Open Access Article
Open Access ArticleThe effect of continuous tubular reactor technologies on the pretreatment of lignocellulosic biomass at pilot-scale for bioethanol production†
José A. Pérez-Pimienta ab,
Gabriela Papac,
John M. Gladden
ab,
Gabriela Papac,
John M. Gladden cd,
Blake A. Simmons
cd,
Blake A. Simmons c and
Arturo Sanchez
c and
Arturo Sanchez *a
*a
aLaboratorio de Futuros en Bioenergía, Unidad Guadalajara de Ingeniería Avanzada, Centro de Investigación y Estudios Avanzados (CINVESTAV), Zapopan, Mexico. E-mail: arturo.sanchez@cinvestav.mx
bDepartment of Chemical Engineering, Universidad Autónoma de Nayarit, Tepic, Mexico
cJoint BioEnergy Institute, Biological Systems and Engineering Division, Lawrence Berkeley National Laboratory, Emeryville, CA, USA
dDepartment of Biomass Science and Conversion Technology, Sandia National Laboratories, Livermore, CA, USA
First published on 12th May 2020
Abstract
A pilot-scale continuous tubular reactor (PCTR) was employed for the isothermal pretreatment of agave bagasse (AG), corn stover (CS), sugarcane bagasse (SC), and wheat straw (WS) with three residence times. The objective was to evaluate the impact of this technology on enzymatic saccharification at low solid loadings (4% w/v) and on sequential saccharification and glucose fermentation (SSF) at high solid loading (20% w/v) for bioethanol production. Deformation in cellulose and hemicellulose linkages and xylan removal of up to 60% were achieved after pretreatment. The shortest residence time tested (20 min) resulted in the highest glucan to glucose conversion in the low solid loading (4% w/v) enzymatic saccharification step for AG (83.3%), WS (82.8%), CS (76.1%) and SC (51.8%). Final ethanol concentrations after SSF from PCTR-pretreated biomass were in the range of 38 to 42 g L−1 (11.0–11.3 kg of ethanol per 100 kg of untreated biomass). Additionally, PCTR performance in terms of xylan removal and sugar release were compared with those from a batch lab-scale autohydrolysis reactor (BLR) under the same process conditions. BLR removed higher xylan amounts than those achieved in the PCTR. However, higher sugar concentrations were obtained with PCTR for SC (13.2 g L−1 vs. 10.5 g L−1) and WS (21.7 g L−1 vs. 18.8 g L−1), whilst differences were not significant (p < 0.05) with BLR for AG (16.0 g L−1 vs. 16.3 g L−1) and CS (18.7 g L−1 vs. 18.4 g L−1).
Introduction
Lignocellulosic biomass from agroindustrial residues is an attractive feedstock for the production of fuels and chemicals due to its high availability, low price, and high polysaccharide content, with an estimate worldwide production of 1 × 1010 ton per year.1,2 However, converting this biomass into chemicals and biofuels using biochemical-platform biorefineries at commercial scale in an efficient and profitable manner is still an open challenge. In particular, the pretreatment stage is one of the most expensive and technically difficult processing stages reaching up to 40% of the total processing cost.3,4 Over the years, many different pretreatments have been developed (e.g. steam explosion, ionic liquids, hot water, dilute acid, alkaline, sulphite pulping, mechanical pulping, and organosolv processes).5,6 Most pretreatments have been reported at laboratory and bench scales (1–10 g h−1), typically in batch mode. However, a continuous operation is preferred, since productivity is a key aspect in industrial processes at commercial scale.7,8Additionally, the evaluation of pilot-scale pretreatment systems at high solids loading (>15% insoluble solids) can provide scale-up data and mimic process capabilities, performance and troubleshooting of envisioned commercial-scale pretreatment systems.9
Among the different configurations for pilot-scale (1–5 kg h−1), pretreatment reactors (i.e. vertical and horizontal oriented systems) including the feeder section (e.g. rotary valves, screw-compression) become particularly difficult to design and operate at high solids loading. As a result, most reports on successful technologies are kept confidential by industrial companies with only few available in the scientific literature.7,9
Scientific reports on continuous pilot-scale pretreatment systems show that this technology may have an impact on biomass recalcitrance as well as sugars conversion.10–12 Most importantly, few of these reports have studied the pretreatment systems up to their impact on ethanol production.13,14 Screw compression feeders and tubular horizontal arrangements have been commonly used in these reports. However, most of them show results referring to the employment of one single feedstock, lacking valuable data regarding reactor design, troubleshooting and ethanol yields at different operation conditions.10,11,13–19 Hence, any meaningful comparison among these reports becomes difficult.
In the Bioenergy Futures Laboratory at CINVESTAV (Mexico), two pilot-scale continuous tubular reactors (PCTRs) with different capacities [5 kg h−1 and 50 kg h−1 of biomass in dry basis (DB)] were designed and built for the pretreatment of agroindustrial residues.
The PCTRs combine three stages (compression, autohydrolysis, and steam explosion), and are capable of handling multiple feedstocks which could increase sugar concentrations (glucose and xylose) in downstream processes and ethanol yields while decreasing CAPEX (capital expenditure) and OPEX (operational expenditure).4
Each of the three stages in the PCTR process contributes with distinctive effects on the biomass. Firstly, continuous compression disrupts the biomass structure by heating, mixing, and shearing and increases its surface area and pore size.20 Secondly, autohydrolysis solubilizes most of the hemicellulose fraction and releases lignin fragments with low molecular weight into the liquor due to water autoionization.21 Finally, the steam explosion causes a rapid explosive decompression and disintegration of the cell wall structure into fine components, leading to improved compression and compaction characteristics of the biomass as well as reducing its shear strength, mean particle size, and bulk density.22,23
These three integrated pilot-scale processes combine the benefits of mechanical and physicochemical effects on the biomass that could improve the effectiveness of this pretreatment technology when compared with a single process. Recent advances in the understanding of flow dynamics inside the tubular body of the PCTR resulted in mathematical models to calculate and control input and output mass flows and xylan depolymerisation in both PCTRs.24,25 The models were validated with agave bagasse (AG), corn stover (CS), sugarcane bagasse (SC), and wheat straw (WS).
The objective of this work was to measure the efficacy of the PCTR technology developed at CINVESTAV in terms of xylan removal and sugar conversion after the isothermal autohydrolysis at different residence times of the four lignocellulosic feedstocks mentioned previously. Sugar concentrations after the enzymatic saccharification step and ethanol production in the alcoholic fermentation step were also measured.
The enzymatic saccharification performance of the four lignocellulosic feedstocks considered was measured first at low glucan loadings (∼1% solids loading) in order to compare the glucose/xylose conversions of untreated and PCTR-pretreated biomass under the same basis. Relatively higher solids loadings were also tested (4% w/v) to measure the sugars production without the limitations of a considerably higher solids loading (i.e. ≥15% w/v) where restrictions such as biomass density, mass transfer, and enzyme/substrate interactions already come into play. The xylan removal and saccharification results were also compared with those obtained using standard batch technologies at lab-scale, in order to elucidate the pretreatment efficiency as a function of scale and mode of operation. Pretreated biomass was characterized by Fourier transform infrared (FTIR) spectroscopy in order to track the chemical changes at molecular level. Ethanol production experiments were then carried out using a simultaneous saccharification and fermentation (SSF) strategy applying a 24 h pre-hydrolysis step to the PCTR-pretreated biomass at high solids loading (20%) in an effort to obtain experimental data at pilot-scale that may lead to decreasing capital and operating costs.26 Finally, mass balances of the pretreatment-SSF processes were carried out in order to measure reactor performance and the PCTR results were compared with similar studies already published in the scientific literature.
Experimental
Materials and sample preparation
The feedstocks were provided by companies of different agro-industrial sectors in western Mexico. Agave bagasse (AG) was obtained from a tequila distillery with a year-round processing capacity (6–15 ton per day) where the agave stems come from defoliated plants aged 6–7 years in a semi-arid climate located near Tequila, Jalisco. The agave stems were cooked in a diffuser at 90 °C during 6 h, then milled and compressed to separate the syrup from wet bagasse. Corn stover (CS) and wheat straw (WS) were obtained from an agricultural company located in Tierras Coloradas, Jalisco. Sugarcane bagasse (SC) was collected from a sugar mill located in Tepic, Nayarit. This sugar mill currently produces standard sugar using the juice obtained from the sugarcane harvested in a semi-humid climate. All samples were milled using an Azteca knife and hammer mill (Proinco, Mexico) equipped with a 1.27 cm screen.PCTR description
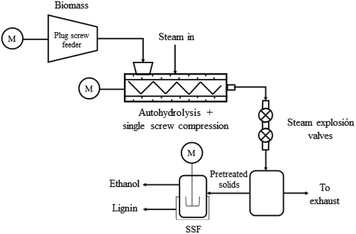
Fig. 1 shows a schematic diagram of the 5 kg biomass DB per h proprietary-technology PCTR employed in the experiments reported in this paper, indicating the compression, autohydrolysis and decompression (steam explosion) stages. Compression is carried out in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 compression screw driven by a 2 HP motor. The tubular reactor consists of a pipe 0.1 m diameter and 1.6 m length, with a screw conveyor operated by a 1 HP motor that can vary the biomass residence times according to the motor speed. The PCTR inner body is heated with saturated steam in order to maintain constant internal pressure and temperature. Biomass is expelled at the end of the reactor using a steam explosion system, consisting of two globe valves operating asynchronously.
2 compression screw driven by a 2 HP motor. The tubular reactor consists of a pipe 0.1 m diameter and 1.6 m length, with a screw conveyor operated by a 1 HP motor that can vary the biomass residence times according to the motor speed. The PCTR inner body is heated with saturated steam in order to maintain constant internal pressure and temperature. Biomass is expelled at the end of the reactor using a steam explosion system, consisting of two globe valves operating asynchronously.
Operation of the reactor is semi-automated. Reactor inner-chamber temperature and pressure, as well as motor torque of the compression and transport stages are controlled and recorded using a supervisory control system.
PCTR operation
In a typical run, samples are first soaked in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 biomass/water ratio at room temperature at least 30 min before starting the PCTR operation.27 The soaked biomass is then fed into the PCTR using the loading hopper and the first screw to compress the biomass into a densified plug, which serves as a pressure-sealing unit inside the reactor. Within the reactor chamber, the densified biomass plug autohydrolyses at constant temperature [in this experiments, 180 °C (∼1013 kPa)]. The biomass is pushed through the reactor until it reaches the two steam explosion valves. The pretreated biomass is then blown out into the reception tank.
10 biomass/water ratio at room temperature at least 30 min before starting the PCTR operation.27 The soaked biomass is then fed into the PCTR using the loading hopper and the first screw to compress the biomass into a densified plug, which serves as a pressure-sealing unit inside the reactor. Within the reactor chamber, the densified biomass plug autohydrolyses at constant temperature [in this experiments, 180 °C (∼1013 kPa)]. The biomass is pushed through the reactor until it reaches the two steam explosion valves. The pretreated biomass is then blown out into the reception tank.
Three residence times (20, 35, and 54 min), namely low (L), medium (M), and high (H), respectively, were tested. These residence times were calculated based on 33%, 50% and 100% of screw maximum speed in order to cover the PCTR sugars depolymerisation range.28
After pretreatment, the samples were cooled to room temperature and weighed, and the moisture content was measured. The three residence times were tested on each experimental run.
Conveyor speeds were changed during each run after the depolymerisation steady state associated with the previous residence time was reached. Samples were collected every 2 min from the biomass collection box according to previous reports.28 After each experimental run, the PCTR was cleaned to remove any chars formed (mainly due to pseudo-lignin generation from furfural/HMF) during the operation that may cause interference on subsequent runs.17
The oligomers and degradation products were measured from the liquid fraction, processed, and analysed as described in the analysis section of this paper. Pretreated solids were washed-up to avoid interference with the liberated sugars during pretreatment and obtaining real yields coming from the solid fraction. The solids were washed with 30 g of deionized (DI) water per g of biomass, milled using a 20-mesh Tyler screen, and stored at 4 °C for their use in the saccharification and fermentation stages.
Batch lab-scale pretreatment
The batch lab-scale autohydrolyses were performed in 1 L stainless steel reactors (model 4520, Parr Instruments Company, Moline, IL, USA). Biomass samples were milled to pass through a 20-mesh Tyler screen in order to obtain a homogeneous feedstock. The temperature and residence time were controlled using a Parr PID controller at 180 °C for 20 min for all feedstocks.25The reactor was loaded with 30 g (dry basis) of biomass and 300 mL of DI water and soaked for 30 min before pretreatment.27 The reactor heating rate was 3.8 °C min−1, reaching 180 °C at 41 min. Experiments were conducted in duplicate, with stirring at 90 rpm using a three-arm, self-centering anchor with polytetrafluoroethylene (PTFE) wiper blades.
After pretreatment, the reactors were immediately cooled and the liquid and solid fractions processed as described in the PCTR procedure. Pretreated samples from the batch lab-scale reactor will be denominated throughout the manuscript as BLR.
Enzymatic saccharification at low solids loading
Untreated and pretreated samples were saccharified at 50 °C for 72 h in a 50 mM citrate buffer (pH 5) containing an enzyme mixture of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v Ctec3 and Htec3 cocktails (Novozymes, Franklington, NC, USA), with 30 rpm constant agitation in a rotary incubator (Enviro-Genie, Scientific Industries, Inc.). The enzyme protein content was measured with bicinchoninic acid (BCA) assay using a Pierce BCA Protein Assay Kit (Thermo Scientific), with BSA (bovine serum albumin) as the protein standard. The CTec 3 and HTec 3 (Novozymes, Franklington, NC, USA) protein contents were 107.7 ± 2.1 mg mL−1 and 80.4 ± 5.4 mg mL−1, respectively. Two saccharification sets were carried out with different biomass loadings.
1 v/v Ctec3 and Htec3 cocktails (Novozymes, Franklington, NC, USA), with 30 rpm constant agitation in a rotary incubator (Enviro-Genie, Scientific Industries, Inc.). The enzyme protein content was measured with bicinchoninic acid (BCA) assay using a Pierce BCA Protein Assay Kit (Thermo Scientific), with BSA (bovine serum albumin) as the protein standard. The CTec 3 and HTec 3 (Novozymes, Franklington, NC, USA) protein contents were 107.7 ± 2.1 mg mL−1 and 80.4 ± 5.4 mg mL−1, respectively. Two saccharification sets were carried out with different biomass loadings.
Firstly, a fixed low solid loading of 5 g glucan per L (i.e. ∼1% solids loading) was used to evaluate the glucan and xylan conversion from PCTR-pretreated biomass at an enzyme dosage of 20 mg g−1 glucan in 15 mL centrifuge tubes a 5 mL as working volume.
Secondly, exploratory reactions at 4% w/v solids loading were conducted to compare sugar release from PCTR- and BLR-pretreated, using an enzyme loading of 20 mg g−1 glucan.
The results from experiments conducted at 4% w/v solids loading were evaluated in terms of glucose and xylose concentration. Reactions were monitored by removing 100 μL of the supernatant at 0, 1, 6, 12, 24, and 72 h. The glucan-to-glucose and xylan-to-xylose conversions were calculated as described elsewhere,29 as the amount of glucose produced after 72 h saccharification divided by the theoretical amount of glucose produced based on the percentage of glucan present in untreated or pretreated samples and then multiplied by 100. All assays were performed in triplicate.
Simultaneous saccharification and fermentation (SSF) at high solids loadings
Saccharomyces cerevisiae strain BY4741 (MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0), was used to carry out the alcoholic fermentation.30 Yeast cells were grown in 2% YPD media at 37 °C and shaken at 200 rpm. After 24 h, the culture broth exhibited an optical density at 600 nm of 3 and the yeast cells were collected by centrifugation at 4000 rpm for 10 min, washed thrice with 0.2% sterile peptone solution and suspended in 0.1 M phosphate buffer solution. The SSFs using a 24 h pre-saccharification and 72 h fermentation steps were conducted at 20% w/v solids loading, as described by the National Renewable Energy Laboratory (NREL) protocol TP-510-42630.31 Pre-saccharification was performed at 50 °C in 120 mL serum bottles containing 6 g of pretreated biomass (dry basis), and 24 mL of citrate buffer plus enzymes. Enzyme cocktail loading (9 CTec3/HTec3, v/v) was 20 mg g−1 glucan. The serum bottles were incubated at 37 °C for 72 h at 90 rpm. Ethanol and sugars during the fermentation were monitored by taking supernatant aliquots at 12, 24, 48 and 72 h. All assays were performed in triplicate.Compositional analysis
The compositional analysis of the untreated and pretreated solids were carried out using the NREL standard two-step sulphuric acid hydrolysis protocol TP-510-42618.32Briefly, 300 mg of biomass and 3 mL of 72% sulphuric acid were incubated at 30 °C for 1 h, then diluted to 4% H2SO4 and autoclaved for 1 h at 121 °C. Carbohydrate contents were determined via high performance liquid chromatography (HPLC). The acid insoluble lignin was quantified gravimetrically using the solid fraction after heating overnight at 105 °C. The liquid filtrates were used to determine the content of acid soluble lignin at 280 nm wavelength using a UV-Vis spectrophotometer (Nanodrop 2000, Thermo Scientific). The total concentration of lignin in the sample was calculated as the sum concentration of acid-soluble lignin and acid-insoluble lignin. All acid hydrolyses were run in triplicate. The solid yield was calculated according to the following equation:
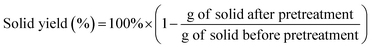 | (1) |
In order to establish the pretreatment efficiency and compare it with previous works not reporting solid yields, xylan removal (%) after pretreatment was calculated as follows:
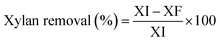 | (2) |
The ash content was determined using a muffle oven (Isotemp 650-14, Fisher Scientific) heated to 550 °C with a temperature ramp.33
Furthermore, for oligomers determination, an aliquot of the pretreatment liquid (PCTR or BLR) was mixed with an equal volume aliquot of 72% sulphuric acid, incubated at 30 °C for 1 h, diluted to 4% sulphuric acid concentration with deionized (DI) water, and autoclaved at 121 °C for 1 h (post-hydrolysis), according to NREL protocol TP-510-42623.34 The oligomer sugar content was defined as the difference between the amount of post-hydrolysis sugars and the initial monomer content.
Carbohydrates, byproducts and ethanol
Glucose, xylose, acetic acid, furfural, 5-hydroxymethylfurfural (HMF), and ethanol were analysed on an Agilent HPLC 1200 Series using an Aminex HPX-87H column, as described elsewhere.35 All samples were centrifuged (5810R, Eppendorf, Inc.) and filtered through 0.45 μm filters and then properly diluted before analysis. The theoretical ethanol yield was calculated taking into consideration that the S. cerevisiae BY4741 consumes only C6 sugars as reported previously.36 The theoretical ethanol from the PCTR-pretreated biomass was calculated based on their glucan content as % of the theoretical, following eqn (3):
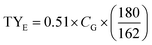 | (3) |
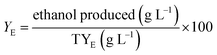 | (4) |
FTIR-ATR spectroscopy analysis
Fourier transform infrared (FTIR) spectroscopy was conducted using a Bruker Optics Vertex system (Billerica, MA, USA) with built-in diamond-germanium ATR (attenuated total reflection) single reflection crystal. Pretreated samples from the PCTR and BLR together with untreated samples were pressed uniformly one at a time against the diamond surface using a spring-loaded anvil. Sample spectra were obtained in triplicate using an average of 32 scans in the NIR range (800–2000 cm−1) with a spectral resolution of 4 cm−1.36 Air and water were used as the background for untreated and pretreated biomass samples. Baseline correction and vector-normalization were conducted using OPUS software from Bruker Optics (Billerica, MA, USA).Statistical analysis
Minitab 18.1 software (Coventry, UK) was used for statistical analysis of xylan removal from the PCTR-pretreated samples, including its subsequent glucan and xylan conversion from specific saccharification reactions and its comparison to the BLR in terms of glucose and xylose release. The data were analysed for statistical significance by a one-way analysis of variance (ANOVA) and Tukey test (p < 0.05).Results and discussion
The morphologic differences between untreated and PCTR-pretreated samples are shown in ESI, Fig. S1.† The initially well-structured, fibrous and compact feedstocks, after pretreatment showed dark and extricated fibres. When comparing the different residence times tested, all the feedstocks looked very similar, except AG, which appeared more fibrous than the others did. The motor speed of the compressor screw was maintained constant along all experiments. However, the input rate to the reactor varied among feedstocks (dry basis) as AG (3.9–4.8 kg h−1), CS (4.2–4.7 kg h−1), SC (6.0–6.8 kg h−1) and WS (6.6–7.7 kg h−1). This behaviour could be related to biomass rheological characteristics (including flowability, bulk density and viscosity, among others).The effects of PCTR pretreatment on lignocellulosic biomass: a compositional analysis of solid and liquid streams
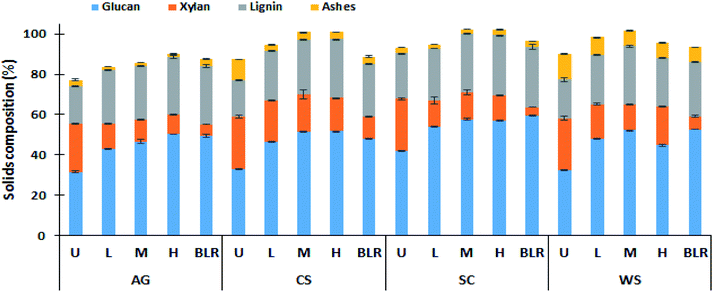
The solids composition of the major plant cell wall from the untreated and PCTR-pretreated biomass are shown in Fig. 2. The untreated biomass composition of the evaluated feedstocks is in the range of 31.6–42.1% glucan, 24.0–25.6% xylan, 18.3–22.7% lignin, and 2.8–12.7% ash, in agreement with previous results.37,38 Nevertheless, important differences are identified in the cell wall components among samples. These differences are more noticeable in AG and SC, with AG exhibiting a low glucan (31.6%) and lignin (18.6%) content, while SC showed the highest (42.1% and 22.7% glucan and lignin, respectively).Ash content was found to be around 3% for AG and SC. Relatively high ash values (>10%) were measured in CS and WS. Xylan (main component of hemicellulose) has been reported to restrict cellulose accessibility for cellulases access to cellulose.39
As expected, the PCTR did not remove lignin from biomass, as seen in Fig. 2. Lignin actually increased after pretreatment due to the formation of pseudo-lignin promoted mainly by the autohydrolysis stage.39
After the feedstocks enter the PCTR system, the compressor screw forms a densified plug feeding the reactor with 45–55% solids content. After pretreatment, the solid recovery of the PCTR-pretreated biomass exhibited high values (62.5–77.6%) depending on the residence time as shown in Table 1.
| Feedstock | Sample code | Solids recovery (%) | Mass flowrate (kg h−1) | Xylan removal (%) |
|---|---|---|---|---|
| Agave bagasse | L | 73.7 | 3.9 | 47.5 |
| M | 67.7 | 4.0 | 54.2 | |
| H | 62.6 | 4.8 | 59.6 | |
| BLR | 61.1 | — | 76.7 | |
| Corn stover | L | 77.6 | 4.3 | 20.2 |
| M | 73.1 | 4.3 | 26.8 | |
| H | 73.7 | 4.7 | 35.4 | |
| BLR | 58.4 | — | 74.6 | |
| Sugarcane bagasse | L | 68.1 | 6.0 | 51.0 |
| M | 62.5 | 6.8 | 47.9 | |
| H | 72.8 | 6.6 | 51.0 | |
| BLR | 68.8 | — | 57.2 | |
| Wheat straw | L | 77.1 | 7.7 | 32.4 |
| M | 71.0 | 6.3 | 50.4 | |
| H | 65.7 | 6.6 | 25.8 | |
| BLR | 55.8 | — | 83.3 |
The recovered PCTR-pretreated samples exhibited reduced xylan and an increment in glucan content compared to the untreated biomass. Interestingly, the xylan removal (calculated using eqn (2)) in AG and SC were in the range of 47.5 to 59.6% and 47.9 to 51.0% respectively, after pretreatment, whereas CS and WS only reached values of up to 35.4% and 50.4%, respectively. As determined by a Tukey test, xylan removal was statistically different (p < 0.05) from AG-L and CS-L as opposed to M and H residence times (Table 1). In contrast, SC and WS pretreated showed statistically significant differences (p < 0.05) depending on the residence time adopted.
As operation conditions become more severe during pretreatment, higher polysaccharides depolymerisations (mainly hemicelluloses) are achieved. Pentoses and hexoses can be dehydrated to downstream inhibition products such as furfural and 5-hydroxymethylfurfural (HMF), detectable in the liquid fraction. Thus, monomer degradation should be minimized during pretreatment.40 Liquid fraction compositions obtained after PCTR pretreatment are shown in Fig. 3. Xylo-oligosaccharides (XylOS) were the main components of the liquors from all three residence times evaluated, with values ranging from 9.5 to 38.1 g kg−1 of biomass.
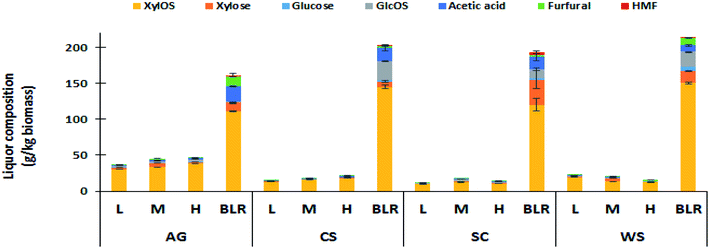 | ||
| Fig. 3 Composition in g kg−1 of biomass of the liquors obtained from post-hydrolysis after PCTR with L, M and H residence times and BLR pretreatments. | ||
The highest values of XylOS were obtained for AG (30.1–38.1 g kg−1 of biomass) while SC exhibited the lowest values (9.5 to 12.4 g kg−1 of biomass).
Gluco-oligosaccharides (GlcOS) were barely produced with CS and WS (0.0–1.5 g kg−1 of biomass), while AG and SC resulted in GlcOS concentrations above 2 g kg−1 of biomass and up to 3.4 g kg−1 of biomass in AG. Xylose was the predominant monosaccharide obtained from pretreated biomass. AG produced the highest xylose values (2.9–5.5 g kg−1 of biomass). Glucose was not detected in either CS or SC, and negligible amounts in WS.
Acetic acid produced from the acetylation of hemicellulose plays an important role in carbohydrate dissolution, but also leads to the further degradation of carbohydrates.41,42 The acetic acid concentration was higher with longer residence time, being more noticeable in AG and CS.
While furfural was obtained in low concentrations, and in some cases, only trace amounts corresponding to 0.2 to 2.3 g kg−1 of biomass, HMF was not detected in any sample. As expected, the highest acetic acid and furfural concentrations were found in H-residence time experiments. This condition suggests the involvements of other mass and energy-related phenomena during PCTR operation. Therefore, these low concentrations of inhibitors could be considered a positive factor for producing XylOS-rich liquors using this PCTR for ethanol fermentation or prebiotic production.43
Comparison of the solids and liquids streams of BLR- and PCTR-pretreated biomass
As previously reported, autohydrolysis is the greatest contributor of the three PCTR stages reflected in biomass digestibility.28 The reactor design, operation mode and scale have a profound effect on the pretreated solids and their subsequent enzymatic saccharification.44 In order to compare the PCTR performance against batch operation mode with L-residence time for all feedstocks, experiments were carried using the BLR as described in the Experimental section.Solids recovery were between 55.8 to 68.8% after BLR pretreatment as shown in Table 1. BLR was more effective in removing xylan than PCTR under the same residence time. When compared to untreated solids, a high xylan removal, in the range of 57.2 to 83.3%, was obtained. Glucan and lignin contents increased as a consequence of xylan extraction, with observed differences among feedstocks due to different plant cell wall constitutions.
In order to have a valid comparison between BLR and PCTR liquid fraction due to scale differences, the comparison was made in terms of sugars and sugars degradation products per kg of biomass. Higher concentrations of XylOS (110.5–150.7 g kg−1 of biomass) and GlcOS (0.3 to 26.2 g GlcOS per kg of biomass) were obtained compared to the PCTR values (Fig. 3).
However, unlike PCTR, formation of HMF occurred during BLR pretreatment which can be attributed to GlcOS dehydration. The BLR liquid fractions contained free sugar monomers especially in those derived from WS (6.4 g glucose per kg of biomass), indicating a higher xylan removal after BLR pretreatment when compared to the PCTR.
However, the concentration of inhibitory compounds was also higher, suggesting a more severe treatment of the biomass at the evaluated residence time and temperature.
FT-IR analysis
Chemical changes after pretreatment were measured by observing a total of seven FT-IR carbohydrate and lignin bands, with two additional bands in the case of AG due to high intensity calcium oxalate peaks (ESI, Table S1†). The normalized FT-IR spectra of untreated, PCTR, and BLR pretreated solids are shown in are shown in ESI, Fig. S2 and S3.† When compared to the untreated samples, a significant decrease in band intensities are observed in all pretreated samples at 1032 cm−1 (C–O stretching in cellulose and hemicellulose), 1245 cm−1 (C–O adsorption resulting from acetyl group cleavage) and 1366 cm−1 (C–H deformation in cellulose and hemicellulose), probably due to hemicellulose removal. The band at 1720 cm−1, associated with carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, also decreased in intensity, specifically in the L-residence time pretreatment, indicating lignin cleavage.36 Furthermore, band positions attributed to calcium oxalate at 1622 and 1321 cm−1 were present only in AG. As expected, these peaks were not found in the other feedstocks.45 The ratio of amorphous crystalline cellulose associated with the ratio intensities at 900 cm−1 and 1032 cm−1 increased for AG, SC, and WS. A decrease was observed only for CS, possibly due to the relatively low pH during pretreatment.46 Additionally, an increase in the intensity of 1235 cm−1 (C
O stretching, also decreased in intensity, specifically in the L-residence time pretreatment, indicating lignin cleavage.36 Furthermore, band positions attributed to calcium oxalate at 1622 and 1321 cm−1 were present only in AG. As expected, these peaks were not found in the other feedstocks.45 The ratio of amorphous crystalline cellulose associated with the ratio intensities at 900 cm−1 and 1032 cm−1 increased for AG, SC, and WS. A decrease was observed only for CS, possibly due to the relatively low pH during pretreatment.46 Additionally, an increase in the intensity of 1235 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O deformation in cellulose and hemicellulose) and 1595 cm−1 (aromatic ring stretch) bands were obtained in the pretreated samples, compared to the untreated samples as a result of the effects of high xylan removal and lignin structural changes.
O deformation in cellulose and hemicellulose) and 1595 cm−1 (aromatic ring stretch) bands were obtained in the pretreated samples, compared to the untreated samples as a result of the effects of high xylan removal and lignin structural changes.
Finally, it is worth noting that L-residence time PCTR-pretreatment exhibited the highest differences in most of the carbohydrate/lignin bands when compared to the other two conditions of PCTR-pretreated solids as well as to BLR samples.
Enzymatic saccharification of pretreated biomass at low solids loading
Table 2 presents glucan and xylan conversion of PCTR-pretreated feedstocks using biomass loading corresponding to 5 g glucan per L or ∼1% solids loading. All four PCTR-pretreated feedstocks exhibited a higher saccharification yield than the corresponding untreated samples. In particular, AG-L showed the largest yield from all pretreated samples when compared to their respective untreated sample, with a 3.1- and 6.2-fold increase in glucan and xylan conversion, respectively. The glucan conversion was significantly higher than the xylan conversion for all samples.| Feedstock | Sample code | % glucan conversion | % xylan conversion |
|---|---|---|---|
| Agave bagasse | Untreated | 26.7 ± 0.2A | 11.5 ± 0.3A |
| L | 83.3 ± 4.8B | 70.9 ± 9.4B | |
| M | 78.4 ± 0.6B | 65.9 ± 0.0B | |
| H | 43.4 ± 2.6C | 46.1 ± 2.3C | |
| Corn stover | Untreated | 38.6 ± 4.1A | 20.0 ± 0.5A |
| L | 76.1 ± 2.2BC | 50.8 ± 1.2B | |
| M | 70.9 ± 1.1C | 39.7 ± 0.5C | |
| H | 78.2 ± 0.3B | 49.5 ± 0.6B | |
| Sugarcane bagasse | Untreated | 25.8 ± 1.1A | 12.2 ± 0.3A |
| L | 51.8 ± 1.3B | 48.5 ± 0.4B | |
| M | 53.7 ± 0.1B | 29.4 ± 0.0C | |
| H | 58.5 ± 0.5C | 29.1 ± 0.6C | |
| Wheat straw | Untreated | 45.4 ± 0.2A | 34.9 ± 0.2A |
| L | 82.8 ± 2.7B | 52.0 ± 1.4B | |
| M | 81.2 ± 2.9B | 44.7 ± 1.3C | |
| H | 48.3 ± 3.0A | 31.4 ± 1.3D |
To evaluate the process conditions during pretreatment for each feedstock, the resulting glucan/xylan conversions were analysed using a one-way ANOVA and Tukey test (p < 0.05). In most feedstocks, L-residence time either produced the highest sugar conversion, or its results were not statistically different from the M-residence time results. Glucan conversion of above 80% was achieved in AG-L (83.3%), WS-L (82.8%) and WS-M (81.2%). However, H-residence time resulted in a decrease of up to 48.3% glucan conversion in WS with respect to L and M-residence times. The same trend occurred in the glucan conversion of AG-H (43.4%) when compared to L and M-residence times with conversions of 83.3 and 78.4%, respectively.
Additionally, xylan conversion values from L-residence time achieved the highest values for all feedstocks (Table 2), indicating the disruption of ligno–polysaccharide interaction even under mild conditions. It is worth noting that the PCTR solids were water-washed to avoid any interference in either compositional analysis or enzymatic saccharification in order to establish the pretreatment efficiency.
A previous study showed the impact of water-washing on PCTR-pretreated solids, measuring a sugar yield reduction of 29% in the presence of inhibitors (0.9 g inhibitors per L) in unwashed solids.23 Further studies must be carried out to evaluate the effects of unwashed solids on sugar yield and the impact of inhibition activity after PCTR pretreatment. In general, L-residence time PCTR-pretreated biomass samples achieved higher glucan and xylan conversion when compared to the other residence times. Therefore, the PCTR samples with L-residence time were chosen for the following saccharification and fermentation experiments.
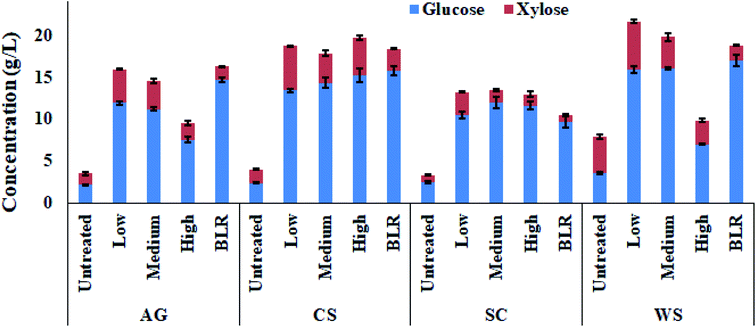
The saccharification experiments performed at 4% w/v solids loading were used to compare the sugars release from BLR- and PCTR-pretreated biomass (Fig. 4). The pretreatment efficiency is evaluated by measuring glucose plus xylose concentration (g L−1) before starting SSF experiments. Interestingly, in accordance with the results obtained at ∼1% solids loading, the four feedstocks pretreated with the L-residence time achieved high sugars concentrations.
 | ||
| Fig. 4 Glucose and xylose concentration from enzymatic saccharification at 4% w/v solids loading from BLR- and PCTR-pretreated biomass. | ||
Note that AG-L (16.0 g L−1), and WS-L (21.7 g L−1) were also considerably higher than those resulting from biomass pretreated with M-residence time (AG: 14.6 g L−1 and WS: 19.8 g L−1) or H (AG: 9.5 g L−1 and WS: 9.9 g L−1). Sugar concentrations of the other biomasses, CS-L (18.7 g L−1) and SC-L (13.2 g L−1), were either not significantly different or slightly higher to the pretreated biomass with M-residence time (CS: 17.9 g L−1 and SC: 13.5 g L−1) or H (CS: 19.7 g L−1 and SC: 13.0 g L−1). It should pointed out that the BLR-pretreated samples achieved similar sugar yields to previous reports at similar conditions.29,47,48 After enzymatic saccharification, the BLR-pretreated samples also produced high sugars concentration in which only AG (16.3 g L−1 vs. 16.0 g L−1) and CS (18.4 g L−1 vs. 18.7 g L−1) were not statistically different (p < 0.05) from the PCTR-pretreated samples at L-residence time.
However, higher total sugar (glucose and xylose) concentrations from the PCTR-pretreated biomass were obtained in SC (10.5 g L−1 vs. 13.2 g L−1) and WS (18.8 g L−1 vs. 21.7 g L−1) when compared to the BLR-pretreated samples, as shown in Fig. 4. Therefore, the enzymatic digestibility of the evaluated feedstocks could be ranked from highest to lowest as WS > CS > AG > SC.
This clearly shows that a high release of sugars can be obtained from PCTR-pretreated biomass from the different feedstocks at mild operational conditions without acid including a low generation of fermentation inhibitors. Moreover, the present comparative study of PCTR technology versus the standard BLR confirms, based on evidence, the potential efficacy of PCTR to provide pretreated solids that can achieve high carbohydrate conversion to sugars without generating inhibitory compounds.
Effect of pretreatment of SSF at high solids loading
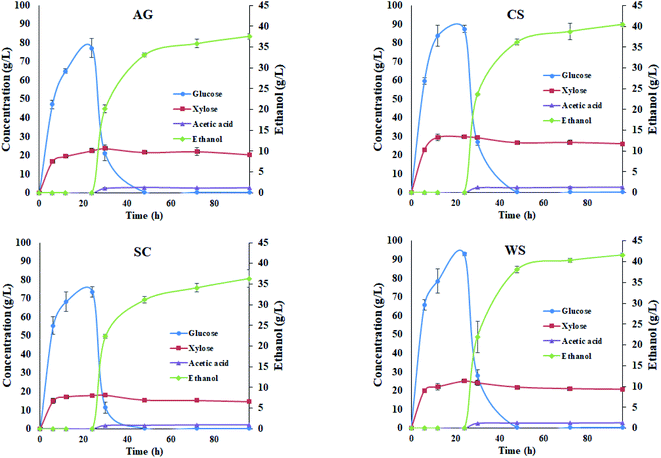
In order to evaluate the effect of PCTR-pretreated biomass for producing ethanol, SSFs experiments at high solids loadings (20% w/v) with a 24 h pre-hydrolysis step were carried out. The SSF kinetics profiles at 20% w/v solids loading from the PCTR-pretreated biomass at L-residence time are shown in Fig. 5.The 24 h pre-hydrolysis of 20% w/v solids of pretreated WS produced the largest sugar concentrations of up to 92.9 g of glucose per L and 25.1 g of xylose per L, whereas SC produced the lowest concentration (73.6 g L−1 glucose and 18.0 g of xylose per L). Results on separate hydrolysis and fermentation (SHF) versus a simultaneous saccharification and fermentation (SSF) with a pre-hydrolysis step, have been previously reported by different authors.49,50 After the inoculation of S. cerevisiae BY4741, the glucose consumption to ethanol was in the ratio of 88 to 97% with a similar behaviour pattern for all feedstocks.
The 72 h fermentation stage generated up to ∼42 g of ethanol per L and 1.59 gEtOH L−1 h−1 in WS, whilst the lowest values were obtained for SC, with ∼38 g of ethanol per L and 1.30 gEtOH L−1 h−1 (ESI, Table S2†), considered to be the minimum concentration (∼4% v/v) for a profitable purification stage.51 Taking into consideration the ethanol yield and glucan content from the PCTR, 352 ± 5.4 g of ethanol was produced from kg of glucan AG. CS and WS showed similar values with 348.4 ± 4.3 and 348.5 ± 3.9 g of ethanol per kg glucan, respectively. Only SC (268.3 ± 15.9 g of ethanol per kg glucan) underperformed and was statistically different (p < 0.05) when compared to the other feedstocks.
The ethanol yield based on the glucan content in the pretreated material as % of the theoretical shows that AG, CS and WS have a similar yield (∼60%) while SC achieved a lower yield (46%).
Mass balances
Mass balances of major components obtained from the PCTR-pretreated biomass (180 °C and 20 min) have been carried out to accurately establish the role of the PCTR on different feedstocks. Process yields during pretreatment, saccharification and fermentation yields were normalized on a 100 kg dry basis of untreated biomass in order to make total yields directly comparable (Fig. 6). When compared side-by-side between feedstocks, the recovered biomass was in the range of 68.1–77.6% after pretreatment, reflecting the discussed differences among feedstocks under the same process conditions. The xylose-rich hydrolysate post-pretreatment could be further transformed into ethanol or other value-added products after detoxification. However, this was not investigated in this study.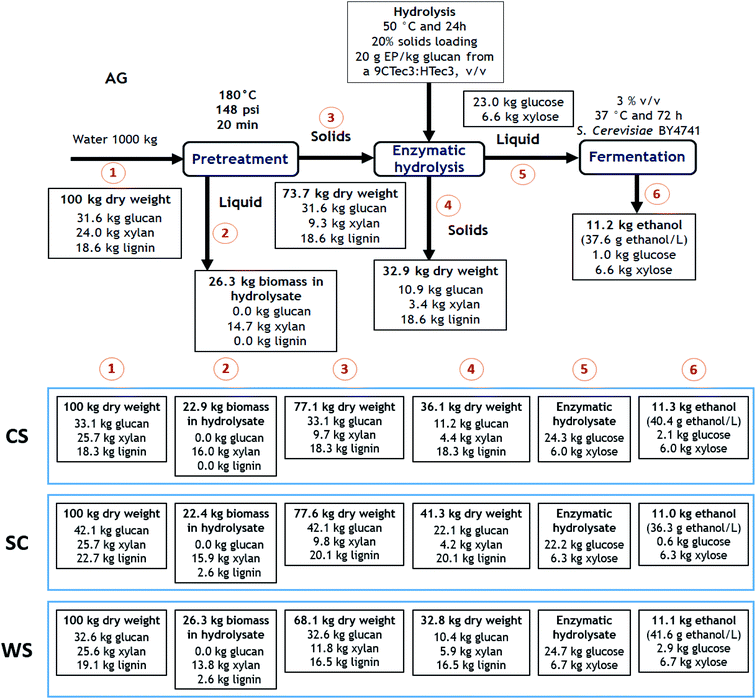 | ||
| Fig. 6 Mass balance of major biomass components on a 100 kg basis of untreated agave bagasse (AG), corn stover (CS), sugarcane bagasse (SC), and wheat straw (WS) for PCTR pretreatment. | ||
The first SSF stage, using commercial enzyme cocktails for saccharification, obtained the highest amount of monomeric sugars from pretreated WS, with 24.7 kg of glucose and 6.7 kg of xylose per 100 kg of initial biomass, while the lowest was achieved with SC (22.2 kg of glucose and 6.3 kg of xylose per 100 kg of initial biomass). The second stage of the SSF, was used to evaluate the ethanol production using the sugars produced during the enzymatic hydrolysis from the PCTR-pretreated feedstocks. All four feedstocks were able to produce 11.0–11.3 kg of ethanol per 100 kg of untreated biomass with a considerable ethanol concentration (∼40 g L−1).
Comparison with other PCTR technologies
Table 3 shows the pretreatment operational conditions, xylan removal, saccharification performances and ethanol concentrations obtained in the present study in comparison with other scientific reports on PCTRs using a variety of feedstocks. Most of the reports used chemicals (sulphuric acid) during pretreatment, obtaining higher xylan removals than with the PCTR subject of this study.| Capacity | Biomass | T (°C) | t (min) | Catalyst | Xylan removal (%) | Solids loading in saccharification (%) | Saccharification | Ethanol production | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Not specified. | |||||||||
| 4.8 kg h−1 | Agave bagasse | 180 | 20 | H2O | 47.9 | 20 | 77.2 g glucose per L and 22.5 g xylose per L in 24 h | 37.6 g L−1 | This study |
| 4.7 kg h−1 | Corn stover | 180 | 20 | H2O | 20.2 | 20 | 87.5 g glucose per L and 29.8 g xylose per L in 24 h | 40.4 g L−1 | This study |
| 6.6 kg h−1 | Sugarcane bagasse | 180 | 20 | H2O | 58.0 | 20 | 73.6 g glucose per L and 18.0 g xylose per L in 24 h | 36.3 g L−1 | This study |
| 6.6 kg h−1 | Wheat straw | 180 | 20 | H2O | 32.4 | 20 | 92.9 g glucose per L and 25.1 g xylose per L in 24 h | 41.6 g L−1 | This study |
| 10 kg h−1 | Rice straw | 162 | 10 | 0.35% H2SO4 | 82.0 | 20 | 83.3 g glucose per L and 31.9 g xylose per L in 48 h | — | 10 |
| 8.3 kg h−1 | Corn stover | 160 | 20 | 2.0% H2SO4 | 85.4 | 1 (glucan) | 81% glucose conversion in 24 h | — | 12 |
| 10 kg h−1 | Eucalyptus | 180 | 15 | 2.4% H2SO4 | 73.6 | 2 (glucan) | 71.8% glucose conversion in 72 h | 18.8 g L−1 from 5% glucan | 13 |
| 50 kg h−1 | Wheat straw | 190 | 6 | 0.7% H2SO4 | 82.0 | 2 | 37.8% glucose and 60% xylose in 24 h | 50% theoretical from 13% solids | 14 |
| 25–30 kg h−1 | Corn stover | 160 | 10 | 0.34% H2SO4 | 82.0 | 20 (glucan) | 91.6 g glucose per L in 96 h | — | 17 |
| 10 kg h−1 | Wheat straw | 160 | 10 | 0.5% H2SO4 | 95.8 | 10 | 80% sugars conversion in 72 h | 26.2 g L−1 from 6% glucan | 18 |
| a | Wheat straw | 198 | 2–4 | H2O | — | 5 | 79.6–88.4% glucose conversion in 48 h | — | 52 |
| a | Sorghum | 180 | 10 | H2O | 23.8 | 10 | 58.1% glucose conversion in 72 h | — | 53 |
| 10 kg h−1 | Switchgrass | 185 | 10 | H2O | 31.6 | 2 | 60.1% sugars conversión in 96 h | — | 54 |
| 5–7 kg h−1 | Rice straw | 160 | 10–30 | 3.0% H2SO4 | — | — | 70% glucose conversion in 72 h | — | 56 |
However, the sugar degradation products are not included in most reports, precluding a proper comparison among technologies. The sugars yields obtained in the enzymatic saccharification stage in the present study are similar or higher than those reported in the literature. Wang et al.12 measured a glucan conversion of ∼80% in 24 h with a 1% glucan loading of corn stover in a 8.3 kg h−1 PCTR using 160 °C, but with 2.0% sulphuric acid and 5 min residence time.
Fang et al.52 presented a glucan conversion in the ratio of 79.6 to 88.4% using WS autohydrolised in an Andritz® 22-in continuous steam explosion pretreatment system at 198 °C (15 bar) and 2–4 min residence time. Kapoor et al.10 reported their results on pretreatment of rice straw in a 10 kg h−1 PCTR (160 °C, 0.35% sulphuric acid, for 10 min). Concentrations of 83.3 g glucose per L and 31.9 g xylose per L were achieved after 72 h saccharification at 20% solids loading. A recent report showed sorghum pretreated in a proprietary technology PCTR at 180 °C and 10 min residence time achieving a glucan conversion below 60% which was improved up to 77.5% by 3-cycles disk milling.53
Bonfiglio et al.54 investigated the pretreatment of switchgrass (Panicum virgatum) by steam explosion in a 10 kg biomass per h semi-continuous pilot reactor (170–200 °C and 5–15 min residence time) achieving after 96 h a 60.1% saccharification yield using pretreated biomass at 185 °C and 10 min. As mentioned previously, limited information is available in the scientific literature related to ethanol production using pilot-scale pretreated biomass. McIntosh et al.13 employed Eucalyptus grandis to produce 11.3 kg of ethanol per 100 kg of untreated biomass using a 150 L horizontal reactor (180 °C, 2.4% sulphuric acid, and 15 min) followed by steam explosion (185 °C, 5 min) in a SSF strategy (24 h pre-hydrolysis and 96 h fermentation). Thomsen et al.14 obtained 7.8–10 kg ethanol per 100 kg of untreated WS using either only water or chemicals (sulphuric acid, ammonia or sodium carbonate) in a PCTR system at 190 °C. Agrawal et al.18 pretreated WS in a 10 kg h−1 mass flowrate PCTR (160 °C, 0.5% sulphuric acid, and 10 min) which was subsequently employed in a SSF strategy with 6% solids loading, achieving ethanol concentration of 19.4–26.2 g L−1. Gladis et al.55 steam-pretreated CS in a batch 10 L reactor using 0.4% phosphoric acid at 190 °C for 10 min. A 60–69% ethanol yield was achieved using a 24-prehydrolysis step in a high solids loading SSF, which was similar to the YE of 60% achieved in this work. Another sequential pretreatment system was employed in rice straw.56 The system consisted of a twin-screw extruder (120 °C, 40 rpm, 3.0% sulphuric acid) feeding the biomass into a steam-pressurized reactor (130–160 °C, reaction time of 10–30 min) followed by the detoxification of hemicellulosic hydrolysates (liquid fraction). The detoxified hydrolysates were fermented with Pichia stipitis reaching 0.44 g of ethanol per g sugars after 72 h.
As shown by Saha et al.57 using dilute acid pretreated WS (160 °C and 20 min), higher ethanol yields (29 kg ethanol per 100 kg of untreated WS) were achieved by SSF at the pilot-scale (100 L Biostat B fermenters) including the utilization of a recombinant Escherichia coli FBR5 for total sugars consumption. Therefore, overall ethanol production from the PCTR pretreated biomass on this study could be improved with additional strategies, such as the utilization of xylose-rich liquid fraction (after detoxification), C5/C6 sugars consumption ethanol strain, bioreactor design for SSF, and strategies for higher solids loading during SSF (fed-batch), among others.58,59
Conclusions
The achieved total ethanol yield (11.0 to 11.3 kg of ethanol per 100 kg of untreated biomass) could be expected at larger-than-pilot scales because this PCTR technology successfully overcame underlying challenges related to biomass recalcitrance. However, differences in final sugar yields and ethanol concentrations were obtained among feedstocks. Therefore, specific process conditions must be considered for each biomass. Xylan removal and structural changes observed using FTIR proved to be important in order to achieve high yields during enzymatic saccharification. These were especially noticeable with L-residence time in which high sugars conversion and low production of fermentation inhibitors were obtained. These results will contribute to enrich the limited information on pilot-scale pretreatment systems required to carry out robust modelling in pretreatment dynamics, reactor design and techno-economic analysis at the demonstration/commercial scale.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Energy Sustainability Fund of the Mexican Secretary of Energy, Grant no. 249564 (Mexican Bioenergy Innovation Centre, Bioalcohols Cluster). This work was part of the DOE Joint BioEnergy Institute (http://www.jbei.org) supported by the U.S. Department of Energy, the Office of Science, and the Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy.References
- N. Akhtar, K. Gupta, D. Goyal and A. Goyal, Environ. Prog. Sustainable Energy, 2016, 35, 489–511 CrossRef CAS
.
- J. K. Saini, R. Saini and L. Tewari, 3 Biotech, 2015, 5, 337–353 CrossRef PubMed
.
- Y. H. Percival Zhang, E. Berson, S. Sarkanen and B. E. Dale, Appl. Biochem. Biotechnol., 2009, 153, 80–83 CrossRef PubMed
.
- A. Sanchez, V. Sevilla-Güitrón, G. Magaña and L. Gutierrez, Fuel, 2013, 113, 165–179 CrossRef CAS
.
- D. P. Maurya, A. Singla and S. Negi, 3 Biotech, 2015, 5, 597–609 CrossRef PubMed
.
- M. Naresh Kumar, R. Ravikumar, S. Thenmozhi, M. Ranjith Kumar and M. Kirupa Shankar, Waste Biomass Valorization, 2018, 1–17 Search PubMed
.
- D. Edwards, Biofuels International, 2012, 44–46 Search PubMed
.
- J. Zhang, W. Hou and J. Bao, Adv. Biochem. Eng./Biotechnol., 2016, 105(Part B), 412–419 Search PubMed
.
- R. T. Elander, Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals, 2013, pp. 417–450 Search PubMed
.
- M. Kapoor, S. Soam, R. Agrawal, R. P. Gupta, D. K. Tuli and R. Kumar, Bioresour. Technol., 2017, 224, 688–693 CrossRef CAS PubMed
.
- W. H. Chen, C. C. Tsai, C. F. Lin, P. Y. Tsai and W. S. Hwang, Bioresour. Technol., 2013, 128, 297–304 CrossRef CAS PubMed
.
- W. Wang, X. Chen, B. S. Donohoe, P. N. Ciesielski, R. Katahira, E. M. Kuhn, K. Kafle, C. M. Lee, S. Park, S. H. Kim, M. P. Tucker, M. E. Himmel and D. K. Johnson, Biotechnol. Biofuels, 2014, 7, 47 CrossRef PubMed
.
- S. McIntosh, Z. Zhang, J. Palmer, H. Wong, W. O. S. Doherty and T. Vancov, Biofuels, Bioprod. Biorefin., 2016, 346–358 CrossRef CAS
.
- M. H. Thomsen, A. Thygesen, H. Jørgensen, J. Larsen, B. H. Christensen and A. B. Thomsen, Appl. Biochem. Biotechnol., 2006, 129–132, 448–460 CAS
.
- Y. L. Cha, J. Yang, S. Il Seo, G. H. An, Y. H. Moon, G. D. You, J. E. Lee, J. W. Ahn and K. B. Lee, Fuel, 2016, 164, 322–328 CrossRef CAS
.
- W. Wang, X. Chen, B. S. Donohoe, P. N. Ciesielski, R. Katahira, E. M. Kuhn, K. Kafle, C. M. Lee, S. Park, S. H. Kim, M. P. Tucker, M. E. Himmel and D. K. Johnson, Biotechnol. Biofuels, 2014, 7, 57 CrossRef PubMed
.
- J. Shekiro III, E. M. Kuhn, N. J. Nagle, M. P. Tucker, R. T. Elander and D. J. Schell, Biotechnol. Biofuels, 2014, 7, 23 CrossRef PubMed
.
- R. Agrawal, A. Satlewal, R. Gaur, A. Mathur, R. Kumar, R. P. Gupta and D. K. Tuli, Biochem. Eng. J., 2015, 102, 54–61 CrossRef CAS
.
- M. Heitz, P. G. Koeberle, J. Gagn, E. Chornet, J. D. Taylor and E. Yu, Bioresour. Technol., 1991, 35, 23–32 CrossRef CAS
.
- R. Wahid, M. Hjorth, S. Kristensen and H. B. Møller, Energy Fuels, 2015, 29, 4030–4037 CrossRef CAS
.
- V. M. Nascimento, C. E. V. Rossell and G. J. de Moraes Rocha, in Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass, ed. H. A. Ruiz, M. Hedegaard Thomsen and H. L. Trajano, Springer International Pub., Cham, 2017, pp. 377–388 Search PubMed
.
- F. Adani, G. Papa, A. Schievano, G. Cardinale, G. D. D'Imporzano and F. Tambone, Environ. Sci. Technol., 2011, 45, 1107–1113 CrossRef CAS PubMed
.
- S. Sharma, R. Kumar, R. Gaur, R. Agrawal, R. P. Gupta, D. K. Tuli and B. Das, Bioresour. Technol., 2015, 175, 350–357 CrossRef CAS PubMed
.
- F. Rodríguez and A. Sánchez, Modelling of biomass residence time distribution and xylan depolimerization kinetics analysis in a Pilot-Scale Pretreatment Continuous Tubular Reactor, Elsevier Masson SAS, 2018, vol. 43 Search PubMed
.
- I. Jaramillo and A. Sanchez, ACS Sustainable Chem. Eng., 2018, 6, 8570–8577 CrossRef CAS
.
- J. Larsen, M. Østergaard Petersen, L. Thirup, H. Wen Li and F. Krogh Iversen, Chem. Eng. Technol., 2008, 31, 765–772 CrossRef CAS
.
- A. Sanchez, P. Hernández-Sánchez and R. Puente, Ind. Crops Prod., 2019, 131, 70–77 CrossRef CAS
.
- F. Rodríguez, L. Amaya-Delgado and A. Sanchez, Ind. Crops Prod., 2019, 134, 62–70 CrossRef
.
- J. A. Perez-Pimienta, C. A. Flores-Gómez, H. A. Ruiz, N. Sathitsuksanoh, V. Balan, L. da Costa Sousa, B. E. Dale, S. Singh and B. A. Simmons, Bioresour. Technol., 2016, 211, 216–223 CrossRef CAS PubMed
.
- G. Papa, S. Rodriguez, A. George, A. Schievano, V. Orzi, K. L. Sale, S. Singh, F. Adani and B. A. Simmons, Bioresour. Technol., 2015, 183, 101–110 CrossRef CAS PubMed
.
- N. Dowe and J. McMillan, Lab. Anal. Proced. Natl. Renew. Energy Lab., Golden, CO, NREL/TP-510-42630.
- A. Sluiter, B. Hames, R. Ruiz and C. Scarlata, Lab. Anal. Proced. Natl. Renew. Energy Lab., Golden, CO, NREL/TP-510-42618.
- A. Sluiter, B. Hames, R. O. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. of Energy, Lab. Anal. Proced. Natl. Renew. Energy Lab., Golden, CO, NREL/TP-510-42622, 2005, pp. 1–6.
- A. Sluiter, B. Hames, R. Ruiz and C. Scarlata, Determination of sugars, byproducts, and degradation products in liquid fraction process samples, Lab. Anal. Proced. Natl. Renew. Energy Lab., Golden, CO, NREL/TP-510-42623, 2006, pp. 1–14 Search PubMed
.
- F. Carvalheiro, M. P. Esteves, J. C. Parajó, H. Pereira and F. M. Gírio, Bioresour. Technol., 2004, 91, 93–100 CrossRef CAS PubMed
.
- J. A. Pérez-Pimienta, A. Vargas-Tah, K. M. López-Ortega, Y. N. Medina-López, J. A. Mendoza-Pérez, S. Avila, S. Singh, B. A. Simmons, I. Loaces and A. Martinez, Bioresour. Technol., 2017, 225, 191–198 CrossRef PubMed
.
- H. Zabed, J. N. Sahu, A. N. Boyce and G. Faruq, Renewable Sustainable Energy Rev., 2016, 66, 751–774 CrossRef CAS
.
- J. A. Pérez-Pimienta, M. G. López-Ortega and A. Sanchez, Biofuels, Bioprod. Biorefin., 2017, 11, 732–748 CrossRef
.
- X. Meng, T. Wells Jr, Q. Sun and A. Ragauskas, Green Chem., 2015, 17, 4239–4246 RSC
.
- H. A. Ruiz, D. S. Ruzene, D. P. Silva, M. A. C. Quintas, A. A. Vicente and J. A. Teixeira, J. Chem. Technol. Biotechnol., 2011, 86, 88–94 CrossRef CAS
.
- Y. Li, W. Liu, Q. Hou, S. Han, Y. Wang and D. Zhou, Ind. Eng. Chem. Res., 2014, 53, 8366–8371 CrossRef CAS
.
- L. J. Rios-González, T. K. Morales-Martínez, M. F. Rodríguez-Flores, J. A. Rodríguez-De la Garza, D. Castillo-Quiroz, A. J. Castro-Montoya and A. Martinez, Bioresour. Technol., 2017, 242, 184–190 CrossRef PubMed
.
- F. Carvalheiro, T. Silva-Fernandes, L. C. Duarte and F. M. Gírio, Appl. Biochem. Biotechnol., 2009, 153, 84–93 CrossRef CAS PubMed
.
- J. J. Lischeske, N. C. Crawford, E. Kuhn, N. J. Nagle, D. J. Schell, M. P. Tucker, J. D. McMillan and E. J. Wolfrum, Biotechnol. Biofuels, 2016, 9, 213 CrossRef PubMed
.
- J. A. Perez-Pimienta, H. M. Poggi-Varaldo, T. Ponce-Noyola, A. C. Ramos-Valdivia, J. A. Chavez-Carvayar, V. Stavila and B. A. Simmons, Biomass Bioenergy, 2016, 91, 48–55 CrossRef CAS
.
- R. Kumar, G. Mago, V. Balan and C. E. Wyman, Bioresour. Technol., 2009, 100, 3948–3962 CrossRef CAS PubMed
.
- F. Carvalheiro, L. C. Duarte, F. Gírio and P. Moniz, in Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, ed. S. I. Mussatto, Elsevier, Amsterdam, 2016, pp. 315–347 Search PubMed
.
- M. Michelin and J. A. Teixeira, Bioresour. Technol., 2016, 216, 862–869 CrossRef CAS PubMed
.
- F. Cotana, G. Cavalaglio, M. Gelosia, V. Coccia, A. Petrozzi, D. Ingles and E. Pompili, Ind. Crops Prod., 2015, 69, 424–432 CrossRef CAS
.
- L. Kang, W. Wang and Y. Y. Lee, Appl. Biochem. Biotechnol., 2010, 161, 53–66 CrossRef CAS PubMed
.
- G. Zacchi and A. Axelsson, Biotechnol. Bioeng., 1989, 34, 223–233 CrossRef CAS PubMed
.
- H. Fang, J. Deng and X. Zhang, BioResources, 2011, 6, 4468–4480 CAS
.
- M. Cheng, B. S. Dien, D. K. Lee and V. Singh, Bioresour. Technol., 2019, 289, 121663 CrossRef PubMed
.
- F. Bonfiglio, M. Cagno, F. Rey, M. Torres, S. Böthig, P. Menéndez and S. I. Mussatto, Biomass Bioenergy, 2019, 121, 41–47 CrossRef CAS
.
- A. Gladis, P. M. Bondesson, M. Galbe and G. Zacchi, Energy Sci. Eng., 2015, 3, 481–489 CrossRef CAS
.
- T. H. Lin, C. F. Huang, G. L. Guo, W. S. Hwang and S. L. Huang, Bioresour. Technol., 2012, 116, 314–319 CrossRef CAS PubMed
.
- B. C. Saha, N. N. Nichols, N. Qureshi, G. J. Kennedy, L. B. Iten and M. Cotta, Bioresour. Technol., 2015, 175, 17–22 CrossRef CAS PubMed
.
- R. Liguori, V. Ventorino, O. Pepe and V. Faraco, Appl. Microbiol. Biotechnol., 2016, 100, 597–611 CrossRef CAS PubMed
.
- R. Wang, P. Unrean and C. J. Franzén, Biotechnol. Biofuels, 2016, 9, 88 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1, macroscopic effect of pretreatment using a PCTR on the agricultural residues; Fig. S2, FTIR spectra of untreated and PCTR-pretreated solids; Fig. S3, FTIR spectra of untreated and BLR-pretreated solids; Table S1, FTIR band assignment; Table S2, comparison of sugar and ethanol yields of PCTR-pretreated biomass using SSF with a total process time of 96 h. See DOI: 10.1039/d0ra04031b |
| This journal is © The Royal Society of Chemistry 2020 |