Open Access Article
Open Access ArticleNew insights into red plant pigments: more than just natural colorants
José A. Fernández-López
 *a,
Vicente Fernández-Lledó
b and
José M. Angosto
a
*a,
Vicente Fernández-Lledó
b and
José M. Angosto
a
aDepartment of Chemical and Environmental Engineering, Technical University of Cartagena (UPCT), Paseo Alfonso XIII 52, E-30203 Cartagena, Murcia, Spain. E-mail: josea.fernandez@upct.es
bHigher Technical School of Telecommunications, Technical University of Madrid (UPM), Madrid, Spain
First published on 29th June 2020
Abstract
Pigments make nature both colorful and attractive. Humans have always incorporated the natural pigments of fruits, vegetables and spices into their dietary requirements. Naturally occurring red pigments in plants are carotenoids, anthocyanins and betacyanins. Natural pigments, apart from colour, provide added properties and are therefore considered to be bioactive constituents. Red natural colorants are one of the most widely used in the food industry. The interest in these pigments lies in the enhancement of the healthy effects of the diet. In this context, attention is given to carotenoids, anthocyanins and betacyanins, with emphasis on the basic chemical and biochemical attributes and wide-ranging health-promoting benefits of these pigments. Thus, in this review, we systematically present the advantages and limitations of these natural pigments as food colorants in relation to their physico-chemical properties, reactivity and bioactivity.
1. Introduction
Plants play a fundamental role in supporting life on Earth. A look at the plant kingdom shows us that the predominant pigments in plants are presented as a palette of colours that covers the entire visible spectrum.1 From a scientific approach, plant pigments should be seen as intermediary products with a chromophore component. This chromophore is responsible for colourful appearance, capturing energy and exciting electrons from lower energy orbitals to a higher orbital while unabsorbed energy is reflected and appears as a colour to the human eye.2 Plant pigments not only attract animals needed for pollination and seed dispersal, to ensure the reproductive success and the permanence of the species, but also participate in critical biological processes for plants, playing essential roles in the maintenance of ecosystems.3,4 In this context, plant pigments are also involved in metabolic and energy generation processes and their role as protectors of other vulnerable biomolecules against oxidative stress and solar radiation are especially noteworthy. Red plant pigments are used as indicator metabolites for evolutionary studies of plant diversity as well as for metabolic studies of plant cell growth and differentiation.5In recent years, plant pigments, in parallel to their role as natural colorants, have begun to be recognized as bioactive substances because of their potential health benefits, which has boosted their commercial demand.6,7
Scientific advances in the interdisciplinary fields of food chemistry and food technology have driven research into the potential applications of plant pigments in functional foods.7–9 Scientific evidence has shown that healthy eating habits, along with a healthy lifestyle, become a key part of promoting quality of life, making society in the 21st century increasingly prefer natural ingredients, which also include natural colorants.7,9,10
Currently, natural pigments are used extensively as additives or supplements in the food industry. Legal restrictions, by the Food and Drug Administration in USA and by the European Food Safety Agency (EFSA) have greatly reduced the colour palette available to the food industry, being the red colour the most affected. This has been due to the confirmed or suspected association of synthetic red colorants with allergic reactions and toxic effects.11–14 Market pressure caused by changes in legislation and consumer preferences, have forced food to be formulated with more natural ingredients, with a growing interest in natural red pigments and in the plants used as source of them.6,15
Fruits and other plant organs contain only a minor percentage of pigments along with other components such as proteins, lipids, carbohydrates, water-insoluble fibers and tannins, among others. Extraction is an essential step not only for the preparation of individual pigments but is also fundamental for obtaining natural colorant extracts. The plant matrix also contains a variety of non-colouring plant components, so the extraction process is a complex one. It is necessary to determine the nature and solubility characteristics of the pigments to be considered before selecting a particular extraction process. The different methods for the extraction of plant pigments are aqueous or solvent extraction, microwave and ultrasonic assisted extraction, enzymatic extraction, fermentation or supercritical fluid extraction.1,9,15 In order to isolate pure pigments, these extracts are then purified by solid phase extraction and subsequent chromatographic separation techniques.
Carotenoids, anthocyanins and betacyanins are the three families of pigments responsible for the natural display of red colours in plants. Although the three groups are structurally well differentiated and present quite diverse traits, all of them are able to provide eye-catching and attractive red colour to plants. These pigments, with colorant and pharmacological properties, are of interest to food scientists, dietitians and food industries because of their beneficial contribution to human health.
2. Carotenoids
2.1 Chemistry and biochemistry of carotenoids
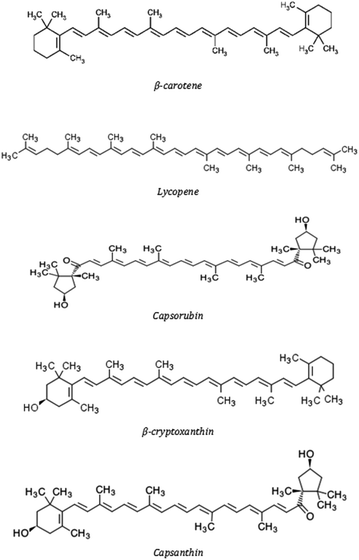
Carotenoids are responsible for yellow, orange and red colours in the plant kingdom. They are the most diverse and widespread pigments found in nature.16,17 This family of over 600 compounds can be subdivided into carotenes, pure hydrocarbons, and xanthophylls, which also contain oxygen, often two or four atoms per molecule. Both groups contain 40 carbon atoms made from eight isoprene units. The most obvious distinguishing feature of the carotenoid molecules is the long polyene chain, which may extend from 3 to 15 conjugated double bonds. The length of this chromophore defines the absorption spectrum of the molecule and consequently its colour at eyesight.18 Larger conjugated systems result in the display of redder hues, whereas short double bond chains, show achromaticity. Cyclization of the carbon skeleton may either occur at one or both ends of the carotenoid molecule,19,20 and also affects the colour appearance.Typical red carotenoid-pigmented fruits are tomato (Solanum lycopersicum), watermelon (Citrullus lanatus) and red pepper (Capsicum annuum) (Fig. 1). Tomato and watermelon are rich in lycopene and β-carotene, whilst red cultivars of Capsicum are particularly noteworthy because they are one of the few examples of plants producting ketocarotenoids as capsanthin and capsorubin21 (Fig. 2).
In higher plants, carotenoids are synthesized and localized in the plastids. The type and availability of carotenoids in fruits and vegetables can be predicted by their external colour. So, yellow-orange fruits and vegetables are generally rich in β-carotene and α-carotene. In orange fruits as papaya, mango and orange zeaxanthin and β-cryptoxanthin are the major constituents.21 In addition, their observed colour is also influenced by pigment concentration.22 For instance, the yellow β-carotene become orange and then red when its content increases. Compositions of major carotenoids in red and orange fruits are given in Table 1.
| α-Carotene | β-Carotene | β-Cryptoxanthin | Zeaxanthin | Lutein | Lycopene | Capsorubin | Capsanthin | |
|---|---|---|---|---|---|---|---|---|
| Mango21 (Mangifera indica) | 445 | 150 | 3170 | |||||
| Papaya23 (Carica papaya) | 30 | 630 | 750 | 410 | 3350 | |||
| Orange23 (Citrus sinensis) | 200 | 151 | 710 | 129 | 205 | |||
| Watermelon23 (Citrullus lanatus) | 324 | 210 | 40 | 4200 | ||||
| Tomato21,23 (Solanum lycopersicum) | 112 | 393 | 130 | 650 | 8050 | |||
| Red pepper24,25 (Capsicum annuum) | 59 | 2379 | 1205 | 350 | 550 | 8600 |
Carotenoids, also called tetraterpenoids, are essential pigments for plant life, contributing to the photosynthesis machinery and providing photo-protection against excess light damage to the photosynthetic reaction center by quenching excited species such as singlet oxygen and free radicals.25 Carotenoids are often considered only as plant pigments, but are also found in fungi, bacteria, algae and animals. In plants, carotenoid biosynthesis occurs universally as a key part of the construction of chloroplasts, but it is also closely associated with the formation of chromoplasts as fruit are mature or flowers bloom.23 Thus, in red pepper, tomato fruit or watermelon, the carotenoid content is increased progressively during ripening.24
The content and composition of carotenoids in plants are influenced by nutritional and environmental factors and can be strongly altered by treatment with a range of chemicals, mainly amines. The biosynthesis of carotenoids differs according to the specific species, although all plants share the common primary metabolic pathway.26,27
2.2 Carotenoids as natural colorants
Carotenoid-rich plant extracts can be applied as natural food colorants and also serve as excellent source of bioactive or functional compounds. Genetic manipulation has succeeded in substantially increasing the carotenoid content of certain plant species, producing carotenoid-rich plants, that can be used as direct dietary sources of nutrients or as raw ingredients for extracting natural red, orange or yellow dyes.28Carotenoids are intracellular compounds and are usually located in membranes of mitochondria, chloroplasts or endoplasmic reticulum. Generally, carotenoids are highly hydrophobic compounds which will therefore tend be associated with lipids or in hydrophobic structures such as membranes.29 The orientation of a particular carotenoid in the membrane, and its stability, depend on features such as the shape and structure of the carotenoid and the presence of functional groups.29,30 So, although carotenoid pigments are largely stable in natural plant environments, they are much more fragile when plant cells are damaged as during chopping and even more fragile when they are extracted or dissolved in organic solvents.
Carotenoids and carotenoid-plant extracts have widely used as colorants in foods. The plant extracts first used were those of paprika, annatto, tomato and saffron. The powdered dried plant and liquid extracts obtained from them have been used for many years in food industries.27 These extracts are not pure carotenoids or simply mixtures of them and can provide certain substances with a strong flavour which will limit their use as food colorants considerably. Genetic engineering has been successful in substantially increasing the carotenoid content of selected plants, producing carotenoid-rich species, which can be used as primary dietary sources of pigments or as raw ingredients to extract natural red, orange or yellow dyes.31,32
In the European Union, E numbers are used as codes for food additives, including natural colorants, according to the EC regulation 1333/2008. All additives are identified by the letter E followed by a number. E-numbers E100–E199 are reserved for colorants, including natural colorants originating from plants or mixtures made from natural foodstuffs. Table 2 summarizes the carotenoid colorants with E number.
| E-number | Carotenoid colorant |
|---|---|
| E160a | Mixed carotenes |
| E160b | Annatto, bixin, norbixin |
| E160c | Paprika extract |
| E160d | Lycopene |
| E160e | β-Apo-8′-carotenal |
| E161b | Lutein |
| E161g | Cantaxanthin |
The plant extracts used as source of red carotenoids are from tomato, red pepper or watermelon. In tomato extracts significant variation in carotenoid profiles can be found, where a number of mutations affecting the total content and diversity of carotenoids are identified,30 although lycopene is the main carotenoid present in these extracts. The colour of lycopene (Fig. 2) depends on its concentration: it is orange in a diluted solvent and bright red when accumulated in the tomato fruit peel. Tomato lycopene extracts and tomato lycopene concentrates are approved and commonly used as red food colorants. Oleoresins, powders, and aqueous-dispersible preparations of lycopene show stable colour over a wide pH and temperature range.1,22,30
Paprika, the powder or oleoresin processed from mature fruit pods of Capsicum annuum, is extensively consumed as food additive throughout the world because of its intense colour and flavor. The red colour intensity is considered as the most important attribute of paprika.25,33 The attractive red color of paprika is mainly due to the presence of ketocarotenoids, (capsorubin and capsanthin) (Fig. 2). They account around 70–80% of its total carotenoid content, with β-carotene, β-cryptoxanthin and lutein also present but in lesser quantities.25,34–36 During processing and storage, the color of paprika can be altered mainly due to degradation of carotenoids and non-enzymatic browning reactions. These degrading reactions are enhanced at higher temperatures and water activities.37–40
Watermelon flesh is also characterized by an attractive red color (Fig. 1.) due to the presence of carotenoids. Around 90% of the total carotenoid content is represented by lycopene, with β-carotene, β-cryptoxanthin and lutein also present.23,41
2.3 Health-promoting benefits of carotenoids
The importance of carotenoids goes beyond their role as natural pigments. Carotenoids are lipophilic molecules and alternative ways of delivering these lipid-soluble compounds include water-dispersible beads, microemulsions, micelles, nanoparticles, artificial liposomes or specialized formulations.42 Biological functions and protective effects have been increasingly attributed to these compounds and a broad spectrum of health-promoting effects is scientifically associated to carotenoids.14,19,21,43The liposolubility of carotenoids is the main characteristic that determines the stages of the process of release, transport and bioassimilation from food. Reported data on carotenoid bioavailability in humans is based mainly on measurement of carotenoid in blood plasma or serum after ingestion. It is noteworthy that at steady state, plasma carotenoids account to approximately 1% of the total body content of carotenoids. The carotenoid level in serum may not be a good indicator of carotenoid status because it depends on several processes of uptake and depletion of the serum for purposes of storage, excretion or bioconversion.44
About metabolic transformations of carotenoids, it is generally assumed that they undergo oxidation cleavage and polyene chain shortening by an analogous breakdown mechanism to β-oxidation of lipids. These transformations are dependent on the specific carotenoid being researched. The metabolic pathway of a number of them leads to direct oxidation products, suggesting that they act as antioxidants in the human body. Lycopene is metabolized in poorly understood pathways, while capsanthin, the red ketocarotenoid of paprika, is associated with lipoproteins in blood plasma, and is quickly eliminated.45
The main established health-promoting function of carotenoids is to be a vital dietary source of vitamin A, and this is mainly found on β-carotene, β-cryptoxanthin and α-carotene.43 The functions of vitamin A include cell growth, immune function, fetal development and vision.45 Together with this function, the antioxidant capacity is described as the most representative, and it is extended to all carotenoids, with or without provitamin A activity. Carotenoids inhibit the process of lipid auto-oxidation, so their presence in the cell membranes avoids the consequent negative processes associated with it. They also react with other radicals of diverse nature, such as those formed during the metabolism of xenobiotic compounds. The development of this function is based on the reduction of the risk of macular degeneration, a disease that causes blindness with a high level of incidence among the elderly population worldwide.43
The antioxidant activity of carotenoids involved in the processes of inhibition or reduction of oxidative stress, is the main mechanism of action of these pigments and hence their characterization as anticarcinogenic and immunoactive agents. So, dietary lycopene consumption is associated with a reduced risk of prostate cancer46 and a possible preventive effect against liver tumorigenesis,47 while β-carotene and other carotenoids are reversively linked to immune activation and inflammatory biomarkers in patients with coronary artery disease.48 These investigations support the physiological functionality of carotenoids that includes their activity as membrane antioxidants, being integrated into the oxidative cycle of the cell, along with other primary antioxidants, and their involvement in the control processes of cell proliferation and differentiation.
Some carotenoids also appear to have effects on cognitive functioning and better neural efficiency, and although the mechanism is not clear, it seems to be associated with their antioxidant activity.49
β-Cryptoxanthin has been reported to have a stimulating effect on bone formation via osteoblast stimulation, so it may be important for bone homeostasis, particularly in post-menopausal women to delay osteoporosis.50
Carotenoids are important as basal protection of the skin against UV light-induced damage and promote skin health care, proper skin hydration and appearance. This effect is associated to the high potential of carotenoids to scavenge reactive oxygen species, such as peroxide radicals or singlet oxygen molecules.43,51
In general, carotenoids are essential components of a healthy diet. They play a key role as antioxidant phytochemicals with multiple health benefits in humans, and further research and clinical studies are being conducted to undoubtedly establish specific carotenoids, doses and health effects.
3. Anthocyanins
3.1 Chemistry and biochemistry of anthocyanins
Anthocyanins are the largest and most important group of vacuolar pigments in the plant kingdom. They are largely responsible for diverse pigmentation from orange to red, pink, purple and blue in flowers and fruits of many plants (strawberries, raspberries, blackberries, elderberries, grapes, cherries, currants, red onions, red cabbages, red lettuces, tulip, roses,…) (Fig. 3). These pigments are easily added to aqueous media, which renders them very interesting in food industry as water-soluble natural colorants.1,9,14Apart from fruits and vegetables, the black grains and cereals are also abundant in anthocyanins. So, coloured cereals, black rice, black beans and black soybean have been subjected to extensive research to evaluate their potentials as natural colorant, nutritional function or functional foods.52,53 At this point, purple sweet potato54 and litchi pericarp55 have also been investigated as potential sources of anthocyanins.
Chemically, anthocyanins are flavonoids, and consequently based on a C15 skeleton with a chromane ring, bearing a second aromatic ring B in position 2 (C6–C3–C6) and with one or more sugar molecules bonded at different hydroxylated positions of the basic structure.56 The basic C6–C3–C6 anthocyanin structure is the origin of all the different colours resulting by its chemical combination with glycosides and/or acyl groups and by its interaction with other molecules. Anthocyanidins are the sugar-free counterparts of anthocyanins.
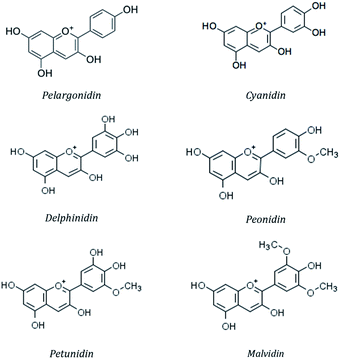
More than 20 anthocyanidins have been reported,57 with differences in the number and position of the hydroxyl and/or methyl ether groups, but six of them (cyanidin, malvidin, peonidin, delphinidin, pelargonidin and petunidin) (Fig. 4) are the most common in nature,58 being present in 80% of pigmented leaves, 69% of fruits and 50% of flowers.59 They are typically linked to a saccharide molecule such as glucose, galactose, arabinose, rhamnose or rutinose, as either 3-glycosides or 3,5-diglycosides.60
Colour differences between anthocyanins are mainly influenced by the substitution pattern of the B-ring of the anthocyanidin, the level of glycosylation and the degree and nature of esterification of the saccharides with aliphatic or aromatic acids and by the pH, temperature, solvent and the presence of co-pigments.60
A major characteristic of anthocyanins is that their colour is pH-dependent. In aqueous solution at pH 1–3 they are red coloured, at pH 5 they form a colourless carbinol pseudo base and, at pH > 6 they are converted in a blue-purple quinoidal base.56 Colour alteration is also influenced by factors such as light, oxygen, metal ions and several enzymes.57,61
The great diversity of anthocyanins present in nature makes them a highly complex and interesting group of phytochemicals. The anthocyanin composition of vegetables and fruits is influenced by cultivar, growing, area, cultivation practices, ripeness, processing and storage.
Table 3 reveals the main anthocyanins in a range of fruits and vegetables. Such is the diversity of these pigments, that, the analysis of the anthocyanin pattern in fruits of different plant species has even made possible to use them as a differential indicator in chemotaxonomical investigations.57,62
| Fruit/vegetable | Main anthocyanin |
|---|---|
| Strawberry | Pelargonidin-3-glucoside |
| Raspberry | Cyanidin-3-sophoroside |
| Sweet cherry | Cyanidin-3-rutinoside |
| Bilberry | Delphinidin-3-galactoside |
| Red grape | Malvidin-3-glucoside |
| Red plum | Cyanidin-3-rutinoside |
| Pomegranate | Delphinidin-3-glucoside |
| Red onion | Cyanidin-3-glucoside |
| Purple carrot | Cyanidin-3-xylosyl-glucosyl-galactoside |
| Red cabbage | Cyanidin 3-diglucoside-5-glucoside |
3.2 Anthocyanins as natural colorants
With the growing consumer demand for natural and safer food ingredients, numerous experiments have been conducted to achieve more effective and selective food colorants. In this context, anthocyanins probably are the most widely studied natural food colorants.9,22,33,56,63Anthocyanins exhibit attractive attributes as natural food colorants and therefore they have been traditionally used in the food industry. It is known that the colour of anthocyanin extracts changes according to the pH of the solution, due to the molecular structure of these pigments has an ionic nature.64 Typical red colour is displayed for pH ranges between 1 and 3.8
Food industry is particularly demanding of water-soluble natural red colorants due to the legal restrictions that have been imposed on certain synthetic red dyes.39 It is because of this situation that natural anthocyanin extracts, referenced in the European Union as additive E163, have become the most widely studied natural food colorants. E163 refers to the food additive, which are not sufficiently characterized and, may contain both the glycosides (anthocyanins) and the aglycones (anthocyanidins). Commercial anthocyanins, namely peonidin-3-glucoside, pelargonidin-3-glucoside and cyanidin-3-glucoside are also used, and their efficiency has been largely tested.66
Use of anthocyanins in food as colorants is rather limited because of their relative unstability and interactions with other food matrix ingredients. As a result of this, the chemical stabilisation of anthocyanins is an important focus of recent studies because their abundance and potential applications.57 The molecular structure itself influences the stability of these molecules. In general, an increase in hydroxylation decreases stability, while an increase in methylation enhances it.67 Thus, the colour of foods containing anthocyanin extracts that are rich in pelargonidin, cyanidin, or delphinidin aglycones is less stable than that of food containing petunidin or malvidin aglycones.68 It is also accepted that the colour of anthocyanins can be preserved and enhanced by copigmentation interactions with non-coloured molecules in pigment solution. Copigmentation is shown by slightly shifting the wavelength of maximum absorbance or by increasing the colour intensity.1,69 Acylation is also known to increase the anthocyanin's stability through intramolecular copigmentation. The increase is mainly from physico- and biochemical reasons.70 Generally, non-acylated anthocyanins occur predominantly in fruits, while acylated anthocyanins are mostly in flowers and vegetables.71 This higher stability of acylated anthocyanins explains the widespread use of vegetable-based anthocyanin extracts as food colorants.71,72
The main factors governing the degradation of anthocyanins are pH, temperature and oxygen concentration. Anthocyanins are remarkably unstable under neutral or basic pH conditions. The typical red colour of the flavylium catium is shown between pH values of 1–3, becoming deprotonated and forming colourless structures and blue-purple quinoidal bases at pH increases. According to this limitations, the main applications of anthocyanins as natural colorants in the food industry are in acid products with pH 4 or lower, such as juices, soft drinks, jellies, jams, ice creams, marmalades and less frequently in meat, pastry, dairy and wines.65,72–74
Anthocyanin stability in foods is significantly reduced by temperature.39,64 Generally, structural characteristics that lead to greater pH stability also lead to greater thermostability. Methylated, glycosylated, or acylated anthocyanidins are more thermostable than highly hydroxylated anthocyanidins.75 Of the natural red food colorants, anthocyanins are recognized as pigments that degrade more easily at high temperatures than carotenoids.39
The presence of double bonds in the anthocyanin structures makes them particularly susceptible to molecular oxygen. It is well known that when grape juice or wine is hot-filled into bottles, the full filling of the bottles will prevent the colour degradation from red-purple to brownish dull. Comparable considerations have been reported with other anthocyanin-rich juices. It is accepted that oxygen may cause its deleterious effects on anthocyanins through a direct oxidative mechanism and/or through the indirect action of oxidizing enzymes such as polyphenol oxidase (PPO). The oxidized constituents of the medium are capable of reacting with the anthocyanins to yield colourless or brown pigmented products.75,76 The beneficial effect of oxygen removal on the colour retention of anthocyanins has also been demonstrated by processing anthocyanin-pigmented fruit juices under nitrogen atmosphere or vacuum.77
Light is another factor that also accelerates the degradation of anthocyanins. This negative outcome has been demonstrated in several fruit juices and red wine. In wine it has been reported that acylated and methylated diglycosides are more resistant than non-acylated ones, with monoglycosides being the most labile anthocyanins.57,77 Hence the food industry's preference for opaque containers or dark glass bottles for anthocyanin-rich juices and drinks.
A variety of methods can be used to increase the stability of anthocyanins, such as oxygen exclusion, light preservation, addition of co-pigments and encapsulation.76 In recent years, the microencapsulation of anthocyanins has allowed the development of new food colorants with increased stability, bioavailability and solubility.78–80
Being aware of the chemical behaviour of anthocyanins allows to achieve a minimum degradation of their colouring capacity through an adequate selection of conditions and by selecting the most suitable anthocyanin pigments and stabilization methods for each potential application.
3.3 Health-promoting benefits of anthocyanins
The trading around the health-promoting benefits of anthocyanins is increasingly being reported, with one of their key attributes the high antioxidant capacity. Nevertheless, this high activity revealed in vitro, is sometimes contradicted by in vivo data on the antioxidant capacity of blood plasma after ingestion of anthocyanin-rich foods by humans.81Anthocyanins and anthocyanidins are said to be low bioavailability molecules, which has been recently estimated to be between 12% and 15%.81–83 Despite this, they have been associated with reduced incidence of major chronic diseases such as cancer, heart disease, diabetes, stroke, cataracts, Alzheimer's disease and deterioration of age-related functions.8,14,57,66
In vivo models have revealed the promising protective effect of anthocyanins on oxidative stress-induced apoptosis.84,85 They are reported to be particularly efficient in scavenging free radicals, exhibiting a key role in prevention of mutagenesis and carcinogenesis.85–87
Anthocyanins have been proved to be effective in inhibiting the initiation, promotion and progression of multiple types of cancers, such as prostate cancer, colorectal cancer, liver cancer, breast cancer, cervical cancer, lung cancer and metastatic melanoma.88 Anticarcinogenic activity of anthocyanins may be ascribed to the aggregative effect of a number of mechanisms. Suggested possible mechanisms are diverse, including inhibition of oxidative DNA damage,89 antiangiongenesis90 and cell cycle detention.91
The anti-inflammatory activity of anthocyanins to overcome inflammation-related diseases such as periodontal disease,92 colitis,93 postprandial inflammation80 and laryngeal reflux94 has also been reported in a number of experimental studies. The dual use of anthocyanins with anti-inflammatory medicines may represent new therapeutic approaches for the treatment of inflammation-related diseases.88
A growing body of evidence suggests that anthocyanins may delay or halt the progression of age-related health disorders and cognitive decline. Several studies reported significant evidence about the neuroprotective effect of anthocyanins to ameliorate memory, cognitive and motor performance. Anthocyanins have been shown to modulate several of the factors contributing to neuronal death, and interest in their use as therapeutics for neurodegeneration processes including Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis, has grown in recent years.95,96
The dietary consumption of anthocyanin-rich extracts and their contribution to the maintenance or improvement of cardiovascular health has been a topic of particular interest to researchers. Different epidemiological studies have revealed the relationship between anthocyanins intake and the reduced risk of developing coronary heart disease.97 It has been reported that supplementation with anthocyanin-rich extracts significantly reduced plasmatic cholesterol and hepatic triglyceride levels with consequent benefits for cardiovascular health.98,99
The state of knowledge about the metabolic processes, uptake and bioavailability of anthocyanins in relation to cardiovascular disease has increased substantially in recent years, however, there are no conclusive studies proving their effect on cardiovascular health. The need for future research in this area is clearly evident.
Some epidemiological studies also correlate anthocyanin intake inversely with the risk of diabetes and obesity. Anthocyanins are able to overcome alterations in glucose and lipid metabolism. Studies with cell lines and animal models and clinical trials in humans suggest that anthocyanins exhibit antidiabetic properties.100 The mechanisms of action in this domain point to a close relationship with the antioxidant activities of these pigments.101 The relation of anthocyanins with the treatment against obesity points out that they improve the lipidic metabolism preventing the excessive accumulation of adipose tissue. A randomised clinical study has revealed that the intake of certain anthocyanin extracts led to a reduction in abdominal fat, cholesterol, triacylglycerol and low-density lipoprotein levels.102
Recent studies have also reported the protective effects of some anthocyanins against the UVB-induced oxidative damage to retinal pigment epithelial cells,103 in addition to positive effect on night vision and dark adaptation.86
The multiple health benefits described above highlight the potential of these pigments as a functional health-enhancing ingredient, supporting their role as natural food colorants, functional ingredients and diet.
4. Betacyanins
4.1 Chemistry and biochemistry of betacyanins
Betacyanins are vacuolar water-soluble nitrogen-containing pigments, responsible for the red pigmentation in fruits, vegetables and flowers,9 associated in most plant families of the order Caryophyllales (Centrospermae) as beets, cacti and amaranths (Fig. 5).A characteristic feature of betacyanins is that never coexist in plants with anthocyanins, they seem to be mutually exclusive.104 This exclusivity remains unexplained. Betacyanins (red-violet pigments) and betaxanthins (yellow-orange pigments) constitute the family of plant pigments known as betalains.
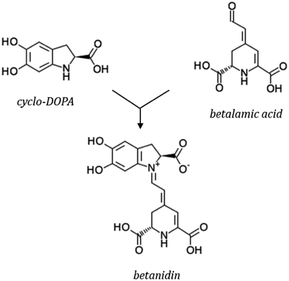
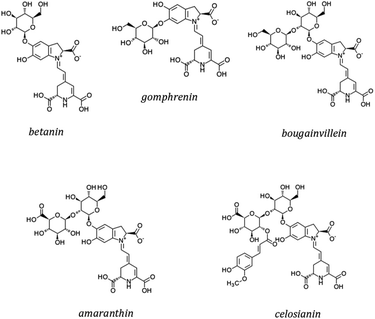
Betalains are secondary metabolites derived from the amino acid L-tyrosine. All betalains have the betalamic acid in their core structure. Condensation of betalamic acid with cyclo-DOPA (cyclo-3,4-dihydroxyphenylalanine) leads to formation of betacyanins39,105 (Fig. 7). The conjugated double bond system of the betalamic acid molecule conforms the chromophore (Fig. 6). Betanidin is the aglycone of most betacyanins. Glycosylation reactions are common in betacyanin biosynthesis, both as part of the central pathway leading to formation of ‘‘simple’’ betacyanins such as betanin (betanidin 5-O-β-glucoside) and gomphrenin (betanidin 6-O-β-glucoside), as well as in subsequent reactions that are responsible of the formation of a large variety of more complex betacyanins.106 Further glycosylation of the 5-O-β-glucoside is very common and as well as esterification with hydroxycinnamic acids.107 The most common betacyanin is betanin from red beet.108
Betacyanins are of great taxonomic significance in higher plants. They can be further classified into subgroups that include betanin-, bougainvillein-, amaranthin- and gomphrenin-type pigments106 (Fig. 7). Table 4 presents the main plant species producing betacyanins. A large number of betacyanins, and in particular within the bougainvillein group, present multiple glycosylations, with sugars such as glucose, xylose, rhamnose and/or apiose. These pigments have been described in several species, most notably in purple bracts of Bougainvillea glabra.106,109
| Family | Plant specie | Main betacyanins |
|---|---|---|
| Chenopodiaceae | Beta vulgaris | Betanin, isobetanin |
| Chenopodium rubrum | Betanin, celosianin | |
| Cactaceae | Opuntia sp. | Betanin, isobetanin |
| Hylocereus polyrhizus | Betanin, isobetanin, phyllocactin, hylocerenin | |
| Amaranthaceae | Amaranthus sp. | Amaranthin, isoamaranthin |
| Gomphrena globosa | Gomphrenin | |
| Iresine sp. | Iresinin | |
| Aizoaceae | Lampranthus productus | Betanidin |
| Nyctaginaceae | Bougainvillea sp. | Bougainvillein, gomphrenin |
Similarly to other plant pigments present in flowers and fruits, betacyanins are likely to play an important role in attracting pollinators and dispersing seeds. In addition, betacyanins are thought to be involved in the defense of plants against various signs of biotic and abiotic stress.110 The involvement of these pigments in the photoprotection of plant organs is also well known. Thus, when plants are exposed to an excess of light or UV radiation it is found that the biosynthesis of betacyanins is stimulated to overcome its effects.111,112 The relationship between betacyanins and the capacity to adapt to extreme conditions is confirmed by the fact that these pigments are found in plants of the order Caryophyllales, especially adapted to the severe conditions of arid and semiarid regions, including the Cactaceae (cacti and hylocereus families), and Aizoaceae (ice-plant family).108
4.2 Betacyanins as natural colorants
Red beet betanin is a well-established natural colorant and it is approved as red food colorant by the European Union (EU no. E162) and under Section 73.40 in the Title 21 of the Code of Federal Regulations (CFR) by the Food and Drug Administration (FDA) in the United States.22,104The availability of commercial preparations of betacyanins is restricted by the legislations of most countries to beet juice concentrates (60–65% total solids) obtained by rotary evaporation under vacuum of fresh red beet juice, or to the solid obtained by spray drying or freeze drying of the concentrated liquid.113 The extraction processes of betacyanins are based on their water-soluble character. The red beet juice is obtained after blanching (for the inactivation of enzymes that can degrade the colour), crushing, pressing and subsequent filtration of the red beet roots. Commercial preparations are not pure and therefore differ in colour depending on the proportion of betacyanins and betaxanthins they contain, often with characteristic earth odours, that are detrimental to the final quality of the colorant, due to the presence of geosmin and pyrazine derivatives. Yields of up to 0.5 grams of betanin per kg of fresh processed root can be achieved.114
Several experiments have been carried out for biotechnological production of red beet from cell cultures for betanin production,115 but this process is economically less efficient, given the low cost of agricultural red beet cultivation.
There are also reported methods for the production of betacyanins from different Opuntia species. Opuntia stricta, is the most promising one, which reaches a yield of 0.8 grams of betanin per kg of fresh fruit.116 In order to reduce the high content of sugars in these colorant extracts, they can be fermented with yeasts, transforming them into ethanol that can be removed by rotary vacuum evaporation, without this having any effect on their concentration of pigments.117
Hylocereus sp.118 and Amaranthus sp.,119 have also been investigated as potential source of betacyanins, with promising results given the great intensity of colour in their fruits and seeds and the affordability of its cultivation.
When betacyanin extracts are used as food colorants, colour stability is the most significant issue. Although betacyanin extracts are stable over a wide pH range, from 3 to 7, betanin is most stable at a pH of 4–5. This pH-dependent stability makes betacyanins very suitable for application in low acid and neutral foods.120–122
Temperature is the most important factor on betacyanin stability during food processing and storage. Different studies confirmed increasing betacyanin degradation rates resulting from increasing temperatures.39,123 Betacyanin extracts were found to be more temperature sensitive than anthocyanin or carotenoid extracts.39 This low thermostability make betacyanins suitable colorants for ice creams, yoghurts, powdered and soft drinks, fruit cocktails, sugar confections, jellies and meat products.121
Betacyanins can undergo a water-dependency hydrolysis, and therefore water activity is also a key factor in the degradation of betacyanins. It has been reported that under conditions with water activity values below 0.63 increase the stability of betacyanins.116,119 Light also has a detrimental effect on betacyanin stability. Due to photolability of these pigments it is advisable to keep them in dark or dim lighting conditions to minimize pigment destruction.124
Several methods have been investigated to prevent the destruction or improve the stability of pigments, including degassing, preservation in darkness, addition of stabilizers, pH control, minimum heat treatment and mainly microencapsulation to coat and isolate the pigment from the external environment to increase its shelf life.121,124 A distinctive characteristic of betacyanin extracts used as natural colorants is that they have a very high colour intensity,121 and the amount of pure pigment required in foods to obtain the desired hue is relatively small, and for most applications does not exceed 50 mg of betanin per kg of fresh food.
The use of betacyanins as food colorants has advantages compared to other natural red pigments. Compared to carotenoids, betacyanins are water-soluble pigments which will facilitate their incorporation into foods, since a very high percentage of the food matrices are of hydrophilic character. If we compare the advantages against anthocyanins, both families of pigments are water-soluble but the solubility of betacyanins is higher than that of anthocyanins. Also their tinctorial strength is significantly greater, with higher extinction molar coefficients (ε ≃ 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and also betacyanins are generally stable in a wider pH range (3 ≤ pH ≤ 7), which makes them more suitable for application in low acidity and neutral foods.124
000) and also betacyanins are generally stable in a wider pH range (3 ≤ pH ≤ 7), which makes them more suitable for application in low acidity and neutral foods.124
The potential commercialization of betacyanin extracts as food colorant depends on the use of plants with a high level of pigmentation, the application of high-performance extraction processes, the protection of the pigment through microencapsulation, the use of cold storage and modified atmosphere, and the control of the application and finishing operations of the final product.125 All these efforts are addressed to increase the possibilities of application of betacyanins in foods to take advantage, not only of their colorant capacity, but also of their numerous health-promoting benefits.
4.3 Health-promoting benefits of betacyanins
There is an increasing interest in the intake of betacyanins due to their potential health-related properties. They are increasingly studied for their physiological roles in protecting higher plants against stress oxidative damage.126The strong free radical scavenging capacity of betacyanins and its modulation by different structural factors is well documented. The cationic structure of betacyanins with a quaternary amine and a conjugated system of double bonds (Fig. 7) provides a high antiradical activity. The presence of hydroxyl groups makes them excellent electron donors and free radical scavengers.112 Glycosylation of betacyanins was demonstrated to reduce their bioactivity due to the blockage of one of the hydroxyl groups.127 Moreover, the position of glycosylation influences the radical-scavenging potential. Thus, 6-O-glycosylated betacyanins show a greater activity than 5-O-glycosylated betacyanins.128 Cai and coworkers tested and categorized the levels of antioxidant activity of some betacyanins from high to low as follows: simple gomphrenins > acylated gomphrenins > betanin/isobetanin > celosianins > iresinins > amaranthin/isoamaranthin.129
Chronic diseases such as cancer, diabetes, cardiovascular and autoimmune disorders are known to associate with free radicals, therefore, given the radical-scavenging activity of betacyanins, it is expected that they behave like bioactive pigments with positive health effects.
In humans, reactive oxygen species (hydroxyl, peroxyl and superoxide anion radicals) and reactive nitrogen species such as nitric oxide, are constantly generated as a result of metabolic reactions. As a consequence, proteins, lipids and DNA will be target of free radical attack, consequently leading to damage and dysfunction of cell membranes, enzymes and genetic material.104,130 In parallel to the intense radical scavenging and antioxidant activities, and closely related to them, in vitro experiments have reported that extracts of red beet betacyanins inhibited linoleic acid peroxidation and low density lipoprotein oxidation.131 From this perspective, it becomes evident that beneficial aspects of betacyanins supporting human defense mechanisms are of crucial interest.
As consequence of the participation in the scavenging of free radicals, betacyanins may consequently prevent the onset of cancer and cardiovascular diseases. Supplementation of human cancer hepatocytes with red beet betacyanins led to a significant increase in cytosolic antioxidants in these cells that induced cell cycle arrest and apoptosis.132 It has also been reported that betacyanin extracts from Opuntia fruits inhibited the growth of human ovarian cancer cells and cervical cancer cells.133 Nevertheless, it should not be overlooked that cell cultures do not behave like real tumours, which means that the efficacy of betacyanins as anti-cancer agents in human clinical trials may not be the same.134
The anti-lipidemic effects of betacyanin extracts have also been proved. One of the essential factors in qualifying these pigments as antioxidants is the protection in vivo against lipid peroxidation. Supporting these benefits, it has been established that when erythrocytes were supplemented with betacyanins from Opuntia fruits they were more resistant to membrane lipid peroxidation and subsequent hemolysis than unfortified ones.135 In another experiment, when hypercholesterolemic rats were fed with betacyanin extract from red pitahaya, their total cholesterol level was reduced by 45%.134 The administration for 21 days of Amaranthus leaf extract to diabetic rats significantly increased high-density lipoproteins, reducing the level of tryglyceride, blood cholesterol and low-density lipoproteins.136
Regarding the anti-inflammatory response of betacyanins, the possible inactivation of the key enzymes involved in the inflammatory response (lipoxygenase and cyclooxygenase enzymes) by natural pure betacyanins has been also reported.137 The authors obtained betanin from roots of Beta vulgaris, and betanidin from violet flowers of Lampranthus productus, and proved that these pigments inhibited the inflammatory activity of the enzymes. As a correspondence between inflammatory response and tumor formation has been described, it has been also reported that betacyanins may also reduce tumor cell proliferation.138
Betanin proved to be a strong inhibitor in the growth of tumor cell lines when used to treat stomach, lung, colon, breast and central nervous system tumor cells. It should be noted that the efficiency of betanin in suppressing the cancer cell growth was significantly decreased when it was administrated in combination with anthocyanins.139
Interestingly, betacyanin extracts have also been characterized as having a differential antimicrobial activity. Opuntia matudae fruit extracts showed the potential to inhibit the growth of Escherichia coli O157:H7 and could provide a natural means of controlling pathogenic contamination, thereby mitigating food safety risks.140 In addition, Amaranthus spinosus extract, used as folk medicinal plant in Burkina Faso, showed significant antimalarial activity in mice.141 In an investigation conducted on the effect of Hylocereus polyrhizus fruit extracts against some food-borne pathogens, it was showed a low, but broad, antimicrobial spectrum, including Gram-positive and Gram-negative bacteria and moulds.142
It is accepted that there is a need for further clinical research to substantiate the effectiveness of betacyanins against the previously reported diseases and other potential health problems.
5. Global considerations
The definition of a food colour, the requirements for approval, the approved colours and their specifications, restrictions for their use and responsibilities for rulemaking and monitoring compliance are different in Europe, in the USA and also in Asian countries.143 Consumer perception and demand have driven the replacement of synthetic colorants with naturally derived alternatives.The importance of colour for food acceptance and the need to satisfy and attract more consumers in an increasingly competitive market has led to increased research into natural red pigments. In many cases natural pigments are incorporated into the food as part of a natural plant extract to avoid possible legal restrictions as it is not considered an additive itself.
Carotenoids, anthocyanins, and betacyanins are responsible of red colour in plants (Fig. 8). The main observable biological function of these pigments is the attraction of animals to act as vectors in the pollination and dissemination of seeds and fruits, thus ensuring the reproductive success and perpetuation of the species. The acquisition of this striking red colour has undoubtedly been selected in the course of evolution and has helped to determine other characteristics. Humans are not indifferent to this attractive phenomenon, so that the food industry, aware of this fact, tries to make foods more attractive, usually through its colour.
Despite the similarity in the shade of colour (Fig. 9), carotenoids, anthocyanins and betacyanins present well differentiated chemical characteristics, the main one being the lipophilic character of carotenoids while anthocyanins and betacyanins are hydrophilic pigments. This will determine both, their cellular emplacement, and also the stages of the processes of release, transport and bioassimilation of each one of them. The process of assimilation leads from the release of the food matrix to the absorption by the intestinal cells. It involves a multifactorial system that selects which pigments are preferably absorbed and in that amount.
For application as food additives it is essential to consider the stability of the pigments both in the food matrix and during industrial processing. Thermal stability will be a key factor at this stage. In general, anthocyanins and betacyanins show a high thermolability, while carotenoids are more thermally resistant.44
Industrial applications of natural pigment extracts are also frequently limited by interactions with other food ingredients, which often result in insufficient colouring and failure to achieve desired hues. In other situations, interaction between natural pigments and other components present in the food matrix is beneficial, since the small molecules are often combined with macromolecules in food (proteins, lipids, DNA,…), indicating stable and promising activities, that could modulate their functional properties, which is also the research topic for food-derived pigments.
The main health benefits of red plant pigments are a consequence of their widespread biological activity and are mainly attributed to their antioxidant and anti-inflammatory properties and more recently to their role in reducing heart disease, cancer and diabetes and also the regulatory role in cell signaling and gene expression pathways. There is no doubt that all these properties strengthen the importance of carotenoids, anthocyanins and betacyanins in our diet.
Diet is an integral part of a healthy lifestyle, and the intestinal microbiota acts as a gateway between nutrition and the promotion/modulation of human health. The bioavailability and health-promoting effects of natural red pigments greatly depend on their transformation by specific components of the intestinal microbiota via dihydroxylation, demethylation, decarboxylation, glucosidase and esterase activities.144 Recent studies have suggested that certain carotenoid metabolites, and not necessarily the native compounds, may be most biologically active, such as certain apo-carotenes (originating after enzymatic cleavage) and other more polar compounds, which act as electrophils more suited to reacting with cellular transcription factors.145 Dietary anthocyanins undergo a complex metabolism after ingestion and interact with endogenous and microbial enzymes, leading to the production of a large number of circulating and excreted anthocyanin metabolites and catabolic products. It is also accepted that the consumption of a diet rich in anthocyanins may be beneficial in approaching a balanced intestinal microbiota, subsequently contributing to the prevention of gastrointestinal disorders and diseases related to oxidative stress and improving the health of the host.146 Betacyanins are characterized by their polyphenolic structure and there are multiple evidences that relate their bioavailability with certain components present in the intestinal microbiota.147
In the field of natural colorants, future technological challenges are focused on the search for new sources of colorants, as well as on improving the colour intensity and stability of pigments, by adding stabilizers, improving emulsion techniques, microencapsulation and copigmentation processes, and designing pigment mixtures to strength the characteristics of the final product.
Consumer demands will continue to be decisive in the evolution of the food industry in the years to come, as people are increasingly demanding with regard to food and their expectations of it are evolving. They include more than just the affordability of food, but also aspects such as quality, health, origin and environmental sustainability. The association of concepts such as natural and healthy food has contributed to increasing public awareness of the positive health benefits of the consumption of natural pigments.
It is important to mention that red pigments in plants are often found in the inevitable part of food, thus, the functional components from food by-products cannot be ignored, including skin, pericarp or seeds. Studies related to the extraction of colorants (mainly carotenoids and anthocyanins) from fruit by-products were conducted, concluding that their processing to obtain natural pigments could be commercially interesting in some particular situations such as grape skins or citrus fruit waste.
Research in the field of natural red pigments as food biocolorants has been productive but industrial applications are yet to be made. Nevertheless, there are good perspectives for more applications of natural red colorants in food products. Consequently, the challenge is to improve natural pigment production systems by searching for plant species with improved characteristics and developing biotechnological production under controlled conditions on an industrial scale.
6. Conclusions
Consumer demands on food quality have become more rigorous over the years. Because of health benefits and eco-friendly nature, public appreciation of naturally-derived food colorants has increased. This is widely associated with the image of good quality, safe and healthy products, which has resulted in a great new challenge for both, food research institutions and food industries.Carotenoids, anthocyanins and betacyanins are regarded as bioactive pigments and their inclusion in the dietary intake may be an alternative to prevent certain diseases such as hypertension, diabetes, cancer and cardiovascular disorders. The wide inter-individual variability reported in clinical trials on the bioactivity of natural red pigments could be related, at least partially, to differences in the composition of intestinal microbiomes and hence in the way intestinal microbiota metabolize these phytochemicals.
It can be concluded that masked by the colour of natural pigments, many health benefits are hidden. The benefits of red natural pigments far exceed the function of providing an attractive, suitable or natural-looking red colour to a food. Let nature attract us with its striking colours and take advantage of the rewards that await us for having been persuaded.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is part of the QUIMYTEC R&D group and is based on the Baccalaureate Research Work of VFL at the IES Juan Carlos I (Murcia, Spain). This paper is dedicated to Juan Fernández Rubio on the celebration of his 90th birthday.References
- G. T. Sigurdson, P. Tang and M. M. Giusti, Annu. Rev. Food Sci. Technol., 2017, 8, 261–280 CrossRef CAS PubMed.
- R. K. Hari, T. R. Patel and A. M. Martin, Food Rev. Int., 1994, 10, 49–70 CrossRef CAS.
- Y. Tanaka, N. Sasaki and A. Ohmiya, Plant J., 2008, 54, 733–749 CrossRef CAS PubMed.
- R. F. Carvalho, M. Takaki and R. A. Azevedo, Acta Physiol. Plant., 2011, 33, 241–248 CrossRef CAS.
- M. Sakuta, Plant Biotechnology Reports, 2014, 8, 37–48 CrossRef.
- H. Y. Leong, P. L. Show, M. H. Lim, C. W. Ooi and T. C. Ling, Food Rev. Int., 2018, 34, 463–482 CrossRef CAS.
- R. H. Liu, Adv. Nutr., 2013, 4, 384S–392S CrossRef CAS PubMed.
- J. He and M. M. Giusti, Annu. Rev. Food Sci. Technol., 2010, 1, 163–187 CrossRef CAS PubMed.
- D. B. Rodriguez-Amaya, Current Opinion In Food Science, 2016, 7, 20–26 CrossRef.
- L. Frewer, J. Scholderer and N. Lambert, Br. Food J., 2003, 105, 714–731 CrossRef.
- S. M. Shim, S. H. Seo, Y. Lee, G. I. Moon, M. S. Kim and J. H. Park, Food Control, 2011, 22, 1054–1060 CrossRef.
- P. B. Pathare, U. L. Opara and F. A. J. Al-Said, Food Bioprocess Technol., 2013, 6, 36–60 CrossRef CAS.
- J. Carreño, A. Martínez, L. Almela and J. A. Fernández-López, Color Res. Appl., 1996, 21, 50–54 CrossRef.
- D. McCann, A. Barret, C. Cooper, D. Crumpler, L. Dalen, K. Grimshaw, E. Kitchin, K. Lok, L. Porteous, E. Prince, E. Sonuga-Barke, J. O. Warner and J. Stevenson, Lancet, 2007, 370, 1560–1567 CrossRef CAS.
- D. B. Rodríguez-Amaya, Food Res. Int., 2019, 124, 200–205 CrossRef PubMed.
- J. C. Varela, H. Pereira, M. Vila and R. León, Photosynth. Res., 2015, 125, 423–436 CrossRef CAS PubMed.
- M. Gong and A. Bassi, Biotechnol. Adv., 2016, 34, 1396–1412 CrossRef CAS PubMed.
- S. M. Rivera and R. Canela-Garayoa, J. Chromatogr. A, 2012, 1224, 1–10 CrossRef CAS PubMed.
- P. D. Fraser and P. M. Bramley, Prog. Lipid Res., 2004, 43, 228–265 CrossRef CAS PubMed.
- D. A. Kopsell and D. E. Kopsell, Trends Plant Sci., 2006, 11, 499–507 CrossRef CAS PubMed.
- R. K. Saini, S. H. Nile and S. W. Park, Food Res. Int., 2015, 76, 735–750 CrossRef CAS PubMed.
- R. E. Wrolstad and C. A. Culver, Annu. Rev. Food Sci. Technol., 2012, 3, 59–77 CrossRef CAS PubMed.
- H. Van den Berg, R. Faulks, H. F. Granado, J. Hirschberg, B. Olmedilla, G. Sandmann, S. Southon and W. Stahl, J. Sci. Food Agric., 2000, 80, 880–912 CrossRef CAS.
- L. Almela, J. A. Fernández-López, M. E. Candela, C. Egea and M. D. Alcázar, J. Agric. Food Chem., 1996, 44, 1704–1711 CrossRef CAS.
- L. Almela, J. M. López-Roca, M. E. Candela and M. D. ALcazar, J. Agric. Food Chem., 1991, 39, 1606–1609 CrossRef CAS.
- M. H. Walter and D. Strack, Nat. Prod. Rep., 2011, 28, 663–692 RSC.
- G. Britton, in Natural Food Colorants, ed. G. A. F. Hendry and J. D. Houghton, Blackie Academic & Professional, London, 2nd edn, 1996, vol. 7, pp. 197–243 Search PubMed.
- A. Z. Mercadante, in Food Colorants. Chemical and Functional Properties, ed. C. Socaciu, CRC Press, 2007, vol. 4.2, pp. 213–240 Search PubMed.
- G. Britton, S. Liaaen-Jensen and H. Pfander, in Carotenoids: Natural Functions, Vol. 4, ed. G. Britton, S. Liaaen-Jensen and H. Pfender, Birkhauser Verlag, 2008, vol. 1, pp. 1–6 Search PubMed.
- G. Farré, G. Sanahuja, S. Naqvi, C. Bai, T. Capell, C. Zhu and P. Christou, Plant Sci., 2010, 179, 28–48 CrossRef.
- E. M. Enfissi, P. D. Fraser and P. M. Bramley, Phytochem. Rev., 2006, 5, 59–65 CrossRef CAS.
- G. Giuliano, R. Tavazza, G. Diretto, P. Beyer and M. A. Taylor, Trends Biotechnol., 2008, 26, 139–145 CrossRef CAS PubMed.
- A. Mortensen, Pure Appl. Chem., 2006, 78, 1477–1491 CAS.
- A. Topuz, LWT--Food Sci. Technol., 2008, 41, 1672–1677 CrossRef CAS.
- M. I. Minguez-Mosquera and D. Hornero-Mendez, J. Agric. Food Chem., 1993, 41, 1616–1620 CrossRef CAS.
- A. Levy, S. Harel, D. Palevitch, B. Akiri, E. Menagem and J. Kanner, J. Agric. Food Chem., 1995, 43, 362–366 CrossRef CAS.
- K. Di Scala and G. Crapiste, LWT--Food Sci. Technol., 2008, 41, 789–795 CrossRef CAS.
- A. Topuz and F. Ozdemir, J. Agric. Food Chem., 2003, 51, 4972–4977 CrossRef CAS PubMed.
- J. A. Fernández-López, J. M. Angosto, P. J. Giménez and G. León, Plant Foods Hum. Nutr., 2013, 68, 11–17 CrossRef PubMed.
- P. J. Giménez, J. A. Fernández-López, J. M. Angosto and J. M. Obón, Plant Foods Hum. Nutr., 2015, 70, 380–387 CrossRef PubMed.
- P. Perkins-Veazie, J. K. Collins, A. R. Davis and W. Roberts, J. Agric. Food Chem., 2006, 54, 2593–2597 CrossRef CAS PubMed.
- H. Tapiero, D. M. Townsend and K. D. Tew, Biomed. Pharmacother., 2004, 58, 100–110 CrossRef CAS PubMed.
- M. Eggersdorfer and A. Wyss, Arch. Biochem. Biophys., 2018, 652, 18–26 CrossRef CAS PubMed.
- C. O. Perera and G. M. Yen, Int. J. Food Prop., 2007, 10, 201–230 CrossRef CAS.
- S. Kim, T. Y. Ha and I. K. Hwang, Food Rev. Int., 2009, 25, 198–213 CrossRef CAS.
- J. L. Rowles, K. M. Ranard, J. W. Smith, R. An and J. W. Erdman, Prostate Cancer Prostatic Dis., 2017, 20, 361–377 CrossRef CAS PubMed.
- B. C. Ip, C. Liu, L. M. Ausman, J. von Lintig and X. D. Wang, Cancer Prev. Res., 2014, 7, 1219–1227 CrossRef CAS PubMed.
- L. Jonasson, A. Wikby and A. G. Olsson, Nutr., Metab. Cardiovasc. Dis., 2003, 13, 120–125 CrossRef CAS.
- Y. P. Jia, L. Sun, H. S. Yu, L. P. Liang, W. Li, H. Ding, X. B. Song and L. J. Zhang, Molecules, 2017, 22, 610 CrossRef PubMed.
- B. J. Burri, M. R. La Frano and C. Zhu, Nutr. Rev., 2016, 74, 69–82 CrossRef PubMed.
- W. Köpcke and J. Krutmann, Photochem. Photobiol., 2008, 84, 284–288 CrossRef PubMed.
- L. Mojica, M. Berhow and E. G. de Mejia, Food Chem., 2017, 229, 628–639 CrossRef CAS PubMed.
- L. Dykes and L. W. Rooney, Cereal Foods World, 2007, 52, 105–111 CAS.
- Z. Zhu, Q. Guan, M. Koubaa, F. J. Barba, S. Roohinejad, G. Cravotto, X. Yang, S. Li and J. He, Food Chem., 2017, 215, 391–400 CrossRef CAS PubMed.
- S. Li, Z. Zhu, C. A. Pinto, F. J. Barba, J. He, D. Montesano and J. A. Saraiva, Int. J. Food Prop., 2017, 20(suppl. 3), S2418–S2428 CrossRef CAS.
- P. Bridle and C. F. Timberlake, Food Chem., 1997, 58, 103–109 CrossRef CAS.
- A. Castañeda-Ovando, M. L. Pacheco-Hernández, M. E. Páez-Hernández, J. A. Rodríguez and C. A. Galán-Vidal, Food Chem., 2009, 113, 859–871 CrossRef.
- J. M. Kong, L. S. Chia, N. K. Goh, T. F. Chia and R. Brouillard, Phytochemistry, 2003, 64, 923–933 CrossRef CAS PubMed.
- D. Ghosh and T. Konishi, Asia Pac. J. Clin. Nutr., 2007, 16, 200–208 CAS.
- Y. P. Jang, J. Zhou, K. Nakanishi and J. R. Sparrow, Photochem. Photobiol., 2005, 81, 529–536 CrossRef CAS PubMed.
- F. L. da Silva, M. T. Escribano-Bailón, J. J. P. Alonso, J. C. Rivas-Gonzalo and C. Santos-Buelga, LWT--Food Sci. Technol., 2009, 40, 374–382 CrossRef.
- E. Gómez-Plaza, R. Gil-Muñoz, J. Carreño-Espín, J. A. Fernández-López and A. Martínez-Cutillas, Eur. Food Res. Technol., 1999, 209, 257–260 CrossRef.
- U. Wissgott and K. Bortlik, Trends Food Sci. Technol., 1996, 7, 298–302 CrossRef CAS.
- P. Bridle and C. F. Timberlake, Food Chem., 1997, 58, 103–109 CrossRef CAS.
- A. Patras, N. P. Brunton, C. O'Donnell and B. K. Tiwari, Trends Food Sci. Technol., 2010, 21, 3–11 CrossRef CAS.
- N. Martins, C. L. Roriz, P. Morales, L. Barros and I. C. F. R. Ferreira, Trends Food Sci. Technol., 2016, 52, 1–15 CrossRef CAS.
- G. Mazza and R. Brouillard, Food Chem., 1987, 25, 207–225 CrossRef CAS.
- H. E. Khoo, A. Azlan, S. T. Tang and S. M. Lim, Food Nutr. Res., 2017, 61, 1361779 CrossRef PubMed.
- L. A. Pacheco-Palencia and S. T. Talcott, Food Chem., 2010, 118, 17–25 CrossRef CAS.
- C. L. Zhao, Y. Q. Yu, Z. J. Chen, G. S. Wen, F. G. Wei, Q. Zheng, C. D. Wang and X. L. Xiao, Food Chem., 2017, 214, 119–128 CrossRef CAS PubMed.
- M. M. Giusti and R. E. Wrolstad, Biochem. Eng. J., 2003, 14, 217–225 CrossRef CAS.
- W. Wiczkowski, D. Szawara-Nowak and J. Topolska, Food Res. Int., 2013, 51, 303–309 CrossRef CAS.
- V. V. de Rosso and A. Z. Mercadante, Innovative Food Sci. Emerging Technol., 2007, 8, 347–352 CrossRef CAS.
- R. E. Wrolstad, R. W. Durst and J. Lee, Trends Food Sci. Technol., 2005, 16, 423–428 CrossRef CAS.
- A. Kirca, M. Özkan and B. Cemeroglu, J. Food Process. Preserv., 2007, 31, 531–545 CrossRef CAS.
- F. Mainente, A. Menin, A. Alberton, G. Zoccatelli and C. Rizzi, Int. J. Food Sci. Technol., 2019, 54, 952–958 CrossRef CAS.
- S. J. Schwartz, J. H. von Elbe and M. M. Giusti, in Fennema's Food Chemistry, ed. S. Damodaran, K. L. Parkin and O. R. Fennema, CRC Press, Boca Raton, 2008, vol. 9, pp. 571–638 Search PubMed.
- R. Cortez, D. A. Luna-Vital, D. Margulis and E. G. de Mejía, Compr. Rev. Food Sci. Food Saf., 2017, 16, 180–198 CrossRef CAS.
- S. A. Mahdavi, S. M. Jafari, E. Assadpoor and D. Dehnad, Int. J. Biol. Macromol., 2016, 85, 379–385 CrossRef PubMed.
- C. A. Nayak and N. K. Rastogi, Drying Technol., 2010, 28, 1396–1404 CrossRef CAS.
- C. B. Pedersen, J. Kyle, A. M. Jenkinson, P. T. Gardner, D. B. McPhail and G. G. Duthie, Eur. J. Clin. Nutr., 2000, 54, 405–408 CrossRef CAS PubMed.
- C. Czank, A. Cassidy, Q. Zhang, D. J. Morrison, T. Preston, P. A. Kroon, N. P. Botting and C. D. Kay, Am. J. Clin. Nutr., 2013, 97, 995–1003 CrossRef CAS PubMed.
- I. A. Ludwig, P. Mena, L. Calani, G. Borges, G. Pereira-Caro, L. Bresciani, D. Del Rio, M. E. Lean and A. Crozier, Free Radical Biol. Med., 2015, 89, 758–769 CrossRef CAS PubMed.
- T. Tsuda, F. Horio and T. Osawa, Biofactors, 2000, 13, 133–139 CrossRef CAS PubMed.
- H. J. Heo and C. Y. Lee, J. Agric. Food Chem., 2005, 53, 1984–1989 CrossRef CAS PubMed.
- S. de Pascual-Teresa and M. T. Sánchez-Ballesta, Phytochem. Rev., 2008, 7, 281–289 CrossRef CAS.
- C. S. Bowen-Forbes, Y. Zhang and M. G. Nair, J. Food Compos. Anal., 2010, 23, 554–560 CrossRef CAS.
- D. Li, P. Wang, Y. Luo, M. Zhao and F. Chen, Crit. Rev. Food Sci. Nutr., 2017, 57, 1729–1741 CrossRef CAS PubMed.
- K. W. Singletary, K. J. Jung and M. Giusti, J. Med. Food, 2007, 10, 244–251 CrossRef CAS PubMed.
- M. K. Kang, S. S. Lim, J. Y. Lee, K. M. Yeo and Y. H. Kang, PLoS One, 2013, 8, e79823 CrossRef PubMed.
- M. C. López de las Hazas, C. Piñol, A. Maciá and M. J. Motilva, J. Agric. Food Chem., 2017, 65, 6467–6476 CrossRef PubMed.
- D. Prasad and R. Kunnaiah, J. Indian Soc. Periodontol., 2014, 18, 428–432 CrossRef PubMed.
- H. Piberger, A. Oehme, C. Hofmann, A. Dreiseitel, P. G. Sand, F. Obermeier, J. Schoelmerich, P. Schreier, G. Kramer and G. Rogler, Mol. Nutr. Food Res., 2011, 55, 1724–1729 CrossRef CAS PubMed.
- I. Edirisinghe, K. Banaszewski, J. Cappozzo, K. Sandhya, C. L. Ellis, R. Tadapaneni, C. T. Kappagoda and B. M. Burton-Freeman, Br. J. Nutr., 2011, 106, 913–922 CrossRef CAS PubMed.
- A. N. Winter and P. C. Bickford, Antioxidants, 2019, 8, 333 CrossRef CAS PubMed.
- S. Subash, M. M. Essa, S. Al-Adawi, M. A. Memon, T. Manivasagam and M. Akbar, Neural Regener. Res., 2014, 9, 1557–1566 CrossRef PubMed.
- T. C. Wallace, Adv. Nutr., 2011, 2, 1–7 CrossRef CAS PubMed.
- A. Mauray, C. Felgines, C. Morand, A. Mazur, A. Scalbert and D. Milenkovic, Genes Nutr., 2010, 5, 343–353 CrossRef CAS PubMed.
- E. Daneshzad, S. Shab-Bidar, Z. Mohammadpour and K. Djafarian, Clin. Nutr., 2019, 38, 1153–1165 CrossRef CAS PubMed.
- I. Muraki, F. Imamura, J. E. Manson, F. B. Hu, W. C. Willett, R. M. van Dam and Q. Sun, BMJ, 2013, 347, f5001 CrossRef PubMed.
- R. A. S. Sancho and G. M. Pastore, Food Res. Int., 2012, 46, 378–386 CrossRef CAS.
- M. Lee, S. R. Sorn, Y. Park and H. K. Park, J. Med. Food, 2016, 19, 995–1003 CrossRef CAS PubMed.
- J. M. Silván, M. Reguero and S. de Pascual-Teresa, Food Funct., 2016, 7, 1067–1076 RSC.
- F. C. Stintzing and R. Carle, Trends Food Sci. Technol., 2004, 15, 19–38 CrossRef CAS.
- M. I. Khan and P. Giridhar, Phytochemistry, 2015, 117, 267–295 CrossRef CAS PubMed.
- G. Polturak and A. Aharoni, Mol. Plant, 2018, 11, 7–22 CrossRef CAS PubMed.
- D. Strack, T. Vogt and W. Schliemann, Phytochemistry, 2003, 62, 247–269 CrossRef CAS PubMed.
- F. Gandía-Herrero and F. García-Carmona, Trends Plant Sci., 2013, 18, 334–343 CrossRef PubMed.
- S. Wybraniec, G. Jerz, N. Gebers and P. Winterhalter, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 538–550 CrossRef CAS PubMed.
- G. Jain and K. S. Gould, Environ. Exp. Bot., 2015, 119, 48–53 CrossRef CAS.
- G. Jain, K. E. Schwinn and K. S. Gould, New Phytol., 2015, 207, 1075–1083 CrossRef CAS PubMed.
- H. M. C. Azeredo, Int. J. Food Sci. Technol., 2009, 44, 2365–2376 CrossRef CAS.
- B. K. Tiwari and P. J. Cullen, in Red Beet Biotechnology, ed. B. Neelwarne, Springer, Boston, 2013, vol. 14, pp. 373–391 Search PubMed.
- K. Ravichandran, N. M. M. T. Saw, A. A. Mohdaly, A. M. Gabr, A. Kastell, H. Riedel, Z. Cai, D. Knorr and I. Smetanska, Food Res. Int., 2013, 50, 670–675 CrossRef CAS.
- D. Pavoković and M. Krsnik-Rasol, Food Technol. Biotechnol., 2011, 49, 145–155 Search PubMed.
- R. Castellar, J. M. Obón, M. Alacid and J. A. Fernández-López, J. Agric. Food Chem., 2003, 51, 2772–2776 CrossRef CAS PubMed.
- M. R. Castellar, J. M. Obón, M. Alacid and J. A. Fernández-López, J. Agric. Food Chem., 2008, 56, 4253–4257 CrossRef CAS PubMed.
- F. C. Stintzing, A. Schieber and R. Carle, Food Chem., 2002, 77, 101–106 CrossRef CAS.
- Y. Z. Cai and H. Corke, J. Food Sci., 2000, 65, 1248–1252 CrossRef CAS.
- A. S. Huang and J. von Elbe, J. Food Sci., 1987, 52, 1689–1693 CrossRef CAS.
- J. M. Obón, M. R. Castellar, M. Alacid and J. A. Fernández-López, J. Food Eng., 2009, 90, 471–479 CrossRef.
- J. A. Fernández-López, M. R. Castellar, J. M. Obón and L. Almela, J. Chromatogr. Sci., 2007, 45, 120–125 Search PubMed.
- K. M. Herbach, F. C. Stintzing and R. Carle, J. Agric. Food Chem., 2006, 54, 390–398 CrossRef CAS PubMed.
- F. Stintzing and R. Carle, Trends Food Sci. Technol., 2007, 18, 514–525 CrossRef CAS.
- F. Delgado-Vargas, A. R. Jiménez and O. Paredes-López, Crit. Rev. Food Sci. Nutr., 2000, 40, 173–289 CrossRef CAS PubMed.
- S. O. Neill, K. S. Gould, P. A. Kilmartin, K. A. Mitchell and K. R. Markham, Plant, Cell Environ., 2002, 25, 539–547 CrossRef CAS.
- Y. Cai, M. Sun and H. Corke, J. Agric. Food Chem., 2003, 51, 2288–2294 CrossRef CAS PubMed.
- Y. Cai, M. Sun, J. Xing, Q. Luo and H. Corke, Life Sci., 2006, 78, 2872–2888 CrossRef CAS PubMed.
- Y. Cai, M. Sun and H. Corke, Trends Food Sci. Technol., 2005, 16, 370–376 CrossRef CAS.
- V. Lobo, A. Patil, A. Phatak and N. Chandra, Pharmacogn. Rev., 2010, 4, 118–126 CrossRef CAS PubMed.
- J. Kanner, S. Harel and R. Granit, J. Agric. Food Chem., 2001, 49, 5178–5185 CrossRef CAS PubMed.
- T. Esatbeyoglu, A. E. Wagner, V. B. Schini-Kerth and G. Rimbach, Mol. Nutr. Food Res., 2015, 59, 36–47 CrossRef CAS PubMed.
- D. M. Zou, M. Brewer, F. Garcia, J. M. Feugang, J. Wang, R. Zang, H. Liu and C. Zou, Nutr. J., 2005, 4, 25 CrossRef PubMed.
- A. Gengatharan, G. A. Dykes and W. S. Choo, LWT--Food Sci. Technol., 2015, 64, 645–649 CrossRef CAS.
- L. Tesoriere, M. Fazzari, M. Allegra and M. A. Livrea, J. Agric. Food Chem., 2005, 53, 7851–7855 CrossRef CAS PubMed.
- A. Clemente and P. V. Desai, Trop. J. Pharm. Res., 2011, 10, 595–602 Search PubMed.
- P. J. Vidal, J. M. López-Nicolás, F. Gandía-Herrero and F. García-Carmona, Food Chem., 2014, 154, 246–254 CrossRef CAS PubMed.
- C. Schneider and A. Pozzi, Cancer Metastasis Rev., 2011, 30, 277–294 CrossRef CAS PubMed.
- M. K. Reddy, R. L. Alexander-Lindo and M. G. Nair, J. Agric. Food Chem., 2005, 53, 9268–9273 CrossRef CAS PubMed.
- S. A. Hayek and S. A. Ibrahim, Int. J. Microbiol., 2012, 368472 Search PubMed.
- A. Hilou, O. G. Nacoulma and T. R. Guiguemde, J. Ethnopharmacol., 2006, 103, 236–240 CrossRef CAS PubMed.
- G. C. Tenore, E. Novellino and A. Basile, J. Funct. Foods, 2012, 4, 129–136 CrossRef CAS.
- S. Lehto, M. Buchweitz, A. Klimm, R. Straßburger, C. Bechtold and F. Ulberth, Food Addit. Contam., Part A, 2017, 34, 335–355 CrossRef CAS PubMed.
- J. M. Laparra and Y. Sanz, Pharmacol. Res., 2010, 61, 219–225 CrossRef CAS PubMed.
- T. Bohn, Int. J. Vitam. Nutr. Res., 2017, 87, 5–9 CrossRef CAS PubMed.
- L. Tian, Y. Tan, G. Chen, G. Wang, J. Sun, S. Ou, W. Chen and W. Bai, Crit. Rev. Food Sci. Nutr., 2019, 59, 982–991 CrossRef CAS PubMed.
- L. Marín, E. M. Miguélez, C. J. Villar and F. Lombó, BioMed Res. Int., 2015, 905215 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |