DOI:
10.1039/D0RA05480A
(Paper)
RSC Adv., 2020,
10, 30062-30068
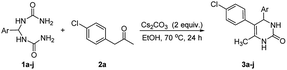
An efficient synthesis of 4,5-diaryl-3,4-dihydropyrimidin-2(1H)-one via a cesium carbonate-promoted direct condensation of 1-aryl-2-propanone with 1,1′-(arylmethylene)diurea†
Received
22nd June 2020
, Accepted 2nd August 2020
First published on 14th August 2020
Abstract
An efficient method for the synthesis of 4,5-diaryl-3,4-dihydropyrimidin-2(1H)-one by using 1,1′-(arylmethylene)diurea and 1-aryl-2-propanone as substrates was developed. The reactions proceeded efficiently in the presence of Cs2CO3 to give the desired products in moderate to good yields with wide substrate scope and good functional group tolerance, serving as an attractive alternative or complement to the previously reported methods for the facile assembly of biologically and pharmaceutically active 3,4-dihydropyrimidin-2(1H)-ones.
1. Introduction
3,4-Dihydropyrimidin-2(1H)-ones (DHPMs) are pivotal structural skeletons which widely exist in a variety of natural products1 and bioactive molecules.2 These compounds exhibit a wide range of biologically and pharmaceutically relevant activities, such as antibacterial, antitumour, antiviral, antimalarial, antidiabetic, and antiepileptic activities.2 Normally, 3,4-dihydropyrimidin-2(1H)-one could be accessed via the Biginelli reaction of aldehyde with ethyl acetoacetate and urea.3,4 With more than one century of development, tremendous achievements have been made in the Biginelli reaction, especially in the field of organic synthesis, medicinal chemistry, polymer chemistry, and material sciences.4,5 In addition, the variants of the Biginelli reaction by using other active methylene compounds (or their equivalents), such as 1,3-cyclohexanedione,6 acetophenone,7 1-tetralone,8 β-oxodithioesters,9 enaminone,10 cyclopentanone,11 alkyl aldehydes,12 and alkynol,13 to replace ethyl acetoacetate for accessing diverse 3,4-dihydropyrimidin-2(1H)-ones have also been reported. Normally, the Biginelli or Biginelli-like reaction should be performed under acidic conditions in the presence of protic or Lewis acid.3–5 In 2010, Ji and co-workers reported an efficient method for the synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1H)-ones via a three-component Biginelli-type condensation of aldehyde with 2-phenylacetophenone and urea/thiourea under basic conditions in the presence of t-BuOK.14 In the studies, it was observed that the reaction using urea as substrate might proceed via the formation of 1,1′-(arylmethylene)diurea as reaction intermediate. Apart from this study, the direct use of pre-prepared 1,1′-(arylmethylene)diurea for the synthesis of 3,4-dihydropyrimidin-2(1H)-one has been rarely investigated. In continuation of our interests in the synthesis of heterocycles, herein we report a cesium carbonate-promoted15 direct condensation of 1-aryl-2-propanone with pre-synthesized 1,1′-(arylmethylene)diurea, leading to the corresponding 4,5-diaryl-3,4-dihydropyrimidin-2(1H)-one in moderate to good yields.
2. Results and discussion
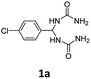
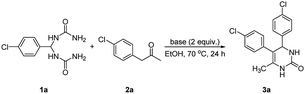
To begin with, we chose 1,1′-((4-chlorophenyl)methylene)diurea (1a) and 1-(4-chlorophenyl)propan-2-one (2a) as model substrates to optimize the reaction conditions by performing the reaction in ethanol at 70 °C for 24 h in the presence of different bases. As shown in Table 1, no desired 4,5-diaryl-3,4-dihydropyrimidin-2(1H)-one 3a was produced when the reaction was performed in the presence of organic bases (entries 1–3). In sharp contrast, the use of inorganic bases (entries 4–7) as reaction promoters were found to promote the condensation with varying performance, with the highest yield (75% yield) being obtained by using Cs2CO3 as reaction promoter (entry 7). However, when only a catalytic amount of Cs2CO3 (0.2 equiv.) was employed in the reaction, the product yield decreased considerably (entry 8). It should be mentioned that, when D-camphorsulfonic acid (CSA) was introduced into the reaction as a promoter, the corresponding product 3a could also be obtained, albeit in a relatively poor yield of 32%. In addition, it should be noted that an alternative method for the synthesis of product 3a via the three-component reaction involving 4-chlorobenzaldehyde (0.5 mmol, 1 equiv.), 1-(4-chlorophenyl)propan-2-one (2a, 1.2 equiv.), and urea (2 equiv.) proceeded sluggishly in the presence of Cs2CO3 (2 equiv.) in ethanol (70 °C, 24 h), leading to the corresponding product 3a only in 3% yield.
Table 1 Optimization of reaction conditions by using different bases
|
| Entry |
Base |
Yielda (%) |
| Isolated yield. Using 0.2 equivalents of Cs2CO3. |
| 1 |
Pyridine |
0 |
| 2 |
DABCO |
0 |
| 3 |
DBU |
0 |
| 4 |
K2CO3 |
51 |
| 5 |
KOH |
60 |
| 6 |
t-BuOK |
61 |
| 7 |
Cs2CO3 |
75 |
| 8 |
Cs2CO3 |
22b |
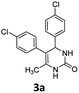
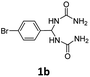
With Cs2CO3 being recognized as the optimum base for the condensation, subsequently we investigated the substrate scope of the reaction by using a range of diureas 1a–j as starting materials. As summarized in Table 2, a variety of aryl substituted diureas could efficiently undergo the intermolecular cyclization under the optimized conditions to afford the expected 4,5-diaryl-3,4-dihydropyrimidin-2(1H)-one 3a–j in 50–86% yields. In addition to phenyl-substituted diureas 1a–h, diureas 1i–j containing naphthenyl and thienyl substituents also efficiently participated in the organic transformation, leading to the anticipated products 3i and 3j in 64% and 85% yields, respectively. Especially noteworthy is that, the mild reaction conditions also allowed the reaction to proceed with the tolerance to various functional groups or substituents (e.g., halogen, nitro, cyano, methyl, and methoxy) in the phenyl ring, which could potentially be utilized at a late stage.
Table 2 Substrate scope study by using different diureas
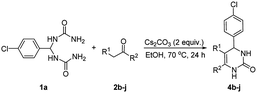
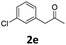
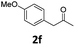
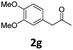
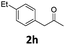
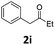
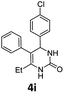
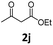
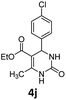
Encouraged by the above results, we continued to study the generality of the reaction by utilizing an array of phenylacetones 2b–i as substrates (Table 3). In all cases, the reactions proceeded efficiently under well-established conditions to deliver the expected products 4b–i in moderate to good yields. Apart from 1-aryl-2-propanones 2b–e bearing electron-withdrawing groups which well reacted with substrate 1a (entries 1–4), substrates 2f–h possessing electron-donating groups in the aryl ring of 1-aryl-2-propanones were also proven to be suitable candidates for the transformation, giving rise to the desired products 4f–h in excellent yields (85–90% yields; entries 5–7). In a same manner, 1-phenylbutan-2-one (2i) could also be applied in the protocol, furnishing the corresponding product 4i in 65% yield (entry 8). Moreover, the method could also be applied to the use of ethyl acetoacetate (2j) as substrate, albeit in a relatively low yield of 26% (entry 9). Analogously, the reaction also showed good compatibility to different functional groups or substituents, including trifluoromethyl, halogen, and methoxy group.
Table 3 Substrate scope study by using various ketones
Mechanistically, the reaction might proceed via the initial formation of an imine-type intermediate 5, which subsequently reacts with 1-aryl-2-propanone 2 in the presence of Cs2CO3 leading to compound 6 (Scheme 1).14 Finally, an ensuing intramolecular cyclization followed by elimination of a hydroxyl group under basic conditions produces the desired product 3 and 4.
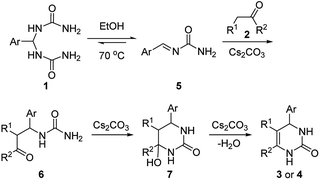 |
| | Scheme 1 Proposed mechanism. | |
3. Conclusions
In summary, we have developed an efficient method for the synthesis of 4,5-diaryl-3,4-dihydropyrimidin-2(1H)-one by using 1,1′-(arylmethylene)diurea and 1-aryl-2-propanone as substrates. The reactions proceeded efficiently in the presence of Cs2CO3, leading to the desired products in moderate to good yields. The transformation exhibited wide substrate scope and good tolerance to various important functional groups or substituents. We believed that the present protocol employing readily available and pre-synthesized diurea as substrate should serve as an attractive alternative or complement to the existing methods for the facile generation of biologically and pharmaceutically active 3,4-dihydropyrimidin-2(1H)-ones.
4. Experimental
4.1 General information
1-Aryl-2-propanone, cesium carbonate, and ethanol were purchased from chemical companies and used directly without further purification (without the need of precautions to exclude air and moisture unless otherwise noted). Starting materials 1a–j were prepared according to reported methods.16 Analytical thin layer chromatography (TLC) was performed using silica gel plate (0.2 mm thickness). Subsequent to elution, plates were visualized using UV radiation (254 nm). Flash chromatography was performed using Merck silica gel (200–300 mesh) for column chromatography with freshly distilled solvents. Columns were typically packed as slurry and equilibrated with the appropriate solvent system prior to use. IR spectra were recorded on a FT-IR spectrophotometer using KBr optics. 1H, 19F, and 13C NMR spectra were recorded in d6-DMSO on Jeol 400 MHz spectrometer. Tetramethylsilane (TMS) served as internal standard for 1H, 19F, and 13C NMR analysis.
4.2 Experimental procedure
General procedure for the preparation of 1,1′-(arylmethylene)diurea:16. To a 250 mL round-bottomed flask was sequentially added aryl aldehyde (30 mmol), urea (90 mmol), a catalytic amount of p-toluenesulfonic acid (3 mmol) and toluene (100 mL). The reaction mixture was refluxed for overnight. After reaction, the precipitate was collected by filtration, washed with saturated NaHCO3 (10 mL), pure water (20 mL), and Et2O (20 mL). It was further dried in oven at 120 °C for half an hour to give the desired product of diurea.
Typical procedure for the condensation of 1,1′-(arylmethylene)diurea with 1-aryl-2-propanone. To a mixture of diurea (0.5 mmol) and 1-aryl-2-propanone (0.6 mmol) was added absolute ethanol (3 mL), and it was stirred at room temperature for 5 minutes. Then Cs2CO3 (1 mmol) was added and the reaction mixture was stirred vigorously at 70 °C for 24 h. After the completion of the reaction, solvent was removed under vacuum. The residue was purified by silica gel column chromatography by using EtOAc/petroleum ether or CH2Cl2/MeOH as eluant to afford the desired product of 4,5-biaryl-3,4-dihydropyrimidin-2(1H)-one.
4.3 Characterization data of products
1,1′-((4-Chlorophenyl)methylene)diurea (1a). 6.6 g. Yield = 91%. White solid. Mp: 190.5–191.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.40 (d, J = 8.5 Hz, 2H), 7.32 (d, J = 8.5 Hz, 2H), 6.79 (d, J = 8.3 Hz, 2H), 6.08 (t, J = 8.3 Hz, 1H), 5.72 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 157.8, 141.9, 131.6, 128.1, 128.0, 58.6 ppm. FTIR (KBr, neat): ν 3453, 3312, 1667, 1608, 866, 817 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C9H12ClN4O2: 243.0643, found: 243.0643.
1,1′-((4-Bromophenyl)methylene)diurea (1b). 4.4 g. Yield = 51%. White solid. Mp: 206.6–207.5 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.53 (d, J = 8.4 Hz, 2H), 7.26 (d, J = 8.4 Hz, 2H), 6.79 (d, J = 8.3 Hz, 2H), 6.06 (t, J = 8.3 Hz, 1H), 5.72 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 158.2, 142.8, 131.4, 128.8, 120.5, 59.1 ppm. FTIR (KBr, neat): ν 3453, 3312, 1667, 1608, 866, 813 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C9H12BrN4O2: 287.0138, found: 287.0137.
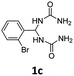
1,1′-((2-Bromophenyl)methylene)diurea (1c). 6.3 g. Yield = 73%. White solid. Mp: 199.2–199.7 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.60 (d, J = 7.8 Hz, 1H), 7.52 (d, J = 6.7 Hz, 1H), 7.40 (t, J = 7.4 Hz, 1H), 7.23 (t, J = 7.0 Hz, 1H), 6.71 (d, J = 6.9 Hz, 2H), 6.25 (t, J = 7.5 Hz, 1H), 5.63 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 157.4, 141.2, 132.7, 129.4, 128.0, 127.5, 122.4, 59.6 ppm. FTIR (KBr, neat): ν 3430, 3331, 1668, 1612, 750, 719 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C9H12BrN4O2: 287.0138, found: 287.0140.
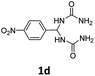
1,1′-((4-Nitrophenyl)methylene)diurea (1d). 6.7 g. Yield = 88%. White solid. Mp: 209.8–210.4 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.21 (d, J = 8.8 Hz, 2H), 7.56 (d, J = 8.6 Hz, 2H), 6.98 (d, J = 8.3 Hz, 2H), 6.17 (t, J = 8.2 Hz, 1H), 5.82 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 157.8, 150.8, 146.5, 127.4, 123.4, 58.8 ppm. FTIR (KBr, neat): ν 3459, 3312, 1682, 1516, 1351, 856, 835 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C9H12N5O4: 254.0884, found: 254.0881.
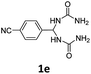
1,1′-((4-Cyanophenyl)methylene)diurea (1e). 6.3 g. Yield = 90%. White solid. Mp: 222.6–222.9 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.82 (d, J = 8.1 Hz, 2H), 7.49 (d, J = 8.3 Hz, 2H), 6.90 (d, J = 8.2 Hz, 2H), 6.12 (t, J = 8.1 Hz, 1H), 5.76 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 158.2, 149.1, 132.6, 127.6, 119.5, 110.1, 59.3 ppm. FTIR (KBr, neat): ν 3352, 3300, 2234, 1656, 862, 825 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C10H12N5O2: 234.0986, found: 234.0987.
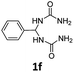
1,1′-(Phenylmethylene)diurea (1f). 4.0 g. Yield = 64%. White solid. Mp: 190.6–191.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.34–7.31 (m, 4H), 7.27–7.23 (m, 1H), 6.72 (d, J = 8.3 Hz, 2H), 6.12 (t, J = 8.3 Hz, 1H), 5.68 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 158.2, 143.2, 128.6, 127.6, 126.5, 59.6 ppm. FTIR (KBr, neat): ν 3420, 3312, 1667, 1535, 747, 699 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C9H13N4O2: 209.1033, found: 209.1033.
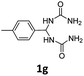
1,1′-(p-Tolylmethylene)diurea (1g). 3.7 g. Yield = 55%. White solid. Mp: 196.2–196.8 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.20 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.1 Hz, 2H), 6.67 (d, J = 8.1 Hz, 2H), 6.08 (t, J = 8.3 Hz, 1H), 5.67 (s, 4H), 2.28 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 158.2, 140.2, 136.6, 129.1, 126.4, 59.4, 21.2 ppm. FTIR (KBr, neat): ν 3458, 3312, 1678, 1532, 864, 814 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C10H15N4O2: 223.1190, found: 223.1191.
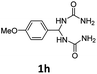
1,1′-((4-Methoxyphenyl)methylene)diurea (1h). 5.2 g. Yield = 73%. White solid. Mp: 186.6–187.3 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.24 (d, J = 8.6 Hz, 2H), 6.90 (d, J = 8.7 Hz, 2H), 6.70 (d, J = 8.0 Hz, 2H), 6.09 (t, J = 8.1 Hz, 1H), 5.70 (s, 4H), 3.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 158.9, 158.3, 135.1, 127.7, 114.0, 59.2, 55.6 ppm. FTIR (KBr, neat): ν 3428, 3308, 1671, 1515, 1261, 1176, 865, 836 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C10H15N4O3: 239.1139, found: 239.1134.
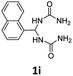
1,1′-(Naphthalen-1-ylmethylene)diurea (1i). 6.8 g. Yield = 88%. White solid. Mp: 221.5–222.7 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.02 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 7.4 Hz, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.64–7.47 (m, 4H), 6.91 (t, J = 7.8 Hz, 1H), 6.82 (d, J = 7.7 Hz, 2H), 5.66 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 157.4, 138.1, 133.5, 130.3, 128.6, 128.0, 126.3, 125.8, 125.2, 123.4, 122.6, 56.5 ppm. FTIR (KBr, neat): ν 3418, 3297, 1667, 1608, 882, 770 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C13H15N4O2: 259.1190, found: 259.1189.
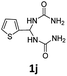
1,1′-(Thiophen-2-ylmethylene)diurea (1j). 4.2 g. Yield = 65%. White solid. Mp: 197.4–198.9 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.41–7.35 (m, 1H), 6.96 (dd, J = 5.0, 3.5 Hz, 1H), 6.91–6.88 (m, 1H), 6.86 (d, J = 8.5 Hz, 2H), 6.32 (t, J = 8.4 Hz, 1H), 5.75 (s, 4H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 157.5, 147.7, 126.8, 124.9, 123.7, 56.2 ppm. FTIR (KBr, neat): ν 3467, 3284, 1671, 1589, 1282, 1123 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C7H11N4O2S: 215.0597, found: 215.0602.
4,5-bis(4-Chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (3a). 124.6 mg. Yield = 75%. Yellow solid. Mp: 214.7–215.8 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.50 (s, 1H), 7.38 (s, 1H), 7.35–7.31 (m, 2H), 7.30–7.26 (m, 2H), 7.18–7.14 (m, 2H), 7.11–7.06 (m, 2H), 5.13 (s, 1H), 1.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.8, 143.0, 136.7, 131.8, 131.2, 130.9, 130.8, 128.7, 128.4, 128.1, 106.8, 58.5, 16.2 ppm. FTIR (KBr, neat): ν 3265, 1693, 1491, 1241, 1091, 758 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15Cl2N2O: 333.0556, found: 333.0558.
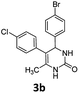
4-(4-Bromophenyl)-5-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (3b). 161.6 mg. Yield = 86%. Brown solid. Mp: 206.8–208.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.51 (s, 1H), 7.46 (d, J = 8.3 Hz, 2H), 7.39 (s, 1H), 7.30–7.24 (m, 2H), 7.14–7.05 (m, 4H), 5.12 (s, 1H), 1.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.8, 143.4, 136.7, 131.3, 131.2, 130.9, 130.8, 129.1, 128.1, 120.4, 106.7, 58.6, 16.2 ppm. FTIR (KBr, neat): ν 3268, 1693, 1489, 1241, 757 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15BrClN2O: 377.0051, found: 377.0051.
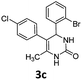
4-(2-Bromophenyl)-5-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (3c). 126.8 mg. Yield = 67%. Yellow solid. Mp: 189.8–191.3 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.56 (s, 1H), 7.49 (d, J = 7.6 Hz, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.40–7.33 (m, 2H), 7.24 (d, J = 8.2 Hz, 2H), 7.13 (t, J = 7.2 Hz, 1H), 7.01 (d, J = 8.3 Hz, 2H), 5.58 (s, 1H), 1.69 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.5, 142.6, 136.2, 132.6, 131.3, 131.1, 131.0, 129.9, 129.5, 128.5, 128.1, 121.9, 106.3, 58.6, 16.1 ppm. FTIR (KBr, neat): ν 3220, 1694, 1493, 1253, 1095, 737 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15BrClN2O: 377.0051, found: 377.0050.
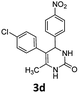
5-(4-Chlorophenyl)-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (3d). 88.8 mg. Yield = 50%. Brown solid. Mp: 200.2–201.7 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.51 (s, 1H), 7.44–7.37 (m, 3H), 7.35–7.30 (m, 2H), 7.16 (d, J = 8.3 Hz, 2H), 7.02 (d, J = 8.3 Hz, 2H), 5.13 (s, 1H), 1.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.8, 148.5, 136.6, 131.1, 131.0, 130.8, 128.1, 126.6, 125.3, 124.4, 107.4, 54.2, 16.3 ppm. FTIR (KBr, neat): ν 2926, 1721, 1491, 1262, 1093, 1014, 831 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15ClN3O3: 344.0796, found: 344.0799.
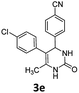
4-(5-(4-Chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidin-4-yl)benzonitrile (3e). 80.3 mg. Yield = 50%. Yellow solid. Mp: 249.3–250.6 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.57 (s, 1H), 7.78–7.72 (m, 2H), 7.47 (s, 1H), 7.33–7.30 (m, 2H), 7.30–7.26 (m, 2H), 7.11–7.06 (m, 2H), 5.24 (s, 1H), 1.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.7, 149.3, 136.4, 132.6, 131.3, 131.2, 130.9, 128.2, 127.8, 118.8, 110.1, 106.2, 58.8, 16.2 ppm. FTIR (KBr, neat): ν 3397, 2226, 1679, 1475, 1242, 759 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C18H15ClN3O: 324.0898, found: 324.0904.
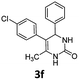
5-(4-Chlorophenyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (3f). 97.4 mg. Yield = 65%. Light yellow solid. Mp: 179.6–180.1 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.45 (s, 1H), 7.34 (s, 1H), 7.29–7.23 (m, 4H), 7.22–7.18 (m, 1H), 7.16 (d, J = 7.9 Hz, 2H), 7.10–7.05 (m, 2H), 5.08 (s, 1H), 1.74 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 153.0, 144.0, 137.0, 131.1, 130.7, 130.7, 128.5, 128.1, 127.3, 126.8, 107.1, 59.2, 16.3 ppm. FTIR (KBr, neat): ν 3055, 1690, 1493, 1014, 744, 698 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H16ClN2O: 299.0946, found: 299.0947.
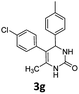
5-(4-Chlorophenyl)-6-methyl-4-(p-tolyl)-3,4-dihydropyrimidin-2(1H)-one (3g). 118.2 mg. Yield = 76%. Light yellow solid. Mp: 215.6–216.8 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.44 (s, 1H), 7.30–7.24 (m, 3H), 7.10–7.03 (m, 6H), 5.04 (s, 1H), 2.23 (s, 3H), 1.74 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.9, 141.1, 137.1, 136.4, 131.1, 130.6, 130.5, 129.0, 128.1, 126.8, 107.2, 58.9, 20.7, 16.3 ppm. FTIR (KBr, neat): ν 3271, 1694, 1491, 1241, 832, cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C18H18ClN2O: 313.1102, found: 313.1102.
5-(4-Chlorophenyl)-4-(4-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (3h). 120.0 mg. Yield = 73%. Yellow solid. Mp: 161.8–163.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.42 (s, 1H), 7.29–7.24 (m, 3H), 7.10–7.06 (m, 4H), 6.85–6.80 (m, 2H), 5.02 (s, 1H), 3.69 (s, 3H), 1.74 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 158.5, 152.9, 137.1, 136.2, 131.1, 130.6, 130.5, 128.1, 128.0, 113.8, 107.3, 58.6, 55.0, 16.3 ppm. FTIR (KBr, neat): ν 3238, 1679, 1510, 1248, 1174, 834 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C18H18ClN2O2: 329.1051, found: 329.1054.
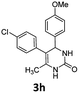
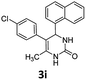
5-(4-Chlorophenyl)-6-methyl-4-(naphthalen-1-yl)-3,4-dihydropyrimidin-2(1H)-one (3i). 112.3 mg. Yield = 64%. Light yellow solid. Mp: 250.4–251.6 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.58 (s, 1H), 8.29–8.19 (m, 1H), 7.92–7.86 (m, 1H), 7.82–7.75 (m, 1H), 7.53–7.40 (m, 4H), 7.36 (s, 1H), 7.15 (d, J = 7.8 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 5.99 (s, 1H), 1.81 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.7, 148.0, 144.0, 138.7, 136.9, 133.7, 131.1, 131.0, 130.6, 130.4, 128.6, 128.1, 127.9, 126.0, 125.9, 125.6, 123.6, 106.8, 16.3 ppm. FTIR (KBr, neat): ν 3230, 1703, 1493, 1253, 776 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C21H18ClN2O: 349.1102, found: 349.1103.
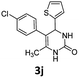
5-(4-Chlorophenyl)-6-methyl-4-(thiophen-2-yl)-3,4-dihydropyrimidin-2(1H)-one (3j). 128.8 mg. Yield = 85%. Light yellow solid. Mp: 193.2–194.4 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.63 (s, 1H), 7.55 (s, 1H), 7.35 (dd, J = 5.0, 1.1 Hz, 1H), 7.33–7.28 (m, 2H), 7.21–7.14 (m, 2H), 6.87 (dd, J = 5.0, 3.5 Hz, 1H), 6.82–6.77 (m, 1H), 5.39 (s, 1H), 1.76 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.9, 148.5, 136.6, 131.1, 131.1, 130.8, 128.1, 126.6, 125.3, 124.4, 107.5, 54.2, 16.3 ppm. FTIR (KBr, neat): ν 3244, 1686, 1490, 1389, 1247, 1091, 839, 698 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C15H14ClN2OS: 305.0510, found: 305.0509.
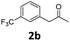
4-(4-Chlorophenyl)-6-methyl-5-(3-(trifluoromethyl)phenyl)-3,4-dihydropyrimidin-2(1H)-one (4b). 132.6 mg. Yield = 72%. Yellow solid. Mp: 109.8–110.4 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.60 (s, 1H), 7.51–7.42 (m, 3H), 7.40–7.31 (m, 4H), 7.23–7.14 (m, 2H), 5.22 (s, 1H), 1.74 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.7, 142.9, 139.0, 133.6, 131.9, 131.7, 129.2, 129.0 (q, J = 31.3 Hz), 128.8, 128.5, 125.7 (q, J = 3.8 Hz), 124.2 (q, J = 271.0 Hz), 123.0 (q, J = 4.1 Hz), 106.6, 58.4, 16.2 ppm. 19F NMR (376 MHz, DMSO-d6): δ −60.98 ppm. FTIR (KBr, neat): ν 3238, 1683, 1490, 1339, 1126, 804, 704 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C18H15ClF3N2O: 367.0820, found: 367.0822.
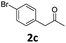
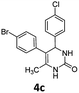
5-(4-Bromophenyl)-4-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4c). 149.6 mg. Yield = 79%. Light yellow solid. Mp: 209.5–209.8 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.51 (s, 1H), 7.42–7.37 (m, 3H), 7.35–7.30 (m, 2H), 7.18–7.14 (m, 2H), 7.04–7.00 (m, 2H), 5.12 (s, 1H), 1.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 152.8, 143.0, 137.1, 131.8, 131.5, 131.0, 130.9, 128.7, 128.4, 119.3, 106.8, 58.4, 16.2 ppm. FTIR (KBr, neat): ν 3269, 1692, 1490, 1240, 1099, 844, 778 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15BrClN2O: 377.0051, found: 377.0050.
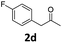
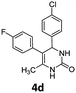
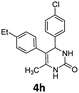
4-(4-Chlorophenyl)-5-(4-fluorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4d). 139.5 mg. Yield = 88%. Yellow solid. Mp: 189.0–190.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.46 (s, 1H), 7.35 (s, 1H), 7.34–7.31 (m, 2H), 7.18–7.14 (m, 2H), 7.11–7.02 (m, 4H), 5.10 (s, 1H), 1.71 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 160.6 (d, J = 241.7 Hz), 152.8, 143.0, 134.1 (d, J = 3.3 Hz), 131.7, 131.3 (d, J = 8.0 Hz), 130.4, 128.7, 128.4, 115.0 (d, J = 21.1 Hz), 107.0, 58.8, 16.2 ppm. 19F NMR (376 MHz, DMSO-d6): δ −115.91 ppm. FTIR (KBr, neat): ν 3259, 1694, 1491, 1242, 1094, 1014, 845, 780 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15ClFN2O: 317.0851, found: 317.0851.
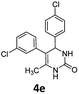
5-(3-Chlorophenyl)-4-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4e). 155.9 mg. Yield = 94%. Yellow solid. Mp: 93.8–95.3 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.59 (s, 1H), 7.45 (s, 1H), 7.32–7.29 (m, 2H), 7.22–7.15 (m, 4H), 7.11 (s, 1H), 7.04–6.98 (m, 1H), 5.17 (s, 1H), 1.75 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 153.1, 142.9, 140.2, 133.0, 132.1, 131.5, 130.1, 129.1, 128.9, 128.6, 128.3, 126.4, 107.0, 58.6, 16.4 ppm. FTIR (KBr, neat): ν 3236, 1682, 1489, 1239, 1091, 785 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C17H15Cl2N2O: 333.0556, found: 333.0556.
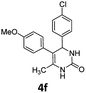
4-(4-Chlorophenyl)-5-(4-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4f). 140.5 mg. Yield = 85%. Yellow solid. Mp: 184.6–186.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.42 (s, 1H), 7.35–7.29 (m, 3H), 7.19–7.14 (m, 2H), 6.99–6.94 (m, 2H), 6.82–6.76 (m, 2H), 5.07 (s, 1H), 3.68 (s, 3H), 1.71 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 157.6, 153.1, 143.3, 131.7, 130.5, 129.8, 129.6, 128.7, 128.3, 113.6, 107.7, 58.9, 54.9, 16.2 ppm. FTIR (KBr, neat): ν 3259, 2924, 1693, 1489, 1249, 842 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C18H18ClN2O2: 329.1051, found: 329.1051.
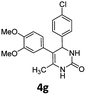
4-(4-Chlorophenyl)-5-(3,4-dimethoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4g). 160.4 mg. Yield = 89%. Yellow solid. Mp: 151.6–152.7 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.36 (s, 1H), 7.34–7.29 (m, 3H), 7.19–7.15 (m, 2H), 6.78 (d, J = 8.3 Hz, 1H), 6.63 (d, J = 2.0 Hz, 1H), 6.50 (dd, J = 8.2, 2.0 Hz, 1H), 5.10 (s, 1H), 3.67 (s, 3H), 3.64 (s, 3H), 1.73 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 153.0, 148.2, 147.2, 143.4, 131.6, 130.2, 129.6, 128.8, 128.3, 121.9, 113.0, 111.4, 108.0, 58.9, 55.4, 55.3, 16.3 ppm. FTIR (KBr, neat): ν 3343, 1698, 1515, 1457, 1251, 1135, 1011, 766 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C19H20ClN2O3: 359.1157, found: 359.1159.
4-(4-Chlorophenyl)-5-(4-ethylphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4h). 146.5 mg. Yield = 90%. Yellow solid. Mp: 229.8–231.2 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.40 (s, 1H), 7.35–7.29 (m, 3H), 7.19–7.14 (m, 2H), 7.06 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 8.2 Hz, 2H), 5.07 (s, 1H), 2.52 (q, J = 7.6 Hz, 2H), 1.73 (s, 3H), 1.12 (t, J = 7.6 Hz, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 153.0, 143.2, 141.6, 135.1, 131.7, 130.1, 129.2, 128.8, 128.4, 127.5, 107.8, 58.7, 27.7, 16.3, 15.3 ppm. FTIR (KBr, neat): ν 3223, 2928, 1686, 1241, 1088, 842 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C19H20ClN2O: 327.1259, found: 327.1260.
4-(4-Chlorophenyl)-6-ethyl-5-phenyl-3,4-dihydropyrimidin-2(1H)-one (4i). 101.8 mg. Yield = 65%. Yellow solid. Mp: 184.8–185.7 °C. 1H NMR (400 MHz, DMSO-d6): δ 8.41 (s, 1H), 7.34–7.30 (m, 3H), 7.23 (t, J = 7.4 Hz, 2H), 7.17–7.12 (m, 3H), 7.03–6.98 (m, 2H), 5.05 (d, J = 2.5 Hz, 1H), 2.04–1.94 (m, 2H), 1.03 (t, J = 7.4 Hz, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 153.2, 143.1, 137.9, 135.5, 131.7, 129.3, 128.7, 128.4, 128.2, 126.5, 107.7, 59.0, 22.7, 13.0 ppm. FTIR (KBr, neat): ν 3235, 1679, 1489, 1227, 1091, 1014, 765, 701 cm−1. HRMS (ESI, m/z): [M + H]+, calcd for C18H18ClN2O: 313.1102, found: 313.1102.
Ethyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4j). 38.2 mg. Yield = 26%. White solid. Mp: 212.3–213.6 °C. 1H NMR (400 MHz, DMSO-d6): δ 9.25 (s, 1H), 7.78 (s, 1H), 7.39 (d, J = 8.4 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 5.14 (d, J = 3.2 Hz, 1H), 3.98 (q, J = 7.0 Hz, 2H), 2.24 (s, 3H), 1.09 (t, J = 7.1 Hz, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 165.3, 152.0, 148.8, 143.8, 131.8, 128.5, 128.3, 98.9, 59.3, 53.5, 17.9, 14.1 ppm. The characterization data of this product is in accordance with the reported ones.17
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We gratefully acknowledge the financial support from Nanjing Tech University (Start-up Grant No. 39837118 and 39837146), National Natural Science Foundation of China (21901087), Natural Science Foundation of Jiangsu Province (BK20180690 and BK20190951), Jiangsu Education Department (19KJB150008), and Nanjing Forestry University.
Notes and references
-
(a) Z. D. Aron and L. E. Overman, J. Am. Chem. Soc., 2005, 127, 3380 CrossRef CAS PubMed;
(b) M. A. Arnold, K. A. Day, S. G. Duron and D. Y. Gin, J. Am. Chem. Soc., 2006, 128, 13255 CrossRef CAS PubMed.
-
(a) R. W. Lewis, J. Mabry, J. G. Polisar, K. P. Eagen, B. Ganem and G. P. Hess, Biochemistry, 2010, 49, 4841 CrossRef CAS PubMed;
(b) X. Zhu, G. Zhao, X. Zhou, X. Xu, G. Xia, Z. Zheng, L. Wang, X. Yang and S. Li, Bioorg. Med. Chem. Lett., 2010, 20, 299 CrossRef CAS PubMed;
(c) K. L. Dhumaskar, S. N. Meena, S. C. Ghadi and S. G. Tilve, Bioorg. Med. Chem. Lett., 2014, 24, 2897 CrossRef CAS PubMed;
(d) T. G. M. Treptow, F. Figueiró, E. H. F. Jandrey, A. M. O. Battastini, C. G. Salbego, J. B. Hoppe, P. S. Taborda, S. B. Rosa, L. A. Piovesan, C. R. M. D'Oca, D. Russowsky and M. G. M. D'Oca, Eur. J. Med. Chem., 2015, 95, 552 CrossRef CAS PubMed;
(e) R. Chikhale, S. Menghani, R. Babu, R. Bansode, G. Bhargavi, N. Karodia, M. V. Rajasekharan, A. Paradkar and P. Khedekar, Eur. J. Med. Chem., 2015, 96, 30 CrossRef CAS PubMed;
(f) U. Rashid, R. Sultana, N. Shaheen, S. F. Hassan, F. Yaqoob, M. J. Ahmad, F. Iftikhar, N. Sultana, S. Asghar, M. Yasinzai, F. L. Ansari and N. A. Qureshi, Eur. J. Med. Chem., 2016, 115, 230 CrossRef CAS PubMed;
(g) K. Singh and T. Kaur, RSC Med. Chem., 2016, 7, 749 RSC.
- P. Biginelli, Gazz. Chim. Ital., 1893, 23, 360 Search PubMed.
-
(a) C. O. Kappe, Tetrahedron, 1993, 49, 6937 CrossRef CAS;
(b) C. O. Kappe, Acc. Chem. Res., 2000, 33, 879 CrossRef CAS PubMed;
(c) C. Simon, T. Constantieux and J. Rodriguez, Eur. J. Org. Chem., 2004, 4957 CrossRef CAS;
(d) D. Dallinger, A. Stadler and C. O. Kappe, Pure Appl. Chem., 2004, 76, 1017 CAS;
(e) L. Z. Gong, X. H. Chen and X. Y. Xu, Chem.–Eur. J., 2007, 13, 8920 CrossRef CAS PubMed;
(f) M. A. Kolosov and V. D. Orlov, Mol. Diversity, 2009, 13, 5 CrossRef CAS PubMed;
(g) H. Nagarajaiah, A. Mukhopadhyay and J. N. Moorthy, Tetrahedron Lett., 2016, 57, 5135 CrossRef CAS;
(h) R. V. Patil, J. U. Chavan, D. S. Dalal, V. S. Shinde and A. G. Beldar, ACS Comb. Sci., 2019, 21, 105 CrossRef CAS PubMed;
(i) A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef CAS PubMed;
(j) Y. Zhao, H. Wu, Z. Wang, Y. Wei, Z. Wang and L. Tao, Sci. China: Chem., 2016, 59, 1541 CrossRef CAS;
(k) R. V. Patil, J. U. Chavan, D. S. Dalal, V. S. Shinde and A. G. Beldar, ACS Comb. Sci., 2019, 21, 105 CrossRef CAS PubMed;
(l) R. Afshari and A. Shaabani, ACS Comb. Sci., 2018, 20, 499 CrossRef CAS PubMed.
-
(a) H. Xue, Y. Zhao, H. Wu, Z. Wang, B. Yang, Y. Wei, Z. Wang and L. Tao, J. Am. Chem. Soc., 2016, 138, 8690 CrossRef CAS PubMed;
(b) T. Mao, G. Liu, H. Wu, Y. Wei, Y. Gou, J. Wang and L. Tao, J. Am. Chem. Soc., 2018, 140, 6865 CrossRef CAS PubMed;
(c) N. Sahota, D. I. AbuSalim, M. L. Wang, C. J. Brown, Z. Zhang, T. J. El-Baba, S. P. Cook and D. E. Clemmer, Chem. Sci., 2019, 10, 4822 RSC;
(d) W. Fan, Y. Queneau and F. Popowycz, Green Chem., 2018, 20, 485 RSC;
(e) N. Li, X.-H. Chen, J. Song, S.-W. Luo, W. Fan and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 15301 CrossRef CAS PubMed;
(f) H. G. O. Alvim, D. L. J. Pinheiro, V. H. Carvalho-Silva, M. Fioramonte, F. C. Gozzo, W. A. da Silva, G. W. Amarante and B. A. D. Neto, J. Org. Chem., 2018, 83, 12143 CrossRef CAS PubMed;
(g) F.-J. Meng, L. Shi, G.-S. Feng, L. Sun and Y.-G. Zhou, J. Org. Chem., 2019, 84, 4435 CrossRef CAS PubMed;
(h) I. L. Gonçalves, L. Davi, L. Rockenbach, G. M. das Neves, L. P. Kagami, R. F. S. Canto, F. Figueiró, A. M. O. Battastini and V. L. Eifler-Lima, Tetrahedron Lett., 2018, 59, 759 CrossRef;
(i) P. Chen and M. Tu, Tetrahedron Lett., 2018, 59, 987 CrossRef CAS;
(j) R. Pathoor, T. Puthiyedath and D. Bahulayan, Tetrahedron Lett., 2019, 60, 191 CrossRef CAS;
(k) S. Zheng, Y. Jian, S. Xu, Y. Wu, H. Sun, G. Zhang, W. Zhang and Z. Gao, RSC Adv., 2018, 8, 8657 RSC;
(l) E. F. Freitas, R. Y. Souza, S. T. A. Passos, J. A. Dias, S. C. L. Dias and B. A. D. Neto, RSC Adv., 2019, 9, 27125 RSC;
(m) N. Li, X.-H. Chen, S.-M. Zhou, S.-W. Luo, J. Song, L. Ren and L.-Z. Gong, Angew. Chem., Int. Ed., 2010, 49, 6378 CrossRef CAS PubMed.
- B. Jauk, T. Pernat and C. O. Kappe, Molecules, 2000, 5, 227 CrossRef CAS.
-
(a) Z. T. Wang, L. W. Xu, C. G. Xia and H. Q. Wang, Tetrahedron Lett., 2004, 45, 7951 CrossRef CAS;
(b) B. Liang, X. T. Wang, J. X. Wang and Z. Y. Du, Tetrahedron, 2007, 63, 1981 CrossRef CAS.
- M. M. Abelman, S. C. Smith and D. R. James, Tetrahedron Lett., 2003, 44, 4559 CrossRef CAS.
-
(a) O. M. Singh and N. S. Devi, J. Org. Chem., 2009, 74, 3141 CrossRef CAS PubMed;
(b) G. C. Nandi, S. Samai and M. S. Singh, J. Org. Chem., 2010, 75, 7785 CrossRef CAS PubMed.
- J. P. Wan and Y. J. Pan, Chem. Commun., 2009, 2768 RSC.
-
(a) H. H. Zhang, Z. Q. Zhou, Z. G. Yao, F. Xu and Q. Shen, Tetrahedron Lett., 2009, 50, 1622 CrossRef CAS;
(b) Z.-L. Zhou, P.-C. Wang and M. Lu, Chin. Chem. Lett., 2016, 27, 226 CrossRef CAS.
- C. D. Bailey, C. E. Houlden, G. L. J. Bar, G. C. Lloyd-Jones and K. I. Booker-Milburn, Chem. Commun., 2007, 2932 RSC.
- S. Yu, J. Wu, H. Lan, L. Gao, H. Qian, K. Fan and Z. Yin, Org. Lett., 2020, 22, 102 CrossRef CAS PubMed.
- Z.-L. Shen, X.-P. Xu and S.-J. Ji, J. Org. Chem., 2010, 75, 1162 CrossRef CAS PubMed.
-
(a) H. Liu, Y. Fang, S.-Y. Wang and S.-J. Ji, Org. Lett., 2018, 20, 930 CrossRef CAS PubMed;
(b) H. Liu, Y. Fang, L. Yin, S.-Y. Wang and S.-J. Ji, J. Org. Chem., 2017, 82, 10866 CrossRef CAS PubMed;
(c) W.-B. Cao, X.-P. Xu and S.-J. Ji, Org. Biomol. Chem., 2017, 15, 1651 RSC;
(d) X. Liu, H. Zhu, S. B. Zhang, Y. Cheng, H. Y. Peng and Z. B. Dong, Tetrahedron Lett., 2018, 59, 3165 CrossRef CAS;
(e) X.-X. Feng, Z. Wu, Q.-D. Wang, B.-Z. Chen, W. Rao, J.-M. Yang and Z.-L. Shen, Appl. Organomet. Chem., 2019, 33, e5110 CrossRef;
(f) T. Xie, G.-Q. Wang, Y.-W. Wang, W. Rao, H. Xu, S. Li, Z.-L. Shen and X.-Q. Chu, iScience, 2020, 23, 101259 CrossRef CAS PubMed.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201 CrossRef CAS PubMed.
-
(a) J. Ma, L. Zhong, X. Peng and R. Sun, Green Chem., 2016, 18, 1738 RSC;
(b) N. Li, Y. Wang, F. Liu, X. Zhao, X. Xu, Q. An and K. Yun, Appl. Organomet. Chem., 2020, 34, e5454 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data of all compounds. See DOI: 10.1039/d0ra05480a |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b,
Haiyan Xu*c and
Zhi-Liang Shen
b,
Haiyan Xu*c and
Zhi-Liang Shen *a
*a