Open Access Article
Open Access ArticleNanocarriers for ocular drug delivery: current status and translational opportunity
Srividya Gorantla†
a,
Vamshi Krishna Rapallia,
Tejashree Waghule a,
Prem Prakash Singhb,
Sunil Kumar Dubey
a,
Prem Prakash Singhb,
Sunil Kumar Dubey a,
Ranendra N. Sahaac and
Gautam Singhvi†
a,
Ranendra N. Sahaac and
Gautam Singhvi† *a
*a
aIndustrial Research Laboratory, Department of Pharmacy, Birla Institute of Technology and Science (BITS), Pilani, Pilani Campus, Rajasthan, India 333031. E-mail: gautam.singhvi@pilani.bits-pilani.ac.in
bFormulation Development, Slayback Pharma India LLP, Hyderabad, Telangana 500072, India
cBirla Institute of Technology & Science (BITS), Pilani, Dubai Campus, UAE
First published on 24th July 2020
Abstract
Ocular diseases have a significant effect on vision and quality of life. Drug delivery to ocular tissues is a challenge to formulation scientists. The major barriers to delivering drugs to the anterior and posterior segments include physiological barriers (nasolacrimal drainage, blinking), anatomical barriers (static and dynamic), efflux pumps and metabolic barriers. The static barriers comprise the different layers of the cornea, sclera, and blood–aqueous barriers whereas dynamic barriers involve conjunctival blood flow, lymphatic clearance and tear drainage. The tight junctions of the blood–retinal barrier (BRB) restrict systemically administered drugs from entering the retina. Nanocarriers have been found to be effective at overcoming the issues associated with conventional ophthalmic dosage forms. Various nanocarriers, including nanodispersion systems, nanomicelles, lipidic nanocarriers, polymeric nanoparticles, liposomes, niosomes, and dendrimers, have been investigated for improved permeation and effective targeted drug delivery to various ophthalmic sites. In this review, various nanomedicines and their application for ophthalmic delivery of therapeutics are discussed. Additionally, scale-up and clinical status are also addressed to understand the current scenario for ophthalmic drug delivery.
1. Introduction
As per a World Health Organization (WHO) report, every five seconds someone in the world goes blind and every minute a child loses their sight.1 The International Classification of Diseases (IDC-11) (2018) states that approximately 1.3 billion people live with some form of vision impairment globally.2 These ocular diseases affect the vision and quality of life of patients. Considerable achievements have been made in the supervision of ocular diseases. In the last decade, extensive research has been done at the preclinical and clinical level for the development of therapeutics for various ocular diseases, including glaucoma, uveitis, age-related macular degeneration (AMD), cataracts, and diabetic retinopathy. Recent advances in the treatment of ophthalmic diseases at the clinical level include anti-vascular endothelial growth factor drugs, gene therapy, laser surgery on the eye, and ocular sealants. To deliver these therapeutics, various drug delivery systems, such as eye drops (solutions, suspensions, emulsions), in situ gels, ocular inserts, contact lenses, punctum plugs, intraocular injections, and implants, have been explored for effective ocular drug delivery.3–5 The unique structural features of the eye and the physiological ocular barriers are major challenges for effective delivery at the disease site.6–10 Recent advances in bioadhesive in situ gelling systems and nanotechnology-based drug delivery systems are gaining substantial attention for overcoming the drug delivery challenges. Nanocarrier-based therapeutic delivery systems have been developed to promote sustained and targeted drug delivery to both the anterior and posterior segments of the eye.11,12 However, translation of nanotechnology-based drug delivery systems from bench to bedside are associated with scale up and quality control challenges.13In this review, we focus on the anatomical and physiological barriers to ocular drug delivery. Further, we discuss the limitations of conventional formulations and other routes of drug delivery. Overcoming the limitations of current therapies, advanced nanocarriers have been shown to be effective in treating ocular diseases. Various nanomedicines and their findings are compiled to understand the impact of nanocarriers in the treatment of ophthalmic diseases. Moreover, this review addresses the current challenges in the translation of nanomedicine, including the large-scale production and quality control aspects of nanomedicine.
2. Anatomical and physiological barriers to ocular drug delivery
The eye can be broadly divided into the anterior and posterior segments. The anterior segment includes the cornea, conjunctiva, iris, ciliary body, lens, and aqueous humour, while the posterior segment includes the sclera, choroid, retina, and vitreous body. The anterior and posterior segments of the eye are affected by several vision-threatening diseases.14 To treat eye diseases, topical administration is the preferred non-invasive technique. However, 90% of currently available conventional ophthalmic formulations are eye drops, which are principally administered into the conjunctival cul-de-sac and exhibit poor ocular bioavailability15 because various anatomical and physiological constraints impede drug delivery to both the anterior and posterior regions of the eye.16 These include physiological barriers (nasolacrimal drainage, lacrimation rate, blinking), anatomical barriers (static and dynamic), efflux pumps, and metabolism in ocular tissues.14 Fig. 1 shows the anatomy of the eye and the physiological barriers to ocular drug delivery.17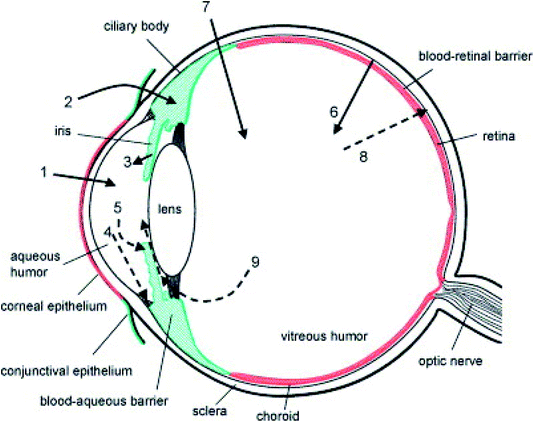 | ||
| Fig. 1 Schematic representation of the anatomy of the eye and physiological barriers to ocular drug delivery (red colour indicates the ocular diffusional barriers whereas green colour indicates routes of elimination). The cornea is the main route for drug penetration on topical administration (1). The conjunctival and scleral route allows some hydrophilic drugs, which further diffuse into the ciliary body (2). Following systemic administration, small compounds diffuse from the iris blood vessels into the anterior segment (3). Further, the drugs in the anterior segment are removed via aqueous humor outflow (4) or diffuse across the iris surface via venous blood flow (5). The retinal pigment epithelium and the retinal capillary endothelium act as major barriers to systemically administered drugs reaching the retina and vitreous humour (6). Instead of these, for effective drug delivery intravitreal injections are used (7). Drugs are removed from the vitreous humour via the blood–retinal barrier (8) or by diffusion into the anterior chamber (9). Reproduced with permission from ref. 17. Copyright 2005, Elsevier. | ||
The tear film also acts as a barrier and prevents drug absorption on topical application. The tear film is composed of an outer lipid layer, a middle aqueous layer, and an inner mucus layer. The tear film can act as a barrier for administered drugs owing to the high tear turnover rate of lacrimal fluid and a gel-like mucus layer. Under physiological conditions, the tear flow is ∼1.2 mL min−1, renewing the tear film for every 5 minutes, but when the eye is irritated by reflex stimulation, lachrymation increases to ∼300 mL min−1. The drug is thus diluted and easily washed away by the tear film. Moreover, mucin present in tear film forms a hydrophilic layer on the glycocalyx of the ocular surface and protects the eye from cell debris and foreign substances, acting as another barrier to administered drugs.18
The anterior segment's static barriers (corneal epithelium, stroma, and blood–aqueous barrier) and dynamic barriers (such as conjunctival blood, lymph flow, and lachrymation) limit drug entry into the anterior chamber of the eye.19 The human cornea comprises five layers, i.e. epithelium, Bowman's membrane, stroma, Descemet's membrane, and endothelium, which each have varying polarity. The cornea epithelium comprises 5–6 layers of close-packed cells with tight junctions that prevent the entry of microbes and drugs. The posterior and anterior chambers of the eye are filled with clear transparent fluid (aqueous humour). The aqueous humour is produced by the epithelium of the ciliary body and provides nutrition to the cornea. The aqueous humour flows from the posterior chamber across the pupil into the anterior chamber.20
However, drug delivery to the posterior segment is limited by the static barriers (sclera, choroid, Bruch's membrane, and blood–retinal barrier) and the dynamic barriers (choroidal blood and lymph flow).19 The sclera is the outermost layer of the eye with irregularly arranged collagen fibers, which prevent the entry of foreign substances to the posterior ocular tissues. Therefore, drugs with high lipophilicity and a high molecular radius can not permeate through the aqueous scleral pores. Additionally, the thickness of the sclera varies from 1 mm at the posterior pole to 25 to 250 nm in the equatorial region in the posterior region, which exhibits low drug permeability. The choroid eliminates administered drugs before they reach Bruch's membrane. The accumulation of cell debris at Bruch's membrane prevents the exchange of nutrients and drugs. The posterior segment consists of tight junctions of the blood–retinal barrier (BRB) with the inner retinal vascular endothelium and outer retinal pigment epithelium. These restrict the penetration of administered drugs into the intraocular chambers.14,16
The conjunctiva is composed of multilayered epithelium and stroma; there are fewer intercellular spaces in the conjunctiva are than in the corneal epithelium. Thus, the cornea and conjunctiva act as the rate-limiting step for hydrophilic drugs. The conjunctival stroma consists of blood capillaries and lymphatics, which leads to drug loss into the systemic circulation. Moreover, efflux proteins prevent the entry of administered antiviral and anti-glaucoma drugs. In contrast, metabolic enzymes prevent the entry of xenobiotics.18 The extent to which the above-mentioned barriers influence drug bioavailability is dependent on the route of administration.
