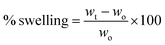Open Access Article
Open Access ArticleWater-based synthesis of photocrosslinked hyaluronic acid/polyvinyl alcohol membranes via electrospinning†
Karine Cappuccio de Castro *a,
Jonny Burga-Sánchez
*a,
Jonny Burga-Sánchez b,
Maria Gabriela Nogueira Campos
b,
Maria Gabriela Nogueira Campos c and
Lucia Helena Innocentini Mei
c and
Lucia Helena Innocentini Mei a
a
aFaculty of Chemical Engineering, Department of Materials Engineering and Bioprocess, University of Campinas, Cidade Universitária Zeferino Vaz, Campinas, SP, Brazil. E-mail: cappuccio.karine@gmail.com
bPiracicaba Dental School, Physiological Science Department, University of Campinas, Piracicaba, SP, Brazil
cNanoScience Technology Center, University of Central Florida, Orlando, USA
First published on 24th August 2020
Abstract
Electrospinning is a versatile and low-cost technique widely used in the manufacture of nanofibrous polymeric membranes applied in different areas, especially in bioengineering. Hyaluronic acid (HA) is a biocompatible natural polymer, but it has rheological characteristics that make the electrospinning process difficult. Thus, its association with another polymer such as poly(vinyl alcohol) (PVA) is an alternative, as PVA has good rheological properties for electrospinning. Based on this, the aim of this work was to produce, by the conventional electrospinning method, cross-linked HA/PVA membranes free from organic solvent with a low degradation rate in PBS 7.4 solution after the photocrosslinking process and without using any organic solvent. The results showed that the electrospinning occurred effectively for all conditions tested, but the best result for complete cross-linking only occurred with 15 and 30% crosslinker, which was evidenced by infrared spectroscopy. The addition of crosslinker favored the stability of the electrospinning jet, especially for 30% crosslinker concentration. The membranes did not show cytotoxicity even after the cross-linking process, which indicates that the material has potential as a drug delivery device.
1. Introduction
Recently, electrospinning has attracted great attention as it is an effective technique to produce nanofibrous membranes for a large variety of applications, especially in the pharmaceutical and medical fields,1 including wound dressings,2 drug delivery systems,3 and tissue engineering scaffolds.4The study of the physical parameters that involve the electrospinning process, such as jet formation, as a function of electrostatic field strength; fluid viscosity; solvent composition, and molecular weight of polymers in solution, is of great importance as they directly impact the characteristics of the formed nanofibers.5,6 In addition to physical properties, polymers suitable for electrospinning should also present the desired characteristics for the final application. Specifically, for wound dressings that are utilized predominantly to improve the cicatrization environment in its various stages; the wound surface must be covered by dressing materials to enhance and speed up the healing process.2,7
Hyaluronic acid (HA) is a natural polysaccharide, commonly found in connective tissues in the body such as vitreous, umbilical cord, joint fluid, presenting very high viscosity and high surface tension in solution, even at very low concentrations.2,8 It consists of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine. Due to its unique rheological properties, biodegradability, and biocompatibility, HA has been the subject of study for many biomedical applications including drug administration, dermatological applications, wound dressings, and cell growth scaffolds.9–11
Poly(vinyl alcohol) (PVA) is a synthetic polymer and has received great attention from several areas due to its low cost, high hydrophilicity, and excellent chemical resistance. PVA has been widely used for several biological applications due to its advantages such as non-toxic, non-carcinogenic, biodegradable, and bio-adhesive characteristics, as well as easy processing. As a result of the aforementioned features, PVA can simulate natural tissues and thus be accepted in body implants.3,12
The production of nanofibers with HA in its native form by the conventional method of electrospinning is not viable due to the rheological properties of the HA solution. Pure HA nanofibers were reported by Um et al. (2004) and by Wang et al. (2005) by a blowing-assisted electrospinning process and the maximum reported HA concentration was 2.7%, even after adapting the process.11,13 In this work, we were able to electrospinning high molecular weight HA at 2% by conventional electrospinning pathways when associated with PVA, which improves the rheological behavior of HA. In addition, PVA also improved the mechanical properties of HA membranes without altering their good compatibility for biological uses.
Electrospinning of the HA/PVA blend has already been reported with the use of surfactants or solvent mixtures.14,15 However, to our knowledge, this is the first study where no organic solvent or surfactant was used to prepare HA and PVA polymeric electrospinnable solutions (only water was used as a solvent), which reduces the toxicity of the final material.
The use of hydrophilic polymers is extremely advisable for making biomedical devices due to their solubility in water as well as the low-toxicity of the system. On the other hand, a highly soluble system will leach very quickly, making it impossible to use effectively when there is a need for contact with body fluids. Therefore the cross-linking process can be an interesting alternative to improve the device's stability and mechanical properties at physiological conditions. As our ultimate goal is to apply the herein prepared membranes as drug carriers for sustained release, we performed a photocrosslinking step using maleic anhydride as a cross-linking agent.
Given this context, this study demonstrated that it is possible to obtain HA/PVA membranes by the conventional electrospinning technique, using high concentration of high molecular weight HA, and only water as solvent. The electrospinning process was stable and enabled obtaining dressings with desirable properties for applications in the biomedical field as drug carriers. This result has not been consolidated in the Literature yet.
2. Materials and methods
2.1. Materials
Photoinitiator 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959, 98%, Mw = 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 g mol−1 Sigma Aldrich), polyvinyl alcohol (PVA, Mw ≈ 130 kg mol−1, 86.5–89.5 mol% hydrolysis, Vetec), hyaluronic acid sodium salt (HA, cosmetic grade, Symbios, Mw > 1.6 MDa, D-glucuronic acid ≥45%, bacterial), maleic anhydride (MA, Mw ≈ 98 g mol−1, 98%, Dinâmica), saline phosphate buffer solution (PBS, pH 7.4 ± 0.2, Dinâmica), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Thermo Fisher Scientific Inc., Oregon, USA), calcein AM (component A) (Thermo Fisher Scientific Inc., Oregon, USA), ethidium homodimer-1 (component B) (Thermo Fisher Scientific Inc., Oregon, USA), were used without further purification.
25 g mol−1 Sigma Aldrich), polyvinyl alcohol (PVA, Mw ≈ 130 kg mol−1, 86.5–89.5 mol% hydrolysis, Vetec), hyaluronic acid sodium salt (HA, cosmetic grade, Symbios, Mw > 1.6 MDa, D-glucuronic acid ≥45%, bacterial), maleic anhydride (MA, Mw ≈ 98 g mol−1, 98%, Dinâmica), saline phosphate buffer solution (PBS, pH 7.4 ± 0.2, Dinâmica), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Thermo Fisher Scientific Inc., Oregon, USA), calcein AM (component A) (Thermo Fisher Scientific Inc., Oregon, USA), ethidium homodimer-1 (component B) (Thermo Fisher Scientific Inc., Oregon, USA), were used without further purification.
2.2. Solution preparation
PVA (6% w/v) was dissolved in distilled water and stirred on a hot plate shaker at 80 °C for 2 h. HA solution (2% w/v) was prepared in NaCl aqueous solution (1% w/v), and at room temperature. PVA and HA were mixed in the proportion of 75PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25HA, under vigorous agitation and at room temperature. Both MA (5%, 15%, 30% by weight of PVA and HA) and Irgacure 2959 (5% by weight of PVA and HA) were added to the final solution and mixed under vigorous stirring overnight and at room temperature before the electrospinning process. The proportion of 75PVA/25HA was chosen after preliminary studies once it showed greater stability and yield in the electrospinning process.
25HA, under vigorous agitation and at room temperature. Both MA (5%, 15%, 30% by weight of PVA and HA) and Irgacure 2959 (5% by weight of PVA and HA) were added to the final solution and mixed under vigorous stirring overnight and at room temperature before the electrospinning process. The proportion of 75PVA/25HA was chosen after preliminary studies once it showed greater stability and yield in the electrospinning process.
2.3. Characterization of solutions
The electrical conductivity of the solutions was determined by an Analion K0392 conductivity meter at 25 °C. Viscosity was measured in a Haake RheoStress 1 rheometer at 25 °C at a speed of 2 rpm to 5 rpm (keeping the torque at 50%). A measurement sensor system having a cylindrical rotor and pot with diameters of 3.6 mm and 4.8 mm respectively was utilized for PVA/HA membranes without crosslinker (spindle SC4-2) and pot with diameters of 3.2 mm and 9.4 mm respectively was utilized for PVA/HA membranes with a crosslinker (spindle SC4-34). Surface tension was measured by the ring method in a K6 Kruss Tensiometer.2.4. Electrospinning step
The prepared electrospinning solutions were loaded in a 10 mL glass syringe with a metal needle (0.80 mm diameter inner). The distance between the needle tip and the aluminum-foil wrapped plane collector (10 × 30 cm) was adjusted to 15 cm (solution with and without a cross-linking agent). A high voltage current of 25 kV (DC) was applied between the needle and the collector to initiate the charged polymer solution jet, which was rate-controlled by the syringe pump. This value was fixed for all solutions, but with the addition of the cross-linking agent, it was possible to have a stable process and without changes in morphology with a voltage of 20 kV. The feed rate was fixed at 0.3 mL h−1 and 1 mL h−1 for solutions without a cross-linking agent and with a cross-linking agent, respectively. All experiments were performed at room temperature and relative humidity of 20 to 30%. The prepared nanofibers were carefully collected and stored at room temperature until the final cross-linking step.2.5. Photocrosslinking reaction
In order to activate the photocrosslinking reaction in the fibers, all membranes were placed in a UV light reactor (BLACK LIGHT 8W F8T5BL – 300 mm at 365 nm – PHILIPS) for 40 min and then stored in a desiccator at room temperature for further analysis.2.6. Characterization of electrospun hyaluronic acid/poly(vinyl alcohol) membranes
where wt and wo are the weights of swollen and dried samples, respectively.
3. Results and discussion
Before defining the PVA/HA best ratio for the electrospinning process, a preliminary study was performed. Three proportions of PVA/HA were studied: 25/75, 50/50, and 75/25. In all proportions, the electrospinning process occurred and nanofibers were formed, but higher proportions of hyaluronic acid left the solution highly viscous, which caused instability in the electrospinning jet and gave rise to beaded nanofibers (Fig. 1). So, the condition chosen for this study was the one that presented the formation of uniform nanofibers and stability in the electrospinning process (75/25).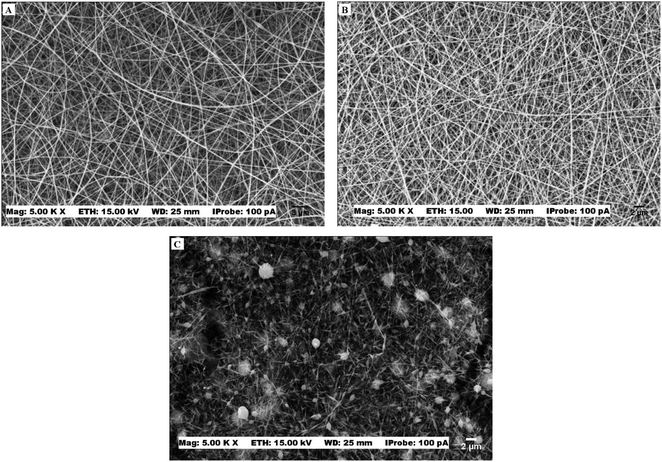 | ||
| Fig. 1 SEM images of PVA/HA membranes with different proportions (A) 75PVA/25HA, (B) 50PVA/50HA (C) 25PVA/75HA. | ||
Table 1 shows the rheological parameters of the cross-linking solutions with maleic anhydride (MA). These parameters play an important role in the properties of the formed nanofibers. Therefore, we carefully discussed their effects on the morphology of the nanofibrous membranes obtained in this work.
| Samples | Superficial tension (mN m−1) | Electric conductivity (mS cm−1) | Viscosity (cP) |
|---|---|---|---|
| 0% MA | 45.3 ± 0.6 | 4.8 ± 0.3 | 4263 ± 18 |
| 5% MA | 41.8 ± 0.8 | 6.0 ± 0.1 | 212 ± 1 |
| 15% MA | 39.3 ± 0.6 | 10.8 ± 0.2 | 167 ± 2 |
| 30% MA | 32.3 ± 0.6 | 15.3 ± 0.2 | 124.5 ± 0.3 |
The addition of MA to the polymeric solution improved the electrospinning parameters, decreased the applied voltage, which was initially 25 kV and went to 20 kV, and the solution ejection flow, which went from 0.3 mL h−1 to 1 mL h−1, since it reduces the solutions' viscosity and surface tension, facilitating its flow by the formation of a more homogeneous and defect-free system. MA addition also increased the solution electrical conductivity, causing it to have sufficient surface loads for Taylor cone generation, and consequently the formation of a stable jet. However, it was observed that above 30% of crosslinker the solution was no longer electrospinnable, resulting in a phenomenon similar to electrospray, i.e., the deposition of small droplets in the collector.
SEM photomicrographs showed that increasing MA concentration decreased nanofiber diameter (Table 2) and made diameter distribution more homogeneous (Fig. 2). Although the average diameters are not statistically different, it is possible to observe a tendency of fiber diameter reduction for high crosslinker concentration (Table 2). In addition, it was possible to observe a greater interconnection of nanofibers with the increase of crosslinker concentration, suggesting that the membrane's porosity also decreased. However, additional studies on pores size distribution are under investigation.
| 0% MA | 5% MA | 15% MA | 30% MA | |
|---|---|---|---|---|
| Diameter (nm) | 308 ± 74 | 247 ± 101 | 226 ± 83 | 217 ± 65 |
| Minimum diameter (nm) | 162 | 110 | 108 | 100 |
| Maximum diameter (nm) | 595 | 576 | 499 | 448 |
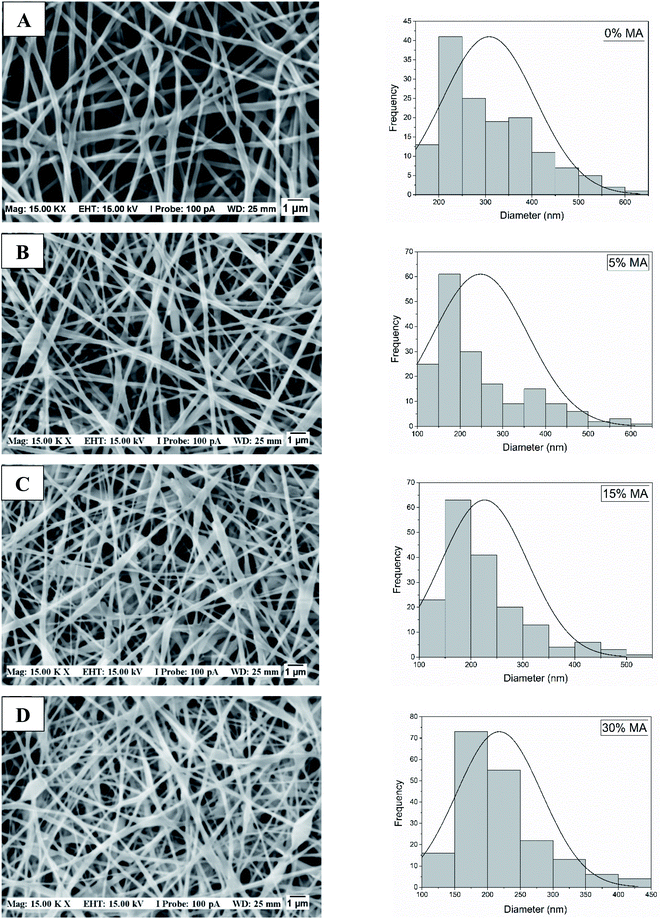 | ||
| Fig. 2 SEM images of PVA/HA membranes with different MA concentrations (A) 0%, (B) 5% (C) 15% and (D) 30%. The relative humidity during the process varied between 20 and 30% for all cases. | ||
Séon-Lutz et al. (2019) found similar fiber diameters (348 ± 51 nm) in their study with the addition of EDC and NHS as PVA–HA–HPβCD membrane's crosslinker, however with a modified electrospinning system.2 In another work developed by Abdel-Mohsen et al. (2019) the fiber diameters were relatively smaller for the PVA/hyaluronan–AgNPs (225 nm) system. In this case, two points must be taken into consideration; first, the electrospinning system was modified and the voltage used was 58 kV; second, hyaluronan served only as reducing and stabilizing agent for the formation of silver nanoparticles, which made its concentration low in the composition.17
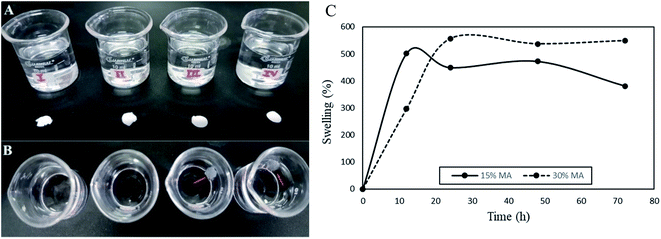
In our work, the membranes were submerged in a PBS 7.4 solution to verify their integrity (Fig. 3a), and as observed the 0 and 5% MA membranes dissolved instantly, while the 15 and 30% remained undissolved after 72 hours (Fig. 3b). Fig. 3c shows the swelling behavior of 15 and 30% MA membranes. Interestingly, in the first 12 hours, 15% MA membrane increased 5 times its initial weight (S% = 501), while 30% MA swelled 296%. However, after 24 hours, 30% MA membrane reached around 550% swelling percentage and maintained it until 72 hours. On the other hand, 15% MA started showing a decrease in the swelling rate, and swelled 381% at 72 hours, suggesting weight loss. Therefore, the degree of cross-linking plays an important role in the membranes' water uptake kinetics and stability.
This is an important result for the application of membranes to prolonged healing wounds. As observed in the SEM micrographs of Fig. 4, the fibers maintained their integrity; however, there were some points of coalescence and fibers swelling, especially after 72 hours. Séon-Lutz et al. (2019) found similar results after 48 h, but in their work, the cross-linking was activated by temperature in the presence of EDC and NHS.2
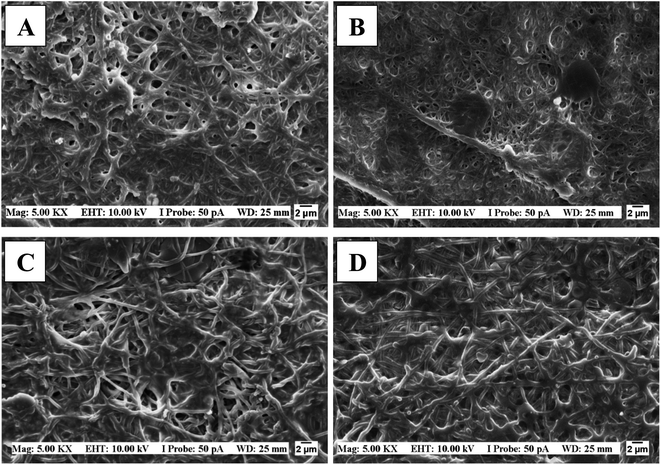 | ||
| Fig. 4 SEM images of PVA/HA membranes: after 48 hours of immersion (A) 15% MA, (B) 30% MA. After 72 hours of immersion (C) 15% MA and (D) 30% MA. | ||
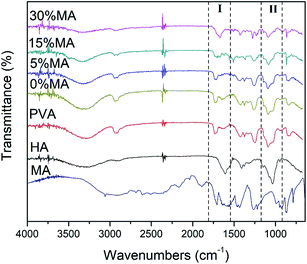
FTIR analysis (Fig. 5) was important to confirm the cross-linking reaction between PVA and HA chains and the effects of crosslinker concentration, by following the functional groups and the bonds present in the sample.
The spectrum revealed the main bands related to PVA and HA, as expected, since they are the major components of the membranes. The broadband around 3200–3400 cm−1 may be related to the lengthening of the –OH bond and also to the lengthening of the –NH, as the cross-linking process increases with the addition of higher concentrations of MA, the intensity of this band decreases.2,18 The weak band close to 2900 cm−1 attributed to the stretching of the –CH vibration, also changes during the cross-linking process. Characteristic bands between 1600 and 1700 cm−1 are related to bond stretching of amide carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and carboxylic acid (–C
O) and carboxylic acid (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups of the HA.19,20 Some characteristic bands near 1434 and 1089 cm−1 for HA are due to amide-type –N–H bending and –C–O–C– bending vibrations, respectively2 and the peak near 1420 cm−1 correspond to the stretching of carboxyl bonds.21,22 All spectra show PVA characteristic peaks near 2945 cm−1, 1734 cm−1, and 1250 cm−1, relative to alkyl, acetyl, and –C–O groups, respectively.23,24 The cross-linking was evidenced by the peak at 1700 cm−1, which is a clear indication of the existence of the –CO–O– (Fig. 5 – Region I) stretching, most evident in both PVA/HA samples with MA concentrations of 15 and 30%.18,25,26 Another proof of cross-linking was the change in intensity at the peak of 1091 cm−1, which is also relative to the cross-linking between –OH of the PVA structure and –C
O) groups of the HA.19,20 Some characteristic bands near 1434 and 1089 cm−1 for HA are due to amide-type –N–H bending and –C–O–C– bending vibrations, respectively2 and the peak near 1420 cm−1 correspond to the stretching of carboxyl bonds.21,22 All spectra show PVA characteristic peaks near 2945 cm−1, 1734 cm−1, and 1250 cm−1, relative to alkyl, acetyl, and –C–O groups, respectively.23,24 The cross-linking was evidenced by the peak at 1700 cm−1, which is a clear indication of the existence of the –CO–O– (Fig. 5 – Region I) stretching, most evident in both PVA/HA samples with MA concentrations of 15 and 30%.18,25,26 Another proof of cross-linking was the change in intensity at the peak of 1091 cm−1, which is also relative to the cross-linking between –OH of the PVA structure and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from MA molecule, giving rise to the –C–O–C– band (Fig. 5 – Region II).26,27 Another interesting observation was the disappearance of the peak at 1640 cm−1, related to the –C
O from MA molecule, giving rise to the –C–O–C– band (Fig. 5 – Region II).26,27 Another interesting observation was the disappearance of the peak at 1640 cm−1, related to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bond in MA molecule, suggesting that it was also involved in the photocrosslinking reaction.28
C– bond in MA molecule, suggesting that it was also involved in the photocrosslinking reaction.28
These two types of reactions are the possible cross-linking routes that can occur in the studied systems (Fig. 6). To better understand these processes, the PVA/MA, HA/MA, and PVA/HA casting membranes were made separately. The casting membranes were prepared using the same concentrations used in the solutions that were electrospun. Membrane PVA/MA: 5 mL of PVA solution (6% w/v) were mixed by magnetic stirring with MA (30% relative to the mass of PVA) for 1 hour and poured into a Petri dish. Membrane HA/MA: 5 mL of HA solution (2% w/v) were mixed by magnetic stirring with MA (30% relative to the mass of HA) for 1 hour and poured into a Petri dish. Membrane PVA/HA: PVA (6% w/v) and HA (2% w/v) were mixed by magnetic stirring at a ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 for 1 hour and poured into a Petri dish. For all formulations, the process was performed under ambient conditions, without the addition of heat or UV light. It was verified that at room temperature the esterification reaction does not occur because the membranes were dissolved in PBS in less than 30 minutes. The same assay was performed with UV radiation and 5% of Irgacure 2959 was added concerning the mass of the polymer in solution. The solutions were then poured into the Petri dish and subjected to UV light for 40 minutes. As a result, the esterification reaction was efficient only for PVA.
25 for 1 hour and poured into a Petri dish. For all formulations, the process was performed under ambient conditions, without the addition of heat or UV light. It was verified that at room temperature the esterification reaction does not occur because the membranes were dissolved in PBS in less than 30 minutes. The same assay was performed with UV radiation and 5% of Irgacure 2959 was added concerning the mass of the polymer in solution. The solutions were then poured into the Petri dish and subjected to UV light for 40 minutes. As a result, the esterification reaction was efficient only for PVA.
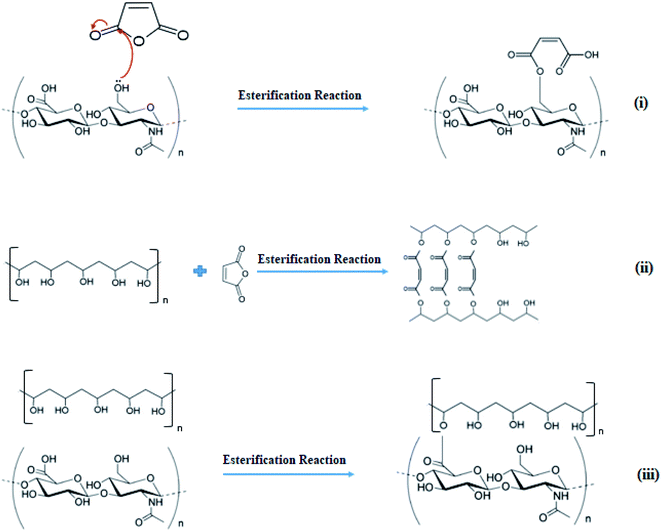 | ||
| Fig. 6 Schematic representation of possible esterification reactions between (i) HA and MA, (ii) PVA and MA, (iii) PVA and HA. | ||
Other aspects that may have contributed to the cross-linking stage were presented by Yang et al. (2008), in their studies, they showed that PVA and MA were efficiently cross-linked during the electrospinning process, without the need of heat treatment or UV radiation. PVA and MA reacted to form mono-esters or bis-esters during electrospinning, where the high electric field can stimulate the chemical activity of the molecules and cause the rapid evaporation of water, which can accelerate the esterification reaction and, as a consequence, increase the equilibrium constant.29 It is noteworthy that the esterification reaction between (ii) PVA and MA is more likely to occur than the esterification reaction between (i) HA and MA or (iii) PVA and HA since the reaction conditions are less favorable to (i) and (iii) than for (ii) (Fig. 6).
The hyaluronic acid cross-linking reaction usually occurs only after HA modification or if the conditions of the medium are favorable, such as the addition of an acidic catalyst together with an activating agent, or in the presence of acid anhydrides in basic medium,30,31 which is not the case of the system studied in this paper.
Although the cross-linking reaction by Fig. 6(ii) occurs in the presence of the electric field, photocrosslinking was carried out to ensure a more effective cross-linking, since we have PVA and HA functional groups competing for the possible active reaction sites.
The HA/PVA membranes did not show direct cytotoxicity against HaCaT cells (Fig. 7). Indeed, they stimulated cell growth in comparison to the negative control of cytotoxicity (no treatment). Comparing the prepared membranes, the one without the cross-linking agent (MA 0%) showed the best cell viability result (290%), while membranes with MA concentrations of 15 and 30% showed similar cell viability (∼225%). The membrane with 5% crosslinker showed less cell viability (120%), which was already expected since the cross-linking reaction was incomplete in this formulation (Fig. 3), as already discussed in the solubility test and FTIR analysis. It is known that unbound chemicals, such as crosslinker agents can be toxic to cells or prevent cell growth.
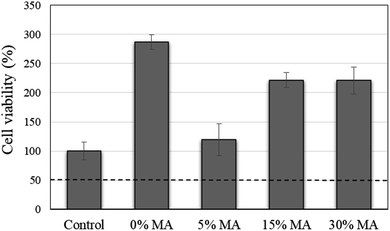 | ||
| Fig. 7 Evaluation of cell viability of HaCaT cells after being cultured with the electrospun membranes for a period of 24 h. | ||
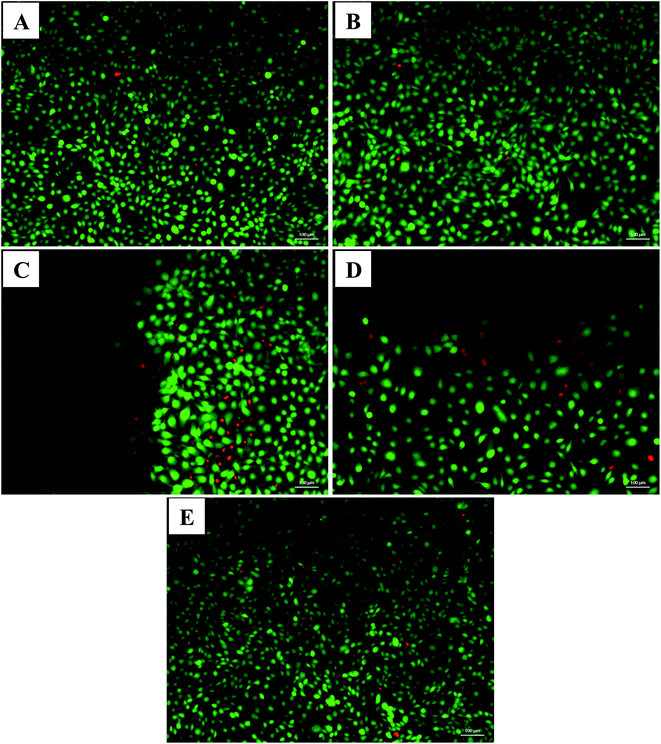
Live/dead assay was performed to analyze the cell survival at the surface of the membranes, by simultaneously staining live (green-labeled) and dead (red-labeled) cells. The images (Fig. 8) show that cells remain viable when in contact with membranes. Using the Fiji supplement from ImageJ® software, it was possible to calculate the percentage of live and dead cells from each sample (Table 3). These results corroborate the data obtained in the MTT assay, thus confirming the biocompatibility of these membranes. As the degree of cross-linking increases, the number of viable cells decreases, this may be related to the fact that the cross-linked membrane decreases water retention and HA, which is the protein present in the skin's ECM and with excellent biological properties, not be free to stimulate migration and cell adhesion.14
| 0% MA | 5% MA | 15% MA | 30% MA | |
|---|---|---|---|---|
| Live cells (%) | 99.5 | 97.1 | 92.4 | 83.9 |
| Dead cells (%) | 0.5 | 2.9 | 7.6 | 16.1 |
The fact that the membranes have been successfully cross-linked and have demonstrated no cytotoxicity against human keratinocytes is an excellent indication for this material to be used as a potential carrier for sustained drug release.
4. Conclusion
The electrospinning of PVA/HA membranes was successful and the addition of a cross-linking agent caused an effective improvement in the rheological properties of the solution and improved the process conditions. This was extremely important especially in increasing the ejection rate of the polymer solution from 0.3 mL L−1 to 1.0 mL h−1, which is related to energy-saving and membrane production time. It was possible to obtain homogeneous nanofibers without the presence of beads, using only water as a solvent. Complete cross-linking reaction occurred with the addition of 15 and 30% crosslinker, meaning that the range of crosslinker concentration between 15 and 30% is interesting to investigate, focusing the application on controlled release modulated devices with different duration times before degrading. In addition, the membranes showed high cell viability, indicating that their use in vivo may be promising.Conflict of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors thank CNPq (140567/2019-4) and CAPES for financial support. They also thank the Faculty of Chemical Engineering at the University of Campinas.References
- L. J. Villarreal-Gómez, J. M. Cornejo-Bravo, R. Vera-Graziano and D. Grande, J. Biomater. Sci., Polym. Ed., 2016, 27, 157–176 CrossRef PubMed.
- M. Séon-Lutz, A.-C. Couffin, S. Vignoud, G. Schlatter and A. Hébraud, Carbohydr. Polym., 2019, 207, 276–287 CrossRef PubMed.
- J. Jalvandi, M. White, Y. Gao, Y. B. Truong, R. Padhye and I. L. Kyratzis, Mater. Sci. Eng., C, 2017, 73, 440–446 CrossRef CAS PubMed.
- B. Jankovic, J. Pelipenko, M. Skarabot, I. Musevic and J. Kristl, Int. J. Pharm., 2013, 455, 338–347 CrossRef CAS PubMed.
- S. Babitha, L. Rachita, K. Karthikeyan, E. Shoba, I. Janani, B. Poornima and K. Purna Sai, Int. J. Pharm., 2017, 523, 52–90 CrossRef CAS PubMed.
- S. Thakkar and M. Misra, Eur. J. Pharm. Sci., 2017, 107, 148–167 CrossRef CAS PubMed.
- N. Bhardwaj and S. C. Kundu, Biotechnol. Adv., 2010, 28, 325–347 CrossRef CAS PubMed.
- J. Li, A. He, C. C. Han, D. Fang, B. S. Hsiao and B. Chu, Macromol. Rapid Commun., 2006, 27, 114–120 CrossRef CAS.
- C. Feng, C. Liu, S. Liu, Z. Wang, K. Yu and X. Zeng, Tissue Eng., Part A, 2019, 25, 1289–1299 CrossRef CAS PubMed.
- G. D. Prestwich, J. Controlled Release, 2011, 155, 193–199 CrossRef CAS PubMed.
- I. C. Um, D. Fang, B. S. Hsiao, A. Okamoto and B. Chu, Biomacromolecules, 2004, 5, 1428–1436 CrossRef CAS PubMed.
- J. A. Quinn, Y. Yang, A. N. Buffington, F. N. Romero and M. D. Green, Polymer, 2018, 134, 275–281 CrossRef CAS.
- X. Wang, I. C. Um, D. Fang, A. Okamoto, B. S. Hsiao and B. Chu, Polymer, 2005, 46, 4853–4867 CrossRef CAS.
- S. P. Miguel, D. Simões, A. F. Moreira, R. S. Sequeira and I. J. Correia, Int. J. Biol. Macromol., 2019, 121, 524–535 CrossRef CAS PubMed.
- L. Vítková, L. Musilová, E. Achbergerová, A. Minařík, P. Smolka, E. Wrzecionko and A. Mráček, Polymers, 2019, 11, 1517 CrossRef PubMed.
- F. Li, Y. Zhao and Y. Song, in Nanofibers, InTech, 2010, p. 438 Search PubMed.
- A. M. Abdel-Mohsen, D. Pavliňák, M. Čileková, P. Lepcio, R. M. Abdel-Rahman and J. Jančář, Int. J. Biol. Macromol., 2019, 139, 730–739 CrossRef CAS PubMed.
- B. Zeytuncu, M. Morcali, S. Akman and O. Yucel, J. Serb. Chem. Soc., 2015, 80, 97–106 CrossRef CAS.
- E. Larrañeta, M. Henry, N. J. Irwin, J. Trotter, A. A. Perminova and R. F. Donnelly, Carbohydr. Polym., 2018, 181, 1194–1205 CrossRef PubMed.
- Y. Zhao, Z. Fan, M. Shen and X. Shi, Adv. Mater. Interfaces, 2015, 2, 1–9 CrossRef PubMed.
- R. Gilli, M. Kacuráková, M. Mathlouthi, L. Navarini and S. Paoletti, Carbohydr. Res., 1994, 263, 315–326 CrossRef CAS PubMed.
- S. A. de Oliveira, B. C. da Silva, I. C. Riegel-Vidotti, A. Urbano, P. C. de Sousa Faria-Tischer and C. A. Tischer, Int. J. Biol. Macromol., 2017, 97, 642–653 CrossRef PubMed.
- S. K. Rai and P. Basak, Int. Conf. Syst. Med. Biol. ICSMB 2010 - Proc., 2010, pp. 360–364 Search PubMed.
- J. M. Gohil, A. Bhattacharya and P. Ray, J. Polym. Res., 2006, 13, 161–169 CrossRef CAS.
- Y. B. Truong, J. Choi, J. Mardel, Y. Gao, S. Maisch, M. Musameh and I. L. Kyratzis, Macromol. Mater. Eng., 2017, 302, 1–9 CrossRef.
- R. F. Majidi, N. Nezafati and M. Pazouki, in 6th International Conference on Nanotechnology (ICN2017), Dubai, 2017, pp. 1–4 Search PubMed.
- M. Pingrui, C. Cuixian, Y. Lixin, L. Jiding and J. Weijun, Tsinghua Sci. Technol., 2000, 5, 172–175 Search PubMed.
- F. J. Liou and Y. J. Wang, J. Appl. Polym. Sci., 1996, 59, 1395–1403 CrossRef CAS.
- E. Yang, X. Qin and S. Wang, Mater. Lett., 2008, 62, 3555–3557 CrossRef CAS.
- R. Buffa, V.Ϋ Velebn, L. Pospisilova, E. Prikopova, M. Pravda, P. Nikodym and L. Palek, WO 2010/105582Al, 2010, pp. 1–18.
- A. V. Sochilina, A. G. Savelyev, P. A. Demina, N. V. Ierusalimsky, D. A. Khochenkov, R. A. Akasov, N. V. Sholina, E. V. Khaydukov and A. N. Generalova, J. Phys.: Conf. Ser., 2018, 1124, 1–6 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra04950f |
| This journal is © The Royal Society of Chemistry 2020 |