Open Access Article
Open Access ArticleRevision of the structure of isochaetoglobosin Db based on NMR analysis and biosynthetic consideration†
Yan-duo Wang a,
Yuan-yuan Lia,
Xiang-mei Tana,
Lin Chenb,
Zhong-qi Weic,
Li Shen*d and
Gang Ding*a
a,
Yuan-yuan Lia,
Xiang-mei Tana,
Lin Chenb,
Zhong-qi Weic,
Li Shen*d and
Gang Ding*a
aKey Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine, Ministry of Education, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100193, People's Republic of China. E-mail: gding@implad.ac.cn
bZhengzhou Key Laboratory of Synthetic Biology of Natural Products, Henan Joint International Research Laboratory of Drug Discovery of Small Molecules, Huanghe Science and Technology College, Zhengzhou, Henan 450063, People's Republic of China
cNanjing Vocational Health College, Nanjing, Jiangsu 210038, People's Republic of China
dInstitute of Translational Medicine, Medical College, Yangzhou University, Yangzhou, Jiangsu 225001, People's Republic of China. E-mail: shenli@yzu.edu.cn
First published on 23rd June 2020
Abstract
Isochaetoglobosin Db is a new chaetoglobosin possessing a unique 3,4-substituted pyrrole ring isolated and named by Qiu et al., and it is different from any one of the 14 sub-types in the macrocyclic ring of chaetoglobosins classified in our previous work. Its chemical shift values, coupling constants and biosynthetic consideration implied that the proposed structure of isochaetoglobosin Db was incorrect. In this report, based on detailed NMR data analysis together with biosynthetic consideration, the structure of isochaetoglobosin Db is suggested to be revised to that of penochalasin C. The NMR spectra of penochalasin C measured in the same solvent (DMSO-d6) as that of isochaetoglobosin Db supported the above conclusion. The results imply that reasonable biosynthetic consideration could complement spectroscopic structural determination, and also support that the 1H-NMR rule of chaetoglobosin summarized in our previous work can provide help for dereplication and rectification.
1. Introduction
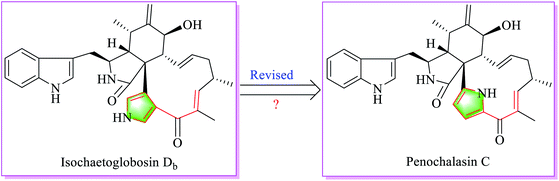
Chaetoglobosins are a large member of mycotoxins originating from a PKS-NRPS hybrid megasynthetase.1–3 The core skeleton mainly consists of three parts: an indol-3-yl, a perhydro-isoindolone and a macrocyclic ring. Other post-modifications mainly including oxygenation, dehydration, and rearrangement increase the chemical diversity of this group of mycotoxins.4,5 According to the possible biosynthesis and structural features, 9 sub-types in the perhydro-isoindolone part and 14 sub-types in the macrocycle ring of chaetoglobosins are classified in our previous work.5 Recently, Qiu et al. described two new chaetoglobosin analogues named isochaetoglobosin Db and cytoglobosin Ab, respectively, from an extreme fungus Chaetomium globosum SNSHI-5.6 The authors elucidated the new structures mainly based on NMR spectra. Structural features of isochaetoglobosin Db revealed that this compound is a new sub-type of chaetoglobosins in the macrocycle ring by possessing a unique 3,4-substituted pyrrole ring. Our group have a longstanding interest in the structural elucidation, biosynthesis and biological effects of chaetoglobosins.5,7,8 The special pyrrole unit in the macrocyclic ring of isochaetoglobosin Db is different from any one of 14 sub-types summarized in our previous work,5 and also the unusual chemical shift values and coupling constants of the 3,4-substituted pyrrole ring in the structure implies incorrect structure determination. Carefully comparing NMR chemical shift values from different analogues, and analyzing coupling constants from different-substituted pyrrole ring also together with considering chaetoglobosin biosynthesis revealed that the structure of isochaetoglobosin Db was incorrect and should be revised to be as penochalasin C (Fig. 1). The NMR spectra of penochalasin C was measured in the same solvent (DMSO-d6) as that of isochaetoglobosin Db, which further supported the above conclusion that isochaetoglobosin Db and penochalasin C are the same structure. In this report, structural revision from isochaetoglobosin Db to penochalasin C is provided based on NMR data analysis and biosynthetic consideration.2. Results and discussion
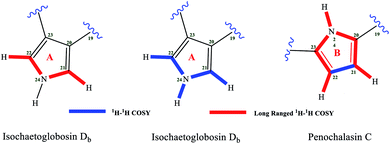
The structure of isochaetoglobosin Db was characterized mainly by NMR spectra including 1H, 13C, 1H–1H COSY, HSQC, and HMBC spectrum.6 In the ESI,† the cross peaks of H-21, H-22 and NH-24 were observed in the 1H–1H COSY spectrum, but in the HMBC spectrum, the cross peaks from H-21, H-22 or NH-24 to C-20, C-21, C-22 or C-23 were not observed.5 The W-long-ranged correlations in conjugated ring system such as pyrrole ring is often observed in 1H–1H COSY spectrum, for example, the correlation of H-21 with H-22 in fragment A. Considering no HMBC correlations from H-21, H-22 and NH-24 to any carbons in the structure of isochaetoglobosin Db (1), actually, the fragment B also conforms to the W-long-ranged correlations (of H-22 with NH-24, and of H-21 with NH-24) in the 1H–1H COSY spectrum. Thus, according to the ESI† provided by authors, there might exist two possible sub-structures about the pyrrole ring: fragments A or B (Fig. 2).If the fragment A is right, the chemical shift values of β-H/C (H/C-21) on the pyrrole ring are not reasonable compared with compounds possessing similar pyrrole units. There is an α,β-unsaturated ketone group in fragment A, and the β-position is connected with a nitrogen atom, which will lead the chemical shift values of β-H/C to be deshielded. The chemical shift values of β-H/C are 7.40/127.3, and 7.67/123.7 in verrucarin E9,10 and azamonosporascone11 with the similar α,β-unsaturated ketone group, whereas chemical shift values of β-H/C in fragment A of isochaetoglobosin Db were 6.60/113.7.6 The differences of β-H/C in similar fragment of three compounds are significant, implying that the structure of fragment A in isochaetoglobosin Db is not right (Fig. 3).
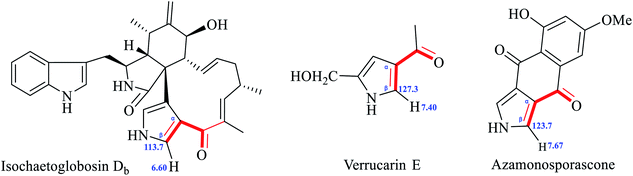 | ||
| Fig. 3 β-H/C chemical shift values of isochaetoglobosin Db6, verrucarin E9,10 and azamonosporascone.11 | ||
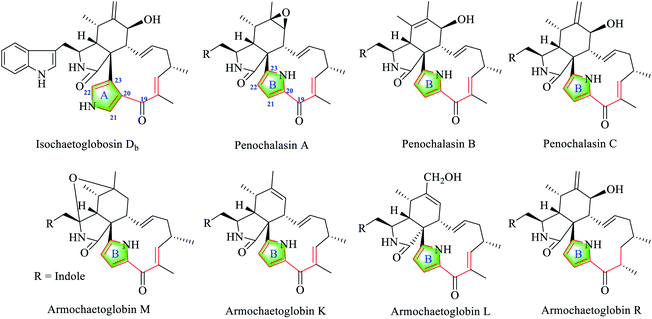
The 13C-NMR chemical shift values of fragment A (including C-19) in isochaetoglobosin Db (1),6 and fragment B in penochalasins A–C,12 armochaetoblobsin K–M, and R were compared and analyzed (Fig. 4).13 Comparison of the 13C chemical shift values of C-19, C-20, C-21, C-22 and C-23 between isochaetoglobosin Db, penochalasins A–C, armochaetoblobsins K–M, and R revealed that the chemical shift values of these carbons were nearly same. They implied that the pyrrole ring (fragment A) in isochaetoglobosin Db should be reassigned as fragment B (Table 1).
| Pos. | 1a | Penochalasin A (CDCl3) | Penochalasin B (CDCl3) | Penochalasin C (CDCl3) | Armochaetoglobsins K (CD3OD) | Armochaetoglobsins L (CDCl3) | Armochaetoglobsins M (CDCl3) | Armochaetoglobsins R (DMSO-d6) |
|---|---|---|---|---|---|---|---|---|
| 19 | 189.3 | 188.47 | 189.49 | 188.04 | 190.5 | 190.6 | 187.6 | 191.1 |
| 20 | 130.1 | 126.79 | 126.81 | 126.90 | 130.4 | 130.5 | 129.7 | 129.5 |
| 21 | 113.7 | 114.92 | 114.46 | 115.07 | 116.4 | 116.4 | 114.8 | 114.4 |
| 22 | 108.0 | 109.48 | 109.47 | 109.17 | 111.3 | 111.2 | 108.9 | 106.4 |
| 23 | 140.7 | 138.90 | 138.50 | 139.81 | 142.3 | 142.2 | 137.2 | 139.1 |
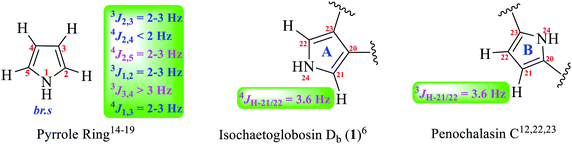
Coupling constants analysis are also diagnostic about the substitution position on a pyrrole ring. If a pyrrole ring is substituted at C-2 and C-5 such as found in penochalasin C, the coupling constants of H-3/H-4 is, usually, more than 3.0 Hz (3J3,4 > 3.0 Hz); If a pyrrole ring is substituted at C-3 and C-4 such as found in isochaetoglobosin Db, the coupling constants of H-2/H-5 (as W-long-ranged correlation) is at 2.0–3.0 Hz (4J2,5 = 2.0–3.0 Hz).14–19 Analysis of the 1H NMR of isochaetoglobosin Db revealed that the coupling constants of H-21/H-22 was 3.6 Hz, which did not conform to the rule mentioned-above. On the contrary, the coupling constants of H-21/H-22 in penochalasin C was also 3.6 Hz (Fig. 5). These analyses further supported that the fragment A in isochaetoglobosin Db (1) should be assigned as fragment B.
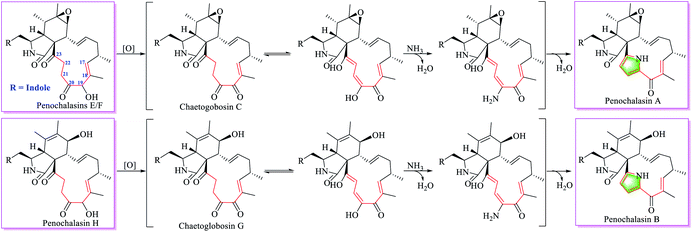
Penochalasins A–C were first isolated from a marine alga symbiotic fungus Penicillium species in 1995, and other analogues including penochalasins D–H, and chaetoglobosin O were later isolated from the same fungus.12,15 It was the first report of chaetoglobosin analogues by possessing a unique pyrrole ring in the macrocylic ring system. From the structural features, the pyrrole ring in penochalasin A (penochalasin B) might be originated from penochalasin E/F (penochalasin H) through the possible intermediate chaetoglobosin C (chaetoglobosin G) by amination and dehydration at C-20, C-21, C-22 and C-23 (Fig. 6).
In 2006, our group isolated five analogues including chaetoglobosins C, E, F, U and penochalasin A from an endophytic fungus Chaetomium globosum IFB-E019.7 Though the structural relationship of these chaetoglobosins were not suggested at that time, the macrocyclic difference in chaetoglobosin C and chaetoglobosin U, penochalasin A implied that the additional cyclopent-2-en-1-one (C-17, C-18, C-19, C-20 and C-21, Fig. S14†) in chaetoglobosin U might be derived from chaetoglobosin C by the intramolecular Michael-addition reaction at C-17 and C-21, whereas the pyrrole ring in penochalasin A could be biosynthesized from chaetoglobosin C by same reactions as those found in Fig. 6.
Recently, Prof Zhang's group also isolated a series of new pyrrole-based chaetoglobosins armochaetoglobins K–R together with other new analogues from Chaetomium globosum (TW1-1).13,20,21 The authors suggested the possible biosynthetic pathway of pyrrole-based chaetoglobosins according to the structural features. When analyzing the structural characteristics, we found the same biosynthetic relationships of these analogues as those found in Fig. 6 and S14.† For example armochaetoglobin X might come from armochaetoglobin U by the intramolecular Michael-addition reaction, which could be originated from isochaetoglobosin J by oxidation and dehydration, whereas the pyrrole ring in armochaetoglobin K might be derived from isochaetoglobosin J by amination and dehydration (Fig. S15†).
Qiu et al. reported two new chaetoglobosin analogues isochaetoglobosin Db and cytoglobosin Ab isolated from an extreme fungus C. globosum SNSHI-5.6 Though the authors did not report known an alogues or possible intermediates from this fungus, according to the structural characteristics, the possible biosynthetic relationship from these chaetoglobosins were suggested, which possesses the same biosynthetic pathway as those found in Fig. 6, and S14–S16.† Thus, the 3,4-substituted pyrrole in isochaetoglobosin Db should be reassigned to be the 2,5-substituted pyrrole in penochalasin C. This result also conforms to the rule summarized in our previous report.
Fortunately, penochalasin C was isolated from an endophytic fungus C. globosum in our lab.22,23 The NMR spectra of penochalasin C were obtained in the same solvent system (DMSO-d6) as that of isochaetoglobosin Db (ESI†). 1H–1H COSY spectrum revealed the correlations H-21, H-22 and 24-NH, and the HMBC correlations from H-21 to C-20, C-22, and C-23, from H-22 to C-20, C-21, and C-23 confirmed a 2,5-substituted pyrrole unit in penochalasin C. The other NMR data including 1H, and 13C data of penochalasin C were the same as those of isochaetoglobosin Db, which further confirmed the conclusion that penochalasin C and isochaetoglobosin Db were the same structure (Table 2).
| Position | Isochaetoglobosin Db (DMSO-d6) | Penochalasin C (DMSO-d6) | Penochalasin C (CDCl3) | |||
|---|---|---|---|---|---|---|
| δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | δH, mult (J in Hz) | δC | |
| a There are some signal assignments are corrected for isochaetoglobosin Db. | ||||||
| 1 | 175.3, C | 175.2, C | 169.87, C | |||
| 2 | 8.16, s | 8.13, s | 5.80, br s | |||
| 3 | 3.30, m | 53.4, CH | 3.29, m | 53.3, CH | 3.54, dt (10.2, 4.0) | 53.16, CH |
| 4 | 2.35, m | 51.6, CH | 2.35, m | 51.5, CH | 2.75, t (4.0) | 53.00, CH |
| 5 | 2.72, m | 31.8, CH | 2.72, m | 31.8, CH | 2.98, qd (6.5, 4.0) | 32.32, CH |
| 6 | 151.6, C | 151.5, C | 147.92, C | |||
| 7 | 3.78, m | 69.3, CH | 3.78, dd (6.0, 10.2) | 69.1, CH | 4.02, br d (10.8) | 68.62, CH |
| 8 | 3.19, m | 47.9, CH | 3.19, t (10.2) | 47.9, CH | 3.05, t (10.0) | 49.82, CH |
| 9 | 49.5, C | 49.4, C | 49.82, C | |||
| 10 | 2.95, m | 33.1, CH2 | 2.94, m | 33.1, CH2 | 2.98, dd (14.0, 10.2) | 34.85, CH2 |
| 2.92, m | 2.94, m | 3.16, dd (14.0, 4.0) | ||||
| 11 | 0.58, d (6.6) | 13.9, CH3 | 0.59, d (6.6) | 13.8, CH3 | 1.24, d (6.5) | 15.10, CH3 |
| 12 | 4.86, s | 112.2, CH2 | 4.86, s | 112.1, CH2 | 5.25, s | 114.62, CH2 |
| 5.16, s | 5.16, s | 5.48, s | ||||
| 13 | 6.16 dd | 132.0, CH | 6.17 dd (9.6, 15.6) | 131.9, CH | 6.67, ddd (15.5, 10.0, 1.6) | 132.63, CH |
| 14 | 5.56, m | 135.1, CH | 5.56, m | 135.0, CH | 5.82, ddd (15.5, 11.5, 3.2) | 138.08, CH |
| 15 | 1.87, m | 41.4, CH2 | 1.87, m | 41.3, CH2 | 2.19 dt (15.5, 11.5); | 41.27, CH2 |
| 2.43, m | 2.43, m | 2.61 dddd (13.5, 4.8, 3.2, 1.6) | ||||
| 16 | 2.76, m | 33.3, CH | 2.76, m | 33.2, CH | 2.91, m | 34.09, CH |
| 17 | 5.29, dd (9.6, 1.5) | 146.1, CH | 5.28, d (9.0) | 145.9, CH | 5.68, dq (9.4, 18) | 142.07, CH |
| 18 | 135.4, C | 135.2, C | 135.08, C | |||
| 19 | 189.3, C | 189.1, C | 188.04, C | |||
| 20 | 130.1, C | 130.0, C | 126.90, C | |||
| 21 | 6.60, d (3.6) | 113.7, CH | 6.59, dd (2.4, 3.6) | 113.5, CH | 7.02, dd (3.9, 2.7) | 115.07, CH |
| 22 | 5.65, t (3.3) | 108.0, CH | 5.66, dd (2.4, 3.6) | 107.9, CH | 6.18, dd (3.9, 2.7) | 109.17, CH |
| 23 | 140.7, C | 140.6, C | 139.81, C | |||
| 24 | 10.53, br s | 10.52, br s | 10.78, br s | |||
| 25 | 0.98, d (6.8) | 19.9, CH3 | 0.97, d (6.6) | 19.8, CH3 | 1.10, d (7.0) | 19.78, CH3 |
| 26 | 1.81, s | 13.2, CH3 | 1.81, s | 13.1, CH3 | 1.95, d (2.0) | 13.68, CH3 |
| 1′ | 10.91, brs | 10.90, s | 8.21, br s | |||
| 2′ | 7.16, d (2.2) | 124.6, CH | 7.15, d (1.8) | 124.5, CH | 7.09, d (2.3) | 122.86, CH |
| 3′ | 110.2, C | 110.1, C | 111.47, C | |||
| 3′a | 128.2, C | 128.1, C | 129.77, C | |||
| 4′ | 7.36, d (9.0) | 118.4, CH | 7.35, d (9.0) | 118.3, CH | 7.55, dd (8.0, 1.0) | 118.44, CH |
| 5′ | 7.05, t (7.1) | 121.3, CH | 7.05, t (7.2) | 121.2, CH | 7.25, td (8.0, 1.0) | 122.60, CH |
| 6′ | 6.94, t (7.7) | 118.9, CH | 6.94, t (7.8) | 118.7, CH | 7.15, td (8.0, 1.0) | 119.99, CH |
| 7′ | 7.34, d (9.0) | 111.9, CH | 7.34, d (9.0) | 111.8, CH | 7.40, dd (8.0, 1.0) | 111.62, CH |
| 7′a | 136.6, C | 136.4, C | 136.51, C | |||
| 7-OH | 4.87, d (5.9) | 4.81, d (5.4) | 2.00, br s | |||
3. Conclusion
In conclusion, isochaetoglobosin Db is revised to be penochalasin C based on NMR data analysis including chemical shift value, coupling constants analysis, and biosynthetic consideration. The results in this report not only confirm that reasonable biosynthetic consideration could complement spectroscopic structural determination, but also further confirm that the 1H-NMR rule chaetoglobosin summarized in our previous work can provide helps for chaetoglobosin dereplication and rectification.4. Experimental section
4.1 General experimental procedures
NMR spectra were acquired with a Bruker AVANCE600 spectrometer. HRESIMS were obtained using a TOF-ESI-MS (Bruker UHR-TOF maXis). Preparation of HPLC was conducted on Agilent 1260 and equipped with a DAD detector (G1315D) and a 4.6 mm × 150 mm i.d., 5 μm, C18 column (HITACHI LaChrom, Tokyo, Japan). Silica gel (200–300 mesh) (Qingdao Marine Chemical Plant) and Sephadex LH-20 (40–70 μm) (Pharmacia Biotech AB, Uppsala, Sweden), chromatographically pure methanol was purchased from TEDIA Chemical Reagent Limited Company. (TEDIA, Ohio, America) and deuterated reagent was purchased from Sigma-Aldrich.4.2 Fungal material and fermentation
The strain of C. globosum was isolated from Imperata cylindrical and identified by Dr Chun-yong Song and deposited in the Institute of Functional Biomolecules of Nanjing University. It was grown on PDA plates at 28 °C for 5 days. Then the fresh mycelium was inoculated into autoclaving sterilized potato liquid media (potato 200 g and glucose 20 g with water 1000 mL) cultivated for 7 days in shaking culture (28 °C, 140 rpm). The resulting seeds were separated 15 mL per bottle and inoculated into autoclaving sterilized solid media (millet 7.5 g, wheat bran 7.5 g, yeast extract 0.5 g, sodium tartrate 0.1 g, sodium glutamate 0.1 g, green grind 0.01 g and corn oil 0.1 mL with water 15 mL in 250 mL flasks) cultivated for 30 days.4.3 Extraction and isolation
After the solid fermentation product was crushed and dried, the culture was extracted with chloroform–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for four times, and the water extract was extracted with ethyl acetate, the solvent was evaporated to afford 26 g of crude extract. The original extract was fractionated on a silica gel column chromatography (CC) using chloroform-methanol gradient elution (100
1) for four times, and the water extract was extracted with ethyl acetate, the solvent was evaporated to afford 26 g of crude extract. The original extract was fractionated on a silica gel column chromatography (CC) using chloroform-methanol gradient elution (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v, each 1000 mL) to get seven fractions (Fr.1 to Fr.7). Fr.2 (3.8 g) was fractionated on a silica gel CC eluted with chloroform–methanol gradient elution (100
100 v/v, each 1000 mL) to get seven fractions (Fr.1 to Fr.7). Fr.2 (3.8 g) was fractionated on a silica gel CC eluted with chloroform–methanol gradient elution (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 100
0, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100
1, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 100
2, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 100
4, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 0
8 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v, each 1000 mL) to get six subfractions (Fr.2-1 to Fr.2-6). Fr.2-2 was fractionated on a silica gel CC eluted with chloroform–methanol gradient elution (100
100 v/v, each 1000 mL) to get six subfractions (Fr.2-1 to Fr.2-6). Fr.2-2 was fractionated on a silica gel CC eluted with chloroform–methanol gradient elution (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v, each 1000 mL) to get Fr. 2-2-2 and Fr. 2-2-3, Fr. 2-2-3 was eluted by Sephadex LH-20 using chloroform–methanol gradient elution to get Fr.2-2-3-3 (1
100 v/v, each 1000 mL) to get Fr. 2-2-2 and Fr. 2-2-3, Fr. 2-2-3 was eluted by Sephadex LH-20 using chloroform–methanol gradient elution to get Fr.2-2-3-3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, each 1000 mL). Fr.2-2-3-3 recrystallization mother liquor was purified by HPLC (55% CH3OH in H2O, v/v, 1.0 mL min−1, 254 nm, tR = 35.1 min) to obtain compound penochalasin C (12 mg).
1 v/v, each 1000 mL). Fr.2-2-3-3 recrystallization mother liquor was purified by HPLC (55% CH3OH in H2O, v/v, 1.0 mL min−1, 254 nm, tR = 35.1 min) to obtain compound penochalasin C (12 mg).
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We acknowledge financial support from the National Key Research and Development Program of China “Research and Development of Comprehensive Technologies on Chemical Fertilizer and Pesticide Reduction and Synergism” (2017YFD0201402), CAMS Initiative for Innovative Medicine (2017-I2M-4-004), and the National Natural Science Foundation of China (31570340).References
- J. Schumann and C. Hertweck, J. Am. Chem. Soc., 2007, 129, 9564–9565 CrossRef PubMed.
- K. Scherlach, D. Boettger, N. Remme and C. Hertweck, Nat. Prod. Rep., 2010, 27, 869–886 RSC.
- E. Skellam, Nat. Prod. Rep., 2017, 34, 1252–1263 RSC.
- Q. Zhang, H. Q. Li, S. C. Zong, J. M. Gao and A. L. Zhang, Mini-Rev. Med. Chem., 2012, 12, 127–148 CrossRef PubMed.
- C. Lin, J. C. Qin, Y. G. Zhang and G. Ding, RSC Adv., 2020, 10, 1946–1955 RSC.
- X. Y. Wang, X. Yan, M. J. Fang, Z. Wu, D. Wang and Y. K. Qiu, Nat. Prod. Res., 2017, 31, 1669–1675 CrossRef CAS PubMed.
- G. Ding, Y. C. Song, J. R. Chen, C. Xu, H. M. Ge, X. T. Wang and R. X. Tan, J. Nat. Prod., 2006, 69, 302–304 CrossRef CAS PubMed.
- T. Jiang, M. Wang, L. Li, J. Si, B. Song, C. Zhou, M. Yu, X. Wang, Y. Zhang, G. Ding and Z. Zou, J. Nat. Prod., 2016, 79, 2487–2494 CrossRef CAS PubMed.
- E. Cherbuliez, S. Jaccard, R. Mouzenidou, J. Rabinowitz and F. E. Cherbuliez, Helv. Chim. Acta, 1966, 49, 233–234 Search PubMed.
- A. Andolfi, A. Boari, A. Evidente and M. Vurro, J. Agric. Food Chem., 2005, 53, 1598–1603 CrossRef CAS PubMed.
- R. D. Stipanovic, J. Zhang, B. D. Bruton and M. H. Wheeler, J. Agric. Food Chem., 2004, 52, 4109–4112 CrossRef CAS PubMed.
- A. Numata, C. Takahashi, Y. Ito, K. Minoura, T. Yamada, C. Matsuda and K. Nomoto, J. Chem. Soc., Perkin Trans. 1, 1996, 3, 239–245 RSC.
- C. Chen, J. Wang, J. Liu, H. Zhu, B. Sun, J. Wang, J. Zhang, Z. Luo, G. Yao, Y. Xue and Y. Zhang, J. Nat. Prod., 2015, 78, 1193–1201 CrossRef CAS PubMed.
- D. B. Stierle and D. J. Faulkner, J. Org. Chem., 1980, 45, 4980–4982 CrossRef CAS.
- M. J. Ortcga, E. Zubia, J. l. Carballo and J. Salva, Tetrahedron, 1997, 53, 331–340 CrossRef.
- H. Duddeck, Magn. Reson. Chem., 2002, 40, 247 CrossRef CAS.
- A. R. Katritzky, N. G. Akhmedov, Z. Wang, V. A. Roznyatovsky, A. A. Shestopalov and C. Dennis Hall, Magn. Reson. Chem., 2003, 41, 908–920 CrossRef CAS.
- W. Zhang, L. Ma, S. Li, Z. Liu, Y. Chen, H. Zhang, G. Zhang, Q. Zhang, X. Tian, C. Yuan, S. Zhang, W. Zhang and C. Zhang, J. Nat. Prod., 2014, 77, 1887–1892 CrossRef CAS PubMed.
- A. Morgenstern, C. Paetz, A. Behrend and D. Spiteller, Chemistry, 2015, 21, 6027–6032 CrossRef CAS PubMed.
- C. Chen, H. Zhu, J. Wang, J. Yang, X. Li, J. Wang, K. Chen, Y. Wang, Z. Luo, G. Yao, Y. Xue and Y. Zhang, Eur. J. Org. Chem., 2015, 46, 3086–3094 CrossRef.
- C. Chen, Q. Tong, H. Zhu, D. Tan, J. Zhang, Y. Xue, G. Yao, Z. Luo, J. Wang, Y. Wang and Y. Zhang, Sci. Rep., 2016, 6, 18711 CrossRef CAS PubMed.
- L. Shen, L. Zhu, Zh. Q. Wei, X. W. Li, M. Li and Y. Ch. Song, China J. Chin. Mater. Med., 2015, 40, 4645–4649 CAS.
- Z. Q. Wei, Master Dissertation, Yangzhou University, Yangzhou, 2014.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra04108d |
| This journal is © The Royal Society of Chemistry 2020 |