSiBr (M)+ 664.2485, found 664.2488.
Synthesis of methyl 2-(dibenzylamino)-1-phenylbutan-1-one 10b (general procedure for the reaction of the amino silyl enol ether with grignard reagents)
Under an argon atmosphere, to a solution of N-bromosuccinimide (19.6 mg, 0.11 mmol) in EtCN (1.0 mL) were successively added a solution of amino silyl enol ether 5 (42.9 mg, 0.10 mmol) in EtCN (1.0 mL), BF3·OEt2 (0.025 mL, 0.20 mmol), and a solution of EtMgBr in Et2O (0.93 M, 0.22 mL, 0.20 mmol) at room temperature. The reaction mixture was stirred for 30 min. It was quenched with sat. aq. NH4Cl (10 mL). The layers were separated, and the aqueous layer was extracted with ethyl acetate (30 mL × 3). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by preparative TLC on silica gel (developed twice with nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt = 15
AcOEt = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 2-(dibenzylamino)-1-phenylbutan-1-one 10b (74%, 25.5 mg).
1) to give 2-(dibenzylamino)-1-phenylbutan-1-one 10b (74%, 25.5 mg).
Yield 74% (25.5 mg); colorless oil; Rf = 0.63 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.93 (t, J = 7.3 Hz, 3H), 1.80–1.89 (m, 1H), 1.93–2.02 (m, 1H), 3.69 (s, 4H), 4.14 (dd, J = 4.9, 8.5 Hz, 1H), 7.20–7.34 (m, 12H), 7.48–7.52 (m, 1H), 7.56–7.58 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 11.5, 17.8, 54.3, 62.6, 127.0, 128.2, 128.2, 128.5, 129.1, 132.6, 137.7, 139.6, 201.4; IR (neat): 3062, 3026, 2978, 2936, 2835, 1684, 1494, 1448, 1378, 1225, 1143, 936, 749, 732, 695 cm−1; HRMS (EI) calcd for C18H20NO (M–C6H5)+ 238.1590, found 238.1589.
1); 1H NMR (400 MHz, CDCl3) δ 0.93 (t, J = 7.3 Hz, 3H), 1.80–1.89 (m, 1H), 1.93–2.02 (m, 1H), 3.69 (s, 4H), 4.14 (dd, J = 4.9, 8.5 Hz, 1H), 7.20–7.34 (m, 12H), 7.48–7.52 (m, 1H), 7.56–7.58 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 11.5, 17.8, 54.3, 62.6, 127.0, 128.2, 128.2, 128.5, 129.1, 132.6, 137.7, 139.6, 201.4; IR (neat): 3062, 3026, 2978, 2936, 2835, 1684, 1494, 1448, 1378, 1225, 1143, 936, 749, 732, 695 cm−1; HRMS (EI) calcd for C18H20NO (M–C6H5)+ 238.1590, found 238.1589.
2-(Dibenzylamino)-1-phenylpropan-1-one 10a
Yield 64% (22.2 mg); colorless oil; Rf = 0.63 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.34 (d, J = 6.6 Hz, 3H), 3.54 (d, J = 13.5 Hz, 2H), 3.68 (d, J = 13.5 Hz, 2H), 4.34 (q, J = 6.6 Hz, 1H), 7.15–7.34 (m, 12H), 7.48–7.52 (m, 1H), 7.58–7.61 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 8.4, 54.3, 57.2, 127.1, 128.0, 128.2, 128.8, 129.2, 132.5, 136.9, 139.3, 202.0; IR (neat) 3062, 3026, 2978, 2936, 2835, 1684, 1494, 1448, 1378, 1225, 1143, 936, 749, 732, 695 cm−1; HRMS (EI) calcd for C17H18NO (M–C6H5)+ 224.1434, found 224.1438.
1); 1H NMR (400 MHz, CDCl3) δ 1.34 (d, J = 6.6 Hz, 3H), 3.54 (d, J = 13.5 Hz, 2H), 3.68 (d, J = 13.5 Hz, 2H), 4.34 (q, J = 6.6 Hz, 1H), 7.15–7.34 (m, 12H), 7.48–7.52 (m, 1H), 7.58–7.61 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 8.4, 54.3, 57.2, 127.1, 128.0, 128.2, 128.8, 129.2, 132.5, 136.9, 139.3, 202.0; IR (neat) 3062, 3026, 2978, 2936, 2835, 1684, 1494, 1448, 1378, 1225, 1143, 936, 749, 732, 695 cm−1; HRMS (EI) calcd for C17H18NO (M–C6H5)+ 224.1434, found 224.1438.
2-(Dibenzylamino)-1-phenylpentan-1-one 10c
Yield 71% (25.4 mg); colorless oil; Rf = 0.64 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.92 (t, J = 7.3 Hz, 3H), 1.29–1.39 (m, 2H), 1.74–1.82 (m, 1H), 1.88–1.97 (m, 1H), 3.70 (s, 4H), 4.24 (dd, J = 5.5, 8.7 Hz, 1H), 7.21–7.34 (m, 12H), 7.48–7.58 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 14.2, 20.1, 27.0, 54.3, 60.5, 127.0, 128.2, 128.2, 128.5, 129.1, 132.6, 137.5, 139.7, 201.7. IR (neat): 3061, 3028, 2957, 2832, 1684, 1494, 1447, 1373, 1245, 1209, 1072, 947, 751, 695 cm−1. HRMS (EI): calcd for C19H22NO (M–C6H5)+ 252.1747, found 252.1753.
1); 1H NMR (400 MHz, CDCl3) δ 0.92 (t, J = 7.3 Hz, 3H), 1.29–1.39 (m, 2H), 1.74–1.82 (m, 1H), 1.88–1.97 (m, 1H), 3.70 (s, 4H), 4.24 (dd, J = 5.5, 8.7 Hz, 1H), 7.21–7.34 (m, 12H), 7.48–7.58 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 14.2, 20.1, 27.0, 54.3, 60.5, 127.0, 128.2, 128.2, 128.5, 129.1, 132.6, 137.5, 139.7, 201.7. IR (neat): 3061, 3028, 2957, 2832, 1684, 1494, 1447, 1373, 1245, 1209, 1072, 947, 751, 695 cm−1. HRMS (EI): calcd for C19H22NO (M–C6H5)+ 252.1747, found 252.1753.
2-(Dibenzylamino)-3-methyl-1-phenylbutan-1-one 10d
Yield 63% (22.5 mg); colorless oil; Rf = 0.63 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.75 (d, J = 6.7 Hz, 3H), 1.24 (d, J = 6.7 Hz, 3H), 2.35–2.46 (m, 1H), 3.34 (d, J = 14.7 Hz, 2H), 4.02 (d, J = 10.4 Hz, 1H), 4.04 (d, J = 14.7 Hz, 2H), 7.21–7.29 (m, 10H), 7.37–7.40 (m, 2H), 7.54–7.57 (m, 1H), 7.65–7.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.3, 20.4, 27.8, 54.3, 65.4, 126.9, 128.1, 128.2, 128.5, 128.6, 133.0, 139.6, 139.9, 203.1; IR (neat): 3059, 3032, 2979, 2950, 2840, 1668, 1494, 1444, 1218, 1093, 976, 839, 737, 730, 699 cm−1; HRMS (EI) calcd for C19H22NO (M–C6H5)+ 252.1747, found 252.1741.
1); 1H NMR (400 MHz, CDCl3) δ 0.75 (d, J = 6.7 Hz, 3H), 1.24 (d, J = 6.7 Hz, 3H), 2.35–2.46 (m, 1H), 3.34 (d, J = 14.7 Hz, 2H), 4.02 (d, J = 10.4 Hz, 1H), 4.04 (d, J = 14.7 Hz, 2H), 7.21–7.29 (m, 10H), 7.37–7.40 (m, 2H), 7.54–7.57 (m, 1H), 7.65–7.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.3, 20.4, 27.8, 54.3, 65.4, 126.9, 128.1, 128.2, 128.5, 128.6, 133.0, 139.6, 139.9, 203.1; IR (neat): 3059, 3032, 2979, 2950, 2840, 1668, 1494, 1444, 1218, 1093, 976, 839, 737, 730, 699 cm−1; HRMS (EI) calcd for C19H22NO (M–C6H5)+ 252.1747, found 252.1741.
2-Cyclopropyl-2-(dibenzylamino)-1-phenylethanone 10e
Yield 35% (12.4 mg); colorless oil; Rf = 0.64 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ −0.02–0.04 (m, 1H), 0.51–0.58 (m, 2H), 0.75–0.84 (m, 1H), 1.28–1.37 (m, 1H), 3.45 (d, J = 9.6 Hz, 1H), 3.79 (d, J = 13.8 Hz, 2H), 3.94 (d, J = 13.8 Hz, 2H), 7.20–7.36 (m, 12H), 7.50–7.61 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 2.9, 5.3, 8.5, 54.7, 66.7, 127.0, 128.1, 128.2, 128.6, 129.0, 132.7, 137.5, 139.8, 201.7; IR (neat) 3081, 3027, 2927, 2840, 1681, 1597, 1495, 1450, 1220, 744, 696 cm−1; HRMS (EI) calcd for C19H20NO (M–C6H5)+ 250.1590, found 250.1591.
1); 1H NMR (400 MHz, CDCl3) δ −0.02–0.04 (m, 1H), 0.51–0.58 (m, 2H), 0.75–0.84 (m, 1H), 1.28–1.37 (m, 1H), 3.45 (d, J = 9.6 Hz, 1H), 3.79 (d, J = 13.8 Hz, 2H), 3.94 (d, J = 13.8 Hz, 2H), 7.20–7.36 (m, 12H), 7.50–7.61 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 2.9, 5.3, 8.5, 54.7, 66.7, 127.0, 128.1, 128.2, 128.6, 129.0, 132.7, 137.5, 139.8, 201.7; IR (neat) 3081, 3027, 2927, 2840, 1681, 1597, 1495, 1450, 1220, 744, 696 cm−1; HRMS (EI) calcd for C19H20NO (M–C6H5)+ 250.1590, found 250.1591.
2-Cyclohexyl-2-(dibenzylamino)-1-phenylethanone 10f
Yield 60% (23.9 mg); colorless solid, mp = 88–89 °C; Rf = 0.65 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 0.76–0.84 (m, 1H), 1.04–1.37 (m, 5H), 1.54–1.56 (m, 1H), 1.63–1.66 (m, 1H), 1.79–1.83 (m, 1H), 2.09–2.17 (m, 1H), 2.42–2.45 (m, 1H), 3.36 (d, J = 14.7 Hz, 2H), 4.05 (d, J = 14.7 Hz, 2H), 4.16 (d, J = 10.4 Hz, 1H), 7.22–7.39 (m, 12H), 7.52–7.55 (m, 1H), 7.63–7.65 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 26.0, 26.1, 26.6, 30.4, 31.0, 37.4, 54.3, 64.2, 126.8, 128.1, 128.2, 128.5, 128.6, 133.0, 139.6, 139.9, 203.4; IR (neat) 3061, 3028, 2926, 2850, 1675, 1494, 1446, 1234, 1201, 907, 844, 735, 698 cm−1; HRMS (EI): calcd for C22H26NO (M–C6H5)+ 292.2060, found 292.2056.
1); 1H NMR (500 MHz, CDCl3) δ 0.76–0.84 (m, 1H), 1.04–1.37 (m, 5H), 1.54–1.56 (m, 1H), 1.63–1.66 (m, 1H), 1.79–1.83 (m, 1H), 2.09–2.17 (m, 1H), 2.42–2.45 (m, 1H), 3.36 (d, J = 14.7 Hz, 2H), 4.05 (d, J = 14.7 Hz, 2H), 4.16 (d, J = 10.4 Hz, 1H), 7.22–7.39 (m, 12H), 7.52–7.55 (m, 1H), 7.63–7.65 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 26.0, 26.1, 26.6, 30.4, 31.0, 37.4, 54.3, 64.2, 126.8, 128.1, 128.2, 128.5, 128.6, 133.0, 139.6, 139.9, 203.4; IR (neat) 3061, 3028, 2926, 2850, 1675, 1494, 1446, 1234, 1201, 907, 844, 735, 698 cm−1; HRMS (EI): calcd for C22H26NO (M–C6H5)+ 292.2060, found 292.2056.
2-(Dibenzylamino)-1,3-diphenylpropan-1-one 10g
Yield 43% (17.4 mg); white solid mp = 84–85 °C; Rf = 0.61 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δ 3.12 (dd, J = 4.3, 13.4 Hz, 1H), 3.36 (dd, J = 9.2, 13.4 Hz, 1H), 3.71 (d, J = 13.4 Hz, 2H), 3.78 (d, J = 13.4 Hz, 2H), 4.54 (dd, J = 4.3, 9.2 Hz, 1H), 7.12–7.14 (m, 5H), 7.18–7.27 (m, 12H), 7.43–7.47 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 30.2, 54.3, 62.9, 126.0, 127.2, 128.1, 128.2, 128.3, 128.6, 129.2, 129.5, 132.6, 137.3, 139.1, 139.2, 199.7; IR (neat) 3060, 3025, 2931, 2815, 1688, 1600, 1494, 1455, 1233, 1116, 1072, 1027, 957, 750, 700 cm−1; HRMS (EI) calcd for C23H22NO (M–C6H5)+ 300.1747, found 300.1761.
1); 1H NMR (400 MHz, CDCl3): δ 3.12 (dd, J = 4.3, 13.4 Hz, 1H), 3.36 (dd, J = 9.2, 13.4 Hz, 1H), 3.71 (d, J = 13.4 Hz, 2H), 3.78 (d, J = 13.4 Hz, 2H), 4.54 (dd, J = 4.3, 9.2 Hz, 1H), 7.12–7.14 (m, 5H), 7.18–7.27 (m, 12H), 7.43–7.47 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 30.2, 54.3, 62.9, 126.0, 127.2, 128.1, 128.2, 128.3, 128.6, 129.2, 129.5, 132.6, 137.3, 139.1, 139.2, 199.7; IR (neat) 3060, 3025, 2931, 2815, 1688, 1600, 1494, 1455, 1233, 1116, 1072, 1027, 957, 750, 700 cm−1; HRMS (EI) calcd for C23H22NO (M–C6H5)+ 300.1747, found 300.1761.
2-(Dibenzylamino)-1-phenyl-2-p-tolylethanone 10i
Yield 46% (18.7 mg); yellow solid, mp = 112–113 °C; Rf = 0.62 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 2.30 (s, 3H), 3.75 (d, J = 13.7 Hz, 2H), 3.96 (d, J = 13.7 Hz, 2H), 5.43 (s, 1H), 7.11–7.31 (m, 16H), 7.42–7.44 (m, 1H), 7.67–7.69 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 21.1, 54.4, 66.5, 126.9, 128.2, 128.3, 128.4, 128.9, 129.2, 129.9, 132.8, 133.3, 136.9, 137.5, 140.1, 201.7; IR (neat): 3059, 3026, 2922, 2844, 1689, 1595, 1494, 1447, 1226, 1138, 961, 804, 747, 700 cm−1; HRMS (EI): calcd for C23H22NO (M–C6H5)+ 300.1747, found 300.1744.
1); 1H NMR (400 MHz, CDCl3) δ 2.30 (s, 3H), 3.75 (d, J = 13.7 Hz, 2H), 3.96 (d, J = 13.7 Hz, 2H), 5.43 (s, 1H), 7.11–7.31 (m, 16H), 7.42–7.44 (m, 1H), 7.67–7.69 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 21.1, 54.4, 66.5, 126.9, 128.2, 128.3, 128.4, 128.9, 129.2, 129.9, 132.8, 133.3, 136.9, 137.5, 140.1, 201.7; IR (neat): 3059, 3026, 2922, 2844, 1689, 1595, 1494, 1447, 1226, 1138, 961, 804, 747, 700 cm−1; HRMS (EI): calcd for C23H22NO (M–C6H5)+ 300.1747, found 300.1744.
2-(Dibenzylamino)-1-phenyl-2-(thiophen-2-yl)ethanone 10j
Yield 64% (25.5 mg); colorless solid, mp = 108−109 °C; Rf = 0.64 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 3.67 (d, J = 13.7 Hz, 2H), 3.91 (d, J = 13.7 Hz, 2H), 5.69 (s, 1H), 6.90–6.92 (m, 1H), 6.96–6.99 (m, 1H), 7.23–7.37 (m, 13H), 7.48–7.52 (m, 1H), 7.67–7.69 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 54.5, 61.3, 126.3, 126.5, 127.1, 128.3, 128.4, 128.6, 129.0, 133.1, 136.5, 137.5, 139.4, 198.9. IR (neat): 3061, 3027, 2928, 2840, 1685, 1595, 1494, 1447, 1214, 957, 750, 699 cm−1; HRMS (EI) calcd for C20H18NOS (M–C6H5)+ 292.1154, found 292.1140.
1); 1H NMR (400 MHz, CDCl3) δ 3.67 (d, J = 13.7 Hz, 2H), 3.91 (d, J = 13.7 Hz, 2H), 5.69 (s, 1H), 6.90–6.92 (m, 1H), 6.96–6.99 (m, 1H), 7.23–7.37 (m, 13H), 7.48–7.52 (m, 1H), 7.67–7.69 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 54.5, 61.3, 126.3, 126.5, 127.1, 128.3, 128.4, 128.6, 129.0, 133.1, 136.5, 137.5, 139.4, 198.9. IR (neat): 3061, 3027, 2928, 2840, 1685, 1595, 1494, 1447, 1214, 957, 750, 699 cm−1; HRMS (EI) calcd for C20H18NOS (M–C6H5)+ 292.1154, found 292.1140.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) and on Innovative Areas “Organic Synthesis Based on Reaction Integration. Development of New Methods and Creation of New Substances” from JSPS and MEXT.Notes and references
- For iminium ion cyclization, see: (a) E. J. Corey and R. D. Balanson, J. Am. Chem. Soc., 1974, 96, 6516–6517 CrossRef CAS; (b) S. Yamada, M. Konda and T. Shioiri, Tetrahedron Lett., 1972, 13, 2215–2218 CrossRef . For N-acyl iminium ion cyclization, see; (c) J. Dijkink, H. E. Shoemeker and W. N. Speckamp, Tetrahedron Lett., 1975, 16, 4043–4046 CrossRef; (d) J. Dijkink and W. N. Speckamp, Tetrahedron Lett., 1975, 16, 4047–4050 CrossRef.
- (a) H. H. Mooiweer, H. Hiemstra and W. N. Speckamp, Tetrahedron, 1989, 45, 4627–4636 CrossRef CAS; (b) H. H. Mooiweer, E. C. Roots, H. Hiemstra and W. N. Speckamp, J. Org. Chem., 1992, 57, 6769–6778 CrossRef; (c) M. Okuda, H. Hioki, W. Miyagi and S. Ito, Tetrahedron Lett., 1993, 34, 6131–6134 CrossRef; (d) J. Yoshida, S. Suga and M. Okajima, Tetrahedron Lett., 2001, 42, 2173–2176 CrossRef; (e) J. Yoshida, S. Suga and M. Watanabe, J. Am. Chem. Soc., 2002, 124, 14824–14825 CrossRef PubMed; (f) P. Knochel, N. Millot, C. Piazza and S. Avolio, Synthesis, 2002, 941–944 Search PubMed; (g) L. W. Bieber and I. H. S. Estevam, Tetrahedron Lett., 2003, 44, 667–670 CrossRef; (h) M. Suginome, L. Uehlin and M. Murakami, Org. Lett., 2004, 6, 1167–1169 CrossRef CAS PubMed; (i) T. Murai, Y. Mutoh, Y. Ohta and M. Murakami, J. Am. Chem. Soc., 2004, 126, 5968–5969 CrossRef CAS PubMed; (j) M. Suginome, L. Uehlin and M. Murakami, J. Am. Chem. Soc., 2004, 126, 13196–13197 CrossRef CAS PubMed.
- For Petasis reactions including the synthesis of α-monosubstituted amino acids, see: (a) N. A. Petasis and I. Akritopoulou, Tetrahedron Lett., 1993, 34, 583–586 CrossRef CAS; (b) N. A. Petasis and I. A. Zavialov, J. Am. Chem. Soc., 1997, 119, 445–446 CrossRef CAS; (c) N. A. Petasis, A. Goodman and I. A. Zavialov, Tetrahedron, 1997, 53, 16463–16470 CrossRef CAS; (d) N. A. Petasis and I. A. Zavialov, J. Am. Chem. Soc., 1998, 120, 11798–11799 CrossRef CAS; (e) N. A. Petasis and S. Boral, Tetrahedron Lett., 2001, 42, 539–542 CrossRef CAS; (f) T. Koolmeister, M. Södelgren and M. Scobie, Tetrahedron Lett., 2002, 43, 5969–5970 CrossRef CAS; (g) H. Jourdan, G. Gouhier, L. V. Hijfte, P. Angibaud and S. R. Piettre, Tetrahedron Lett., 2005, 46, 8027–8031 CrossRef CAS; (h) T. J. Southwood, M. C Curry and C. A. Hutton, Tetrahedron, 2006, 62, 236–242 CrossRef CAS . For asymmetric Petasis reaction: ; (i) S. Lou and S. E. Schaus, J. Am. Chem. Soc., 2008, 130, 6922–6923 CrossRef CAS PubMed.
- (a) Y. Niwa and M. Shimizu, J. Am. Chem. Soc., 2003, 125, 3720–3721 CrossRef CAS PubMed; (b) M. Shimizu, H. Itou and M. Miura, J. Am. Chem. Soc., 2005, 127, 3296–3297 CrossRef CAS PubMed; (c) T. Iwao and M. Shimizu, Heterocycles, 2009, 77, 767–772 CrossRef CAS; (d) M. Shimizu, H. Itou, T. Iwao and Y. Umeda, Chem. Lett., 2009, 38, 732–733 CrossRef CAS; (e) M. Shimizu, I. Hachiya and I. Mizota, Chem. Commun., 2009, 874–889 RSC; (f) S. Hata, H. Koyama and M. Shimizu, J. Org. Chem., 2011, 76, 9670–9677 CrossRef CAS PubMed; (g) M. Shimizu, T. Kusunoki, M. Yoshida, K. Kondo and I. Mizota, Chem. Lett., 2011, 40, 351–353 CrossRef CAS; (h) I. Mizota and M. Shimizu, Chem. Rec., 2016, 16, 688–702 CrossRef CAS PubMed; (i) M. Shimizu, H. Imazato, I. Mizota and Y. Zhu, RSC Adv., 2019, 9, 17341–17346 RSC; (j) M. Shimizu, M. Mushika, I. Mizota and Y. Zhu, RSC Adv., 2019, 9, 23400–23407 RSC; (k) M. Shimizu, Y. Furukawa, I. Mizota and Y. Zhu, New J. Chem., 2020, 44, 152–161 RSC; (l) M. Shimizu, T. Morimoto, Y. Yanagi, I. Mizota and Y. Zhu, RSC Adv., 2020, 10, 9955–9963 RSC.
- We have already reported the formation of the iminium salt by 1H and 13C NMR spectra and a possible ionic mechanism by the ineffectiveness of radical scavengers.4b.
- Effects of the silyl substituents on the reactivity of silyl enol ethers, see: (a) I. Kuwajima and E. Nakamura, Acc. Chem. Res., 1985, 18, 181–187 CrossRef CAS; (b) M. B. Boxer and H. Yamamoto, J. Am. Chem. Soc., 2006, 128, 48–49 CrossRef CAS PubMed; (c) M. Akakura, M. B. Boxer and H. Yamamoto, ARKIVOC, 2007, 337–347 Search PubMed.
- For the diastereoselecitivity of the Mannich reaction, see: (a) A. Ting and S. E. Schaus, Eur. J. Org. Chem., 2007, 5797–5815 CrossRef CAS; (b) J. M. M. Verkade, L. J. C. van Hemert, P. J. L. M. Quaedflieg and F. P. J. T. Rutjes, Chem. Soc. Rev., 2008, 37, 29–41 RSC; (c) Y. Gnas and F. Glorius, Synthesis, 2006, 1899–1930 CAS; (d) B. T. Hahn, R. Fröhlich, K. Harms and F. Glorius, Angew. Chem., Int. Ed., 2008, 47, 9985–9988 CrossRef CAS PubMed.
- The reaction of enolates and enols with DDQ was reported, see: (a) J. M. Williams, G. Marchesini, R. A. Reamer, U.-H. Dolling and E. J. J. Grabowski, J. Org. Chem., 1995, 60, 5337–5340 CrossRef CAS; (b) A. Bhattacharya, L. M. DiMichele, U.-H. Dolling, A. W. Douglas and E. J. J. Grabowski, J. Am. Chem. Soc., 1988, 110, 3318–3319 CrossRef CAS; (c) H.-D. Becker, J. Org. Chem., 1965, 30, 989–994 CrossRef CAS.
- (a) M. Bandini, A. Melloni, S. Tommasi and A. Umani-Ronchi, Synlett, 2005, 1199 CrossRef CAS; (b) J. C. Badenock and G. W. Gribble, Adv. Heterocycl. Chem., 2016, 120, 99–136 CrossRef CAS , and references cited therein.
- For determination of the relative stereochemistry, see: (a) J. Delbos-Krampe, N. Risch and U. Florke, Z. Naturforsch., B: J. Chem. Sci., 2004, 59, 414–423 CAS; (b) M. Feroci, J. Lessard, M. Orsini and A. Inesi, Tetrahedron Lett., 2005, 46, 8517–8519 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05768a |
| This journal is © The Royal Society of Chemistry 2020 |