Open Access Article
Open Access ArticleA guanosine-based 2-formylphenylborate ester hydrogel with high selectivity to K+ ions†
Hongwei Qiao‡
a,
Jiakun Bai‡b,
Sichun Zhang a and
Chao Li
a and
Chao Li *b
*b
aDepartment of Chemistry, Tsinghua University, Beijing 100084, P. R. China
bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: lichao@mail.buct.edu.cn
First published on 3rd August 2020
Abstract
Guanosine-based supramolecular hydrogels are particularly of interest for biomaterial and biomedical purposes, as they are generally biocompatible and stimuli-responsive. We found a strong and long-life transparent hydrogel made by mixing guanosine (G) with 1 equiv. of 2-formylbenzeneboronic acid (2FPB) and KOH. Alkali cations can assist the stacking of individual G-quartet to give extended nanowires, but only K+ ion induces the formation of a stable and self-supporting network hydrogel for a couple of months. Data from variable temperature NMR indicated that guanosine 2-formylbenzeneborate ester and G are the key components of the self-assembly. Further, G-2FPB-K+ hydrogel solution can induce berberine (BBR) fluorescence, showing high selectivity to K+ ion and anti-ion interference capability. A good linear relationship between fluorescent intensity and K+ concentration allowed us to directly detect K+ levels in human blood serum.
Supramolecular hydrogels composed of biomolecular building blocks are of particular interest for tissue engineering, controlled release of bioactive substances, and targeted drug delivery.1 Among them, guanosine-derived supramolecular hydrogel has received a perennial attention due to its unique self-assembly properties and potential biomedical applications.2 Since the earliest G-quartet, a layered tetramer was reported by Davies in 1962,3 many studies have focused primarily on developing novel gelators such as guanosine derivatives or binary components, for improving the lifetime stability of guanosine hydrogels.4 Recently, Davis et al. developed a G-quartet hydrogel with excellent lifetime stability utilizing borate ester chemistry.5 The hydrogels formed in the presence of K+ ions by stabilizing the G-quartet and the anionic borate diesters were stronger than those formed in the presence of other alkali cations. Guanosine borate diesters were considered as the key components of the G4·K+ hydrogel. In the next few years, more systems based on guanosine borate ester were widely studied.6 Shi group described a supramolecular hydrogel composed of guanosine, 2-formylboronic acid, and tris(2-aminoethyl)amine in the presence of KCl, achieving zero-order drug-release.7 Very recently, Das et al. reported a G-quadruplex hydrogel self-assembled by guanosine and 1-naphthaleneboronic acid. The injectable, shape-supported, printable, and self-healable G-quadruplex hydrogel was used as a promising drug delivery agent.8
In this paper, a simple G-quartet hydrogel construct that consists of equimolar guanosine (1) and 2-formylphenylboronic acid (2) was reported. On reaction with 2 (50 mM) in the present of KOH, 1 formed covalent guanosine 2-formylphenylborate ester (3) that self-assembled to form G-2FPB-K+ hydrogel (Fig. 1). The equimolar OH− was required to dissolve guanosine and stabilize the tetravalent boron atom. Interestingly, the guanosine-derived stacked assembly showed the extreme selectivity to K+ ions. The K+-stabilized system has remained a self-supporting transparent hydrogel for a couple of months, while other alkali cations (Li+, Na+, Rb+, and Cs+) system gave to liquid rather than gel (Fig. 1, inverted bottle). This could be because the size of the K+ ion (1.52 Å) is only suitable to fit inside the G4 cavities formed by guanosine and guanosine 2-formylphenylborate ester and results into the formation of a strong hydrogel. The sizes of Li+ (0.90 Å) and Cs+ (1.81 Å) are too small and too large to fit inside the cavities, respectively, and the sizes of Na+ (1.16 Å) and Rb+ (1.66 Å) are also not suitable to form a G-quadruplex, which reflects precipitation instead of hydrogelation.4b,8 The correct stoichiometry of each component was crucial for self-assembly and increased hydrogel lifetime. All of the guanosine did not dissolve in the mixture with insufficient OH−; with addition of excess OH− (>1.2 equiv.), the hydrogel became weak until viscous solution. The pH of the stable G-2FPB-K+ system was 8–9, and which was independent on the concentration of the hydrogel.
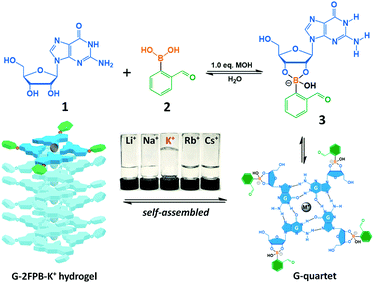 | ||
| Fig. 1 Self-assembled hydrogel G-2FPB-K+ formed by guanosine (1) and 2-formylbenzeneboronic acid (2) in the presence of K+ ions. | ||
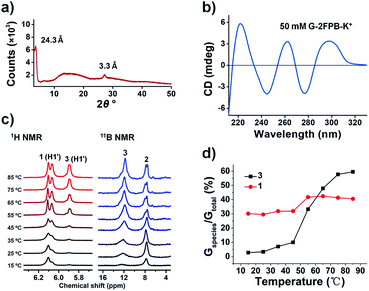
We firstly showed powder X-ray diffraction (PXRD) data of a freeze-dried 50 mM G-2FPB-K+ hydrogel (1, 2 and KOH 50 mM each) that consistent with the stacking of G-quartets in the hydrogel (Fig. 2a). In the wide-angle region, a well-resolved peak appears at 2θ ≈ 26.8° (d = 3.3 Å), which corresponds to the π–π stacking distance between two planar G-quartets.9 Additionally, there is a signal at 2θ ≈ 3.6° with a corresponding distance of 24.3 Å, in line with the width of a single G-quartet. Subsequently, we used circular dichroism (CD) spectrometry to assign the polarity of stacked G-quartets, thus confirming the formation of guanosine-based assemblies. The CD spectrum of 50 mM G-2FPB-K+ hydrogel showed positive peaks at 262 and 298 nm and negative peaks at 244 and 277 nm (Fig. 2b). The previous report revealed that C4-symmetric G8 octamer (head-to-tail stacking of G-quartets) displayed bands of opposite signal at 240 and 260 nm, whereas the CD signals for a D4-symmetric G8 octamer (head-to-head stacking) were shifted to 260 and 290 nm.10 The CD signals of G-2FPB-K+ hydrogel indicated the G-quartets are stacked in both head-to-tail and head-to-head orientations. It was hard to form self-supporting hydrogel when K+ ion was replaced by other alkali metal ions, but the CD spectra of these G-2FPB-M+ solution also showed the formation of G-quartets as the concentration increased (Fig. S1†). Fourier transform infrared (FTIR) spectroscopy of the hydrogel system was performed as shown in Fig. S2.† The amide vibration of G-2FPB-K+ hydrogel appeared at 1693, 1531 and 1481 cm−1 instead of amide vibration (ν(CO), δ(NH2)) of free G at 1726 cm−1, which indicated the formation of G-quartets.6b The decreased wavenumber was due to intermolecular H-bonding between guanosines. The stretching vibration of the B–OC bond at 1103 cm−1 suggested the formation of boronate ester in the hydrogel network.6a The broad bands centered at 3332 and 3135 cm−1 were assigned to the OH and NH stretching frequency. There was no significant different in the FTIR spectra between G-2FPB-Na+ and G-2FPB-K+.
Variable temperature solution-state 1H NMR and 11B NMR (VT-NMR) experiments were next carried out, and allowed us to monitor the species in solution and indirectly determine the structural information on the composition of the gel phase. Compared with the multiple components (diastereomeric borate diesters, monoester, and guanosine) of G4·K+ system from Davis work, we suspected that the G-2FPB-K+ hydrogel was constructed by a simple two-component system, guanosine 1 and guanosine 2-formylphenylborate ester 3.
The sample was prepared with 50 mM G-2FPB-K+ in D2O and followed with an increasing at temperature with a gradient of 10 °C ranging from 15 °C to 85 °C. 2,2,3,3-(d4)-3-(Trimethylsilyl)propionic acid sodium salt (0.31 mM) was used as an internal standard for VT 1H NMR. As shown in Fig. 2c (left), although the H1′ of the ribose around 6.0 ppm was distinguished well from other proton, the diffusion-ordered NMR (DOSY) experiment was performed to better assign H1′ peaks from 1 or 3 (Fig. S4a†). The diffusion coefficient for the peak at δ 5.89 (3.882 × 10−10 m2 s−1) was smaller than that at δ 6.10 (5.546 × 10−10 m2 s−1), which indicated that the peak at δ 6.10 was the smaller species in solution. Thus, the peaks at δ 5.89 and δ 6.10 were assigned to H1′ from 3 and 1, respectively. Notably, in weak alkaline environment 2FPBA easily formed an intramolecular borate ester upon nucleophilic addition of hydroxide to the aldehyde,11 which was verified by 1H NMR spectra (Fig. S3†). Thus, the peak at δ 6.06 was assigned to the proton of –CH–(OR)2, giving the same diffusion coefficient 3.882 × 10−10 m2 s−1 with H1′ of 3. The detailed assignment for other NMR-visible species in DOSY spectra was shown in Fig. S4b.†
Below 45 °C, almost no 3 existed in solution where the main species was free 1. However, when the temperature was above 55 °C, the peak at δ 5.89 ppm appeared and increased in relative intensity, indicating 3 was gradually released from melted hydrogel. The contents of 1 and 3 in solution were calculated by integrating the NMR signals as shown in Fig. 2d. About 30% 1 and 7% 3 (Gspecies/Gtotal) were observed in solution below 35 °C respectively. As the temperature increased, more NMR-visible species including both 1 and 3 appeared in the solution. Assuming the hydrogel melted completely at 85 °C where 40% 1 and 60% 3 (Gspecies/Gtotal) were obtained, we can figure out that below 35 °C 1 (10%) and 3 (53%) with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 participated in the assembly of hydrogel. For other M+-stabilized system, the contents of 3 in 50 mM G-2FPB-M+ solution at different temperature were also calculated and shown in Fig. S5.† Below 45 °C, more 3 in the tested solution were observed and less 3 were released at 85 °C compared with G-2FPB-K+ system, suggesting that stable assemblies were difficult to form in the presence of other alkali metal ions.
5 participated in the assembly of hydrogel. For other M+-stabilized system, the contents of 3 in 50 mM G-2FPB-M+ solution at different temperature were also calculated and shown in Fig. S5.† Below 45 °C, more 3 in the tested solution were observed and less 3 were released at 85 °C compared with G-2FPB-K+ system, suggesting that stable assemblies were difficult to form in the presence of other alkali metal ions.
Subsequently, VT 11B NMR spectra were performed with BF3·O(C2H5)2 as an external standard, as shown in Fig. 2c (right). At 15 °C, almost the only peak appeared at 7.8 ppm was assigned to 2. With the increase of temperature, a gradually growing peak was observed around 12.0 ppm, which was assigned to 3. The result indicated that 3 was the key component of the stacked hydrogel, which was in line with that obtained from the VT 1H NMR.
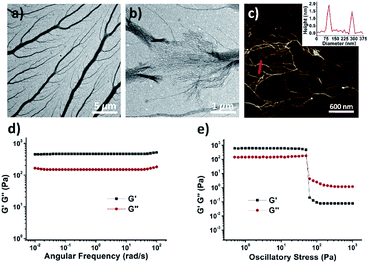
The morphology of xerogel G-2FPB-K+ hydrogel was visualized by transmission electron microscopy (TEM) and atomic force microscopy (AFM). The image of TEM revealed that the well-organized and dendritic fibrillar structures with high aspect ratio existed in a 10 mM hydrogel solution (Fig. 3a). The thickest trunk was near 1 μm, from which lots of tiny branches stretched out. When the sample was diluted to 5 mM, the fractured trunk presented a dense mass of nanowires, suggesting the thick fiber was probably bundled from these thin wires (Fig. 3b). The AFM image showed that the height of a single nanowire perpendicular to the image plane was about 2 nm corresponding to the predicted diameter of G-quartet (Fig. 3c), and the average width was around 50–70 nm. These individual assembly fibers could prefer to huddle together through π–π stacking of the peripheral phenyl.
To quantitatively assess the mechanical properties of hydrogels, we then carried out rheological measurements performed with a 50 mM G-2FPB-K+ hydrogel. As shown in Fig. 3d, the storage modulus (G′) remained larger than its loss modulus (G′′) as it was examined as a function of frequency, exhibiting solid-like rheology. What's more, dynamic frequency sweeps showed that G′ was independent of frequency, indicating that the fibrous network didn't relax even over long time scales. Oscillatory stress sweeps on the G-2FPB-K+ showed that G′ rapidly decreased after the shear stress exceeded about 50 Pa, and G′ and G′′ were inverted, resulting in a quasi-liquid state, indicating a gel-to-sol transition.12,13
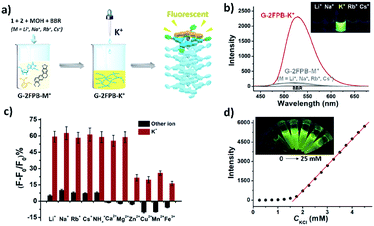
Inspired by some specific fluorogenic dyes to probe the formation of G-quadruplex,14 we explored whether the current system can also induce emission enhancement of certain fluorescent sensors. Interestingly, we discovered that the fluorescence of berberine (BBR) can be selectively increased during the self-assembled process of G-2FPB-K+ hydrogel. Adding BBR (31 μM) to a 50 mM G-2FPB-M+ solution (M+ = Li+, Na+, Rb+, or Cs+), almost no fluorescence was obtained. Despite the evidence that G-quartet and few assemblies existed in G-2FPB-M+ solution, the fluorescence of BBR was not enhanced. When K+ ions were added in the mixture above, network hydrogel was formed simultaneous with the increasing fluorescence (Fig. 4a). In Fig. 4b, the BBR in 50 mM G-2FPB-K+ hydrogel showed an ∼18 fold enhancement in its relative fluorescence intensity over the other G-2FPB-M+ solution with the same concentration. The inset image visually presented a significant difference between alkali cations, indicating the extremely high selective to K+ ions. The UV absorption spectra of the G-2FPB-K+/BBR were obviously different from G-2FPB-K+ hydrogel, indicating the strong interaction between the hydrogel system and berberine (Fig. S6†).
Subsequently, the anti-ion interference capability of G-2FPB-K+/BBR system was investigated by calculating the change in signal-to-background ratio (ΔF/F0) of G-2FPB-Na+ solution containing various cations in the absence or presence of K+ ions (Fig. S7†). As shown in Fig. 4c, adding different monovalent cations to a 100 mM G-2FPB-Na+/BBR solution (1, 2, NaOH, 100 mM each; BBR, 31 μM), all the ΔF/F0 were below 10%. However, when 0.2 mM K+ ions was added in the above solution (100 equiv. Li+, Na+ and Cs+, 10 equiv. NH4+, 1 equiv. Rb+), the ΔF/F0 sharply increased to 60%, indicating the strong ability to anti-ion interference. For divalent Zn2+, Cu2+, Mn2+ and trivalent Fe3+, the basic fluorescence intensity of G-2FPB-Na+/BBR solution reduced obviously (negative ΔF/F0). The decreased solubility of guanosine in these water-insoluble systems resulted in the decrease in fluorescence. Upon adding 0.2 mM K+ ions to the above solution, less than 30% ΔF/F0 was obtained. In addition, the fluorescence intensity of G-2FPB-K+/BBR system was positively related to the concentration of hydrogel (Fig. 4d inset), which also strictly depended on K+ ion concentration. When K+ ions was added in 100 mM G-2FPB-Na+/BBR solution, the fluorescence intensity at 523 nm increased gradually as K+ concentration increasing, and an excellent linear relationship (y = 340.5x − 70.0, R2 = 0.9956) between ΔF and K+ concentration was obtained in the range of 0.1 to 1.0 mM (linear range depends on the solution concentration), as shown in Fig. 4d.
Encouraged by the above experiment results, the G-2FPB-K+/BBR system was used to detect K+ ion in real blood samples. Considering normal serum K+ ion levels ranged from 3.8 mM to 5.4 mM,15 the samples were diluted 10-fold to fit in the linear range. Five different human blood serum samples were detected, and their K+ concentration was calculated according to the linear calibration equation. Compared with the ion-selective electrode (ISE) method, consistent values were determined by the proposed method with acceptable standard deviations (Table 1). These data demonstrated that the proposed approach has the potential to be applied in clinical K+ ion detection based on K+-dependent formation of G-quartet self-assembly.
| Sample | ISEa (mM) | Proposed method (meanb ± SDc) (mM) | Recoveryd (%) |
|---|---|---|---|
| a Ion-selective electrode (ISE) experiments were conducted in China-Japan Friendship Hospital, see Fig. S8 for detailed reports.b Mean of three determinations.c Standard deviation.d Percent recovery compared to ISE method.e Adding 4.0 mM KCl to the sample 4. All human blood samples used in this work were provided by the clinical laboratory of China-Japan Friendship Hospital. | |||
| 1 | 3.4 | 3.36 ± 0.12 | 98.8 |
| 2 | 3.8 | 3.82 ± 0.17 | 100.5 |
| 3 | 4.2 | 4.18 ± 0.08 | 99.5 |
| 4 | 4.6 | 4.56 ± 0.16 | 99.1 |
| 5 | 6.2 | 6.31 ± 0.05 | 101.8 |
| 6e | 8.6 | 8.77 ± 0.23 | 101.9 |
We have described a transparent and stable G-2FPB-K+ hydrogel formed from equimolar guanosine and 2-formylbenzeneboronic acid in the presence of KOH. This system showed excellent selectivity for K+ without interference from other alkali metal ions. Berberine can be used to selectively sense formation of G-2FPB-K+ hydrogel during the self-assembly process. The good linear relationship between fluorescent intensity and K+ concentration allowed us to detect K+ ion in human blood serum samples, which was comparable to the standard ISE approach. We expect that the current work can inspire some new ideas for design of guanosine-based hydrogel and application in serum K+ detection.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The work was supported by the National Natural Science Foundation of China (21974078, 21672021, and 21572018).Notes and references
- (a) L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089–6102 RSC; (b) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed; (c) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed; (d) A. G. Cheetham, R. W. Chakroun, W. Ma and H. Cui, Chem. Soc. Rev., 2017, 46, 6638–6663 RSC.
- (a) G. M. Peters and J. T. Davis, Chem. Soc. Rev., 2016, 45, 3188–3206 RSC; (b) T. Bhattacharyya, P. Saha and J. Dash, ACS Omega, 2018, 3, 2230–2241 CrossRef CAS PubMed; (c) J. L. Huppert, Chem. Soc. Rev., 2008, 37, 1375–1384 RSC; (d) J. T. Davis and G. P. Spada, Chem. Soc. Rev., 2007, 36, 296–313 RSC.
- M. Gellert, M. N. Lipsett and D. R. Davies, Proc. Natl. Acad. Sci. U. S. A., 1962, 48, 2013–2018 CrossRef CAS PubMed.
- (a) R. N. Das, Y. P. Kumar, S. Pagoti, A. J. Patil and J. Dash, Chem.–Eur. J., 2012, 18, 6008–6014 CrossRef CAS PubMed; (b) B. Adhikari, A. Shah and H. B. Kraatz, J. Mater. Chem. B, 2014, 2, 4802–4810 RSC; (c) Z. Li, L. E. Buerkle, M. R. Orseno, K. A. Streletzky, S. Seifert, A. M. Jamieson and S. J. Rowan, Langmuir, 2010, 26, 10093–10101 CrossRef CAS PubMed.
- (a) G. M. Peters, L. P. Skala, T. N. Plank, B. J. Hyman, G. N. M. Reddy, A. Marsh, S. P. Brown and J. T. Davis, J. Am. Chem. Soc., 2014, 136, 12596–12599 CrossRef CAS PubMed; (b) G. M. Peters, L. P. Skala and J. T. Davis, J. Am. Chem. Soc., 2016, 138, 134–139 CrossRef CAS PubMed; (c) T. N. Plank and J. T. Davis, Chem. Commun., 2016, 52, 5037–5040 RSC; (d) T. N. Plank, L. P. Skala and J. T. Davis, Chem. Commun., 2017, 53, 6235–6238 RSC; (e) S. Pieraccini, M. Campitiello, F. Carducci, J. T. Davis, P. Mariani and S. Masiero, Org. Biomol. Chem., 2019, 17, 2759–2769 RSC.
- (a) T. Bhattacharyya, Y. P. Kumar and J. Dash, ACS Biomater. Sci. Eng., 2017, 3, 2358–2365 CrossRef CAS; (b) V. Venkatesh, N. K. Mishra, I. Romero-Canelon, R. R. Vernooij, H. Y. Shi, J. P. C. Coverdale, A. Habtemariam, S. Verma and P. J. Sadler, J. Am. Chem. Soc., 2017, 139, 5656–5659 CrossRef CAS PubMed; (c) J. J. Li, H. L. Wei, Y. Peng, L. F. Geng, L. M. Zhu, X. Y. Cao, C. S. Liu and H. Pang, Chem. Commun., 2019, 55, 7922–7925 RSC; (d) A. Biswas, S. Malferrari, D. M. Kalaskar and A. K. Das, Chem. Commun., 2018, 54, 1778–1781 RSC.
- Y. Li, Y. Liu, R. Ma, Y. Xu, Y. Zhang, B. Li, Y. An and L. Shi, ACS Appl. Mater. Interfaces, 2017, 9, 13056–13067 CrossRef CAS PubMed.
- T. Ghosh, A. Biswas, P. K. Gavel and A. K. Das, Langmuir, 2020, 36, 1574–1584 CrossRef CAS PubMed.
- C. Arnal Hérault, A. Banu, M. Barboiu, M. Michau and A. van der Lee, Angew. Chem., Int. Ed., 2007, 46, 4268–4272 CrossRef PubMed.
- (a) S. Masiero, R. Trotta, S. Pieraccini, S. De Tito, R. Perone, A. Randazzo and G. P. Spada, Org. Biomol. Chem., 2010, 8, 2683–2692 RSC; (b) J. Y. Lee, J. Yoon, H. W. Kihm and D. S. Kim, Biochemistry, 2008, 47, 3389–3396 CrossRef CAS PubMed; (c) P. Zhou and H. Li, Dalton Trans., 2011, 40, 4834–4837 RSC.
- N. J. Gutierrez-Moreno, F. Medrano and A. K. Yatsimirsky, Org. Biomol. Chem., 2012, 10, 6960–6972 RSC.
- (a) S. R. Raghavan and J. F. Douglas, Soft Matter, 2012, 8, 8539–8546 RSC; (b) Y. E. Shapiro, Prog. Polym. Sci., 2011, 36, 1184–1253 CrossRef CAS.
- X. Huang, S. R. Raghavan, P. Terech and R. G. Weiss, J. Am. Chem. Soc., 2006, 128, 15341–15352 CrossRef CAS PubMed.
- (a) A. C. Bhasikuttan and J. Mohanty, Chem. Commun., 2015, 51, 7581–7597 RSC; (b) J. Mohanty, N. Barooah, V. Dhamodharan, S. Harikrishna, P. I. Pradeepkumar and A. C. Bhasikuttan, J. Am. Chem. Soc., 2013, 135, 367–376 CrossRef CAS PubMed; (c) A. Renaud de la Faverie, A. Guedin, A. Bedrat, L. A. Yatsunyk and J. L. Mergny, Nucleic Acids Res., 2014, 42, e65 CrossRef CAS PubMed.
- L. Yang, Z. H. Qing, C. H. Liu, Q. Tang, J. S. Li, S. Yang, J. Zheng, R. H. Yang and W. H. Tan, Anal. Chem., 2016, 88, 9285–9292 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation, rheology procedure, morphological, CD, FTIR, UV-Vis, fluorescence and PXRD, 1H, 11B NMR and DOSY spectra assay of G-2FPB-K+ hydrogel; CD spectra of 50 mM G-2FPB-M+ and the contents of guanosine 2-formylphenylborate ester 3 in 50 mM G-2FPB-M+ at different temperature. See DOI: 10.1039/d0ra05254j |
| ‡ H. W. Qiao and J. K. Bai contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |