Open Access Article
Open Access Article1,3-Dipolar cycloaddition of isatin N,N′-cyclic azomethine imines with α,β-unsaturated aldehydes catalyzed by DBU in water†
Zhan-Yong Wang a,
Ting Yangb,
Rongxiang Chena,
Xueji Maa,
Huan Liua and
Kai-Kai Wang
a,
Ting Yangb,
Rongxiang Chena,
Xueji Maa,
Huan Liua and
Kai-Kai Wang *a
*a
aCollege of Chemistry and Chemical Engineering, Xinxiang University, Xinxiang 453003, P. R. China. E-mail: zhanyongw@126.com
bMedical College, Xinxiang University, Xinxiang 453003, P. R. China
First published on 25th June 2020
Abstract
A simple and green procedure was established by [3 + 3] cycloaddition reaction of isatin derived cyclic imine 1,3-dipoles with α,β-unsaturated aldehydes, giving the desired spiro heterocyclic oxindoles with aza-quaternary centers in good yields and diastereoselectivities. It should be noted that water can be employed as a suitable solvent for the improvement of diastereoselectivity.
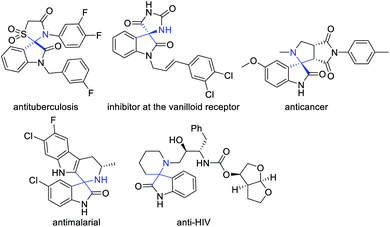
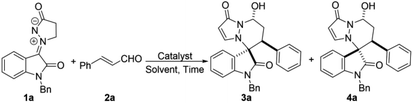
Aza-quaternary centers are pivotal structural units, which exist in a variety of bioactive molecules and natural products.1 In particular, spirooxindoles at the C3 position bearing a quaternarized N-heterocycle have attracted considerable attention because of their privileged structural units with attractive bioactivities,2 for example, antimalarial,3 anti-HIV,4 antitumor,5 anticancer,6 inhibitor at the vanilloid receptor,7 antituberculosis,8 etc. (Fig. 1). Due to their remarkable biological importance, great efforts have been made to access spiro heterocyclic oxindoles with aza-quaternary centers. These methods include cycloaddition of imines,9 1,3-dipolar cycloaddition,10 multicomponent cyclization reaction11 and metal-catalyzed cycloaddition.12 Among them, 1,3-dipolar cycloaddition is one of the most powerful tools for the construction of diverse spirooxindole fused N-heterocyclic scaffolds. Of these, N,N′-cyclic azomethine imines were widely studied for constructing various types of N-heterocyclic skeletons with spirooxindole as a stable and easily accessed 1,3-dipoles. In 2013, Wang's group reported their pioneering studies on Et3N-catalyzed diastereoselective [3 + 3] annulation of N,N′-cyclic azomethine imines with isothiocyanatooxindoles to build 3,3′-triazinylspirooxindoles. In 2017, Wang et al. developed a new isatin-derived N,N′-cyclic azomethine imine 1,3-dipoles, and successfully applied in the [3 + 2] cycloaddition reaction for the construction of spirooxindoles bearing N-heterocycles (Scheme 1a).10c Very recently, Jin's group reported a Cs2CO3-catalyzed [3 + 4] annulation of isatin-derived 1,3-dipole with aza-oQMs (Scheme 1b).10d Furthermore, Moghaddam and coworkers developed an efficient method for the synthesis of pyridazine-fused spirooxindole scaffolds by 1,3-dipolar [3 + 3] cycloadditions (Scheme 1c).10e On the other hand, α,β-unsaturated aldehydes and their analogs as readily available substrates are also important building blocks in the synthesis of heterocyclic compounds which are widely applied in N-heterocyclic carbenes catalysis and other organocatalysis.13 Inspired by these great works and our continuing efforts towards green synthesis of spirooxindole skeletons. We envisioned a quick and efficient way of [3 + 3] cyclization reaction of α,β-unsaturated aldehydes with the new isatin N,N′-cyclic azomethine imine 1,3-dipoles via oxindole C3 umpolung. We wish to disclose herein that a green and practical access to synthesize pharmacologically interesting spirooxindole derivatives by involving isatin N,N′-cyclic azomethine imine 1,3-dipole as nucleophiles and various α,β-unsaturated aldehydes in water using DBU as organocatalyst. Our initial examinations were carried out using isatin derivated cyclic imine 1,3-dipole 1a (0.1 mmol) and α,β-unsaturated aldehyde 2a (0.12 mmol) as the model substrates, the results of condition optimization are shown in Table 1. At the outset, without catalyst condition and with catalysts were investigated at room temperature in dichloromethane (DCM) (Table 1, entries 1–10). The results show that catalyst had a significant effect on the yields. However, it has negligible effect on the diastereoselectivities. Organic bases, such as DABCO, DMAP, Et3N, DIPEA (N,N-diisopropylethylamine), DBU, were compared and found that DBU could improve the yield obviously with 75% yield (Table 1, entry 6 vs. entries 1–5). While the inorganic bases were used, such as Cs2CO3, KOBut, failed to improve the reaction yields (Table 1, entries 7, 8). Other catalysts were also tested, but no better results were found (Table 1, entries 9, 10). When the catalyst loading was reduced to 10 mol%, the yield decreased with increasing the reaction time (Table 1, entry 11). Subsequently, a series of solvents were further investigated (Table 1, entries 12–17). Solvents such as THF and CHCl3 slightly improved the yields but no positive results were obtained for the diastereoselectivities (Table 1, entries 14, 15). A higher reaction temperature gave no better result (Table 1, entry 16). When DBU or Na2CO3 was used in ethanol also gave no satisfactory results (Table 1, entries 17, 18). With the hope of further improving diastereoselectivity, water was chosen as the solvent, the diastereoselectivity was significantly improved but the yield was decreased to 61% (Table 1, entry 19). Finally the optimum process conditions were carried out as follows: 1a/2a/DBU = 1.0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 molar ratio, in water at room temperature (Table 1, entry 19).
1.0 molar ratio, in water at room temperature (Table 1, entry 19).
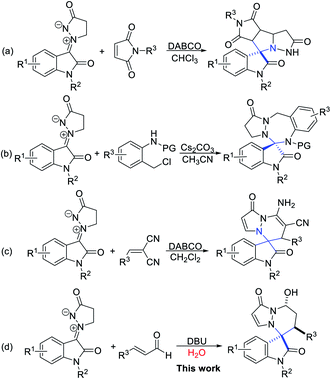 | ||
| Scheme 1 Isatin-derived N,N′-cyclic azomethine imine 1,3-dipoles participated in the construction of N-heterocyclic skeletons with C3-spirooxindole. | ||
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) | 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ac 4ac |
|---|---|---|---|---|---|
| a Otherwise specified, all reactions were carried out using 1a (0.1 mmol), 2a (0.12 mmol), catalyst (0.1 mmol), solvent (1 ml).b Isolated yields of diastereoisomeric mixture.c Determined by 1H NMR.d Catalyst (0.01 mmol).e Performed at reflux. | |||||
| 1 | — | DCM | 24 | — | — |
| 2 | DABCO | DCM | 24 | Trace | — |
| 3 | DMAP | DCM | 24 | Trace | — |
| 4 | NEt3 | DCM | 3 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.4 3.4 |
| 5 | DIPEA | DCM | 24 | Trace | — |
| 6 | DBU | DCM | 0.1 | 75 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Cs2CO3 | DCM | 1 | 34 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4 |
| 8 | KOBut | DCM | 0.1 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4 |
| 9 | PPh3 | DCM | 24 | Trace | — |
| 10 | Pyrrolidine | DCM | 24 | 59 | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11d | DBU | DCM | 24 | 61 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | DBU | Toluene | 0.2 | 75 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | DBU | CH3CN | 0.1 | 20 | 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | DBU | THF | 0.1 | 85 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | DBU | CHCl3 | 0.1 | 86 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16e | DBU | CHCl3 | 0.1 | 86 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | DBU | EtOH | 24 | 62 | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 18 | Na2CO3 | EtOH | 24 | 46 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
| 19 | DBU | H2O | 24 | 61 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
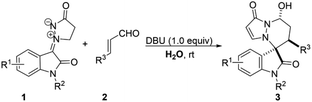
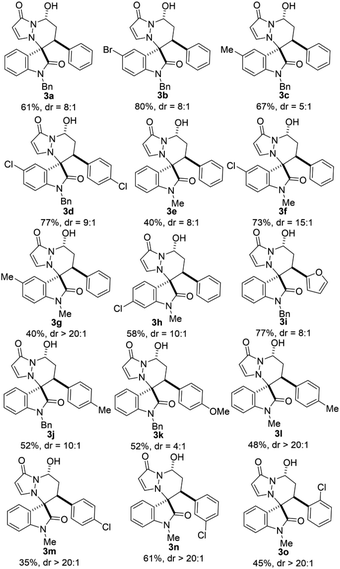
Under the optimal reaction conditions, the generality of this reaction was next investigated. As can be seen from Table 2, all reactions proceeded well to give the desired products 3 in moderate to good yields with good to high diastereoselectivities under identical conditions. The scope of isatin derivated cyclic imine 1,3-dipoles 1 were examined under the optimal reaction conditions, both N-Bn 1a and N-Me 1e substituted isatin derivated cyclic imine 1,3-dipoles could proceed smoothly and gave the desired products with moderate results (3a and 3e). The 5-substituted electron-withdrawing groups on the aromatic ring of isatin derivated cyclic imine 1,3-dipoles 1 gave better yields compared with the electron-donating counterparts (3b vs. 3c, and 3f vs. 3g). Subsequently, the electronic characteristics of α,β-unsaturated aldehydes 2 were studied, while both electron-donating (3j, 3k and 3l) and mildly electron-withdrawing groups (3d, 3m) on phenyl ring had only a slight impact on yields and diastereoselectivities. Reaction involving heteroaryl aldehyde such as 2-furanacrolein 2i also gave product 3i in 77% yield with high diastereoselectivity (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Sterically hindered substituent on α,β-unsaturated aldehyde 2o had little influence on the yield and diastereoselectivity.
1 dr). Sterically hindered substituent on α,β-unsaturated aldehyde 2o had little influence on the yield and diastereoselectivity.
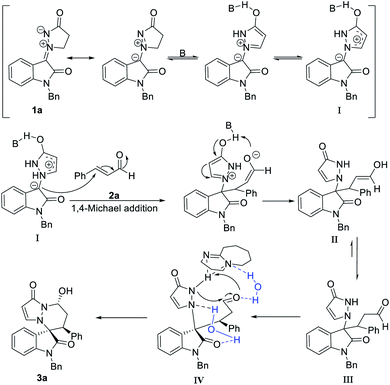
Based on our results and previous studies, a plausible catalytic cycle is proposed in Scheme 2. 1a was promoted by a base to form more stable intermediate I. After this, intermediate I underwent 1,4-Michael addition with α,β-unsaturated aldehyde 2a to form II. Next, keto–enol tautomerism occurred to form intermediate III. To avoid the steric hindrance, the intermediate III attack preferentially to the Re-face of aldehyde, leading to the formation of the major product 3a.
In conclusion, we have disclosed a novel metal-free DBU-catalyzed [3 + 3] cycloaddition reaction via C3 umpolung strategy of oxindole. Varieties of isatin derivated cyclic imine 1,3-dipoles and α,β-unsaturated aldehydes were compatible with this protocol under mild conditions, and afforded spiro heterocyclic oxindoles with aza-quaternary center in good yields with good to high diastereoselectivities. Notably, water as a green solvent had positive effect on the diastereoselectivities.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21801214, 21702176), Foundation of University Student Innovation Program (201911071001, S201911071007).Notes and references
- (a) J. Clayden, M. Donnard, J. Lefranc and D. J. Tetlow, Quaternary centres bearing nitrogen (α-tertiary amines) as products of molecular rearrangements, Chem. Commun., 2011, 47, 4624 RSC; (b) A. K. Mailyan, J. A. Eickhoff, A. S. Minakova, Z. Gu, P. Lu and A. Zakarian, Cutting-Edge and Time-Honored Strategies for Stereoselective Construction of C-N Bonds in Total Synthesis, Chem. Rev., 2016, 116, 4441 CrossRef CAS PubMed; (c) A. Hager, N. Vrielink, D. Hager, J. Lefranc and D. Trauner, Synthetic approaches towards alkaloids bearing α-tertiary amines, Nat. Prod. Rep., 2016, 33, 491 RSC; (d) G. Dake, Recent Approaches to the Construction of 1-Azaspiro[4.5]decanes and Related 1-Azaspirocycles, Tetrahedron, 2006, 62, 3467 CrossRef CAS; (e) M. C. Jennings, K. P. C. Minbiole and W. M. Wuest, Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance, ACS Infect. Dis., 2015, 1, 288 CrossRef CAS; (f) B. Trost, J. Tracy and T. Saget, Direct catalytic enantioselective amination of ketones for the formation of tri-and tetrasubstituted stereocenters, Chem. Sci., 2018, 9, 2975 RSC; (g) C. J. Pierce, M. Nguyen and C. H. Larsen, Copper/titanium catalysis forms fully substituted carbon centers from the direct coupling of acyclic ketones, amines, and alkynes, Angew. Chem., Int. Ed., 2012, 51, 12289 CrossRef CAS PubMed.
- (a) R. Dalpozzo, G. Bartoli and G. Bencivenni, Recent advances in organocatalytic methods for the synthesis of disubstituted 2-and 3-indolinones, Chem. Soc. Rev., 2012, 41, 7247 RSC; (b) J. Bariwal, L. G. Voskressensky and E. V. Van der Eycken, Recent advances in spirocyclization of indole derivatives, Chem. Soc. Rev., 2018, 47, 3831 RSC; (c) Q. Zhao, C. Peng, H. Huang, S.-J. Liu, Y.-J. Zhong, W. Huang, G. He and B. Han, Asymmetric synthesis of tetrahydroisoquinoline-fused spirooxindoles as Ras-GTP inhibitors that inhibit colon adenocarcinoma cell proliferation and invasion, Chem. Commun., 2018, 54, 8359 RSC; (d) J. P. MacDonald, J. J. Badillo, G. E. Arevalo, A. Silva-Garcia and A. K. Franz, Catalytic stereoselective synthesis of diverse oxindoles and spirooxindoles from isatins, ACS Comb. Sci., 2012, 14, 285 CrossRef CAS PubMed; (e) N. Ye, H. Chen, E. A. Wold, P.-Y. Shi and J. Zhou, Therapeutic potential of spirooxindoles as antiviral agents, ACS Infect. Dis., 2016, 2, 382 CrossRef CAS PubMed; (f) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Strategies for the enantioselective synthesis of spirooxindoles, Org. Biomol. Chem., 2012, 10, 5165 RSC.
- (a) M. Rottmann, C. McNamara, B. K. Yeung, M. C. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia and J. Tan, Spiroindolones, a potent compound class for the treatment of malaria, Science, 2010, 329, 1175 CrossRef CAS PubMed; (b) B. K. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki and C. Fischli, Spirotetrahydro β-Carbolines (Spiroindolones): A New Class of Potent and Orally Efficacious Compounds for the Treatment of Malaria, J. Med. Chem., 2010, 53, 5155 CrossRef CAS PubMed.
- (a) A. K. Ghosh, G. Schiltz, R. S. Perali, S. Leshchenko, S. Kay, D. E. Walters, Y. Koh, K. Maeda and H. Mitsuya, Design and synthesis of novel HIV-1 protease inhibitors incorporating oxyindoles as the P2′-ligands, Bioorg. Med. Chem. Lett., 2006, 16, 1869 CrossRef CAS PubMed; (b) G. Kumari, M. Modi, S. K. Gupta and R. K. Singh, ChemInform Abstract: Rhodium(II) Acetate-Catalyzed Stereoselective Synthesis, SAR and anti-HIV Activity of Novel Oxindoles Bearing Cyclopropane Ring, Eur. J. Med. Chem., 2011, 46, 1181 CrossRef CAS PubMed.
- R. F. George, N. S. M. Ismail, J. Stawinski and A. S. Girgis, Design, synthesis and QSAR studies of dispiroindole derivatives as new antiproliferative agents, Eur. J. Med. Chem., 2013, 68, 339 CrossRef CAS PubMed.
- (a) Y. Arun, G. Bhaskar, C. Balachandran, S. Ignacimuthuand and P. T. Perumal, Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line, Bioorg. Med. Chem. Lett., 2013, 23, 1839 CrossRef CAS PubMed; (b) A. Czarna, B. Beck, S. Srivastava, G. M. Popowicz, S. Wolf, Y. Huang, M. Bista, T. A. Holak and A. Dömling, Robust Generation of Lead Compounds for Protein-Protein Interactions by Computational and MCR Chemistry: p53/Hdm2 Antagonists, Angew. Chem., Int. Ed., 2010, 49, 5352 CrossRef CAS PubMed.
- K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey and K. Krajewski, Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction, J. Med. Chem., 2006, 49, 3432 CrossRef CAS PubMed.
- V. V. Vintonyak, K. Warburg, H. Kruse, S. Grimme, K. Hübel, D. Rauh and H. Waldmann, Identification of Thiazolidinones Spiro-Fused to Indolin-2-ones as Potent and Selective Inhibitors of the Mycobacterium tuberculosis Protein Tyrosine Phosphatase B, Angew. Chem., Int. Ed., 2010, 49, 5902 CrossRef CAS PubMed.
- (a) M.-X. Zhao, L. Jing, H. Zhou and M. Shi, Cinchona alkaloid thiourea mediated asymmetric Mannich reaction of isocyanoacetates with isatin-derived ketimines and subsequent cyclization: enantioselective synthesis of spirooxindole imidazolines, RSC Adv., 2015, 5, 75648 RSC; (b) Y. Zhu, Y. Li, Q. Meng and X. Li, An organocatalytic enantioselective vinylogous Mannich reaction of α,α-dicyanoolefins with isatin N-Boc ketimines, Org. Chem. Front., 2016, 3, 709 RSC; (c) Y.-M. Wang, H.-H. Zhang, C. Li, T. Fan and F. Shi, Catalytic asymmetric chemoselective 1,3-dipolar cycloadditions of an azomethine ylide with isatin-derived imines: diastereo-and enantioselective construction of a spiro[imidazolidine-2,3′-oxindole] framework, Chem. Commun., 2016, 52, 1804 RSC; (d) B. Li, F. Gao, X. Feng, M. Sun, Y. Guo, D. Wen, Y. Deng, J. Huang, K. Wang and W. Yan, Highly efficient enantioselective synthesis of bispiro[benzofuran-oxindole-pyrrolidine]s through organocatalytic cycloaddition, Org. Chem. Front., 2019, 6, 1567 RSC; (e) M.-C. Yang, C. Peng, H. Huang, L. Yang, X.-H. He, W. Huang, H.-L. Cui, G. He and B. Han, Organocatalytic Asymmetric Synthesis of Spiro-oxindole Piperidine Derivatives That Reduce Cancer Cell Proliferation by Inhibiting MDM2-p53 Interaction, Org. Lett., 2017, 19, 6752 CrossRef CAS PubMed.
- (a) P. Saraswat, G. Jeyabalan, M. Z. Hassan, M. U. Rahman and N. K. Nyola, A Review of Synthesis and Various Biological Activities of Spiro Heterocyclic Compounds Comprising Oxindole and Pyrrolidine Moities, Synth. Commun., 2016, 46, 1643 CrossRef CAS; (b) G. Zhu, W. Sun, C. Wu, G. Li, L. Hong and R. Wang, Base-catalyzed diastereoselective [3+3] annulation of 3-isothiocyanatooxindoles and azomethine imines, Org. Lett., 2013, 15, 4988 CrossRef CAS PubMed; (c) X. Wang, P. Yang, Y. Zhang, C.-Z. Tang, F. Tian, L. Peng and L.-X. Wang, Isatin N,N′-Cyclic Azomethine Imine 1,3-Dipole and Abnormal [3+2]-Cycloaddition with Maleimide in the Presence of 1,4-Diazabicyclo [2.2.2] octane, Org. Lett., 2017, 19, 646 CrossRef CAS PubMed; (d) Q. Jin, J. Zhang, C. Jiang, D. Zhang, M. Gao and S. Hu, Self [3+4] cycloadditions of isatin N,N′-cyclic azomethine imine 1,3-dipole with N-(o-chloromethyl) aryl amides, J. Org. Chem., 2018, 83, 8410 CrossRef CAS; (e) F. M. Moghaddam, M. Eslami, A. Siahpoosh and G. Hoda, Diastereoselective construction of a functionalized dihydro-pyridazine-based spirooxindole scaffold via C-3 umpolung of isatin N,N′-cyclic azomethine imine, New J. Chem., 2019, 43, 10318 RSC; (f) S. Hu, J. Zhang and Q. Jin, DMAP-catalyzed alkylation of isatin N,N′-cyclic azomethine imine 1,3-dipoles with Morita-Baylis-Hillman carbonates, New J. Chem., 2018, 42, 7025 RSC; (g) C. Yin, L. Lin, D. Zhang, J. Feng, X. Liu and X. Feng, Asymmetric [3+2] Cycloaddition of Methyleneindolinones with N,N′-Cyclic Azomethine Imines Catalyzed by a N,N′-Dioxide-Mg(OTf)2 Complex, J. Org. Chem., 2015, 80, 9691 CrossRef CAS.
- (a) J. Yue, S. Chen, X. Zuo, X.-L. Liu, S.-W. Xu and Y. Zhou, Diversity-oriented one-pot multicomponent synthesis of chromanone-based 3,3′-pyrrolidinyl-spirooxindoles via a 1,3-dipolar cycloaddition reaction, Tetrahedron Lett., 2019, 60, 137 CrossRef CAS; (b) N. Zohreh and A. Alizadeh, Uncatalyzed one-pot synthesis of highly substituted pyridazines and pyrazoline-spirooxindoles via domino SN/condensation/aza-ene addition cyclization reaction sequence, ACS Comb. Sci., 2013, 15, 278 CrossRef CAS PubMed; (c) Z. Tang, Z. Liu, Y. An, R. Jiang, X. Zhang, C. Li, X. Jia and J. Li, Isocyanide-based multicomponent bicyclization with substituted allenoate and isatin: synthesis of unusual spirooxindole containing [5.5]-fused heterocycle, J. Org. Chem., 2016, 81, 9158 CrossRef CAS PubMed.
- (a) H.-W. Zhao, L.-R. Wang, J.-M. Guo, W.-Q. Ding, X.-Q. Song, H.-H. Wu, Z. Tang, X.-Z. Fan and X.-F. Bi, Formal [5+3] Cycloaddition of Vinylethylene Carbonates with Isatin-Based α-(Trifluoromethyl) imines for Diastereoselective Synthesis of Medium-Heterocycle-Fused Spirooxindoles, Adv. Synth. Catal., 2019, 361, 4761 CrossRef CAS; (b) H.-W. Zhao, B. Li, H.-L. Pang, T. Tian, X.-Q. Chen, X.-Q. Song, W. Meng, Z. Yang, Y.-D. Zhao and Y.-Y. Liu, Diastereoselective 1,3-Dipolar Cycloadditions of N,N′-Cyclic Azomethine Imines with Iminooxindoles for Access to Oxindole Spiro-N,N-bicyclic Heterocycles, Org. Lett., 2016, 18, 848 CrossRef CAS PubMed.
- (a) X. Chen, Q. Liu, P. Chauhan and D. Enders, N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations, Angew. Chem., Int. Ed., 2018, 57, 3862 CrossRef CAS; (b) L.-L. Zhao, X.-S. Li, L.-L. Cao, R. Zhang, X.-Q. Shi and J. Qi, Access to dihydropyridinones and spirooxindoles: application of N-heterocyclic carbene-catalyzed [3+3] annulation of enals and oxindole-derived enals with 2-aminoacrylates, Chem. Commun., 2017, 53, 5985 RSC; (c) Y. Reddi and R. B. Sunoj, Origin of Stereoselectivity in Cooperative Asymmetric Catalysis Involving N-Heterocyclic Carbenes and Lewis Acids toward the Synthesis of Spirooxindole Lactone, ACS Catal., 2017, 7, 530 CrossRef CAS; (d) Z.-Y. Wang, T. Yang, K.-K. Wang, R. Chen, M. Liu and H. Liu, Oxidative N-heterocyclic carbene-catalyzed [3+3] annulation reaction of enals with benzofuran-3-ones: efficient access to benzofuran-fused δ-lactones, Org. Chem. Front., 2020, 7, 1011 RSC; (e) Y. Liu, X. Zhang, R. Zeng, Y. Zhang, Q.-S. Dai, H.-J. Leng, X.-J. Gou and J.-L. Li, Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis, Molecules, 2017, 22, 1882 CrossRef PubMed; (f) Y. Li, C. Barløse, J. Jørgensen, B. D. Carlsen and K. A. Jørgensen, Asymmetric Catalytic Aza-Diels-Alder/Ring-Closing Cascade Reaction Forming Bicyclic Azaheterocycles by Trienamine Catalysis, Chem.–Eur. J., 2017, 23, 38 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data, NMR spectra for products. CCDC 1991911 and 1915292. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03806g |
| This journal is © The Royal Society of Chemistry 2020 |