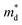Open Access Article
Open Access ArticleImprovement in the thermoelectric performance of highly reproducible n-type (Bi,Sb)2Se3 alloys by Cl-doping†
Nadra Nasir‡
a,
Kyu Hyoung Lee‡b,
Sang-il Kim c,
Hyun-Sik Kimd,
Jae-Hong Lime,
Liangwei Fu*a and
Sung Wng Kim
c,
Hyun-Sik Kimd,
Jae-Hong Lime,
Liangwei Fu*a and
Sung Wng Kim *af
*af
aDepartment of Energy Science, Sungkyunkwan University, Suwon 16419, South Korea. E-mail: fulw@skku.edu; kimsungwng@skku.edu
bDepartment of Materials Science and Engineering, Yonsei University, Seoul 03722, South Korea
cDepartment of Materials Science and Engineering, University of Seoul, Seoul 02504, South Korea
dDepartment of Materials Science and Engineering, Hongik University, Seoul 04066, South Korea
eDepartment of Materials Science and Engineering, Gachon University, Seongnam 13120, South Korea
fCenter for Integrated Nanostructure Physics, Institute of Basic Science, Suwon 16419, South Korea
First published on 29th June 2020
Abstract
(Bi,Sb)2Se3 alloys are promising alternatives to commercial n-type Bi2(Te,Se)3 ingots for low-mid temperature thermoelectric power generation due to their high thermoelectric conversion efficiency at elevated temperatures. Herein, we report the enhanced high-temperature thermoelectric performance of the polycrystalline Cl-doped Bi2−xSbxSe3 (x = 0.8, 1.0) bulks and their sustainable thermal stability. Significant role of Cl substitution, characterized to enhance the power factor and reduce the thermal conductivity synergetically, is clearly elucidated. Cl-doping at Se-site of both Bi1.2Sb0.8Se3 and BiSbSe3 results in a high power factor by carrier generation and Hall mobility improvement while maintaining converged electronic band valleys. Furthermore, point defect phonon scattering originated from mass fluctuations formed at Cl-substituted Se-sites reduces the lattice thermal conductivity. Most importantly, spark plasma sintered Cl-doped Bi2−xSbxSe3 bulks are thermally stable up to 700 K, and show a reproducible maximum thermoelectric figure of merit, zT, of 0.68 at 700 K.
1. Introduction
Bi2Te3-based alloys are the only commercialized thermoelectric materials for solid-state cooling and low-mid temperature (473–873 K) power generation, and their ingot-type materials are widely used due to a high thermoelectric figure of merit (zT = S2σT/κtot, where S, σ, κtot, and T are the Seebeck coefficient, electrical conductivity, total thermal conductivity, and the absolute temperature, respectively) of about 1.0 near room-temperature.1 However, ingots of Bi2Te3-based alloys have a poor mechanical reliability (fracture strength of ∼10 MPa) because of 00l-oriented structure weakly bonded by van der Waals forces,2 which limits their wider applications such as in automobile thermoelectric generator (ATEG). To address this, polycrystalline bulk form materials have been intensively studied, and an improved mechanical strength (∼80 MPa) with a higher zT ∼1.1 at 300 K has been obtained in micro-grained p-type Bi2−xSbxTe3 prepared by ball milling (BM) and spark plasma sintering (SPS).2 Its n-type counterpart with a comparable mechanical strength and zT is required to construct thermoelectric module with improved mechanical reliability as well as high performance, however, no marked improvement in zT from n-type micro-grained materials was achieved in BMed and SPSed Bi2Te2.7Se0.3 (∼0.63 at 300 K).3 Furthermore, a severe reproducibility problem was also found in this polycrystalline sample owing to the uncontrollable defect structures such as vacancies (Te- or Se-site) and antisite defects. Polycrystalline bulk of n-type Bi2Te2.7Se0.3 with high zT (∼0.98 at 300 K) and improved reproducibility has been demonstrated by combining a cold deformation and a hot extrusion benefitting from precise control of point defects,4 however, a simpler and yet easily scalable approach is always sought after.Bi2Se3 is a narrow-bandgap layered semiconductor (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m-D53d) with tetradymite structure and it has singly degenerate conduction band. The conduction band minimum (CBM) is observed at the center of the Brillouin zone (Γ-point) and the second conduction band is located 150–250 meV (Z-point) above the CBM,5–7 thus the zT of pristine Bi2Se3 is very low (<0.1 at 300 K) mainly due to low S (∼−40 μV K−1 at 300 K).8 High κtot ∼2.4 W m−1 K−1 at 300 K is another reason for the low zT of Bi2Se3. Very recently, Te-free (Bi,Sb)2Se3-based alloys have been received attention as promising alternatives to Bi2(Te,Se)3-based alloys especially for low-mid temperature power generation applications. High zT values of ∼1.0 and ∼1.4 were obtained at 800 K in micro-grained I- and Br-doped BiSbSe3, respectively.5,9 One main origin of the high thermoelectric performance of these compounds was the simultaneous improvement of electronic (enlarged S) and thermal transport properties (reduced lattice thermal conductivity (κlat)) due to a structural transition.5 A phase transition triggered the convergence of conduction band resulting in largely increased density of states (DOS) effective mass
m-D53d) with tetradymite structure and it has singly degenerate conduction band. The conduction band minimum (CBM) is observed at the center of the Brillouin zone (Γ-point) and the second conduction band is located 150–250 meV (Z-point) above the CBM,5–7 thus the zT of pristine Bi2Se3 is very low (<0.1 at 300 K) mainly due to low S (∼−40 μV K−1 at 300 K).8 High κtot ∼2.4 W m−1 K−1 at 300 K is another reason for the low zT of Bi2Se3. Very recently, Te-free (Bi,Sb)2Se3-based alloys have been received attention as promising alternatives to Bi2(Te,Se)3-based alloys especially for low-mid temperature power generation applications. High zT values of ∼1.0 and ∼1.4 were obtained at 800 K in micro-grained I- and Br-doped BiSbSe3, respectively.5,9 One main origin of the high thermoelectric performance of these compounds was the simultaneous improvement of electronic (enlarged S) and thermal transport properties (reduced lattice thermal conductivity (κlat)) due to a structural transition.5 A phase transition triggered the convergence of conduction band resulting in largely increased density of states (DOS) effective mass 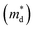 . Additionally, κlat was reduced due to the phonon softening and substantial lattice anharmonicity benefitting from weakened interchain interaction in orthorhombic phase. The high zTs were obtained by enhanced power factor (S2σ) of I- and Br-doped BiSbSe3 (by an order of magnitude), resulted from the increase in carrier concentration (nc). However, intrinsic drawbacks of I- and Br-doped BiSbSe3, which included solubility limit of I (∼3 at%) and Hall mobility (μH) deterioration with Br doping, should be resolved to enhance the low σ value (<300 S cm−1 at 300 K) in order to increase the efficiency of the thermoelectric power generation module. Moreover, the thermal stability and reproducibility of high-temperature thermoelectric performance was still elusive, demanding a clear criterion for the temperature limit of the module.
. Additionally, κlat was reduced due to the phonon softening and substantial lattice anharmonicity benefitting from weakened interchain interaction in orthorhombic phase. The high zTs were obtained by enhanced power factor (S2σ) of I- and Br-doped BiSbSe3 (by an order of magnitude), resulted from the increase in carrier concentration (nc). However, intrinsic drawbacks of I- and Br-doped BiSbSe3, which included solubility limit of I (∼3 at%) and Hall mobility (μH) deterioration with Br doping, should be resolved to enhance the low σ value (<300 S cm−1 at 300 K) in order to increase the efficiency of the thermoelectric power generation module. Moreover, the thermal stability and reproducibility of high-temperature thermoelectric performance was still elusive, demanding a clear criterion for the temperature limit of the module.
Chlorine (Cl) is a commonly used doping element especially at Se-site of various selenides such as In4Se3−x, PbSe, AgPb18SbSe20, and SnSe2 to increase nc.10–13 Moreover, a large difference in atomic mass between Se (MSe = 78.971) and Cl (MCl = 35.45) is advantageous to further reduce κlat by mass-defect phonon scattering. In this work, we fabricated the polycrystalline bulks of Cl-doped Bi2−xSbxSe3 (x = 0.8, 1.0) and evaluated their electronic and thermal transport properties in an effort to develop (Bi,Sb)2Se3-based alloys with high σ and zT, at the same time. We found that Cl was an effective doping element to facilitate the carrier transport in Bi1.2Sb0.8Se3, thus high μH of ∼27.3 cm2 V−1 s−1 was observed even in highly Cl-doped Bi1.2Sb0.8Se2.76Cl0.24 with high nc (∼9.0 × 1019 cm−3). Compared to I-doped Bi1.2Sb0.8Se2.91I0.09 (σ ∼80 S cm−1 and zT ∼0.53 at 700 K),5 higher σ (∼165 S cm−1 at 700 K) and higher zT (∼0.67 at 700 K) were obtained in Bi1.2Sb0.8Se2.76Cl0.24. Moreover, we confirmed the thermal stability of the sample by a cyclic measurement.
2. Experimental methods
High purity elements of bismuth (Bi shot, 99.99%, 5N Plus), antimony (Sb shot, 99.99%, 5N Plus), selenium (Se shot, 99.99%, iTASCO), and antimony trichloride (SbCl3 crystalline, 99.99%, Sigma Aldrich) were used as starting raw materials. Stoichiometric (BiSbSe3−yCly (y = 0, 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0, 0.12, 0.18, 0.24, 0.3)) amount of these materials was weighted and sealed into a quartz tube under vacuum (∼10−3 Pa), and the mixtures were melted at 1173 K for 12 h. After melting, the quartz tubes were water quenched and annealed at 673 K for 48 h. The acquired ingots were pulverized into powders via BM, and compacted bulks (13 mm in diameter and 11 mm in thickness) were fabricated by using SPS for 2 min at 773 K under a pressure of 40 MPa.Phase formation behavior in SPSed bulks was analyzed by X-ray diffraction (XRD, SmartLab (9 kW), Rigaku, Japan) with CuKα radiation (λ = 1.5418 Å). The microstructures of the fractured surface of the SPSed bulks were investigated by scanning electron microscopy (SEM, JSM-7600F, JEOL, Japan). The temperature dependences of S and σ were measured using a commercial measurement system (ZEM-3, Ulvac-Riko, Japan) from 300–700 K under a He atmosphere. The κtot (=D × Cp × ρ, where D, Cp, and ρ are the thermal diffusivity, specific heat capacity, and the density, respectively) was calculated from the separate measurement of D and ρ. Temperature-dependent D was measured by laser flash method (TC-1200RH, Ulvac-Riko, Japan) from 300–700 K under a vacuum and ρ was measured by the Archimedes principle (MD-300S, Alfa Miracle, Japan). Temperature dependence of Cp was estimated from the reported values.5 The rectangular bar-type sample (10 mm × 3 mm × 3 mm) and square plate-type sample (10 mm × 10 mm × 1 mm) were cut in a plane perpendicular and parallel to the SPS press direction, respectively. In this manner, electronic (S, σ) and thermal (D) transport properties can be measured in the same direction. The Hall coefficient (RH) was measured by the van der Pauw method via a commercial Hall effect measurement system (8400 Series, LakeShore, USA) at room temperature. The nc and μH were calculated by nc = e−1RH−1 and μH = σRH.
3. Results and discussion
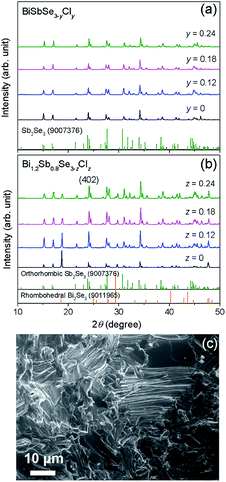
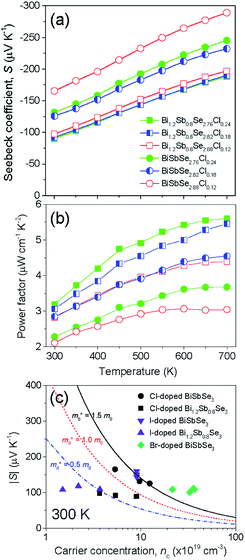
In the present study, we selected two different matrixes; (1) BiSbSe3 with an orthorhombic phase and (2) Bi1.2Sb0.8Se3 with orthorhombic and rhombohedral phases.5 Fig. 1a shows the XRD patterns for BiSbSe3−yCly samples. All the peaks can be indexed as a pure orthorhombic structure of Sb2Se3 without any secondary phases, suggesting the Cl substitution at Se-site. Structure factors including lattice parameters of BiSbSe3-based compounds with orthorhombic phase (Fig. S1†) and those of Bi1.2Sb0.8Se3-based compounds with mixed (orthorhombic and rhombohedral) phases (Fig. S3†) were extracted by the Rietveld refinement (GSAS II suite) after refinements with different structural models at the condition of convergence with the best pattern match. The slight decrease in lattice parameters (a and c) of BiSbSe3 after the Cl doping is another evidence for Cl substitution due to the smaller ionic radius of Cl− (167 pm) when compared to that of Se2− (184 pm) (Fig. S1†). On the other hand, as reported in the previous report,14 both orthorhombic and rhombohedral phases are clearly detected in Bi1.2Sb0.8Se3−zClz samples as shown in Fig. 1b. Peaks for the rhombohedral structure of Bi2Se3 were observed at 2θ ∼18.58° and ∼29.35°. The strongest intensity of (402) indicates the preferred crystal orientation generated during the SPS process. Oriented grain structure is also found in SEM images for the fractured surface of SPSed Bi1.2Sb0.8Se3−zClz (Fig. 1c and S2†). The mole fraction of rhombohedral phase estimated by Rietveld refinement is about 0.74, and this value does not show significant change with Cl doping contents (see the Table S1†). And slight decrease in cell volume by Cl-doping is observed in orthorhombic phase (Fig. S3†). Cl-related impurity phase (Bi3Se4Cl) was observed in Bi1.2Sb0.8Se2.7Cl0.3, suggesting that the solubility limit of Cl for Se-site is about 8 at% in Bi1.2Sb0.8Se3 (Fig. S4†).The temperature dependences of σ for both BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24) samples are plotted in Fig. 2a. All thermoelectric transport properties (σ, S, and κ) are measured perpendicular to SPS pressing direction since the electrical transport is dominant along the in-plane direction. The σ values of BiSbSe3 and Bi1.2Sb0.8Se3 are effectively increased by Cl-doping. Interestingly, the σ values of Cl-doped Bi1.2Sb0.8Se3 are higher than those of Cl-doped BiSbSe3 in the whole measured temperature range. The σ values of the BiSbSe2.76Cl0.24 are 132 S cm−1 and 61.2 S cm−1 at 300 K and 700 K, respectively, while those of Bi1.2Sb0.8Se2.76Cl0.24 are 397 S cm−1 and 159 S cm−1 at 300 K and 700 K, respectively. To clarify this, we estimated the nc and μH of both Cl-doped BiSbSe3 and Bi1.2Sb0.8Se3 at 300 K (Fig. 2b). The improvement in σ by Cl-doping is resulted from the increase of μH as well as nc both in BiSbSe3 and Bi1.2Sb0.8Se3. It is noted that μH values of Cl-doped Bi1.2Sb0.8Se3 are much higher than those of Cl-doped BiSbSe3. The μH values of BiSbSe3−yCly (y = 0.12, 0.18, 0.24) at 300 K is ranged from 8.44 to 9.01 cm2 V−1 s−1, whereas that of Bi1.2Sb0.8Se2.88Cl0.12 is ∼50.4 cm2 V−1 s−1. Moreover, the μH value of highly Cl-doped BiSbSe2.76Cl0.24 is retained in value about 27.3 cm2 V−1 s−1 despite of the high nc ∼9.0 × 1019 cm−3. This high μH has been also reported in I-doped Bi1.2Sb0.8Se3.5
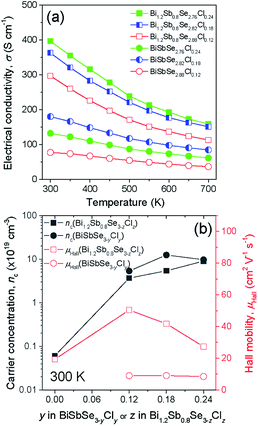 | ||
| Fig. 2 (a) Temperature dependence of electrical conductivity and (b) carrier concentration and Hall mobility for BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24). | ||
Unexpected difference between electronic transport properties of Cl-doped Bi1.2Sb0.8Se3 and those of I-doped Bi1.2Sb0.8Se3 is observed in S. Fig. 3a depicts the temperature dependences of S for both BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24) samples. The S values of the all samples are negative in the whole measured temperature range, indicating n-type semiconductors. The large |S| values of Cl-doped BiSbSe3 samples due to the convergence of conduction band by phase transition are well demonstrated both in I-doped and Br-doped BiSbSe3.5,9 To investigate the change in band structure by Cl-doping especially in Bi1.2Sb0.8Se3, we calculate the 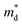 by using measured S and nc at 300 K based on the following eqn (1):1
by using measured S and nc at 300 K based on the following eqn (1):1
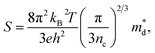 | (1) |
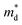 values are listed in Table 1 together with those for I-doped BiSbSe3 and Bi1.2Sb0.8Se3 samples, which are estimated from eqn (1) by using previously reported data.5 Large
values are listed in Table 1 together with those for I-doped BiSbSe3 and Bi1.2Sb0.8Se3 samples, which are estimated from eqn (1) by using previously reported data.5 Large 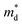 values of 1.55m0 and 1.67m0 are obtained both in BiSbSe2.82Cl0.18 and BiSbSe2.91I0.09, respectively, with pure orthorhombic phase benefiting from converged electronic band valleys. However, the
values of 1.55m0 and 1.67m0 are obtained both in BiSbSe2.82Cl0.18 and BiSbSe2.91I0.09, respectively, with pure orthorhombic phase benefiting from converged electronic band valleys. However, the 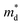 values of Cl-doped Bi1.2Sb0.8Se3 and those of I-doped Bi1.2Sb0.8Se3 are smaller than those of Cl- and I-doped BiSbSe3 mainly due to the large mole fraction of rhombohedral phase (Table S1†) with singly-degenerate conduction band. It is noted that
values of Cl-doped Bi1.2Sb0.8Se3 and those of I-doped Bi1.2Sb0.8Se3 are smaller than those of Cl- and I-doped BiSbSe3 mainly due to the large mole fraction of rhombohedral phase (Table S1†) with singly-degenerate conduction band. It is noted that 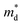 value of Bi1.2Sb0.8Se2.76Cl0.24 reaches value about 0.90m0, which results in a large S even in Bi1.2Sb0.8Se3 systems. Fig. 3c shows the Pisarenko plots (nc–|S|) for both BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24) samples at 300 K. Those for I-doped BiSbSe3, I-doped Bi1.2Sb0.8Se3, and Br-doped BiSbSe3 samples are also shown for comparison.5,9
value of Bi1.2Sb0.8Se2.76Cl0.24 reaches value about 0.90m0, which results in a large S even in Bi1.2Sb0.8Se3 systems. Fig. 3c shows the Pisarenko plots (nc–|S|) for both BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24) samples at 300 K. Those for I-doped BiSbSe3, I-doped Bi1.2Sb0.8Se3, and Br-doped BiSbSe3 samples are also shown for comparison.5,9
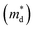 values of Cl-doped BiSbSe3 and Bi1.2Sb0.8Se3. Those of I-doped BiSbSe3 and Bi1.2Sb0.8Se3, which are estimated from the reported data ref. 5, are also shown for comparison
values of Cl-doped BiSbSe3 and Bi1.2Sb0.8Se3. Those of I-doped BiSbSe3 and Bi1.2Sb0.8Se3, which are estimated from the reported data ref. 5, are also shown for comparison
| Compositions (nominal) | (m0) | Compositions (nominal) | (m0) |
|---|---|---|---|
| BiSbSe2.88Cl0.12 | 1.17 | BiSbSe2.97I0.03 | 1.50 |
| BiSbSe2.82Cl0.18 | 1.55 | BiSbSe2.94I0.06 | 1.63 |
| BiSbSe2.76Cl0.24 | 1.38 | BiSbSe2.91I0.09 | 1.67 |
| Bi1.2Sb0.8Se2.88Cl0.12 | 0.54 | Bi1.2Sb0.8Se2.97I0.03 | 0.33 |
| Bi1.2Sb0.8Se2.82Cl0.18 | 0.66 | Bi1.2Sb0.8Se2.94I0.06 | 0.65 |
| Bi1.2Sb0.8Se2.76Cl0.24 | 0.90 | Bi1.2Sb0.8Se2.91I0.09 | 0.43 |
As clearly shown in Fig. 3c, similar value of S is obtained in Bi1.2Sb0.8Se2.76Cl0.24 despite of the large increase in nc when compared to that of I-doped Bi1.2Sb0.8Se3 samples. Resultantly, a maximum power factor values of ∼3.19 μW cm−1 K−2 and ∼5.61 μW cm−1 K−2 at 300 K and 700 K, respectively, are obtained in Bi1.2Sb0.8Se2.76Cl0.24 (Fig. 3b), which ensures the enhanced zT especially at higher temperatures. This beneficial characteristic feature for the realization of highly-efficient low-mid temperature thermoelectric power generation system is only found in Cl-doped Bi1.2Sb0.8Se3 among other (Bi,Sb)2Se3-based alloys.
Fig. 4a shows the temperature dependence of κtot for BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24) samples. The κtot values of Cl-doped Bi1.2Sb0.8Se3 are higher than those of Cl-doped BiSbSe3. The room temperature κtot values of both Cl-doped BiSbSe3 and Bi1.2Sb0.8Se3 are ∼0.58–0.62 W m−1 K−1 and ∼0.72–0.83 W m−1 K−1, respectively. This is considered to be related to the increased electronic contribution (κele) originated from the higher σ of Cl-doped Bi1.2Sb0.8Se3. On the other hand, as shown in Fig. 4a, the κtot of all the samples gradually decrease with temperature, suggesting the small contribution of bipolar thermal conduction (κbp). We estimated the κlat and κele by using the relationship of κtot = κele + κlat. Details for the calculation are described in Section 6 of ESI.†
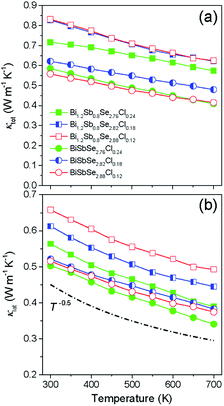 | ||
| Fig. 4 Temperature dependence of (a) total thermal conductivity and (b) lattice thermal conductivity for BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24). | ||
Fig. 4b shows the temperature dependence of κlat for both Cl-doped BiSbSe3 and Bi1.2Sb0.8Se3. The temperature dependence of κlat shows roughly κlat ∝ T−0.5, indicating the additional point defect phonon scattering from the mass difference between host Se (MSe = 78.971) and dopant Cl (MCl = 35.45) by Cl substituted at Se-site. The κlat values of Cl-doped BiSbSe3 are lower than those of Cl-doped Bi1.2Sb0.8Se3 mainly due to the soft bonding in orthorhombic phase, however, κlat reduction effect by Cl-doping is relatively small compared to that of Cl-doped Bi1.2Sb0.8Se3 due to cumulative phonon scattering by soft bonding and point defect. Thus the significantly reduced κlat (∼0.56 W m−1 K−1 at 300 K and ∼0.39 W m−1 K−1 at 700 K) is obtained in Bi1.2Sb0.8Se2.76Cl0.24 mainly due to the intensified mass-defect phonon scattering. The slightly higher κlat of BiSbSe2.82Cl0.18 than that of BiSbSe2.88Cl0.12 is considered to be related with the difference in preferred orientation (Fig. 1b).
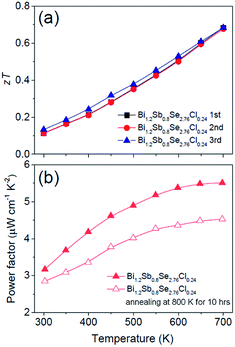
Fig. 5a and b show the temperature dependent zT of BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and that of Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24), respectively. The Cl-doping effectively enhances the zT both in BiSbSe3 and Bi1.2Sb0.8Se3 due to improvement of electronic and thermal transport properties. High zT of Cl-doped BiSbSe3 with pure orthorhombic phase is mainly due to the enlarged 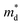 benefitting from the increased valley degeneracy and flattened band, which results in a larger S. Reduced κlat by the bond softening in orthorhombic phase is another origin for high zT of Cl-doped BiSbSe3. On the other hand, higher zT found in Cl-doped Bi1.2Sb0.8Se3 despite of high rhombohedral phase fraction (∼0.74) is attributed to the simultaneous improvement of electronic (enlarged
benefitting from the increased valley degeneracy and flattened band, which results in a larger S. Reduced κlat by the bond softening in orthorhombic phase is another origin for high zT of Cl-doped BiSbSe3. On the other hand, higher zT found in Cl-doped Bi1.2Sb0.8Se3 despite of high rhombohedral phase fraction (∼0.74) is attributed to the simultaneous improvement of electronic (enlarged 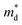 and improved μH) and thermal (reduced κlat) transport properties by Cl-doping. The highly-reproducible maximum zT reaches in value about 0.68 ± 0.04 at 700 K for three different Bi1.2Sb0.8Se2.76Cl0.24 samples. Moreover, high σ values of 397 S cm−1 at 300 K and 159 S cm−1 at 700 K make this material a promising candidate for practical applications.
and improved μH) and thermal (reduced κlat) transport properties by Cl-doping. The highly-reproducible maximum zT reaches in value about 0.68 ± 0.04 at 700 K for three different Bi1.2Sb0.8Se2.76Cl0.24 samples. Moreover, high σ values of 397 S cm−1 at 300 K and 159 S cm−1 at 700 K make this material a promising candidate for practical applications.
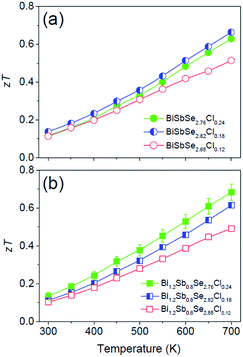 | ||
| Fig. 5 Temperature dependence of dimensionless figure of merit zT for (a) BiSbSe3−yCly (y = 0.12, 0.18, 0.24) and (b) Bi1.2Sb0.8Se3−zClz (z = 0.12, 0.18, 0.24). | ||
We verify the thermal stability of Bi1.2Sb0.8Se2.76Cl0.24 via the cyclic measurement of zT within temperature range from 300 K to 700 K (Fig. 6a) and remeasurement of power factor after annealing at 800 K for 10 h (Fig. 6b). Fig. 6a and b indicate that the Cl-doped Bi1.2Sb0.8Se3 alloys are chemically stable up to 700 K.
4. Conclusions
In summary, temperature dependent thermoelectric transport properties of Te-free Cl-doped BiSbSe3 and Bi1.2Sb0.8Se3 are systematically investigated. Improved Seebeck coefficient and electrical conductivity are simultaneously obtained due to the enlarged density-of-states effective mass by high content Cl-doping (∼8 at%), while maintaining intrinsic high mobility of Bi1.2Sb0.8Se3-based alloys. This provides the optimized power factor of ∼5.61 μW cm−1 K−2 at 700 K. Additionally, despite of the weaker phonon scattering owing to decreased bond softening effect in Bi1.2Sb0.8Se3 compared to that in BiSbSe3, lattice thermal conductivity is effectively reduced in value about 0.39 W m−1 K−1 at 700 K by 8 at% Cl-doping from the intensified mass-defect phonon scattering. This synergetic effect contributes to a high electrical conductivity of ∼159 S cm−1 and the high zT ∼0.68 at 700 K.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (NRF-2017R1A2B3011949) and Global Frontier Program through the Global Frontier Interface Materials (GFHIM) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013M3A6B1078870).References
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 CrossRef CAS PubMed.
- J. Jiang, L. Chen, S. Bai, Q. Yao and Q. Wang, Scr. Mater., 2005, 52, 347 CrossRef CAS.
- K. H. Lee, S. I. Kim, H. Mun, B. Ryu, S. M. Choi, H. J. Park, S. Hwang and S. W. Kim, J. Mater. Chem. C, 2015, 3, 10604 RSC.
- S. J. Jung, B. H. Lee, B. K. Kim, S. S. Lim, S. K. Kim, D. I. Kim, S. O. Won, H. H. Park, J. S. Kim and S. H. Baek, Acta Mater., 2018, 150, 153 CrossRef CAS.
- S. Wang, Y. Sun, J. Yang, B. Duan, L. Wu, W. Zhang and J. Yang, Energy Environ. Sci., 2016, 9, 3436 RSC.
- S. K. Mishra, S. Satpathy and O. Jepsen, J. Phys.: Condens. Matter, 1997, 9, 461 CrossRef CAS.
- P. Larson, V. A. Greanya, W. C. Tonjes, R. Liu, S. D. Mahanti and C. G. Olson, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 85108 CrossRef.
- W. Liu, K. C. Lukas, K. McEnaney, S. Lee, Q. Zhang, C. P. Opeil, G. Chen and Z. Ren, Energy Environ. Sci., 2013, 6, 552 RSC.
- X. Liu, D. Wang, H. Wu, J. Wang, Y. Zhang, G. Wang, S. J. Pennycook and L. D. Zhao, Adv. Funct. Mater., 2019, 29, 106558 Search PubMed.
- J. S. Rhyee, K. Ahn, K. H. Lee, H. S. Ji and J. H. Shim, Adv. Mater., 2011, 23, 2191 CrossRef CAS PubMed.
- Q. Zhang, H. Wang, W. Liu, H. Wang, B. Yu, Q. Zhang, Z. Tian, G. Ni, S. Lee, K. Esfarjani, G. Chen and Z. Ren, Energy Environ. Sci., 2012, 5, 5246 RSC.
- Q. Zhang, Y. Lan, S. Yang, F. Cao, M. Yao, C. Opeil, D. Broido, G. Chen and Z. Ren, Nano Energy, 2013, 2, 1121 CrossRef CAS.
- S. I. Kim, S. Hwang, S. Y. Kim, W. J. Lee, D. W. Jung, K. S. Moon, H. J. Park, Y. J. Cho, Y. H. Cho, J. H. Kim, D. J. Yun, K. H. Lee, I. Han, K. Lee and Y. Sohn, Sci. Rep., 2016, 6, 19733 CrossRef CAS PubMed.
- V. G. Kuznetsov, K. K. Palkina and A. A. Reshchikova, Izv. Akad. Nauk SSSR, Neorg. Mater., 1968, 4, 670 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra04065g |
| ‡ These authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2020 |