Open Access Article
Open Access ArticlePhoto-controllable rotational motion of cholesteric liquid crystalline droplets in a dispersion system†
Yota Sakaia,
Woon Yong Sohn b and
Kenji Katayama
b and
Kenji Katayama *ac
*ac
aDepartment of Applied Chemistry, Chuo University, Tokyo 112-8551, Japan. E-mail: kkata.33g@g.chuo-u.ac.jp; Tel: +81-3-3817-1913
bDepartment of Chemistry, Chungbuk National University, Cheongju, Chungbuk 28644, South Korea
cPRESTO, Japan Science and Technology Agency (JST), Saitama 332-0012, Japan
First published on 3rd June 2020
Abstract
A photo-controllable rotational motion was demonstrated for an isolated cholesteric liquid crystalline droplet in a surfactant solution. The droplet showed unidirectional rigid-body rotation with UV light irradiation and the rotational rate could be controlled by the light intensity. Furthermore, the rotational direction could be controlled by the chirality of dopants. The motion was explained by the rotational torque induced by the photo-induced gradient of chemical concentrations and/or temperature inside the droplet from the theory of the Lehmann effect, and the possible mechanisms are discussed.
Introduction
Active matter, referring to objects spontaneously moving around with external energy sources, is one of the hot topics in biology, physics, and chemistry.1–5 In recent years, active matters made of liquid crystals (LCs) or remaining in an LC medium have been reported, with active motion of an LC shell induced by biological energy,6 bacteria motion control inside an LC or a patterned LC,4,7 etc.In recent years, a new category of active matters was introduced; chemicals in droplets were gradually dissolved into a surfactant solution, causing the convective flow inside and outside the droplets and they could move around.8–10 Several types of this system using LCs have been reported; different types of motion were switched at some velocities depending on the surfactant concentrations,11 an instantaneous helical motion was demonstrated for the cholesteric LC droplet in a surfactant solution.12,13 We demonstrated photo-controllable active LC droplets made of N-(4-methoxybenzylidene)-4-butylaniline (MBBA),14 and 4-cyano-4′-pentylbiphenyl (5CB) with photo-responsive dyes.15 Interestingly, the motion direction can be controlled by the variation of the doped dyes because of the different solubilization behavior of dyes under the light illumination.
Cholesteric LCs (CLCs), where the molecular alignment is twisted with a constant pitch defined by the chiral dopant concentration, have been intensively studied for the application of the color control,16 lasing,17 etc. When the CLC was formed into droplets, various color and color patterns were demonstrated.18,19 It was applied for the biosensing by the detection of the color change20 or the pattern change.21
Contrary to various applications of the CLC droplets, there has been a mystery about the motion induced by the thermal gradient, which was initially discovered by Lehmann in 1900.22 The CLC droplet showed a rotational motion under the thermal gradient. This phenomenon was theoretically explained long after the discovery by Leslie.23 However, the Lehmann effect has not been studied well because of the poor reproducibility of the experiments until recently. Oswald, et al. found the stable rotation conditions in an isotropic–cholesteric coexistence system under the thermal gradient.24 In this experiment, a droplet was formed with contacts with the top and bottom substrates, where the anchoring conditions were controlled under the planer and homeotropic boundary conditions.25 Double twisted structure,26 and different helical axis directions were demonstrated.27 Recently, Tabe, et al. demonstrated that a rotation can be induced for a similar system including the photo-responsive molecules by the UV light irradiation.28 Araoka, et al. demonstrated the Lehmann motion in a surfactant solution for an isolated CLC droplet.29
Since the light energy is superior in the controllability of the position, direction, and energy input compared with the other control methods such as the temperature or the chemical concentrations, we utilize the light for the control of the LC active matter. In this study, we found the photo-controllable rotational motion of an isolated CLC droplet in a surfactant solution, which was made of photo-responsive molecules, and the rotational rate could be controlled by the light intensity and the rotational direction could be switched by the chiral dopants. The possible mechanisms of these motions were discussed.
