Open Access Article
Open Access ArticleLanthanide complexes based on a new bis-chelating carbacylamidophosphate (CAPh) scorpionate-like ligand†
Iryna Olyshevets *a,
Vladimir Ovchynnikova,
Nataliia Kariakaa,
Viktoriya Dyakonenkob,
Svitlana Shishkina
*a,
Vladimir Ovchynnikova,
Nataliia Kariakaa,
Viktoriya Dyakonenkob,
Svitlana Shishkina b,
Tatiana Slivaa,
Małgorzata Ostrowskac,
Aleksandra Jedyńczuk
b,
Tatiana Slivaa,
Małgorzata Ostrowskac,
Aleksandra Jedyńczuk c,
Elżbieta Gumienna-Kontecka
c,
Elżbieta Gumienna-Kontecka c and
Vladimir Amirkhanova
c and
Vladimir Amirkhanova
aTaras Shevchenko National University of Kyiv, Department of Chemistry, 64/13, Volodymyrska Street, Kyiv 01601, Ukraine. E-mail: olishevetsirina@gmail.com
bInstitute for Single Crystals, National Academy of Science of Ukraine, Nauky ave. 60, Kharkiv 61001, Ukraine
cFaculty of Chemistry, University of Wroclaw, F. Joliot-Curie 14, Wroclaw, 50383, Poland
First published on 29th June 2020
Abstract
The novel bis-chelating carbacylamidophosphate type ligand, tetramethyl[pyridine-2,6-diyldi(iminocarbonyl)]diamidophosphate (H2L), and its sodium salt, NaHL, have been synthesized and their structural properties have been investigated. Coordination compounds of lanthanides [Ln(HL)2NO3]·i-PrOH (Ln = Eu3+, Tb3+) were obtained for the first time, isolated in the individual state and characterized by means of IR and NMR spectroscopies, electrospray ionization mass spectrometry (ESI-MS), potentiometric titration, and elemental, thermal gravimetric and X-ray diffraction analyses. It was shown that H2L behaves like a scorpionate type ligand and in a mono-deprotonated form coordinates in a tridentate manner via the oxygen atoms of phosphoryl and carbonyl groups with formation of a mononuclear metal complex. The protonation constants of H2L and stability constants of Eu3+ and Tb3+ complexes have been determined. According to the results of X-ray diffraction analysis the H2L and [Ln(HL)2NO3]·i-PrOH molecules have monomeric structure but NaHL is a dimer. The Hirshfeld surface and fingerprint plots of the compounds have been used to analyze various hydrogen bonds and intermolecular interactions displayed in the crystal structure.
Introduction
Recently, the research of coordination compounds of lanthanides has been intensively developing, which is associated with the prospect of their practical application in the latest technologies, biology and medicine.1 Because of numerous unique physical properties of lanthanide containing compounds, including the ability of lanthanide complexes to radiate in a rather narrow spectral range, a large number of studies have been aimed at creating new luminescent materials on their basis.2 Lanthanide based complexes can also be used in medicine as contrast agents for MRI trials,3 luminescent probes4 etc.Over the past decades, many papers have been devoted to the use of lanthanide complexes in the technology of organic electroluminescent diodes (OLED),2a,5 a large part of which involves the study of β-diketonate complexes of lanthanides with “antenna” ligands, capable of transforming light energy from the UV region into the visible one due to excitation of f-electrons.6 Lanthanide complexes with P, N-hetero-substituted structural analogues of β-diketones – carbacylamidophosphates (CAPh) – compounds containing the chelating fragment C(O)NHP(O) – have some essential advantages.7 The presence of a phosphorus atom in the functional fragment provides synthetic possibilities (compared with β-diketones) for the introduction of additional functional groups-antenna. Moreover, compared with β-diketones, replacing one of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups with the P
O groups with the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the CAPh ligands leads to a decrease of vibration energy of Ln3+ coordinated fragments (from ∼1600 cm−1 to ∼1250 cm−1) and replacement of the carbon atom of chelating moiety with nitrogen one eliminates high-frequency C–H vibrations in chelating ring. These factors decrease the multiphoton quenching of the lanthanide emission in Ln3+ CAPh based complexes.
O group in the CAPh ligands leads to a decrease of vibration energy of Ln3+ coordinated fragments (from ∼1600 cm−1 to ∼1250 cm−1) and replacement of the carbon atom of chelating moiety with nitrogen one eliminates high-frequency C–H vibrations in chelating ring. These factors decrease the multiphoton quenching of the lanthanide emission in Ln3+ CAPh based complexes.
The compounds of the bis-β-diketonate series are promising chelating ligands, and their complexes with lanthanide ions have excellent photoluminescence properties6b,8 and can be used as markers in various variants of time-resolved fluorescence analysis. The syntheses of chelates based on bis-β-diketones are rather cheap and have high yields; also rather high stability constants of such complexes allow conducting measurements on the solid phase and in solution. β-Diketonate type complexones are also used for the detection of rare-earth elements, their extraction and concentration.9
Another promising type of ligands is “scorpionates”. Scorpionate ligands are the tridentate ligands which bind to a metal in a fac-manner. As an example, among the most popular class of scorpionates one can mention the pyrazol-based borates.10 The advantage of scorpionate complexes is that their easily tuneable electronic and steric features lead to the formation of very stable complexes with interesting and unique properties.11 As it will be shown in this research, the monodeprotonated bis-chelating CAPh ligand, due to its specific steric structure, behaves like a scorpionate ligand binding a metal by two oxygen atoms of deprotonated chelating core, like the pincers of a scorpion, and by the third oxygen atom of phosphoryl group of non-deprotonated arm of the ligand, which is situated over the plane formed by the metal and the two donor oxygen atoms of deprotonated chelating core (Fig. 1).
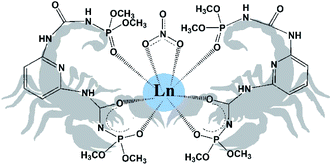 | ||
| Fig. 1 Schematic representation of obtained scorpionate complexes [Ln(HL)2NO3]·i-PrOH (2-propanol molecule is omitted). | ||
In this paper we report synthesis, structures, spectral and thermal properties of new bis-CAPh type ligand tetramethyl[pyridine-2,6-diyldi(iminocarbonyl)]diamidophosphate (H2L), it's sodium salt, and europium(III) and terbium(III) complexes of general formula [Ln(HL)2NO3]·i-PrOH.
Experimental section
Materials and methods
All of the chemicals used in this work were of analytical grade and used without further purification. Solutions of NaOH, HCl, NaCl, Eu(NO3)3·6H2O, and Tb(NO3)3·6H2O were prepared from pure commercial products (Sigma Aldrich). Stock solutions were standardized by ICP-AES along with the complexometric titration against EDTA using xylenol orange as an indicator. HCl solution was titrated by standardized NaOH solution. Carbonate-free NaOH solution was standardized by titration with potassium hydrogen phthalate.IR measurements were performed on a Perkin-Elmer Spectrum BX spectrometer on samples in form of KBr pellets.
1H NMR spectra in DMSO-d6 solutions were obtained on an AVANCE 400 Bruker NMR spectrometer at room temperature.
Elemental analyses (C, H, N) were performed on an EL III Universal CHNOS Elemental Analyzer.
Thermal gravimetric analysis was performed using a synchronous TG/DTA analyzer Shimadzu DTG-60H. The typical sample of 5–10 mg was heated to 800 °C in the alumina crucible under steady argon flow (300 ml min−1), with the heating rate of 10 °C min−1. The crystalline powdery Al2O3 was used as a standard compound.
X-ray diffraction data for structures H2L, NaHL·i-PrOH and [Ln(HL)2NO3]·i-PrOH (Ln = Eu3+, Tb3+) were collected on an Xcalibur-3 diffractometer (graphite monochromated MoKα radiation, CCD detector, φ and ω-scanning). The structures were solved by direct method and refined against F2 by full-matrix least-squares method using the SHELXTL package.12 All non-hydrogen atoms were refined within anisotropic approximation. The H atoms were located from the electron density difference maps and refined by ‘‘riding’’ model with Uiso = nUeq of the carrier atom (n = 1.5 for methyl and hydroxyl groups and n = 1.2 for other hydrogen atoms). The crystal and structure refinement data for H2L, NaHL·i-PrOH and [Ln(HL)2NO3]·i-PrOH (Ln = Eu3+, Tb3+) are given in Table S1.† Final atomic coordinates, geometrical parameters and crystallographic data have been deposited into the Cambridge Crystallographic Data Center, 11 Union Road, Cambridge, CB2 1EZ, UK (E-mail: E-mail: deposit@ccdc.cam.ac.uk; fax: +44 1223 336033).
Electrospray ionization mass spectrometry (ESI-MS) data were recorded on a BrukerQ-FTMS spectrometer. The instrumental parameters were: scan range, m/z 200–1600; dry gas, nitrogen; temperature, 170 °C; capillary voltage, 4500 V; ion energy, 5 eV. The capillary voltage was optimized to the highest signal-to-noise ratio. The spectra were recorded in the positive negative ion modes. MeOH/H2O solvent (50/50 v/v) was used to prepare solutions of ligand with concentrations of 10−4 to 10−5 M and metal-to-ligand molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with pH 3 and 8.
3 with pH 3 and 8.
The potentiometric titrations were performed at a constant temperature of 25 °C in an atmosphere of argon as an inert gas, using an automatic titrator system Titrando 905 (Methrom), connected to a combined glass electrode (Mettler Toledo InLab Semi-Micro) calibrated daily for hydrogen ion concentration using HCl. All the titrations were performed on 0.1 M solutions, using NaCl as a background electrolyte. Starting volumes of ca. 2 mM ligand solutions were 3 ml, and the exact concentration of the ligand was determined using the method of Gran.13 Metal-to-ligand molar ratios were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for all metal ions. The potentiometric data, collected over a pH range 2.0–11.0, were refined with the SUPERQUAD14 program, which use nonlinear least-squares methods. The species distribution diagrams were generated using a computer HYSS program.15
3 for all metal ions. The potentiometric data, collected over a pH range 2.0–11.0, were refined with the SUPERQUAD14 program, which use nonlinear least-squares methods. The species distribution diagrams were generated using a computer HYSS program.15
The three-dimensional Hirshfeld surfaces (HSs) and two-dimensional fingerprint plots were generated using Crystal Explorer 3.0.16,17 The dnorm plots were mapped with color scale in between – 0.649 au (blue) and 1.554 au (red). The fingerprint plots were displayed by using the expanded 0.4–2.4–3.0 Å view with the de and di distance scales displayed on the graph axes. When the cif files were uploaded into the CrystalExplorer software, all bond lengths to hydrogen were automatically modified to typical standard neutron values, i.e., d(C–H) = 1.083 and d(O–H) = 0.983 Å.
Synthetic procedures
0.018 mol of 2,6-diaminopyridine was dissolved in 40 ml of dioxane. The mixture was added dropwise to a solution of 0.036 mol of dimethyl ester of isocyanatophosphate acid in 40 ml of diethyl ether, with vigorous stirring and cooling. Yield 80%, M.P. = 155 °C. The compound is soluble in alcohols, acetone and water.
Compound H2L. Analysis for C11H19N5O8P2, %: calculated – C 32.13, H 4.66, N 17.02; found – C 32.41, H 4.36, N 16.98.
IR (KBr pellet, cm−1): 1689, 1729 (ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1256 (ν(P
O)), 1256 (ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)).
O)).
1H NMR (400 MHz, DMSO-d6, 25 °C): d = 3.69–3.72 (d, 12H, 3 J P-H = 11.5 Hz, OCH3), 7.40 (d, 2Hb, C5H3N), 7.71 (t, 1Hc, C5H3N), 8.8 (s, 2H, NH(CO)), 9.25 (s, 2H, NH(P)).
Compound NaHL. Analysis for C11H18NaN5O8P2, %: calculated – C 30.50, H 4.20, N 16.17; found – C 30.44, H 4.11, N 16.19.
IR (KBr pellet, cm−1): 1606, 1699 (ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1223 (ν(P
O)), 1223 (ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)).
O)).
1H NMR (400 MHz, DMSO-d6, 25 °C): d = 3.61–3.64 (d, 12H, 3 J P–H = 11.5 Hz, OCH3), 7.05 (d, 2Hb, C5H3N), 7.50 (t, 1Hc, C5H3N), 8.8 (s, 2H, NH(CO)), 9.23 (s, 1H, NH(P)).
Analysis for C25H44N11O20P4Eu, %: calculated – C 27.43, H 4.05, N 14.08; found – C 27.01, H 4.13, N 14.12. IR (KBr pellet, cm−1): 1556, 1652 (ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1215 (ν(P
O)), 1215 (ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1299, 1507 (NO3−).
O)), 1299, 1507 (NO3−).
Analysis for C25H44N11O20P4Tb, %: calculated – C 27.26, H 4.03, N 13.99; found – C 27.41, H 4.13, N 14.02. IR (KBr pellet, cm−1): 1556, 1650 (ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1190 (ν(P
O)), 1190 (ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1299, 1507 (NO3−).
O)), 1299, 1507 (NO3−).
They are soluble in water, methanol, soluble poorly in 2-propanol, acetone, DMSO and insoluble in nonpolar aromatic solvents.
Results and discussion
Description of the crystal structure
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯P
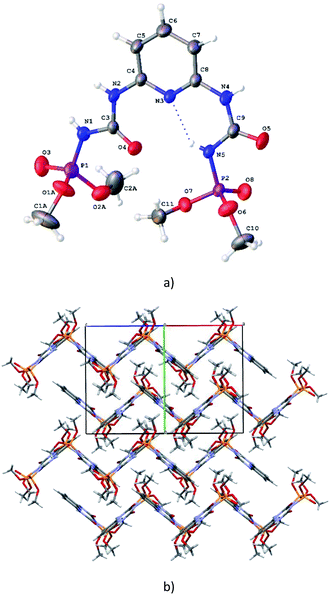
C⋯P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O pseudo-torsion angles are −55.8(1)° and −162.8(1)°). The both of these conformations are characteristic for CAPh.7a It should be noted that orientation of two substituents in H2L are stabilized additionally by the N5–H⋯N3/O4 bifurcated intramolecular hydrogen bonds (Table S3†). The bond lengths of the chelating fragments are d(PO)syn = 1.458(2) Å, d(CO)syn = 1.226(2) Å, d(PO)anti = 1.456(1) Å, d(CO)anti = 1.203(1) Å (Table S2†). In the crystal phase H2L molecules are bind by the N–H⋯O intermolecular hydrogen bonds (Table S3†) forming corrugated layers parallel to the ac crystallographic plane (Fig. 2b).
O pseudo-torsion angles are −55.8(1)° and −162.8(1)°). The both of these conformations are characteristic for CAPh.7a It should be noted that orientation of two substituents in H2L are stabilized additionally by the N5–H⋯N3/O4 bifurcated intramolecular hydrogen bonds (Table S3†). The bond lengths of the chelating fragments are d(PO)syn = 1.458(2) Å, d(CO)syn = 1.226(2) Å, d(PO)anti = 1.456(1) Å, d(CO)anti = 1.203(1) Å (Table S2†). In the crystal phase H2L molecules are bind by the N–H⋯O intermolecular hydrogen bonds (Table S3†) forming corrugated layers parallel to the ac crystallographic plane (Fig. 2b).
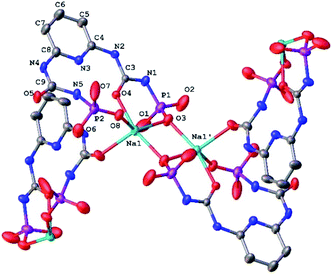 | ||
| Fig. 3 The fragment of molecular structure of NaHL·i-PrOH (H-atoms and CH3 of methoxy groups are omitted for clarity; the numbering scheme is given for the asymmetrical part of the unit cell). | ||
The Na–O bond lengths in the coordination polyhedron vary within 2.265(4) ÷ 2.400(4) Å and the O–Na–O bond angles vary within 79.15(2) ÷ 103.28(1)° and 95.97(2) ÷ 137.70(1)° in the axial and equatorial directions, respectively (Table S2†).
The bond lengths and bond angles of the NaHL ligand are in good agreement with the values for the free ligand (Table S2†). But the conformations of both substituents are changed due to rotation of both dimethoxyphosphoryl groups around the N–P bonds (Fig. 3). The N5–H⋯N3/O4 bifurcated intramolecular hydrogen bonds are very close to ones in the free ligand H2L (Table S3†).
In the crystal phase, (NaHL)n polymeric chains and isopropanol solvent molecules form the alternating layers parallel to the bc crystallographic plane (Fig. 4). The ligand molecule and propanol solvate molecules are bind by N–H⋯O and O–H⋯N intermolecular hydrogen bonds (Table S3†).
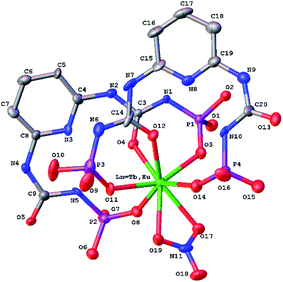 | ||
| Fig. 5 Molecular structure of [Ln(HL)2(NO3)] (Ln = Eu3+, Tb3+) (H-atoms and CH3 of methoxy groups are omitted for clarity). | ||
The conformation of deprotonated HL− ligand is very close to one in the sodium polymeric complex in the both lanthanide complexes. The N–H⋯N/O bifurcated intramolecular bonds are revealed in the both ligands between the neutral and deprotonated substituents in the [Ln(HL)2(NO3)]·i-PrOH complexes (Table S3†).
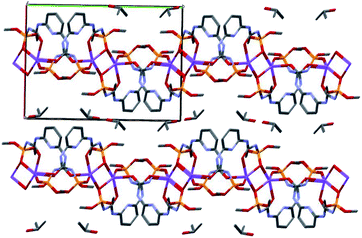
In the crystal phase, the [Ln(HL)2(NO3)] and i-PrOH solvate molecules form the layers parallel to the (−101) crystallographic plane. The coordination complex and solvate molecules are bind by the N–H⋯O and O–H⋯N intermolecular hydrogen bonds (Table S3†) and (Fig. 7).
Hirshfeld surface analysis and fingerprint plots
Hirshfeld surface analysis is an effective tool for exploring packing modes and intermolecular interactions in molecular crystals, as they provide a visual picture of intermolecular interactions and of molecular shapes in a crystalline environment. Surface features characteristic of different types of intermolecular interactions can be identified, and these features can be revealed by color coding distances from the surface to the nearest atom exterior (de plots) or interior (di plots) to the surface. This gives a visual picture of different types of interactions present, and also reflects their relative contributions from molecule to molecule. Further, 2D fingerprint plots (FP), in particular the breakdown of FP into specific atom⋯atom contacts in a crystal, provide a quantitative idea of the types of intermolecular contacts experienced by molecules in the bulk and presents this information in a convenient color plot.Hirshfeld surfaces comprising dnorm surface plots and FP were generated and analyzed for the crystal of H2L, NaHL·i-PrOH, [Tb(HL)2(NO3)]·i-PrOH, [Eu(HL)2(NO3)]·i-PrOH in order to explore their packing modes and intermolecular interactions. The dnorm surfaces of molecules are shown in Fig. 8, and the FP for the overall contacts and individual atom⋯atom contacts in compounds synthesized are shown in Fig. 9. The dark-red spots on the dnorm surface arise as a result of the short interatomic contacts, i.e., strong hydrogen bonds, while the intermolecular interactions appear as light-red spots. The analysis of the dnorm surface and FP gives a pictorial conformation (both qualitatively as well as quantitatively) to the nature and geometries of the hydrogen bonds and intermolecular interactions described in the crystal structure of the compounds.
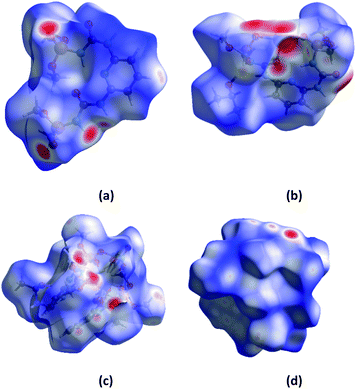 | ||
| Fig. 8 The dnorm surfaces of molecules of H2L (a), NaHL·i-PrOH (b), [Tb(HL)2NO3]·i-PrOH (c), [Eu(HL)2NO3]·i-PrOH (d). | ||
The dnorm and FP are consistent with the observed intermolecular interactions. The light red spots on the dnorm surface located near the oxygen, nitrogen atoms and NH group (Fig. 8) can be attributed to the N⋯H and O⋯H contacts displayed in H2L, NaHL·i-PrOH, [Tb(HL)2(NO3)]·i-PrOH, [Eu(HL)2(NO3)]·i-PrOH molecules.
The analysis of the fingerprint plots (Fig. 9) shows that the major contribution to the Hirshfeld surface is from H⋯H contact, with a contribution 39.2% for H2L, 51.7% for NaHL·i-PrOH, 48.9% for [Tb(HL)2(NO3)]·i-PrOH and 50.2% for [Eu(HL)2(NO3)]·i-PrOH, the closest H⋯H contact occurs at di + de is a little over 1 Å. The contribution of H⋯H interactions increases when comparing H2L with sodium salt and lanthanide complexes, which can be explained by a change in the conformation of ligands when coordinated to metal ions and by the fact that the number of oxygen atoms capable of participating in intermolecular interactions due to coordination with the central atom decreases.
These findings are supported by an analysis of the second largest contribution comes from the closest O⋯H contact, with a 35.9% for H2L, 24.5% for NaHL·i-PrOH, 38.0% for [Tb(HL)2(NO3)]·i-PrOH and 36.7% for [Eu(HL)2(NO3)]·i-PrOH. Where the decrease in the value for NaHL·i-PrOH when compared with H2L is due to a change in the conformation of the molecule and the screening of coordinated oxygen atoms from intermolecular interactions and an inverse increase for [Tb(HL)2(NO3)]·i-PrOH and [Eu(HL)2(NO3)]·i-PrOH due to the presence of three oxygen atoms of nitrate groups in their composition. The contributions of N⋯H and C⋯H of intermolecular interactions naturally decrease when comparing H2L with sodium salt and lanthanide complexes, the contributions of H⋯H increase, while the contributions of N⋯H and C⋯H decrease (Table 1).
| H–H | O–H | N–H | C–H | |
|---|---|---|---|---|
| H2L | 39.2 | 35.9 | 8.7 | 12.7 |
| NaHL·i-PrOH | 51.7 | 24.5 | 3.4 | 9.9 |
| [Tb(HL)2NO3]·i-PrOH | 48.9 | 38.0 | 4.1 | 4.5 |
| [Eu(HL)2NO3]·i-PrOH | 50.2 | 36.7 | 3.9 | 4.8 |
Thus, the Hirshfeld surface and FP analysis give conclusive evidence, both qualitatively and quantitatively, of the various hydrogen bonds/intermolecular interactions displayed in the crystal structure.
Thermal gravimetric analyses
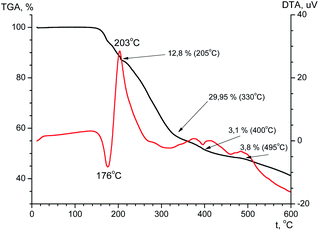
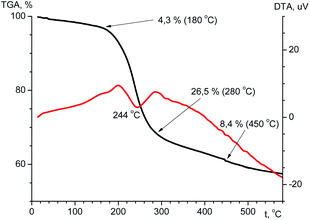
The thermal stability is an important feature that determines the possibility of compound practical applications. The thermal stability of the ligand and terbium coordination compound has been investigated using TGA in the temperature range of 20–600 °C (Fig. 10 and Fig. 11). According to the TGA curve (Fig. 10) for H2L there is no obvious weight loss of up to 150 °C. If heated above 150 °C, the sample melts (endothermic effect at 176 °C) with simultaneous decomposition accompanied by weight loss near 6% and upon further heating decomposes abruptly. The decomposition is accompanied by one exothermic effect at 203 °C and two endothermic effects at 400 and 495 °C. The total weight loss after heating of the sample to 600 °C is 58%, and at the boundary value of the experimental temperature, the mass of the sample has not reached a constant value.From the TGA data for the terbium complex one can conclude that significant weight loss occurs in one step (Fig. 11). Up to 180 °C there is a slight (∼4.3%) loss of mass accompanied by endothermic effect, which may be due to evaporation of solvent. When heated above 180 °C, the compound begins to decompose. Considerable weight loss continues to a temperature of ∼500 °C. The total weight loss is 42.3% and at the boundary value of the experimental temperature, the mass of the sample has not reached a constant value.
The decomposition of the complex is an endothermic process. The DTA curve shows an endothermic effect at a temperature of 244 °C.
Analysis of the IR spectrum of the solid residual in the crucible after TGA investigation reveals the formation of a mixture of terbium(III) orthophosphate and metaphosphate.
ESI-MS spectrometry
Analysis of the ESI-MS spectra of H2L showed the formation of monomeric and oligomeric species {[H2L] + H+}+ (m/z 412.08), {[(H2L)2] + Na+}+ (m/z 845.14), {[(H2L)3] + Na+}+ (m/z 1256.22) (Fig. S1†).Complex formation and the compositions of species formed in solution upon mixing H2L ligand with Eu3+ and Tb3+ ions were first monitored using ESI-MS. Although this technique is not able to distinguish the ionizable protons in the species, the method can be successfully applied to determine the metal-to-ligand stoichiometry directly from the m/z values and has been used successfully by us when previously describing analogous systems.20 Analysis of the ESI-MS spectra of the reaction mixture of Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2L, with a metal-to-ligand molar ratio 1
H2L, with a metal-to-ligand molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showed the formation of monomeric and oligomeric species successfully attributed to mononuclear, [Eu(HL) + Cl−]+ (m/z 597.96), [Eu[(HL)2]2−]+(m/z 973.06), [Eu[(HL)2]2−(H2L)]+(m/z 1384.14), and dinuclear [Eu2[(L)2]2− + Cl−]+(m/z 1156.94), species (Fig. S2†). It seems that the multinuclear complexes are adducts that arise during ESI-MS experiments; additional ESI-MS experiments showed that the multinuclear species are not resistant to the application of an increased SID voltage. All peak assignments were based on the comparison between the calculated and experimental isotope patterns (Fig. S2†).
1, showed the formation of monomeric and oligomeric species successfully attributed to mononuclear, [Eu(HL) + Cl−]+ (m/z 597.96), [Eu[(HL)2]2−]+(m/z 973.06), [Eu[(HL)2]2−(H2L)]+(m/z 1384.14), and dinuclear [Eu2[(L)2]2− + Cl−]+(m/z 1156.94), species (Fig. S2†). It seems that the multinuclear complexes are adducts that arise during ESI-MS experiments; additional ESI-MS experiments showed that the multinuclear species are not resistant to the application of an increased SID voltage. All peak assignments were based on the comparison between the calculated and experimental isotope patterns (Fig. S2†).
ESI-MS spectra of the Tb3+-H2L is dominated by the signal corresponding to the [Tb[(HL)2]2−]+ (m/z 979.06) complex (Fig. S2†). Additionally, in the spectra there are signals corresponding to mononuclear [Tb(HL) + Cl−]+ (m/z 603.96), and [Tb[(HL)2]2−(H2L)]+(m/z 1390.13) species.
Acid–base properties of the ligand
To evaluate the coordination capabilities of the ligand toward lanthanide ions, first the acid–base properties of H2L were determined. The deprotonated form of the ligand, [L2−], may attach three protons in the measured pH range, two amide nitrogens and pyridine nitrogen. The protonation constants determined are presented in Table 2 (Fig. S3†). The first constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1 = 9.07) corresponds probably to the protonation of amide NH group near the phosphorous atom of one arm of the ligand, the second (log
K1 = 9.07) corresponds probably to the protonation of amide NH group near the phosphorous atom of one arm of the ligand, the second (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2 = 7.77) corresponds to the protonation of the same amide group of the second arm. As it was observed for one-arm analogous ligand, deprotonation of amide group located between carbonyl and phosphoryl group is preferable to another one.21 The third (log
K2 = 7.77) corresponds to the protonation of the same amide group of the second arm. As it was observed for one-arm analogous ligand, deprotonation of amide group located between carbonyl and phosphoryl group is preferable to another one.21 The third (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K3 = 2.29) relates to the protonation of pyridine N atom. Low value of protonation constant of pyridine nitrogen atom, compared to similar systems,22 may contribute to the formation of its intramolecular hydrogen bonds with N–H and may be influenced by an inductive effect of two arms and the possibility of formation of bifurcate hydrogen bonds.23
K3 = 2.29) relates to the protonation of pyridine N atom. Low value of protonation constant of pyridine nitrogen atom, compared to similar systems,22 may contribute to the formation of its intramolecular hydrogen bonds with N–H and may be influenced by an inductive effect of two arms and the possibility of formation of bifurcate hydrogen bonds.23
| Species | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β β |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
|---|---|---|
| a 25 °C, I = 0.1 M NaCl. | ||
| [LH]− | 9.07(1) | 9.07 |
| LH2 | 16.85(2) | 7.77 |
| [LH3]+ | 19.14(3) | 2.29 |
Potentiometric titrations of Ln3+ complexes (Ln = Eu, Tb)
The stability constants and stoichiometry of the complexes obtained upon potentiometric titration of Eu3+ and Tb3+ with H2L performed with metal-to-ligand molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 are listed in Table 3.
3 are listed in Table 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) of the Eu3+ and Tb3+ complexes with H2La
β) of the Eu3+ and Tb3+ complexes with H2La
| Eu3+ | Tb3+ | |||
|---|---|---|---|---|
| Species | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β β |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β β |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
| a 25 °C, I = 0.1 M NaCl. | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
||||
| [MLH]2+ | 12.79(4) | — | 12.40(7) | — |
| [ML]+ | 4.90(2) | 7.89 | 5.26(2) | 7.14 |
| [MLH-1] | −3.20(2) | 8.10 | — | — |
| [MLH-2]− | −11.21(2) | 8.01 | −10.22(3) | — |
| [MLH-3]2− | −20.25(3) | 9.04 | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3 3 |
||||
| [MLH]2+ | 12.83(4) | — | 13.30(4) | — |
| [MLH-2]− | −11.53(2) | — | −10.12(2) | — |
| [MLH-3]2− | −20.63(2) | 9.10 | −18.78(2) | 8.66 |
| [M(LH)2]+ | 25.08(3) | — | 25.57(5) | — |
| [ML2H] | 17.40(3) | 7.68 | 18.29(3) | 7.28 |
| [ML2]− | 9.00(2) | 8.40 | 10.26(3) | 8.03 |
The data obtained for the Eu3+/H2L system with metal-to-ligand molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggest the formation of five species of stoichiometry 1
1 suggest the formation of five species of stoichiometry 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
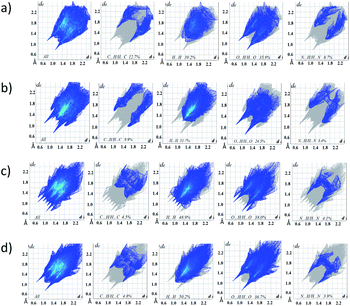
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with different protonation state: [EuLH]2+, [EuL]+, [EuLH-1], [EuLH-2]− and [EuLH-3]2−. The complexation process starts at pH 4 with the formation of the [EuLH]2+ species (Fig. 12a) in which, amide group of one arm of ligand is still protonated. The log
1 with different protonation state: [EuLH]2+, [EuL]+, [EuLH-1], [EuLH-2]− and [EuLH-3]2−. The complexation process starts at pH 4 with the formation of the [EuLH]2+ species (Fig. 12a) in which, amide group of one arm of ligand is still protonated. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks values for the deprotonation reaction of the [EuLH]2+ to [EuLH-3]2− range from 7.89 to 9.04 and taking into account fact that in the bis-complexes (vide infra) amide group of only one arm of ligand is deprotonated one can suppose that described here deprotonation steps are probably associated with the deprotonation of metal coordinated water molecules to complete the coordination sphere of Eu3+ ions. The calculated log
Ks values for the deprotonation reaction of the [EuLH]2+ to [EuLH-3]2− range from 7.89 to 9.04 and taking into account fact that in the bis-complexes (vide infra) amide group of only one arm of ligand is deprotonated one can suppose that described here deprotonation steps are probably associated with the deprotonation of metal coordinated water molecules to complete the coordination sphere of Eu3+ ions. The calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks lay in the range typical for the deprotonation of water molecule coordinated to lanthanide ions.24 When the excess of ligand was used, the simultaneous formation of complexes with 1
Ks lay in the range typical for the deprotonation of water molecule coordinated to lanthanide ions.24 When the excess of ligand was used, the simultaneous formation of complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal-to-ligand stoichiometry was observed. As it was earlier, the coordination process starts with the formation of the [EuLH]2+ species at pH around 4 (Fig. 12b). Due to the formation of bis-complexes starting from pH 6 it is not possible to detect all of the complexes of stoichiometry 1
2 metal-to-ligand stoichiometry was observed. As it was earlier, the coordination process starts with the formation of the [EuLH]2+ species at pH around 4 (Fig. 12b). Due to the formation of bis-complexes starting from pH 6 it is not possible to detect all of the complexes of stoichiometry 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as it was for the first system. In the first complex of stoichiometry 1
1, as it was for the first system. In the first complex of stoichiometry 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [Eu(LH)2]+, amide groups of one arm in both ligands are still protonated. As it is observed in the crystal structure of [Eu(HL)2(NO3)]·i-PrOH, the coordination of Eu3+ ion proceeds through the two oxygen atoms (Ocarbonyl, Ophosphoryl) of the deprotonated arm and monodentaly via Ophosphoryl of the second arm of each ligand. The next deprotonation steps leading to the formation of [ML2H] and [ML2]− species are probably related to the deprotonation of two water molecules coordinated to the Eu3+ ion, in place of NO3− present in the crystal structure.
2, [Eu(LH)2]+, amide groups of one arm in both ligands are still protonated. As it is observed in the crystal structure of [Eu(HL)2(NO3)]·i-PrOH, the coordination of Eu3+ ion proceeds through the two oxygen atoms (Ocarbonyl, Ophosphoryl) of the deprotonated arm and monodentaly via Ophosphoryl of the second arm of each ligand. The next deprotonation steps leading to the formation of [ML2H] and [ML2]− species are probably related to the deprotonation of two water molecules coordinated to the Eu3+ ion, in place of NO3− present in the crystal structure.
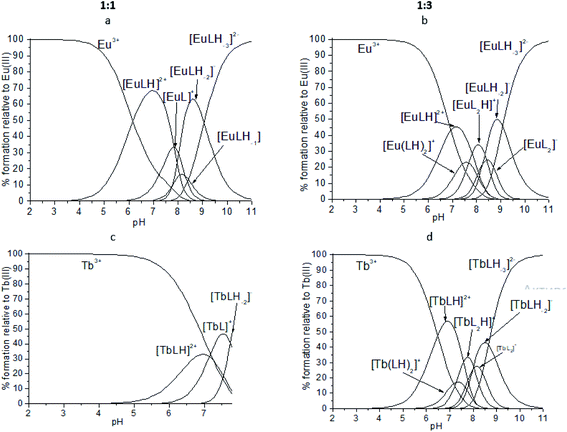 | ||
Fig. 12 Representative distribution diagrams for the systems Eu3+/ (a and b) H2L and Tb3+/ (c and d)H2L. Metal-to-ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, [L] = 2 mM, I = 0.1 M NaCl. 3, [L] = 2 mM, I = 0.1 M NaCl. | ||
For the Tb3+/H2L system very similar behavior was observed. The best fitting of the potentiometric experimental and calculated curves for metal-to-ligand molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained for the model containing complexes of 1
1 was obtained for the model containing complexes of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with different protonation state (Fig. 12c). Unfortunately, above pH 7.7 precipitation was observed. Calculated model and distribution diagram calculated for the titration with the excess of the ligand is identical as for Eu3+ system (Table 3, Fig. 12d).
1 stoichiometry with different protonation state (Fig. 12c). Unfortunately, above pH 7.7 precipitation was observed. Calculated model and distribution diagram calculated for the titration with the excess of the ligand is identical as for Eu3+ system (Table 3, Fig. 12d).
Conclusions
New bis-chelating carbacylamidophosphate (CAPh) ligand H2L and its monodeprotonated sodium salt NaHL have been synthesized and characterized for the first time. The novel coordination compounds [Ln(HL)2NO3]·i-PrOH of europium and terbium have been obtained and their structural, IR spectral and thermal properties have been characterized. The protonation constants of H2L and stability constants of Eu3+ and Tb3+ complexes with H2L have been determined. It was shown that the coordination process starts with the formation of the [LnLH]2+ species at pH around 4.X-ray structural studies of the compounds obtained showed that the H2L molecule has a monomeric structure and in crystals H2L molecules are bonded in the chain via intermolecular hydrogen bonds through the P(O) group and the hydrogens of NH groups. Sodium salt NaHL in the crystal state has a dimer structure, which is realized due to the bridge function of the oxygen atoms of the PO of the deprotonated arm of CAPh ligand. The deprotonated chelate core is coordinated to the sodium ion in the bidentate manner via the oxygen atoms of the carbonyl and phosphoryl groups. The undeprotonated ligand arm is coordinated to the sodium ion in a monodentate manner through the oxygen atom of the P(O) group.
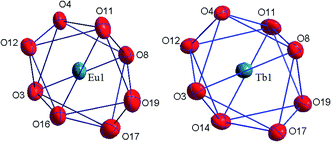
The coordination compounds [Eu(HL)2(NO3)]·i-PrOH and [Tb(HL)2(NO3)]·i-PrOH are isostructural and have a monomeric structure. Nitrate anion is coordinated to the lanthanide ion in the bidentate manner supplementing the coordination number of the lanthanide ion to 8. The structure of the coordination polyhedron of lanthanide ion can be interpreted as a distorted bicapped trigonal prism. There are numerous weak intermolecular and intramolecular contacts in the crystalline structure of all the compounds obtained.
The thermal properties of the complexes synthesized are investigated and their high thermal stability is shown (the decay start is about 200 °C), which indicates the prospect of the complexes for practical use in electroluminescent diode technology. And good solubility of the obtained complexes in water opens prospective for their practical application in biology and medicine.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding sources: this work was supported by the Ministry of Education and Science of Ukraine (Project no. 19BF037-05) and Ministry of Science and Higher Education of Poland (research subsidy ZB2A-2020). The publication contains the results of studies conducted by President's of Ukraine grant for competitive projects (Project no. 19BF037-06).References
- (a) J.-C. G. Bünzli, Chem. Rev., 2010, 110(5), 2729–2755 CrossRef PubMed; (b) J.-C. G. Bunzli, Handbook on the Physics and Chemistry of Rare Earths, 2016, vol. 50, pp. 141–176 Search PubMed; (c) G. Aromı and R. Olivier, Handbook on the Physics and Chemistry of Rare Earths, 2019, vol. 56, pp. 1–54 Search PubMed.
- (a) J. Kido and Y. Okamoto, Chem. Rev., 2002, 102, 2357–2368 CrossRef CAS PubMed; (b) G. F. De Sa, O. L. Malta, C. De Mello Donega, A. M. Simas, R. L. Longo, P. A. Santa-Cruz and E. F. Da Silva, Coord. Chem. Rev., 2000, 196, 165 CrossRef CAS; (c) K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS PubMed.
- H. Ur. Rashid and K. Yu, J. Struct. Chem., 2013, 54(1), 223–249 CrossRef CAS.
- B. H. Bakker, M. Goes and N. Hoebe, et al., Coord. Chem. Rev., 2000, 208(1), 3–16 CrossRef CAS.
- F. Zinna, M. Pasini, F. Galeotti, C. Botta, L. DiBari and U. Giovanella, Adv. Funct. Mater., 2016, 27(1), 1603719 CrossRef.
- (a) A. N. Gusev, M. Hasegawa, T. Shimizu, T. Fukawa, S. Sakurai, G. A. Nishchymenko, V. F. Shul'gin, S. B. Meshkova and W. Linert, Inorg. Chim. Acta, 2013, 406, 279–284 CrossRef CAS PubMed; (b) J. Shi, Y. Hou, W. Chu, X. Shi, H. Gu, B. Wang and Z. Sun, Inorg. Chem., 2013, 52(9), 5013–5022 CrossRef CAS PubMed.
- (a) P. Gawryszewska and P. Smolenski, Ligands. Synthesis, Characterization and Role in Biotechnology, Nova Science Publishers, 2014, p. 295 Search PubMed; (b) O. O. Litsis, I. O. Shatrava, O. V. Amirkhanov, V. A. Ovchynnikov, T. Y. Sliva, S. V. Shishkina, V. V. Dyakonenko, O. V. Shishkin and V. M. Amirkhanov, Struct. Chem., 2016, 27(1), 341–355 CrossRef CAS.
- (a) N. Sayyadi, A. Care, R. E. Connally, A. C. Try, P. L. Bergquist and A. Sunna, Sci. Rep., 2016, 6(1), 27564 CrossRef CAS PubMed; (b) H. F. Li, G. M. Li, P. Chen, W. B. Sun and P. F. Yan, Spectrochim. Acta, Part A, 2012, 97, 197–201 CrossRef CAS PubMed.
- K. Binnemans, Rare-Earth Beta-Diketonates, in Handbook on the Physics and Chemistry of Rare Earths, ed. Gschneidner K. A. Jr, Bünzli J.-C. G. and Pecharsky V. K., Elsavier, Amsterdam, 2005, pp. 107–272 Search PubMed.
- (a) S. Trofimenko, Scorpionates. The Coordination Chemistry of Polypyrazolylborate Ligands, Imperial College Press, London, 1999 CrossRef; (b) C. Pettinari, Scorpionates II: Chelating Borate Ligands, Imperial College Press, London, 2008 Search PubMed.
- M. Bortoluzzia, G. Paoluccia, S. Polizzia, L. Bellottoa, F. Enrichib, S. Ciorba and B. S. Richards, Inorg. Chem. Commun., 2011, 14, 1762 CrossRef.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. Gran, Acta Chem. Scand., 1950, 4(4), 559–577 CrossRef CAS.
- P. Gans, A. Sabatini and A. Vacca, J. Chem. Soc., Dalton Trans., 1985,(6), 1195–1200 RSC.
- L. Alderighi, P. Gans, A. Ienco, D. Peters, A. Sabatini and A. Vacca, Coord. Chem. Rev., 1999, 184, 311–318 CrossRef CAS.
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, Crystal Explorer 3.0. University of Western Australia, Perth, 2012 Search PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- M. A. Porai-Koshits and L. A. Aslanov, J. Struct. Chem., 1972, 13, 244–253 CrossRef.
- SHAPE v2.1, Continuous Shape Measures Calculation, 2013 Electronic Structure Group, Universitat de Barcelona, contact: llunell@ub.edu Search PubMed.
- I. O. Shatrava, V. A. Ovchynnikov, K. E. Gubina, T. Yu. Sliva, O. V. Severinovskaya, A. G. Grebenyuk, S. V. Shishkina and V. M. Amirkhanov, Polyhedron, 2018, 139, 98–106 CrossRef CAS.
- I. Shatrava, V. Ovchynnikov, K. Gubina, S. Shishkina, O. Shishkin and V. Amirkhanov, Struct. Chem., 2016, 27(5), 1413–1425 CrossRef CAS.
- J. Gałęzowska, P. Kafarski, H. Kozłowski, P. Młynarz, V. M. Nurchi and T. Pivetta, Inorg. Chim. Acta, 2009, 362, 707–713 CrossRef.
- E. Gumienna-Kontecka, I. A. Golenya, A. Szebesczyk, M. Haukka, R. Krämer and I. O. Fritsky, Inorg. Chem., 2013, 52(13), 7633–7644 CrossRef CAS PubMed.
- J. Gałęzowska, R. Janicki, A. Mondry, R. Burgada, T. Bailly, M. Lecouvey and H. Kozłowski, Dalton Trans., 2006, 36, 4384–4394 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1984336–1984339. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra04714g |
| This journal is © The Royal Society of Chemistry 2020 |