3. Benefits and limitations of ocular drug administration routes
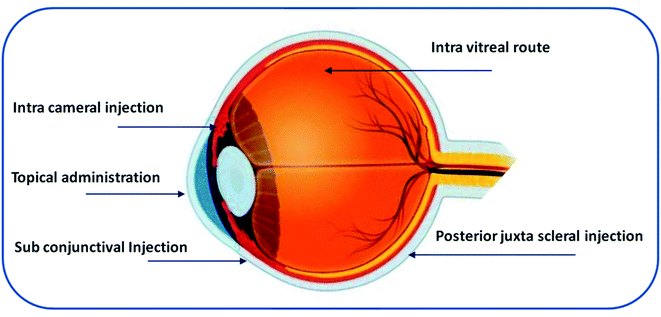
There are multiple routes of drug delivery to the eye: systemic administration (oral, parenteral), topical administration, and ocular injections (subconjunctival, periocular, and intravitreal).21 Fig. 2 shows the routes of administration for ocular drug delivery.213.1. Systemic administration
Parenteral and oral dosing are considered under systemic administration methods for ocular drug delivery. The eye has a low blood supply compared with the whole body, and the tight junctions of the retinal pigment epithelial cells allow only 1–2% of an administered drug to reach the retina and vitreous region, necessitating frequent drug administration to get the desired therapeutic effect. Frequent administration of high doses may lead to systemic side effects and poor adherence to therapy.22,23 In some cases, the infants and children are mostly affected by systemic side effects because of the inefficiency of multi-resistant protein (P-glycoprotein) in the BRB and the immature blood–brain barrier.24,25 Hence, it is a challenge to deliver drugs to the posterior segment by systemic administration.3.2. Topical administration
Topical application is the preferred way to treat ophthalmic diseases. Conventional formulations such as eye drops, suspensions, and ointments are administered via the topical route owing to good patient compliance. Mostly, the administered drug is absorbed by the corneal and conjunctival route. Owing to tear dynamics, precorneal loss factors, and lachrymation, only 5% of the drug is absorbed through the ocular surface.26 Additionally, the presence of anatomical barriers prevents drug absorption and causes low bioavailability.15 Therefore, eye drops have to be administered frequently to maintain the drug concentration on the ocular surface. Ointments can increase the residence time on the ocular surface but, due to blurred vision, patient compliance is compromised.26 In inflammation, excessive secretion of lacrimal fluid leads to dilution and rapid elimination of the administered drug from the site.26–28 Moreover, drug absorption by several other routes is possible, such as nasolacrimal drainage, in which 80% of the drug is absorbed via nasal mucosa.24 Although eye drops are effective in treating anterior eye diseases to some extent, they are less efficient for treating posterior eye diseases, even when following a frequent dosage regimen.293.3. Ocular injections
Intravitreal injection is the most common route for the administration of 20–100 μL of solution or suspension using a 27 or 30 gauge needle into the vitreous cavity.30 The vitreous region consists of viscous liquid. Drug distribution to this region depends on the molecular weight of the drug and the pathophysiological conditions of the vitreous region.31 Smaller drug molecules (molecular weight < 500 Da) exhibit short retention whereas large linear molecules (>40 kDa), and globular molecules (>70 kDa) exhibit greater retention in the vitreous region. The vitreous region consists of hyaluronan, a negatively charged glycosaminoglycan that can interact with cationic charged molecules.32 Intravitreal injection is an invasive surgical procedure that involves penetration of a needle through all the layers of the eyeball and can lead to severe complications, such as cataracts, retinal detachment, infection, and vitreous haemorrhage.33 The subconjunctival, intracameral, and posterior juxta scleral routes are less invasive than intravitreal injections. However, the major drawback of these routes is the shorter retention time.33,34 Table 1 provides a summary of multiple routes of administration, benefits, and challenges in ocular drug delivery.23,35,36| Route | Benefits | Challenges | References |
|---|---|---|---|
| Topical eye drop | High patient compliance, self-administrable and non-invasive | Higher tear dilution and turnover rate, cornea acts as a barrier, efflux pumps, bioavailability (BA) < 5% | 23 |
| Oral/systemic | Patient compliance | Blood–aqueous barrier (BAB), BRB, high dosing causes toxicity, BA < 2% | 23 |
| Intravitreal | Direct delivery to posterior region (vitreous and retina), sustains drug levels, evades BRB | Retinal detachment, haemorrhage, cataracts, endophthalmitis, intraocular damage, patient compliance | 23 |
| Intracameral | Provides higher drug levels in the anterior chamber, eliminates the use of topical drops, reduces corneal and systemic side effects seen with topical steroid therapy | TASS (toxic anterior segment syndrome), TECCDS (toxic endothelial cell destruction syndrome) | 35 |
| Subconjunctival | Delivery to the anterior and posterior segment, a site for depot formulations | Conjunctival and choroidal circulation, trans scleral diffusion of the drug | 36 |
4. Importance of nanomedicine in ocular drug delivery systems
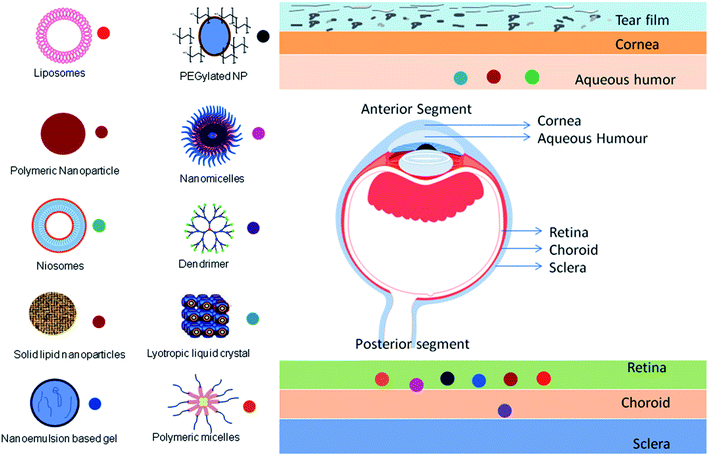
Drug delivery to the ocular surface is challenging for formulation scientists owing to its anatomical barriers and the limitations of conventional ocular therapy have been already discussed above. However, the chronic nature of many ocular diseases requires frequent administration of drugs.37 Nanocarriers are designed to overcome the limitations associated with current ocular therapy and ensure targeted and controlled drug delivery.38,39Nanocarriers are distinct particulate systems with a particle size in the nanometer range (10–1000 nm) with a specific surface charge. The varying size range provides numerous applications in the area of biomedicine. Various nanocarriers have been explored for their use in ocular applications. Moreover, the surface charge of nanocarriers contributes to their conjugation and retention at the specific site.40 This surface charge is measured as zeta potential. The zeta potential is defined as the potential difference between the surface charge of the nanocarrier and those of opposite charge derived from the medium that is arranged around the particle. This zeta potential is responsible for the stability of the nanodispersion.41 If two particles have a high zeta potential of the same charge, they repel each other owing to the repulsive forces, preventing the aggregation of particles. In the case of ophthalmic delivery, the cornea and conjunctiva have a negative charge on the surface; thus, cationic nanoparticles can be attracted owing to electrostatic interactions. This leads to the retention of cationic nanoparticles on negatively charged ocular tissues and achieves topical drug delivery to the anterior eye region. On the contrary, intravitreal administration of cationic nanoparticles leads to clustering of nanoparticles in the vitreous region without diffusion, whereas anionic nanoparticles are capable of diffusing into the retina.41 With their nanosize and surface properties, nanocarriers have the potential to overcome the ocular barriers and can deliver therapeutics at the targeted site.41 Different nanocarrier systems and their targeting ability are presented in Fig. 3.
Generally, nanomedicines are categorized as polymer–drug conjugates and nanoparticulate systems. Owing to the extensive development of drug delivery systems, the difference between these is not clear and there is a large amount of overlap. However, the advances with respect to nanocarriers are gaining importance because of the feasibility of scale-up. Drug delivery systems such as microemulsions, nanosuspensions, nanomicelles, solid lipid nanoparticles, nanostructured lipid carriers, polymeric nanoparticles, liposomes, niosomes, discomes, cubosomes, nanowafers, dendrimers, polymer–drug conjugates, and nanocarrier-loaded gel systems have been investigated as carriers for ocular drug delivery.42–44 A comparative description of the various nanocarriers with their pros and cons is provided in Table 2.21,45–59 Some examples of recent findings with the above-mentioned nanocarriers for the treatment of ophthalmic diseases are compiled in Table 3.60–79
| Drug delivery system | Benefits | Disadvantages | References |
|---|---|---|---|
| Microemulsions | • Clear, thermodynamically stable formulations with a droplet size of 100 nm | • Stabilization of microdroplets requires large concentration of surfactant and co-surfactant so chances of ocular irritation | 45 |
| • Improves solubility and prolongs release of drug, therefore reducing dosing frequency | |||
| • The presence of a surfactant and co-surfactant enhances the corneal membrane permeability | |||
| Nanosuspensions | • Colloidal dispersion system of hydrophobic drugs in dispersion medium which is stabilized by surfactant and polymer with size range of 10 nm to 1000 nm | • Physical stability, sedimentation | 46 |
| • Increases solubility, thus enhancing the bioavailability of ocular drugs | |||
| • Enhances the residence time in the cul-de-sac and prolongs drug release owing to its ability to enhance the inherent solubility of poorly water-soluble drugs in lacrimal fluid | |||
| Surfactant nanomicelles | • Normal micelles can form clear aqueous formulations of hydrophobic drugs, reduce drug degradation, and minimize toxicity with a nano size range typically less than 100 nm | • Ionic surfactants cause toxic effects. E.g. Cremophor EL causes hypersensitivity | 47 |
| • Enhances the penetration of topically applied ophthalmic drugs through the cornea, thus improving the bioavailability of the administered drug | |||
| • Targeted drug delivery to ocular tissues, which enhances the drug bioavailability | |||
| Polymeric nanomicelles | • Solubilizes hydrophobic drugs, with size less than 200 nm | • Difficulty in loading | 48 |
| • Improved permeability of ocular drugs across ocular barriers | • Undergoes deformation and disassembly leading to drug leakage and burst release of drug | ||
| • Suitable candidates for active targeting approach | • Lack of scale-up ability owing to the high cost | ||
| • Biocompatible with reduced toxicity and lower side effects | |||
| Polyion complex nanomicelles | • Oppositely charged polyion copolymer and ionic drug can self-assemble in solution and form a polyion complex with size less than 100 nm | • Chance of flocculation owing to hydrophobic attractions between the neutral coacervates | 49 |
| • Effective for the delivery of ionic macromolecules | |||
| • Target-specific i.e., selective drug accumulation at the ocular pathological site | |||
| • Cost-effective manufacturing techniques provide high industrial acceptance | |||
| Solid lipid nanoparticles | • Non-toxic carrier with size of 10 nm to 500 nm | • Drug expulsion upon long-term storage | 50 |
| • Prevents degradation of lipophilic drugs and offers long-term stability | |||
| • Targeted drug delivery with easy surface modification | • Inadequate loading capacity | ||
| • Large-scale production is possible | |||
| Nanostructured lipid carriers | • Prepared using a blend of solid and liquid lipids, biocompatible and stable with size of 50 to 1000 nm | • Cytotoxic effects associated with the nature of the matrix and concentration | 51 and 52 |
| • Prevents drug expulsion upon storage | |||
| • Enhances bioavailability to ocular tissue | |||
| Polymeric nanoparticles | • Nanoparticles with size range typically <400 nm are suitable for ophthalmic use | • Low drug loading and particle aggregation | 53 |
| • Target-specific drug delivery to ocular tissues, avoids non-specific distribution, and improves therapeutic efficacy | • Burst release of drugs owing to high surface area | ||
| • Protects drug from degradation | • Cytotoxicity issue | ||
| • Imparts sustained drug release | • Lack of scale-up techniques | ||
| • Elevates intracellular penetration, thus increasing drug absorption | |||
| Liposomes | • Liposomes size range is of 0.08 to 10.00 μm | • Lack of scalability potential owing to its low stability | 54 and 55 |
| • Encapsulate both hydrophilic and lipophilic drugs | • Production costs are very high | ||
| • Biocompatible and non-toxic | • Leakage of encapsulated drug | ||
| • Improves corneal permeability | |||
| • Decreases dosing frequency | |||
| Niosomes | • Niosomes are 10 to 1000 nm in size | • Inefficient drug loading | 21 |
| • Less toxic, biodegradable, biocompatible, and mucoadhesive because they are composed of nonionic surfactants | • Leaching of encapsulated drug | ||
| • Controlled drug release and targeted delivery to ocular tissues, hence enhanced bioavailability of drug | • Aggregation and fusion of vesicles | ||
| • High-cost and specialized equipment is required | |||
| Discomes | • Discomes are giant niosomes (size nearly 20 μm) containing poly-24 ethylene cholesteryl ether, which prevents systemic drainage | • Ineffective drug loading | 21 |
| • Disc shape favours discomes fitting into the cul-de-sac, thus improving the drug residence time | |||
| Cubosomes | • Self-assembled liquid crystalline nanoparticles with size less than 500 nm | • Exhibits low entrapment efficiency for hydrophilic drugs compared to hydrophobic drug | 56 |
| • Incorporation of hydrophilic, lipophilic, and amphiphilic therapeutics is feasible with high loading capacity | |||
| • Increases ocular residence time | |||
| • Improves bioavailability of ocular drugs | |||
| Nanowafers | • Nanowafers are nanosized drug-loaded circular discs administered on to the eye surface | • Inadequate drug loading | 57 |
| • Effective for the treatment of corneal neovascularization | |||
| • Improves drug stability and diminishes the toxicity of encapsulated drug | |||
| • Prolongs drug duration on the ocular surface, therefore enhancing therapeutic efficacy and patient compliance | |||
| Dendrimers | • Highly branched star-shaped polymeric macromolecule with 5–20 nm size range | • Synthesis procedure involves multiple steps hence difficult scalability | 58 |
| • Feasible for delivering lipophilic and hydrophilic drugs | • Causes chemical modifications to drug molecule, leading to cytotoxicity issue | ||
| • Enriches drug solubility and exhibits high drug loading and sustained drug release | • Low encapsulation efficiency and storage ability | ||
| • Polyamidoamine (PAMAM) has been commercialized for the preparation of dendrimers | |||
| Polymer–drug conjugates | • Hydrolysable chemical bonds connect the functional groups of the polymeric backbone with the drug | • Early release of the drug causes unwanted toxicity | 59 |
| • Size range from approximately 10 nm to 100 nm | • Considered as new chemical entities | ||
| • Enhances the solubility and stability of drugs in biological fluids |
| Drug | Type of formulation | Polymer/lipid | Technique used | Observation | Reference |
|---|---|---|---|---|---|
| Timolol maleate | Bioadhesive liposomes | Chitosan | Ammonium sulfate gradient coupled with a pH-gradient method | Compared to eye drops, retained for longer on the corneal surface. Significant mucoadhesion and corneal permeation were observed | 60 |
| Ampicillin and ofloxacin | Supercritical-assisted liposome | Soybean L-α phosphatidylcholine | SuperLip (supercritical-assisted liposome formation) | Controlled drug release for 3 to 4 months and the formed liposomes were stable for 3 months | 61 |
| Azithromycin | Liposomes | Cholesterol hemisuccinate | Solvent evaporation method | Azithromycin liposomes showed enhanced corneal permeation compared to the azithromycin solution | 62 |
| pDNA | Liposomes | Polyethylenimine (PEI)-associated liposomes | Detergent removal method | Nucleic acid-loaded liposomes as eye drops to treat posterior eye disorders. They observed high encapsulation efficiency and good cellular uptake by ARPE-19 cells, and they also expect that alteration of the ligand on the RPE cells to the liposomes may improve gene delivery | 63 |
| Avastin | Liposomes | Annexin A5 | Lipid film hydration technique | Upon topical administration of avastin into rats and rabbits, significant concentrations (127 ng g−1 and 18 ng g−1, respectively) were observed in the retina of both the animals. Hence, they expect that lipidic drugs can cross the ophthalmic barriers by endocytosis when associated with annexin A5 | 64 |
| Natamycin | SLNs | Precirol ATO 5® (SLNs) | Emulsification and ultrasonication technique | Enhanced corneal penetration and antifungal activity. No cytotoxicity effect on the corneal tissues | 65 |
| Triamcinolone acetonide (TA) | SLNs and in situ gel loaded SLNs | Glyceryl monostearate and Compritol® 888ATO | Hot homogenization and ultrasonication method | TA-SLNs and TA-SLNs-in situ gel demonstrated a 10- and 9.3-fold increase in transcorneal permeability in comparison to TA suspension | 66 |
| Voriconazole | SLNs | Compritol® 888ATO, stearic acid | Probe ultrasonication method | The dissolution rate and bioavailability were enhanced | 67 |
| Fluorometholone | In situ gel nanoparticles | PLGA RG 503H, Poloxamer 188 (P188) and P407 | Solvent displacement method | In situ gel nanoparticles enhanced the bioavailability by improving the precorneal residence time and reached deeper layers of the aqueous humour in comparison to the commercial formulation | 68 |
| Fluconazole | (a) Niosomal gel | (a) Span® 60 and cholesterol, Carbopol® 934 | (a) Thin-film hydration method | The niosomal gel and microemulsion showed controlled release for 12 h and enhanced bioavailability in comparison to solution form. A 2-fold increase in bioavailability was shown by niosomal gel compared to the microemulsion | 69 |
| (b) Microemulsion | (b) Isopropyl myristate (IPM) | (b) Aqueous phase titration method | |||
| Propranolol hydrochloride | NLCs | Compritol ATO 888, oleic acid (NLCs) | Cold homogenization | They demonstrated that the surfactant/lipid ratio played the main role in drug loading and corneal permeation. Initial burst release and sustained release for 48 h were observed | 70 |
| Nile red (model lipophilic drug) | NLCs | Lecithin, cetyl palmitate or glyceryl behenate, gelucire 44/14 (NLCs) | Hot, high-pressure homogenization | Nile red-loaded NLCs of 40 nm size showed good corneal penetration. PEG-coated NLCs and positively charged NLCs showed improved mucoadhesion and higher porcine corneal epithelial cell uptake | 71 |
| Baicalin (model drug) | Hybrid genipin-crosslinked dual-sensitive hydrogel NLCs | Compritol 888 ATO, Miglyol 812 N, genpin | Melt emulsification with ultrasonication technique | Hybrid baicalin NLCs showed controlled drug release and demonstrated a 4.46-fold increase in the apparent permeability coefficient in comparison to the eye drops | 72 |
| Moxifloxacin | NLC loaded in situ gel | Glyceryl monostearate (GMS) and Capmul MCM mixture | Hot homogenization ultrasonication method | Ex vivo permeation studies demonstrated that moxifloxacin-loaded NLCs in situ gel showed a 2-fold increase in permeation and retention compared to free drug-loaded in situ gel. No corneal tissue damage was observed | 73 |
| Spironolactone | Nanomicelles | Methoxy-poly(ethylene glycol)-dihexyl-substituted-poly(lactic acid) (mPEGdihexPLA) di block co polymer | Sonication | 0.1% spironolactone nanomicellar solution enhanced the re-epithelialization | 74 |
| Cyclosporine A | Polymeric nanomicelles | Poly(ethylene glycol)–poly(lactide) polymer (mPEG–PLA) | Solvent casting | The lyophilized cyclosporin A-loaded nanomicelles enhanced the solubility and bioavailability of cyclosporine A. In vivo studies revealed a 4.5-fold increase in retention at the eye compared with 0.05% cyclosporine A emulsion | 75 |
| Ketorolac tromethamine | Nanosuspension | Eudragit RL-100 | Combined nanoprecipitation and probe sonication method | In vitro study showed biphasic drug release profile (immediate release followed by prolonged release) | 76 |
| Dexamethasone | Dendrimers | Hydroxyl-functionalized ethylenediamine core generation four PAMAM dendrimers | Synthesized dendrimer–dexamethasone conjugates | Compared to free dexamethasone, the dendrimer–dexamethasone conjugate attenuated corneal inflammation by reducing macrophage infiltration and proinflammatory cytokine expression | 77 |
| Cysteamine | Nanowafer | Poly(vinyl alcohol), poly(dimethylsiloxane) (PDMS) imprints | Hydrogel template strategy | In vivo studies in cystinosin knockout mice showed that the cysteamine nanowafers had enhanced efficacy with half the drug dose strength compared to eye drops. They enhanced cysteamine stability at room temperature | 78 |
| Brimonidine tartrate | Cubosomes | Glyceryl monooleate and poloxamer 407 | Melt dispersion emulsification technique | In vivo pharmacodynamic study revealed a 9.1-fold increase in residence time relative to marketed Alphagan® P | 79 |
4.1. Liposomes
Liposomes are lipid-based spherical vesicles that are composed of phospholipids and cholesterol with size ranging from 0.08 to 10.00 μm. They are biocompatible, biodegradable, flexible, and can encapsulate both hydrophilic and hydrophobic drugs simultaneously in a single system. Various attempts have been made with liposomes to improve their bioavailability, corneal penetration, stability, and targeted action.27,54 Liposomes have been investigated for prolonged drug release and targeted delivery for various drugs. Fahmy et al. prepared liposomes of latanoprost and thymoquinone by thin-film hydration method and administered them as a subconjunctival injection to treat glaucoma. The drug-loaded liposomes showed a particle size of less than 0.2 μm with 88% encapsulation efficiency. In vitro and in vivo drug release studies revealed that drug-loaded liposomes showed a significant reduction in intraocular pressure up to 84 h compared to test formulations.80Surface modification of liposomes can provide targeted drug delivery with improved bioavailability. Lin et al. prepared doxorubicin (DOX)-loaded liposomes by solvent evaporation method to treat posterior eye diseases. The surface of the prepared liposomes was modified with hyaluronic acid. The surface-modified liposomes showed a mean particle size varying from 134 to 517 nm with an entrapment efficiency of 86.5% by using a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of phospholipid and cholestenone. An in vivo study on rabbit eyes demonstrated a 1.4-fold higher drug concentration in the rabbit aqueous humour with DOX-loaded liposomes compared to free DOX. Moreover, the confocal microscope examination results suggested that surface modification with hyaluronic acid can enhance the ocular bioavailability of DOX compared to free DOX.81
1 ratio of phospholipid and cholestenone. An in vivo study on rabbit eyes demonstrated a 1.4-fold higher drug concentration in the rabbit aqueous humour with DOX-loaded liposomes compared to free DOX. Moreover, the confocal microscope examination results suggested that surface modification with hyaluronic acid can enhance the ocular bioavailability of DOX compared to free DOX.81
Jin and co-workers prepared nanoliposomes of brinzolamide (Brz) to lower the intraocular pressure (IOP) without corneal damage. The surface modification of the nanoliposomes was done using D-alpha-tocopheryl poly(ethylene glycol 1000) succinate (TPGS). The modified nanoliposomes exhibited a mean particle size of 96.87 ± 4.43 nm, with a zeta potential of −1.17 ± 1.91 mV and 95.41 ± 3.03% entrapment efficiency. The results revealed that compared to the Brz-loaded simple liposomes and commercial Brz ophthalmic solution, the TPGS surface-modified liposomal formulation exhibited sustained drug release, improved permeation, prolonged precorneal retention, and reduced IOP without cytotoxicity to ocular tissues.82 Zhan et al. developed tetrodotoxin and dexmedetomidine-loaded liposomes surface-modified using succinyl-concanavalin A. The results illustrated that, compared to the free drug solutions, the liposomal formulation gave sustained drug release behavior and prolonged liposomal persistence on the corneal surface. Therefore, such modified liposomes can provide a longer period of extreme analgesia under topical anesthesia.83
4.2. Solid lipid nanoparticles and nanostructured lipid carriers
Solid lipid nanoparticles (SLNs) are colloidal carrier systems made up of lipids dispersed in an aqueous surfactant system with a particle size of 10 nm to 500 nm. They are most suitable for the delivery of hydrophobic drugs.84 SLNs have been shown to have improved retinal permeation and sustained drug release for a longer duration at the ocular site. They can reduce the toxicity related to the repetitive administration of a high dose. Ahmed et al. prepared etoposide-loaded SLNs by melt emulsification and ultrasonication technique for intravitreal administration to treat retinal diseases. The particle size of the prepared SLNs was found to be 239.43 ± 2.35 nm with 80.96 ± 2.21% entrapment efficiency. The prepared formulation exhibited biphasic drug release, i.e. initial burst release and sustained release for 7 days in the vitreous region. Histopathological studies revealed reduced toxicity in the retinal region.84 Li et al. prepared anionic (TET-NP) and cationic SLNs of tetrandrine (TET-CNP) by emulsion evaporation–solidification method to treat glaucoma and retinopathy. The prepared TET-NP and TET-CNP showed particle sizes of 18.77 ± 1.23 nm and 15.29 ± 1.34 nm with zeta potentials of −8.71 ± 1.23 mV and 5.11 ± 1.03 mV, respectively. Flow cytometry and confocal microscopy analysis demonstrated that the negatively charged nanoparticles were efficiently internalized by cells and showed significantly higher cellular uptake than cationic nanoparticles on human lens epithelial cells (SRA 01/04). This study showed that anionic SLNs diffused faster into the vitreous region and provided greater penetration in the inner retinal layers compared to cationic SLNs.85SLN-based nanocarriers exhibit limitations such as poor loading capacity, drug expulsion by lipid crystallization, and conversion of alpha to beta confirmation upon storage. Nanostructured lipid carriers (NLCs) have been investigated as next-generation lipid nanocarriers that can provide improved drug loading and stability. NLCs are developed with a combination of solid and liquid lipids in a nanocarrier system. NLCs have an asymmetric structure, which prevents drug expulsion and results in comparatively slow drug release. NLCs are an ideal drug delivery system for the posterior region of the eye owing to their lipid character, efficient drug-loading capacity and good permanence.86–88 Lakhani et al. prepared an NLC formulation of curcumin using an organic solvent-free hot-melt emulsification technique. The entrapment efficiency of curcumin was found to be 96 ± 1.6%. The curcumin NLCs were further tested for transcorneal permeation and toxicity across excised rabbit corneas. The results indicated that the prepared curcumin NLCs were safe and showed a 2.5-fold increase in corneal permeability compared to a propylene glycol-based curcumin suspension. Thus, NLCs have the potential to treat various anterior segment diseases.89
Puglia et al. prepared stable NLCs of palmitoylethanolamide (PEA) to treat diabetic retinopathy using two different methods. In one method, the NLCs were prepared using a combination of high-shear homogenization (HSH) and ultrasonication (HSH/US), while in the other method only HSH was used. The results showed that the particles prepared using the combination technique had good physical stability with enhanced entrapment efficiency (from 20.6% to 82.3%), and drug loading (from 0.08% to 0.32%) compared to HSH alone. In vivo pharmacokinetic studies with PEA-NLC on male Sprague-Dawley rats revealed that the PEA-loaded NLCs reached the retinal tissue on topical administration and notably inhibited the retinal tumor necrosis factor-α levels in streptozotocin-induced diabetic rats compared to PEA as a suspension.90 Rathod et al. prepared non-steroidal anti-inflammatory drug-loaded NLCs using dynasan 114 as the solid lipid and miglyol 840 as the liquid lipid for the treatment of postoperative ocular inflammation. In in vitro drug release studies, the NLCs exhibited sustained drug release up to 12 h.91
The NLC surface can be modified with a suitable polymer to make it bioadhesive and enhance its retention at the disease site. Selvaraj et al. developed chitosan-coated itraconazole-loaded NLCs using a high-pressure homogenization method. The prepared NLCs showed an average particle size of 86.75 nm, zeta potential of +17.2 mV, and entrapment efficiency of 98% ± 1.02%. The results revealed that the chitosan coating resulted in excellent mucoadhesion and promoted longer retention on the ocular surface by interacting with the negatively charged mucous membrane of the eye. The prepared NLCs concomitantly inhibited the vascular endothelial growth factors (VEGF-165) and showed an anti-neovascularization effect on vascular endothelial growth factor-induced diabetic retinopathy rats.92 The incorporation of hydrophilic polyethylene glycol (PEG) and PEG derivatives on NLCs can increase their dispersion stability and uptake by cells. Patil et al. developed PEGylated NLCs of natamycin for ocular application. Their in vivo biodistribution study revealed that PEGylated NLCs exhibited two-fold higher concentrations in the cornea and iris ciliary body compared to the marketed suspension formulation (Natacyn®). Thus, NLCs could be a potential alternative to conventional suspension for the treatment of fungal keratitis.93
Salamouni et al. carried out a comparative study with ocular delivery of brimonidine via SLNs, NLCs, and conventional eye drops. In their ex vivo permeation study, the NLCs showed a 1.27-fold increase in permeability coefficient in comparison to SLNs because the liquid lipid possesses a stronger affinity towards the cell membrane compared to the solid lipid. Moreover, NLCs sustained the drug release and lowered the intraocular pressure in comparison to SLNs and eye drops.94
4.3. Polymeric nanoparticles
Polymeric nanoparticles are made up of natural and synthetic polymers and are categorized as nanospheres and nanocapsules with sizes ranging from 10 to 1000 nm. They provide the advantages of increased bioavailability, adherence and residence time.56,68,95–97 Li et al. prepared betaxolol hydrochloride-loaded montmorillonite-chitosan nanoparticles to treat glaucoma. In this method, betaxolol hydrochloride was intercalated into the interlayer gallery of montmorillonite and then further inlayed by chitosan. The mean particle diameter was found to be 460 ± 0.6 nm. The particles exhibited a positive surface charge of 29 ± 0.18 mV. Hence, it was expected that they would form a tight contact with the negatively charged corneal mucin. This resulted in a prolonged residence time and improved bioavailability on the corneal surface.98Alvarez-Trabado and his team prepared sorbitan ester nanoparticles (SENS) and hyaluronic acid-coated SENS of cyclosporine for topical ocular drug delivery. The prepared nanoparticles were studied for tissue distribution and cyclosporine penetration tests through ex vivo porcine cornea. The results demonstrated that the hyaluronic acid-coated SENS broadly distributed throughout the epithelial layers and reached the corneal stroma whereas the SENS nanoparticles accumulated on top of the epithelium owing to the negative charge on the surface. In the cyclosporine penetration study, SENS and hyaluronic acid-coated SENS exhibited 1.3- and 2.1-fold increased ocular tissue penetration compared to the commercial formulation (Sandimmune®). In addition, ex vivo stimulated lymphocytes studies revealed that both the nanoparticles demonstrated the same reduction in interleukin (IL-2) levels as Sandimmune®. This confirmed that the immunosuppressive efficacy of both the nanoparticles was the same as that of Sandimmune®.99
Solanki and his colleagues conducted studies on humanin derivative nanoparticles for treating AMD. Their results demonstrated the potential impact of nanoparticles on the suppression of inflammatory IL-6 cytokine receptors and reduced retinal apoptosis.95 Liu et al. prepared hyaluronic acid-based surface-modified lipid–polymer hybrid nanoparticles. Their results showed accelerated cellular uptake of surface-modified nanoparticles by receptor-mediated endocytosis, and higher fluorescence intensity was observed in the cornea and conjunctiva. The designed nanoparticles exhibited extended precorneal retention, ocular bioavailability and better corneal permeability with a targeted approach.100
4.4. Polymeric micelles
Nanomicelles are amphoteric self-assembling structures in the range of 10 to 100 nm.101 The nanomicelles can solubilize hydrophobic drugs in the hydrophobic core and form clear aqueous formulations.102 These polymeric micelles encapsulate hydrophobic drugs, which can protect the drug from degradation and enhance drug stability. Alvarez-Rivera et al. prepared polymeric nanomicelles with alpha-lipoic acid in Soluplus® to treat diabetes-associated corneal diseases. The designed nanomicelles exhibited a 10-fold increase in the solubility of alpha-lipoic acid and enhanced corneal residence time compared to commercial eye drops.103 In one study, curcumin-loaded nanomicelles were prepared using polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol to treat ocular inflammation. The designed nanomicelles showed improved curcumin solubility, chemical stability, in vitro cellular uptake, and in vivo corneal permeation compared to free curcumin solution.102 Correspondingly, nanomicelles prepared using polyoxyl 40 stearates, polysorbate 80, D-alpha-tocopheryl polyethylene glycol succinate, octoxynol-40 and hydrogenated castor oil-40 showed significantly improved drug retention on the retina and choroid as well as negligible cytotoxicity to rabbit corneal epithelial cells (rPCEC) and human retinal pigment epithelial cells (D407).484.5. Nanocarrier-loaded gels
Nanocarrier-loaded in situ gels have gained attention for their targeted delivery with stimuli-responsive behavior. The ophthalmic in situ gel consists of environmentally responsive polymers that change structurally in response to small changes in specific circumstances like temperature, pH, and ionic strength in the environment. Additionally, the loading of nanoparticles into the gel improves the burst release problems observed with nanoparticles and gives prolonged drug release.26,104 Studies have revealed that temperature-sensitive polymers like Pluronic (PF-127 & PF-68), poloxamer 407 and poloxamer 188, Carbopol 934P, sodium alginate, and hydroxypropyl methylcellulose (HPMC) K4M provide prolonged drug release, increased ocular bioavailability and sustained drug release for about 8 h.26Morsi et al. investigated nanoemulsion-based ion-sensitive in situ gels for the delivery of acetazolamide. The stability of nanoemulsions depends on the surfactant and co-surfactant concentration. The nanoemulsion formulation was prepared by mixing oil (peanut oil), surfactants (Tween 80 and Cremophor EL), and Transcutol P as a co-surfactant and then integrating into the gellan gum alone and in combination with xanthan gum, HPMC or Carbopol. The results showed that the combination of gellan/xanthan and gellan/HPMC gave good stability and enhanced therapeutic efficacy of acetazolamide compared to commercial eye drops and oral tablets.105 Patel and team members prepared cationic nanoemulsion-based in situ ophthalmic gel of loteprednol etabonate (LE), which demonstrated a 2.54-fold increase in bioavailability compared to the marketed formulation.106 Phua et al. reported a 12-fold increased residence time with a liposome-loaded hydrogel compared to free liposomes.107 Thus, the delivery of therapeutics using nanocarriers can provide improved permeation and prolonged retention at ocular sites owing to its nanoscale.
Further, surface modification of nanocarriers can improve their targeting ability and mucoadhesion at the ocular surface, which can improve both therapeutic efficacy and patient compliance.
5. Current challenges in the translation of ophthalmic nanomedicine
Nanomedicine has to cross many hurdles to reach clinical trials. The current obstacles in the translation of nanomedicine concerning nanopharmaceutical design include large-scale production to Good Manufacturing Practice standards and quality control assays for characterization.13 Fig. 4 shows the key components in the stages of product development, starting from preformulation (lab scale) to commercialization (production scale). The marketed product should be within acceptable limits for safety, efficacy, stability, and patient acceptance. The method used during formulation should be within the standards and it should be reproducible.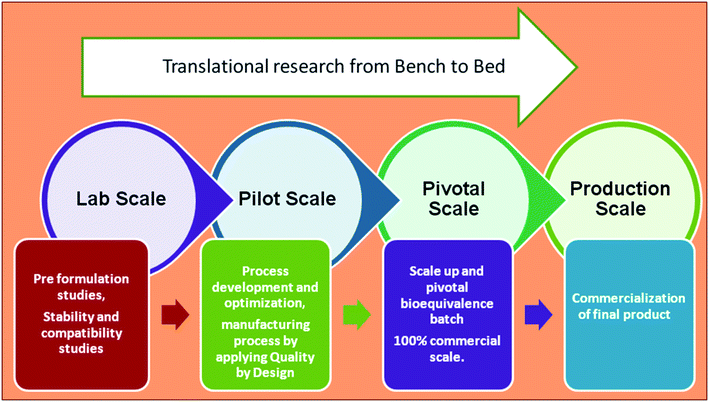 | ||
| Fig. 4 Key components in the stages of product development, starting from preformulation (lab scale) to commercialization (production scale). | ||
5.1. Scale-up of nanomedicine
Translation from laboratory scale to commercial scale is challenging in the case of nanomedicine.108 The success of these formulations depends on their scalability and reproducibility. A scalable formulation should always be robust at the lab, pilot, and industrial levels. Advances in scale-up technology and Quality by Design (QbD) concepts have led to significant progress and smoothed the process of commercialization of nanomedicine.109Recent advances in formulation development have led to the entry of many nanoformulations, such as liposomes, solid lipid nanoparticles, nanostructured lipid particles, nanoparticles, micelles, and nano in situ gels, for preclinical and clinical testing. However, efforts are still being made to bring these advanced nanomedicine formulations to the market.
Nanocrystals have been explored well for commercialization. Currently, 15 nanocrystal based products are available in the market for different routes of administration, among which Ilevro® (nepafenac ophthalmic suspension, 0.3%) is available for ophthalmic administration.110 During the production of nanocrystals, the size, shape, and other process parameters can be controlled.111 Two main methodologies are used for the preparation of nanocrystals, bottom-up (precipitation process) and top-down (including wet-bead milling, nanospray dryer, and high-pressure homogenization). In a study, nanocrystals of steroid drugs fluorometholone and dexamethasone were prepared by using nano Spray Dryer B-90 technology (Büchi®). The authors observed that the particle size changed depending on the mesh aperture size. The results revealed that with mesh aperture sizes of 4.0, 5.5, and 7.0 μm, the average particle sizes for fluorometholone nanocrystals were found to be 620 ± 268, 795 ± 285, and 856 ± 344 nm and for dexamethasone nanocrystals were found to be 833 ± 402, 1118 ± 573, and 1344 ± 857 nm, respectively.112
Nanoparticles can be crystalline or amorphous, depending on the manufacturing technique and material employed. The production of nanoparticles is a challenging task in terms of reproducibility of size and polydispersity index. Critical process parameters involved in preparation techniques greatly influence the particle characteristics. Galindo-Rodriguez et al. prepared nanoparticles using three different methods: emulsion-based, salting out, and nanoprecipitation methods. The particle size ranges changed with the different methods and variations in the physicochemical properties of the aqueous and organic phases. Particle size range of 123–710 nm, 108–715 nm and 147–245 nm were observed with salting out, emulsification-diffusion, and nanoprecipitation methods, respectively.113
Colombo et al. conducted a study to investigate the essential scale-up parameters for nanocapsule production. They employed the emulsification-diffusion method for the preparation of nanocapsules and assessed at pilot scale by increasing the laboratory batch volume by 33-fold, i.e. from 60 mL to 2 L. They observed that increasing the impeller speed and duration of agitation led to a slight decrease in emulsion size.111
Nanoparticle preparation from micro/nanoemulsions using low-energy approaches, such as phase inversion temperature, phase inversion composition, and emulsion inversion point methods, has led to scale-up issues including variation in physicochemical properties and also requires a large amount of surfactant. During the scale-up of solid lipid nanoparticles by a hot-melt extrusion process, the process parameters such as feed rate, extruder diameter, and heat transfer play an important role as the change in the feed rate may change the residence time of the material in the barrel.114 High-energy approaches, such as ultrasonication, high-pressure homogenization, and microfluidization, have been explored to control the characteristics and reproducibility of nanoparticles. The major problem observed with sonication and homogenization is the recoalescence of new droplets, leading to the formation of thermodynamically unstable formulations.115,116 In one study, a pilot-scale batch of nanoparticles was prepared by ionotropic gelation method. It was observed that increasing the homogenization speed from 500 to 900 rpm contributed to an increase in the heat and kinetic energy of the molecule, which in turn led to agglomeration of the emulsion.117 This showed the huge difference between lab-scale and industrial-scale equipment. Formulation scientists need to optimize all the process parameters extensively in the scale-up of nanocarrier preparation using homogenizers.
For achieving adequate particle size, scalability, and reproducibility, microfluidization has become a potential approach for commercial applications. Microfluidic technology is an optimum technique to support, speed up, and favour clinical translation of nanomedicine by reducing the batch to batch variability. This technique involves the rapid mixing of lipids and the aqueous phase in a micronized chamber (chip) within milliseconds. The accurate mixing of fluids in the microchannel facilitates precise control over the physicochemical properties of the nanoparticles. The nanoparticles are prepared by nanoprecipitation, and self-assembly with continuous flow gave the same quality over time for the produced nanoparticles, avoiding the batch to batch variability. This is the most important feature for industrialization from the lab scale.118 Ali et al. prepared a hydrocortisone nanosuspension using microfluidic nanoprecipitation and wet milling. The particle size range of 295 ± 32 nm (simple mixing) and 300 nm (with 105 minutes mixing) was observed with y-junction microfluidic and wet milling technique, respectively. The results revealed that both the nanosuspensions showed sustained action and enhanced 1.8-fold bioavailability compared to the commercial solution. Microfluidic nanoprecipitation is advantageous over milling owing to the low energy of the process. The microfluidic technique reduces both the production time and the economic costs involved in nanoparticle production.119 Thus, by controlling a few parameters, such as the flow rate and organic phase ratio, uniform-sized nanoparticles can be produced on a large scale. Gdowski et al. synthesized a nanolipomer formulation using a high-flow microfluidic system and observed batch to batch uniformity upon increasing to pilot-scale production.120
Other advanced technologies such as BUONAPART-E (better scale-up and optimization of nanoparticles and nanostructure production using electrical discharges) can be used to synthesize high-purity metallic nanoparticles. This technique utilizes the arc and spark discharge method. It is a stable and low-cost process with a high production rate and energy efficiency. This technique can be further scaled up with multiple parallel electrodes set to increase the nanoparticle production.121
The supercritical solvent technique has also been explored for scale-up of nanocarrier preparation. Jung et al. prepared nanoparticles at three different scales (0.5 L, 4 L, and 50 L) by using a supercritical anti-solvent process. They observed no change in particle size distribution and no loss of residual solvent and percent yield.122 Pham et al. determined the scale-up ability of liposomes and niosomes by using a syringe pump at the lab scale and then membrane contractors at pilot scale. They prepared 30 mL of liposomes and 20 mL of niosomes by using a syringe pump at a laboratory scale to pilot-scale production of 750 mL of liposomes and 1000 mL niosomes using a membrane contactor. They reported that reproducible results were observed concerning size and entrapment efficiency.123
Recent advances in nanocarrier development for ocular delivery include particle replication in non-wetting template (PRINT) technology and hydrogel template method.124,125 In PRINT technology, the particles are produced by manufacturing roll-to-roll with the required particular size, shape, and modulus. It includes three steps, fabrication of micro molds with a precise cavity, molding of suitable material into the cavities, and separation of the formed particles. The continuous production of particles with the desired size, shape, and surface can be achieved by altering the composition of the material matrix and post functionalization.124 This technique can be utilized with a large number of biocompatible polymers and therapeutic agents, including peptide molecules, nucleic acids, proteins, and antibodies.124 The hydrogel template method can be widely used for nano- and micro-size particle development. This template was utilized for the production of a nanowafer containing a nano-reservoir as an ultra-thin lens.125 In a study, a silicon-based wafer template was fabricated using e-beam lithography and polyvinyl alcohol was used to prepare the template with different arrays of wells. The drug solution was loaded into the arrays to form nanowafers. The particle size and drug loading can be controlled by using these drug reservoirs, and these are utilized as a lens by placing on the ocular surface.126
The success of nanomedicine is governed by the reproducibility of the physicochemical characteristics at the industrial scale. Recent advances in technology such as microfluidic, high-pressure homogenization, PRINT, and hydrogel template methods have provided hope for commercialization.127 Further, guideline progression and clinical acceptance of nanocarriers from regulatory agencies will accelerate the development of nanomedicine for ophthalmic therapy.
5.2. Quality control of nanomedicine
The quality and cost-effective production are important considerations in pharmaceutical product development. For quality, the stability and the manufacturing process are the main aspects for nanoformulations. The challenges in nanoparticle preparation include batch to batch reproducibility, dispersion stability and the safety of the nanomaterials. A small change in the process parameters may lead to changes to the particle size and total yield and may influence various formulation parameters, such as particle size distribution, encapsulation efficiency, drug release, and therapeutic efficacy.128 Further, changes in the specifications of the nanoparticles may alter the pharmacokinetic and pharmacological properties of encapsulated drugs.129Quality control testing ensures the safety and efficacy of the pharmaceutical product during the process (in-process quality control testing) and at the end of the process (finished product testing). The common tests performed for all ophthalmic formulations include description, identification, assay, impurities, pH, isotonicity/osmolality, viscosity, clarity, particulate matter, particle size, bacterial endotoxins, sterility test, uniformity of dosage form, and weight loss.130,131
Regulatory agencies have taken steps for the commercial approval of safe and effective nanotechnology-based product development. These agencies have provided guidelines for product specification and characterization required for the product approval process. In the year 2017, the United States Food and Drug Administration (US FDA) published “Drug Products, Including Biological Products, that Contain Nanomaterials” draft guidelines for industry and suggested some attributes to be described and measured for nanoformulations.132 The guidelines comprised characteristics of nanoformulations, including chemical composition, average particle size, particle size distribution (describing d10, d50, d90 or polydispersity; modality), general shape and morphology (aspect ratio), physical stability (e.g., aggregation and agglomeration or separation) and chemical stability. Further, the guidelines include specific studies, such as distribution of the active ingredient associated with the nanomaterial, free in solution, structural attributes (lamellarity, core–shell structure), surface properties (surface area, surface charge, chemical reactivity, ligand hydrophobicity, and roughness), coating properties of nanomaterials, porosity (a function related to the drug-loading capacity), particle concentration, in vitro release, crystal form, impurities, sterility and endotoxin levels. The above-mentioned guidelines also provide factors to be selected and specific characterization methods for nanoformulations.132
The US FDA also provided specific guidelines for “Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation” in the year 2018 for liposomal product development.133 In 2018, the US FDA drafted another guideline, “Selected FDA Publications Related to the Application, Characterization, Effects, and Evaluation of Nanotechnology” for characterization and evaluation of nanoparticulate-based drug delivery systems.134
Recently, in October 2019, Indian regulatory bodies (Central Drug Standard Control Organization, Indian Council of Medical Research, Department of Biotechnology, and Ministry of Health and Family Welfare) issued the “Guidelines for Evaluation of Nanopharmaceuticals” with details of the evaluation parameters required for quality control for nanopharmaceuticals, similar to the US FDA guidelines.135 Additionally, these guidelines suggested that the product should meet specifications for analytical method validation, stability studies of nanoformulations, comparative analysis of innovator product if applicable, preclinical data, clinical data, and sample testing protocols.135
The efforts of regulatory bodies will certainly ensure the safety and efficacy of products with stringent product specification for nanocarriers for ophthalmic drug delivery.
5.3. Safety and toxicity concerns for nanocarriers
Safety and toxicity are the main issues for clinical approval of ophthalmic formulations. At the preclinical level, researchers have investigated the ocular toxicity of designed nanoformulations using the Draize eye test on rabbits and in vitro human corneal epithelial cells for the determination of acute ocular toxicity issues.136 De et al. prepared brimonidine-loaded polycarboxylic (polyacrylic and polyitaconic) acid nanoparticles for the treatment of glaucoma. They studied the biocompatibility of prepared nanoparticles with human corneal epithelial cells. The results revealed that the polyacrylic acid nanoparticles were safe, i.e., biocompatible, compared to the polyitaconic acid nanoparticles.137 Vega et al. prepared flurbiprofen-loaded poly(lactic/glycolic acid) nanoparticles. Topical application of the prepared nanoparticles to rabbit eyes showed no sign of toxicity.138 Prow et al. compared the safety and toxicity of chitosan, amphiphilic polyphosphoester, and magnetic nanoparticles. They reported that intravitreal administration of chitosan causes inflammation of the eye.139Among the different nanomaterials, lipid-based nanocarriers including nanoemulsions and liposomes were found to be more biocompatible and safer for interaction with biological membranes and showed their presence in the market.140
6. Drugs approved and undergoing clinical trials
Substantial investigations have been done on nanocarriers like nanoparticles and nanomicelles for the alleviation of anterior and posterior ocular disorders. They have entered into clinical trials and shown positive results in patients. The current ophthalmic nanostructured marketed products are Restasis® (cyclosporine A nanoemulsion), Cyclokat® (cyclosporine A cationic nanoemulsion), Cequa® (cyclosporine A ophthalmic nano micellar solution), VISUDYNE® (verteporfin liposomal injection), Lacrisek® (vitamin A palmitate and vitamin E liposomal spray), and Artelac Rebalance® (vitamin B12 liposomal eye drops), which are used for the treatment of dry eye syndrome. Liposome-based ocular products have gained more attention owing to their biodegradable, biocompatible, non-toxic, and non-immunogenic nature. Moreover, Ikervis® (cyclosporine A cationic nanoemulsion) has been approved for treating severe keratitis in dry eye disease patients.141 Sun Pharmaceuticals developed Cequa® (cyclosporine A ophthalmic nano micellar solution) 0.09%, which is intended to improve drug delivery and penetration to ocular tissues. Cequa® has been investigated for efficacy and safety in treating keratoconjunctivitis sicca (dry eye). The company conducted phase III trials with Cequa® on 744 patients and the study design involved two 12 week randomized and vehicle-controlled trials. The results exhibited a statistically significant increase in the Schirmer score (≥10 mm) from baseline with Cequa® relative to vehicle in tear production with twice-daily dosing. Moreover, adverse events were reported by greater than 5% of patients, i.e., 22% of patients showed pain at the instillation site and 6% of patients showed conjunctival hyperemias, which are most common for the drugs analyzed in this category. In 2018, Cequa® was the first nanotechnology-based product to be approved by the US FDA for treating dry eye.142Kala Pharmaceuticals developed an innovative treatment with nanosized mucus penetrating particles (MPP) to improve drug delivery to the back of the eye. MPP allow effective penetration through the tear film and mucin layer, which prevents drugs from being trapped by the tear film and clearance by blinking. Kala Pharmaceuticals applied this MPP platform technology for loteprednol etabonate (KPI-121, 1% and KPI-121, 0.25%) for the treatment of several ocular diseases (NCT02163824) (NCT02813265). The phase III clinical trial results for 1% KPI-121 demonstrated a significant decrease in both pain and inflammation with twice a day dosing.143 In addition to this, particle replication in non-wetting template (PRINT) technology offers a distinctive capability to reproduce and fabricate particles of any size.124 The clinical-stage pharmaceutical companies Envisia Therapeutics and Aerie Pharmaceuticals combined PRINT particle engineering technology with biodegradable polymer science.144,145 With this technology, various different ocular formulations, including subconjunctival implants, intravitreal implants, intracameral implants, and nanosuspensions, have been developed for controlled delivery of the drug into the ocular tissue. Some clinical trials are underway, including a multi-center open-labeled study to evaluate the safety and efficacy study of ENV 515 (travoprost) for treating glaucoma and ocular hypertension (NCT02371746). AR-1105 (NCT03739593) and AR-13503 (NCT03835884) designed using PRINT technology as intravitreal implants for the treatment of age-related macular degeneration and diabetic macular edema are in clinical trials. Nanomedicines currently undergoing clinical trials are outlined in Table 4.146–163
| Clinicaltrials.gov identifier | Drug | Phase | Disease | Nanomedicine | Sponsor | Reference |
|---|---|---|---|---|---|---|
| NCT00738361 | Paclitaxel | II | Intraocular melanoma | Nanoparticles | Ohio State University Comprehensive Cancer Center | 146 |
| NCT01987323 | Liposomal latanoprost | I/II | Ocular hypertension | Subconjunctival injection of liposomal egg PC | Singapore National Eye Centre | 147 |
| NCT03001466 | Urea | II | Cataracts | Nanoparticles | Assiut University-Faculty of medicine | 148 |
| NCT03093701 | TLC399 (Pro Dex) | II | Macular edema | Pro Dex | Taiwan Liposome Company, Taiwan | 149 |
| NCT03617315 | Crosslinked hyaluronic acid | Not applicable | Dry eye syndrome (dysfunction of meibomian gland) | Liposome | University of Seville | 150 |
| NCT03785340 | Brimonidine tartrate (0.2% nanoemulsion eye drops) | III | Dry eye disease | Nanoemulsion | Ocugen | 151 |
| NCT03249740 | Sunitinib malate (GB-102) | I | Age-related macular degeneration | Intravitreal injection of micro particles | Graybug Vision | 152 |
| NCT03140111 | LAMELLEYE for the treatment of dry eye symptoms in primary Sjögren's syndrome patients | NA | Dry eye | Liposome | NHS Greater Glasgow & Clyde | 153 |
| NCT03598699 | AXR-159 ophthalmic solution | II | Dry eye | Micelles | Andover Eye Associates. AxeroVision, Inc. ORA, Inc. | 154 |
| NCT02163824 | KPI-121 (1% and 0.25% loteprednol etabonate) | III | Ocular infection, irritation and inflammation | Mucus penetrating particles (submicron suspension) | Kala Pharmaceuticals, Inc. | 155 |
| NCT02813265 | KPI-121 (1% and 0.25% loteprednol etabonate) | III | Dry eye disease and keratoconjunctivitis sicca | Submicron suspension | Kala Pharmaceuticals, Inc. | 156 |
| NCT03739593 | AR-1105 (dexamethasone) | II | Macular edema due to retinal vein occlusion | Intravitreal implant (PRINT technology) | Aerie Pharmaceuticals, Inc. | 157 |
| NCT03835884 | AR-13503 implant alone and in combination with aflibercept | I | Neovascular age-related macular degeneration, diabetic macular edema | Intravitreal implant (PRINT technology) | Aerie Pharmaceuticals | 158 |
| NCT02908282 | Topical omega-3 fatty acids (REMOGEN® OMEGA) | NA | Dry eye | Microemulsion | TRB Chemedica AG | 159 |
| NCT02420834 | Dry eye treatment with artificial tears | NA | Dry eye | Liposomal spray | Aston University | 160 |
| NCT02371746 | ENV 515 travoprost extended release (XR) | II | Glaucoma and ocular hypertension | Intracameral implant (PRINT technology) | Envisia Therapeutics | 161 |
| NCT04008771 | SeeQ CdSe 655 alt nanoparticles | NA | Retinitis pigmentosa | Intravitreal injection of nanoparticles | 2C Tech Corp | 162 |
| NCT04130802 | OCS-01 – dexamethasone cyclodextrin nanoparticle ophthalmic suspension 1.5% | II | Inflammation corneal pain, postoperative | Nanoparticles | Oculis | 163 |
7. Conclusion
Targeted drug delivery to the anterior and posterior segments of the eye using nanomedicine to overcome the limitations of conventional formulations has gained remarkable attention. Advances in nanomedicine have facilitated controlled and target-specific drug delivery to ocular tissues to treat ocular diseases. Therapeutic delivery through nanocarriers can reduce the overall administration frequency of intravitreal injections, enhance the therapeutic efficacy, significantly reduce treatment costs and improve the quality of life of ocular disease patients. Further, technological advances related to easy scale-up and reproducibility bring more hope for the cost-effective commercialization of nanomedicines. Various methods have been developed for the production of nanomedicines with reproducible characteristics. Promising methods, for instance microfluidizers, supercritical fluid technology, and membrane extrusion, show scale-up capabilities. Certain ophthalmic nanomedicines, such as nanocrystals, nanoemulsions, liposomes, advanced PRINT, and hydrogel technologies, have scale-up potential and products of these technologies are on the market. Regulatory agencies have drafted guidelines for nanoformulation specifications to ensure their safety and efficacy.8. Future perspective
Anterior segment diseases such as cataracts, glaucoma, dry eye, and other infectious can ultimately lead to poor eyesight or blindness. For treating these conditions, eye drops are mostly prescribed but tear fluid generation, lacrimal drainage, and barrier functions limit the efficacy of the administered drugs. Drug delivery through films, hydrogels, and implants may improve the ocular residence time. However, these approaches can obstruct the vision and cause inconvenience to patients. Long-term contact with the carrier material in implants may compromise the safety of the ocular tissue. The reported preclinical and clinical outcomes for nanocarriers provide future hope for safer and effective drug delivery for treating anterior segment diseases. However, targeting nanocarriers to the posterior part is challenging and there is a need to deliver therapeutics via a suitable delivery system that can overcome the ocular barriers. Currently, injectable formulations are administered to treat chronic retinal disorders. For example, AMD requires anti-VEGF treatment by intravitreal injection, and it requires repeated administration, and expert medical supervision, while lacking patient compliance. Intravitreal nanomedicine injection can deliver the drug directly to the retina by overcoming the clearance of the drug molecule in the vitreous region. This nanomedicine can stay in the vitreous region and provide prolonged salutary drug concentrations to the target site. Intravitreal delivery of nanomedicines can reduce the dose and dosing frequency, and employing less invasive techniques can reduce the medical burden for patients and healthcare professionals. The delivery of nanocarriers larger than 300 nm to the posterior part of the eye increases aggregation and causes disturbance to the vision. A particle size of less than 300 nm not only decreased the chances of aggregation but also improved the release and drug loading, which are of major importance. All these considerations are the bottleneck for the ocular delivery of nanoparticles.In addition to the scale-up of nano-drug delivery systems, controlled drug release from implants, nano wafers, and 3D printed hydrogel technology has also been explored for the treatment of retinal diseases. The safety, scale-up, and reproducibility of nanomedicine are essential for reaching commercial scale. Most biodegradable and natural polymers are considered safe for drug delivery but detailed toxicity studies need to be performed before commercial acceptance. Some nanocarrier preparation techniques have been investigated for scale-up and large-scale production but there is a need to further explore more techniques that are simple, feasible, and provide regulatory acceptable nanomedicines.
Nanotechnology has the potential to add innovative functionality and provide superior therapeutic efficacy over conventional ocular drug delivery systems. The overall global ophthalmic drugs market was valued at approximately USD 25.03 billion in 2017 and is expected to generate revenue of around USD 34.52 billion by the end of 2024, growing at a compound annual growth rate of around 4.7% between 2018 and 2024.164 This reflects the huge scope of ocular products for abbreviated new drug applications in future.
Conflicts of interest
Authors declare no conflict of interest.References
- WHO, https://www.who.int/blindness/causes/priority/en/index1.html, 2018.
- WHO, International Classification of Diseases, 11th Revision (ICD-11), https://www.who.int/classifications/icd/en/, 2019 Search PubMed.
- K.-J. Cheng, C.-M. Hsieh, K. Nepali and J.-P. Liou, J. Med. Chem., 2020 DOI:10.1021/acs.jmedchem.9b01033
.
- U. Patel, M. Boucher, L. de Léséleuc and S. Visintini, Voretigene Neparvovec: An Emerging Gene Therapy for the Treatment of Inherited Blindness, Canadian Agency for Drugs and Technologies in Health, 2016 Search PubMed
.
- ReSure® Sealant – Ocular Therapeutix, https://www.ocutx.com/products/resure-sealant/, accessed 30 June 2020 Search PubMed.
- I. P. Kaur and R. Smitha, Drug Dev. Ind. Pharm., 2002, 28, 353–369 CrossRef CAS PubMed
.
- T. Ye, K. Yuan, W. Zhang, S. Song, F. Chen and X. Yang, et al., Asian J. Pharm. Sci., 2013, 8, 207–217 CrossRef
.
- S. P. Vyas, N. Mysore, V. Jaitely and N. Venkatesan, Pharmazie, 1998, 53, 466–469 CAS
.
- D. D.-S. Tang-Liu, J. B. Richman, R. J. Weinkam and H. Takruri, J. Pharm. Sci., 1994, 83, 85–90 CrossRef CAS PubMed
.
- Y. Liu, J. Liu, X. Zhang, R. Zhang, Y. Huang and C. Wu, AAPS PharmSciTech, 2010, 11, 610–620 CrossRef CAS PubMed
.
- J. Salamzadeh, Iran. J. Pharm. Res., 2018, 17, 1 Search PubMed
.
- B. K. Mann, D. L. Stirland, H. Lee and B. M. Wirostko, Adv. Drug Delivery Rev., 2018, 126, 195–203 CrossRef CAS PubMed
.
- A. S. Narang, R. K. Chang and M. A. Hussain, J. Pharm. Sci., 2013, 102, 3867–3882 CrossRef CAS PubMed
.
- D. Yadav, L. T. Varma and K. Yadav, in Drug Delivery for the Retina and Posterior Segment Disease, Springer International Publishing, Cham, 2018, pp. 51–67 Search PubMed
.
- A. Patel, K. Cholkar, V. Agrahari and A. K. Mitra, World J. Pharmacol., 2013, 2, 47 CrossRef PubMed
.
- E. Sánchez-López, M. Espina, S. Doktorovova, E. B. Souto and M. L. García, Eur. J. Pharm. Biopharm., 2017, 110, 70–75 CrossRef PubMed
.
- M. Hornof, E. Toropainen and A. Urtti, Eur. J. Pharm. Biopharm., 2005, 60, 207–225 CrossRef CAS PubMed
.
- E. Mannermaa, K. S. Vellonen and A. Urtti, Adv. Drug Delivery Rev., 2006, 58, 1136–1163 CrossRef CAS PubMed
.
- K. Cholkar, S. R. Dasari, D. Pal and A. K. Mitra, in Ocular Transporters and Receptors: Their Role in Drug Delivery, Elsevier Ltd., 2013, pp. 1–36 Search PubMed
.
- M. Goel, R. G. Picciani, R. K. Lee and S. K. Bhattacharya, Open Ophthalmol. J., 2010, 4, 52–59 CrossRef CAS PubMed
.
- R. Bachu, P. Chowdhury, Z. Al-Saedi, P. Karla and S. Boddu, Pharmaceutics, 2018, 10, 28 CrossRef PubMed
.
- K. G. Janoria, S. Gunda, S. H. Boddu and A. K. Mitra, Expert Opin. Drug Delivery, 2007, 4, 371–388 CrossRef CAS PubMed
.
- S. S. Shah, L. V. Denham, J. R. Elison, P. S. Bhattacharjee, C. Clement and T. Huq, et al., Expet Rev. Ophthalmol., 2010, 5, 75–93 CrossRef CAS PubMed
.
- S. Sethi, M. A. Malik, S. Goswami, P. Saxena, A. Srivastava and S. Kashyap, et al., Tumor Biol., 2014, 35, 11735–11740 CrossRef CAS PubMed
.
- A. Farkouh, P. Frigo and M. Czejka, Clin. Ophthalmol., 2016, 10, 2433–2441 CrossRef CAS PubMed
.
- P. L. Destruel, N. Zeng, M. Maury, N. Mignet and V. Boudy, Drug Discovery Today, 2017, 22, 638–651 CrossRef CAS PubMed
.
- K. Cholkar, S. P. Patel, A. D. Vadlapudi and A. K. Mitra, J. Ocul. Pharmacol. Ther., 2013, 29, 106–123 CrossRef CAS PubMed
.
- A. K. Mitra, Ophthalmic drug delivery systems, Marcel Dekker, 2003 Search PubMed
.
- C. Baudouin, A. Labbé, H. Liang, A. Pauly and F. Brignole-Baudouin, Prog. Retinal Eye Res., 2010, 29, 312–334 CrossRef CAS PubMed
.
- R. K. Vadlapatla, A. D. Vadlapudi, D. Pal and A. K. Mitra, Curr. Drug Metab., 2014, 15, 680–693 CrossRef CAS PubMed
.
- A. Urtti, Adv. Drug Delivery Rev., 2006, 58, 1131–1135 CrossRef CAS PubMed
.
- A. C. Amrite and U. B. Kompella, J. Pharm. Pharmacol., 2005, 57, 1555–1563 CrossRef CAS PubMed
.
- R. T. Addo, in Ocular drug delivery: advances, challenges and applications, Springer International Publishing, Cham, 2018 Search PubMed
.
- D. Kwatra and A. K. Mitra, World J. Pharmacol., 2013, 2, 78–83 CrossRef
.
- T. J. Shah, M. D. Conway and G. A. Peyman, Clin. Ophthalmol., 2018, 12, 2223–2235 CrossRef CAS PubMed
.
- S. Raghava, M. Hammond and U. B. Kompella, Expert Opin. Drug Delivery, 2004, 1, 99–114 CrossRef PubMed
.
- K. Nayak and M. Misra, Biomed. Pharmacother., 2018, 107, 1564–1582 CrossRef CAS PubMed
.
- V. Girdhar, S. Patil, S. Banerjee and G. Singhvi, Curr. Nanomed., 2018, 8, 88–99 CrossRef CAS
.
- G. Singhvi, S. Patil, V. Girdhar and S. K. Dubey, Nanosci. Nanotechnol., 2019, 9, 329–336 CAS
.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres and L. S. Acosta-Torres, et al., J. Nanobiotechnol., 2018, 16, 17 CrossRef PubMed
.
- C. H. Tsai, P. Y. Wang, I. C. Lin, H. Huang, G. S. Liu and C. L. Tseng, Int. J. Mol. Sci., 2018, 19, 2830 CrossRef PubMed
.
- G. Singhvi, S. Banerjee and A. Khosa, in Org. Mater. as Smart Nanocarriers Drug Deliv., William Andrew Publishing, 2018, pp. 471–517 Search PubMed
.
- S. Jain, S. Krishna Cherukupalli, A. Mahmood, S. Gorantla, V. Krishna Rapalli, S. Kumar Dubey and G. Singhvi, J. Appl. Pharm. Sci., 2019, 9, 130–143 CAS
.
- V. K. Rapalli, S. Gorantla, T. Waghule, A. Mahmood, P. P. Singh and S. K. Dubey, et al., Recent Pat. Drug Delivery Formulation, 2019, 13(4), 283–290 CrossRef CAS PubMed
.
- N. Gautam and K. Kesavan, Ther. Delivery, 2017, 8, 313–330 CrossRef CAS PubMed
.
- S. Jacob, A. B. Nair and J. Shah, Biomater. Res., 2020, 24, 3 CrossRef CAS PubMed
.
- K. Cholkar, A. Patel, A. Dutt Vadlapudi and A. K. Mitra, Recent Pat. Nanotechnol., 2012, 2, 82–95 CAS
.
- A. Mandal, R. Bisht, I. D. Rupenthal and A. K. Mitra, J. Controlled Release, 2017, 248, 96–116 CrossRef CAS PubMed
.
- I. Insua, A. Wilkinson and F. Fernandez-Trillo, Eur. Polym. J., 2016, 81, 198–215 CrossRef CAS PubMed
.
- F. Wang, M. Zhang, D. Zhang, Y. Huang, L. Chen and S. Jiang, et al., J. Biomed. Res., 2018, 32, 411–423 Search PubMed
.
- P. Jaiswal, B. Gidwani and A. Vyas, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 27–40 CrossRef CAS PubMed
.
- R. Liu, Z. Liu, C. Zhang and B. Zhang, J. Pharm. Sci., 2012, 101, 3833–3844 CrossRef CAS PubMed
.
- H. Almeida, M. H. Amaral, P. Lobão, A. C. Silva and J. M. S. Lobo, J. Pharm. Pharm. Sci., 2014, 17, 278–293 Search PubMed
.
- J. Y. Hwang, Z. Li and X. J. Loh, RSC Adv., 2016, 6, 70592–70615 RSC
.
- G. Singhvi, V. K. Rapalli, S. Nagpal, S. K. Dubey and R. N. Saha, in Nanoscience in Medicine, Springer International Publishing, Cham, 2020, pp. 51–88 Search PubMed
.
- G. Singhvi, S. Banerjee and A. Khosa, in Organic Materials as Smart Nanocarriers for Drug Delivery, Elsevier, 2018, pp. 471–517 Search PubMed
.
- X. Yuan, D. C. Marcano, C. S. Shin, X. Hua, L. C. Isenhart, S. C. Pflugfelder and G. Acharya, ACS Nano, 2015, 9, 1749–1758 CrossRef CAS PubMed
.
- M. G. Lancina III and H. Yang, Can. J. Chem., 2017, 95, 897–902 CrossRef PubMed
.
- P. Taskar, A. Tatke and S. Majumdar, Expert Opin. Drug Delivery, 2017, 14, 49–63 CrossRef CAS PubMed
.
- G. Tan, S. Yu, H. Pan, J. Li, D. Liu, K. Yuan, X. Yang and W. Pan, Int. J. Biol. Macromol., 2017, 94, 355–363 CrossRef CAS PubMed
.
- R. Campardelli, P. Trucillo and E. Reverchon, J. CO2 Util., 2018, 25, 235–241 CrossRef CAS
.
- T. Ren, X. Lin, Q. Zhang, D. You, X. Liu and X. Tao, et al., Mol. Pharm., 2018, 15, 4862–4871 CrossRef CAS PubMed
.
- Y. Takashima, T. Tsuchiya, Y. Igarashi, T. Kanazawa, H. Okada and A. Urtti, J. Pharm. Soc. Jpn., 2012, 132, 1365–1370 CrossRef CAS PubMed
.
- B. M. Davis, E. M. Normando, L. Guo, L. A. Turner, S. Nizari and P. O'Shea, et al., Small, 2014, 10, 1575–1584 CrossRef CAS PubMed
.
- A. Khames, M. A. Khaleel, M. F. El-Badawy and A. O. H. El-Nezhawy, Int. J. Nanomed., 2019, 14, 2515–2531 CrossRef CAS PubMed
.
- A. Tatke, N. Dudhipala, K. Yadav Janga, S. Prachetan Balguri, B. Avula and M. M. Jablonski, et al., Nanomaterials, 2018, 9(1), 33 CrossRef PubMed
.
- A. Khare, I. Singh, P. Pawar and K. Grover, J. Drug Delivery, 2016, 1–11 CAS
.
- R. Gonzalez-Pizarro, P. Carvajal-Vidal, L. Halbault Bellowa, A. C. Calpena, M. Espina and M. L. García, Colloids Surf., B, 2019, 175, 365–374 CrossRef CAS PubMed
.
- O. A. E.-A. Soliman, E. A. Mohamed and N. A. A. Khatera, Pharm. Dev. Technol., 2019, 24, 48–62 CrossRef CAS PubMed
.
- B. Sharif Makhmal Zadeh, H. Niro, F. Rahim, G. Esfahani, B. Sharif Makhmal Zadeh and H. Niro, et al., Sci. Pharm., 2018, 86, 16 CrossRef PubMed
.
- P. Niamprem, S. P. Srinivas and W. Tiyaboonchai, Colloids Surf., B, 2019, 176, 371–378 CrossRef CAS PubMed
.
- Y. Yu, R. Feng, J. Li, Y. Wang, Y. Song and G. Tan, et al., Asian J. Pharm. Sci., 2018, 14, 423–434 CrossRef PubMed
.
- R. Kesarla, T. Tank, P. A. Vora, T. Shah, S. Parmar and A. Omri, Drug Delivery, 2015, 23, 1–8 CrossRef PubMed
.
- N. Dahmana, T. Mugnier, D. Gabriel, V. Kaltsatos, T. Bertaim and F. Behar-Cohen, et al., Mol. Pharm., 2018, 15, 1192–1202 CrossRef CAS PubMed
.
- C. Guo, Y. Zhang, Z. Yang, M. Li, F. Li and F. Cui, et al., Sci. Rep., 2015, 5, 1–14 Search PubMed
.
- P. A. Jadhav and A. V. Yadav, Ther. Delivery, 2019, 10, 585–597 CrossRef CAS PubMed
.
- U. Soiberman, S. P. Kambhampati, T. Wu, M. K. Mishra, Y. Oh and R. Sharma, et al., Biomaterials, 2017, 125, 38–53 CrossRef CAS PubMed
.
- D. C. Marcano, C. S. Shin, B. Lee, L. C. Isenhart, X. Liu and F. Li, et al., Mol. Pharm., 2016, 13, 3468–3477 CrossRef CAS PubMed
.
- A. E. Eldeeb, S. Salah and M. Ghorab, J. Drug Delivery Sci. Technol., 2019, 52, 236–247 CrossRef CAS
.
- H. M. Fahmy, E. A. E.-M. S. Saad, N. M. Sabra, A. A. El-Gohary, F. F. Mohamed and M. H. Gaber, Int. J. Pharm., 2018, 548, 597–608 CrossRef CAS PubMed
.
- J. Lin, H. Wu, Y. Wang, J. Lin, Q. Chen and X. Zhu, Drug Delivery, 2016, 23, 1144–1151 CAS
.
- Q. Jin, H. Li, Z. Jin, L. Huang, F. Wang and Y. Zhou, et al., Int. J. Pharm., 2018, 553, 21–28 CrossRef CAS PubMed
.
- C. Zhan, C. M. Santamaria, W. Wang, J. B. McAlvin and D. S. Kohane, Biomaterials, 2018, 181, 372–377 CrossRef CAS PubMed
.
- I. Ahmad, J. Pandit, Y. Sultana, A. K. Mishra, P. P. Hazari and M. Aqil, Mater. Sci. Eng., C, 2019, 100, 959–970 CrossRef CAS PubMed
.
- J. Li, X. Guo, Z. Liu, C. I. Okeke, N. Li and H. Zhao, et al., Drug Dev. Ind. Pharm., 2014, 40, 980–987 CrossRef CAS PubMed
.
- T. Waghule, V. K. Rapalli, G. Singhvi, P. Manchanda, N. Hans and S. K. Dubey, et al., J. Drug Delivery Sci. Technol., 2019, 52, 303–315 CrossRef CAS
.
- V. K. Rapalli, V. Kaul, S. Gorantla, T. Waghule, S. K. Dubey and M. M. Pandey, et al., Spectrochim. Acta, Part A, 2020, 224, 117392 CrossRef CAS PubMed
.
- V. K. Rapalli, G. Singhvi, S. Gorantla, T. Waghule, S. K. Dubey and R. N. Saha, et al., J. Sep. Sci., 2019, 42, 3413–3420 CrossRef CAS PubMed
.
- P. Lakhani, A. Patil, P. Taskar, E. Ashour and S. Majumdar, J. Drug Delivery Sci. Technol., 2018, 47, 159–166 CrossRef CAS PubMed
.
- C. Puglia, D. Santonocito, C. Ostacolo, E. Maria Sommella, P. Campiglia and C. Carbone, et al., Nanomaterials, 2020, 10, 287 CrossRef CAS PubMed
.
- V. R. Rathod, D. A. Shah and R. H. Dave, Drug Dev. Ind. Pharm., 2020, 46, 443–455 CrossRef CAS PubMed
.
- K. Selvaraj, G. Kuppusamy, J. Krishnamurthy, R. Mahalingam, S. K. Singh and M. Gulati, Assay Drug Dev. Technol., 2019, 17, 178–190 CrossRef CAS PubMed
.
- A. Patil, P. Lakhani, P. Taskar, K. W. Wu, C. Sweeney and B. Avula, et al., J. Pharm. Sci., 2018, 107, 2160–2171 CrossRef CAS PubMed
.
- N. S. El-Salamouni, R. M. Farid, A. H. El-Kamel and S. S. El-Gamal, J. Microencapsulation, 2018, 35, 102–113 CrossRef CAS PubMed
.
- A. Solanki, R. Smalling, A. H. Parola, I. Nathan, R. Kasher, Y. Pathak and V. Sutariya, Curr. Drug Delivery, 2019, 16, 226–232 CrossRef CAS PubMed
.
- G. Singhvi, N. Hans, N. Shiva and S. Kumar Dubey, in Natural Polysaccharides in Drug Delivery and Biomedical Applications, Elsevier, 2019, pp. 121–144 Search PubMed
.
- G. Singhvi, S. Patil, V. Girdhar, D. K. Chellappan, G. Gupta and K. Dua, Panminerva Med., 2018, 60, 170–173 Search PubMed
.
- J. Li, S. Tian, Q. Tao, Y. Zhao, R. Gui and F. Yang, et al., Int. J. Nanomed., 2018, 13, 3975–3987 CrossRef CAS PubMed
.
- J. Alvarez-Trabado, A. López-García, M. Martín-Pastor, Y. Diebold and A. Sanchez, Int. J. Pharm., 2018, 546, 20–30 CrossRef CAS PubMed
.
- D. Liu, Y. Lian, Q. Fang, L. Liu, J. Zhang and J. Li, Int. J. Biol. Macromol., 2018, 116, 1026–1036 CrossRef CAS PubMed
.
- R. Trivedi and U. B. Kompella, Nanomedicine, 2010, 5, 485–505 CrossRef CAS PubMed
.
- M. Li, M. Xin, C. Guo, G. Lin and X. Wu, Drug Dev. Ind. Pharm., 2017, 43, 1846–1857 CrossRef CAS PubMed
.
- F. Alvarez-Rivera, D. Fernández-Villanueva, A. Concheiro and C. Alvarez-Lorenzo, J. Pharm. Sci., 2016, 105, 2855–2863 CrossRef CAS PubMed
.
- S. Gorantla, T. Waghule, V. K. Rapalli, P. P. Singh, S. K. Dubey, R. N. Saha and G. Singhvi, Recent Pat. Drug Delivery Formulation, 2019, 13(4), 291–300 CrossRef CAS PubMed
.
- N. Morsi, M. Ibrahim, H. Refai and H. El Sorogy, Eur. J. Pharm. Sci., 2017, 104, 302–314 CrossRef CAS PubMed
.
- N. Patel, H. Nakrani, M. Raval and N. Sheth, Drug Delivery, 2016, 23, 3712–3723 CrossRef CAS PubMed
.
- J. Phua, A. Hou, Y. Lui, T. Bose, G. Chandy and L. Tong, et al., Int. J. Mol. Sci., 2018, 19, 2977 CrossRef PubMed
.
- V. Agrahari, Nanomedicine, 2017, 12, 819–823 CrossRef CAS PubMed
.
- V. K. Rapalli, A. Khosa, G. Singhvi, V. Girdhar, R. Jain and S. K. Dubey, Pharm. Qual. by Des., 2019, pp. 255–296 Search PubMed
.
- M. L. Chen, M. John, S. L. Lee and K. M. Tyner, AAPS J., 2017, 19, 642–651 CrossRef CAS PubMed
.
- A. P. Colombo, S. Briançon, J. Lieto and H. Fessi, Drug Dev. Ind. Pharm., 2001, 27, 1063–1072 CrossRef CAS PubMed
.
- K. Baba and K. Nishida, Pharmaceutics, 2013, 5, 107–114 CrossRef CAS PubMed
.
- S. Galindo-Rodriguez, E. Allémann, H. Fessi and E. Doelker, Pharm. Res., 2004, 21, 1428–1439 CrossRef CAS PubMed
.
- H. Patil, R. V. Tiwari and M. A. Repka, AAPS PharmSciTech, 2016, 17, 20–42 CrossRef CAS PubMed
.
- S. M. Jafari, E. Assadpoor, Y. He and B. Bhandari, Food Hydrocolloids, 2008, 22, 1191–1202 CrossRef CAS
.
- V. Jenning, A. Lippacher and S. H. Gohla, J. Microencapsulation, 2002, 19, 1–10 CrossRef CAS PubMed
.
- A. Fàbregas, M. Miñarro, E. García-Montoya, P. Pérez-Lozano, C. Carrillo and R. Sarrate, et al., Int. J. Pharm., 2013, 446, 199–204 CrossRef PubMed
.
- D. Liu, H. Zhang, F. Fontana, J. T. Hirvonen and H. A. Santos, Adv. Drug Delivery Rev., 2018, 128, 54–83 CrossRef CAS PubMed
.
- H. S. M. Ali, P. York, A. M. A. Ali and N. Blagden, J. Controlled Release, 2011, 149, 175–181 CrossRef CAS PubMed
.
- A. Gdowski, K. Johnson, S. Shah, I. Gryczynski, J. Vishwanatha and A. Ranjan, J. Nanobiotechnol., 2018, 16, 12 CrossRef PubMed
.
- C. A. Charitidis, P. Georgiou, M. A. Koklioti, A.-F. Trompeta and V. Markakis, Manuf. Rev., 2014, 1, 11 Search PubMed
.
- J. Jung, J. Y. Clavier and M. Perrut, Mater. Sci., 2003, 3, 1683–1688 Search PubMed
.
- T. T. Pham, C. Jaafar-Maalej, C. Charcosset and H. Fessi, Colloids Surf., B, 2012, 94, 15–21 CrossRef CAS PubMed
.
- J. L. Perry, K. P. Herlihy, M. E. Napier and J. M. Desimone, Acc. Chem. Res., 2011, 44, 990–998 CrossRef CAS PubMed
.
- G. Acharya, C. S. Shin, M. McDermott, H. Mishra, H. Park, I. C. Kwon and K. Park, J. Controlled Release, 2010, 141, 314–319 CrossRef CAS PubMed
.
- T. Meng, V. Kulkarni, R. Simmers, V. Brar and Q. Xu, Drug Discovery Today, 2019, 24, 1524–1538 CrossRef CAS PubMed
.
- S. Soares, J. Sousa, A. Pais and C. Vitorino, Front. Chem., 2018, 6, 360 CrossRef PubMed
.
- R. Paliwal, R. J. Babu and S. Palakurthi, AAPS PharmSciTech, 2014, 15, 1527–1534 CrossRef CAS PubMed
.
- S. Hua, M. B. C. de Matos, J. M. Metselaar and G. Storm, Front. Pharmacol., 2018, 9, 1–9 CrossRef PubMed
.
- FDA, Center for drug evaluation and research application number: 210913orig1s000 summary review, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210913Orig1s000SumR.pdf Search PubMed.
- M. S. Uddin, A. Al Mamun, M.T. Kabir, J. R. Setu, S. Zaman, Y. Begum and M. S. Amran, J. Adv. Med. Pharm. Sci., 2017, 14(2), 1–17 Search PubMed
.
- FDA, CDER, Yeaton and Ayse, Drug Products, Including Biological Products, that Contain Nanomaterials - Guidance for Industry, https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm Search PubMed.
- FDA and CDER, Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry, 2018, http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm Search PubMed.
- Selected FDA Publications Related to the Application, Characterization, Effects, and Evaluation of Nanotechnology, FDA, https://www.fda.gov/science-research/nanotechnology-programs-fda/selected-fda-publications-related-application-characterization-effects-and-evaluation-nanotechnology, accessed 31 March 2020 Search PubMed.
- Guidelines for Evaluation of Nanopharmaceuticals in India, 2019, http://www.dbtindia.gov.in/sites/default/files/uploadfiles/Guidelines_For_Evaluation_of_Nanopharmaceuticals_in_India_24.10.19.pdf Search PubMed.
- J. H. Draize, J. Pharmacol. Exp. Ther., 1944, 82, 377–390 CAS
.
- T. K. De, E. J. Bergey, S. J. Chung, D. J. Rodman, D. J. Bharali and P. N. Prasad, J. Microencapsulation, 2004, 21, 841–855 CrossRef CAS PubMed
.
- E. Vega, M. A. Egea, O. Valls, M. Espina and M. L. Garcia, J. Pharm. Sci., 2006, 95, 2393–2405 CrossRef CAS PubMed
.
- T. W. Prow, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 317–333 CrossRef CAS
.
- E. Beltrán-Gracia, A. López-Camacho, I. Higuera-Ciapara, J. B. Velázquez-Fernández and A. A. Vallejo-Cardona, Cancer Nanotechnol., 2019, 10, 1–40 CrossRef
.
- Y. I. Eroglu, J. Mark. Access Health Policy, 2017, 5, 1336043 CrossRef PubMed
.
- Sun Pharma Launches Cequa for the Treatment of Dry Eye Disease in the US – Eyewire News, https://www.eyewire.news/articles/sun-pharma-launches-cequa-for-the-treatment-of-dry-eye-disease-in-the-us/, accessed 29 March 2020 Search PubMed.
- MPP Platform - Kala Pharmaceuticals, https://www.kalarx.com/technology/mpp-platform/, accessed 29 March 2020 Search PubMed.
- Aerie Pharmaceuticals Announces Drug Delivery Asset Acquisition to Further Advance Its Retinal Disease Program | Business Wire, https://www.businesswire.com/news/home/20171005005490/en/Aerie-Pharmaceuticals-Announces-Drug-Delivery-Asset-Acquisition, accessed 30 March 2020 Search PubMed.
- Envisia Therapeutics Secures $16.5 Million In Additional Series A Financing, https://www.prnewswire.com/news-releases/envisia-therapeutics-secures-165-million-in-additional-series-a-financing-300240151.html, accessed 28 March 2020 Search PubMed.
- Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Metastatic Melanoma of the Eye That Cannot Be Removed By Surgery - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT00738361, accessed 31 July 2019 Search PubMed.
- Safety and Efficacy of Liposomal Latanoprost in Ocular Hypertension - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT01987323, accessed 29 July 2019 Search PubMed.
- A Randomized Controlled Trial Comparing Urea Loaded Nanoparticles to Placebo: a New Concept for Cataract Management - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03001466, accessed 29 July 2019 Search PubMed.
- TLC399 (ProDex) in Subjects With Macular Edema Due to Retinal Vein Occlusion (RVO) - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03093701, accessed 28 August 2019 Search PubMed.
- Crosslinked Hyaluronic Acid With Liposomes and Crocin in Dry Eye - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03617315, accessed 8 February 2020 Search PubMed.
- Search of: NCT03785340 - List Results - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/results?cond=%26term=NCT03785340%26cntry=%26state=%26city=%26dist=, accessed 28 March 2020.
- A Depot Formulation of Sunitinib Malate (GB-102) in Subjects With Neovascular (Wet) Age-related Macular Degeneration - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03249740, accessed 28 March 2020 Search PubMed.
- LAMELLEYE for the Treatment of Dry Eye Symptoms in pSS Patients - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03140111, accessed 28 March 2020 Search PubMed.
- A Study to Evaluate the Safety, Tolerability, Systemic Pharmacokinetics, and Pharmacodynamics of AXR-159 Ophthalmic Solution in Patients With Dry Eye Disease (DED) - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03598699, accessed 28 March 2020 Search PubMed.
- Safety and Efficacy of KPI-121 in Subjects With Postsurgical Inflammation - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02163824, accessed 28 March 2020 Search PubMed.
- Safety and Efficacy of KPI-121 in Subjects With Dry Eye Disease - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02813265, accessed 28 March 2020 Search PubMed.
- Study Assessing AR-1105 in Subjects With Macular Edema Due to Retinal Vein Occlusion (RVO) - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03739593, accessed 28 March 2020 Search PubMed.
- A Study Assessing AR-13503 Implant Alone and in Combination With Aflibercept in Subjects With nAMD or DME - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT03835884, accessed 28 March 2020 Search PubMed.
- Topical Omega-3 Fatty Acids (REMOGEN® OMEGA) in the Treatment of Dry Eye - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02908282, accessed 28 March 2020 Search PubMed.
- Dry Eye Treatment With Artificial Tears - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02420834, accessed 28 March 2020 Search PubMed.
- Safety and Efficacy of ENV515 Travoprost Extended Release (XR) in Patients With Bilateral Ocular Hypertension or Primary Open Angle Glaucoma - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02371746, accessed 28 March 2020 Search PubMed.
- Intravitreal Injection of SeeQ CdSe 655 Alt Nanoparticles for Patients With Degenerative Retinal Diseases - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT04008771, accessed 29 March 2020 Search PubMed.
- OCS-01 in Treating Inflammation and Pain in Post-cataract Patients - Full Text View - ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT04130802, accessed 29 March 2020 Search PubMed.
- Global Ophthalmic Drugs Market Expected to Reach USD 34.52 Billion By 2024: Zion Market Research, https://www.globenewswire.com/news-release/2018/09/21/1574283/0/en/Global-Ophthalmic-Drugs-Market-Expected-to-Reach-USD-34-52-Billion-By-2024-Zion-Market-Research.html, accessed 31 July 2019 Search PubMed.
Footnote |
| † Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |