Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of methoxy- or cyano-substituted thiophene/phenylene co-oligomers for lasing application†
Takumi Matsuo *abc,
Carina Rössigerb,
Jasmin Herrb,
Richard Göttlichb,
Derck Schlettweinc,
Hitoshi Mizunoa,
Fumio Sasakid and
Hisao Yanagia
*abc,
Carina Rössigerb,
Jasmin Herrb,
Richard Göttlichb,
Derck Schlettweinc,
Hitoshi Mizunoa,
Fumio Sasakid and
Hisao Yanagia
aGraduate School of Science and Technology, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan. E-mail: matsuo.takumi.mp2@ms.naist.jp
bInstitute of Organic Chemistry, Justus-Liebig-University Giessen, Heinrich-Buff-Ring 17, 35392 Giessen, Germany
cInstitute of Applied Physics, Justus-Liebig-University Giessen, Heinrich-Buff-Ring 16, 35392 Giessen, Germany
dElectronics and Photonics Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Umezono, Tsukuba, Ibaraki 305-8568, Japan
First published on 23rd June 2020
Abstract
As new candidates of thiophene/phenylene co-oligomer (TPCO) species, 5,5′′-bis(4′-methoxy-[1,1′-biphenyl]-4-yl)-2,2′:5′,2′′-terthiophene (BP3T-OMe) and 4′,4′′′-([2,2′:5′,2′′-terthiophene]-5,5′′-diyl)bis(([1,1′-biphenyl]-4-carbonitrile)) (BP3T-CN) were synthesized for lasing applications. Although most unsubstituted TPCO species crystallize in monoclinic form, BP3T-OMe and BP3T-CN crystallized in orthorhombic and triclinic forms, respectively. Since the unsubstituted species, 5,5′′-bis(4-biphenylyl)-2,2′:5′,2′′-terthiophene (BP3T), shows unique and superior lasing performance in single crystals, the newly synthesized BP3T-OMe and BP3T-CN have possibilities to show different or improved optoelectronic characteristics. Amplified spontaneous emission (ASE) and optically pumped lasing were observed from both of the single crystals based on their well-shaped crystalline cavity and high group refractive index values of 3.7–5.3 for excellent light confinement. The lasing threshold for the BP3T-OMe crystal was lower than that for the BP3T-CN crystal, which was attributed to their different molecular orientation, standing in the former and inclining in the latter.
Introduction
Since the realization of an organic electroluminescence (EL) device composed of an anthracene single crystal reported by M. Pope et al. in 1963,1 organic light emissive π-conjugated compounds have been paid great attention toward practical applications because of their tunability of light emission wavelengths by changing the molecular structure and their good processability. Afterwards, optically pumped gain-narrowed emissions and lasing have been reported for various organic materials such as π-conjugated polymers,2 distyrylbenzene (DSB) derivatives,3 thiophene/phenylene co-oligomers (TPCO)4–10 etc. Recently, carbon-bridged oligo-para-phenylenevinylene (COPV) derivatives were synthesized to realize low threshold lasing and especially high optoelectronic durabilities.11,12 In addition, unique light amplification characteristics based on gain systems using excited state intramolecular proton transfer (ESIPT)13 or Förster resonance energy transfer (FRET)14 have been realized or proposed to construct efficient gain systems. Of these, gain systems using FRET was also reported toward the realization of electrically pumped lasing, by constructing a host-guest energy transfer system from triplet excited state to singlet excited state.14 Beyond optical pumping conditions, electrically pumped gain-narrowed emissions have been recently reported from both organic light emitting diode (OLED) devices15 and organic light emitting field-effect transistor (OLEFET) devices.16Among a large number of organic light emissive π-conjugated compounds, thiophene/phenylene co-oligomers (TPCO) which are composed of linearly chained thiophene and phenylene units, are promising materials as active media for lasing under both electrically and optically pumped conditions owing to their high carrier mobilities, high thermal stabilities and high refractive indices.17–20 Of these, 5,5′′-bis(4-biphenylyl)-2,2′:5′,2′′-terthiophene (BP3T) has been frequently used as an active medium in OLEFET devices, and electrically pumped gain narrowed emissions were observed due to their ambipolar carrier transport characteristics with high carrier mobilities and high internal quantum efficiency.21 Although single crystals of BP3T have been prepared by physical vapor transport (PVT) process, and gain narrowed emissions were successively observed under both optical and electrical pumping, lasing behaviours by self-cavity effect have hardly been investigated due to their curved crystal edges. However, BP3T single crystals show unusual light amplification behaviour, which cannot be explained by the conventional stimulated emission (SE) process. Stimulated resonant Raman scattering (SRRS) was observed from a BP3T single crystal at 12 K,22 which indicates an existence of coherent molecular vibrations. The SRRS has also been observed for other TPCO single crystals even at room temperature with emission lines more narrow than that of ASE even without any cavity structure,22,23 and their light emission wavelength can be tuned by changing the excitation wavelength.
Moreover, the BP3T single crystal showed pulse-shaped light emission with several tens picoseconds delay relative to the excitation pulse at room temperature.24,25 This time-delayed emission in contrast to the SE strongly suggests a cooperative light amplification process similar to superfluorescence or polariton lasing.25 As an origin of the delay time, phase relaxation to form a macroscopic ensemble of correlated emitters was expected among highly oriented molecules. According to literature,26 BP3T crystallizes in monoclinic form, and the molecular long axis is perpendicular to the crystal basal plane. Since the π–π* transition dipole moments are oriented parallel to the molecular long axis, the emitted light propagates in a transverse magnetic (TM) mode, and the light emission is confined strongly inside a platelet single crystal. This condition of light confinement seems favourable for the formation of aforementioned macroscopic ensemble. Although it's still controversial to interpret the relationships between structure – light amplification characteristics, several reports have shown the fact that amplified spontaneous emission (ASE) or lasing characteristics are critically changed by molecular arrangement depending on the π-electronic interactions,6 which is controlled by the modification of constituting molecules in organic crystals.
Here, we report on the synthesis of BP3T derivatives with methoxy- or cyano-groups at the para-positions of the terminating ends for further investigations of the aforementioned unique light amplification characteristics. The introduction of substituents leads to various intermolecular interactions, which result in different crystal structures, molecular packings and molecular orientations.
Results and discussion
Here, we synthesized 5,5′′-bis(4′-methoxy-[1,1′-biphenyl]-4-yl)-2,2′:5′,2′′-terthiophene (BP3T-OMe) and 4′,4′′′-([2,2′:5′,2′′-terthiophene]-5,5′′-diyl)bis(([1,1′-biphenyl]-4-carbonitrile)) (BP3T-CN), following a previously published report.26 XRD measurements revealed crystal structures for both single crystals of BP3T-OMe (CCDC-1991924) and BP3T-CN (CCDC-1991925).† BP3T-OMe crystallized in an orthorhombic form (a = 65.19 Å, b = 7.43 Å, c = 5.80 Å, space group: Cmc21, Z value: 4, cell volume: 2808 Å3). Same as in the case of BP3T, the π-electronic transition dipole moment is oriented parallel to the molecular long axis. As shown in Fig. 1(a)–(c), BP3T-OMe takes perfectly perpendicular molecular orientation to the crystal bottom plane (bc-plane), which is ideal to confine the cavity photon inside a single crystal with in-plane direction. Although the orientation of the molecular long axis of BP3T is also perpendicular to the crystal bottom plane (ab-plane), however, it's a little inclined with a tilting angle of 2.6°.26 Therefore, the orientation of BP3T-OMe seems more favourable to confine photons in a cavity consisting of a single crystal, comparing to the case of BP3T. The origin of the perpendicularly aligned molecular orientation in the BP3T-OMe single crystal can be attributed to the H-bonding interaction between methoxy-groups of adjacent molecules as shown in Fig. 1(d). In a previous report about other methoxy-substituted TPCO compounds, 2,5-bis(4′-methoxybiphenyl-4-yl)thiophene (BP1T-OMe) showed the same perpendicular orientation as a consequence of the hydrogen bonding between the methoxy-groups.On the other hand, BP3T-CN crystallized in a triclinic form (a = 9.49 Å, b = 14.92 Å, c = 19.58 Å, α = 82.73°, β = 87.51°, γ = 89.92°, space group: P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z value: 4, cell volume: 2746 Å3). The molecular axis of BP3T-CN is tilted by about 45° against the bottom plane (ab-plane) as shown in Fig. 2(a) and (b). An origin of the obliquely lying orientation is attributed to the electrostatic interaction between cyano-substituents of neighbouring molecules, as revealed in a previous report for 2,5-bis(4′-cyanobiphenyl-4-yl)thiophene (BP1T-CN).10 The cyano-groups are oppositely oriented at the intermolecular interface, therefore, the C(δ+)–N(δ−) interaction is formed between the adjacent molecular terminals as shown in Fig. 2(d). These lying molecular orientation is suitable for the realization of vertical cavity surface emitting lasers (VCSELs) using distributed Bragg reflector (DBR) mirrors since the tilted transition dipole moments allow sufficient emission components to the crystal surface direction.28,29 Moreover, organic polariton lasers have recently been investigated using VCSEL structures to realize low threshold lasing devices. Actually, gain-narrowed emissions from VCSEL structures were observed using cyano-substituted TPCOs as active media in VCSELs.28,29
, Z value: 4, cell volume: 2746 Å3). The molecular axis of BP3T-CN is tilted by about 45° against the bottom plane (ab-plane) as shown in Fig. 2(a) and (b). An origin of the obliquely lying orientation is attributed to the electrostatic interaction between cyano-substituents of neighbouring molecules, as revealed in a previous report for 2,5-bis(4′-cyanobiphenyl-4-yl)thiophene (BP1T-CN).10 The cyano-groups are oppositely oriented at the intermolecular interface, therefore, the C(δ+)–N(δ−) interaction is formed between the adjacent molecular terminals as shown in Fig. 2(d). These lying molecular orientation is suitable for the realization of vertical cavity surface emitting lasers (VCSELs) using distributed Bragg reflector (DBR) mirrors since the tilted transition dipole moments allow sufficient emission components to the crystal surface direction.28,29 Moreover, organic polariton lasers have recently been investigated using VCSEL structures to realize low threshold lasing devices. Actually, gain-narrowed emissions from VCSEL structures were observed using cyano-substituted TPCOs as active media in VCSELs.28,29
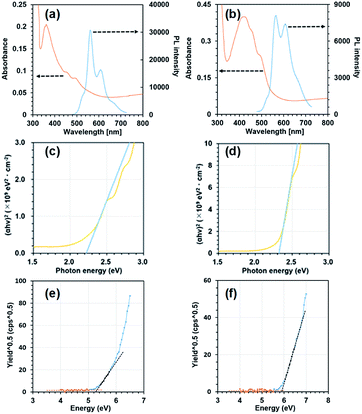
Next, we measured absorption and photoluminescence (PL) spectra using vapour deposited thin films as shown in Fig. 3(a) and (b). Fig. 3(c) and (d) show plots of (αhν)2 as a function of photon energy where α and h and ν represent the absorption coefficient, Plank's constant and the frequency, respectively. The values of α were obtained from the respective absorbance divided by sample thickness. The frontier orbital gap value (Eg) was obtained by extrapolating the linear slope of (αhν)2 to the zero absorption coefficient (α = 0). The obtained Eg values were 2.22 eV and 2.32 eV for BP3T-OMe and BP3T-CN, respectively. Fig. 3(e) and (f) show plots of (photon yield)0.5 as a function of incident photon energy in the measurements of photoelectron spectroscopy in air (PESA). The highest occupied molecular orbital (HOMO) levels estimated from the threshold photon energy in Fig. 3(e) and (f) were −5.31 and −5.91 eV for BP3T-OMe and BP3T-CN, respectively. The lowest unoccupied molecular orbital (LUMO) levels calculated by the HOMO levels and aforementioned frontier orbital gap energy values were −3.09 and −3.59 eV for BP3T-OMe and BP3T-CN, respectively. The HOMO and LUMO levels of BP3T estimated by the same procedures were −5.78 eV and −3.40 eV, respectively. These results show that the methoxy- and cyano-group act as electron donating and electron withdrawing substituents, therefore, p-type and n-type semiconducting characteristics can be expected in BP3T-OMe and BP3T-CN, respectively.
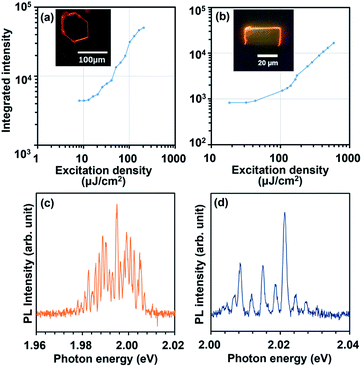
Lasing characteristics of their derivative crystals were investigated under optical pumping using a Nd:YAG pulsed laser as an excitation source. As the excitation density is elevated, the emission intensity was nonlinearly increased at a certain threshold both for BP3T-OMe and BP3T-CN crystals, as shown in Fig. 4(a) and (b). The threshold excitation density was estimated to be 17 μJ cm−2 for BP3T-OMe and 152 μJ cm−2 for BP3T-CN, respectively. The threshold for the BP3T-OMe single crystal was lower than that of the BP3T-CN single crystal, which can be attributed to their different molecular orientation, as discussed in Results and discussion part. The perpendicular orientation to the crystal bottom plane in the BP3T-OMe single crystal is favourable for the efficient stimulated emission along the planar cavity compared to the inclining orientation in the BP3T-CN single crystal. As shown in Fig. 4(c) and (d), longitudinal multi-mode lasing spectra were observed both for BP3T-OMe and BP3T-CN single crystal, owing to the self-cavity effect in a well-shaped single crystal. Group refractive index (ng) values were calculated using an equation of ng = 1/2LΔλ−1, where L is the cavity length estimated by the fluorescence micrograph shown in Fig. 4(a) and (b), Δλ−1 is the mode intervals at each peaks of longitudinal multi-mode lasing spectra. The calculated ng value was 4.8 for BP3T-OMe while that of a BP3T-CN single crystal was 3.7–5.3. As both single crystals of BP3T-OMe and BP3T-CN showed lasing performances based on those high group refractive indices as similarly reported for other TPCO single crystals, these newly synthesized BP3T derivatives are strongly expected as promising organic lasing media.
Experiments
Synthesis
To the best of our knowledge, only BP3T-OMe has been synthesized via coupling reactions, so far.30 Here we synthesized both BP3T-OMe and BP3T-CN via a Negishi coupling reaction as shown in Scheme 1. | ||
| Scheme 1 Synthesis protocol of BP3T-OMe and BP3T-CN where X = methoxy, Y = bromide for BP3T-OMe, and X = cyano, Y = iodide for BP3T-CN. | ||
2,2′:5′,2′′-Terthiophene (681 mg, 2.74 mmol) was mixed with anhydrous THF (10 mL) and N,N,N′,N′-tetramethylethylenediamine (1.00 mL, 6.68 mmol). To this solution, 4 mL of 1.6 M n-butyllithium in cyclohexane was added dropwise at 0 °C and the reaction mixture was refluxed for 30 minutes and successively cooled down to −10 °C. Anhydrous zinc chloride (1.233 g, 9.045 mmol) dispersed in anhydrous tetrahydrofuran (15 mL) was added and the reaction solution was kept at 25 °C for 2 hours. Tetrakis(triphenylphosphine)-palladium(0) (106.5 mg, 0.09220 mmol) was immediately added and subsequently 4-bromo-4′-methoxybiphenyl (1.743 g, 6.624 mmol) dispersed in anhydrous tetrahydrofuran (5 mL) was added. The reaction mixture was warmed to 50 °C and kept for 23 hours to yield a precipitate. The precipitate was washed with 50 mL THF, 50 mL H2O, 10 mL 10% HCl, 50 mL H2O, 50 mL acetone and 250 mL hot 1,2,4-trichlorobenzene, yielding 377 mg (0.615 mmol, 22%).
Due to the low solubility in any solvents for BP3T-OMe and BP3T-CN, identification by 1H-NMR or 13C-NMR were unavailable. However, both of the target compounds were identified by MALDI-TOF-MS, found [M] = 612 and 602, for BP3T-OMe and BP3T-CN respectively. Almost all of TPCO series were identified by mass spectroscopy instead of using NMR spectroscopy in previous reports.27,30
Preparation of single crystals and vapour deposited thin films
Single crystals for X-ray diffraction measurements were prepared via a modified sublimation process. Purified powder of BP3T-OMe or BP3T-CN was put in a quartz crucible, and the molecules were sublimed and collected into another quartz crucible facing each other in a vacuum chamber (2 × 10−4 to 4 × 10−4 Pa). Single crystals of BP3T-CN for PL measurements were prepared by the same process used in X-ray diffraction measurements. Single crystals of BP3T-OMe for PL measurements were prepared via a solution growth process. First, a saturated BP3T-OMe solution was prepared at 150 °C using 1,2,4-trichlorobenzene as solvent. The solution was heated to 190 °C, then, successively cooled down to 40 °C for 18 hours. Thin films of BP3T-OMe and BP3T-CN for optical and photoelectron spectroscopy measurements were prepared by vapour deposition onto glass substrates. Purified powder of BP3T-OMe or BP3T-CN was evaporated from a resistively heated a quartz crucible under a vacuum condition (2 × 10−4 to 4 × 10−4 Pa). Their deposition rate and film thickness were monitored by a quartz crystal microbalance.Optical characterizations
Absorption and PL spectra were measured for the vapour-deposited thin films of BP3T-OMe and BP3T-CN using an ultraviolet-visible (UV-vis) spectrometer (JASCO V-530). The work functions of BP3T-OMe and BP3T-CN were measured by photo-electron spectroscopy in air (PESA, Riken Keiki AC-3) at the vapour-deposited thin films on ITO/glass substrates. Fluorescence images as well as PL spectra of single-crystal samples were measured using a fluorescence microscope (Olympus BX-51) equipped with a charge coupled device (CCD) spectrometer (Hamamatsu PMA-50) through a 10× objective lens.Observation of optically pumped lasing
Single crystals of BP3T-OMe grown via the solution process were transferred onto glass substrates by immersing the substrates into the solution, and then dried at atmosphere. Single-crystals of BP3T-CN were transferred onto glass substrates using a metal needle. Their fluorescence images were taken under ultraviolet excitation. Optically pumped PL measurements were performed using a Nd:YAG pulsed laser (λex = 355 nm, pulse frequency: 1.2 kHz, pulse duration: 1.1 ns) at room temperature under ambient conditions. The excitation beam shaped into a stripe of 0.24 × 0.04 cm2 through a cylindrical lens was focused on the single crystal surface with an incident angle of ∼45°. Photoemission radiated from the single-crystal edge was detected using a CCD spectrometer (Hamamatsu PMA-50) through an optical fiber.Conclusions
BP3T-OMe and BP3T-CN were successfully synthesized and their crystal structures were identified. BP3T-OMe and BP3T-CN crystallized in orthorhombic and triclinic forms, respectively. The molecular orientation of BP3T-OMe is perfectly perpendicular to the crystal basal plane, suggesting more efficient light confinement in the single-crystal cavity in a TM mode comparing to that of BP3T single crystals with slightly inclined orientation. On the other hand, a molecular orientation of BP3T-CN was oblique to the crystal basal plane, which is suitable for surface-emitting devices. The HOMO and LUMO levels of BP3T-OMe and BP3T-CN estimated by absorption and photoelectron spectroscopies revealed that the methoxy-group and cyano-groups act as electron-donating and electron-withdrawing groups, leading to expected p- and n-type semiconduction, respectively. Single crystals of BP3T-OMe and BP3T-CN showed optically pumped lasing with especially high group refractive index values, indicating the possibility to serve as good active media for organic lasers, especially in OLED structures due to their p- and n-type characteristics. Furthermore, the lasing threshold for the BP3T-OMe crystal was lower than that for the BP3T-CN crystal, which was attributed to their different molecular orientation, standing in the former and inclining in the latter.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japanese Society for the Promotion of Science (JSPS) KAKENHI No. 19H02172. This work was also partly supported by a grant-in-aid from the Graduate School of Science and Technology, Nara Institute of Science and Technology.References
- M. Pope, H. P. Kallmann and P. Magnante, J. Chem. Phys., 1963, 38, 2042–2043 CrossRef CAS.
- H. Xia, C. Hu, T. Chen, D. Hu, M. Zhang and K. Xie, polymers, 2019, 11, 443 CrossRef PubMed.
- S. Varghese, S.-J. Yoon, S. Casado, R. Fischer, R. Wannemacher, S.-Y. Park and J. Gierschner, Adv. Opt. Mater., 2014, 2, 542–548 CrossRef CAS.
- M. Ichikawa, R. Hibino, M. Inoue, T. Haritani, S. Hotta, K. Araki, T. Koyama and Y. Taniguchi, Adv. Mater., 2005, 17, 2073–2077 CrossRef CAS.
- M. Ichikawa, K. Nakamura, M. Inoue, H. Mishima, T. Haritani, R. Hibino, T. Koyama and Y. Taniguchi, Appl. Phys. Lett., 2005, 87, 221113 CrossRef.
- J. Gierschner, S. Varghese and S. Y. Park, Adv. Opt. Mater., 2016, 4, 348–364 CrossRef CAS.
- S. Hotta, Polym. Int., 2017, 66, 223–236 CrossRef CAS.
- H. Yanagi, Y. Marutani, F. Sasaki, Y. Makino, T. Yamao and S. Hotta, Appl. Phys. Express, 2011, 4, 062601 CrossRef.
- H. Mizuno, U. Haku, Y. Marutani, A. Ishizumi, H. Yanagi, F. Sasaki and S. Hotta, Adv. Mater., 2012, 24, 5744–5749 CrossRef CAS PubMed.
- H. Mizuno, T. Maeda, H. Yanagi, H. Katsuki, M. Aresti, F. Quochi, M. Saba, A. Mura, G. Bongiovanni, F. Sasaki and S. Hotta, Adv. Opt. Mater., 2014, 2, 529–534 CrossRef CAS.
- X. Zhu, H. Tsuji, L. J. T. Navarrete, J. Casado and E. Nakamura, J. Am. Chem. Soc., 2012, 134, 19254–19259 CrossRef CAS PubMed.
- M. Morales-Vidal, P. G. Boj, J. M. Villalvilla, J. A. Quintana, Q. Yan, N.-T. Lin, X. Zhu, N. Ruangsupapichat, J. Casado, H. Tsuji, E. Nakamura and M. A. Diaz-Garcia, Nat. Commun., 2015, 6, 8458 CrossRef CAS PubMed.
- S. Park, O.-H. Kwon, S. Kim, S. Park, M.-G. Choi, M. Cha, S. Y. Park and D.-J. Chang, J. Am. Chem. Soc., 2005, 127, 10070–10074 CrossRef CAS PubMed.
- Y. Jiang, P. Lv, J.-Q. Pan, Y. Li, H. Lin, X.-W. Zhang, J. Wang, Y.-Y. Liu, Q. Wei, G.-C. Xing, W.-Y. Lai and W. Huang, Adv. Funct. Mater., 2019, 29, 1806719 CrossRef.
- S. Sandanayaka, T. Matsushima, F. Bencheikh, S. Terakawa, W. J. Potscavage Jr, C. Qin, T. Fujihara, K. Goushi, J. Ribierre and C. Adachi, Appl. Phys. Express, 2019, 12, 061010 CrossRef.
- T. Kanagasekaran, H. Shimotani, K. Kasai, S. Onuki, R. D. Kavthe, R. Kumashiro and K. Tanigaki, arXiv:1903.08869, 2019.
- W. Takahashi, K. Maruyama, J. Li, M. Imakawa and T. Takenobu, Jpn. J. Appl. Phys., 2014, 53, 02BB02 CrossRef.
- Y. Sakurai, W. Hayashi, T. Yamao and S. Hotta, Jpn. J. Appl. Phys., 2014, 53, 02BB01. CrossRef.
- T. Yamao, Y. Okuda, Y. Makino and S. Hotta, J. Appl. Phys., 2011, 110, 053113 CrossRef.
- T. Yamao, N. Sakamoto, S. Hotta, H. Mizuno and H. Yanagi, Jpn. J. Appl. Phys., 2012, 51, 5111PD03 CrossRef.
- S. Kanazawa, M. Ichikawa, T. Koyama and Y. Taniguchi, ChemPhysChem, 2006, 7, 1881–1884 CrossRef CAS PubMed.
- H. Yanagi, Y. Marutani, N. Matsuoka, T. Hiramatsu, A. Ishizumi, F. Sasaki and S. Hotta, Appl. Phys. Lett., 2013, 103, 243301 CrossRef.
- H. Yanagi, A. Yoshiki, S. Hotta and S. Kobayashi, J. Appl. Phys., 2004, 96, 4240–4244 CrossRef CAS.
- N. Matsuoka, T. Hiramatsu, H. Yanagi, F. Sasaki and S. Hotta, Jpn. J. Appl. Phys., 2010, 49, 01AD05 Search PubMed.
- T. Matsuo, H. Mizuno, F. Sasaki and H. Yanagi, Jpn. J. Appl. Phys., 2020, 59, SDDB02 CrossRef CAS.
- S. Hotta, M. Goto, R. Azumi, M. Inoue, M. Ichikawa and Y. Taniguchi, Chem. Mater., 2004, 16, 237–241 CrossRef CAS.
- T. Katagiri, S. Ota, T. Ohira, T. Yamao and S. Hotta, J. Heterocycl. Chem., 2007, 44, 853–862 CrossRef CAS.
- K. Yamashita, U. Huynh, J. Richter, L. Eyre, F. Deschler, A. Rao, K. Goto, T. Nishimura, T. Yamao, S. Hotta, H. Yanagi, M. Nakayama and R. H. Friend, ACS Photonics, 2018, 5, 2182–2188 CrossRef CAS.
- R. Hatano, K. Goto, K. Yamashita, F. Sasaki and H. Yanagi, Jpn. J. Appl. Phys., 2017, 56, 04CL02 CrossRef.
- Y. Inada, M. Koda, Y. Urabe, T. Katagiri, T. Yamao, Y. Yoshida and S. Hotta, Sci. Rep., 2019, 9, 9739 CrossRef PubMed.
Footnote |
| † CCDC 1991924–1991925. For crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra04742b |
| This journal is © The Royal Society of Chemistry 2020 |