Experiment
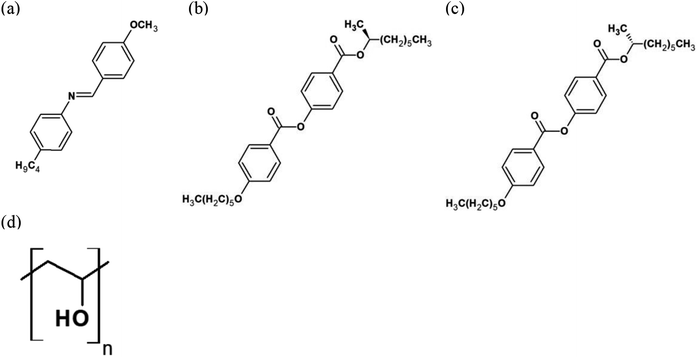
Photo-responsive CLC droplets were prepared using a microfluidic device.30 A schematic drawing of the setup of the device and a picture is shown in Fig. S1 in ESI.† A square glass capillary (inner diameter: 0.90 × 0.90 mm2) was adhered to a glass slide, and a cylindrical glass capillary (inner diameter: 0.70 mm, outer diameter: 0.87 mm) with the tip diameter 50 μm, was inserted into it. The tapered tip was prepared by a micropipette puller (P-1000, Sutter Instrument). Two syringe needles were attached to both the edges of the square capillary, and two syringe pumps and syringe needles were connected via a micro-tube, then the two types of liquids (inner fluid and outer fluid) were introduced. The inner fluid (CLC) was introduced from the inlet side of the square glass capillary to the outlet side, and the inner fluid was sheared by the outer fluid introduced from another side of the square glass capillary. The CLC droplets were formed in the inlet of the tapered cylindrical capillary. The typical size of the droplets was 100 μm in diameter.N-(4-Methoxybenzylidene)-4-butylaniline (MBBA, nematic phase: 22–48 °C) was used both as a host LC and a photo-responsive LC (Fig. 1(a)). Two different chiral dopants were mixed in MBBA for the chirality control. The chiral dopants were (S)-2-octyl 4-[4-(hexyloxybenzoyloxy)]benzoate (S811, 2.5 wt%) and (R)-2-octyl 4-[4-(hexyloxybenzoyloxy)]benzoate (R811, 2.5 wt%) as shown in Fig. 1(b) and (c), respectively. Polyvinyl alcohol (PVA, 1 wt%, 25 °C) (Fig. 1(d)) solution was used as a surfactant solution and it was also used as the outer fluid. PVA work as a protective agent for the droplets in the concentration range from 0.1–1.0 wt% and no obvious solubilization was recognized by naked eye. When it is higher than 1.5 wt%, the LC droplets cannot keep the shape because LC molecules were solubilized as micelles. There was no dependence on the rotational behavior itself on the PVA concentration.
The schematic drawing of the observation setup is shown in Fig. S2 in ESI.† A rubber spacer (thickness: 0.2 mm) with an open space was sandwiched with two coverslips (thickness: 0.12–0.17 mm), and a PVA solution including the CLC droplets was pipetted into the open space. The cell was placed on an inverted optical microscope (IX71, OLYMPUS), and the behavior of the CLC droplets under the UV light irradiation was observed. A non-polarized UV-LED (Execure LH-1V, HOYA, center wavelength: 360 nm) was used as a UV light source with an intensity of 25 mW cm−2 unless otherwise stated, and was irradiated from the top side of the CLC droplet. The illumination light was an LED (M530L3-C1, Thorlabs, center wavelength: 530 nm), and was irradiated from the bottom side of the cell and the reflected image was observed with a CCD camera (DCU223C, Thorlabs).
Result and discussions
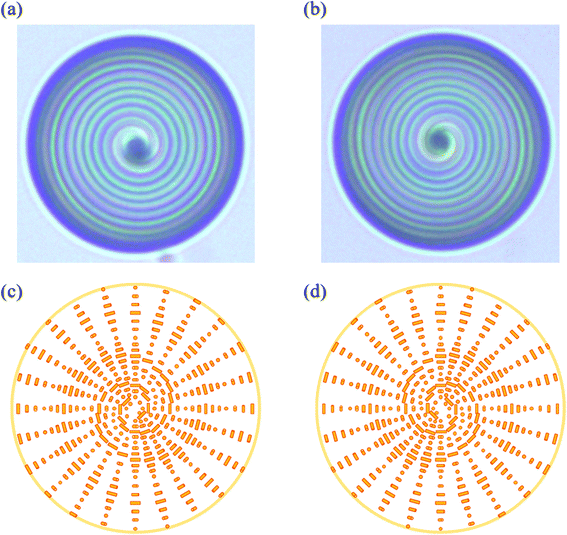
The bright-field images of an S811-doped and an R811-doped CLC droplet in a PVA solution are shown in Fig. 2(a) and (b), respectively. The double spiral pattern in the counter-clockwise direction was observed for the S811-doped CLC droplet, while the R811-doped CLC droplet showed the same pattern in the opposite direction. It was noticed that LC had a periodic color change in a radial direction and it indicates a spatial variation of the refractive index due to the orientation difference of the LC molecules.31 It is known that PVA molecules adsorb on the LC/water interface with a random coil conformation, imposing the tangential anchoring (planer orientation) of the LC molecules on the interface.32 Since the director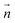 of the cholesteric LC rotates and the orientation of LC molecules changed in the radial direction, the LC molecules are subject to twist in the radial direction starting from the tangentially aligned molecules at the interface. Based on these considerations, the LC alignments in the two-dimensional plane for the S811-doped CLC droplet and the R811-doped CLC droplet are shown in Fig. 2(c) and (d), respectively.
of the cholesteric LC rotates and the orientation of LC molecules changed in the radial direction, the LC molecules are subject to twist in the radial direction starting from the tangentially aligned molecules at the interface. Based on these considerations, the LC alignments in the two-dimensional plane for the S811-doped CLC droplet and the R811-doped CLC droplet are shown in Fig. 2(c) and (d), respectively.
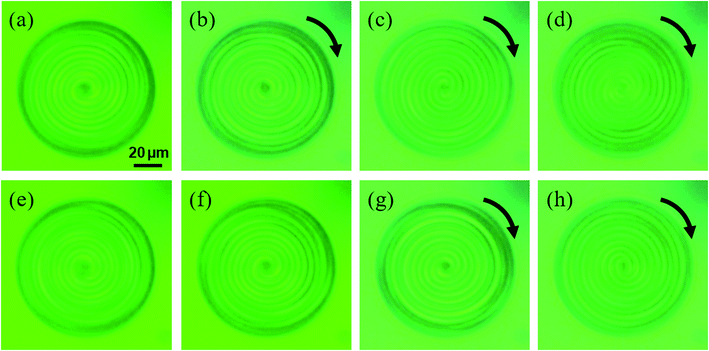
Next, the behavior under the light irradiation is shown for the S811-doped CLC droplet. We found that it started the unidirectional rotation by the UV light irradiation. The snapshots of the droplet rotation are shown in Fig. 3 (the video of the motion is included in Mov. S1(a) in ESI†). During the rotation, the droplet gradually expanded the pitch, and the double spiral pattern started to whirl in the clockwise direction. The LC pattern became gradually unclear while rotating. The motion stopped immediately when the light was turned off and returned to the original state for about 40 seconds. This rotation and the pattern change could be observed repeatedly when the UV light was turned on and off.
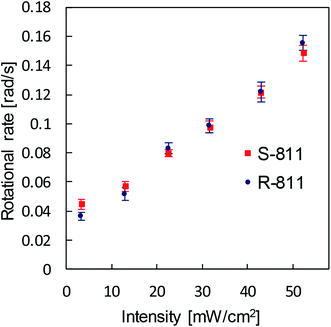
The effect of the chirality of the dopant on the photo-induced rotation was studied. The droplets including the opposite chirality showed the opposite direction of motion; clockwise and counter-clockwise rotation for the left-handed and right-handed dopants, respectively. The sequence of the snapshots for the R811-doped CLC droplet is shown in Fig. 4 (the video for the R811-doped CLC droplet is included in Mov. S1(b) in ESI†). Except for the rotational direction, the photo-induced behavior was the same for both types of droplets.
 | ||
| Fig. 4 The snapshots of the R811-doped CLC droplet in a PVA solution under the UV light irradiation: (a) before irradiation (b) 10 s (c) 20 s (d) 30 s after the UV was turned on. | ||
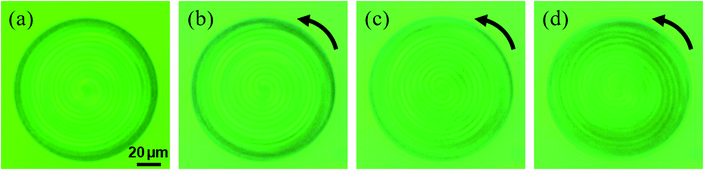
The dependence of the rotational rate of the LC droplets on the UV light intensity was investigated for the S811-doped and R811-doped CLC droplets. In both cases, the velocity was linearly proportional as the UV light intensity as shown in Fig. 5, though there is a threshold intensity for the rotating motion (∼3.3 mW cm−2) possibly because there is a threshold energy input to overcome the adherence on the substrate.
 | ||
| Fig. 5 The dependence of the rotational rate of the S811-doped and R811-doped CLC droplets on the UV light intensity. | ||
We investigated if this observation was due to the rigid-body motion of the LC droplet because only the molecular orientation change could cause a pattern change. To clarify this issue, the same droplet (S811-doped CLC droplet) including micron-sized polystyrene particles (D = 1 μm) was prepared and traced their motion under the UV light irradiation during the motion. The snapshots of the LC droplet motion under the UV irradiation is shown in Fig. 6 (the movie is included in Mov. S2 in ESI†). Aggregates of the polystyrene particles rotated with the textual pattern rotation of the droplet. It indicates that the droplet showed a rigid-body rotation similar to the previous study of Tabe, et al.33
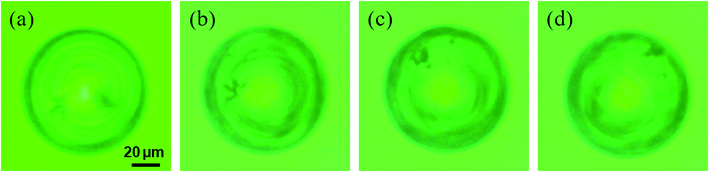 | ||
| Fig. 6 The snapshots of S811-doped CLC droplet including polystyrene particles under the UV irradiation is shown; (a) before irradiation (b) 60 s (c) 120 s (d) 180 s. | ||
In recent years, the stable rotation was observed for the CLC droplets by imposing the thermal gradient. However, in the previous studies,34–38 most of the demonstrations were for a cholesteric droplet in an isotropic medium consisted of the same LC, except that Araoka, et al. demonstrated a CLC droplet motion in a surfactant solution under a thermal gradient,39 and also Oswald, et al. further studied on a CLC droplet under a thermal gradient and explained the mechanism by the Marangoni convection theory.40 We could demonstrate a novel type of light-controllable rotation in a dispersion system (isolated droplet) by light irradiation.
In the Lehmann effect, the absence of inversion symmetry in cholesterics allows a thermomechanical torque along the thermal gradient as35
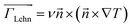 | (1) |
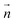 is the director vector. This equation indicates that the director perpendicular to the temperature gradient induces the torque around the axis of the temperature gradient. In recent years, Tabe, et al. demonstrated the Lehmann effect not only by the thermal gradient but also by the gradient of the chemical potential due to concentrations, which was controlled by the photo-isomerization reaction.28 In this study, since the Lehmann coefficients for the cis and trans isomers are different, the net torque could be generated.
is the director vector. This equation indicates that the director perpendicular to the temperature gradient induces the torque around the axis of the temperature gradient. In recent years, Tabe, et al. demonstrated the Lehmann effect not only by the thermal gradient but also by the gradient of the chemical potential due to concentrations, which was controlled by the photo-isomerization reaction.28 In this study, since the Lehmann coefficients for the cis and trans isomers are different, the net torque could be generated.
To confirm whether the photo-induced concentration gradient of cis/trans isomers in the light propagation direction, the optical penetration depth of MBBA at the illumination wavelength of 365 nm was studied, and it was 40 μm (Fig. S3 in ESI†), and it indicates that the light absorption had a gradient inside the droplet with a typical size of 100 μm. The illumination induced the photo-isomerization of MBBA, and the concentration gradient of the cis isomer should be formed inside the droplet. This could be the origin of the Lehmann force applied to the droplet. Similarly, the temperature gradient generated in the reaction and relaxation of the photo-isomerization is another possible origin of the Lehmann force. This light-induced gradient of chemical concentration and/or temperature could induce the torque of the rotation.
This motion could be considered as an analogy of the motion of cholesteric fingers under a thermal gradient,41 where the cholesteric region in the isotropic media shows a spiral pattern and the spiral pattern rotates due to the net Lehmann torque. In this case, the Lehmann force induces the drift of the finger in the outside or inside the direction of the spiral, depending on the Lehmann coefficient.
However, as the rigorous review by Oswald, et al. indicated,42 the actual mechanism for the rotation is still controversial. Based on his review, one of the possible mechanisms for a CLC droplet in a surfactant solution is caused by the Marangoni effect (H model), where the surface tension gradient in the vertical direction induces a convective flow inside and outside the droplet and the flow coupled with the orientation could generate a torque. However, this mechanism is denied in our case because we did not observe any flow outside the droplet when the micro-sized polystyrene particles remained around the droplet during the motion (the movie is included in Mov. S3 in ESI†). Another possibility is the classical theory on the thermomechanical coupling between the gradient force and the gradient of the chemical potential (TM3 model). As Oswald pointed out, the magnitude of the rotational rate cannot be explained without the gradient in the direction of the rotational axis, and the theoretical equation indicates that the orthoradial component of the temperature or the concentration is necessary for the magnitude of the observed rotational rate. The third possibility is the melting-growth model (MG model), where the impurities (in this case, PEG) and LC molecules could circulate by melting and contacting could induce a torque if there is a twist of the droplet in the vertical direction. This could be possible because the light irradiation induces the melting of MBBA and surfactants at the same time,14 and a similar convective process could happen. At this moment, we could not decide one of the mechanisms yet and will further study with a theoretical consideration.
From a different point of view, we could observe the pitch expansion during the rotation. It could be induced by the replacement of the trans to cis isomers, which possess different pitch. Since the increase in the pitch indicates the decrease in the twisted energy, the elastic energy was released along the spiral line and it could cause the rotational torque for the spirally aligned LC molecules. As a result, the aligned LC molecules could prefer to whirl.
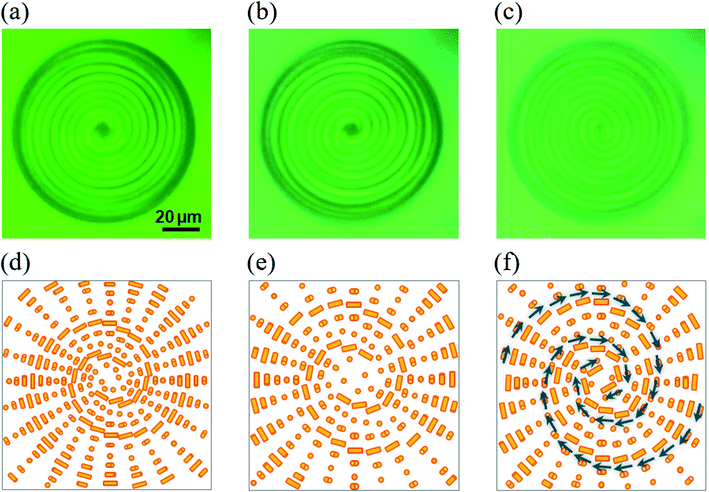
At this moment, the following processes for the photo-induced rotation of the CLC droplet were confirmed. Several snapshots for the S811-doped CLC droplet under the light irradiation were shown in Fig. 7; before the irradiation, during the pitch expansion (5 s after irradiation) and during the macroscopic rotation (15 s after irradiation), and the corresponding proposed schematic drawings of LC molecular alignment change are given in each process. The photo-absorption gradient was generated inside the LC droplet when the UV light was irradiated from the top side. It caused the concentration gradient of the cis-isomer and/or the temperature gradient due to the reaction of the photo-isomerized molecules. These gradients can induce the torque for the LC molecules, then the macroscopic pitch expansion was observed (Fig. 7(b) and (e)), followed by the macroscopic rotational motion in a clockwise direction (Fig. 7(c) and (f)). During the rotation, the droplet gradually expanded the pitch, and the double spiral pattern started to whirl in the clockwise direction. The LC pattern became gradually unclear while rotating.
Conclusion
We successfully demonstrated the photo-controllable rotation of an isolated CLC droplet in a surfactant solution for the first time. The droplet started unidirectional rigid-body rotation with a pattern change of the CLC droplet by the UV irradiation. We also found that the rotational direction could be controlled by the chirality of dopants and the rotational rate could be controlled by the light intensity. This system can be regarded as a novel type of the Lehmann effect and photo-active soft matter since it can be repeatedly rotated and stopped and controlled from a remote distance by the light irradiation, and it could help the further study of the rotational motion of CLC droplets and their application. We suppose that the motion mechanism in this rotation could be explained by the rotational torque due to the photo-induced gradient of chemical concentration and/or temperature inside the droplet, which could be explained by the theory of the Lehmann effect, though there are several possible mechanisms and not clarified fully yet.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was financially supported by the Institute of Science and Engineering, Chuo University, JST PRESTO (#JPMJPR1675), the research was financially supported by the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan.References
- S. Sánchez, L. Soler and J. Katuri, Angew. Chem., Int. Ed., 2015, 54, 1414–1444 CrossRef PubMed.
- S. J. Ebbens and J. R. Howse, Langmuir, 2011, 27, 12293–12296 CrossRef CAS.
- S. Sanchez, A. A. Solovev, Y. Mei and O. G. Schmidt, J. Am. Chem. Soc., 2010, 132, 13144–13145 CrossRef CAS PubMed.
- R. Dong, Q. Zhang, W. Gao, A. Pei and B. Ren, ACS Nano, 2016, 10, 839–844 CrossRef CAS PubMed.
- Y. Xiao, S. Zarghami, K. Wagner, P. Wagner, K. C. Gordon, L. Florea, D. Diamond and D. L. Officer, Adv. Mater., 2018, 1801821 CrossRef PubMed.
- F. C. Keber, E. Loiseau, T. Sanchez, S. J. DeCamp, L. Giomi, M. J. Bowick, M. C. Marchetti, Z. Dogic and A. R. Bausch, Science, 2014, 345, 1135 CrossRef CAS PubMed.
- C. Peng, T. Turiv, Y. Guo, Q.-H. Wei and O. D. Lavrentovich, Science, 2016, 354, 882 CrossRef CAS PubMed.
- C. Jin, C. Krüger and C. C. Maass, Proc. Natl. Acad. Sci., 2017, 114, 5089–5094 CrossRef CAS PubMed.
- S. Herminghaus, C. C. Maass, C. Krüger, S. Thutupalli, L. Goehring and C. Bahr, Soft Matter, 2014, 10, 7008–7022 RSC.
- C. Krüger, C. Bahr, S. Herminghaus and C. C. Maass, Eur. Phys. J. E, 2016, 39, 64 CrossRef PubMed.
- M. Suga, S. Suda, M. Ichikawa and Y. Kimura, Phys. Rev. E, 2018, 97, 062703 CrossRef CAS PubMed.
- T. Yamamoto and M. Sano, Soft Matter, 2017, 13, 3328–3333 RSC.
- C. Krüger, G. Klös, C. Bahr and C. C. Maass, Phys. Rev. Lett., 2016, 117, 048003 CrossRef PubMed.
- Y. Dogishi, Y. Sakai, W. Y. Sohn and K. Katayama, Soft Matter, 2018, 14, 8085–8089 RSC.
- Y. Sakai, W. Y. Sohn and K. Katayama, Soft Matter, 2019, 15, 7159–7165 RSC.
- L. Wang and Q. Li, Adv. Funct. Mater., 2016, 26, 10–28 CrossRef CAS.
- K.-J. Che, Y.-J. Yang, Y.-L. Lin, Y.-W. Shan, Y.-H. Ge, S.-S. Li, L.-J. Chen and C. J. Yang, Lab Chip, 2019, 19, 3116–3122 RSC.
- J. Fan, Y. Li, H. K. Bisoyi, R. S. Zola, D. Yang, T. J. Bunning, D. A. Weitz and Q. Li, Angew. Chem., Int. Ed., 2015, 54, 2160–2164 CrossRef CAS PubMed.
- S. S. Lee, S. K. Kim, J. C. Won, Y. H. Kim and S.-H. Kim, Angew. Chem., Int. Ed., 2015, 54, 15266–15270 CrossRef CAS PubMed.
- H.-G. Lee, S. Munir and S.-Y. Park, ACS Appl. Mater. Interfaces, 2016, 8, 26407–26417 CrossRef CAS PubMed.
- J. Kim, M. Khan and S.-Y. Park, ACS Appl. Mater. Interfaces, 2013, 5, 13135–13139 CrossRef CAS PubMed.
- O. Lehmann, Ann. Phys., 1900, 307, 649–705 CrossRef.
- F. M. Leslie, Proc. Roy. Soc. Lond. Math. Phys. Sci., 1968, 307, 359–372 CAS.
- P. Oswald and A. Dequidt, Phys. Rev. Lett., 2008, 100, 217802 CrossRef PubMed.
- T. Yamamoto, M. Kuroda and M. Sano, Europhys. Lett., 2015, 109, 46001 CrossRef.
- J. Yoshioka, F. Ito, Y. Suzuki, H. Takahashi, H. Takizawa and Y. Tabe, Soft Matter, 2014, 10, 5869–5877 RSC.
- F. Ito, J. Yoshioka and Y. Tabe, J. Phys. Soc. Jpn., 2016, 85, 114601 CrossRef.
- S. Bono, S. Sato and Y. Tabe, Soft Matter, 2017, 13, 6569–6575 RSC.
- J. Yoshioka and F. Araoka, Nat. Commun., 2018, 9, 1–11 CrossRef CAS PubMed.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef CAS PubMed.
- I. Muševič, H. Peng, M. Nikkhou and M. Humar, Proc. SPIE, 2014, 8960, 896016 CrossRef.
- D. Wang, S.-Y. Park and I.-K. Kang, J. Mater. Chem. C, 2015, 3, 9038–9047 RSC.
- K. Nishiyama, S. Bono, Y. Maruyama and Y. Tabe, J. Phys. Soc. Jpn., 2019, 88, 063601 CrossRef.
- P. Oswald and A. Dequidt, Phys. Rev. Lett., 2008, 100, 217802 CrossRef PubMed.
- P. Oswald and A. Dequidt, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 051706 CrossRef PubMed.
- T. Yamamoto, M. Kuroda and M. Sano, Europhys. Lett., 2015, 109, 46001 CrossRef.
- J. Yoshioka, F. Ito, Y. Suzuki, H. Takahashi, H. Takizawa and Y. Tabe, Soft Matter, 2014, 10, 5869–5877 RSC.
- F. Ito, J. Yoshioka and Y. Tabe, J. Phys. Soc. Jpn., 2016, 85, 114601 CrossRef.
- J. Yoshioka and F. Araoka, Nat. Commun., 2018, 9, 1–11 CrossRef CAS PubMed.
- P. Oswald, J. Ignés-Mullol and A. Dequidt, Soft Matter, 2019, 15, 2591–2604 RSC.
- P. Oswald and A. Dequidt, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 051706 CrossRef PubMed.
- P. Oswald, A. Dequidt and G. Poy, Liq. Cryst. Rev., 2019, 7, 142–166 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03465g |
| This journal is © The Royal Society of Chemistry 2020 |