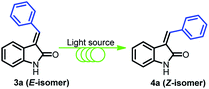
Open Access Article
Open Access ArticleE–Z isomerization of 3-benzylidene-indolin-2-ones using a microfluidic photo-reactor†
Chada Raji Reddy *ab,
Veeramalla Ganeshab and
Ajay K. Singh
*ab,
Veeramalla Ganeshab and
Ajay K. Singh *a
*a
aDivision of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, India. E-mail: rajireddy@iict.res.in; ajaysingh015@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, Uttar Pradesh, India
First published on 3rd August 2020
Abstract
Here, we report controlled E–Z isomeric motion of the functionalized 3-benzylidene-indolin-2-ones under various solvents, temperature, light sources, and most importantly effective enhancement of light irradiance in microfluidic photoreactor conditions. Stabilization of the E–Z isomeric motion is failed in batch process, which might be due to the exponential decay of light intensity, variable irradiation, low mixing, low heat exchange, low photon flux etc. This photo-μ-flow light driven motion is further extended to the establishment of a photostationary state under solar light irradiation.
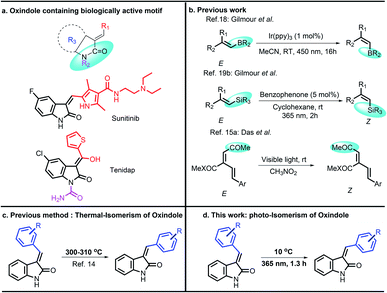
Functionalized 3-benzylidene-indolin-2-ones are an important structural motif in organic chemistry and are embedded in many naturally occurring compounds.1 They found wide applications in molecular-motors,2 energy harvesting dyes,3 pharmaceutical chemistry (sunitinib, tenidap),4 protein kinase inhibitors,5 pesticides,6 flavors,7 and the fragrance industry.8 In the last few decades, numerous protocols have been developed for the synthesis of novel indolin-2-ones. For instance, palladium (Pd)-catalysed intramolecular hydroarylation of N-arylpropiolamides,9 Knoevenagel condensation of oxindole and aldehyde,10 two-step protocols such as Ni-catalyzed CO2 insertion followed by coupling reaction,11 Pd-catalysed C–H functionalization/intramolecular alkenylation,12 Pd(0)/monophosphine-promoted ring–forming reaction of 2-(alkynyl)aryl isocyanates with organoboron compound, and others.13
Knoevenagel condensation is one of the best methods for the preparation of 3-benzylidene-indolin-2-ones, but often it gives mixture of E/Z isomeric products. Otherwise, noble metal-catalysed protocols received enormous interest. However, the limited availability, high price, and toxicity of these metals diminished their usage in industrial applications. Therefore, several research groups have been engaged in search of an alternative greener and cleaner approach under metal-free conditions. To address the diastereoisomeric issue, Tacconi et al. reported a thermal (300–310 °C) isomerization reaction of 3-arylidene-1,3-dihydroindol-2-ones,14 which suffers from poor reaction efficiency and E/Z selectivity. Therefore, transformations controlling E/Z ratio of 3-benzylidene-indolin-2-ones remains a challenging task and highly desirable (Scheme 1).
 | ||
| Scheme 1 Functionalized 3-benzylidene-indolin-2-ones and alkenes in bioactive compounds and the accessible methods. | ||
On the other hand, selective E/Z stereo-isomerization of alkenes has been well established using various methods in the presence of light stimuli,15a cations,15b halogens or elemental selenium,16 palladium-hydride catalyst,10 cobalt-catalyst,17 Ir-catalyst,18 organo-catalysts.19 Among these, light-induced photostationary E/Z stereoisomerization is very attractive, due to its close proximity towards the natural process. In recent years, several light-driven molecular motors (controlled motion at the molecular level), molecular propellers,20 switches,21 brakes,22 turnstiles,23 shuttles,24 scissors,25 elevators,26 rotating modules,27 muscles,28 rotors,29 ratchets,30 and catalytic self-propelled objects have been developed.31 Further, equipment's relying on molecular mechanics were rapidly developed, particularly in the area of health care.
Till date, controlled photo-isomerization of functionalized 3-benzylidene-indolin-2-ones is one of the puzzling problems to the scientific community. Photochemical reactions in batch process have serious drawbacks with limited hot-spot zone due to inefficient light penetration with increasing light path distance through the absorbing media, and the situation becomes poorer when the reactor size increases.32,33 In contrast, the capillary microreactor platform has emerged as an efficient the artificial tool with impressive advantages, such as excellent photon flux, uniform irradiation, compatibility with multi-step syntheses, excellent mass and heat transfer, which lead to significant decrease the reaction time with improved yield or selectivity over batch reactors.33a,34 To address the aforementioned challenges, it is essential to develop a highly efficient photo-microchemical flow approach for the controlled isomerization of functionalized 3-benzylidene-indolin-2-ones in catalyst-free and an environment friendly manner.
Results and discussion
The required substrate, (E)-3-benzylideneindolin-2-one (3a), was synthesised following the literature Knoevenagel condensation procedure (details in ESI†).14 As most of the E to Z isomerization of alkenes are contra-thermodynamic in nature, to appropriateness for the photostationary state, electromagnetic radiation, irradiance time, temperature, solvent, and aptness of reactor needs to be optimized. Accordingly, we were interested to find the suitable electromagnetic radiation which can interact with the starting materials. In this regard, the UV-Visible absorption spectrum of the 3a in DMF is tested, which showed absorption peak close to output spectrum of the medium pressure Hg lamp (Fig. 1). It suggests that the medium pressure Hg lamp is suitable for the photo-chemical reaction. Next, the residence time in photo-flow condition is optimized, using a solution of 3a (0.25 M) in DMF solvent and infuse into a homemade photo-flow reactor through the leak-proof syringes to the synthesis of (Z)-3-benzylideneindolin-2-one 4a (Table 1, entry 1). The solution 3a, passing through a commercially available high purity perfluoroalkoxy (PFA) tubing (inner diameter (id) = 1000 μm, length = 2 meter, volume = 1.57 mL) held at 30 °C was irradiated with 250 W medium pressure UV lamp at a 100 μL min−1 flow rate (irradiation time = 15.7 min) which typically leads to the formation of the brownish colour solution having 12% (yield) of the 4a product (Table 1, entry 1). Next, the reactions at various temperatures and residence times were carried out to find the optimal condition, with an improved yield of 4a (Table 1, entries 2 to 6). To our delight, 4a was obtained in 76% yield at 10 °C with 20 μL min−1 flow rate in DMF solvent along with 3a (24%), separable by column chromatography (Table 1, entry 7, Fig. S2†). Moreover, the increment of the residence time 78.5 to 157 min failed to increase the yield of 4a (Table 1, entry 8, Fig. 2) by providing the energy-efficient photo-flow system. Further, when the medium pressure 250 W Hg lamp was replaced with blue LED 4 W, the desired product 4a formed only in 18% yield (Table 1, entry 9). The reaction in batch process under the flow-optimized stock solution condition failed to produce any desired product 4a (Table 1, entry 10), which may be due to the exponential decay of light intensity in bulk solution, low light irradiation, low mixing, low heat exchange and low photon flux.35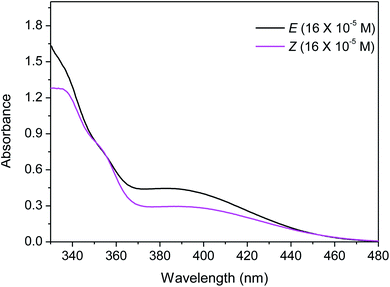 | ||
| Fig. 1 UV-Vis spectroscopy of the (E)-3-benzylideneindolin-2-one (3a) and (Z)-3-benzylideneindolin-2-one (4a) in DMF solvent. | ||
| Entry | Flow rate (μL min−1) | Residence time (min) | Temp. (°C) | Yielde (%) | |
|---|---|---|---|---|---|
| 4a (Z-isomer) | 3a (E-isomer) | ||||
| a Reaction conditions: 3a: 0.25 M in dimethylformamide (DMF) solvent, reactor volume 1.57 mL (PFA tubing id 1000 μm and 2 meter length), light source medium pressure 250 W Hg lamp.b Reaction conditions: Light source blue LED (4 W, light out-put 465 nm; 10 lux per cm2, brand name Lizone).c Reaction conditions: Batch process.d Reaction conditions: Reactor volume 7.85 mL.e Reaction conditions: Yields are based on the LC-MS.f Isolated yield in parenthesis. | |||||
| 1 | 100 | 15.7 | 30 | 12 | 88 |
| 2 | 200 | 7.8 | 30 | 7 | 93 |
| 3 | 50 | 31.4 | 30 | 18 | 82 |
| 4 | 20 | 78.5 | 30 | 44 | 56 |
| 5 | 10 | 157 | 30 | 44 | 56 |
| 6 | 20 | 78.5 | 20 | 56 | 44 |
| 7 | 20 | 78.5 | 10 | 76 (71)f | 24 (19)f |
| 8 | 10 | 157 | 10 | 76 | 24 |
| 9b | 20 | 78.5 | 10 | 18 | 82 |
| 10c | NA | 960 | 10 | NA | 100 |
| 11d | 100 | 78.5 | 10 | 76 | 24 |
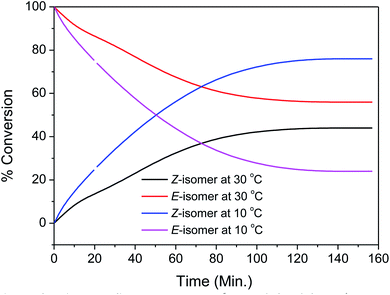 | ||
| Fig. 2 Time profile. Reaction condition: 3a: 0.25 M in DMF, reactor volume 1.57 mL (PFA tubing) light source medium pressure 250 W Hg lamp; conversions are based on LC-MS analysis. | ||
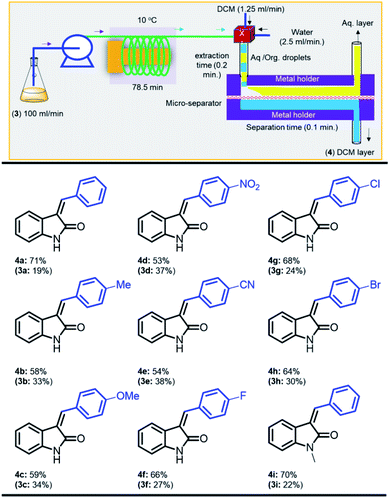
In order to improve the photo-flow reaction efficiency, we next investigated the effect of solvents (Table S1†) and found that both. DMF and DMA as good solvents to provide a decent yield in the isomerization at 10 °C in 1.3 h, while hexane failed to stabilize the photo stationary state. Having the optimized controlled-photostationary state in hand on milligram scale, we next moved toward the process intensification reactor in gram scale. The scale-up synthesis of 4a was examined using 7.85 mL volume reactor by the extension ((inner diameter (id) = 1000 μm, length = 10 meter) of PFA tubing (Table 1, entry 11). The long tubing reactors were irradiated with a medium pressure lamp to maintain the adequate light intensity, and the solution of 3a was passed to the reactor with a flow rate of 100 μL min−1, although maintaining 10 °C to produce 4a in 76% yield, resulting in ca. 6.0 g d−1 productivity (see ESI Video S1†).
Having the preliminary optimized photo-flow process system for the isomerization reaction of 3-benzylideneindolin-2-one (3a), we turned our attention towards integrating one-flow process to reduce the laborious extraction and the work-up to get 4a from the DMF solution, as depicted in Fig. 3.33a,36 Due to high boiling point of the solvent, DMF (153 °C), it is difficult to evaporate to obtain the product using rotary evaporator. Thus, we switched to extract the product with low boiling solvent, (dichloromethane (DCM)/diethyl ether (DEE)/and industrial favourable t-butyl methyl ether (MTBE)), to favourably isolate the product as a separable mixture of 4a and 3a. It is well-known phenomenon that DMF (hydrophilic solvent) prefers to move into aqueous-phase and is partially miscible with DCM (relatively hydrophobic solvent). As a result, water (flow rate = 1.25 mL min−1) and extracting solvent DCM (flow rate = 1.25 mL min−1) were infused into an X-junction to the DMF mixture that flowed from the exit of the photo-reactor to form the aqueous-organic droplet in the PTFE capillary to accomplish the extraction of isomer 4a into the DCM droplet within 0.2 min residence time (see ESI Video S1†). Next, to separate the aqueous organic droplet, we were adapted our previously designed and commercialized continuous flow liquid–liquid micro-separator.37 Notably, the completion of the DCM–water separation obtained in 0.1 min of the residence time. Notably, additional sodium sulphate or magnesium sulphate is not needed to dry the product.
After the successful development of an integrated system for the photostationary state for the conversion of 3-benzylideneindolin-2-one (3a) to 4a, we next examined the scope and limitations of the continuous flow system as well as the effect of the directing group. Toward this, a few electron-releasing and electron-withdrawing 3-benzylideneindolin-2-one derivatives were prepared using substituted aldehydes. Notably, the methyl (3b), methoxy (3c), nitro (3d), cyano (3e), fluoro (3f), chloro (3g), bromo (3h) substituted 3-benzylideneindolin-2-ones underwent photostationary state to afford the corresponding Z-isomer derivatives 4b to 4h, in good yield (53–71%).
Having explored the photostationary state for E–Z isomerization under fixed wavelength, as an artificial light source, temperature, and solvent conditions, we became interested to develop the photostationary state under the sunlight irradiation (to save the power and present domain application).33a The homemade cylindrical shape capillary reactor is suitable only for the laboratory-controlled condition. Hence, a novel arrangement of capillary microreactor needed for highly efficient solar light harvesting, which is unprecedented. The newly designed solar panel microreactor (details fabrication is provided in ESI, Fig. S3†) was placed on roof-top to expose to daylight (the average amount of solar emission 5.25 kW h per m2per day in Hyderabad, India, manually controlled light intensity > 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux) to verify the photostationary state of 3a.38 As shown in Fig. 4, the solution of 3a in DMF (0.25 M) infused into the solar panel PFA capillary microreactor with a syringe pump. It was observed that the optimal conversion to 4a was 50% (isolated yield) in the presence of sunlight. Though the isomerization is low, the overall strategy minimized the laborious extraction and separation process with 0.166 g h−1 productivity.
000 lux) to verify the photostationary state of 3a.38 As shown in Fig. 4, the solution of 3a in DMF (0.25 M) infused into the solar panel PFA capillary microreactor with a syringe pump. It was observed that the optimal conversion to 4a was 50% (isolated yield) in the presence of sunlight. Though the isomerization is low, the overall strategy minimized the laborious extraction and separation process with 0.166 g h−1 productivity.
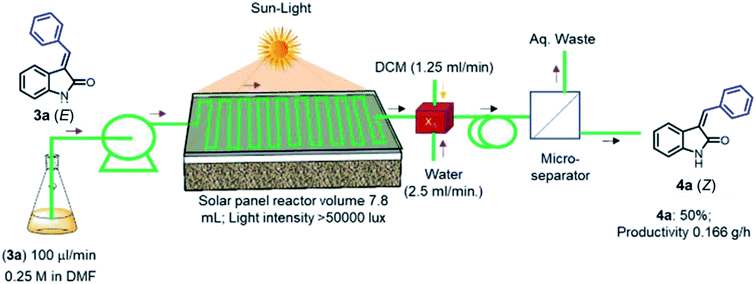 | ||
| Fig. 4 Isomerization under the solar irradiation. Reactor volume 7.8 mL (PFA tubing id 1000 μm and 10 meter length) light source sunlight; yield is based on isolated yields. | ||
In summary, the photostationary state for E to Z isomerization of functionalised 3-benzylideneindolin-2-one has been accomplished under controlling of solvent, temperature, and enhanced irradiation in microfluidic condition. This is a first integrated photostationary state system operated under controlled condition as well mixture of light (solar irradiance) condition to form the desired functionalised (Z)-3-benzylideneindolin-2-ones. This integrated platform can be useful to those syntheses where the photostationary state for E–Z isomerization involves in drug discovery, natural products, biology, energy, and environmental chemistry. Genuine-time reaction control for photostationary state for E–Z isomerization under unstable irradiance (cloudy or rainy days) work is under progress in our laboratory.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
One of the authors (V. G.) is thankful to the Council of Scientific & Industrial Research (CSIR), New Delhi, for providing a University Grant Commission-Senior Research Fellowship (UGC-SRF). A. K. S. thanks Department of Science and Technology, New Delhi (Govt. of India) (DST), New Delhi, for the INSPIRE Faculty Award (DST/INSPIRE/04/2016/000247) and Early Carrier Research Award (ECR/2017/000208). CSIR-IICT Communication No. IICT/Pubs./2020/116.References
- X. Ma, X. Xie, L. Liu, R. Xia, T. Li and H. Wang, Chem. Commun., 2018, 54, 1595–1598 RSC.
- D. Roke, M. Sen, W. Danowski, S. J. Wezenberg and B. L. Feringa, J. Am. Chem. Soc., 2019, 141, 7622–7627 CrossRef CAS PubMed.
- Y. S. Tingare, M.-T. Shen, C. Su, S.-Y. Ho, S.-H. Tsai, B.-R. Chen and W.-R. Li, Org. Lett., 2013, 15, 4292–4295 CrossRef CAS PubMed.
- A. Millemaggi and R. J. K. Taylor, Eur. J. Org. Chem., 2010, 2010, 4527–4547 CrossRef.
- F. A. Al-Obeidi and K. S. Lam, Oncogene, 2000, 19, 5690–5701 CrossRef CAS PubMed.
- L.-S. Huang, D.-Y. Han and D.-Z. Xu, Adv. Synth. Catal., 2019, 361, 4016–4021 CrossRef CAS.
- J. Xu, L.-D. Shao, D. Li, X. Deng, Y.-C. Liu, Q.-S. Zhao and C. Xia, J. Am. Chem. Soc., 2014, 136, 17962–17965 CrossRef CAS PubMed.
- M. Zhu, C. Zheng, X. Zhang and S.-L. You, J. Am. Chem. Soc., 2019, 141, 2636–2644 CrossRef CAS PubMed.
- T.-S. Jiang, R.-Y. Tang, X.-G. Zhang, X.-H. Li and J.-H. Li, J. Org. Chem., 2009, 74, 8834–8837 CrossRef CAS PubMed.
- W. Zhang and M.-L. Go, Bioorg. Med. Chem., 2009, 17, 2077–2090 CrossRef CAS PubMed.
- B. Miao, Y. Zheng, P. Wu, S. Li and S. Ma, Adv. Synth. Catal., 2017, 359, 1691–1707 CrossRef CAS.
- S. Ueda, T. Okada and H. Nagasawa, Chem. Commun., 2010, 46, 2462–2464 RSC.
- T. Miura, T. Toyoshima, Y. Takahashi and M. Murakami, Org. Lett., 2008, 10, 4887–4889 CrossRef CAS PubMed.
- A. C. Coda, A. G. Invernizzi, P. P. Righetti, G. Tacconi and G. Gatti, J. Chem. Soc., Perkin Trans. 2, 1984, 615–619 RSC.
- (a) S. R. Chowdhury, S. Mondal, D. K. Maiti, S. Mishra and I. Das, Org. Lett., 2019, 21, 1578–1582 CrossRef PubMed; (b) R. Dorel, C. Miro, Y. Wei, S. J. Wezenberg and B. L. Feringa, Org. Lett., 2018, 20, 3715–3718 CrossRef CAS PubMed.
- S. Ortgies and A. Breder, ACS Catal., 2017, 7, 5828–5840 CrossRef CAS.
- (a) H. Liu, M. Xu, C. Cai, J. Chen, Y. Gu and Y. Xia, Org. Lett., 2020, 22, 1193–1198 CrossRef CAS PubMed; (b) Q.-Y. Meng, T. E. Schirmer, K. Katou and B. König, Angew. Chem., Int. Ed., 2019, 58, 5723–5728 CrossRef CAS PubMed.
- J. J. Molloy, J. B. Metternich, C. G. Daniliuc, A. J. B. Watson and R. Gilmour, Angew. Chem., Int. Ed., 2018, 57, 3168–3172 CrossRef CAS PubMed.
- (a) K. Livingstone, M. Tenberge, F. Pape, C. G. Daniliuc, C. Jamieson and R. Gilmour, Org. Lett., 2019, 21, 9677–9680 CrossRef CAS PubMed; (b) S. I. Faßbender, J. J. Molloy, C. Mück-Lichtenfeld and R. Gilmour, Angew. Chem., Int. Ed., 2019, 58, 18619–18626 CrossRef PubMed.
- N. L. Mutter, J. Volarić, W. Szymanski, B. L. Feringa and G. Maglia, J. Am. Chem. Soc., 2019, 141, 14356–14363 CrossRef CAS PubMed.
- M. Suda, Y. Thathong, V. Promarak, H. Kojima, M. Nakamura, T. Shiraogawa, M. Ehara and H. M. Yamamoto, Nat. Commun., 2019, 10, 2455 CrossRef PubMed.
- R. Portman, Nat. Nanotechnol., 2008 DOI:10.1038/nnano.2008.177.
- M. K. J. ter Wiel, R. A. van Delden, A. Meetsma and B. L. Feringa, J. Am. Chem. Soc., 2003, 125, 15076–15086 CrossRef CAS PubMed.
- T. van Leeuwen, A. S. Lubbe, P. Štacko, S. J. Wezenberg and B. L. Feringa, Nat. Rev. Chem., 2017, 1, 0096 CrossRef CAS.
- M. Zaremba and V. Siksnys, Proc. Natl. Acad. Sci., 2010, 107, 1259–1260 CrossRef CAS.
- S. Silvi, M. Venturi and A. Credi, Chem. Commun., 2011, 47, 2483–2489 RSC.
- M. Guentner, M. Schildhauer, S. Thumser, P. Mayer, D. Stephenson, P. J. Mayer and H. Dube, Nat. Commun., 2015, 6, 8406 CrossRef CAS PubMed.
- (a) Y. Liu, A. H. Flood, P. A. Bonvallet, S. A. Vignon, B. H. Northrop, H.-R. Tseng, J. O. Jeppesen, T. J. Huang, B. Brough, M. Baller, S. Magonov, S. D. Solares, W. A. Goddard, C.-M. Ho and J. F. Stoddart, J. Am. Chem. Soc., 2005, 127, 9745–9759 CrossRef CAS PubMed; (b) L. Greb and J.-M. Lehn, J. Am. Chem. Soc., 2014, 136, 13114–13117 CrossRef CAS PubMed.
- N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152–155 CrossRef CAS PubMed.
- B. Lau, O. Kedem, J. Schwabacher, D. Kwasnieski and E. A. Weiss, Mater. Horiz., 2017, 4, 310–318 RSC.
- B. L. Feringa, Adv. Mater., 2020, 32, 1906416 CrossRef CAS PubMed.
- S. Protti and M. Fagnoni, Photochem. Photobiol. Sci., 2009, 8, 1499–1516 RSC.
- (a) S. Kumar, D. Aand, S. Pabbaraja, D.-P. Kim and A. K. Singh, ACS Sustainable Chem. Eng., 2019, 7, 19605–19611 CrossRef CAS; (b) X.-J. Wei, W. Boon, V. Hessel and T. Noël, ACS Catal., 2017, 7, 7136–7140 CrossRef CAS PubMed.
- (a) D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed; (b) D. Aand, B. Mahajan, S. Pabbaraja and A. K. Singh, React. Chem. Eng., 2019, 4, 812–817 RSC; (c) J. Mateos, A. Cherubini-Celli, T. Carofiglio, M. Bonchio, N. Marino, X. Companyó and L. Dell'Amico, Chem. Commun., 2018, 54, 6820–6823 RSC.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS PubMed.
- D. Aand, S. Karekar, B. Mahajan, A. B. Pawar and A. K. Singh, Green Chem., 2018, 20, 4584–4590 RSC.
- Micro-separator available with Amar Equipment, http://www.amarequip.com/docs/Flow-chemistry-catalog.pdf.
- Hyderabad solar irradiation intensity, http://www.synergyenviron.com/tools/solar-irradiance/india/telangana/Hyderabad, accessed, Nov 27, 2018 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05288d |
| This journal is © The Royal Society of Chemistry 2020 |