Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
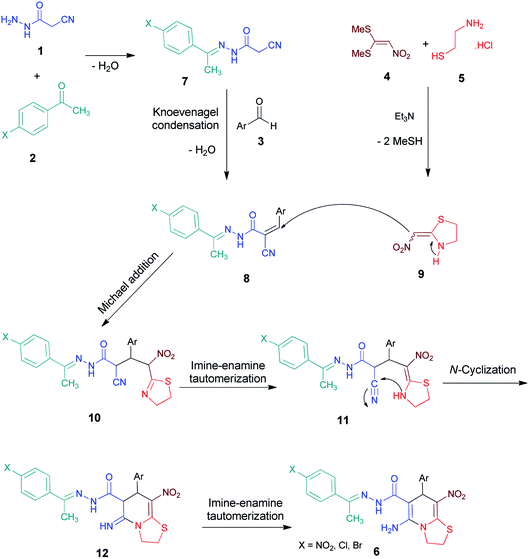
Synthesis of highly functionalized thiazolo[3,2-a]pyridine derivatives via a five-component cascade reaction based on nitroketene N,S-acetal†
Zohreh Sahhaf Razavi,
Mohammad Bayat * and
Hajar Hosseini
* and
Hajar Hosseini
Department of Chemistry, Faculty of Science, Imam Khomeini International University, Qazvin, Iran. E-mail: bayat_mo@yahoo.com; m.bayat@sci.ikiu.ac.ir
First published on 21st August 2020
Abstract
A highly efficient and straightforward synthesis of N-fused heterocyclic compounds including 5-amino-7-(aryl)-8-nitro-N'-(1-(aryl)ethylidene)-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide derivatives is successfully achieved via a five-component cascade reaction utilizing cyanoacetohydrazide, various acetophenones, aromatic aldehydes, 1,1-bis(methylthio)-2-nitroethylene and cysteamine hydrochloride in ethanol at reflux conditions. The new approach involves domino N,S-acetal formation, Knoevenagel condensation, Michael reaction, imine–enamine tautomerization and N-cyclization sequences. The prominent advantages of this protocol include: facility of operation, available and economical starting materials, no need for toxic solvents, high yields and tolerance of a wide variety of functional groups.
Introduction
The thiazolopyridine moiety is found in a wide spectrum of biologically active compounds. Thiazolo[3,2-a]pyridines are an important category with notable antibacterial and antifungal activity1 and other considerable bioactivities including as a beta-amyloid production inhibitor,2 potent CDK2-cyclin A inhibitor,3 potential uterus stimulant,4 coronary dilator, antihypertensives, and muscle relaxant.5 Also they are useful for chemotherapy of various cancers, such as leukemia, lung cancer, and melanoma.6–8 Some biologically active compounds with this nucleus are presented in Fig. 1.9–11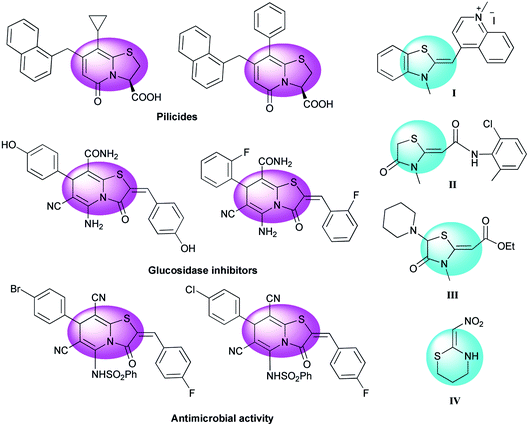 | ||
| Fig. 1 Selected examples of thiazolo[3,2-a]pyridines with biological and pharmacological activities and examples of drugs, insecticides and probes having the ketene N,S-acetal structure. | ||
Obviously, the synthesis of new classes of thiazolo[3,2-a]pyridines may give a library of compounds as possible candidates for various biological activities.
Cyclic ketene N,S-acetal structures are as such used as drugs for the treatment of hypertension diseases and usually employed as probes for nucleic acids to study the interaction between G4 (G-quadruplex) and its ligands (Fig. 1, I–III).12 It's interesting that the cyclic nitroketene N,S-acetal nithiazine IV was the first reported compound of neonicotinoid insecticides13 and is widely used as a common insecticide around the world (Fig. 1). Synthetically, the cyclic nitroketene N,S-acetals have a rigid structure and act as Michael donor 1,3-N,C di-nucleophiles for the generation of nitrogen-containing heterocyclic compounds. The ethylene motif has a polarized push–pull type of alkene, therefore the one end expands an electrophilic character, whereas the other end develops a nucleophilic character. This feature of nitroketene N,S-acetals make them highly useful to apply in the Michael addition, annulation and multicomponent reactions.14 Today, multicomponent reactions (MCRs) have become a prominent strategy and are selected over stepwise synthesis due to the following reasons: reduced synthetic time, labor and cost, minimal utilization of toxic and harmful chemicals, simple workup of products, high yields, straight forward and simplicity of experimental procedures and economic viability; therefore, MCRs are a powerful approach to promotion of green chemistry by reducing the formation of large quantities of waste.15–19
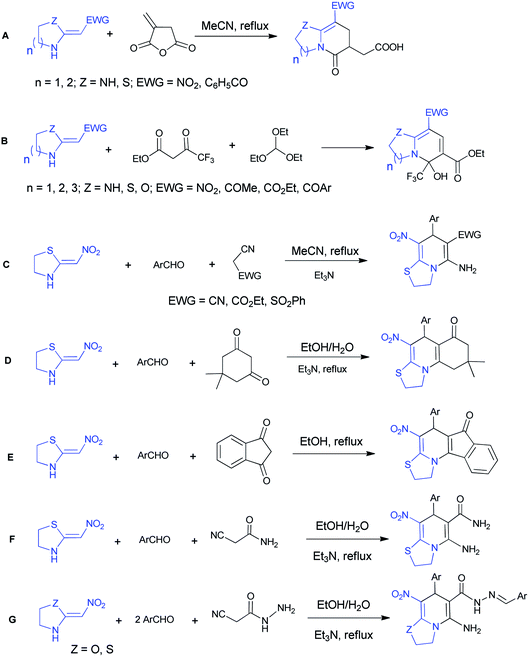
The five-membered cyclic nitroketene N,S-acetal and commercially available six-membered nithiazine have been remarkably explored in the literature and their reactions with different Michael acceptors are most expected. Here we report the some synthesis of thiazolo[3,2-a]pyridine compounds performed with cyclic ketene N,S-acetals (Scheme 1). In 2005, Chakrabarti et al. described the reactions between diverse cyclic N,S- and N,N-ketene acetals and itaconic anhydride (A).20 In 2010, Yan et al. reported one-pot synthesis of functionalized bicyclic pyridines under solvent- and catalyst-free conditions with triethoxymethane, ethyl 4,4,4-trifluoro-3-oxobutanoate and various ketene aminals (B).21 In 2011, Altug et al. developed the synthesis of thiazolo[3,2-a]pyridines via a one-pot reaction between 2-(nitromethylene)thiazolidine, aromatic aldehydes and ethyl 2-cyanoacetate, malononitrile or 2-(phenylsulfonyl)acetonitrile (C).22
In 2018, our research group synthesized fused thiazolo[3,2-a]pyridines utilizing the five-membered cyclic nitroketene N,S-acetal, dimedone and different aromatic aldehydes (D).23 Also we reported the synthesis of indenone-fused thiazolo[3,2-a]pyridines via a one-pot reaction between 2-(nitromethylene)thiazolidine, aromatic aldehydes and 1,3-indandione (E).24 In addition, we were able to produce the desired products using cyanoacetamide, aromatic aldehydes and 2-(nitromethylene)thiazolidine (F).25 Moreover, in 2018, the reaction of cyanoacetohydrazide with aromatic aldehydes and 2-(nitromethylene)thiazolidine/oxazolidine resulted in functionalized thiazolo/oxazolo pyridine derivatives (G).26
Following our efforts to synthesize the new heterocyclic compounds using cyanoacetohydrazide and based on previous works, we designed new reactions utilizing 2-(nitromethylene)thiazolidine as heterocyclic ketene aminal. In this article we report an efficient synthesis of highly functionalized 2H-thiazolo[3,2-a]pyridine-6-carbohydrazide compounds via a one-pot five-component domino reaction. To the best of our knowledge, there is no report on the synthesis of these structures.
Results and discussion
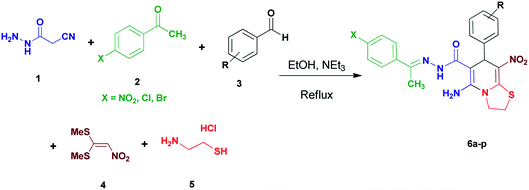
We have developed an efficient synthesis of new functionalized thiazolo[3,2-a]pyridine structures 6 by using of cyanoacetohydrazide 1, acetophenone derivatives 2, aromatic aldehydes 3, 1,1-bis(methylthio)-2-nitroethene 4 and cysteamine hydrochloride 5 in the presence of triethylamine in ethanol at reflux conditions (Scheme 2).Optimization of the conditions
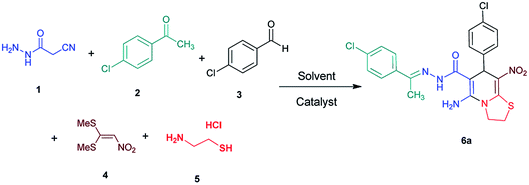
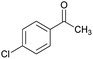
Initially, cyanoacetohydrazide 1, 4-chloroacetophenone 2, 4-chlorobenzaldehyde 3, 1,1-bis(methylthio)-2-nitroethene 4 and cysteamine hydrochloride 5 were used as model substrates to achieve the best yield.In general, due to the variable reactivity of cyanoacetohydrazide (based on its specific structure) and on the other hand due to the five-component nature of the defined reactions, great efforts were made to obtain the desired products with high purity. At first ethanol was examined and the experimental results showed when ethanol was used as solvent with triethylamine at reflux conditions, the yield of desired product 6a was 93% (Table 1, entry 1). It should be noted that the catalyst used (NEt3) is not working on the rate-limiting step. To prepare 2-(nitromethylene)thiazolidine solution (from 1,1-bis(methylthio)-2-nitroethene and cysteamine hydrochloride, which is mentioned in the Experimental section), it is necessary to use triethylamine to separate cysteamine from its salt.23,27 No reaction will occur without the use of triethylamine (entry 4). The use of other catalysts is related to the whole reaction.
| Entry | Solvent | Catalyst (mol%) | Time (h) | Temp (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: cyanoacetohydrazide (1 mmol), 4-chloroacetophenone (1 mmol), 4-chlorobenzaldehyde (1 mmol), 1,1-bis(methylthio)-2-nitroethene (1 mmol), cysteamine hydrochloride (1 mmol), solvent (20 mL), catalyst (1 mmol). | |||||
| 1 | EtOH | NEt3 | 24 | 78 | 93 |
| 2 | EtOH | Piperidine | 24 | 78 | 80 |
| 3 | EtOH | AcOH | 24 | 78 | No reaction |
| 4 | EtOH | — | 24 | 78 | No reaction |
| 5 | H2O | NEt3 | 24 | 100 | No reaction |
| 6 | H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
NEt3 | 24 | 78 | 40 |
| 7 | CH3CN | NEt3 | 24 | 82 | No reaction |
| 8 | CHCl3 | NEt3 | 24 | 61 | No reaction |
| 9 | MeOH | NEt3 | 24 | 65 | No reaction |
| 10 | DMF | NEt3 | 24 | 153 | No reaction |
In order to increase the reaction rate, two types of catalysts were used. With piperidine, the reaction efficiency decreased slightly (entry 2) and with acetic acid, the product did not form (entry 3). According to the investigations, it was determined that in basic and acidic medium, other products are formed (two-, three- and four-component products). The percentage of each was different for various derivatives. In general, it was found that the slightest change in the reaction conditions (even in ethanol amount) leads to a decrease in the efficiency of the desired product or often its non-formation. In addition, we observed the formation of a four-component by-product in two cases, which are described in the general procedure section. However, we also studied the effect of other solvents. The use of water or acetonitrile did not result in the desired product (entry 5 and 7), and when the mixture of water and ethanol was used (overall 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), the efficiency decreased (entry 6). With chloroform, methanol and DMF, in reflux conditions the desired products were not formed (entry 8, 9 and 10).
1, v/v), the efficiency decreased (entry 6). With chloroform, methanol and DMF, in reflux conditions the desired products were not formed (entry 8, 9 and 10).
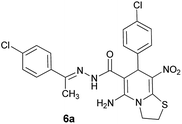
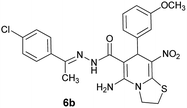
With information obtained from optimization conditions table, we could synthesize target compounds (E)-5-amino-7-(aryl)-8-nitro-N'-(1-(aryl)ethylidene)-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide 6a–p in good to high yields (70–95%) using cyanoacetohydrazide 1, acetophenone derivatives 2, various aromatic aldehydes 3, 1,1-bis(methylthio)-2-nitroethene 4 and cysteamine hydrochloride 5 as starting materials (Scheme 2).
The reactions were completed after 24 h to afford the corresponding heterocyclic structures. The results are summarized in Table 2.
| Entry | Aromatic aldehyde | Acetophenone derivative | Product | Yield (%) | Mp (°C) |
|---|---|---|---|---|---|
| a The reactions were performed using cyanoacetohydrazide (1 mmol), acetophenone derivatives (1 mmol), aromatic aldehydes (1 mmol), 1,1-bis(methylthio)-2-nitroethene, (1 mmol), cysteamine hydrochloride (1 mmol), triethylamine (1 mmol), EtOH (20 mL). | |||||
| 1 | 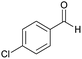 |
 |
 |
93 | 252–254 |
| 2 |  |
 |
 |
76 | 230–233 |
| 3 |  |
 |
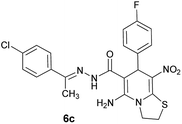 |
86 | 239–241 |
| 4 |  |
 |
 |
80 | 222–224 |
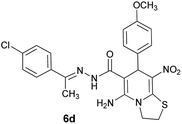
| 5 |  |
 |
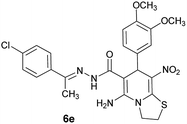 |
75 | 217–219 |
| 6 | 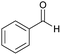 |
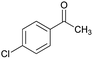 |
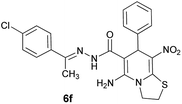 |
84 | 248–250 |
| 7 |  |
 |
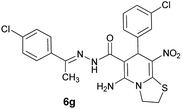 |
82 | 237–240 |
| 8 |  |
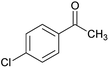 |
 |
78 | 225–227 |
| 9 | 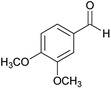 |
 |
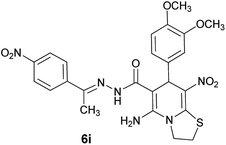 |
80 | 203–205 |
| 10 | 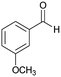 |
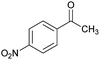 |
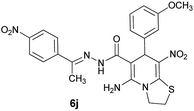 |
78 | 234–236 |
| 11 | 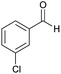 |
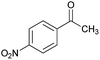 |
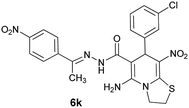 |
90 | 218–220 |
| 12 | 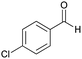 |
 |
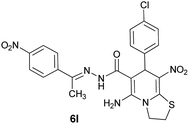 |
95 | 244–246 |
| 13 |  |
 |
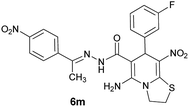 |
85 | 242–245 |
| 14 |  |
 |
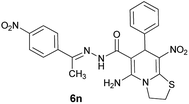 |
87 | 244–246 |
| 15 | 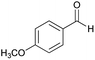 |
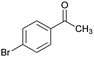 |
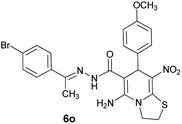 |
75 | 253–255 |
| 16 | 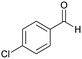 |
 |
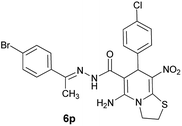 |
70 | 262–264 |
Scope and limitations
This reaction was performed with ortho derivatives of benzaldehyde (2-chloro, 2-hydroxy and 2-nitro) under the same conditions, which did not result in the product probably due to steric effects. Also the use of acetophenone and 4-methoxyacetophenone did not lead to the favorable products. The reaction was also used with aliphatic ketones instead of acetophenone derivatives and aliphatic aldehydes instead of aromatic aldehydes which resulted in no product formation.It was found that the major by-product of this reaction is a four-component structure that was previously synthesized using two equivalents of aldehyde26 which will prevent its formation by performing the correct reaction steps (see Experimental section).
Structure determination
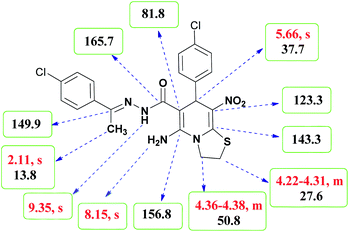
The structures of all new compounds 6a–p were supported by means of IR, 1H NMR, 13C NMR spectroscopic and mass spectrometric data (see the ESI†).As a representative case the key signals of 1H and 13C NMR chemical shifts of (E)-5-amino-7-(4-chlorophenyl)-N'-(1-(4-chlorophenyl)ethylidene)-8-nitro-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide 6a are presented in Fig. 2.
The 1H NMR spectrum of 6a showed NH group at δ 9.35 ppm. The NH2 group appeared at δ 8.15 ppm. The proton of CH at pyridine ring was seen at δ 5.66 ppm. Four protons of two methylene groups appeared at δ 4.22 to 4.38 ppm as two multiplets. The signal at δ 2.11 ppm was related to methyl group.
The 1H-decoupled 13C NMR spectrum of 6a indicated 18 distinct resonances in accordance to desired structure. The characteristic signals of four aliphatic carbons (CH3, CH and two CH2 groups) were seen at δ 13.8, 37.7, 27.6 and 50.8 ppm respectively. Characteristic signal at δ 81.8 ppm was related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO. The carbonyl group appeared at δ 165.7 ppm (Fig. 2).
C–CO. The carbonyl group appeared at δ 165.7 ppm (Fig. 2).
The IR spectrum of 6a showed absorption bands at 3141 and 3284 cm−1 due to NH and NH2 groups, strong absorption of carbonyl group at 1626 and C–N band at 1237 cm−1. Two absorption bands due to nitro group appeared at 1514 and 1302 cm−1.
Mechanism
A general plausible mechanism for the formation of thiazolo[3,2-a]pyridine carbohydrazides is shown in Scheme 3. The condensation of cyanoacetohydrazide 1 with acetophenone 2 leads to the hydrazide-hydrazone structures 7. On the basis of well-established chemistry of 1,1-bis(methylthio)-2-nitroethene, on the other hand, addition of cysteamine hydrochloride 5 to 1,1-bis(methylthio)-2-nitroethene 4 leads to the formation of ketene N,S-acetal 9.23,27 The formation of β-nitrothiazolidine 9 occurs in the presence of an equivalent amount of triethylamine base for releasing cysteamine salt. Further, with adding aldehyde 3, the Knoevenagel condensation affords intermediate 8. Then, Michael addition of nitroenamine 9 to adduct 8 leads to the intermediate 10, which undergoes successive imine–enamine tautomerization followed by an intramolecular cyclization via nucleophilic addition of –NH to nitrile group. Finally, imine–enamine tautomerization leads to thiazolo[3,2-a]pyridine products 6 (Scheme 3).Conclusion
A green and efficient approach to easy synthesis of novel and highly substituted fused 1,4-dihydropyridines, 5-amino-7-(aryl)-8-nitro-N'-(1-(aryl)ethylidene)-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazides, has been developed based on a one-pot five-component condensation via annulation of cyclic nitroketene N,S-acetal, β-nitrothiazolidine, and a three-component product of cyanoacetohydrazide, acetophenone derivatives and different aromatic aldehydes. The reactions are completed within 24 h in EtOH at reflux conditions. The present synthesis shows significant properties such as high regioselectivity, cascade one-pot methodology, high yields, simple purification of products, and high atom economy.Experimental
Materials
All commercially available reagents and other solvents were purchased from Aldrich and Merck chemical Co. and used without further purification. The NMR spectra were recorded with a Bruker DRX-300 AVANCE instrument (300 MHz for 1H and 75.4 MHz for 13C) with DMSO-d6 as solvent. Chemical shifts are given in ppm (δ) relative to internal TMS, and coupling constant (J) are reported in Hertz (Hz). Melting points were measured with an electrothermal 9100 apparatus. Mass spectra were recorded with an Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. IR spectra were measured with Bruker Tensor 27 spectrometer. Elemental analyses for C, H and N were performed using a PerkinElmer 2004 series [II] CHN elemental analyzer.General procedure of the synthesis of 5-amino-7-(aryl)-8-nitro-N'-(1-(aryl)ethylidene)-3,7-dihydro-2H-thiazolo[3,2-a]pyridine-6-carbohydrazide derivatives
A mixture of cysteamine hydrochloride (0.113 g, 1 mmol), 1,1-bis(methylthio)-2-nitroethylene (0.165 g, 1 mmol), Et3N (140 μL, 1 mmol) and 10 mL EtOH in a 50 mL flask was refluxed for 5 hours. In another 50 mL flask the stoichiometric mixture of cyanoacetohydrazide (1 mmol, 0.099 g) and acetophenone derivative (1 mmol) in EtOH (10 mL) was refluxed for 3–5 hours depending on the type of acetophenone. After these times, TLC shows the consumption of the starting components. Then, aromatic aldehyde (1 mmol) and the first solution (HKA), were added to the second mixture simultaneously. The progress of the reaction was monitored by TLC using ethyl acetate/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After completion of the reaction (24 hours), without the need for chromatography or recrystallization, the precipitated product was collected by filtration and washed with warm ethanol to give the pure products 6a–p in excellent yield.
1). After completion of the reaction (24 hours), without the need for chromatography or recrystallization, the precipitated product was collected by filtration and washed with warm ethanol to give the pure products 6a–p in excellent yield.
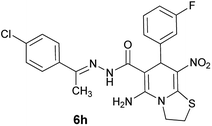
To achieve the pure products, it was necessary to complete the reaction of cyanoacetohydrazide and acetophenone derivatives in ethanol at reflux conditions in sufficient time (3 hours for 4-nitroacetophenone and 5 hours for 4-chloro and 4-bromoacetophenone), then with no need for product separation, nitroenamine solution and aromatic aldehyde were added to two-component hydrazone mixture at the same time. We found two distinct cases (6g and 6h) that led to a mixture of two products: the desired product and the product without participation of acetophenone derivative.26
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 123.3 (C–NO2), 124.3, 127.7, 128.1, 128.2, 129.7, 131.3, 133.5, 137.1 (Ar), 143.3 (C
C–CO), 123.3 (C–NO2), 124.3, 127.7, 128.1, 128.2, 129.7, 131.3, 133.5, 137.1 (Ar), 143.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 149.9 (C
C–S), 149.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.8 (C–NH2), 165.7 (C
N), 156.8 (C–NH2), 165.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C22H19Cl2N5O3S: C, 52.39; H, 3.80; N, 13.88. Found: C, 52.7; H, 3.5; N, 13.7.
O); anal. calcd for C22H19Cl2N5O3S: C, 52.39; H, 3.80; N, 13.88. Found: C, 52.7; H, 3.5; N, 13.7.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 111.4, 114.4, 120.0 (Ar), 123.5 (C–NO2), 127.7, 128.2, 129.4, 133.5, 137.1 (Ar), 145.8 (C
C–CO), 111.4, 114.4, 120.0 (Ar), 123.5 (C–NO2), 127.7, 128.2, 129.4, 133.5, 137.1 (Ar), 145.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 149.0 (Ar), 150.0 (C
C–S), 149.0 (Ar), 150.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.5 (C–NH2), 159.0 (CAr–OMe), 165.3 (C
N), 156.5 (C–NH2), 159.0 (CAr–OMe), 165.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C23H22ClN5O4S: C, 55.25; H, 4.44; N, 14.01. Found: C, 55.6; H, 4.7; N, 14.3.
O); anal. calcd for C23H22ClN5O4S: C, 55.25; H, 4.44; N, 14.01. Found: C, 55.6; H, 4.7; N, 14.3.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 114.8, 114.9 (Ar), 123.4 (C–NO2), 127.7, 128.2, 129.7, 129.8, 133.5, 137.1, 149.7 (Ar), 140.6 (C
C–CO), 114.8, 114.9 (Ar), 123.4 (C–NO2), 127.7, 128.2, 129.7, 129.8, 133.5, 137.1, 149.7 (Ar), 140.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 149.9 (C
C–S), 149.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.6 (C–NH2), 159.9, 161.7 (Ar), 165.7 (C
N), 156.6 (C–NH2), 159.9, 161.7 (Ar), 165.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C22H19ClFN5O3S: C, 54.15; H, 3.92; N, 14.35. Found: C, 54.3; H, 3.6; N, 14.1.
O); anal. calcd for C22H19ClFN5O3S: C, 54.15; H, 3.92; N, 14.35. Found: C, 54.3; H, 3.6; N, 14.1.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 113.7 (Ar), 124.0 (C–NO2), 127.8, 128.3, 129.0, 133.7, 136.3, 136.8 (Ar), 141.5 (C
C–CO), 113.7 (Ar), 124.0 (C–NO2), 127.8, 128.3, 129.0, 133.7, 136.3, 136.8 (Ar), 141.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 149.0 (C
C–S), 149.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.0 (C–NH2), 158.2 (CAr–OMe), 165.6 (C
N), 150.0 (C–NH2), 158.2 (CAr–OMe), 165.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); m/z (%) = 474 (0.1), 439,2 353,2 305,18 288 (100), 257 (94), 218,17 186,24 167 (31), 138 (79), 117,10 103 (77), 77 (43), 61 (38); anal. calcd for C23H22ClN5O4S: C, 55.25; H, 4.44; N, 14.01.
O); m/z (%) = 474 (0.1), 439,2 353,2 305,18 288 (100), 257 (94), 218,17 186,24 167 (31), 138 (79), 117,10 103 (77), 77 (43), 61 (38); anal. calcd for C23H22ClN5O4S: C, 55.25; H, 4.44; N, 14.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 112.5, 112.6, 120.4 (Ar), 123.9 (C–NO2), 124.0, 127.4, 137.2 (Ar), 144.9 (C
C–CO), 112.5, 112.6, 120.4 (Ar), 123.9 (C–NO2), 124.0, 127.4, 137.2 (Ar), 144.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 147.6 (C
C–S), 147.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 148.3 (CAr–OMe), 148.6 (CAr–OMe), 150.8 (C–NH2), 156.7 (C
N), 148.3 (CAr–OMe), 148.6 (CAr–OMe), 150.8 (C–NH2), 156.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C24H24N6O7S: C, 53.33; H, 4.48; N, 15.55.
O); anal. calcd for C24H24N6O7S: C, 53.33; H, 4.48; N, 15.55.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 111.5, 114.3, 120.0 (Ar), 123.4 (C–NO2), 127.0, 129.4, 131.6, 144.4 (Ar), 145.8 (C
C–CO), 111.5, 114.3, 120.0 (Ar), 123.4 (C–NO2), 127.0, 129.4, 131.6, 144.4 (Ar), 145.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 147.1 (C
C–S), 147.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.3 (C–NH2), 156.6 (CAr–OMe), 159.0 (C
N), 150.3 (C–NH2), 156.6 (CAr–OMe), 159.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C23H22N6O6S: C, 54.11; H, 4.34; N, 16.46.
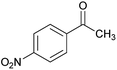
O); anal. calcd for C23H22N6O6S: C, 54.11; H, 4.34; N, 16.46.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 123.0 (C–NO2), 123.4, 126.6, 126.8, 127.0, 127.7, 130.2, 132.6 (Ar), 144.4 (C
C–CO), 123.0 (C–NO2), 123.4, 126.6, 126.8, 127.0, 127.7, 130.2, 132.6 (Ar), 144.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 146.7, 147.2 (Ar), 148.1 (C
C–S), 146.7, 147.2 (Ar), 148.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.3 (CAr–NO2), 157.0 (C–NH2), 165.7 (C
N), 150.3 (CAr–NO2), 157.0 (C–NH2), 165.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); m/z (%) = 509 (0.02), 471 (0.5), 432 (0.2), 387 (0.1), 326 (48), 311 (100), 292 (78), 261 (59), 222,25 179 (46), 149 (42), 117 (95), 103 (41), 77 (70), 61 (78); anal. calcd for C22H19ClN6O5S: C, 51.31; H, 3.72; N, 16.32.
O); m/z (%) = 509 (0.02), 471 (0.5), 432 (0.2), 387 (0.1), 326 (48), 311 (100), 292 (78), 261 (59), 222,25 179 (46), 149 (42), 117 (95), 103 (41), 77 (70), 61 (78); anal. calcd for C22H19ClN6O5S: C, 51.31; H, 3.72; N, 16.32.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 123.9 (C–NO2), 127.5, 128.6, 130.1, 139.4, 143.8, 144.9 (C
C–CO), 123.9 (C–NO2), 127.5, 128.6, 130.1, 139.4, 143.8, 144.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 147.6, 147.9 (Ar), 148.5 (C
C–S), 147.6, 147.9 (Ar), 148.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.0 (CAr–NO2), 157.7 (C–NH2), 166.5 (C
N), 151.0 (CAr–NO2), 157.7 (C–NH2), 166.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); m/z (%) = 509 (0.01), 453 (0.4), 410 (0.2), 368 (0.8), 326,18 311 (46), 292 (100), 261 (69), 222,20 205,10 179 (40), 149 (55), 117 (38), 103 (53), 77 (90), 61 (70); anal. calcd for C22H19ClN6O5S: C, 51.31; H, 3.72; N, 16.32.
O); m/z (%) = 509 (0.01), 453 (0.4), 410 (0.2), 368 (0.8), 326,18 311 (46), 292 (100), 261 (69), 222,20 205,10 179 (40), 149 (55), 117 (38), 103 (53), 77 (90), 61 (70); anal. calcd for C22H19ClN6O5S: C, 51.31; H, 3.72; N, 16.32.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 113.7 (d, 2JCF = 21 Hz, CH of Ar), 114.6 (d, 2JCF = 21 Hz, CH of Ar), 123.2 (C–NO2), 123.5, 124.0, 127.0 (Ar), 130.1 (d, 3JCF = 8 Hz, CH of Ar), 144.4 (C
C–CO), 113.7 (d, 2JCF = 21 Hz, CH of Ar), 114.6 (d, 2JCF = 21 Hz, CH of Ar), 123.2 (C–NO2), 123.5, 124.0, 127.0 (Ar), 130.1 (d, 3JCF = 8 Hz, CH of Ar), 144.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 147.2, 147.2 (Ar), 148.1 (C
C–S), 147.2, 147.2 (Ar), 148.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.4 (CAr–NO2), 157.0 (C–NH2), 162.0 (d, 1JCF = 242 Hz, C–F), 165.8 (C
N), 150.4 (CAr–NO2), 157.0 (C–NH2), 162.0 (d, 1JCF = 242 Hz, C–F), 165.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); m/z (%) = 495 (0.02), 455 (0.4), 438 (0.2), 394 (0.1), 351 (0.5), 326,7 311,12 293,19 276 (34), 245 (36), 222,3 206,15 179,17 149 (38), 117 (40), 103 (47), 77 (100), 61 (97); anal. calcd for C22H19FN6O5S: C, 53.01; H, 3.84; N, 16.86.
O); m/z (%) = 495 (0.02), 455 (0.4), 438 (0.2), 394 (0.1), 351 (0.5), 326,7 311,12 293,19 276 (34), 245 (36), 222,3 206,15 179,17 149 (38), 117 (40), 103 (47), 77 (100), 61 (97); anal. calcd for C22H19FN6O5S: C, 53.01; H, 3.84; N, 16.86.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 123.4 (C–NO2), 123.8, 126.8, 126.9, 127.8, 128.2, 144.3 (Ar), 144.4 (C
C–CO), 123.4 (C–NO2), 123.8, 126.8, 126.9, 127.8, 128.2, 144.3 (Ar), 144.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–S), 147.1 (Ar), 147.4 (C
C–S), 147.1 (Ar), 147.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 150.3 (CAr–NO2), 156.5 (C–NH2), 165.6 (C
N), 150.3 (CAr–NO2), 156.5 (C–NH2), 165.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); m/z (%) = 480 (0.03) [M]+, 437,1 392 (0.2), 345,1 326,16 311,27 275 (54), 258 (100), 227 (80), 179,22 149 (41), 117 (30), 103 (48), 77 (71), 61 (45); anal. calcd for C22H20N6O5S: C, 54.99; H, 4.20; N, 17.49.
O); m/z (%) = 480 (0.03) [M]+, 437,1 392 (0.2), 345,1 326,16 311,27 275 (54), 258 (100), 227 (80), 179,22 149 (41), 117 (30), 103 (48), 77 (71), 61 (45); anal. calcd for C22H20N6O5S: C, 54.99; H, 4.20; N, 17.49.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.Notes and references
- G. El-Hag Ali, A. Khalil, R. Lamphon and A. El-Maghraby, Phosphorus, Sulfur Silicon Relat. Elem., 2005, 180, 1909 CrossRef CAS.
- A. El-Maghraby, G. El-Hag Ali, A. H. A. Ahmed and M. S. A. El-Gaby, Phosphorus, Sulfur Silicon Relat. Elem., 2002, 177, 293 CrossRef CAS.
- S. Vadivelan, B. N. Sinha, S. J. Irudayam and S. A. Jagarlapudi, J. Chem. Inf. Model., 2007, 47, 1526 CrossRef CAS.
- F. M. Manhi and G. A. Soliman, Bull. Fac. Pharm., 1993, 31, 265 CAS.
- M. A. Terzidis, J. S. Stephanatou, C. A. Tsoleridis, A. Terzis and C. P. Raptopoulou, Tetrahedron, 2010, 66, 947 CrossRef CAS.
- R. M. Acheson, Adv. Heterocycl. Chem., 1963, 1, 125 CrossRef CAS.
- M. S. A. El-Gaby, A. G. Al-Sehemi, Y. A. Mohamed and Y. A. Ammar, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 631 CrossRef CAS.
- R. M. Acheson and N. F. Elmore, Adv. Heterocycl. Chem., 1978, 23, 263 CrossRef CAS.
- V. Aberg, M. Sellstedt, M. Hedenstro, J. S. Pinkner, J. S. Hultgrenband and R. Almqvista, Bioorg. Med. Chem., 2006, 147, 563 Search PubMed.
- H. Park, K. Y. Hwang, K. H. Oh, Y. H. Kim, J. Y. Lee and K. Kim, Bioorg. Med. Chem., 2008, 16, 284 CrossRef CAS PubMed.
- G. A. El-Hag Ali, A. Khalil, A. H. A. Ahmed and M. S. A. El-Gaby, Acta Chim. Slov., 2002, 49, 365 CAS.
- (a) X. Fei, Y. Gu, Y. Ban, Z. Liu and B. Zhang, Bioorg. Med. Chem., 2009, 17, 585 CrossRef CAS PubMed; (b) A. K. Jain, A. Vaidya, V. Ravichandran, S. K. Kashaw and R. K. Agrawal, Bioorg. Med. Chem., 2012, 20, 3378 CrossRef CAS PubMed; (c) P. Agarwala, S. Pandey and S. Maiti, Org. Biomol. Chem., 2015, 13, 5570 RSC.
- (a) P. Jeschke, R. Nauen and M. E. Beck, Angew. Chem., Int. Ed., 2013, 52, 9464 CrossRef CAS PubMed; (b) M. Tomizawa and J. E. Casida, J. Agric. Food Chem., 2011, 59, 2883 CrossRef CAS PubMed.
- (a) Saigal, S. Khan, R. Habibur, Shafiullah and Md. Musawwer Khan, RSC Adv., 2019, 9, 14477 RSC; (b) I. Yavari, N. Zahedi, L. Baoosi and S. Skoulika, Mol. Diversity, 2018, 22, 11 CrossRef CAS PubMed.
- L. M. Ramos, M. O. Rodrigues and B. A. D. Neto, Org. Biomol. Chem., 2019, 17, 7260 RSC.
- I. A. Ibarra, A. Islas-Jacome and E. Gonzalez-Zamora, Org. Biomol. Chem., 2018, 16, 1402 RSC.
- G. Mari, M. Verboni, L. D. Crescentini, G. Favi, S. Santeusanio and F. Mantellini, Org. Chem. Front., 2018, 5, 2108 RSC.
- (a) X. Chang, X. Zhang and Z. Chen, Org. Biomol. Chem., 2018, 16, 4279 RSC; (b) S. Yu, R. Hua, X. Fu, G. Liu, D. Zhang, S. Jia, H. Qiu and W. Hu, Org. Lett., 2019, 21, 5737 CrossRef CAS PubMed.
- (a) G. L. Wu and Q. P. Wu, Adv. Synth. Catal., 2018, 360, 1949 CrossRef CAS; (b) H. G. O. Alvim, J. R. Correa, J. A. F. Assumpcao, W. A. da Silva, M. O. Rodrigues, J. L. de Macedo, M. Fioramonte, F. C. Gozzo, C. C. Gatto and B. A. D. Neto, J. Org. Chem., 2018, 83, 4044 CrossRef CAS PubMed.
- S. Chakrabarti, K. Panda, N. C. Misra, H. Ila and H. Junjappa, Synlett, 2005, 1437 CAS.
- S.-J. Yan, Y.-L. Chen, L. Liu, N.-Q. He and J. Lin, Green Chem., 2010, 12, 2043 RSC.
- C. Altug, A. K. Burnett, E. Caner, Y. Dürüst, M. C. Elliott, R. P. J. Glanville, C. Gu and A. D. Westwell, Tetrahedron, 2011, 67, 9522 CrossRef CAS.
- M. Bayat, F. S. Hosseini and S. Nasri, J. Sulfur Chem., 2018, 39, 99 CrossRef CAS.
- S. Nasri, F. S. Hosseini and M. Bayat, Tetrahedron, 2018, 74, 4409 CrossRef CAS.
- F. S. Hosseini, S. Nasri and M. Bayat, J. Sulfur Chem., 2018, 39, 1 CrossRef.
- M. Bayat and F. S. Hosseini, J. Sulfur Chem., 2018, 39, 1 CrossRef.
- Z. T. Huang and X. Shi, Synthesis, 1990, 162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03910a |
| This journal is © The Royal Society of Chemistry 2020 |