Open Access Article
Open Access ArticleCu(II) immobilized on Fe3O4@HNTs–tetrazole (CFHT) nanocomposite: synthesis, characterization, investigation of its catalytic role for the 1,3 dipolar cycloaddition reaction, and antibacterial activity†
Zoleikha Hajizadeh,
Fereshte Hassanzadeh-Afruzi,
Diana Fallah Jelodar,
Mohammad Reza Ahghari and
Ali Maleki *
*
Catalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir; Fax: +98-21-73021584; Tel: +98-21-73228313
First published on 15th July 2020
Abstract
In the present study, Cu(II) immobilized on an Fe3O4@HNTs–tetrazole (CFHT) nanocomposite was designed and prepared. For this, halloysite nanotubes (HNTs) as natural mesoporous substances were modified during several chemical reactions. The synthesis of the CFHT nanocomposite was investigated step by step with the required physicochemical techniques such as FT-IR, EDX, SEM, TEM, XRD, VSM, TGA and CHNS analyses. After ensuring that the nanocomposite was successfully prepared, its catalytic application in the synthesis of the 5-substituted 1H-tetrazole derivatives via multicomponent reactions (MCRs) between aromatic aldehydes, malononitrile, and sodium azide was assessed. According to the experimental results, the prepared nanocomposite exhibited excellent capability for conducting this MCR reaction. All desired products were obtained in a short reaction time (30–40 min) with high productivity (90–97%) and without a complicated workup procedure. Furthermore, the magnetic property of the synthesized heterogeneous nanocomposite empowers it to be recovered and reused in five times successive reactions without any significant reduction in reaction efficiency. Moreover, the remarkable antibacterial activity of the nanocomposite against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) was evaluated by agar diffusion and plate-count methods. The zones of inhibition were around 16 and 20 mm for E. coli and S. aureus bacteria, respectively. Also, colony analysis confirms the killing of bacteria by using the CFHT nanocomposite.
1. Introduction
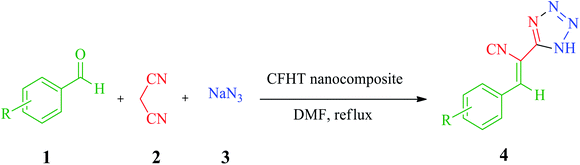
Nanotubes have been a novel and fast-growing field of nanotechnology in recent research. The properties of organic or inorganic nanotubes can be altered by functionalizing the surface of these materials by covalent or non-covalent interactions.1 Halloysite is a natural aluminosilicate clay with a nanotube structure that possesses positive and negative charges on the inner and outer surfaces. The presence of negative and positive charges is associated with the existence of silicate and alumina groups in this material.2 HNTs are known as mesoporous compounds with an average outer diameter and an inner diameter of about 50 nm and 12 nm, respectively. Under acidic, alkaline or thermal conditions, the size of the porous of HNTs could be altered.3 HNTs, with interesting features such as biocompatibility, chemical modification, high water uptake, and affordability are widely employed in diverse fields such as biomedical, tissue engineering, absorbents, and cosmetics.4–7 The existence of a great deal of hydroxyls as active functional groups on the inner and outer walls of HNTs permits them to be chemically modified. In recent years, the modification of HNTs by magnetic nanoparticles has been frequently reported in the scientific literature.8–10 The modified nanocomposites have been utilized in some areas such as reduction of nitrophenol, dyes removal.11,12 The metals-containing nanocatalysts with their applicable properties have been mostly employed to assist diverse chemical reactions, such as oxidation and reduction reactions, coupling, cross-coupling, multicomponent reactions, click and cycloaddition reactions under the convenient and benign procedure. Copper is one of those intermediate metals that has gained much attention with appreciated features such as availability, biocompatibility, affordable price, catalytic efficiency for some reactions and its outstanding antimicrobial activity against various bacterial strains.11 Several hybrid composites have recently been fabricated by immobilizing copper on the organic, inorganic or hybrid compounds and then utilized as efficient catalytic or antibacterial systems.12–16 MCRs are one-pot methods in which simple initial materials incorporated into the direct manufacture of complex structures.17,18 Atomic economy, high efficiency, convergence, high bond-forming index are such outstanding features of MCRs. Therefore, the synthesis of many complicated heterocycles compound, intricate pharmaceutical molecules, and natural products are accomplished using these kinds of reactions.19–21 Tetrazoles and their derivatives are an important member of N-containing heterocycles family which potential application in coordination chemistry, material science, organometallic chemistry, pharmacology, imaging materials, and military.22 The close acidity of this functional group and the carboxylic group resulted in their application as potential pharmaceutical material and the preparation of some compounds such as losartan, irbesartan, tomelukast, and PTZ (2). 5-Substituted tetrazoles also possess biological and pharmacological activities, such as antimicrobial, antidiabetic, antiviral and antihypertensive and have potential therapeutic effects against cerebral ischemia and schizophrenia. They are potential antibacterial and antifungal activities and have shown variable growth inhibitory effects on Gram-positive and Gram-negative bacteria. Some researches focused on the evaluation of these compounds suitability for antibiotics and their cytotoxicity.23–25 The synthesis of tetrazoles has been developed by various methods and diverse catalysts due to the importance of these compounds as one of the main constituents of organic compounds and their biological and pharmaceutical properties. Although most of the reported methods offered advantages, there are still some issues such as unapproachability or toxicity of reagents, non-recyclable catalysts, unproductive efficiency and long reaction times.In this study, CFHT nanocomposite was introduced as an effectiveness heterogeneous nanocatalyst in connection with our recent research on the design and development of natural material based hybrid catalysts. This heterogeneous nanocatalyst was designed, prepared by several step chemical reactions, characterized successfully and its catalytic application was assessed for speeding up the synthesis of 5-substituted 1H-tetrazoles derivatives. The CFHT nanocomposite preparation pathway and production of 5-substituted 1H-tetrazoles derivatives via MCRs between aromatic aldehydes (1), malononitrile (2) and sodium azide (3) have been schematically illustrated in Fig. 1.
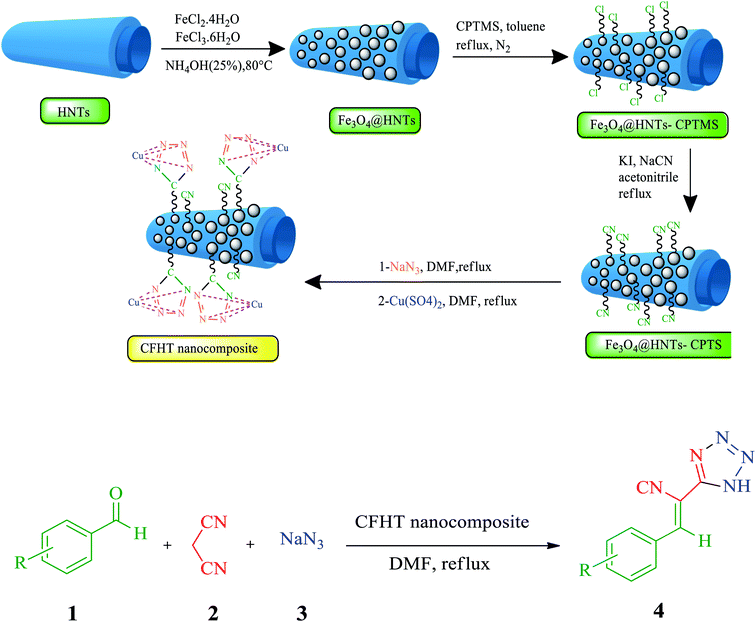 | ||
| Fig. 1 The preparation pathway of the CFHT nanocomposite and production of 5-substituted 1H-tetrazoles derivatives. | ||
2. Result and discussion
The catalyst synthesis process involves several steps as follows:(1) Preparation of the Fe3O4@HNTs through simple chemical precipitation of HNTs and two kinds of iron chloride salts in alkaline condition.
(2) Surface modification of the Fe3O4@HNTs with (3-chloropropyl)triethoxysilane (CPTMS) to prepare Fe3O4@HNTs–CPTMS.
(3) The reaction of the Fe3O4@HNTs–CPTMS with NaCN to produce the Fe3O4@HNTs–CPTS nanocomposite.
(4) The preparation of Fe3O4@HNTs–tetrazole through the 1,3 dipolar cycloaddition reaction between the Fe3O4@HNTs–CPTS and NaN3.
(5) And the final step was immobilization of Cu(II) onto the Fe3O4@HNTs–tetrazole by using an aqueous solution of Cu(SO4)2 to obtain CFHT nanocomposite.
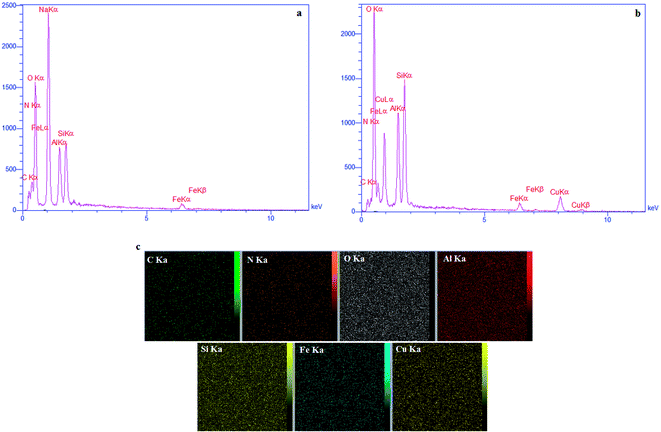
2.1. Characterization of the CFHT nanocomposite
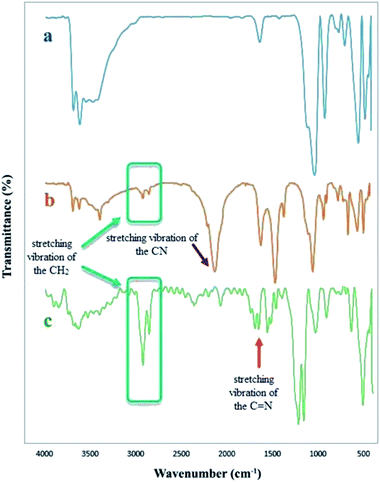
Various physicochemical identification analyses have employed to study the structural and distinctive characteristics of the synthesized catalysts (CFHT nanocomposite), and the results are delicately discussed.As expected, several new bands become apparent at 2117, 2850 and 2916 cm−1 that were corresponded to the stretching vibrations of the CN functional group and aliphatic CH2 bond, respectively, approval of surfaces chemical modification of the Fe3O4@HNTs with the (3-cyanopropyl)triethoxysilane (CPTS). The FT-IR of CFHT nanocomposite was observed in the spectrum (c). This nanocomposite was the result of two steps reaction of the Fe3O4@HNTs–CPTS. The first one was the click reaction with sodium azide to create Fe3O4@HNTs–tetrazole composite and the next one was the coordination of Cu(II) onto the tetrazole ring of Fe3O4@HNTs–tetrazole. What was expected to be observed is that the strong absorption at 2117 cm−1 that existed in the spectrum (b) (signifying the presence of the nitrile group) must be either eliminated or reduced in intensity. As seen in the spectrum (c), that absorption was disappeared. In addition, a new absorption band appeared in 1652 cm−1 is related to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in the tetrazole ring of the CFHT nanocomposite approving the construction of this nanocomposite.
N bond in the tetrazole ring of the CFHT nanocomposite approving the construction of this nanocomposite.
| Sample | % C | % H | % N |
|---|---|---|---|
| Fe3O4@HNTs–CPTS | 3.09 | 1.53 | 2.35 |
| Fe3O4@HNTs–tetrazole | 6.29 | 1.54 | 16.69 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to +10
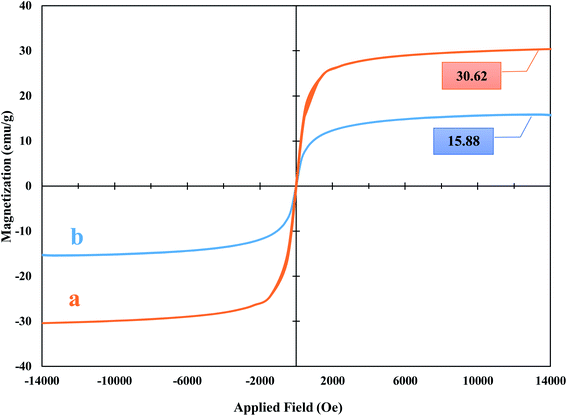
000 to +10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 oersted, the observed saturation magnetic of the CFHT nanocomposite was approximately 15.88 emu g−1, which decreased compared to the Fe3O4@HNTs with 30.62 emu g−1 under similar conditions. Since the Fe3O4@HNTs has been modified by several chemical reactions using non-magnetic materials, this decline seems reasonable. The catalyst is sufficiently magnetized to be collected from the reaction mixture by a magnet. But what is the point of a superparamagnetic heterogeneous catalyst being prepared? The striking benefits of utilizing magnetic heterogeneous catalysts in organic reactions include providing a high specific surface area, the ability to be easily separated from the reaction medium and their repeated usability, which is fundamental in terms of time, cost and energy savings at the industrial scale.
000 oersted, the observed saturation magnetic of the CFHT nanocomposite was approximately 15.88 emu g−1, which decreased compared to the Fe3O4@HNTs with 30.62 emu g−1 under similar conditions. Since the Fe3O4@HNTs has been modified by several chemical reactions using non-magnetic materials, this decline seems reasonable. The catalyst is sufficiently magnetized to be collected from the reaction mixture by a magnet. But what is the point of a superparamagnetic heterogeneous catalyst being prepared? The striking benefits of utilizing magnetic heterogeneous catalysts in organic reactions include providing a high specific surface area, the ability to be easily separated from the reaction medium and their repeated usability, which is fundamental in terms of time, cost and energy savings at the industrial scale.
2.2. Catalytic application of the CFHT nanocomposite in organic synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, various solvents, temperature, catalytic quantity were tested. Experimental information on the optimization process has been provided in Table 2. At first, the reaction was carried out under various conditions without catalyst, the data showed that the reaction efficiency was negligible after 100 minutes in all of these experiments. The details of these experiments are presented in Table 2 (data 1 to 2). Therefore, the catalyst has a crucial effect to conduct and boost this reaction. The reaction was then carried out in ethanol and DMF in the presence of different quantities of catalyst. The results showed that 30 mg of catalyst was adequate to conduct the reaction, lower values resulted in reduced efficiency of the reaction. By increasing the catalyst content to 50 mg, not only does not the reaction efficiency improve, but it also decreased slightly. The existence of 30 mg CFHT catalyst in DMF under refluxing conditions was the best condition reaction for the synthesis of the 5-substituted 1H-tetrazoles derivatives by MCRs.
1.2, various solvents, temperature, catalytic quantity were tested. Experimental information on the optimization process has been provided in Table 2. At first, the reaction was carried out under various conditions without catalyst, the data showed that the reaction efficiency was negligible after 100 minutes in all of these experiments. The details of these experiments are presented in Table 2 (data 1 to 2). Therefore, the catalyst has a crucial effect to conduct and boost this reaction. The reaction was then carried out in ethanol and DMF in the presence of different quantities of catalyst. The results showed that 30 mg of catalyst was adequate to conduct the reaction, lower values resulted in reduced efficiency of the reaction. By increasing the catalyst content to 50 mg, not only does not the reaction efficiency improve, but it also decreased slightly. The existence of 30 mg CFHT catalyst in DMF under refluxing conditions was the best condition reaction for the synthesis of the 5-substituted 1H-tetrazoles derivatives by MCRs.
| Entry | Solvent | Amount of catalyst (mg) | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aromatic aldehyde (1 mmol), malononitrile (1 mmol) and sodium azide (1.2 mmol), the CFHT nanocatalyst (10–50 mg).b The yields relate to the isolated product. | |||||
| 1 | Solvent-free | — | r.t. | 100 | Trace |
| 2 | Water | — | r.t. | 100 | Trace |
| 3 | Solvent-free | — | Reflux | 100 | Trace |
| 4 | Ethanol | — | r.t. | 100 | Trace |
| 5 | Ethanol | — | Reflux | 100 | 20 |
| 6 | Ethanol | 30 | Reflux | 40 | 38 |
| 7 | DMF | 10 | Reflux | 40 | 56 |
| 8 | DMF | 20 | Reflux | 40 | 72 |
| 9 | DMF | 30 | Reflux | 40 | 97 |
| 10 | DMF | 50 | Reflux | 40 | 89 |
Different substituted benzaldehydes with electron drawings and electron-donating groups were tested to evaluate the generality and limitation of this method and some 5-substituted 1H-tetrazoles derivatives have made by the Knoevenagel condensation reaction and the 1,3 dipolar cycloaddition reaction of aldehydes (1), malononitrile (2) and sodium azide (3). The nature of substitution on the aromatic aldehydes had no significant difference in the productivity of the related products or the reaction time. Both types of benzaldehydes possessing either electron-withdrawing or electron-donating substitutions in the reaction with malononitrile and sodium azide, made desired products with 90–97% efficiency just after 30–40 min (Table 3).
| Entry | R | Product | Time (min) | Yielda (%) | Mp (°C) | |
|---|---|---|---|---|---|---|
| Observed | Literature | |||||
| a The yields relate to the isolated product. | ||||||
| 1 | H | 4a | 40 | 97 | 170–171 | 168–170 (ref. 27) |
| 2 | 4-NO2 | 4b | 30 | 93 | 167–169 | 166–168 (ref. 27) |
| 3 | 4-Br | 4c | 40 | 90 | 166–168 | 165–167 (ref. 27) |
| 4 | 4-OH | 4d | 40 | 92 | 160–162 | 159–161 (ref. 28) |
| 5 | 4-F | 4e | 30 | 94 | 178–179 | 176–179 (ref. 29) |
| 6 | 4-OMe | 4f | 40 | 90 | 153–154 | 153–155 (ref. 30) |
| 7 | 4-Me | 4g | 40 | 91 | 190–192 | 189–191 (ref. 30) |
| 8 | 3-Me | 4h | 40 | 95 | 132–134 | 130–132 (ref. 30) |
| 9 | 4-Cl | 4i | 30 | 92 | 164–166 | 165–168 (ref. 29) |
2.3. Recyclability of the CFHT nanocomposite
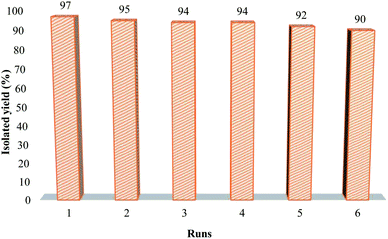
The superparamagnetic behavior was one of the beneficial features of the constructed nanocomposite which verified by characterization analyzes. This feature, in addition to being a facilitator in the laboratory work due to its simple separation from the reaction mixture, is also important at the industrial application. Furthermore, conducting chemical reactions using retrievable and reusable catalysts is a substantial issue in terms of conserving the environment. In this work, the capacity of the catalyst to be re-used was investigated, in which the catalyst was isolated using magnet after finishing the reaction, washed several times with acetone and dried at room temperature. Then it was reused for five sequential runs in the synthesis of 4a derivative. As presented in Fig. 9, the reaction efficiency reduced from 97% to 90% after five times of catalyst reuse.2.4. A mechanism for the synthesis of the 5-substituted 1H-tetrazoles derivatives
The suggested mechanism of the synthesis of 5-substituted 1H-tetrazoles derivatives is illustrated in Fig. 10. First, the nitrile group of malononitrile and the carbonyl group of aromatic aldehyde were activated by the CFHT nanocatalyst, the Knoevenagel condensation reaction between activated malononitrile and aromatic aldehyde resulted in the formation of arylidenemalononitrile (I). Subsequently, by the interaction of Cu from the CFHT nanocatalyst with the N of molecule (I), accelerated [3 + 2] cycloaddition reaction between arylidenemalononitrile and sodium azide. Afterward, the catalyst was isolated from a complex (II) using acidic conditions workup (aqueous solution of HCl) and the molecule (III) was made. Eventually, through the tautomerization of molecule of (III), the 5-substituted 1H-tetrazoles (4a–h) as a more stable tautomer was accomplished.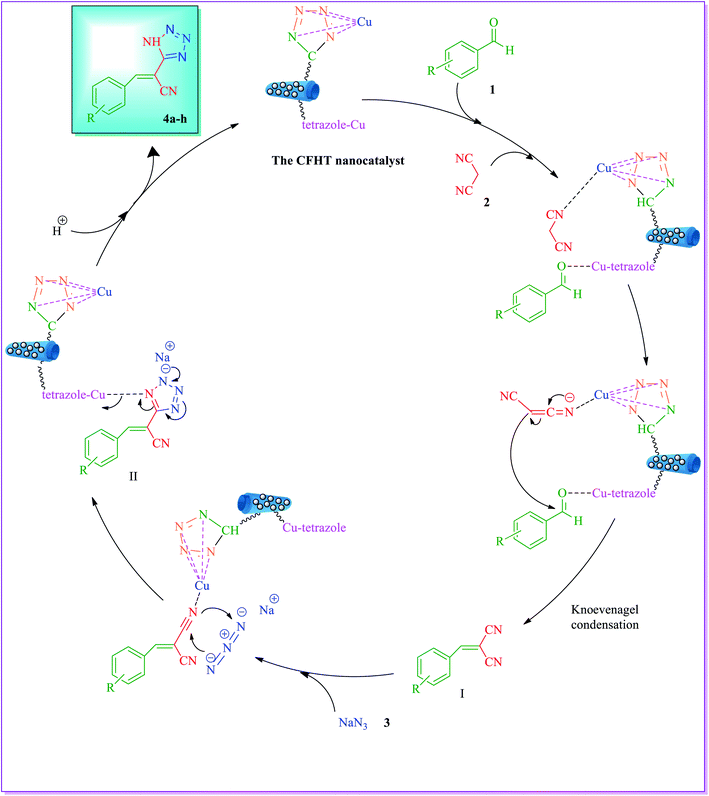 | ||
| Fig. 10 Suggested mechanism for the synthesis of 5-substituted 1H-tetrazoles derivatives (4a–h) in the presence of the CFHT nanocatalyst. | ||
2.5. Antibacterial activity of the CFHT nanocomposite
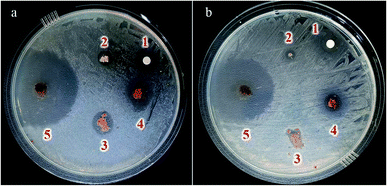 | ||
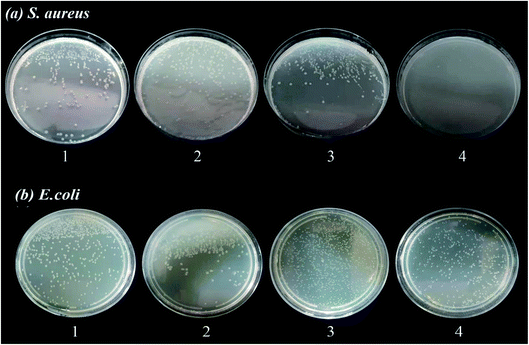
| Fig. 11 Inhibition zones of (1) Cu(II), (2) 1H-tetrazoles, (3) Fe3O4@HNTs–CPTS, (4) Fe3O4@HNTs–tetrazole and (5) CFHT nanocomposite against (a) S. aureus and (b) E. coli bacteria for 24 h. | ||
| Sample | Inhibition zone (diameter), mm | Inhibition zone (diameter), mm |
|---|---|---|
| S. aureus | E. coli | |
| Cu(II) | 3 | 4 |
| 5-Substituted 1H-tetrazoles | 2 | 2 |
| Fe3O4@HNTs–CPTS | 4 | 3 |
| Fe3O4@HNTs–tetrazole | 8 | 7 |
| CFHT nanocomposite | 20 | 16 |
3. Experimental
3.1. General
All consumed chemicals, reagents and solvents were bought from Sigma Aldrich and Merck companies. Monitoring the progress of catalytic reactions was done by thin-layer chromatography (TLC). Melting points of all synthesized derivatives were measured with an Electrothermal 9100 apparatus. FT-IR spectra were recorded on a Shimadzu IR-470 spectrometer in the range of 400 to 4000 cm−1 by KBr pellets. 1H and 13C NMR spectra were recorded on a Bruker DRX-500 spectrometer at 500 and 125 MHz, respectively. Elemental analysis of prepared samples was performed by EDX analysis recorded on Numerix JEOL-JDX 8030 (30 kV, 20 mA). The XRD pattern of the fabricated composites were obtained by Bruker D8 Advance X-ray diffractometer. The morphology and structure of the nanocatalyst were studied by SEM, VEGA2 TESCAN instrument. TEM image was achieved by a Philips CM120 instrument. The magnetic properties of the prepared samples were identified at room temperature using VSM analysis, accomplished by LBKFB model-magnetic Kashan Kavir, thermogravimetric analysis (TGA) was performed using a Bahr-STA 504 instrument, and Eager 300 for EA1112 was used for CHNS analysis.3.2. Catalyst synthesis pathway
As mentioned, the synthesis of CFHT nanocomposite was performed by some following steps.3.3. A typical procedure for the preparation of the 5-substituted 1H-tetrazoles derivatives
CFHT nanocatalyst (0.03 g), aromatic aldehyde (1.00 mmol), malononitrile (1.00 mmol), and NaN3 (1.20 mmol) were mixed and stirred in 5 mL DMF, then the mixture was refluxed. The reaction development was checked by TLC (n-hexane–ethyl acetate, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), after completion of the reaction, the reaction mixture was filtered. Then, to get the precipitate of the product, a 20 mL aqueous solution of HCl (2 M) was poured to the filtrate under extreme stirring. The obtained precipitate was again filtered and dried in an oven to provide the 5-substituted 1H-tetrazoles derivatives. Spectral and analytical analyses structures of some synthesized products confirmed their structures.
1), after completion of the reaction, the reaction mixture was filtered. Then, to get the precipitate of the product, a 20 mL aqueous solution of HCl (2 M) was poured to the filtrate under extreme stirring. The obtained precipitate was again filtered and dried in an oven to provide the 5-substituted 1H-tetrazoles derivatives. Spectral and analytical analyses structures of some synthesized products confirmed their structures.
3.4. Procedure for antibacterial studies
Evaluation of the antibacterial activity of the prepared samples was carried out through using the standard agar diffusion and the colony counter methods against E. coli as a Gram-negative (ATCC 9637) and S. aureus as a Gram-positive (ATCC 12600) bacteria. All the instruments and glassware were sterilized at 121 °C for 10 min in an autoclave before each examination. Agar was employed as a base medium and a solid growth medium plus nutrients microorganisms. Firstly, for disk diffusion tests, the suspensions with 0.5 McFarland turbidity of S. aureus and E. coli were prepared. Then the 0.01 g of samples (Fe3O4@HNTs–CPTS, Fe3O4@HNTs–tetrazole, and CFHT nanocomposite), 0.001 g of 1H-tetrazole and Cu(II) filter paper disc were added to Muller-Hinton agar culture medium containing bacteria, separately. The results of their antibacterial properties were checked by keeping the Petri dishes at 37 °C in an incubator for 24 h. To assess the relative antibacterial effects of the nanocomposite against two kinds of bacteria, the inhibition zones around the disk were measured. Next, for the colony counter method, at first S. aureus and E. coli 0.5 McFarland turbidity standard was added to 0.2 mL DMSO and a 0.1 g of the samples. The samples were stirred for 1 h and the following, 0.01 mL of the solution was added to Mueller Hinton agar media. The dishes were kept at 37 °C for 24 h for growing bacteria and form different colonies.4. Conclusion
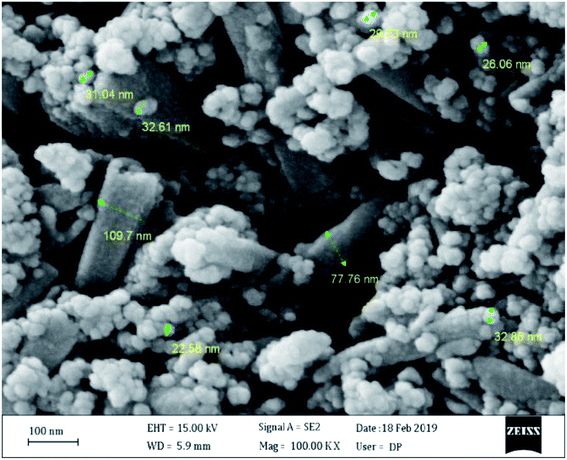
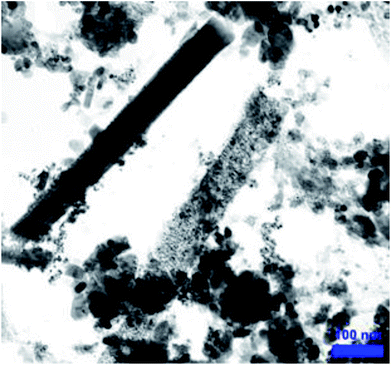
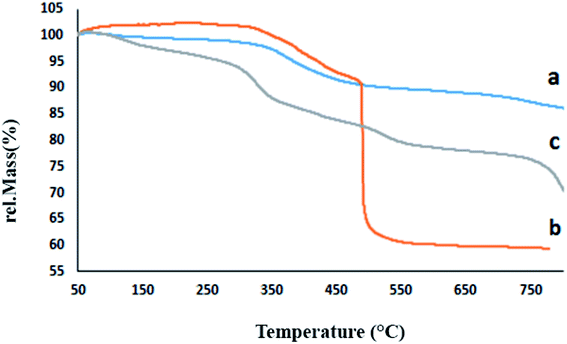
The CFHT nanocomposite was prepared based on HNTs as a naturally occurring mesoporous material, inexpensive and readily available salts and reagents through several chemical reactions. The conventional analyses identified the nanocomposites and confirmed the fabrication of the CFHT nanocomposite. The VSM analysis shows that the magnetic saturation of the final nanocomposite is adequate to be easily separated from the reaction medium which can be beneficial to save time, cost and energy at the industrial scale. Thermogravimetric study investigations have revealed that although by the formation of tetrazole on the surface of the magnetic HNTs, the thermal resistance decreases, this is well compensated by the coordination of the Cu(II) on the tetrazole rings. The CFHT nanocomposite retains about 70% of its weight up to 800 °C. The SEM and TEM images of this nanocomposite demonstrated that the morphology of the HNTs has not changed over some modification reactions to generate the desired functional groups. Evaluation of catalytic performance of the prepared nanocomposite in the synthesis of 5-substituted 1H-tetrazoles derivatives showed that it was an effectives catalyst for obtaining desired products with high yield (90–97%) in short reaction time (30–40 min). According to the presented suggested mechanism, the prepared nanocomposite played a vital role in both the Knoevenagel condensation and [3 + 2] cycloaddition reaction. Furthermore, the zone of inhibition around 16 and 20 mm for E. coli and S. aureus bacteria revealed the considerable antibacterial efficacy of CFHT nanocomposite.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
All authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology (IUST).References
- S. Barrientos-Ramírez, G. M. de Oca-Ramírez, E. Ramos-Fernández, A. Sepúlveda-Escribano, M. Pastor-Blas and A. González-Montiel, Appl. Catal., A, 2011, 406, 22–33 CrossRef.
- S. Hillier, R. Brydson, E. Delbos, T. Fraser, N. Gray, H. Pendlowski, I. Phillips, J. Robertson and I. Wilson, Clay Miner., 2016, 51, 325–350 CrossRef CAS.
- Z. Hajizadeh and A. Maleki, Mol. Catal., 2018, 460, 87–93 CrossRef CAS.
- D. Rawtani and Y. K. Agrawal, Rev. Adv. Mater. Sci., 2012, 30, 282–295 CAS.
- Z. Hajizadeh, K. Valadi, R. Taheri-Ledari and A. Maleki, ChemistrySelect, 2020, 5, 2441–2448 CrossRef CAS.
- Y. M. Lvov, D. G. Shchukin, H. Mohwald and R. R. Price, ACS Nano, 2008, 2, 814–820 CrossRef CAS PubMed.
- R. F. Fakhrullin and Y. M. Lvov, Nanomedicine, 2016, 11, 2243–2246 CrossRef CAS PubMed.
- A. Maleki, Z. Hajizadeh and P. Salehi, Sci. Rep., 2019, 9, 1–8 CrossRef CAS PubMed.
- A. Maleki, Z. Hajizadeh and R. Firouzi-Haji, Microporous Mesoporous Mater., 2018, 259, 46–53 CrossRef CAS.
- A. Maleki and Z. Hajizadeh, Silicon, 2019, 11, 2789–2798 CrossRef CAS.
- D. Marković, C. Deeks, T. Nunney, Ž. Radovanović, M. Radoičić, Z. Šaponjić and M. Radetić, Carbohydr. Polym., 2018, 200, 173–182 CrossRef PubMed.
- A. H. Gemeay, M. E. El-Halwagy, R. G. El-Sharkawy and A. B. Zaki, J. Environ. Chem. Eng., 2017, 5, 2761–2772 CrossRef CAS.
- W. Yuan, C. Zhang, H. Wei, Q. Wang and K. Li, RSC Adv., 2017, 7, 22825–22835 RSC.
- A. Zarnegaryan, M. Moghadam, S. Tangestaninejad, V. Mirkhani and I. Mohammdpoor-Baltork, New J. Chem., 2016, 40, 2280–2286 RSC.
- M. Rezaei, K. Amani and K. Darvishi, Catal. Commun., 2017, 91, 38–42 CrossRef CAS.
- D. Solairaj, P. Rameshthangam, P. Muthukumaran and J. Wilson, Int. J. Biol. Macromol., 2017, 101, 668–679 CrossRef CAS PubMed.
- M. Zhang, Y. H. Liu, Z. R. Shang, H. C. Hu and Z. H. Zhang, Catal. Commun., 2017, 88, 39–44 CrossRef CAS.
- M. A. Maleki, Z. Hajizadeh and H. Abbasi, Carbon Lett., 2018, 27, 42–49 Search PubMed.
- A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef CAS PubMed.
- A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi and M. S. Esmaeili, Mater. Sci. Eng., C, 2020, 109, 110502 CrossRef CAS PubMed.
- A. Maleki, Z. Varzi and F. Hassanzadeh-Afruzi, Polyhedron, 2019, 171, 193–202 CrossRef CAS.
- C. G. Neochoritis, T. Zhao and A. Dömling, Chem. Rev., 2019, 119, 1970–2042 CrossRef CAS PubMed.
- M. Esmaeilpour, A. R. Sardarian and H. Firouzabadi, Appl. Organomet. Chem., 2018, 32, e4300 CrossRef.
- T. Jin, F. Kitahara, S. Kamijo and Y. Yamamoto, Chem.–Asian J., 2008, 3, 1575–1580 CrossRef CAS PubMed.
- N. Ahmed and Z. N. Siddiqui, RSC Adv., 2015, 5, 16707–16717 RSC.
- M. Jafarzadeh, E. Soleimani, P. Norouzi, R. Adnan and H. Sepahvand, J. Fluorine Chem., 2015, 178, 219–224 CrossRef CAS.
- Z. N. Tisseh, M. Dabiri, M. Nobahar, H. R. Khavasi and A. Bazgir, Tetrahedron, 2012, 68, 1769–1773 CrossRef CAS.
- M. Bakherad, R. Doosti, A. Keivanloo, M. Gholizadeh and K. Jadidi, J. Iran. Chem. Soc., 2017, 14, 2591–2597 CrossRef CAS.
- P. Akbarzadeh, N. Koukabi and E. Kolvari, Res. Chem. Intermed., 2019, 45, 1009–1024 CrossRef CAS.
- J. Safaei-Ghomi and S. Paymard-Samani, Chem. Heterocycl. Compd., 2015, 50, 1567–1574 CrossRef CAS.
- A. S. Kritchenkov, A. R. Egorov, I. S. Krytchankou, N. V. Dubashynskaya, O. V. Volkova, T. V. Shakola and Y. A. Skorik, Int. J. Biol. Macromol., 2019, 132, 340–350 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra. See DOI: 10.1039/d0ra04772d |
| This journal is © The Royal Society of Chemistry 2020 |