Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Natural product diversity from the endophytic fungi of the genus Aspergillus†
Seham S. El-hawarya,
Abeer S. Moawadb,
Hebatallah S. Bahrc,
Usama Ramadan Abdelmohsen *de and
Rabab Mohammed*b
*de and
Rabab Mohammed*b
aDepartment of Pharmacognosy, Faculty of Pharmacy, Cairo University, 11936 Cairo, Egypt
bDepartment of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, 62514 Beni-Suef, Egypt. E-mail: rababmohammed@pharm.bsu.edu.eg
cDepartment of Pharmacognosy, Faculty of Pharmacy, Nahda University, 62513 Beni-Suef, Egypt
dDepartment of Pharmacognosy, Faculty of Pharmacy, Minia University, 61519 Minia, Egypt. E-mail: usama.ramadan@mu.edu.eg
eDepartment of Pharmacognosy, Faculty of Pharmacy, Deraya University, Universities Zone, P. O. Box 61111 New Minia City, Minia, Egypt
First published on 9th June 2020
Abstract
The endophytic fungus Aspergillus is considered as an enormous source of chemical leads with promising biological activities. Different Aspergillus species have proved their ability to produce plenty of secondary metabolites including butenolides, alkaloids, terpenoids, cytochalasins, phenalenones, ρ-terphenyls, xanthones, sterols, diphenyl ether and anthraquinone derivatives with diverse biological activities, such as anti-cancer, antifungal, anti-bacterial, anti-viral, anti-inflammatory, antitrypanosomal and antileishmanial activities. From January 2015 until December 2019, three hundred and sixty-one secondary metabolites were reported from different endophytic Aspergillus species. This review discusses the isolated secondary metabolites from different endophytic Aspergillus species reported from January 2015 to December 2019 along with their reported biological activities and structural aspects whenever applicable.
1. Introduction
Natural products (secondary metabolites) are heterogeneous low molecular mass molecules. Secondary metabolites differ from primary nutrients in that they are not essential for growth, but they play a vital role in the survival and adaptation of producing organisms in nature.1 Scientists are fascinated by finding novel classes of secondary metabolites from new natural sources, such as marine invertebrates and plant endophytes in order to treat various diseases and improve pharmaceutical products.Plant endophytes are an interesting source of bioactive secondary metabolites, they are able to produce metabolites similar to those produced by the plant itself or novel compounds rather than those produced by the plant.2 De Bary first defined the term endophyte in 1866 that described the presence of bacterial or fungal microorganisms in the internal tissues of healthy plants, which are capable of living and colonizing without causing noticeable symptoms of diseases.66 The relationship between the endophyte and its host plant is still ambiguous, but sometimes can be identified as a mutualistic relationship; in which the plant acts as a protector and source of nutrition for the microorganisms, which in turn are capable of yielding bioactive substances that play a role in promoting plant growth and survival under different environmental conditions that may be drastic to the plant.3
There are as many as one million different fungal endophytic species present in the investigated plants as approximated by Hawksworth and Rossman in 1987.65 Among the most dominated fungal genera, Aspergillus has been described as a rich source for a plethora of bioactive compounds with diverse chemical structures and biological activities.4
Aspergillus is one of the most familiar filamentous fungi belonging to Ascomycetes (family Trichocomaceae). There are about 378 species as reported by the World of Microorganisms Information Center (WDCM).5 Aspergillus members are highly aerobic fungi growing in oxygen-rich environments and many of them are capable of growing in vital nutrients-depleted environments.6 Furthermore, many species have been successfully cultivated over a wide range of temperatures (10–50 °C), pH (2–11) and salinity (0–34%).7 They have similar aspergillum-like morphological features and microscopical characteristics; they exist in nature as endophytes, saprophytes, parasites, and human pathogens. Traditionally, Aspergillus species were identified and classified depending on their morphological, physiological and biochemical methods. Recently, molecular techniques, such as ribosomal DNA (rDNA) sequences analysis, random amplified polymorphic DNA (RAPD) and restriction fragment length polymorphism (RFLP) are used.8
Endophytic Aspergillus species have proved their ability to produce plenty of active secondary metabolites, such as butenolides, alkaloids, terpenoids, cytochalasins, phenalenones, ρ-terphenyls, xanthones, sterols, diphenyl ether and anthraquinones derivatives of great importance in pharmaceutical and commercial industries. The metabolites produced by different endophytic Aspergillus species showed various biological activities, such as anti-inflammatory, anti-cancer, anti-bacterial and anti-viral activities.9 This review focuses on the natural products isolated from different endophytic Aspergillus species that exhibited diverse bioactivities. In the ESI,† a list of other endophytic Aspergillus metabolites with no bioactivity was presented in Table S1.† In this table, the name, chemical nature, Aspergillus species, isolation source, and references were discussed. This review covers the period from January 2015 to December 2019.
2. Aspergillus allahabadii
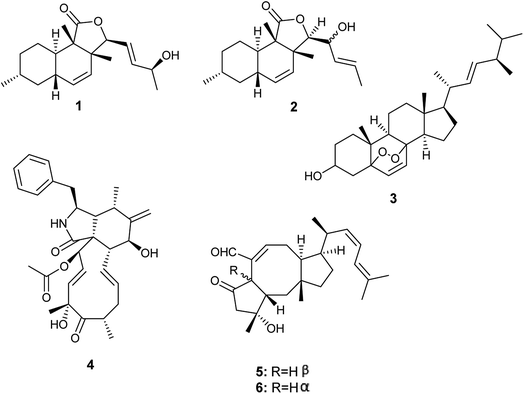
From 2015 until 2019, A. allahabadii was isolated only once from the roots of Cinnamomum subavenium and fermented, which resulted in the isolation of two polyketide derivatives identified as allahabadolactones A (1) and B (2), in addition to, a sterol named (22E)-5α-8α-epidioxyergosta-6,22-dien-3β-ol (3), and a cytochalasin named cytochalasin D (4) (Fig. 1). Allahabadolactones A (1) and B (2) displayed cytotoxicity against NCI-H187 (human small-cell lung cancer) with IC50 values of 17.78 and 30.51 μg mL−1 respectively, and against non-cancerous Vero cells with IC50 values of 31.50 and 21.00 μg mL−1 respectively. Moreover, allahabadolactones B (2) showed antibacterial activity against Bacillus cereus with an IC50 value of 12.50 μg mL−1. (22E)-5α-8α-Epidioxyergosta-6,22-dien-3β-ol (3) showed cytotoxicity against NCI-H187 (human small-cell lung cancer) and non-cancerous Vero cells with IC50 values of 24.24 and 15.76 μg mL−1, respectively. Cytochalasin D (4) showed good cytotoxicity against non-cancerous Vero cells with an IC50 value of 0.58 μg mL−1.43. Aspergillus calidoustus
A. calidoustus is a well-known human pathogenic fungus that was isolated once by Carvalho et al. from the leaves of Acanthospermum australe.11 Bioassay-guided fractionation of the fermented culture extract led to the isolation of the sesterterpene derivatives, ophiobolin K (5) and 6-epi-ophiobolin K (6) (Fig. 1). Ophiobolin K (5) displayed antifungal activities against Colletotrichum acutatum, Colletotrichum gloeosporioides, Fusarium oxysporum and Phomopsis viticola. Moreover, ophiobolin K (5) displayed cytotoxicity to TK-10 tumor cells with an IC50 value of 0.51 μM. On the other hand, 6-epi-ophiobolin K (6) showed antifungal activity against Phomopsis obscurans, and exhibited cytotoxicity to the tumor cell line MCF-7 with an IC50 value of 2.97 μM.114. Aspergillus capensis
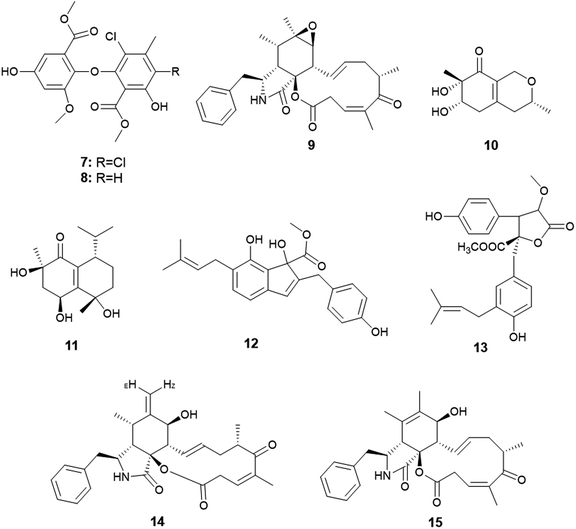
Qin et al. were the first to report the isolation of the endophytic fungus A. capensis from the plant Brassica napus L. (oilseed rape).12 Chemical investigation of the fermented culture resulted in the isolation of two diphenyl ether derivatives identified as methyl dichloroasterrate (7) and penicillither (8) together with the cytochalasin derivative; rosellichalasin (9) (Fig. 2). The three metabolites exhibited varying antifungal activities against the plant pathogenic fungi; Botrytis cinerea, Monilinia fructicola, Sclerotinia sclerotiorum and Sclerotinia trifoliorum with EC50 values ranging from 2.46 to 65.00 μg mL−1; Methyl dichloroasterrate (7) was the most effective compound against B. cinerea with an EC50 value of 9.33 ± 1.66 μg mL−1 while penicillither (8) was more effective against S. trifoliorum with an EC50 value of 9.93 ± 1.97 μg mL−1. On the other hand, rosellichalasin (9) was the most effective compound against the fungus S. sclerotiorum with an EC50 value of 2.46 ± 0.18 μg mL−1.125. Aspergillus clavatus
Only one A. clavatus strain was isolated from the leaves of Tripterygium hypoglaucum (Level.) Hutch. Chemical investigation resulted in the isolation of the metabolites aspergillusones C (10) and D (11) (Fig. 2). Aspergillusone C (10) displayed cytotoxicity to the tumor cell lines MCF-7 and A549 with IC50 values of 2.5 and 41.9 μM, respectively. Aspergillusone D (11) showed potent cytotoxic activity against the tumor cell line A549 (IC50 0.2 μM), and good cytotoxic activity against MCF-7 tumor cell line (IC50 5.9 μM).136. Aspergillus flavipes
The novel indene derivative, methyl 2-(4-hydroxy benzyl)-1,7-dihydroxy-6-(3-methyl but-2-enyl)-1H-indene-1-carboxylate (12) along with the butenolide, 2-O-methylbutyrolactone I (13) as well as the cytochalasins; cytochalasin Z16 (14), cytochalasin Z17 (15) and rosellichalasin (9) were isolated from the fungus A. flavipes associated with the stem of the plant Suaeda glauca (Bunge) (Fig. 2). The compounds; methyl 2-(4-hydroxy benzyl)-1,7-dihydroxy-6-(3-methyl but-2-enyl)-1H-indene-1-carboxylate (12), cytochalasin Z16 (14), cytochalasin Z17 (15) and rosellichalasin (9) showed antibacterial activities against Pseudomonas aeruginosa and Klebsiella pneumonia with the same MIC values (32 μg mL−1) while, 2-O-methylbutyrolactone I (13) showed antibacterial activity against K. pneumonia only with MIC value of 32 μg mL−1.147. Aspergillus flavus
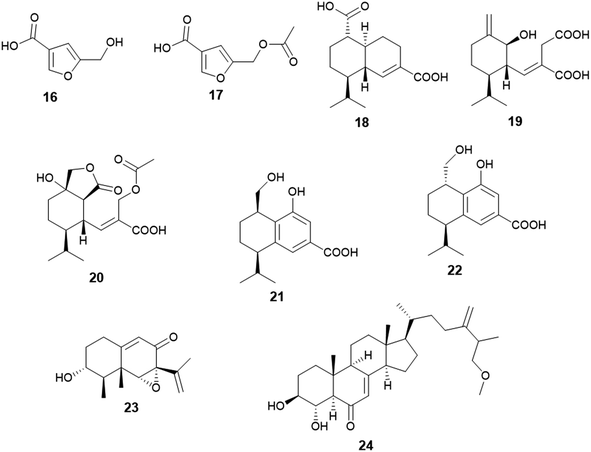
Two furan derivatives identified as 5-hydroxymethylfuran-3-carboxylic acid (16) and 5-acetoxymethylfuran-3-carboxylic acid (17) were isolated as a result of fermentation of the culture of A. flavus, the endophyte hosted in the stem of Cephalotaxus fortunei (Fig. 3). Both furans exhibited antibacterial activity against Staphylococcus aureus with MIC values of 31.3 and 15.6 μg mL−1, respectively. Moreover, 5-acetoxymethylfuran-3-carboxylic acid (17) showed moderate antioxidant activity with an IC50 value of 237 μg mL−1, while 5-hydroxymethylfuran-3-carboxylic acid (16) displayed weak antioxidant activity with an IC50 value of 435 μg mL−1 using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals assay.15The sesquiterpenoids; (1S,6R,7R,10S)-aspergilloid D (18), (1S,6S,7R)-aspergilloid E (19), (1S,6S,7R,10S)-aspergilloid E (20), (7R,10R)-aspergilloid G (21), (7R,10S)-aspergilloid H (22) and sporogen AO-1 (23) along with the sterol 3β,4α-dihydroxy-26-methoxyergosta-7,24(28)-dien-6-one (24) were isolated from the endophyte A. flavus associated with the leaves of the toxic medicinal plant Tylophora ovata (Fig. 3). All isolated compounds at 10 μM demonstrated hepatoprotective effect on acetaminophen (APAP)-induced damage model of HepG2 cells. They improved the HepG2 cell survival rates from 24.0% (APAP, 8 mM) to 33.2–41.6%. Furthermore, the sterol 3β, 4α-dihydroxy-26-methoxyergosta-7,24(28)-dien-6-one (24) exhibited cytotoxicity against MCF-7 breast cancer cells with an IC50 value of 2.6 μM.16
8. Aspergillus flocculus
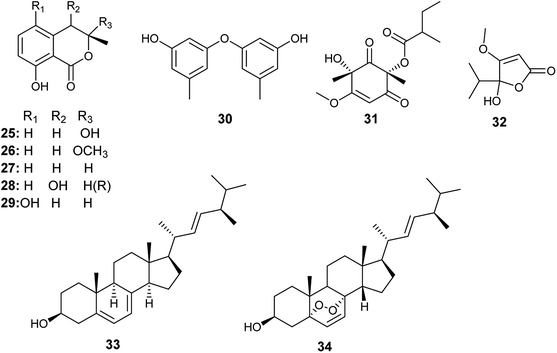
Tawfike et al. applied recent metabolomic technologies to the fermented culture extract of A. flocculus, the endophyte associated with the stem of the medicinal plant Markhamia platycalyx, to identify bioactive anticancer and anti-trypanosome secondary metabolites.17 Bioactivity-guided fractionation resulted in the isolation of the isocoumarin derivatives, 3-hydroxymellein (25), botryoisocoumarin A (26), mullein (27), cis-4-hydroymellein (28) and 5-hydroxymellein (29) along with diocrinol (30), phomaligol A1 (31), dihydropenicillic acid (32), ergosterol (33) and ergosterol peroxide (34) (Fig. 4). 3-Hydroxymellein (25) showed antitrypanosomal activity against Trypanosoma brucei with inhibition 56%. Diocrinol (30) displayed a 97% inhibitory effect against T. brucei (MIC 108 μM). On the other hand, phomaligol A1 (31) and dihydropenicillic acid (32) demonstrated moderate antitrypanosomal activity against T. brucei with MIC values of 88 and 145.3 μM, respectively. The sterols; ergosterol (33) and ergosterol peroxide (34) were strongly active antitrypanosomal agents with MIC values of 31.6 and 7.3 μM, respectively. Finally, the mellein's analogues, botryoisocoumarin A (26), mullein (27), cis-4-hydroymellein (28) and 5-hydroxymellein (29), showed cytotoxicity against K562 cancer cell line at conc. 30 μM.179. Aspergillus fumigatus
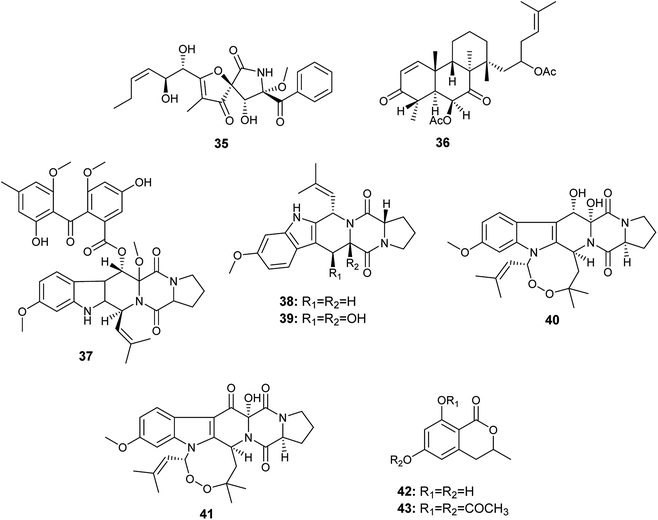
Aspergillus fumigatus is a frequently isolated Aspergillus species that has produced the largest number of bioactive secondary metabolites; twenty-nine bioactive metabolites have been isolated and identified.An A. fumigatus strain was isolated from the internal tissues of the stem of Erythrophleum fordii Oliv. Further chemical investigation led to the isolation of the alkaloid pseurotin A (35) (Fig. 5), which demonstrated indirect anti-inflammatory activity with IC50 value of 5.20 μM.18
Chemical investigation of the fermented culture of the fungal strain A. fumigatus, the endophyte isolated from the plant Diphylleia sinensis. L., resulted in the isolation of the metabolite 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione (36) in addition to the alkaloids; fumitremorgin D (37), fumitremorgin C (38), 12,13-dihydroxyfumitremorgin C (39), verruculogen (40) and 13-oxoverruculogen (41) (Fig. 5). The alkaloid 12,13-dihydroxyfumitremorgin C (39) showed the highest cytotoxicity against human hepatocellular carcinoma (HepG2) cell line with an IC50 value of 4.5 μM, and followed in potency by verruculogen (40) with IC50 value of 9.8 μM. On the other hand, fumitremorgin D (37) and 13-oxoverruculogen (41) showed mild cytotoxic activity against HepG2 cell line with IC50 values of 47.5 and 44.9 μM, respectively. Moreover, the compounds 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20, 21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione (36) and fumitremorgin C (38) showed weak cytotoxicity to HepG2 cell line with IC50 values of 139.9 and 156.5 μM, respectively. From the previous, it is clear that the strongest cytotoxic activity against HepG2 cell line is for the compounds 12,13-dihydroxyfumitremorgin C (39) and verruculogen (40). 12,13-dihydroxyfumitremorgin C (39) is more cytotoxic (IC50 4.5 μM) than verruculogen (40) (IC50 9.8 μM) despite the lack of the macrocyclic linking at 1-N and 3-C in 12, 13-dihydroxyfumitremorgin C (39), thus the existence of the macrocyclic linking at 1-N and 3-C does not play a major role in increasing activity. On the other hand, both active compounds 12,13-dihydroxyfumitremorgin C (39) and verruculogen (40) contain adjacent hydroxyl groups at positions C-12 and C-13 that is absent in others, so the increased activity is related to the presence of the hydroxyl group at positions C-12 and C-13 simultaneously.19
6-Hydroxymellein (42) is an isochromenone isolated from the endophyte A. fumigatus colonized in the leaves of Bacopa monnieri. Furthermore, its diacetyl derivative (43) was laboratory synthesized (Fig. 5). 6-Hydroxymellein (42) and its diacetyl derivative (43) showed antioxidant activity in DPPH scavenging assay with IC50 values 185.66 and 106.6 μg mL−1, respectively.20
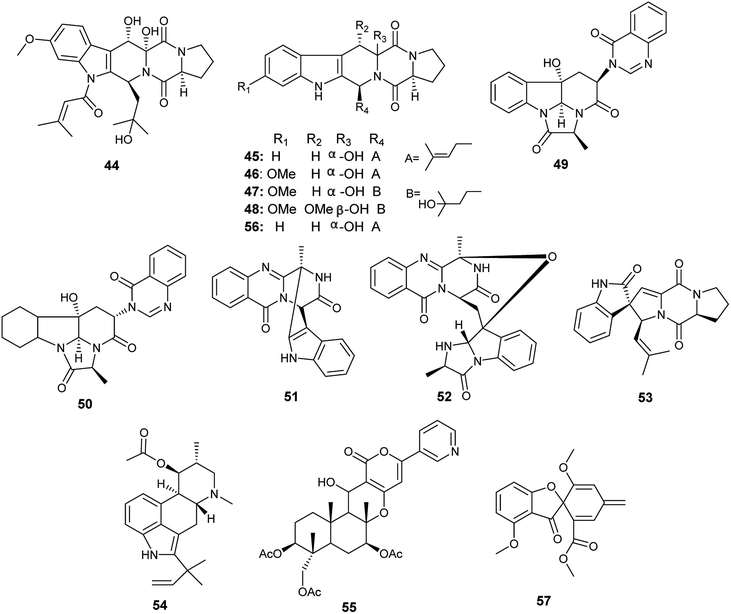
The alkaloids; asperfumigatin (44), demethoxyfumitremorgin C (45), fumitremorgin C (38), cyclotryprostatin C (46), 12,13-dihydroxyfumitremorgin C (39), verruculogen TR-2 (47), 20-hydroxycyclotryprostatin B (48), chaetominine (49), isochaetominine (50), fumiquinazoline J (51), fumiquinazoline C (52), spirotryprostatin B (53), fumigaclavine C (54), pyripyropene A (55) and 13-dehydroxycyclotryprostatin C (56) together with the metabolite trypacidin (57) were obtained from the fungus A. fumigatus, the endophyte isolated from the Chinese liverwort Heteroscyphus tener (Steph.) Schiffn. (Fig. 6). The compounds (44), (45), (38), (46), (39), (47), (48), (49), (50), (51), (52), (53), (54), (56) and (57) displayed weak anticancer activity against human prostate cancer (PC3) cell line with IC50 values ranging from 19.9 ± 0.5 to 38.9 ± 0.5 μM. On the other hand, the compounds (38), (39), (55), (56) and (57) showed weak cytotoxicity to multiple drug resistance (PC3D) cells with IC50 values ranging from 27.7 ± 0.7 to 39.9 ± 1.3 μM. Furthermore, the two alkaloids pyripyropene A (55) and trypacidin (57) have weak cytotoxic activity to human lung adenocarcinoma epithelial (A549) cell line with IC50 values of 38.3 ± 0.8 and 33.8 ± 0.8 μM, respectively. Finally, the alkaloids fumiquinazoline J (51), fumiquinazoline C (52) and trypacidin (57) displayed weak cytotoxicity against human lung cancer (NCI-H 460) cell line with IC50 values of (26.9 ± 0.6), (33.4 ± 0.7) and (31.0 ± 0.5) μM, respectively.21
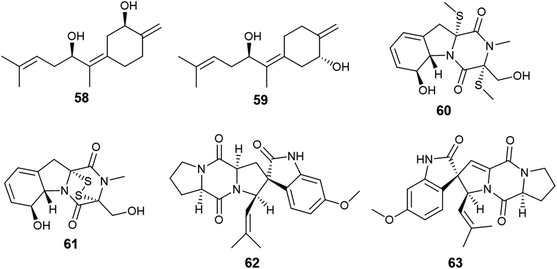
Fumagillene A (58) and B (59) are sesquiterpenoids obtained from Ligusticum wallichii roots associated endophytic A. fumigatus fungus (Fig. 7). Both compounds (58) and (59) possessed moderate cytotoxicity against MV4-11 cell line with IC50 values of 8.4 ± 2.9 μg mL−1 and 11.2 ± 3.6 μg mL−1, respectively, as well as against MDA-ME-231 cell line with IC50 values of 14.3 ± 5.8 μg mL−1 and 17.3 ± 6.4 μg mL−1, respectively.22
Bioassay-guided fractionation of the fermented culture of the fungus A. fumigatus, the endophyte isolated from the leaves of Edgeworthia chrysantha, led to the isolation of the alkaloids identified as isdethiobis (methylthio) gliotoxin (60), gliotoxin (61), pseurotin A (35), spirotryprostatins A (62) and spirotryprostatins G (63) (Fig. 7). The isolates showed potent antimicrobial activities against the human pathogenic microbes Escherichia coli, Staphyloccocus aureus, and Candida albicans with MIC values ranging from 0.39 to 12.5 μg mL−1. (Methylthio) gliotoxin (60) and spirotryprostatin G (63) are the most active inhibitors of C. albicans (MIC 0.39 μg mL−1). Pseurotin A (35) and spirotryprostatin A (62) demonstrated the highest antibacterial efficacy against S. aureus (MIC 0.39 μg mL−1). Spirotryprostatin A (62) showed the highest antibacterial activity against E. coli (MIC 0.39 μg mL−1).23
10. Aspergillus micronesiensis
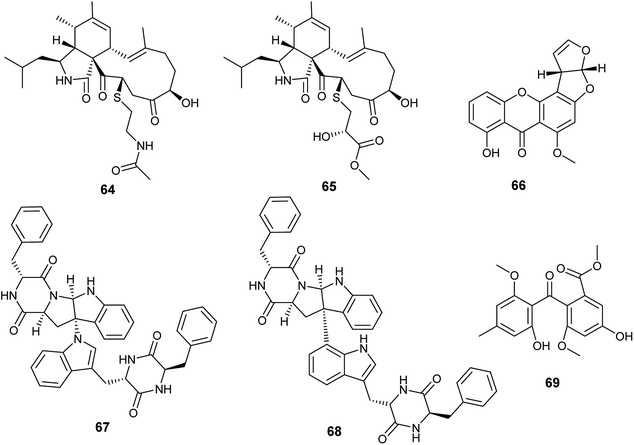
Cyschalasins A (64) and B (65) are new types of merocytochalasin, which are originally cytochalasin derivatives obtained from the fungus A. micronesiensis, the endophyte isolated from the roots of the Chinese medicinal plant Phyllanthus glaucus (Fig. 8). Cyschalasins A (64) exhibited strong cytotoxicity against the human cancer cell line SW480 with IC50 value of 10.1 ± 0.3 μM. Additionally, it displayed cytotoxic effects against the human cancer cell lines Hep3β and MCF-7 with IC50 values of 19.9 ± 0.6 μM and 16.1 ± 0.7 μM, respectively, and moderate cytotoxicity against HL-60 cell line (IC50 9.3 ± 0.2 μM). Furthermore, cyschalasin A (64) showed antimicrobial activities against Staphyloccocus aureus, methicillin-resistant Staphyloccocus aureus (MRSA) and Candida albicans with MIC values of (40.8 ± 5.0), (17.5 ± 0.3) and (43.3 ± 1.5) μg mL−1. Thus, the highest antibacterial activity was against MRSA with MIC50, and MIC90 values of 17.5 ± 0.3 and 28.4 ± 0.1 μg mL−1, respectively. Cyschalasin B (65) showed potent cytotoxicity against the human cancer cell line Hep3β (8.2 ± 0.1 μM) and moderate cytotoxicity against the cancer cell lines HL-60, MCF-7, SW480 and A549 with IC50 values of (3.0 ± 0.0), (17.1 ± 0.2), (13.6 ± 1.1) and (16.7 ± 0.9) μg mL−1, respectively. Cyschalasin B (65) possessed antimicrobial activities against S. aureus, MRSA and C. albicans with MIC values of (14.5 ± 0.4), (10.6 ± 0.1) and (94.7 ± 1.3); the highest activity was against MRSA with MIC50 and MIC90 values of 10.6 ± 0.1 and 14.7 ± 0.0 μg mL−1, respectively.2411. Aspergillus nidulans
Recently in 2019, Sana et al. isolated the endophytic fungus Aspergillus nidulans associated with Nyctanthes arbor-tristis Linn.25 Chemical investigation of the fermented culture of Aspergillus nidulans resulted in the isolation of the xanthone sterigmatocystin (66) (Fig. 8), which was tested against the human cancer cell lines lung (NCI-H460), breast (MCF-7) and uterine cervix (HeLa). Sterigmatocystin (66) demonstrated cytotoxic activity against breast (MCF-7) cell line only with IC50 value of 50 ± 2.5 μM mL−1.2512. Aspergillus niger
Asperazine A (67) and asperazine (68) are diketopiperazine heterodimer alkaloids isolated as a result of fermentation of the culture broth of the endophytic fungus A. niger hosted in the liverwort Heteroscyphus tener (Steph.) Schiffn. (Fig. 8). Both compounds (67) and (68) exhibited weak cytotoxicity against A2780 cell line with the IC50 values of 56.7 and 56.3 μM, respectively.26Methylsulochrin (69) is a diphenyl ether derivative isolated from the fermented culture of the endophytic fungus A. niger associated with the stems of the plant Acanthus montanus (Fig. 8). It showed antibacterial activities against the microbes Staphylococcus aureus, Enterobacter cloacae and Enterobacter aerogenes with MIC values of 15.6, 7.8 and 7.8 μg mL−1, respectively.27
13. Aspergillus oryzae
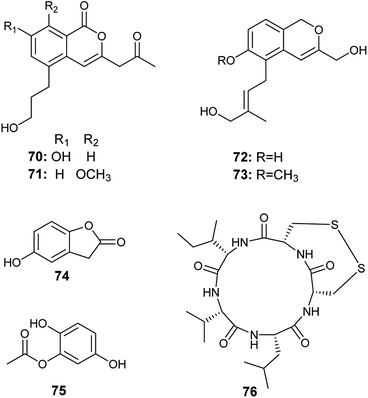
The isocoumarin derivatives oryzaeins A–D (70–73) were obtained from the solid culture of the fungus A. oryzae, the endophyte hosted in the rhizome of Paris polyphylla var. yunnanensis (Fig. 9). The four compounds showed anti-tobacco mosaic virus activity with inhibition rates ranging from 30.6% to 22.4% at the concentration of 20 μM. Oryzaeins A (70) and oryzaeins B (71) displayed the highest anti-tobacco mosaic virus activities with inhibition rates of 28.4% and 30.6%, respectively. Besides, the four compounds exhibited moderate inhibitory activities against the tumor cell lines NB4, A549, SHSY5Y, PC3 and MCF7 with IC50 values ranging from 2.8–8.8 μM. Oryzaein B (71) was the most active compound against the tumor cell lines NB4, A549, SHSY5Y, PC3 and MCF7 with IC50 values of 2.8, 4.2, 3.5, 4.8 and 3.0 μM, respectively.28The metabolites 5-hydroxy-benzofuran-2 (3H)-one (74) and 2, 5-di-hydroxyphenyl acetate (75) were isolated from the culture of A. oryzae, the endophyte harboring in the plant Lycium ruthenicum Murr. (Fig. 9). They showed antioxidant activity in DPPH radical scavenging assay with IC50 of 4.58 and 7.00 μg mL−1.29
14. Aspergillus tamarii
Malformin E (76) is a cyclic pentapeptide isolated from the culture broth of A. tamarii, the endophyte colonized in the roots of Ficus carica (Fig. 9). It is a potent cytotoxic agent against the human cancer cell lines MCF-7 and A549 with IC50 values of 0.65 and 2.42 μM, respectively. Besides, it exhibited noticeable antimicrobial activities against Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Penicillium chrysogenum, Candida albicans, and Fusarium solani with MIC values of 0.91, 0.45, 1.82, 0.91, 3.62, 7.24, and 7.24 μM, respectively.815. Aspergillus terreus
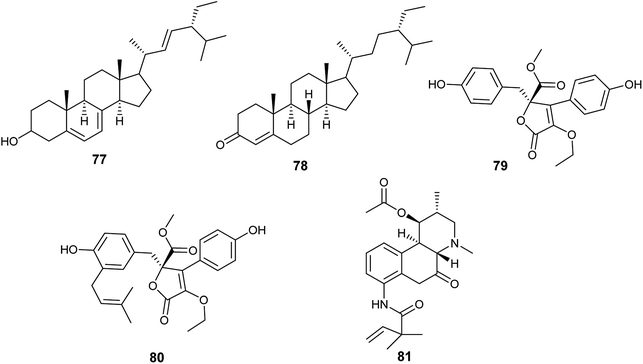
Until 2019, A. terreus was the most frequently isolated endophytic fungus, from which twenty-three bioactive compounds were produced.In 2015, Aspergillus terreus was derived from the roots of Carthamus lanatus and its fermentation led to the isolation of, the sterols, (22E,24R)stigmasta-5,7,22-trien-3-β-ol (77) and stigmast-4-ene-3-one (78) in addition to, the butenolides, terrenolide S (79) and aspernolide F (80) (Fig. 10). (22E,24R)Stigmasta-5,7,22-trien-3β-ol (77) showed antimicrobial activities against Staphylococcus aureus and Cryptococcus neoformans with IC50 values of 28.54 and 4.38 μg mL−1, respectively. Additionally, (22E,24R)stigmasta-5,7,22-trien-3β-ol (77) exhibited potent antibacterial activity against methicillin-resistant Staphyloccocus aureus (MRSA) with IC50 0.96 μg mL−1. (22E,24R). Stigmasta-5,7,22-trien-3β-ol (77) and stigmast-4-ene-3-one (78) demonstrated anti-leishmanial activity against Leishmania donovani with IC50 values of 4.61 and 6.31 μg mL−1, and IC90 values of 6.02 and 16.71 μg mL−1, respectively. Terrenolide S (79) exhibited antileishmanial activity against L. donovani with IC50 value of 27.27 μM and IC90 of 167.03 μM. Aspernolides F (80) showed mild antibacterial activity against MRSA with an IC50 value of 6.39 μg mL−1, and mild antifungal activity against C. neoformans with IC50 value of 5.19 μg mL−1.30,31 Moreover, aspernolide F (80) possessed a cardioprotective effect against DOX-induced cardiotoxicity.32
The alkaloid fumigaclavine I (81) was obtained from the endophytic fungus A. terreus isolated from the stem of rice (Fig. 10). It showed mild cytotoxic activity against human hepatocarcinoma cell line (SMMC-7721) with IC50 value of 7.62 μg mL−1.33
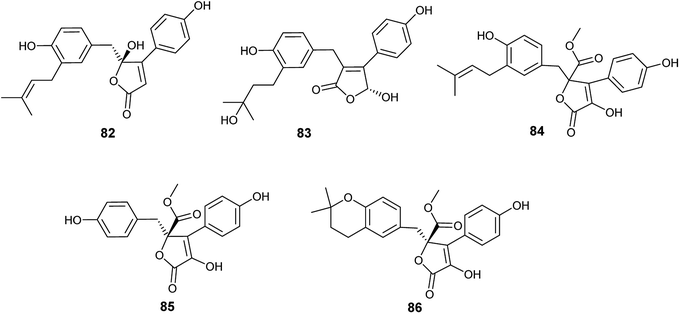
Five bioactive butenolides were isolated from the fermented culture of the endophytic fungus A. terreus obtained from the leaves of Camellia sinensis var. assamica, identified as asperteretal A (82) and C (83) in addition to butyrolactones I (84) and II (85) along with aspernolides A (86) (Fig. 11). Asperteretal A (82), C (83) and butyrolactone I (84) showed potent anti-inflammatory activity with IC50 values of 26.64, 16.80 and 17.21 μM, respectively while, butyrolactone II (85) and aspernolide A (86) displayed good anti-inflammatory activity with IC50 values of 44.37 and 45.37 μM, respectively. Furthermore, butyrolactone I (84) and aspernolide A (86) exhibited moderate cytotoxicity against human leukemia (HL-60) cell line with IC50 values of 18.85 and 39.36 μM, respectively.34
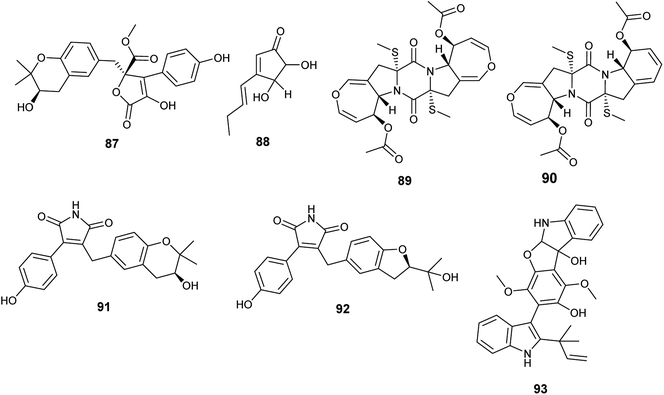
Butyrolactone I (84), butyrolactone V (87) and terrein (88) are butenolides isolated from the endophytic fungus A. terreus obtained from Hyptis suaveolens (L.) Poit. (Fig. 12). Butyrolactone I (84) possessed antioxidant effect and exhibited strong anticancer activity against the breast cancer cell line MCF-7 with IC50 value of 17.4 μM. Additionally, butyrolactone I (84) showed schistosomicidal activity against Schistosoma mansoni adult worms and antimicrobial activity against Escherichia coli. Butyrolactone V (87) displayed strong antioxidant activity, antischistosomal activity, and potent cytotoxicity against the breast cancer cell line MDA-MB-231 with IC50 value of 22.2 μM. Terrein (88) showed intermediate antioxidant activity and antischistosomal activity.35 The antioxidant activity of these compounds is related to the presence of the hydroxyl groups in lactone and aromatic rings; with resonance, hydrogen atoms can be easily released from the hydroxyl groups to stabilize free radicals.36
Bisdethiodis(methylthio)-acetylaranotin (89) and bisdethiodis(methylthio)-acetylapoaranotin (90) are epipolythiodiketopiperazines alkaloids (ETPs) isolated from A. terreus PR-P-2 hosted in Camellia sinensis var. assamica (Fig. 12). Bisdethiodis(methylthio)-acetylaranotin (89) was the most powerful compound in inhibiting human promyelocytic leukemia cells carcinoma (HL-60) with IC50 value of 9.34 μmol L−1 followed by bisdethiodis(methylthio)-acetylapoaranotin (90) with IC50 value of 16.30 μmol L−1.37 Reports declared that the diverse bioactivities of ETPs are strongly related to the presence of the disulfide bridge in the diketopiperazine structure.38
New butenolides with maleimide core named asperimides C (91) and D (92) along with butyrolactone I (84) were isolated from the fermented culture of the endophyte Aspergillus terreus derived from the tropical plant Suriana maritima L. (Fig. 12). Asperimides C (91), D (92) and butyrolactone I (84) showed potent anti-inflammatory activity with IC50 values of 0.78, 1.26 and 24.2 μM, respectively.39
Giluterrin (93) is a prenyl indole alkaloid obtained as a result of fermentation of the solid culture of the fungus A. terreus isolated from the roots of the grass Axonopus leptostachyus (Fig. 12). It displayed cytotoxicity against the human cancer cell lines 786-0 (kidney) and PC3 (prostate) with IC50 values of 22.93 and 48.55 μM, respectively.40
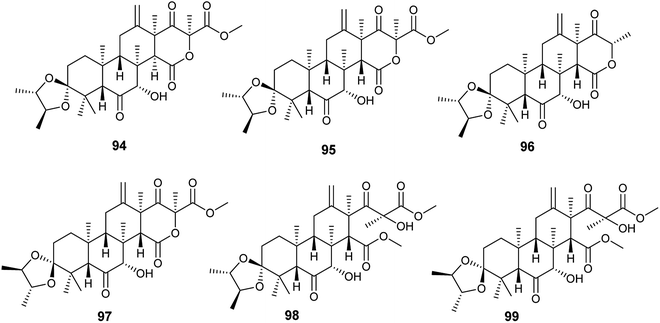
Spiroterreusnoids A–F (94–99) are meroterpenoid derivatives obtained from the endophytic fungus A. terreus isolated from the roots of Tripterygium wilfordii Hook. f. (Fig. 13). The isolated spiroterreusnoids exhibited anti-Alzheimer's activity for its capability to inhibit β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) with IC50 values ranging from 5.86 to 27.16 μM, and inhibit acetylcholinesterase enzyme (AchE) with IC50 values ranging from 22.18 to 32.51 μM. Spiroterreusnoids A–F (94–99) share the same spiro-dioxolane moiety, which is responsible for the BACE1 and AchE inhibitory potential.41
16. Aspergillus tubingensis
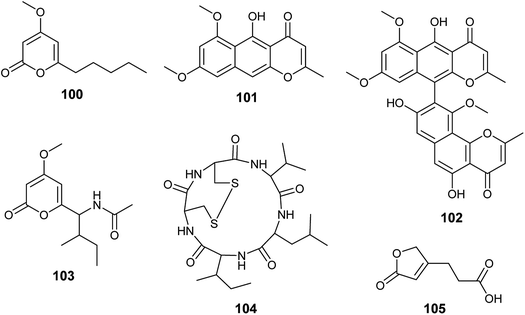
Chemical investigation of the fermented culture of the endophytic fungus A. tubingensis isolated from the plant Lycium ruthenicum resulted in the isolation of four pyrone derivatives identified as 6-isovaleryl-4-methoxy-pyran-2-one (100), rubrofusarin B (101), asperpyrones A (102) and campyrone A (103) (Fig. 14). Rubrofusarin B (101) possessed strong antibacterial activity against Escherichia coli with MIC value of 1.95 μg mL−1 while, the metabolites (100), (102) and (103) showed weak antibacterial activities against Escherichia coli, Pseudomonas aeruginosa, Streptococcus lactis and Staphylococcus aureus with MIC values ranging from 62.5 to 500 μg mL−1. Besides, all the isolated compounds displayed weak antioxidant activity in DPPH free radical scavenging assay (IC50 = 109.34–711.3 μg mL−1); asperpyrones A (102) was most active compound as an antioxidant (IC50 = 109.34 μg mL−1), which may be because it contains the largest number of hydroxyl groups attached to aromatic rings.42,36Malformin A1 (104) is a cyclic penta-peptide produced by the endophyte A. tubingensis isolated from the stem of Brucea javanica (L.) Merr. (Fig. 14). A strong inhibitory effect was shown by malformin A1 (104) against infection and replication of the tobacco mosaic virus (TMV) using local lesion assay and leaf-disk method with IC50 values of 19.7 and 45.4 μg mL−1, respectively.43
3-(5-oxo-2,5-Dihydrofuran-3-yl)propanoic acid (105) is a furan derivative isolated from A. tubingensis, the endophyte associated with the stems of Decaisnea insignis (Griff.) Hook. f. (Fig. 14). It showed potent antifungal activity against Fusarium graminearum (MIC 16 μg mL−1) and moderate antibacterial activity against Streptococcus lactis (MIC 32 μg ML−1).44
17. Aspergillus versicolor
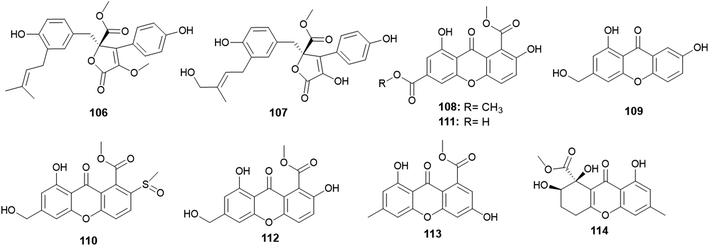
A. versicolor strain was frequently isolated as much as A. terreus, but A. versicolor outperformed A. terreus in the number of isolated metabolites. Twenty-five new bioactive metabolites were isolated and identified.Aspernolides C (106) and D (107) are butenolides obtained from A. versicolor, the endophyte colonized in the rhizomes of Paris polyphylla var. yunnanensis (Fig. 15). Aspernolides C (106) and D (107) displayed moderate anti-tobacco mosaic virus (anti-TMV) activity with IC50 values of 64.2 and 88.6 μM, respectively.45
The xanthones, named huperxanthones A–C (108–110), along with 1,7-dihydroxy-8(methoxycarbonyl)xanthone-3-carboxylic acid (111), β-diversonolic acid methyl ester (112), 4-hydroxyvertixanthone (113) and sydowinin B (114) were obtained from the endophytic fungus A. versicolor isolated from the stems of the medicinal plant Huperzia serrata (Fig. 15). All isolates showed weak inhibitory activity against α-glucosidase enzyme; however, 1,7-dihydroxy-8-(methoxycarbonyl)xanthone-3-carboxylic acid (111) was the most potent α-glucosidase inhibitor with an IC50 value of 0.24 μM.46
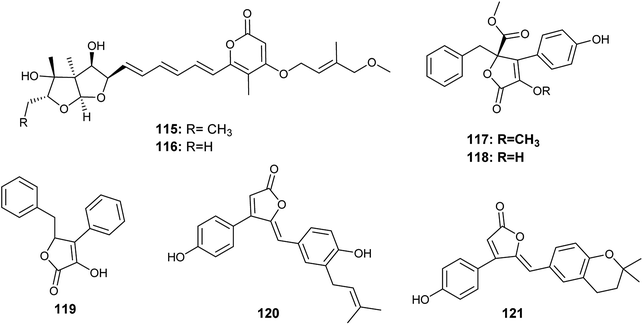
Avertoxins B (115) and C (116) are prenyl asteltoxin derivatives obtained from the culture of A. versicolor, the endophyte isolated from the leaves of Huperzia serrata (Fig. 16). Avertoxins B (115) showed cytotoxicity against HCT 116 cell line with an IC50 value of 9 μM. In addition, avertoxins B (115) was an acetylcholinesterase (AchE) inhibitor with an IC50 value of 14.9 μM. Avertoxin C (116) exhibited cytotoxicity against the cancer cell lines Hela and HCT 116 with IC50 values of 11 and 21 μM, respectively.47
K. Zhou et al., reported the isolation of five butyrolactones identified as versicolactones E (117) and F (118) together with, xenofuranone B (119), rubrolide R (120) and S (121) from the fermented broth of the endophytic fungus A. versicolor isolated from the rhizome of Paris polyphylla var. yunnanensis48 (Fig. 16). The isolated butyrolactones displayed moderate inhibitory activities against tobacco mosaic virus (TMV) with an inhibition rates ranging from 14.6% to 18.8%.48
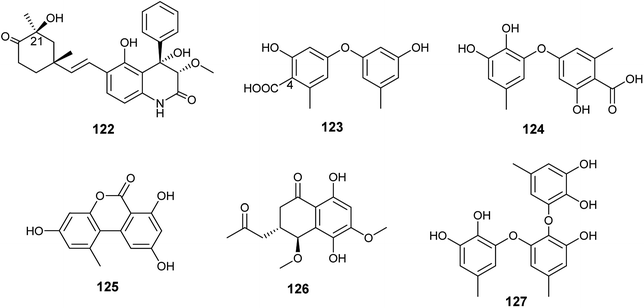
Ebada et al., reported the isolation of different classes of metabolites from the endophytic fungus A. versicolor colonized in the Egyptian aquatic plant Eichhornia crassipes.7 Among them, the alkaloid aflaquinolone H (122) together with the diphenyl ether derivatives, 4-carboxydiocrinol (123) and gerfelin (124), as well as the xanthones, sterigmatocystin (66) and aternariol (125) (Fig. 17). Aflaquinolone H (122) is a new dihydro-quinolone alkaloid that showed moderate proliferative inhibitory activity against mouse lymphoma (L5178Y) cell line with an IC50 value of 10.3 μM. Aflaquinolone H (122) was the only active compound among the isolated alkaloids, which was supposed to be due to presence of OH group at C-21. Moreover, 4-carboxydiocrinol (123) showed high cytotoxicity against mouse lymphoma (L5178Y) cell line with an IC50 value of 0.36 μM. Gerfelin (124) exhibited weak anti-proliferative activity against mouse lymphoma (L5178Y) cell line with IC50 value > 30 μM. It is noticeable that 4-carboxydiocrinol was the most active compound among the tested diphenyl ether derivatives; therefore, the presence of the carboxylic group at position C-4 is critical for cytotoxic activity. Sterigmatocystin (66) is a toxic xanthone derivative that has potent cytotoxicity to mouse lymphoma cell line with IC50 value of 2.2 μM. Alternariol (125) is a pyrano xanthone derivative exhibited moderate cytotoxicity to mouse lymphoma (L5178Y) cell line with IC50 value of 16.3 μM.7
Abdelwahab et al., tried OSMAC approach and co-cultivation of Bacillus subtilis bacterium with the endophytic fungus A. versicolor, which led to the isolation of aspvanicin B (126), sydowiol B (127) and sterigmatocystin (66)49 (Fig. 17). Aspvanicin B (126) is a new dihydronaphthalenone derivative, it displayed mild to weak cytotoxicity against mouse lymphoma (L5178Y) cell line with IC50 value of 22.8 μM. Sydowiol B (127) is a diphenyl ether derivative that showed weak antibacterial activity against Staphylococcus aureus with MIC value of 50 μM. Sterigmatocystin (66) is a xanthone derivative, it displayed strong anti-proliferative effect against mouse lymphoma cell line (L5178Y) with IC50 value of 2.2 μM.49
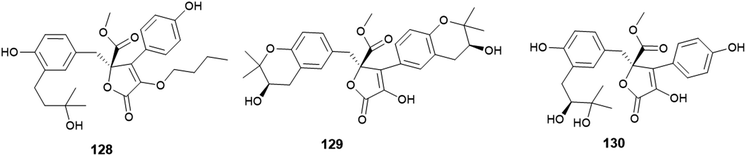
The γ-butyrolactone derivatives; aspernolides L (128) and M (129) along with butyrolactones I (84) and VI (130) were obtained from A. versicolor, the endophytic fungus hosted in the roots of Pulicaria crispa (Asteraceae) (Fig. 18). Aspernolide L (128) exhibited moderate antimicrobial activities against Candida albicans, Aspergillus fumigatus, Escherichia coli and Pseudomonas aeruginosa with IC50 values ranging from 2.78 to 6.04 mM; also, it showed anti-leishmanial activity against Leishmania donovani with IC50 value of 2.31 mM and IC90 value of 5.67 mM. Furthermore, the radioligand displacement affinity of aspernolide L (128) on human cannabinoid and opioid receptors was estimated, it showed a good binding affinity towards the CB1 receptor with displacement value of 71.2%. Aspernolide L (128) displayed cytotoxicity against SK-MEL cell line with IC50 value of 0.70 mM. Aspernolide M (129) displayed moderate antimicrobial activities against C. albicans, A. fumigatus, E. coli and P. aeruginosa with IC50 values ranging from 2.60 to 6.04 mM. It showed anti-leishmanial activity against L. donovani with IC50 value of 3.47 mM and IC90 value of 3.89 Mm, and antimalarial activity against D6 and D6S1 clones (chloroquine-sensitive strains of Plasmodium falciparum) with IC50 values of 2.16 and 1.43 mM, respectively. Moreover, aspernolide M (129) displayed a good binding affinity towards CB1 receptor with displacement value of 80.5%, and affinity to d-, k- and m-receptors with displacement values of 61.2, 73.5 and 61.3% respectively. Furthermore, aspernolide M (129) exhibited the highest cytotoxicity against the cancer cell lines KB, SK-MEL, BT-549 and SKOV-3 with IC50 values of 1.2, 0.9, 0.1 and 0.8 mM, respectively. Butyrolactone I (84) possessed moderate antimicrobial activities against Cryptococcus neoformans and Aspergillus fumigates with IC50 values of 7.90 and 9.75 mM, respectively. Butyrolactones VI (130) showed a good binding affinity towards the CB1 receptor with a displacement value of 66.1%.50
18. Aspergillus sp.
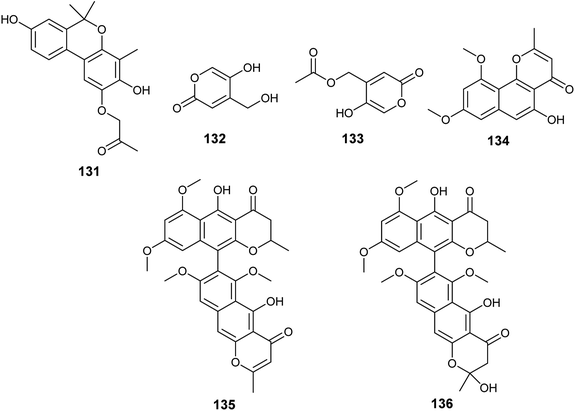
Several unidentified Aspergillus species were isolated and produced many secondary metabolites with exciting biological activities.The metabolites, 1-(3,8-dihydroxy-4,6,6-trimethyl-6H-benzochromen-2-yloxy)propane-2-one (131), 5-hydroxy-4-(hydroxymethyl)-2H-pyran-2-one (132) and (5-hydroxy-2oxo-2H-pyran-4-yl)methyl acetate (133) were obtained from the fermented culture of the endophyte Aspergillus sp. (SbD5) isolated from the leaves of Andrographis paniculata (sambiloto) (Fig. 19). The isolated metabolites showed mild to moderate antibacterial activities against Staphylococcus aureus, Escherichia coli, Shigella dysenteriae and Salmonella typhi with zone of inhibition diameters ranging from 8.1 ± 0.3 to 12.1 ± 0.3 mm at a concentration 500 μg mL−1. Compound (131) showed the highest antibacterial activity against the tested strains followed by the compound (132) then (133). The increased activity among the isolated metabolites is associated with the presence of free hydroxyl groups in the compound; whenever hydroxyl groups increase the antibacterial activity increases.51
Flavasperone (134), rubrofusarin B (101), aurasperone A (135) and fonsecinone D (136) are polyketide metabolites produced by the fungus Aspergillus sp. associated with the seeds of the edible fruit Limonia acidissima L. (Fig. 19). They showed moderate activity in the brine shrimp assay with LD50 values of 32, 50, 9 and 40 ppm, respectively.52
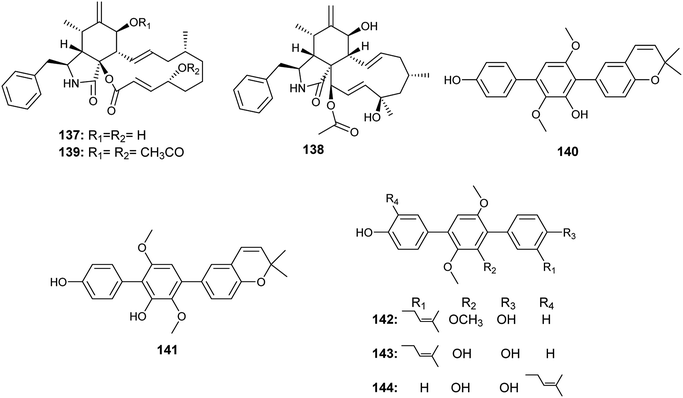
The cytochalasins, cytochalasin B (137) and H (138) were isolated from an Aspergillus sp. EJC08 obtained from Bauhinia guianensis. Furthermore, 7,20-diacetyl-cytochalasin B (139) was synthesized by acetylation of cytochalasin B (137). Cytochalasin B (137) and its diacetate derivative (139) showed high lethality against Artemia salina with LC50 values of 24.9 and 22.8 ± 2.85 μg mL−1, respectively. Cytochalasin H (138) showed good lethality against Artemia salina with LC50 value of 69.1 ± 2.24 μg mL−1.53
In 2017, Yan et al. reported the isolation of the ρ-terphenyls, named prenylterphenyllin D (140), prenylterphenyllin E (141), 2′-O-methylprenylterphen (142), prenylterphenyllin (143) and prenylterphenyllin B (144), as a result of fermentation of an Aspergillus sp. derived from Ginkgo biloba leaves54(Fig. 20). The compounds (140), (141), (142) and (143) exhibited the same antibacterial activities against Xanthomonas oryzae and Erwinia amylovora with MIC value of 20 μg mL−1. Prenylterphenyllin B (144) showed antibacterial activities against Erwinia amylovora and Xanthomonas oryzae with MIC values of 10 and 20 μg mL−1, respectively.54
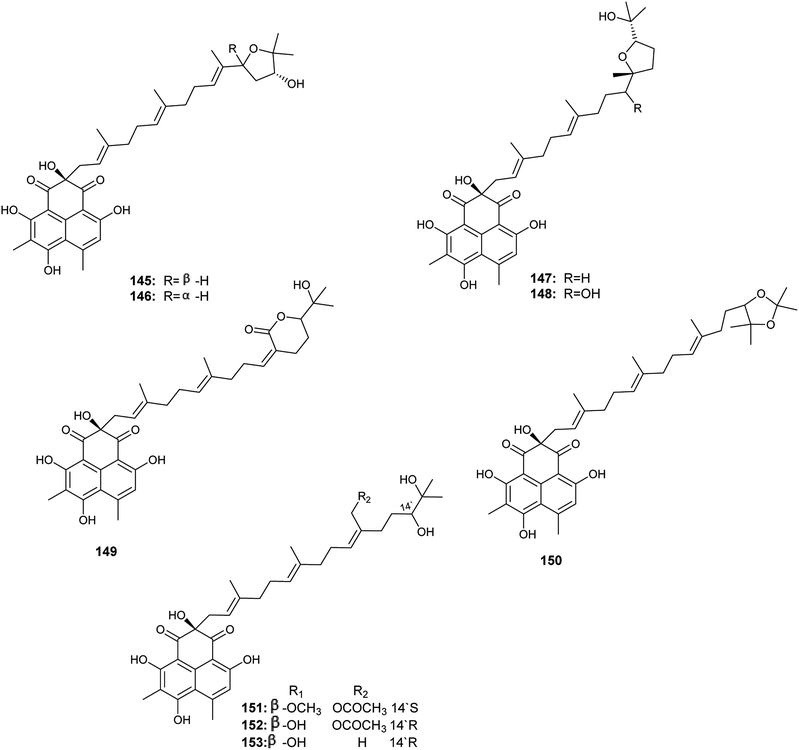
Aspergillussanones D–L (145–153) are phenalenones isolated by Gombodorj et al. from the endophytic fungus Aspergillus sp. obtained from the tubers of Pinellia ternata55 (Fig. 21). All the identified aspergillussanones have the same phenalenone moiety and differ in the acyclic diterpenoid moiety. Aspergillussanone L (153) showed high antibacterial activities against Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis with MIC50 values of 1.87, 2.77 and 4.8 μg mL−1 respectively. Aspergillussanone H (149), I (150) and K (152) showed antibacterial activity against P. aeruginosa with MIC50 values of 8.59, 12.0 and 6.55 μg mL−1 respectively. Aspergillussanones E (146), F (147), H (149) and J (151) showed antibacterial activity against Escherichia coli with MIC50 values of 7.83, 3.93, 5.87 and 5.34 μg mL−1. Aspergillussanones D (145) and G (148) possessed weak antibacterial activities against P. aeruginosa with MIC50 values of 38.47 and 24.46 μg mL−1, respectively, and against S. aureus with MIC50 values of 29.91 and 34.66 μg mL−1, respectively.55
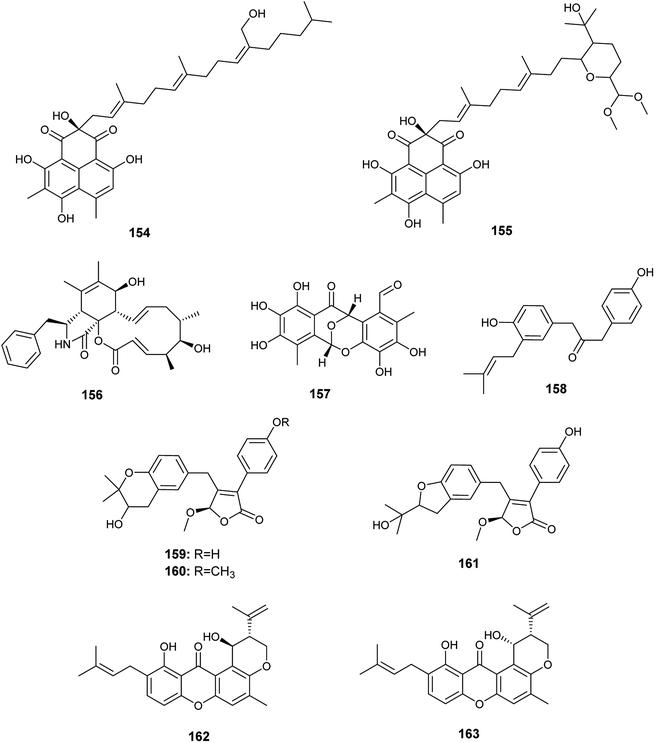
Pang et al. reported the isolation of a large diversity of secondary metabolites from the endophyte Aspergillus sp. CPCC 400735 derived from Kadsura longipedunculata, identified as the phenalenones, asperphenalenones A (154) and D (155) together with cytochalasin Z8 (156) and the phenyl derivative, epicocconigrone A (157)56 (Fig. 22). Asperphenalenones A (154) and D (155) exhibited activity against human immunodeficiency virus (HIV) with IC50 values of 4.5 μM and 2.4 μM respectively. Cytochalasin Z8 (156) and epicocconigrone A (157) displayed anti-HIV activity with IC50 values of 9.2 and 6.6 μM respectively.56
In 2018, Qi et al. isolated the butenolide derivatives; terrusnolides A–D (158–161), from an Aspergillus sp. derived from Tripterygium wilfordii57 (Fig. 22). The four terrusnolides are potent anti-inflammatory agents with IC50 values ranging from 16.21 to 38.15 μM.57
The pyrano xanthone derivatives, isoshamixanthone (162) and epiisoshamixanthone (163) were isolated from the fermented culture of the endophytic fungus Aspergillus sp. ASCLA, which is derived from the leaves of the medicinal plant Callistemon subulatus (Fig. 22). Isoshamixanthone (162) and epiisoshamixanthone (163) showed moderate antimicrobial activities against Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Saccharomyces cerevisiae, Bacillus cereus and Bacillus subtilis ATCC 6633 with zone of inhibition diameters ranging from 6 to 11 mm.10
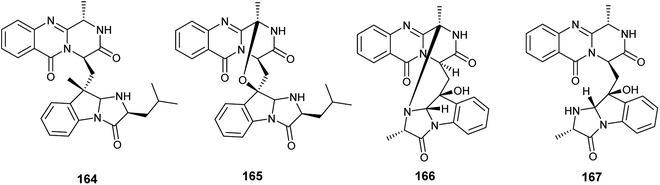
In 2018, six quinazoline alkaloids identified as fumiquinazoline J (51), fumiquinazoline I (164), fumiquinazoline C (52), fumiquinazoline H (165), fumiquinazoline D (166) and fumiquinazoline B (167) were isolated from the fermented culture of the fungus Aspergillus sp., the endophyte hosted in the roots of Astragalus membranaceus (Fig. 23). The isolated alkaloids were evaluated for their antimicrobial activities against the four bacterial strains, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, as well as the three fungi, Candida albicans, Fusarium solani, and Penicillium chrysogenum. Fumiquinazoline D (166) showed the highest antibacterial activity against the tested strains of bacteria with MIC values of 0.5, 1, 1 and 0.5 μg mL−1, respectively; in addition, it showed strong antifungal activities against the tested fungal strains with MIC values of 4, 4 and 2 μg mL−1, respectively. The metabolites, fumiquinazoline J (51), fumiquinazoline C (52) and fumiquinazoline H (165) showed strong antibacterial and antifungal activities against the tested strains with MIC values ranges from 0.5 to 16 μg mL−1. However, fumiquinazoline I (164) and fumiquinazoline B (167) possessed the least moderate antibacterial and antifungal activities with MIC values ranging from 4 to 32 μg mL−1.58
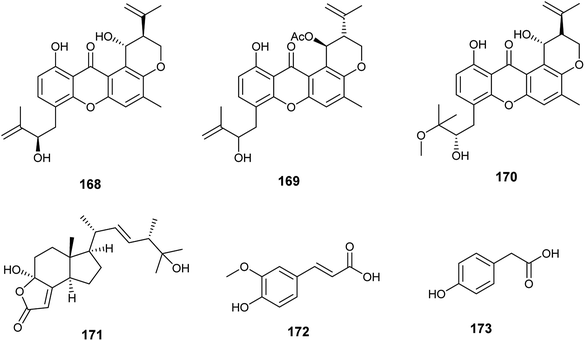
The xanthones, ruguloxanthone C (168), asperanthone (169) and tajixanthone hydrate (170), together with the butenolide, salimyxin B (171), and the sterol, ergosterol (33), were produced by the endophytic fungus Aspergillus sp. TJ23 harboring in the leaves of the medicinal plant Hypericum perforatum L. (Fig. 24). Ruguloxanthone C (168) displayed cytotoxic activities against the human cancer cell lines B16, HepG2, and LLC with IC50 values of 35.6, 29.5, and 32.7 μM. Besides, the compounds 169, 170 and 171 exhibited cytotoxicity against HepG2 cancer cell line with IC50 values of 35.5, 36.8, and 9.87 μM, respectively. Ergosterol (33) showed potent inhibitory activities against the cancer cell lines HepG2, B16, LLC, MDA-MB-231 and 4T1 with IC50 values of (5.31 ± 0.03), (6.52 ± 0.02), (7.31 ± 0.02), (7.36 ± 0.03) and (12.3 ± 0.04) μM respectively.59
Ferulic acid (172) and ρ-hydroxyphenyl acetic acid (173) are phenolic acids isolated from the fermented culture of the endophytic fungus Aspergillus sp. associated with the leaves of the plant Moringa oleifera (Fig. 24). Ferulic acid (172) exhibited mild antifungal activity against Aspergillus niger with MIC value of 2 mm (at conc. 500 μg mL−1), and strong antioxidant activity (at conc. 250 μg mL−1) with inhibition of 90.4%. ρ-Hydroxyphenyl acetic acid (173) exhibited a mild antioxidant activity (at conc. 250 μg mL−1) with inhibition of 35.4%.60
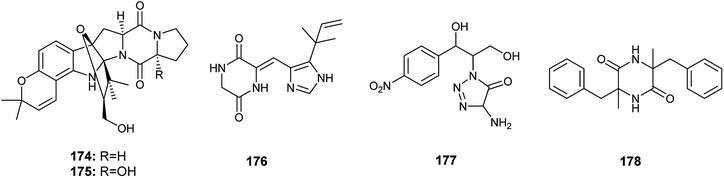
Gartryprostatins A (174), B (175) and C (176) are indolyl diketopiperazines alkaloids obtained from the fermented culture of the endophyte Aspergillus sp. GZWMJZ-258 isolated from the fruits of Garcinia multiflora (Fig. 25). The three alkaloids (174), (175) and (176) exhibited good cytotoxicity against MV4-11 cell line with IC50 values of 7.2, 10.0, and 0.22 μM, respectively. In addition, they showed weak cytotoxic activities against K562, HL-60, and A549 cell lines with IC50 values more than 10.0 μM. It was reported that the strong cytotoxicity of gartryprostatins C (176) against the MV4-11 tumor cells (IC50 0.22 μM) is due to its structural similarity to plinabulin, a phase 3 drug against febrile neutropenia and non-small-cell lung cancer.61
The alkaloids, 4-amino-1-(1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl)-1H-1,2,3-triazole-5(4H)one (177) and 3,6-dibenzyl-3,6-dimethylpiperazine-2,5-dione (178) were produced by an Aspergillus sp. isolated from the rhizome of Zingiber cassumunar Roxb. (Fig. 25). 4-Amino-1-(1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl)-1H-1,2,3-triazole-5(4H)one (177) showed strong antibacterial activities against Xanthomonas oryzae, Bacillus subtilis and Escherichia coli with zone of inhibition diameters 37, 30 and 27 mm, respectively. 3,6-Dibenzyl-3,6-dimethylpiperazine-2,5-dione (178) displayed moderate antibacterial activities against Escherichia coli and Xanthomonas oryzae with zone of inhibition diameters 21 and 16 mm.62
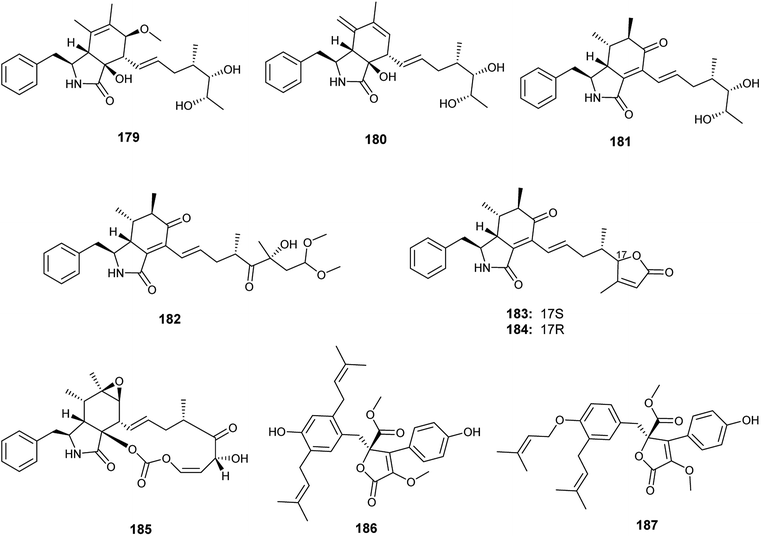
Recently in 2019, Xin et al. reported the isolation of the new seco-cytochalasins, asperchalasins A–F (179–184), along with the cytochalasins, cytochalasin E (185), cytochalasin Z17 (15) and rosellichalasin (9), as well as the butenolides, asperlactone G (186) and H (187), from the fermented culture of the endophyte Aspergillus sp. obtained from Pinellia ternata tubers63 (Fig. 26). Asperchalasin E (183) and F (184) are unusual seco-cytochalasins as their side chains contain an α,β-unsaturated furanone structure. All isolated compounds demonstrated cytotoxicity against the human lung cancer (A-549) cell line with IC50 values ranging from 7.8 to 65.3 μM; cytochalasin E (185) was the most active compound with an IC50 value of 7.8 μM. On the other hand, asperchalasin D (182) enhanced the effect of doxorubicin on doxorubicin-resistant human breast cancer at conc. 16 μM.63
19. Conclusion and future perspectives
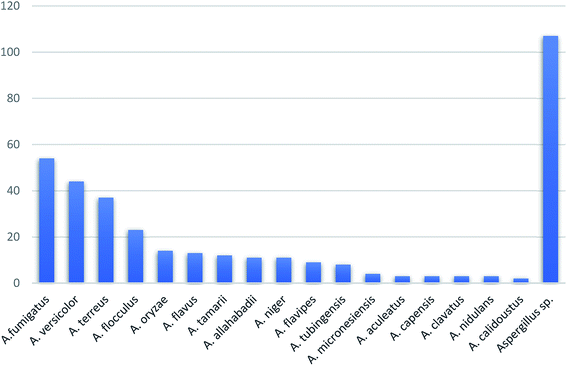
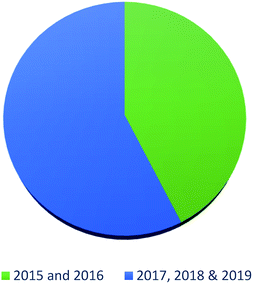
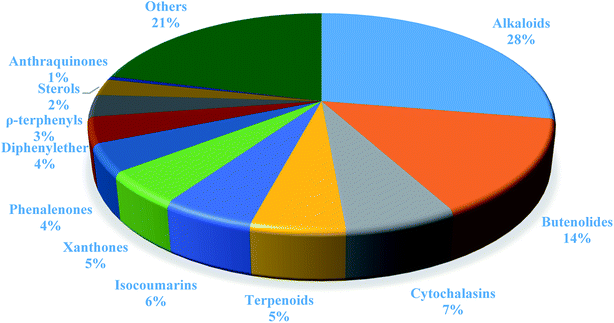
In summary, fungal endophytes are the fungal population of the internal tissues of plants causing no apparent symptoms of disease. These endophytic fungal organisms are able to produce certain phytochemicals originally specific to the host plant, which is thought to be due to the genetic recombination between the endophyte and the host plant that occurred in the evolutionary period. In addition, fungal endophytes are reported to produce new secondary metabolites totally different from those characteristic to the host plant, so it is a precious source of novel bioactive natural products and an alternative source for phytochemicals initially produced by higher plants. The genus Aspergillus is among the well-known genera of fungal endophytes. Aspergillus endophytes are considered as a rich source of new secondary metabolites with valuable biological activities and the secured way for drug discovery. There are many different species of Aspergillus. In this review, seventeen Aspergillus species were reported. Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus oryzae, Aspergillus tubingensis, Aspergillus terreus, and Aspergillus versicolor are among the mostly isolated and identified endophytic Aspergillus species. This review revealed that A. fumigatus, A. versicolor and A. terreus are the predominant endophytes associated with different plants and are the most productive of secondary metabolites as shown in Fig. 27. The last review published in 2015 reported the isolation of 162 secondary metabolites in the period (2004–2015).64 In this review, which covers the period from January 2015 until December 2019, three hundred sixty-two secondary metabolites were reported, among them one hundred eighty-seven showed diverse biological activities, which ensures that the curiosity in studying Aspergillus endophytes is increased. The reported Aspergillus metabolites differs from year to year (Fig. 28); in 2015 and 2016, 152 compounds were illustrated. In 2017, 2018 and 2019 there was a significant increase in the newly identified compounds to be 210. The recent advances in chemical tools such as LC-MS could explain increased chances in the discovery of new metabolites. Metagenomics studies showed the chances for discovery of new species within the genus Aspergillus as well as new gene clusters. Alkaloids are the most prolific chemical class produced by different Aspergillus species followed by butenolides and cytochalasins as illustrated in Fig. 29. It was observed that A. fumigatus is the major producer of alkaloids while, butenolides are produced mainly by A. terreus. The isolated compounds showed anti-microbial, anti-leishamanial, anti-inflammatory, anti-HIV, anti-tobacco mosaic virus, schistosomicidal and anti-cancer activities in addition to α-glucosidase inhibition. Further investigations are recommended concerning endophytes to achieve promising scientific discoveries; these investigations should be directed towards fungal endophytes associated with the higher plants of valuable medicinal uses in order to produce the same phytochemicals isolated from those plants in an economical way. In addition, fungal endophytes can be manipulated genetically in order to produce high concentrations of certain natural products with high medicinal importance. Finally, intensive attention should be paid to the study of the mechanism of action of the frequently isolated fungal metabolites, such as butenolides, cytochalasins and alkaloids.AuthorContributions
H. S. B. collected a complete survey of all compounds isolated from the genus Aspergillus; H. S. B. and A. S. M. wrote the manuscript; U. R. A. interpreted and revised the results, and wrote the manuscript; S. S. E., R. M. and U. R. A. discussed the results scientifically and contributed to editing of the paper.Conflicts of interest
The authors declare that they have no conflicts of interest.References
- J. Soltani, New Futur. Dev. Microb. Biotechnol. Bioeng., Elseiver, 2016, pp. 275–292 Search PubMed
.
- H. Nisa, A. N. Kamili, I. A. Nawchoo, S. Sha, N. Shameem and S. A. Bandh, Microb. Pathog., 2015, 82, 50–59 CrossRef CAS PubMed
.
- R. M. P. Gutierrez, A. M. N. Gonzalez and A. M. Ramirez, Curr. Med. Chem., 2012, 19, 2992–3030 CrossRef CAS PubMed
.
- K. Sadorn, S. Saepua, N. Boonyuen, P. Laksanacharoen, P. Rachtawee, S. Prabpai, P. Kongsaeree and P. Pittayakhajonwut, Tetrahedron, 2016, 72, 489–495 CrossRef CAS
.
- V. Vadlapudi, N. Borah, K. R. Yellusani, S. Gade, P. Reddy, M. Rajamanikyam, L. N. S. Vempati, S. P. Gubbala, P. Chopra, S. M. Upadhyayula and R. Amanchy, Sci. Rep., 2017, 7, 1–10 CrossRef CAS PubMed
.
- Y. M. Lee, M. J. Kim, H. Li, P. Zhang, B. Bao, K. J. Lee and J. H. Jung, Mar. Biotechnol., 2013, 15, 499–519 CrossRef CAS PubMed
.
- S. S. Ebada, M. El-neketi, W. Ebrahim, A. Mándi, T. Kurtán, R. Kalscheuer, W. E. G. Müller and P. Proksch, Phytochem. Lett., 2018, 24, 88–93 CrossRef CAS
.
- Y.-M. Ma, X. Liang, H. Zhang and R. Liu, J. Agric. Food Chem., 2016, 64, 3789–3793 CrossRef CAS PubMed
.
- P. Wang, J. Yu, K. Zhu, Y. Wang, Z. Cheng, C. Jiang, J.-G. Dai, J. Wu and H. Zhang, Fitoterapia, 2018, 127, 322–327 CrossRef CAS PubMed
.
- R. A. Kamel, A. S. Abdel-razek, A. Hamed, R. Reham, H. G. Stammler, M. Frese, N. Sewald, R. Ibrahim, H. G. Stammler, M. Frese, N. Sewald and M. Shaaban, Nat. Prod. Res., 2018, 34, 1080–1090 CrossRef PubMed
.
- C. R. De Carvalho, M. D. L. Almeida, C. L. Cantrell, D. E. Wedge, T. M. A. Alves, C. L. Zani, R. S. Pimenta, P. A. Sales, S. M. F. Murta, A. J. Romanha, A. C. Rosa and L. H. Rosa, Nat. Prod. Res., 2015, 30, 37–41 Search PubMed
.
- J. Qin, A. Lyu, L. Yang, J. Zhang, M. De Wu and G. Li, Mol. Biol. Rep., 2019, 46, 3451–3460 CrossRef CAS
.
- J. Wang, G. Bai, Y. Liu, H. Wang, Y. Li, W. Yin, Y. Wang and F. Lu, Chem. Lett., 2015, 44, 1148–1149 CrossRef CAS
.
- N. Akhter, C. Pan, Y. Liu, Y. Shi and B. Wu, Nat. Prod. Res., 2019, 33, 2939–2944 CrossRef CAS
.
- Y. Ma, C. Ma, T. Li and J. Wang, Nat. Prod. Res., 2016, 30, 79–84 CrossRef CAS PubMed
.
- Z. Liu, J. Zhao, S. Sun, Y. Li, J. Qu, H. Liu and Y. Liu, J. Nat. Prod., 2019, 82, 1063–1071 CrossRef CAS PubMed
.
- A. F. Tawfike, M. Romli, C. Clements, G. Abbott, L. Young, M. Schumacher, R. Edrada-Ebel, M. Diederich, M. Farag and R. Edrada-Ebel, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2019, 1106, 71–83 CrossRef PubMed
.
- Y. Shi, Y. Zhang, X. Chen, N. Zhang and Y. Liu, Molecules, 2015, 20, 10793–10799 CrossRef CAS PubMed
.
- Z. Liang, T. Zhang, X. Zhang, J. Zhang and C. Zhao, Molecules, 2015, 20, 1424–1433 CrossRef PubMed
.
- J. P. Thakur, R. Haider, D. K. Singh, S. Kumar, P. G. Vasudev, A. Kalra, D. Saikia and A. S. Negi, Microbiol. Res., 2015, 6, 5800 Search PubMed
.
- X. Li, J. Zhou, Q. Xu, X. Wang, H. Yuan and H. Lou, Chem. Biodiversity, 2015, 12, 1313–1321 CrossRef PubMed
.
- S. Li, J. Chen, L. Qin, X. Li, Z. Cao, Y. Gu, D.-L. Guo and Y. Deng, J. Asian Nat. Prod. Res., 2019, 20, 1–6 CrossRef PubMed
.
- H. Zhang, C. Ruan, X. Bai, J. Chen and H. Wang, Chem. Nat. Compd., 2018, 54, 411–414 CrossRef CAS
.
- Z. Wu, X. Zhang, W. Hasan, A. Anbari, Q. Zhou, P. Zhou, M. Zhang, F. Zeng, C. Chen, Q. Tong, J. Wang, H. Zhu and Y. Zhang, J. Nat. Prod., 2019, 82, 2653–2658 CrossRef CAS PubMed
.
- T. Sana, B. S. Siddiqui, S. Shahzad, A. D. Farooq, F. Siddiqui, S. Sattar and S. Begum, Med. Chem., 2019, 15, 352–359 CrossRef CAS PubMed
.
- X. Li, Y. Li, J. Zhou, H. Yuan, N. Wang and H. Lou, J. Asian Nat. Prod. Res., 2015, 17, 182–187 CrossRef CAS PubMed
.
- I. K. Mawabo, C. Nkenfou, A. Notedji, J. B. Jouda, P. Lunga, P. Eke, V. T. Fokou and J.-R. Kuiate, Issues Biol. Sci. Pharm. Res., 2019, 7, 7–15 CrossRef
.
- M. Zhou, K. Zhou, P. He, K.-M. Wang, R.-Z. Zhu, Y.-D. Wang, W. Dong, G.-P. Li, H.-Y. Yang, Y.-Q. Ye, G. Du, X.-M. Li and Q.-F. Hu, Planta Med., 2016, 82, 414–417 CrossRef CAS PubMed
.
- X. Yang, P. Wang, Y. Ma and Q. Jia, Tianran Chanwu Yanjiu Yu Kaifa, 2015, 27, 1554–1557 CAS
.
- E. S. Elkhayat, S. R. M. Ibrahim, G. A. Mohamed and S. A. Ross, Nat. Prod. Res., 2015, 30, 814–820 CrossRef PubMed
.
- S. R. M. Ibrahim, E. S. Elkhayat, G. A. Mohamed, A. I. M. Khedr, M. A. Fouad, M. H. R. Kotb and S. A. Ross, Phytochem. Lett., 2015, 14, 84–90 CrossRef CAS
.
- D. S. El-agamy, S. R. M. Ibrahim, N. Ahmed, S. Khoshhal, H. M. Abo-haded, M. A. Elkablawy, N. Aljuhani and G. A. Mohamed, Int. Immunopharmacol., 2019, 72, 429–436 CrossRef CAS PubMed
.
- S. Li, Z. H. U. Li, L. U. O. Qian, L.
I. Xiao-wen, X. I. Ju-qun, K. Gui-mei and S. Yong-chun, Chin. J. Nat. Med., 2015, 13, 937–941 CrossRef PubMed
.
- F. Guo, Z. Li, X. Xu, K. Wang, M. Shao, F. Zhao, H. Wang, H. Hua, Y. Pei and J. Bai, Fitoterapia, 2016, 113, 44–50 CrossRef CAS PubMed
.
- I. P. da Silva, E. Brissow, L. Claudio, K. Filho, J. Senabio, D. Crispim, T. Lizandra, G. Magalhães, P. Ademar, S. Junior, A. Helena and M. A. Soares, World J. Microbiol. Biotechnol., 2017, 33, 62 CrossRef PubMed
.
- M. S. Brewer, Compr. Rev. Food Sci. Food Saf., 2011, 10, 221–247 CrossRef CAS
.
- J. Bai, F. Guo, R. Wang, G. Chen, Z. Li, M. Shao, C. Xue and H. Hua, Chin. Chem. Lett., 2018, 29, 535–537 CrossRef CAS
.
- N. Boyer, K. C. Morrison, J. Kim, P. J. Hergenrother and M. Movassaghi, Chem. Sci., 2013, 4, 1646–1657 RSC
.
- G. Liao, P. Wu, J. Xue, L. Liu, H. Li and X. Wei, Fitoterapia, 2018, 131, 50–54 CrossRef CAS PubMed
.
- J. R. Gubiani, M. C. S. Oliveira, R. A. R. Neponuceno, M. J. Camargo, W. S. Garcez, A. R. Biz, M. A. Soares, A. R. Araujo, V. d. S. Bolzani, H. C. F. Lisboa, P. T. De Sousa, L. G. De Vasconcelos, T. A. N. Ribeiro, J. M. De Oliveira, T. P. Banzato, C. A. Lima, G. B. Longato, J. M. Batista, R. G. S. Berlinck and H. L. Teles, Phytochem. Lett., 2019, 32, 162–167 CrossRef CAS
.
- C. Qi, Q. Zhou, W. Gao, M. Liu, C. Chen, X.-N. Li, Y. Lai, Y. Zhou, D. Li, Z. Hu, H. Zhu and Y. Zhang, Phytochemistry, 2019, 165, 112041 CrossRef CAS PubMed
.
- Y. Ma, T. Li and C. Ma, Nat. Prod. Res., 2015, 30, 1–6 Search PubMed
.
- Q. Tan, F. Gao, F. Wang and Q. Chen, Int. J. Mol. Sci., 2015, 16, 5750–5761 CrossRef CAS PubMed
.
- X. Yang, N. Wang, Y. Kang and Y. Ma, Nat. Prod. Res., 2019, 33, 2777–2783 CrossRef CAS PubMed
.
- M. Zhou, J. Lou, Y. Li, Y. Wang, K. Zhou, B. Ji, W. Dong, X. Gao, G. Du and Q. Hu, J. Braz. Chem. Soc., 2015, 26, 545–549 CAS
.
- T. Ma, W. Shan, Y. Ying, L. Ma, W. Liu and Z.-J. Zhan, Helv. Chim. Acta, 2015, 98, 4–7 CrossRef
.
- M. Wang, M. Sun, H. Hao and C. Lu, J. Nat. Med., 2015, 78, 3067–3070 CAS
.
- K. Zhou, L. Zhu, X. Wang, T. Zhang, Y. Wang, W. Dong, B. Ji, H. Yang, G. Du, Q. Hu and M. Zhou, Chem. Nat. Compd., 2016, 52, 591–594 CrossRef CAS
.
- M. F. Abdelwahab, T. Kurtán, A. Mándi, W. E. G. Müller, M. A. Fouad, M. S. Kamel, Z. Liu, W. Ebrahim, G. Daletos and P. Proksch, Tetrahedron Lett., 2018, 59, 2647–2652 CrossRef CAS
.
- S. R. Mohamed and H. Z. Asfour, Int. J. Pharmacol., 2018, 14, 437–443 Search PubMed
.
- E. Elfita, M. Munawar, M. Muharni and I. Ivantri, Microbiol. Indones., 2015, 9, 82–88 CrossRef
.
- A. M. D. A. Siriwardane, N. S. Kumar, L. Jayasinghe and Y. Fujimoto, Nat. Prod. Res., 2015, 29, 1384–1387 CrossRef CAS PubMed
.
- A. D. O. Feitosa, A. Cristina, S. Dias, C. Ramos, H. R. Bitencourt, J. Edson, S. Siqueira, P. Santana, B. Marinho, A. Barison, F. M. M. Ocampos, A. Moacir and R. Marinho, Rev. Argent. Microbiol., 2016, 48, 259–263 Search PubMed
.
- W. Yan, Wuringege, S. Li, Z. Guo, W. Zhang, W. Wei, R.-X. Tan and R.-H. Jiao, Bioorg. Med. Chem. Lett., 2017, 27, 51–54 CrossRef CAS PubMed
.
- S. Gombodorj, M. Yang, Z. Shang, R. Liu, T. Li, G. Yin and L. Kong, Fitoterapia, 2017, 120, 72–78 CrossRef CAS PubMed
.
- X. Pang, J. Zhao, X. Fang, T. Zhang, D. Zhang, H. Liu, J. Su, S. Cen and L. Yu, J. Nat. Prod., 2017, 80, 2595–2601 CrossRef CAS PubMed
.
- C. Qi, W. Gao, J. Wang, M. Liu, J. Zhang, C. Chen, Z. Hu, Y. Xue, D. Li, Q. Zhang, Y. Lai, Q. Zhou, H. Zhu and Y. Zhang, Fitoterapia, 2018, 130, 134–139 CrossRef CAS PubMed
.
- R. Liu, H. Li, J. Yang and Z. An, Chem. Nat. Compd., 2018, 54, 683–685 Search PubMed
.
- Y. Qiao, K. Tu, W. Feng, J. Liu, Q. Xu, L. Tao, H. Zhu, C. Chen, J. Wang, Y. Xue and Y. Zhang, Chem. Biodiversity, 2018, 15, e1800395 Search PubMed
.
- D. O. Abonyi, P. M. Eze, C. C. Abba, N. T. Ujam, P. Proksch, F. B. C. Okoye and C. O. Esimone, Eur. J. Biol. Res., 2018, 8, 157–167 Search PubMed
.
- W. He, Y. Xu, P. Fu, M. Zuo, W. Liu, Y. Jiang, L. Wang and W. Zhu, J. Agric. Food Chem., 2019, 67, 10660–10666 CrossRef CAS PubMed
.
- S. S. Y. Hnin, M. M. Thu, Y. Y. Thu and A. Pe, J. Myanmar. Acad. Arts Sci., 2019, 17, 287–304 Search PubMed
.
- X. Xin, Y. Chen, H. Zhang, Y. Li, M. Yang and L. Kong, Fitoterapia, 2019, 132, 53–59 CrossRef CAS PubMed
.
- H. Zhang, Y. Tang, C. Ruan and X. Bai, Rec. Nat. Prod., 2016, 10, 1–16 CAS
.
- D. C. Hawksworth and A. Y. Rossman, Phytopathology, 1987, 87, 888–891 CrossRef PubMed
.
- A. De Bary, Morphologie und physiologie der pilze, flechten und myxomyceten, Handbook of Physiological Botany, W. Engelmann, Leipzig, 1866, p. 2 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: a list of other endophytic Aspergillus isolated metabolites with no bioactivity. See DOI: 10.1039/d0ra04290k |
| This journal is © The Royal Society of Chemistry 2020 |