Open Access Article
Open Access ArticlePalladium nanoparticles immobilized on a nano-silica triazine dendritic polymer: a recyclable and sustainable nanoreactor for C–S cross-coupling†
Amir Landarani-Isfahani,
Iraj Mohammadpoor-Baltork *,
Valiollah Mirkhani*,
Majid Moghadam
*,
Valiollah Mirkhani*,
Majid Moghadam ,
Shahram Tangestaninejad
,
Shahram Tangestaninejad and
Hadi Amiri Rudbari
and
Hadi Amiri Rudbari
Department of Chemistry, University of Isfahan, Isfahan, 81746-73441, Iran. E-mail: imbaltork@sci.ui.ac.ir; mirkhani@sci.ui.ac.ir; Fax: +98 031 36689732
First published on 3rd June 2020
Abstract
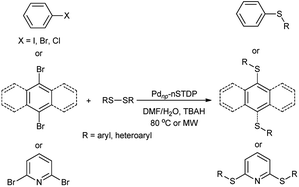
Dendrimers are of great interest due to their special structural topology and chemical versatility. Owing to their properties, dendrimers have found practical applications in catalytic processes as efficient nanoreactors. Therefore, we herein report an environmentally attractive strategy and highly efficient route for the synthesis of a wide variety of diaryl sulfides using palladium nanoparticles immobilized on a nano-silica triazine dendritic polymer (Pdnp-nSTDP) as a nanoreactor. In this manner, different diaryl or aryl heteroaryl sulfides and bis(aryl/heteroarylthio)benzene/anthracene/pyridine derivatives were prepared via C–S cross-coupling reactions of aryl halides with diaryl/diheteroaryl disulfides under thermal conditions and microwave irradiation. The catalyst could be easily recovered and reused several times without any significant loss of its activity.
Introduction
Over the last few years, chemists have tried to understand the principles and significance of nanoscale reaction systems. Since the introduction of the concept of supramolecular nanoreactors by Breslow,1 nanoreactors have been investigated increasingly in the field of catalysis.2 The most significant supramolecular nanoreactors that have been established so far for the stabilization of catalytically active nanoparticles are cucurbiturils, porphyrins, metal–organic frameworks, micelles, colloidosomes and dendrimers.3–9 Along this line, dendrimers, which are highly branched and tree-like macromolecules with 3D structures, chemical functionalities and internal cavities, can act as excellent hosts for encapsulation of a variety of metal ions, complexes and nanoparticles.10–15Diarylsulfides are important intermediates in a wide variety of organic synthesis and play a significant role in many biologically and pharmaceutically active compounds.16 These moieties are used for treatment of inflammation,17 cancer,18,19 immunodeficiency virus (HIV),17 and Alzheimer's and Parkinson's diseases.20 Because of their importance and useful properties, different approaches have been developed for the synthesis of diarylsulfides via C–S cross-coupling reactions.21,22 Generally, in these coupling reactions, thiols (ArSH) are reacted with aryl halides or aryl boronic acids as aryl donors in the presence of different catalytic systems including Fe,23–25 Cu,26–28 Ni,29,30 Co,31,32 In (ref. 33) and Pd.34–38
However, thiols have offensive odors and are easily oxidized to the corresponding diaryl disulfides (ArSSAr) in air. Thus, diaryl disulfides are often produced as a by-product in the above mentioned methods.39 To solve this problem and for the effective conversion of thiols to diaryl sulfides, aryl donors are usually used in excess.40 On the contrary, diaryl disulfides are air stable, and easy to handle. Nevertheless, little attention has been paid to the cross-coupling of diaryl disulfides as sulfur nucleophile with aryl donors.41–45 Accordingly, development of convenient methods and catalysts for the synthesis of diaryl sulfides via such a cross-coupling reactions is still critically needed.
Based on the green chemistry concept and principal, synthetic approach and process should be premeditated to use and generate substances which exhibit little or no hazard to human body and the natural environment. Moreover, these chemical transformations are often performed with high yields, at low temperatures, in short reaction times, and in the presence of low amount of the catalyst in water or aqueous media.46
Nano-silica triazine dendritic polymer (nSTDP) can be effectively applied for nanoscale organic transformations. During the course of our research on the application of this nanoreactors47–50 and also our interest in Pd-catalyzed coupling reactions, herein we disclose a convenient method for mono- and di C–S cross-coupling reactions with diaryl or diheteroaryl disulfides using Pdnp-nSTDP (Fig. 1) as an eco-friendly nanoreactor under conventional heating and microwave irradiation (Scheme 1). As far as we know, the use of palladium nanoparticles-based dendritic catalyst for such a coupling reactions is reported for the first time and could be considered as an exclusive feature of nanoscience and green chemistry.
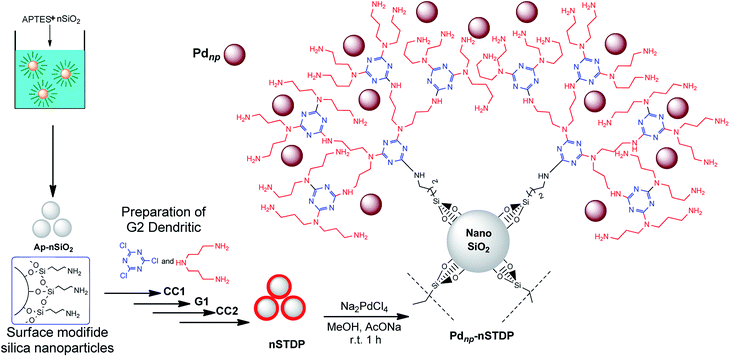 | ||
| Fig. 1 Schematic illustration of preparation of palladium nanoparticles immobilized on nano silica triazine dendritic polymer (Pdnp-nSTDP) catalyst. | ||
Results and discussion
Fig. 1 illustrates the synthetic pathway of palladium nanoparticles immobilized on nano silica triazine dendritic polymer (Pdnp-nSTDP).47 Initially, nano-silica was functionalized with 3-aminopropyltrimethoxysilane (APTES) to afford aminopropyl-functionalized nano-silica (AP-nSiO2). Then, treatment of AP-nSiO2 with cyanuric chloride (CC) in the presence of diisopropylethylamine (DIPEA) at room temperature afforded CC1-nSiO2 which upon reaction with bis(3-aminopropyl)amine gave G1. After that, the reaction of G1 with cyanuric chloride (CC) and DIPEA provided CC2-nSiO2 which in turn was transformed to G2 (nSTDP) by the reaction with bis(3-aminopropyl)amine. Finally, the Pdnp-nSTDP catalyst was prepared by reduction of Na2Pd2Cl6 (prepared in situ from PdCl2 and NaCl)51 in methanol and sodium acetate at 60 °C in the presence of nSTDP (ESI†). The palladium loading of the catalyst was measured by ICP analysis and it was found that the amount of Pd in the catalyst is 1.27% (0.12 mmol g−1 of nSTDP).Initially, for screening experiments, the coupling reaction of 4-bromoanisole (1 mmol) with di-p-tolyl disulfide (0.5 mmol) was carried out using Pdnp-nSTDP catalyst for determination of effective factors such as types of base and solvent, temperature, catalyst loading and MW power. The results are shown in Table 1. Primarily, different bases such as NEt3, piperidine, DBU (1,8-diazabicyclo[5.4.0]undec-7-ene), NaOH, Na2CO3, K2CO3 and 20% solution of tetrabutylammonium hydroxide in water (TBAH) were examined. Amongst, TBAH was found as the most effective base in terms of both reaction time and yield. The effect of solvent was also checked in the model reaction and highest yield was obtained in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O–DMF (Table 1, entries 7–12). Next, we investigated the catalyst loading (0.08–0.12 mol% Pd) and 0.1 mol% (8 mg) of the catalyst was found to be the optimum amount for completion of this reaction (Table 1, entries 7, 13 and 14). Finally, the effect of temperature on the activity of this catalyst was studied in the range of 70 to 100 °C. As can be seen in Table 1, by increasing the temperature to 80 °C, the yield of the reaction was improved but its increasing from 80 to 100 °C did not influence the yield of the product. Therefore, 0.1 mol% (8 mg) of the catalyst and TBAH base in a mixture of H2O–DMF (1
1 mixture of H2O–DMF (Table 1, entries 7–12). Next, we investigated the catalyst loading (0.08–0.12 mol% Pd) and 0.1 mol% (8 mg) of the catalyst was found to be the optimum amount for completion of this reaction (Table 1, entries 7, 13 and 14). Finally, the effect of temperature on the activity of this catalyst was studied in the range of 70 to 100 °C. As can be seen in Table 1, by increasing the temperature to 80 °C, the yield of the reaction was improved but its increasing from 80 to 100 °C did not influence the yield of the product. Therefore, 0.1 mol% (8 mg) of the catalyst and TBAH base in a mixture of H2O–DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C are the most appropriate reaction conditions for this transformation (Table 1, entry 7). It is worth mentioning that aqueous DMF has been used as a green solvent in different organic transformations, especially in cross coupling reactions.52
1) at 80 °C are the most appropriate reaction conditions for this transformation (Table 1, entry 7). It is worth mentioning that aqueous DMF has been used as a green solvent in different organic transformations, especially in cross coupling reactions.52
| Entry | Base | Catalyst (mol% Pd) | Solventa | Method | Time (h) | Yieldb (%) | TONc | TOFd (h−1) |
|---|---|---|---|---|---|---|---|---|
| a The reaction was performed using 1 mL of solvent and 1 mmol of base.b Isolated yield.c TON = turnover numbers.d TOF = turnover frequency.e DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene.f TBAH = 20% tetrabutylammonium hydroxide in water. | ||||||||
| 1 | Et3N | 0.1 | DMF | 80 °C | 24 | 15 | 150 | 6.25 |
| 2 | Piperidine | 0.1 | DMF | 80 °C | 24 | 40 | 400 | 16.7 |
| 3 | DBUe | 0.1 | DMF | 80 °C | 24 | 28 | 280 | 11.7 |
| 4 | NaOH | 0.1 | DMF | 80 °C | 12 | 64 | 640 | 53.3 |
| 5 | K2CO3 | 0.1 | DMF | 80 °C | 12 | 60 | 600 | 50.0 |
| 6 | Na2CO3 | 0.1 | DMF | 80 °C | 12 | 83 | 830 | 69.2 |
| 7 | TBAHf | 0.1 | H2O/DMF | 80 °C | 5 | 95 | 950 | 190.0 |
| 8 | TBAH | 0.1 | H2O/EtOH | 75 °C | 12 | 51 | 510 | 42.5 |
| 9 | TBAH | 0.1 | H2O/DMSO | 80 °C | 12 | 86 | 860 | 71.7 |
| 10 | TBAH | 0.1 | H2O/dioxane | 80 °C | 12 | 85 | 850 | 70.8 |
| 11 | TBAH | 0.1 | H2O/toluene | 80 °C | 12 | 30 | 300 | 25.0 |
| 12 | TBAH | 0.1 | — | 80 °C | 12 | 45 | 450 | 37.5 |
| 13 | TBAH | 0.08 | H2O/DMF | 80 °C | 5 | 53 | 625 | 125.0 |
| 14 | TBAH | 0.12 | H2O/DMF | 80 °C | 5 | 95 | 792 | 158.3 |
| 15 | TBAH | 0.1 | H2O/DMF | 100 °C | 5 | 95 | 950 | 190.0 |
| 16 | TBAH | 0.1 | H2O/DMF | 70 °C | 8 | 74 | 740 | 92.5 |
| 17 | TBAH | 0.1 | H2O/DMF | 170 W, 50 °C | 15 min | 85 | 850 | 226.6 |
| 18 | TBAH | 0.1 | H2O/DMF | 200 W, 70 °C | 10 min | 90 | 900 | 5400 |
| 19 | TBAH | 0.1 | H2O/DMF | 230 W, 80 °C | 10 min | 95 | 950 | 5700 |
| 20 | TBAH | 0.15 | H2O/DMF | 230 W, 80 °C | 10 min | 73 | 487 | 2920 |
| 21 | TBAH | 0.08 | H2O/DMF | 230 W, 80 °C | 10 min | 62 | 775 | 4650 |
In order to evaluate the effect of microwave irradiation (MW) on this transformation, the model reaction was carried out under MW at different conditions (Table 1, entries 17–21). The highest yield was obtained with an applied power of 230 W at 80 °C (Table 1, entry 19).
The scope and generality of this protocol were then investigated in the C–S cross-coupling of various aryl halides with different diaryl disulfides (Table 2). The reaction of a series of aryl iodides and bromides with different diaryl disulfides proceeded effectively in the presence of Pdnp-nSTDP catalyst under the optimized conditions and the desired diaryl sulfides were produced in 93–96% yields (Table 2, entries 1–11).
| Entry | R1 | X | R2 | Thermal | MW | ||
|---|---|---|---|---|---|---|---|
| Time (h) | Yieldb (%) | Time (min) | Yieldb (%) | ||||
a Reaction conditions: aryl halide (1 mmol), diaryl disulfide (0.5 mmol), TBAH (1 mmol), Pdnp-nSTDP (0.1 mol% Pd, 8 mg), H2O/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL), 80 °C or MW (230 W, 80 °C).b Isolated yield. 1, 1 mL), 80 °C or MW (230 W, 80 °C).b Isolated yield. |
|||||||
| 1 | 4-Me | I | H | 3 | 96 | 5 | 95 |
| 2 | 4-Me | I | 4-Me | 3 | 94 | 5 | 95 |
| 3 | 4-OMe | I | 4-Me | 3 | 96 | 5 | 96 |
| 4 | 4-Me | I | 4-Ac | 3 | 95 | 5 | 96 |
| 5 | H | I | 4-Me | 3 | 93 | 5 | 95 |
| 6 | 4-Me | Br | 4-Ac | 5 | 95 | 10 | 92 |
| 7 | 4-Me | Br | H | 5 | 95 | 10 | 93 |
| 8 | H | Br | 4-Me | 5 | 94 | 10 | 89 |
| 9 | 3-OMe | Br | 4-OMe | 5 | 96 | 10 | 93 |
| 10 | 4-Me | Br | 4-OMe | 5 | 95 | 10 | 95 |
| 11 | 4-Me | Br | 4-CHO | 5 | 95 | 10 | 90 |
| 12 | 4-Me | Cl | 2-Me | 10 | 85 | 25 | 82 |
| 13 | 4-Me | Cl | 4-CHO | 10 | 90 | 25 | 86 |
| 14 | 4-Me | Cl | 4-Ac | 10 | 87 | 25 | 90 |
| 15 | 4-Me | Cl | H | 10 | 83 | 25 | 85 |
The applicability of this palladium-catalyzed C–S cross-coupling reactions was also examined using the less reactive but cheaper and more readily available aryl chlorides instead of their bromide and iodide counterparts. As the data revealed, the cross-coupling of aryl chlorides with diaryl disulfides proceeded smoothly in the presence of Pdnp-nSTDP to afford the corresponding products in 83–90% yields (Table 2, entries 12–15). The results in Table 2 disclose that the dendritic polymer accelerates the C–S cross coupling reactions due to its cavities that isolate the catalytic active sites from the surrounding environment and provide an efficient nanoreactors.
The C–S cross-coupling of these aryl halides with diaryl disulfide was also investigated under microwave irradiation, in which the desired products were provided in 82–96% yields within 5–25 min (Table 2). These results clearly showed that microwave irradiation as an eco-friendly technology has the evident advantage of very short reaction times over the conventional heating mode.
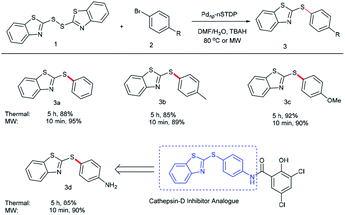
2-(Arylthio)-1,3-benzothiazoles are essential building blocks which are found in a large number of biologically and pharmaceutically active molecules.53,54 Accordingly, we decided to explore the potential of this catalytic system in the synthesis of 2-(arylthio)-1,3-benzothiazoles via C–S cross-coupling of arylbromides with 2,2′-dithiobis(benzothiazole) under conventional heating and microwave irradiation. In this manner, different arylbromides such as bromobenzene, p-tolylbromobenzene and p-methoxybromobenzene were converted to their corresponding sulfides in high to excellent yields (Table 3). One interesting example is 4-(1,3-benzothiazol-2-ylthio)aniline (3d) which is the precursor of cathepsin-D inhibitor. So, this simplified strategy can be potentially utilized for accessing a broad range of pharmaceutically important molecules.55
In order to further broaden the applicability of this method, we studied the one-pot C–S cross-coupling reactions of dibromoarenes with diaryl disulfides or 2,2′-dithiobis(benzothiazole) using this catalytic system. As can be seen, two-fold C–S cross-coupling reaction of 1,4-dibromobenzene, 9,10-dibromoanthracene or 2,6-dibromopyridine with di-p-tolyl disulfide, di-m-methoxyphenyl disulfide or 2,2′-dithiobis(benzothiazole) in the presence of Pdnp-nSTDP catalyst was performed efficiently under conventional heating and microwave irradiation and provided excellent yields of the desired coupling products (Table 4).
| Dibromoarene | Product | Yieldb (%) (time) | |
|---|---|---|---|
| Thermal | MW | ||
a Reaction conditions: dibromoarene or 2,6-dibromopyridine (1 mmol), diaryl or 2,2′-dibenzothiazyl disulfide (1 mmol), TBAH (2 mmol), Pdnp-nSTDP (0.2 mol% Pd, 16 mg), H2O/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL), 80 °C or MW (230 W, 80 °C).b Isolated yield. 1, 2 mL), 80 °C or MW (230 W, 80 °C).b Isolated yield. |
|||
 |
 |
85 (10 h) | 81 (10 min) |
 |
 |
82 (6 h) | 90 (45 min) |
 |
 |
81 (8 h) | 85 (15 min) |
 |
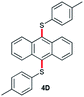 |
90 (6 h) | 94 (12 min) |
 |
 |
90 (6 h) | 93 (10 min) |
 |
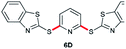 |
75 (6 h) | 89 (10 min) |
 |
 |
85 (6 h) | 85 (10 min) |
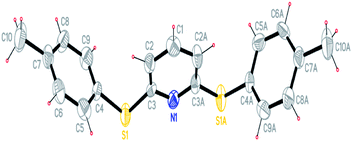
The products were characterized by different analytical tools such FT-IR, 1H NMR, and 13C NMR and elemental analysis. In addition, the compound 5D was characterized by X-ray crystallographic analysis (CCDC 1029634,† Fig. 2).
The recovery and reuse of a catalyst is of practical importance and is economically as well as environmentally attractive. In this manner, the recovery of Pdnp-nSTDP was investigated in model reaction. After completion of the reaction, ethyl acetate was added and the catalyst was separated by centrifugation. The recovered catalyst was washed with DMF, dried and reused for subsequent reactions. As shown in Fig. 3, Pdnp-nSTDP could be recycled and reused at least five times without any significant loss of its activity. The yields of the desired product after six consecutive runs were 93 and 89% under conventional heating and MW irradiation, which show the high efficiency of this method. Moreover, the amount of palladium leached from Pdnp-nSTDP catalyst, measured by ICP-OES, indicated very low leaching of palladium.
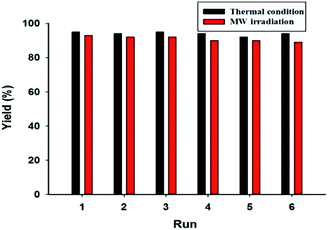 | ||
| Fig. 3 Reusability of the Pdnp-nSTDP catalyst in the C–S cross coupling reaction of 4-bromoanisole and di-p-tolyl disulfide. | ||
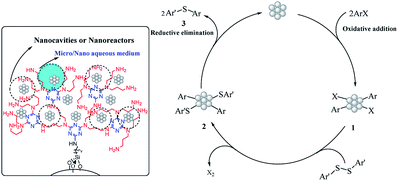
In addition, little increase in the yield of product in the hot filtration test confirmed the low leaching of the Pd species. This means that the leached Pd species are restabilized by the dendritic polymer. Therefore, it seems that the reaction mechanism is identical as a “Cocktail” of catalysts in which the Pd species are leached into the reaction medium, and then C–S coupling reaction occurs in the solution phase. After the corresponding product forms via reductive elimination, the leached Pd species are restabilized on the surface of the dendritic polymer.56–58
Based on these findings, the C–S cross-coupling mechanism is shown in Scheme 2. First, the aryl halide (ArX) is added to palladium nanoparticles via an oxidative-addition pathway to form the organopalladium species 1. Then, aromatic disulfide is added to intermediate 1 to produce the intermediate 2 and released X2.58 Finally, the desired C–S cross-coupling product 3 is formed by a reductive-elimination process and restores the Pdnp-nSTDP catalyst for the next cycle.
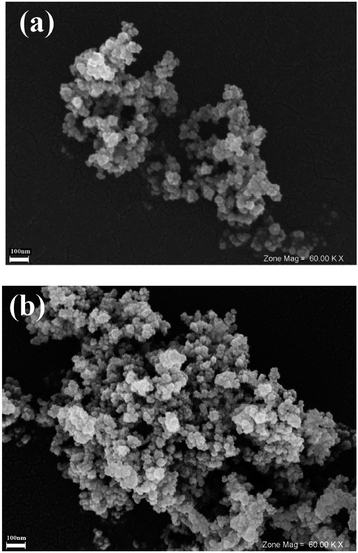
The structure of the catalyst after the final run was also investigated by SEM and compared with the fresh Pdnp-nSTDP catalyst. As shown in Fig. 4, the shape and morphology of Pdnp-nSTDP did not show any significant change after 6th run.
The applicability and reactivity of Pdnp-nSDTP catalyst was also compared with some other previously reported catalysts. As presented in Table 5, the Pdnp-nSDTP catalyst is superior in terms of TOF (h−1), reaction times and amount of catalyst.
| Catalyst and conditions | Aryl halide | Yield (%) | TOF (h−1) | Ref. |
|---|---|---|---|---|
| Cu2S (1 mol%), Fe, K2CO3, DMSO, 110 °C, 18 h |  |
95 | 5.28 | 45 |
| CuFe2O4 (5 mol%), Cs2CO3, DMSO, 100 °C, 24 h | 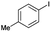 |
90 | 0.75 | 44 |
| PdCl2(dppf) (5 mol%), Zn, THF, reflux, 24 h | 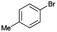 |
68 | 0.57 | 43 |
| Pdnp-nSDTP (0.1 mol%), TBAH, DMF/H2O, 80 °C, 3–5 h | 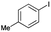 |
93 | 310 | Present work |
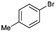 |
94 | 188 |
Experimental
General information
The Pdnp-nSTDP catalyst was synthesized by our reported method.47 Melting points were determined with a Stuart Scientific SMP2 apparatus. FT-IR spectra were recorded on a Nicolet-Impact 400D spectrophotometer. 1H and 13C NMR (400 and 100 MHz) spectra were recorded on a Bruker Avance 400 MHz spectrometer using CDCl3 or DMSO-d6 as solvent. Elemental analysis was done on a LECO, CHNS-932 analyzer. The microwave system used in these experiments includes the following items: Micro-SYNTH labstation, equipped with a glass door, a dual magnetron system with pyramid shaped diffuser, 1000 W delivered power, exhaust system, magnetic stirrer, ‘quality pressure’ sensor for flammable organic solvents, and a ATCFO fiber optic system for automatic temperature control.General procedure for synthesis of diaryl sulfides via C–S cross-coupling catalyzed by Pdnp-nSTDP
A mixture of aryl halide (1 mmol), diaryl disulfide or 2,2′-dithiobis(benzothiazole) (0.5 mmol), TBAH (1 mmol) and Pdnp-nSTDP (0.1 mol% Pd, 8 mg) in H2O/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) was stirred at 80 °C or irradiated with MW (230 W, 80 °C) for the time indicated in Tables 2 and 3. The reaction progress was followed by TLC (eluent: ether/ethyl acetate, 5
1, 1 mL) was stirred at 80 °C or irradiated with MW (230 W, 80 °C) for the time indicated in Tables 2 and 3. The reaction progress was followed by TLC (eluent: ether/ethyl acetate, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). At the end of the reaction, the mixture was cooled to room temperature, ethyl acetate (10 mL) was added and the catalyst was separated by centrifugation. The organic phase was washed with water (2 × 10 mL) and dried over anhydrous MgSO4. The solvent was evaporated and the resulting crude material was purified by recrystallization from ether and ethyl acetate (5
1). At the end of the reaction, the mixture was cooled to room temperature, ethyl acetate (10 mL) was added and the catalyst was separated by centrifugation. The organic phase was washed with water (2 × 10 mL) and dried over anhydrous MgSO4. The solvent was evaporated and the resulting crude material was purified by recrystallization from ether and ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure product.
1) to afford the pure product.
General procedure for synthesis of disulfides via two-fold C–S cross-coupling catalyzed by Pdnp-nSTDP
A mixture of 1,4-dibromobenzene, 9,10-dibromoanthracene or 2,6-dibromopyridine (1 mmol), diaryl/2,2′-dibenzothiazyl disulfide (1 mmol), TBAH (2 mmol) and Pdnp-nSTDP (0.2 mol% Pd, 16 mg) in H2O/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 mL) was stirred at 80 °C or exposed to microwave irradiation (230 W, 80 °C) for the time mentioned in Table 4. The reaction progress was check by TLC (eluent: ether/ethyl acetate, 3
1, 2 mL) was stirred at 80 °C or exposed to microwave irradiation (230 W, 80 °C) for the time mentioned in Table 4. The reaction progress was check by TLC (eluent: ether/ethyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). When the reaction was completed, the mixture was cooled to room temperature, ethyl acetate (10 mL) was added and the catalyst was separated by centrifugation. The organic phase was washed with water (2 × 10 mL), dried over anhydrous MgSO4. Evaporation of the filtrate followed by purification of the crude material by recrystallization from ethyl acetate provided the pure product.
1). When the reaction was completed, the mixture was cooled to room temperature, ethyl acetate (10 mL) was added and the catalyst was separated by centrifugation. The organic phase was washed with water (2 × 10 mL), dried over anhydrous MgSO4. Evaporation of the filtrate followed by purification of the crude material by recrystallization from ethyl acetate provided the pure product.
Conclusions
In conclusion, we have developed an eco-friendly and convenient method for the preparation of a series of diaryl sulfides via C–S cross-coupling of aryl halides with diaryl/diheteroaryl disulfides catalyzed by Pdnp-nSTDP under conventional heating and microwave irradiation. This catalytic system was also efficiently used for the one-pot two-fold C–S cross-coupling reactions of dibromoarenes in high yields. The high performance as well as the recyclability of this dendritic catalyst as a nanoreactor makes this method a valid contribution compared to the existing methodologies.Conflicts of interest
There is no conflict of interest.Acknowledgements
The authors are thankful to the Research Council of the University of Isfahan for financial support of this work.Notes and references
- R. Breslow and L. E. Overman, J. Am. Chem. Soc., 1970, 92, 1075–1077 CrossRef CAS PubMed.
- S. H. Petrosko, R. Johnson, H. White and C. A. Mirkin, J. Am. Chem. Soc., 2016, 138, 7443–7445 CrossRef CAS PubMed.
- W. L. Mock, in Supramolecular Chemistry II—Host Design and Molecular Recognition, Springer, 1995, pp. 1–24 Search PubMed.
- D. M. Vriezema, M. Comellas Aragonès, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan and R. J. M. Nolte, Chem. Rev., 2005, 105, 1445–1490 CrossRef CAS PubMed.
- G. Stephenson, R. M. Parker, Y. Lan, Z. Yu, O. A. Scherman and C. Abell, Chem. Commun., 2014, 50, 7048–7051 RSC.
- D. G. Shchukin and G. B. Sukhorukov, Adv. Mater., 2004, 16, 671–682 CrossRef CAS.
- H.-L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351–3370 RSC.
- S. Rezaei, A. Landarani-Isfahani, M. Moghadam, S. Tangestaninejad, V. Mirkhani and I. Mohammadpoor-Baltork, RSC Adv., 2016, 6, 92463–92472 RSC.
- A. Hamel, M. Sacco, N. Mnasri, F. Lamaty, J. Martinez, F. De Angelis, E. Colacino and C. Charnay, ACS Sustainable Chem. Eng., 2014, 2, 1353–1358 CrossRef CAS.
- F. Vögtle, G. Richardt and N. Werner, Dendrimer chemistry: concepts, syntheses, properties, applications, John Wiley & Sons, 2009 Search PubMed.
- D. Astruc and F. Chardac, Chem. Rev., 2001, 101, 2991–3024 CrossRef CAS PubMed.
- K. Yamamoto, T. Imaoka, M. Tanabe and T. Kambe, Chem. Rev., 2020, 120, 1397–1437 CrossRef PubMed.
- G. E. Oosterom, J. N. Reek, P. C. Kamer and P. W. van Leeuwen, Angew. Chem., 2001, 113, 1878–1901 CrossRef.
- D. Astruc, M.-C. Daniel and J. Ruiz, Chem. Commun., 2004, 2637–2649 RSC.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- F. Minghao, T. Bingqing, H. L. Steven and J. Xuefeng, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef PubMed.
- M.-L. Alcaraz, S. Atkinson, P. Cornwall, A. C. Foster, D. M. Gill, L. A. Humphries, P. S. Keegan, R. Kemp, E. Merifield and R. A. Nixon, Org. Process Res. Dev., 2005, 9, 555–569 CrossRef CAS.
- A. Gangjee, Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk and S. F. Queener, J. Med. Chem., 2007, 50, 3046–3053 CrossRef CAS PubMed.
- S. Pasquini, C. Mugnaini, C. Tintori, M. Botta, A. Trejos, R. K. Arvela, M. Larhed, M. Witvrouw, M. Michiels, F. Christ, Z. Debyser and F. Corelli, J. Med. Chem., 2008, 51, 5125–5129 CrossRef CAS PubMed.
- G. Liu, J. R. Huth, E. T. Olejniczak, R. Mendoza, P. DeVries, S. Leitza, E. B. Reilly, G. F. Okasinski, S. W. Fesik and T. W. von Geldern, J. Med. Chem., 2001, 44, 1202–1210 CrossRef CAS PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337–2364 CrossRef CAS.
- J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651–2710 CrossRef CAS PubMed.
- A. Correa, M. Carril and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 2880–2883 CrossRef CAS PubMed.
- S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586–5587 CrossRef CAS PubMed.
- Y.-Y. Lin, Y.-J. Wang, C.-H. Lin, J.-H. Cheng and C.-F. Lee, J. Org. Chem., 2012, 77, 6100–6106 CrossRef CAS PubMed.
- A. K. Verma, J. Singh and R. Chaudhary, Tetrahedron Lett., 2007, 48, 7199–7202 CrossRef CAS.
- M. Carril, R. SanMartin, E. Domínguez and I. Tellitu, Chem.–Eur. J., 2007, 13, 5100–5105 CrossRef CAS PubMed.
- A. Sujatha, A. M. Thomas, A. P. Thankachan and G. Anilkumar, ARKIVOC, 2015, 1, 1–28 Search PubMed.
- X.-B. Xu, J. Liu, J.-J. Zhang, Y.-W. Wang and Y. Peng, Org. Lett., 2013, 15, 550–553 CrossRef CAS PubMed.
- K. Yang, Y. Wang, X. Chen, A. A. Kadi, H.-K. Fun, H. Sun, Y. Zhang and H. Lu, Chem. Commun., 2015, 51, 3582–3585 RSC.
- Y.-C. Wong, T. T. Jayanth and C.-H. Cheng, Org. Lett., 2006, 8, 5613–5616 CrossRef CAS PubMed.
- R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed.
- V. P. Reddy, K. Swapna, A. V. Kumar and K. R. Rao, J. Org. Chem., 2009, 74, 3189–3191 CrossRef CAS PubMed.
- T. Scattolin, E. Senol, G. Yin, Q. Guo and F. Schoenebeck, Angew. Chem., Int. Ed., 2018, 57, 12425–12429 CrossRef CAS PubMed.
- Z. Qiao, J. Wei and X. Jiang, Org. Lett., 2014, 16, 1212–1215 CrossRef CAS PubMed.
- C. Valente, M. Pompeo, M. Sayah and M. G. Organ, Org. Process Res. Dev., 2014, 18, 180–190 CrossRef CAS.
- J. Xu, R. Y. Liu, C. S. Yeung and S. L. Buchwald, ACS Catal., 2019, 9, 6461–6466 CrossRef CAS PubMed.
- A. Landarani-Isfahani, I. Mohammadpoor-Baltork, V. Mirkhani, M. Moghadam, A. R. Khosropour, S. Tangestaninejad, M. Nasr-Esfahani and H. A. Rudbari, Synlett, 2014, 25, 645–652 CrossRef.
- R. Luque, J. H. Clark, K. Yoshida and P. L. Gai, Chem. Commun., 2009, 5305–5307 RSC.
- Y. Feng, H. Wang, F. Sun, Y. Li, X. Fu and K. Jin, Tetrahedron, 2009, 65, 9737–9741 CrossRef CAS.
- N. Taniguchi, J. Org. Chem., 2004, 69, 6904–6906 CrossRef CAS PubMed.
- O. Baldovino-Pantaleón, S. Hernández-Ortega and D. Morales-Morales, Adv. Synth. Catal., 2006, 348, 236–242 CrossRef.
- S.-i. Fukuzawa, D. Tanihara and S. Kikuchi, Synlett, 2006, 2006, 2145–2147 CrossRef.
- K. Swapna, S. N. Murthy, M. T. Jyothi and Y. V. D. Nageswar, Org. Biomol. Chem., 2011, 9, 5989–5996 RSC.
- H. Wang, L. Jiang, T. Chen and Y. Li, Eur. J. Org. Chem., 2010, 2010, 2324–2329 CrossRef.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- A. Landarani Isfahani, I. Mohammadpoor-Baltork, V. Mirkhani, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and R. Kia, Adv. Synth. Catal., 2013, 355, 957–972 CrossRef.
- M. Nasr-Esfahani, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, V. Mirkhani, S. Tangestaninejad and H. Amiri Rudbari, J. Org. Chem., 2014, 79, 1437–1443 CrossRef CAS PubMed.
- A. Daneshvar, M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor-Baltork and A. Khalili, Organometallics, 2016, 35, 1747–1755 CrossRef CAS.
- M. Zakeri, M. Moghadam, V. Mirkhani, S. Tangestaninejad, I. Mohammadpoor-Baltork and Z. Pahlevanneshan, RSC Adv., 2016, 6, 104608–104619 RSC.
- R. Bernini, S. Cacchi, G. Fabrizi, G. Forte, F. Petrucci, A. Prastaro, S. Niembro, A. Shafir and A. Vallribera, Green Chem., 2010, 12, 150–158 RSC.
- C. Liu, Q. Ni, F. Bao and J. Qiu, Green Chem., 2011, 13, 1260–1266 RSC.
- J. Kočí, V. Klimešová, K. Waisser, J. Kaustová, H.-M. Dahse and U. Möllmann, Bioorg. Med. Chem. Lett., 2002, 12, 3275–3278 CrossRef.
- G. P. Nagaraju and B. Reddy, Exploring pancreatic metabolism and malignancy, Springer, 2019 Search PubMed.
- T.-Y. Lin and H. R. Williams, J. Biol. Chem., 1979, 254, 11875–11883 CAS.
- A. Ohtaka, M. Kawase, A. Usami, S. Fukui, M. Yamashita, K. Yamaguchi, A. Sakon, T. Shiraki, T. Ishida and S. Nagata, ACS Omega, 2019, 4, 15764–15770 CrossRef CAS PubMed.
- D. B. Eremin and V. P. Ananikov, Coord. Chem. Rev., 2017, 346, 2–19 CrossRef CAS.
- M. V. Polynski and V. P. Ananikov, ACS Catal., 2019, 9, 3991–4005 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectrum. CCDC 1029634. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra00719f |
| This journal is © The Royal Society of Chemistry 2020 |