Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A dual-sensitized luminescent europium(III) complex as a photoluminescent probe for selectively detecting Fe3+†
Yafeng Zhao‡
a,
Yanhong Xu‡a,
Bing Xuc,
Peipei Cen*b,
Weiming Song*a,
Lijuan Duana and
Xiangyu Liu *ad
*ad
aCollege of Agriculture, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China. E-mail: xiangyuliu432@126.com; songwm@nxu.edu.cn
bCollege of Public Health and Management, Ningxia Medical University, Yinchuan 750021, China. E-mail: 13895400691@163.com
cSchool of Chemistry and Chemical Engineering, Xi'an University of Architecture & Technology, Xi'an 710055, China
dState Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, 210023, China
First published on 25th June 2020
Abstract
A new luminescent EuIII complex, namely [Eu2(BTFA)4(OMe)2(dpq)2] (1), in which BTFA = 3-benzoyl-1,1,1-trifluoroacetone and dpq = dipyrido [3,2-d:2′,3′-f] quinoxaline, has been designed and synthesized by employing two different ligands as sensitizers. Crystal structure analysis reveals that complex 1 is composed of dinuclear EuIII units crystallized in the monoclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Notably, 1 exhibits high thermal stability up to 270 °C and excellent water stability. The photoluminescence property of the complex is investigated. Further studies show 1 can recognize Fe3+ ions with high selectivity from mixed metal ions in aqueous solution through the luminescence quenching phenomenon. Furthermore, the recyclability and stability of 1 after sensing experiments are observed to be adequate. By virtue of the superior stability, detection efficiency, applicability and reusability, the as-prepared EuIII complex can be a promising fluorescent material for practical sensing.
space group. Notably, 1 exhibits high thermal stability up to 270 °C and excellent water stability. The photoluminescence property of the complex is investigated. Further studies show 1 can recognize Fe3+ ions with high selectivity from mixed metal ions in aqueous solution through the luminescence quenching phenomenon. Furthermore, the recyclability and stability of 1 after sensing experiments are observed to be adequate. By virtue of the superior stability, detection efficiency, applicability and reusability, the as-prepared EuIII complex can be a promising fluorescent material for practical sensing.
Introduction
As one of the most essential trace elements in the human body, iron ions play essential roles in many biochemical processes including cellular metabolism, oxygen uptake, DNA/RNA synthesis and enzyme catalysis.1–3 The permissible limit of iron in drinking water given by the World Health Organization (WHO) is 0.3 mg mL−1.4 Both a deficiency and excess of Fe3+ from the normal permissible limit will break cellular homeostasis and cause serious biological disorders like microcytic hypochromic anemia and Alzheimer's disease.5–7 Thus it is of great urgency to obtain effective methods for probing the trace of Fe3+ ions. To date, existing techniques have been put forward for Fe3+ detection including liquid chromatography, inductively coupled plasma atomic mass spectrometry (ICP-MS) and atomic absorption spectrophotometry (AAS).8–10 While all the above-mentioned ways offer advantages, none is ideal due to certain features such as lack of portability, being time-consuming, high cost and requiring expertise.Compared to traditional instrumental and chemical methods, chemsensors have received wide attention due to their short response time, excellent sensitivity, simplicity and low cost. Organic–inorganic hybrid LnIII complexes have been attracted as promising luminescent probes for sensing applications due to their unique spectroscopic and chemical properties such as long luminescence lifetime, large Stokes shift, sharp emission bands, as well as good mechanical, thermal, and chemical stability.11–13 However the Laporte-forbidden 4f–4f transition prevents direct excitation of lanthanide luminescence, LnIII ions always require sensitization by suitable organic chromophores. Furthermore, for practical applications, LnIII ion must be incorporated into highly stable coordinated complexes. The efficiency of ligand-to-metal energy transfer, which requires compatibility between the energy levels of the ligand excited states and accepting levels of LnIII ions, is crucial in the design of high performance luminescent molecular devices. The β-diketone ligand is one of the important “antennas”,14 from which the energy can be effectively transferred to LnIII ions for high harvest emissions and has the following advantages. The β-diketone ligand has strong absorption within a large wavelength range for its π–π* transition, consequently, being targeted for its ability to sensitize the luminescence of the LnIII ions. Meanwhile, it has ability to form stable and strong adducts with LnIII ions, which can have practical usage.15,16 Therefore, 3-benzoyl-1,1,1-trifluoroacetone (BTFA) is selected as the main ligand. Besides, the advantages of the mixed ligands synthetic strategy inspired us to introduce other versatile bridging linkers into the reaction system. Herein, dipyrido [3,2-d:2′,3′-f] quinoxaline (dpq) is selected as an auxiliary ligand. On the one hand, it has four potential coordination domains to coordinate with the Ln ions. On the other hand, it is born with conjunctive aromatic rings, which not only increases the rigidity of the structures but also provides a powerful absorbing sensitizer.17–20
Using this strategy, we prepare a novel luminescent EuIII complex [Eu2(BTFA)4(OMe)2(dpq)2] (1) based on the BTFA ligand and dpq coligand (Scheme 1). Single-crystal structure analysis demonstrates that 1 is a bimetallic construction in which the EuIII ion is best described as an eight-coordinated square antiprism geometry (SAPR-8, D4d, 0.626) with moderate distortions from the ideal geometry.21 The complex integrates the advantages of two functional ligands and the photoelectric units of EuIII, exhibiting favorable stability and superior fluorescence properties. Moreover, 1 is indicative of a highly selective luminescence quenching action for Fe3+ cation in aqueous media. The strategy here for preparing stable EuIII complex is very simple and feasible for large-scale synthesis, which would open a new way towards the functional applications in chemical sensor.
Experimental
Materials and physical measurements
All chemicals and solvents were of reagent grade, obtained from commercial sources without further purification. Fourier transform infrared (FT-IR) spectra were recorded in the range 400–4000 cm−1 using KBr pellets on an EQUINOX55 FT/IR spectrophotometer. Elemental analysis (C, H, N) was performed with a PerkinElmer 2400 CHN elemental analyzer. The phase purity of the bulk or polycrystalline samples was confirmed by powder X-ray diffraction (PXRD) measurements executed on a Rigaku RU200 diffractometer at 60 kV, 300 mA, and Cu Kα radiation (λ = 1.5406 Å), with a scan speed of 5° min−1 and a step size of 0.02° in 2θ. Thermal gravimetric analyses (TGA) were performed by using a NETZSCH STA 449F3 instrument at a heating rate of 10 °C min−1 from 30 to 800 °C and under a N2 stream using an empty Al2O3 crucible as the standard. The fluorescence spectra were obtained using a Hitachi F-7000 fluorescence spectrophotometer at room temperature. UV-Vis spectroscopic studies were collected on shimadzu WFH-203B spectrophotometer.Synthesis of [Eu2(BTFA)4(OMe)2(dpq)2] (1)
A mixture of Et3N (0.014 mL, 0.2 mmol) and BTFA (0.0648 g, 0.3 mmol) in methanol (20 mL) was kept stirring for an hour, to which EuCl3·6H2O (0.1 mmol) and dpq (0.0464 g, 0.2 mmol) were introduced. After stirring for 4 h, the resulting mixture was immediately filtrated, and the filtrate was left to stand at room temperature for slow evaporation. Colorless crystals were generated by slow evaporation of the filtrate after several days, obtain a 55.3% yield of 1 (based on EuIII). Elemental analysis: (%) calcd for C70H46Eu2F12N8O10 (1691.07): C, 49.72; H, 2.74; N, 6.63. Found: C, 49.70; H, 2.72; N, 6.70. Main IR (KBr, cm−1): 3424 (w), 1638 (m), 1611 (s), 1576 (s), 1530 (m), 1403 (w), 1321 (m), 1294 (s), 1184 (s), 1141 (s), 944 (w), 767 (s), 701 (m), 580 (m).Crystallographic data collection and refinement
The X-ray experiments were implemented on a Bruker SMART APEX-CCD-based diffractometer (Mo Kα radiation, λ = 0.71073 Å) at low temperature. Using Olex2,22 the structure of 1 is solved with the ShelXT23 structure solution program by using Intrinsic Phasing, and refined with the ShelXL24 refinement package by using Least Squares Minimisation. All the non-hydrogen atoms are refined anisotropically. All the hydrogen atoms of complex 1 are located from difference maps by the program Olex2. Crystallographic data and refinement parameters are listed in Table 1, while selected interatomic distances and angles for complex 1 are given in Table S1.†| Complex | 1 |
| Empirical formula | C70H46Eu2F12N8O10 |
| Formula weight | 1691.07 |
| Temperature | 100(10) K |
| Crystal system | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 10.2450(12) |
| b (Å) | 12.4101(10) |
| c (Å) | 14.5367(13) |
| α (°) | 112.234(8) |
| β (°) | 106.909(10) |
| γ (°) | 94.287(8) |
| V (Å3) | 1600.8(3) |
| Z | 1 |
| D (g cm−3) | 1.754 |
| Mu (mm−1) | 2.045 |
| F(0 0 0) | 836.0 |
| Unique reflections | 7443 |
| Observed reflections | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 478 478 |
| Rint | 0.0635 |
| Final R indices [I > 2σ(I)] | R1 = 0.0714 wR2 = 0.1437 |
| R indices (all data) | R1 = 0.0979 wR2 = 0.1600 |
| Goodness-of-fit on F2 | 1.011 |
Fluorescence measurements
The powder sample (3 mg) of 1 was respectively immersed in 0.01 mol L−1 of various M(NO3)x (M = Co2+, NH4+, Mg2+, Cr3+, Pb2+, Ag+, Na+, Cd2+, Zn2+, Cu2+, Li+, Ca2+, K+, Mn2+, Ni2+ and Fe3+) aqueous solutions (5 mL) for 2 days at room temperature. Then, all the mixtures should be subjected to ultrasonication for 30 min to produce a stable suspension. Subsequently, the fluorescence spectrum of the MOF suspension was recorded. For selective experiments, 3 mg of the powder sample of 1 was submerged into kinds of aqueous solutions containing Fe3+ ions and other metal ions (5 mL, 0.01 mol L−1 of Co2+, NH4+, Mg2+, Cr3+, Pb2+, Ag+, Na+, Cd2+, Zn2+, Cu2+, Li+, Ca2+, K+, Mn2+ and Ni2+) for 2 days. Also, we used an identical method to treat the mixed solutions before the fluorescence test. For the detection of Fe3+ ions, 3 mg of the powder sample of 1 was respectively submerged into aqueous solutions (5 mL) with various concentrations of Fe3+ ions for 2 days; then, its fluorescence intensity was measured after obtaining a uniform MOF suspension.Recyclable luminescence experiments
The recyclability of 1 towards sensing Fe3+ was also investigated. After the first quenching experiment, the powder of 1 was centrifuged and washed several times with deionized water. The recovered solid was collected and then used in the successive quenching experiments.Results and discussion
Crystal structure of 1
Single-crystal X-ray structural analysis reveals that complex 1 is centrosymmetric and crystallizes in the monoclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Table 1). As shown in Fig. 1a, 1 features dinuclear units, consisting of two EuIII ions, four BTFA ligands, two dpq motif and two methanol molecules. The coordination environments of two EuIII ions are equivalent to each other. Each EuIII ion has a [EuN2O6] coordination sphere with two nitrogen atoms (N1 and N2) from one dpq motif ligand, four oxygen atoms (O1, O2, O3 and O4) from two BTFA ligands moiety, and two oxygen atoms (O5 and O5A) from two methanol molecules. Two Eu centers are dual-bridged by oxygen atoms from two methanol molecules, forming a dinuclear unit with Eu1⋯Eu1A distances of 3.770 Å and Eu1–O2–Eu1A angles of 109.19. In the crystal packing (Fig. S1†), the dinuclear Eu2 unit is further strengthened through π⋯π (3.5301(3) Å) interactions. The shortest intermolecular Eu⋯Eu distances are 9.838 Å, suggesting spatial isolation of the dinuclear motifs.25 To ascertain the precise geometries around the metallic centers and the degree of the distortion from the ideal model for Eu complex, the geometric spheres of EuIII cations are calculated by using the SHAPE 2.1 program26 based on the structural parameters; the typical geometric polyhedrons are depicted in Fig. 1b. In principle, the data extracted from the package tend to zero, responding to the optimal geometry, whereas a greater value presents a major deviation from the optimal polyhedron.27 As shown in Table S2,† the calculation indicates that EuIII centers are best described as eight-coordinated square antiprism geometry (CshMs parameters: 0.626).
space group (Table 1). As shown in Fig. 1a, 1 features dinuclear units, consisting of two EuIII ions, four BTFA ligands, two dpq motif and two methanol molecules. The coordination environments of two EuIII ions are equivalent to each other. Each EuIII ion has a [EuN2O6] coordination sphere with two nitrogen atoms (N1 and N2) from one dpq motif ligand, four oxygen atoms (O1, O2, O3 and O4) from two BTFA ligands moiety, and two oxygen atoms (O5 and O5A) from two methanol molecules. Two Eu centers are dual-bridged by oxygen atoms from two methanol molecules, forming a dinuclear unit with Eu1⋯Eu1A distances of 3.770 Å and Eu1–O2–Eu1A angles of 109.19. In the crystal packing (Fig. S1†), the dinuclear Eu2 unit is further strengthened through π⋯π (3.5301(3) Å) interactions. The shortest intermolecular Eu⋯Eu distances are 9.838 Å, suggesting spatial isolation of the dinuclear motifs.25 To ascertain the precise geometries around the metallic centers and the degree of the distortion from the ideal model for Eu complex, the geometric spheres of EuIII cations are calculated by using the SHAPE 2.1 program26 based on the structural parameters; the typical geometric polyhedrons are depicted in Fig. 1b. In principle, the data extracted from the package tend to zero, responding to the optimal geometry, whereas a greater value presents a major deviation from the optimal polyhedron.27 As shown in Table S2,† the calculation indicates that EuIII centers are best described as eight-coordinated square antiprism geometry (CshMs parameters: 0.626).
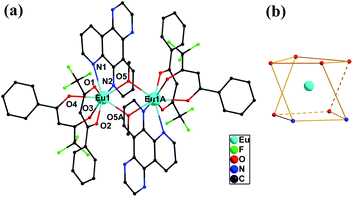 | ||
| Fig. 1 Molecular structure of 1 (a) and coordination geometry around the Eu ion for 1. (b) All hydrogen atoms are omitted for clarity. | ||
PXRD and thermal analysis
The phase purity of the synthesized complex 1 is demonstrated by PXRD. As displayed in Fig. S2,† all experimental peaks are in agreement with those in the simulated profile derived from single-crystal diffraction data. The result demonstrates the high phase purity of 1. Aiming to investigate the thermal stabilities of 1, the thermal gravimetric (TG) analyses of 1 is performed (Fig. S3†). According to the TGA curve, 1 almost do not lose any obvious weight until 270 °C, suggesting no guest molecules in the lattice, as confirmed by the single crystal X-ray diffraction analysis. Subsequently, the framework begins to decompose, implying a certain thermostability existing in the complex.Water stability
Most MOFs have shown limitations in practical applications due to low stability, especially when exposed to water. To further explore the water stability of MOFs is significant to practical applications. The stability experiments of 1 are executed via immersing crystals of 1 in water for three days at room temperature. The obtained PXRD patterns remain intact, indicating that no phase transition or framework collapse occurred during the treatments (Fig. S4†). After immersing in water for 3 days, no signals observed in the NMR spectrum showed that there is a poor solubility in water. Moreover, CHN analysis (C, 49.74%; H, 2.75%; N, 6.68%) after prolonged exposure to water has been carried out, further confirming that the MeO-bridges could not be exchangeable with the OH bridges. As shown in Fig. S5,† NMR experiment in manual operation reveals that the MeO groups still exist in the complex, confirming that the complex can be dissolved in MeOH and is thus soluble. The results obtained reveal that 1 possesses satisfactory water stability.Fluorescence properties
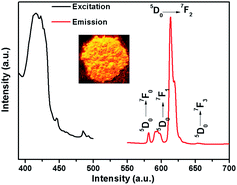
Because of partial filling of the 4f shells of Ln3+, Ln-complexes have shown good luminescence properties including sharp feature emissions, large Stokes shifts, relatively long luminescence lifetimes, and bright luminescent colors, especially for the Eu-complexes.28 In order to determine the luminescent properties of the system, the solid-state excitation and emission spectra of 1 was studied. The incorporation of the two ligands shifts the excitation window towards a favorable longer wavelength region. As shown in Fig. 2, the solid fluorescence spectra of 1 showed characteristic emission peaks of EuIII under excitation at 420 nm. The peaks at 581, 593, 613 and 653 nm could be assigned to 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, and 5D0 → 7F3 transitions, respectively. In dual-sensitized [Eu2(BTFA)4(OMe)2(dpq)2] (1), the energy transfer from the dual antenna to the EuIII is more facile because of efficient intersystem crossing (ISC) and optimal ET from the ligand triplet states to the emissive excited state (5D0) of EuIII. This results in a characteristic brilliant red luminescence due to 5D0 → 7FJ (J = 0–3) transitions dominated by electric dipole (ED)-induced hypersensitive 5D0 → 7F2 (613 nm) transitions.29,30 The 5D0 → 7F1 transition for Eu-MOF at 593 nm was attributed to the magnetic dipole transition, and the intensity was independent of the local environment of the Eu3+. The most powerful emission at 613 nm was attributed to the electric dipole induced 5D0 → 7F2 transition, which was hypersensitive to the coordination environment of Eu3+.31 The luminescence intensity at 613 nm (5D0 → 7F2 transitions) is stronger than at 593 nm (5D0 → 7F1 transitions), which indicates that Eu3+ ions take up non-inversion center. For an effective ET process in EuIII, the 3T energy level of the ligands should be higher than the 5D0 level of EuIII (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1) and ideally the hydration number, q, should be zero. An optimal L → LnIII ET process depends on ligands that can efficiently transfer energy to the triplet states through ISC. Moreover, the energy gap (DE) between the ligand 3T states (e.g., 3n–π*, 3π–π*) and the emissive excited states of EuIII (5D0) ideally should be ≥2500 cm−1.32 The singlet (S1) and triplet (T1) energy levels of the BTFA (31
200 cm−1) and ideally the hydration number, q, should be zero. An optimal L → LnIII ET process depends on ligands that can efficiently transfer energy to the triplet states through ISC. Moreover, the energy gap (DE) between the ligand 3T states (e.g., 3n–π*, 3π–π*) and the emissive excited states of EuIII (5D0) ideally should be ≥2500 cm−1.32 The singlet (S1) and triplet (T1) energy levels of the BTFA (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 996 and 28
996 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488 cm−1) and dpq (29
488 cm−1) and dpq (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 23
400 and 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) are provided to semi-quantitively evaluate the ET processes of the EuIIII complex.20 The schematic ET processes for the complexes are shown in Fig. 3. On account of the superior water stability, thermal stability and luminescence properties, 1 can be identified as a feasible complex that exhibits potential application performance in fluorescence sensing.
500 cm−1) are provided to semi-quantitively evaluate the ET processes of the EuIIII complex.20 The schematic ET processes for the complexes are shown in Fig. 3. On account of the superior water stability, thermal stability and luminescence properties, 1 can be identified as a feasible complex that exhibits potential application performance in fluorescence sensing.
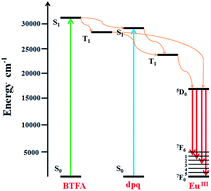 | ||
| Fig. 3 Schematic energy-level diagram showing the intramolecular ET between the ligands and EuIII; S1 = first excited singlet state; T1 = first excited triplet state. | ||
Detection of metal ions
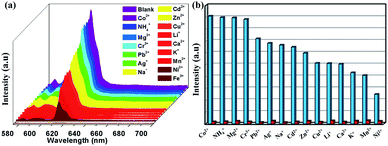
Considering the desired excellent water stability and fluorescence performance of 1, we investigate the sensing capability of this Eu-complex towards various metal ions in water. The powder samples of 1 are immersed in 0.01 mol L−1 of various M(NO3)x aqueous solutions, including Co2+, NH4+, Mg2+, Cr3+, Pb2+, Ag+, Na+, Cd2+, Zn2+, Cu2+, Li+, Ca2+, K+, Mn2+, Ni2+, Fe3+, respectively. The mixtures are sonicated before fluorescence measurements. Compared with the original spectra, the metal ions cause different changes in the fluorescence intensity of 1. As depicted in Fig. 4a, the fluorescence intensity of 1 remains steady for Co2+, NH4+, Mg2+ and Cr3+ ions; Pb2+, Ag+, Na+, Cd2+, Zn2+, Cu2+, Li+, Ca2+, K+, Mn2+ and Ni2+ ions produce moderate quenching; whereas Fe3+ ions represent manifest quenching with an efficiency of as much as 97% in contrast to that of itself.Furthermore, competitive experiments were also performed to investigate the effect of mixed cations on emission intensity. Therefore, the sample of 1 was submerged into kinds of aqueous solutions containing Fe3+ ions and other metal ions (Co2+, NH4+, Mg2+, Cr3+, Pb2+, Ag+, Na+, Cd2+, Zn2+, Cu2+, Li+, Ca2+, K+, Mn2+ and Ni2+), respectively. The quenching phenomenon of Fe3+ ions on 1 is not affected by other competitive anions, revealing that 1 can be considered as a potential luminescent probe to detect Fe3+ ions among the above-mentioned cations (Fig. 4b).
To further investigate the luminescence quenching of Fe3+ ions in 1, titration experiments were executed (Fig. 5). Specifically, with the increase of Fe3+ ions concentration, the luminescence intensity gradually decreases. Quantitatively, quenching efficiency is calculated using the Stern–Volmer (S–V) equation: (I0/I) = 1 + Ksv[Q],33,34 where I0 and I are the luminescence intensity before and after adding metal ions, respectively, [Q] refers to the molar concentration of metal ions and Ksv is the quenching constant, which is an important indicator of the sensing ability of a fluorescent sensor. The Ksv values are found to be 1.1 × 104 M−1 for 1, signifying that Fe3+ has high-efficiency quenching effect on the luminescence emission of 1. According to the ratio of 3δ/Ksv (δ: standard error),35,36 the detection limit of 3.5 × 10−5 M is superior to that of most known probes with good Fe3+ sensing properties (Table S3†).
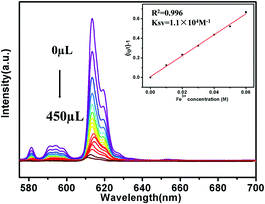 | ||
| Fig. 5 Luminescence spectra of 1 in aqueous solutions with Fe3+ at different concentrations (The inset is Stern–Volmer plot). | ||
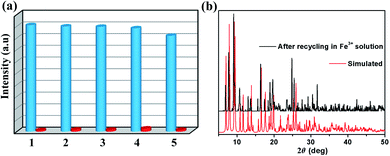
Repeatability is an important parameter to measure the practicability of a luminescence probe.37 Therefore, the recyclability experiment to detect Fe3+ was measured as follows: samples of 1 were immersed in water for several minutes to form 1-Fe3+, and then 1-Fe3+ were washed several times and centrifuged. Subsequently, the reclaimed samples were performed by luminescence spectra. As shown in Fig. 6a, 1 can be simply and quickly regenerated and reused at five times. Meanwhile, the PXRD patterns proved that the crystallinity and structural integrity of 1 exhibited no change after the detection test (Fig. 6b), suggesting excellent recyclability.
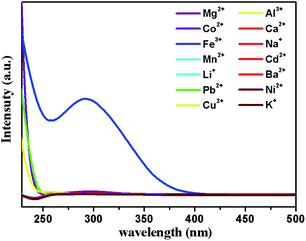
The mechanism of luminescence quenching includes several mechanisms possibilities which have been revealed by previous studies.38–41 To understand the underlying sensing mechanism, further analyses were carried out. First, the PXRD patterns of 1 before and after the fluorescence experiments were consistent, excluding the possibility of structural damage collapse of the framework (Fig. 6b). Second, the absorption spectrum of Fe3+ shows intense absorption bands in the ranges of 230–260 and 260–390 nm, and the excitation wavelength of 1 is 366 nm, which reveals that Fe3+ can significantly absorb the energy of the excitation wavelength, resulting in the luminescence quenching of 1 (Fig. 7). Third, the intensity ratio (I0/I) and Fe3+ concentration can be well fitted into the (I0/I) = 1 + Ksv[Q] equation, suggesting that luminescence quenching can be put down to the dynamic process (the collision interactions). In conclusion, not only the overlap of absorption spectrum between Fe3+ and 1 but also the collision interactions collectively result in the luminescence quenching.
Conclusions
In summary, a novel Eu-complex assembled from mixed ligands of BTFA and dpq have been successfully isolated and structurally characterized. The stability and luminescence properties of the synthetic Eu-complex are investigated. The complex presents a potential for highly sensitive and selective detection of Fe3+ in water. The quenching efficiency of Fe3+ is 97% which is the highest one compared to that of other metal cations. Moreover, verified by the quenching testes and titration experiments, complex 1 possesses satisfactory sensitivity and selectivity indicated by the large quenching constant of 1.1 × 104 M−1 and prominent detection limit of 3.5 × 10−5 M, respectively. Most importantly, the solid products of Eu-complex can keep its original network and be recycled at least five times in the detecting experiments, which can be used as chemical sensors for practical applications. Additionally, the possible fluorescence quenching mechanism has been discussed. The successful assembly of this case may provide a good guideline to construct new LnIII-complexes functional materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (21863009, 21463020 and 41561106), the Natural Science Foundation of Ningxia Province (2020AAC02005 and 2020AAC03118), the research project of Ningxia Medical University (XT2019011), the National Undergraduate Innovative and Entrepreneurial Training Program (G2020107490004), the discipline project of Ningxia (NXYLXK2017A04), and the Major Innovation Projects for Building First-class Universities in China’s Western Region (No. ZKZD2017003).References
- S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponic and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195–7227 RSC.
- W. Yan, C. Zhang, S. Chen, L. Han and H. Zheng, ACS Appl. Mater. Interfaces, 2017, 9, 1629–1634 CrossRef CAS PubMed.
- S. R. Lynch, Nutr. Rev., 1997, 55, 102–110 CrossRef CAS PubMed.
- W. H. Organization, Guidelines for drinking-water quality: recommendations, World Health Organization, 2004 Search PubMed.
- N. C. Andrews, N. Engl. J. Med., 1999, 341, 1986–1995 CrossRef CAS PubMed.
- L.-Z. Liu, Y. Wang, R.-Y. Lin, Z.-Z. Yao, Q.-J. Lin, L.-H. Wang, Z.-J. Zhang and S.-C. Xiang, Dalton Trans., 2018, 47, 16190–16196 RSC.
- P. Cheng, H.-J. Chen, P. Fan, X.-X. Tu, H. Min, X.-Y. Yu, X.-F. Li, J.-L. Zeng and S.-W. Zhang, Chem.–Asian J., 2019, 14, 3611 CrossRef.
- F. Séby, S. Charles, M. Gagean, H. Garraud and O. Donard, J. Anal. At. Spectrom., 2003, 18, 1386–1390 RSC.
- D. Yuan, D. Fu, R. Wang and J. Yuan, Spectrochim. Acta, Part A, 2008, 71, 276–279 CrossRef PubMed.
- Z. Sun and P. Liang, Microchim. Acta, 2008, 162, 121–125 CrossRef CAS.
- J. Zhao, S. Wang, S. Lu, J. Sun and X. Yang, Nanoscale, 2018, 10, 7163–7170 RSC.
- Y. Cui, J. Zhang, H. He and G. Qian, Chem. Soc. Rev., 2018, 47, 5740–5785 RSC.
- L.-J. Duan, C.-C. Zhang, P.-P. Cen, X.-Y. Jin, C. Liang, J.-H. Yang and X.-Y. Liu, CrystEngComm, 2020, 22, 1695–1704 RSC.
- (a) J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1990, 29, 1304–1319 CrossRef; (b) X.-H. Ma, Y.-R. Liu, W.-M. Song, Z. Wang, X.-Y. Liu, G. Xie, S.-P. Chen and S.-L. Gao, Dalton Trans., 2018, 47, 12092–12104 RSC; (c) G.-F. de Sa, O. L. Malta, C. de. Mello Donega, A. M. Simas, R. L. Longo, P. A. Santa-Cruz and E. F. da Silva Jr, Coord. Chem. Rev., 2000, 196, 165–195 CrossRef CAS.
- (a) L.-N. Sun, J.-B. Yu, G.-L. Zheng, H.-J. Zhang, Q.-G. Meng, C.-Y. Peng, L.-S. Fu and F.-Y. Liu, Eur. J. Inorg. Chem., 2006, 19, 3962–3973 CrossRef; (b) J. Yu, L. Zhou, H. Zhang, Y. Zheng, H. Li, R. Deng, Z. Peng and Z. Li, Inorg. Chem., 2005, 44, 1611–1618 CrossRef CAS PubMed; (c) A. Fratini, G. Richards, E. Larder and S. Swavey, Inorg. Chem., 2008, 47, 1030–1036 CrossRef CAS PubMed; (d) Y.-W. Wu, J. Xi, J.-H. Yang, W.-M. Song, S. Luo, Z. Wang and X.-Y. Liu, CrystEngComm, 2020, 22, 2297–2303 RSC; (e) S. V. Eliseeva, M. Ryazanov, F. Gumy, S. I. Troyanov, L. S. Lepnev, J.-C. G. Buünzli and N. P. Kuzmina, Eur. J. Inorg. Chem., 2006, 23, 4809–4820 CrossRef.
- (a) S. Biju, D. A. Raj, M. L. P. Reddy and B. M. Kariuki, Inorg. Chem., 2006, 45, 10651–10660 CrossRef CAS PubMed; (b) L. Yang, Z. Gong, D. Nie, B. Lou, Z. Bian, M. Guan, C. Huang, H. J. Lee and W. P. Baik, New J. Chem., 2006, 30, 791–796 RSC; (c) X.-Y. Liu, H.-X. Liu, P.-P. Cen, X.-Y. Chen, H.-L. Zhou, W.-M. Song and Q.-L. Hu, Inorg. Chim. Acta, 2016, 447, 12–17 CrossRef CAS; (d) R. Pavithran, N. S. Saleesh Kumar, S. Biju, M. L. P. Reddy, S. Alves Jr and R. O. Freire, Inorg. Chem., 2006, 45, 2184–2192 CrossRef CAS PubMed; (e) K. Binnemans, Rare-earth beta-diketonates, Handbook on the Physics and Chemistry of Rare Earths, 2005, vol. 35, pp. 107–272 Search PubMed.
- (a) G. Bobba, Y. BretonniHre, J. C. Frias and D. Parker, Org. Biomol. Chem., 2003, 1, 1870–1872 RSC; (b) X.-Y. Liu, L. Sun, H.-L. Zhou, P.-P. Cen, X.-Y. Jin, G. Xie, S.-P. Chen and Q.-L. Hu, Inorg. Chem., 2015, 54, 8884–8886 CrossRef CAS PubMed; (c) G. Yi, H.-H. Cui, C. Zhang, W. Zhao, L. Chen, Y.-Q. Zhang, X.-T. Chen, Y. Song and A. Yuan, Dalton Trans., 2020, 49, 2063–2067 RSC.
- R. A. Poole, G. Bobba, M. J. Cann, J. C. Frias, D. Parker and R. D. Peacock, Org. Biomol. Chem., 2005, 3, 1013–1024 RSC.
- X.-Y. Liu, X.-F. Ma, W.-Z. Yuan, P.-P. Cen, Y.-Q. Zhang, J. Ferrando-Soria, S.-P. Chen and E. Pardo, Inorg. Chem., 2018, 57, 14843–14851 CrossRef CAS PubMed.
- S. Dasari, S. Singh, S. Sivakumar and A. K. Patra, Chem.–Eur. J., 2016, 22, 17387–17396 CrossRef CAS PubMed.
- X.-Y. Liu, F.-F. Li, X.-H. Ma, P.-P. Cen, S.-C. Luo, Q. Shi, S.-R. Ma, Y.-W. Wu, C.-C. Zhang, Z. Xu, W.-M. Song, G. Xie and S.-P. Chen, Dalton Trans., 2017, 46, 1207–1217 RSC.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., 2015, 71, 3–8 CrossRef PubMed.
- D. A. Pantazis, X.-Y. Chen, C. R. Landis and F. J. Neese, J. Chem. Theory Comput., 2008, 4, 908–919 CrossRef CAS PubMed.
- Y.-W. Wu, D.-N. Tian, J. Ferrando-Soria, J. Cano, L. Yin, Z.-W. Ouyang, Z.-X. Wang, S.-C. Luo, X.-Y. Liu and E. Pardo, Inorg. Chem. Front., 2019, 6, 848–856 RSC.
- M. Llunell, D. Casanova, J. Cirera, P. Alemany and S. Alvarez, SHAPE, v2.1, Universitat de Barcelona Barcelona, Spain, 2013 Search PubMed.
- X.-H. Ma, C. Cai, W.-J. Sun, W.-M. Song, Y.-L. Ma, X.-Y. Liu, G. Xie, S.-P. Chen and S.-L. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 9233–9238 CrossRef CAS PubMed.
- (a) A. Thibon and V. C. Pierre, Anal. Bioanal. Chem., 2009, 394, 107–120 CrossRef CAS PubMed; (b) M. Delbianco, V. Sadovnikova, E. Bourrier, G. Mathis, L. Lamarque, J. M. Zwier and D. Parker, Angew. Chem., Int. Ed., 2014, 53, 10718–10722 CrossRef CAS PubMed.
- (a) V. Divya, M. L. P. Reddy and R. Pavithran, Dalton Trans., 2013, 42, 15249–15262 RSC; (b) V. Divya, V. Sankar, K. G. Raghu and M. L. P. Reddy, Dalton Trans., 2013, 42, 12317–12323 RSC; (c) D. B. Ambili Raj, S. Biju and M. L. P. Reddy, Inorg. Chem., 2008, 47, 8091–8100 CrossRef CAS PubMed.
- (a) J. Yu, L. Zhou, H. Zhang, Y. Zheng, H. Li, R. Deng, Z. Peng and Z. Li, Inorg. Chem., 2005, 44, 1611–1618 CrossRef CAS PubMed; (b) J. Li, H. Li, P. Yan, P. Chen, G. Hou and G. Li, Inorg. Chem., 2012, 51, 5050–5057 CrossRef CAS PubMed; (c) J. Sun, B. Song, Z. Ye and J. Yuan, Inorg. Chem., 2015, 54, 11660–11668 CrossRef CAS PubMed; (d) J. Shi, Y. Hou, W. Chu, X. Shi, H. Gu, B. Wang and Z. Sun, Inorg. Chem., 2013, 52, 5013–5022 CrossRef CAS PubMed.
- X. Wang, T. Qin, S.-S. Bao, Y.-C. Zhang, L.-M. Zheng and D.-R. Zhu, J. Mater. Chem. A, 2016, 42, 16484–16489 RSC.
- G. Bobba, Y. Bretonnière, J. C. Frias and D. Parker, Org. Biomol. Chem., 2003, 1, 1870–1872 RSC.
- D. Zhao, X.-H. Liu, Y. Zhao, P. Wang, Y. Liu, M. Azam, S. I. AlResayes, Y. Lu and W.-Y. Sun, J. Mater. Chem. A., 2017, 5, 15797–15807 RSC.
- Z.-Q. Liu, Y. Zhao, X.-D. Zhang, Y.-S. Kang, Q.-Y. Lu, M. Azam, S. I. Al-Resayes and W.-Y. Sun, Dalton Trans., 2017, 46, 13943–13951 RSC.
- R.-C. Gao, F.-S. Guo, N.-N. Bai, Y.-L. Wu, F. Yang, J.-Y. Liang, Z.-J. Li and Y.-Y. Wang, Inorg. Chem., 2016, 55, 11323–11330 CrossRef CAS PubMed.
- (a) H. Xu, C.-S. Cao and B. Zhao, Chem. Commun., 2015, 51, 10280–10283 RSC; (b) K.-G. Qu, J.-S. Wang, J. Ren and X.-G. Qu, Chem.–Eur. J., 2013, 19, 7243–7249 CrossRef CAS PubMed.
- H. Xu, H.-C. Hu, C.-S. Cao and B. Zhao, Inorg. Chem., 2015, 54, 4585–4587 CrossRef CAS.
- P. Mahata, S. K. Mondal, D. K. Singha and P. Majee, Dalton Trans., 2017, 46, 301–328 RSC.
- J. C. Sanchez, A. G. DiPasquale, A. L. Rheingold and W. C. Trogler, Chem. Mater., 2007, 19, 6459–6470 CrossRef CAS.
- M.-M. Guo, S.-L. Liu, H.-D. Guo, Y.-Y. Sun, X.-N. Guo and R.-P. Deng, Dalton Trans., 2017, 46, 14988–14994 RSC.
- G.-X. Wen, Y.-P. Wu, W.-W. Dong, J. Zhao, D.-S. Li and J. Zhang, Inorg. Chem., 2016, 55, 10114–10117 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S4, Tables S1 and S2, crystal structure of 1 in CIF format. CCDC 1982394 (1). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03821k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |