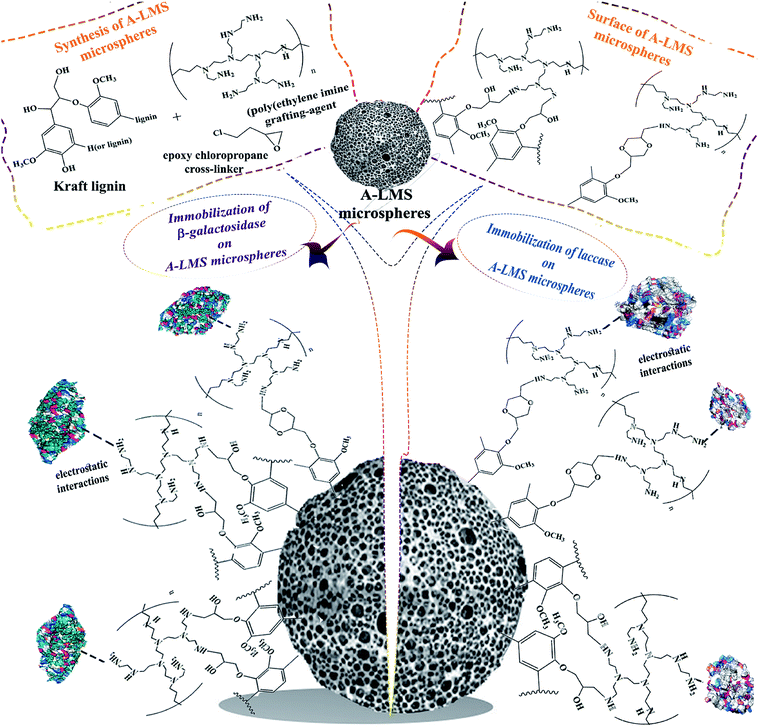
Open Access Article
Open Access ArticleAmino-modified kraft lignin microspheres as a support for enzyme immobilization†
Jelena Bebića,
Katarina Banjanac *ab,
Jelena Rusmirovićc,
Marija Ćorovićd,
Ana Milivojevićb,
Milica Simovićd,
Aleksandar Marinkoviće and
Dejan Bezbradicad
*ab,
Jelena Rusmirovićc,
Marija Ćorovićd,
Ana Milivojevićb,
Milica Simovićd,
Aleksandar Marinkoviće and
Dejan Bezbradicad
aDirectorate of Measures and Precious Metals, Mike Alasa 14, 11000 Belgrade, Serbia
bInnovation Centre of Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, 11000 Belgrade, Serbia. E-mail: kbanjanac@tmf.bg.ac.rs
cMilitary Technical Institute, Ratka Resanovića 1, 11000 Belgrade, Serbia
dDepartment of Biochemical Engineering and Biotechnology, Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, 11000 Belgrade, Serbia
eDepartment of Organic Chemistry, Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, 11000 Belgrade, Serbia
First published on 4th June 2020
Abstract
In this research, it has been demonstrated that amino-modified microspheres (A-LMS) based on bio-waste derived material, such as kraft lignin, have good prospects in usage as a support for enzyme immobilization, since active biocatalyst systems were prepared by immobilizing β-galactosidase from A. oryzae and laccase from M. thermophila expressed in A. oryzae (Novozym® 51003) onto A-LMS. Two types of A-LMS were investigated, with different emulsifier concentrations (5 wt% and 10 wt%), and microspheres produced using 5 wt% of emulsifier (A-LMS_5) showed adequate pore shape, size and distribution for enzyme attachment. The type of interactions formed between enzymes (β-galactosidase and laccase) and A-LMS_5 microspheres demonstrated that β-galactosidase is predominantly attached via electrostatic interactions while attachment of laccase is equally governed by electrostatic and hydrophobic interactions. Furthermore, the A-LMS_5-β-galactosidase exhibited specificity towards recognized prebiotics (galacto-oligosaccharides (GOS)) synthesis with 1.5-times higher GOS production than glucose production, while for environmental pollutant lindane degradation, the immobilized laccase preparation exhibited high activity with a minimum remaining lindane concentration of 22.4% after 6 days. Thus, this novel enzyme immobilization support A-LMS_5 has potential for use in green biotechnologies.
Introduction
Immobilization is a commonly used technique for improving the possible extensive industrial application of enzymes. Immobilized enzymes are more stable at usually non favourable process conditions, can be used in continuous operations and more easily separated from the reaction media by non-chemical means, thus preventing product contamination by enzymes.1–3 The selection of the carrier is based on the most important parameters, which are immobilization capacity and activity recovery and stability.So far, a large variety of carriers was used for the immobilization of different enzymes. In the selection of a support, several characteristics should be considered, such as high affinity for protein, large internal and external surface area, high density of reactive groups with minimized steric hindrances with enzyme reactive groups and sufficient mechanical stability, for the intended use, inert surface of the carrier after the enzyme separation, non-toxicity and biodegradability.4 The commonly used supports for enzyme immobilization include solid beads, porous particles, capsules, membranes and gels. Regarding the surface and the shape of the carrier, spherical carriers have been investigated in recent years, particularly nano- and micro-sized materials, with differing porosity, pore distribution, mechanical properties and chemical scaffold. Preferably, they are made of biocompatible materials, and can be, for example, porous microspheres, microcapsules, smart gel microspheres, nanospheres or magnetic beads.5 For mesoporous and macroporous microspheres, in order to have high enzyme loadings and to reduce diffusion limitations, the larger pore sizes are more preferred, as well as the available surface inside the sphere. Materials that have been used so far, for this types of carriers can be organic, inorganic and hybrid or composite, like inorganic particles (glass beads, zeolites, mesoporous silicas), carbon-based materials, polystyrene and polymethyl meth-acrilate resins, polystyrene, biopolymers and others.5 Since most of commercial supports used for enzyme immobilization (acrylic resins, synthetic polymers, active membranes and exchange resins) are synthetic polymers, they are not always safe for usage in the food processing, pharmaceutical and cosmetic industry due to the possibility of their chemical components leakage. Novel research in this area is based on new materials, as support for enzyme immobilization, which are natural, non-toxic and inexpensive such as materials of organic origin, hybrid and composite materials. Regardless of the material type, for the formation of stable and efficient biocatalytic systems, the optimization of the attachment technique must be performed individually, for the specific enzyme, the specific material and the intended biocatalytic process.6
In present study, the research will be focused on exploring the possibility to exploit the amino-modified lignin microspheres (A-LMS) with abundant amine functional groups as a carrier for enzyme immobilization (Scheme 1). As a precursor for synthesis of A-LMS, kraft lignin is used. The kraft lignin is a type of industrial lignin which is obtained as residue from kraft pulping process. In the pulping process, the lignin macromolecules are fractured resulting in decrease of the molecular weight by dissolving the lignin in alkaline solution, making the solution turn dark brown (“black liquor”). The kraft lignin molecules are isolated by pH controlled precipitation of the “black liquor”. Since chemical structure of native lignin is complex and depends on plant species from which it is isolated, the complexity of its chemical structure becomes more pronounced by applied delignification process with the kraft pulping process being of no exception. In general, chemically, native lignin consists of highly complex phenyl-propanoid (C9) polyphenols oligomers, which are linked by arylglycerol ether bonds between phenolic para-coumaryl alcohol (H-type), coniferyl alcohol (G-type), and sinapyl alcohol (S-type) units.7 The chemical structure of used sample of a kraft lignin is presented on Scheme 1. Since, the 70% to 75% of the hydroxyl groups of kraft lignin are sulfonated, the kraft lignin express high solubility in polar organic solvents, as well as in alkali and basic solutions. It should be emphasized that the kraft lignin is relatively cheap bio-waste derived material which has a positive impact with regard to the economic aspects of A-LMS synthesis.
In course of A-LMS microspheres formation, the process of copolymerization between kraft lignin, poly(ethylene imine) grafting-agent and epoxy chloropropane cross-linker is optimized and described in study of Popovic et al.8 In their study, the structural characterization (the particle size, distribution and morphology) of the synthetized A-LMS, has been done by using field emission scanning electron microscopy (FESEM) by Tescan Mira 3 FEG, the specific surface area of the adsorbents has been calculated according to the Brunauer–Emmett–Teller (BET) while for the calculation of the pore volume the Barrett–Joyner–Halenda (BJH) method has been used.8 So far, these microspheres, produced from a natural material-kraft lignin, have demonstrated good adsorption properties and potential use in bioremediation.8,9 The authors of Popovic et al.'s study8 were kindly enough to provide A-LMS microspheres in order to be used in this research, since created A-LMS microspheres with defined properties give us the possibility of combining the undoubted advantages of kraft lignin precursor, such as biocompatibility and non-toxicity, in the process of enzyme immobilization. The presence of amino functional groups in the surface of A-LMS increases their affinity to biomolecules. Moreover, as an immobilization support, lignin has been used so far in chitin-lignin novel matrix for immobilization of lipase by adsorption, and silica-lignin matrix as a stable and reusable biocatalytic system, but there are no reports in the literature concerning the use of amino-modified microspheres based on kraft lignin in enzyme immobilization.10,11
The main goal of the present study was to investigate the possibility of use of the A-LMS microspheres as a novel matrix for immobilization of the β-galactosidase from Aspergillus oryzae and laccase from Myceliophthora thermophila expressed in Aspergillus oryzae (Novozym 51003® laccase) (Scheme 1). Two types of A-LMS microspheres (A-LMS_5 and A-LMS_10), with different characteristics were selected to be examined in preliminary experiments as supports for two mentioned enzymes. These two enzymes were chosen to be used as study models due to the fact that in the previous published studies it was demonstrated that both of these enzymes have exhibited affinity toward supports with free amino groups on their surfaces.12–14 The immobilization on A-LMS was studied by determination of most important immobilization parameters such as adsorption kinetics, protein immobilization yield, activity of immobilized preparations and activity immobilization yield. Furthermore, the type of interactions between enzymes (laccase and β-galactosidase) and A-LMS were investigated and discussed to evaluate the potential of A-LMS as a carrier. Additionally, the possibility of produced laccase and β-galactosidase immobilized preparations application in the degradation of pesticide lindane (γ-hexachlorocyclohexane) and in galacto-oligosaccharide (GOS) synthesis, respectively, were also investigated.
Experimental
Commercial laccase from M. thermophila expressed in A. oryzae (Novozym® 51003) was kind gift of Novozymes (Basgvaerd, Denmark). β-Galactosidase from A. oryzae, Bradford reagent, 4-hydroxy-3,5-dimethoxybenzaldehyde azine (syringaldazine), o-nitrophenol-β-D-galactopyranoside (o-NPG), lactose, methanol, acetone and hexane were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Lindane PESTANAL CRM, 99.8%, was purchased from Sigma-Aldrich GmbH Germany and produced by Riedel-de Haën. All others chemicals were of analytical grade and used without further purification.Both types of A-LMS (A-LMS_5 and A-LMS_10), that are used in this study, were kindly provided by our colleagues from Department of Organic Chemistry, Faculty of Technology and metallurgy, University of Belgrade, Serbia. The synthesis procedure and characterization of used A-LMS, are in detail described and discussed in their previously reported study.8 Since in that study,8 it has been demonstrated that this bio-waste based amino-modified microspheres have excellent adsorption properties and have good potential use in bioremediation, our intention was to expand their application potential. Additionally, the applied synthesis procedure and instrumental methods that are relevant for characterization of synthetized A-LMS are provided in the ESI (Fig. 1 and 2).†
β-Galactosidase and laccase immobilization procedures
In order to select adequate carrier for β-galactosidase immobilization, the 20 mg of A-LMS_5 and A-LMS_10 were mixed with 5 mg of β-galactosidase powder dissolved in 1 mL of 0.1 M buffer. The several different buffers were used in range of pH 3.2 to pH 7.2. For laccase immobilization experiments, the 10 mg of A-LMS_5 and A-LMS_10 were mixed with 1 mL of enzyme preparation dissolved in buffer. The different buffers used were in range of pH 4.0 to pH 7.0. For pH 4.0 and 5.5, 0.1 M Na-acetate buffer was applied, while for pH 6.0 and 7.0, it was 0.1 M Na-phosphate buffer. The immobilization of both enzymes on these two supports was performed under stirring within an orbital shaker at a speed of 150 rpm and at temperature of 25 °C. After immobilization, obtained supernatants were assayed for protein concentration and activity, and the activity of immobilized preparations were measured after being washed three times with buffer used. After the selection of immobilization carrier, the offered protein concentration (2 mg–175 mg proteins per g support for β-galactosidase and 28 mg–140 mg proteins per g of support for laccase) and optimum pH and immobilization time (1 h–7 h) were examined. All experiments regarding immobilization of both enzymes were performed in duplicate, and the mean values of obtained results (the activity of immobilized preparations (IU per g support), the protein immobilization yields (%), and the specific activities (IU per mg of proteins)) were presented.Enzyme activity assay
The hydrolytic and oxidative activities of the free and immobilized enzymes on A-LMS_5 and A-LMS_10 were determined using 10 mM o-nitrophenol-β-D-galactopyranoside (o-NPG) in 0.1 M acetate buffer (pH 4.5) as a substrate for β-galactosidase, and 0.216 mM solution of syringaldazine in HPLC methanol as a substrate for laccase. The reaction mixture contains 0.1 mL free enzyme solution or supernatant and 0.9 mL substrate.The hydrolytic activity of free β-galactosidase in reaction with o-NPG (IU mL−1) is measured at pH 4.5, since in our previously reported study, it was determined that pH–activity optimum for this enzyme–substrate complex is at pH 4.5.15 In the case of free laccase (Novozym® 51003), the activity measurements (IU mL−1) are preformed at pH 6.5, whereas this is the recommended pH according to used standard activity assay procedure.16
In the case of immobilized β-galactosidase, reaction mixture contains 20 mg of immobilized preparation with 2 mL of substrate (10 mM o-NPG dissolved in 0.1 M acetate buffer (pH 4.5)) under vigorous magnetic stirring. The reaction was allowed to proceed for at least 3 minutes. The o-nitrophenol (o-NP) released in reaction was measured on spectrophotometer each 30 seconds at 410 nm. One unit is defined as the amount of the enzyme that catalyses the liberation of 1 μmol o-NP per min under the assay conditions.
In the case of immobilized laccase, 10 mg of immobilized preparation was suspended in 40.5 mL of 0.1 M Na-phosphate buffer (pH 6.5) and then the 4.5 mL of substrate solution was added. The reaction mixture (total volume of 50 mL) is placed on magnetic stirrer. The activity of immobilized enzyme (IU per g support) was monitored by sampling 1 mL of reaction mixture and measuring absorbance at 530 nm every 15 seconds for 2 min. After each measurement, the sample was returned to the reaction mixture to avoid the potential impact of the reaction mixture volume reduction on the measurement results.
At start of each the activity measurement of immobilized preparations, the absorbance of sample which consisted of suspended A-LMS microspheres in substrate solution (blank sample) was measured in order to eliminate the error due to potential light scattering. This means that with the null-point correction based on wavelength-independent scattering error, the corrected absorption spectra for each activity measurement of immobilized preparations were obtained.
The activities of immobilized preparations are measured at the same pH as in case of the free enzymes due to the fact that preliminary performed experiments demonstrated that the immobilization did not cause changes in pH–activity profile of enzymes (data not shown).
Protein assay
Concentration of proteins in enzyme solutions and supernatants was assayed according to well established procedure of Bradford using bovine serum albumin as the standard.17 The amount of bound enzyme was determined indirectly from the deference between the amount of enzyme introduced into the reaction mixture and the amount of enzyme remain in the supernatant after the immobilization.For investigation of the relationship between the amounts of enzyme absorbed onto A-LMS_5, and the equilibrium concentration of enzyme, the Langmuir adsorption isotherm model, described by the following equation, was used:18,19
| qe = qmbCe/(1 + bCe) | (1) |
For investigation of enzyme diffusion from solution onto support surface the Lagergren's rate equation (pseudo-first-order kinetic model), described by following equation, is used:20,21
| qt = qe(1 − e−k1t) | (2) |
Desorption assay
To investigate the type of interactions formed between β-galactosidase or laccase and selected support (A-LMS_5), the immobilized preparations obtained under previously determined optimal conditions were treated separately with 1% Triton X-100 and 1 M CaCl2. For both enzymes the same procedure was applied: the 20 mg of the immobilized enzyme was suspended in 1 mL of the desorption solution (Triton X-100 or CaCl2) and incubated for one hour under stirring in an orbital shaker at a speed of 150 rpm and at temperature of 25 °C. After desorption treatment, the immobilized preparation was washed thoroughly with immobilization buffer and then the residual activity (%) was determined.β-Galactosidase-catalyzed GOS synthesis
The synthesis of GOS with free and immobilized enzyme is performed according to established and described procedure in our previously reported study.13 In short, the reaction of GOS synthesis was performed in flasks (50 mL) placed on an orbital shaker at 200 rpm at 50 °C for 24 h. The reaction mixture was composed of 400 g L−1 lactose solution in 0.1 M acetate buffer pH 4.5 and 60 mg of immobilized enzyme. The procedures for sampling, sample treatment as well as the quantitative analysis (HPLC analysis) of samples are provided in the ESI.†Laccase-catalyzed lindane degradation
The reaction of lindane degradation with free and immobilized laccase on A-LMS_5 is monitored in the same way as described in our previously published study.14 Detailed procedure is also provided in the ESI.†FT-IR analysis
Fourier transforms-infrared (FT-IR) spectra of the samples were recorded in absorbance mode using a Nicolet™ iS™ 10 FT-IR Spectrometer (Thermo Fisher SCIENTIFIC) with Smart iTR™ Attenuated Total Reflectance (ATR) Sampling accessories, within a range of 400–4000 cm−1, at a resolution of 4 cm−1 and in 20 scan mode.Results and discussion
Characterization and selection of support
It is well known that the available surface for enzyme attachment and the interaction forces formed between functional groups present on support surface and surface of enzyme molecule are crucial for enzyme immobilization onto solid supports.22 Moreover, morphological and physical characteristics of support have significant effect on enzyme immobilization process as well as on its catalytic properties, since during immobilization process the support and enzyme are in direct contact.23 One of the desired properties of support is the porosity because of the large surface areas and greater number of pores will lead to higher protein loading per unit mass of support and the immobilized enzyme will be protected from the environment. However, the size of pores should be sufficient to allow unhampered substrate diffusion to the enzyme active site as well as higher enzyme flexibility inside of pores.24 Due to these facts, two types of A-LMS microspheres (A-LMS_5 and A-LMS_10), with different morphology, porosity and amino group content (Table 1, ESI Fig. 1 and 2†), are selected to be examined in preliminary experiments as supports for β-galactosidase and laccase immobilization. In previously published study of Popovic et al.,8 it has been shown that the main influence on textural/morphological properties of A-LMS has the initial amount of emulsifier (sodium alginate solution) added in polymerization mixture. Also, it has been proven that the emulsifier will stabilize growth of new particles while, on the other hand, the emulsifier will prevent aggregation/flocculation and coalescence.8 As a result of this finding, for synthesis of A-LMS_5 and A-LMS_10, the same procedure was applied, the only difference was in amount of used alginate emulsifier solution: A-LMS_5 were obtained by utilization of 5 wt% of emulsifier, while during synthesis of A-LMS_10, 10 wt% of emulsifier was used.| Sample | Specific surface area SBET, m2 g−1 | Mean diameter, μm | Average pore diameter Dmn, nm | Maximum pore diameter Dmax, nm | Amino group content, mmol g−1 |
|---|---|---|---|---|---|
| A-LMS_5 | 7.68 | 800 ± 80 | 12.36 | 25–50 | 7.7 |
| A-LMS_10 | 2.38 | 800 ± 80 | 22.22 | 20.32–59.77 | 6.5 |
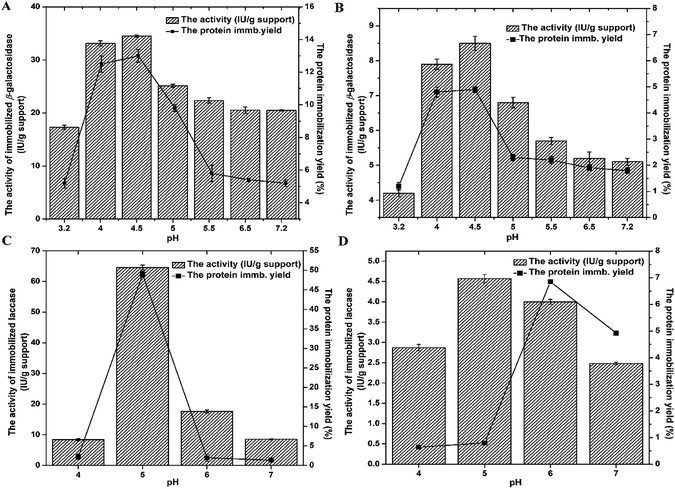
The preliminary screening of A-LMS_5 and A-LMS_10 as supports for enzyme immobilization was performed on the basis of immobilization efficiency parameters (the protein immobilization yield (%) and the activity of immobilized preparation (IU per g support)) obtained when the immobilization is performed at different pH (Fig. 1).
Based on results presented on Fig. 1, it is evident that during immobilization on A-LMS_5 and A-LMS_10, the protein immobilization yields and the activities of both immobilized enzymes (β-galactosidase and laccase) exhibited the same trend throughout examined pH range. Also, for both enzymes, the highest yields of immobilized proteins per mass of microspheres and activities of immobilized preparations are achieved when immobilization is performed on the A-LMS_5 microspheres. If we compare the characteristics of these two microspheres, given in Table 1, ESI Fig. 1 and 2,† with respect of immobilization efficiency, although, the microspheres A-LMS_5 and A-LMS_10 are equal in size of 800 μm ± 80 μm, the A-LMS_5 have 3 times higher specific surface area then A-LMS_10 meaning that this microspheres have larger surface available for enzyme attachment which resulted in higher achieved protein immobilization yields.
Generally, it can be presumed that immobilization of enzymes on amino-modified microspheres (A-LMS) occurs by adsorption via electrostatic interactions due to abundance of amino groups, as well as by hydrogen bonding, van-der-Waals forces and hydrophobic interactions (Scheme 1).
Additionally, from Fig. 1 it can be also seen that there is the significant influence of pH on adsorption process. At pH values above enzyme isoelectric point (pI), the enzyme molecules will bear negative charge meaning that they will be more prone to interact with positively charged functional groups of support.25 The pI is around 4.6 for β-galactosidase from A. oryzae and 4.0 for Novozym® 51003 laccase.13,14 Since surfaces of the both supports (A-LMS_5 and A-LMS_10) are positively charged throughout examined pH range due to the presence of surface NH2 functional groups, which in an acidic environment can undergo protonation to NH3+, it can be assumed that the difference in distribution and amount of introduced amino groups on A-LMS_5 and A-LMS_10, 7.7 mmol g−1 and 6.5 mmol g−1, respectively, cause large discrepancies in efficiency of immobilization on A-LMS_5 and A-LMS_10 in case of both enzymes. Namely, the results obtained for the activity of both enzymes are several times lower in the case of A-LMS_10 as a carrier, than for the immobilization on A-LMS_5. Beside the influence of above mentioned characteristics of microspheres on immobilization process, distribution of negatively charged residues and properties of residues in their vicinity on enzyme molecule have great impact on occupied orientation and three-dimensional structure of immobilized enzymes and are crucial to ensure high enzyme activity.26 Generally, the substantial changes in the surface microenvironment, conformation and protein refolding could occur to enzyme molecule as result of immobilization process.6 This could also explain the dramatically reduced activity of both enzymes on A-LMS_10. The immobilized β-galactosidase exhibited the highest activities and protein immobilization yields at pH 4–4.5 during immobilization on A-LMS_5, meaning that enzyme occupied favourable enzyme conformational structure followed by the convenient amount of immobilized molecules per g of support. These findings are in accordance with previously reported studies in which it is reported that optimal pH for immobilization of β-galactosidase on amino supports was in pH range 4.0–5.0.13 The immobilized laccase exhibited significant activity and protein immobilization yield at pH value 5.0 for immobilization on A-LMS_5. The activity of immobilized laccase at pH 5.0 was 6 and 3 times higher than at pH 4.0 and pH 6.0, respectively. Thus the pH value of 5.0 was adopted as the optimum for immobilization of laccase from M. thermophila on A-LMS_5 which is in accordance with our previously reported study where immobilization of laccase on amino-modified fumed silica nanoparticles was examined.14 Based on all obtained data, for both enzymes, further experiments were performed at pH value 4.5 for β-galactosidase and at pH value 5.0 for laccase, for immobilization on A-LMS_5 as a selected support.
Immobilization of β-galactosidase on amino-modified microspheres with 5 wt% of alginate
In general, there are essentially three consecutive steps in the adsorption of enzymes from solution onto porous supports such as A-LMS_5, namely bulk diffusion, film diffusion and pore diffusion. Each of these steps can be “rate-limiting” in adsorption, but both pore and film diffusion were considered to be the major factors controlling rates of adsorption from solution onto porous supports.30
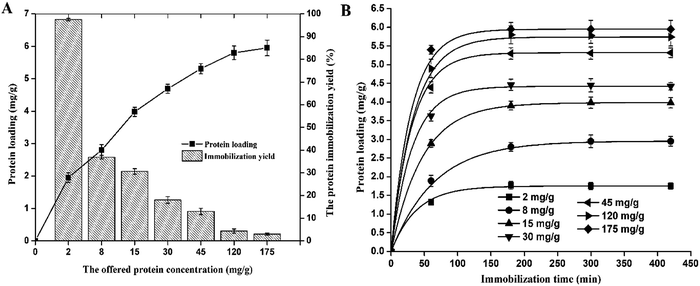
β-Galactosidase adsorption on A-LMS_5 could be described by a Langmuir adsorption model (given by eqn (1)) with constants of qm = 6.03 mg g−1, b = 88.46 and regression coefficient R2 = 0.986 indicating unrestricted monolayer adsorption (ESI Fig. 3†).
Furthermore, in order to describe the rate of enzyme diffusion from solution onto support surface (bulk diffusion), the experimental data for adsorption kinetics of β-galactosidase on A-LMS_5 are fitted by pseudo-first-order kinetic model (eqn (2)).19 The fitted curves are presented on Fig. 2B and obtained parameters were given in ESI Table 1.† The obtained high values of regression coefficient R2 (0.999–1) indicate that the applied model successfully describes the rate of β-galactosidase diffusion from solution onto A-LMS_5. Moreover, it can be seen that value of the rate constant increase with the increase of the offered protein concentration from 0.0188 min−1 to 0.0390 min−1 meaning that the number of vacant binding cites for β-galactosidase attachment is limited after initial stage of adsorption process was finished.
In order to get more information concerning the adsorption mechanism, the data were further investigated by applying of intra-particle diffusion model which is represented by the following Weber and Morris equation:31
| qt = kid × t1/2 + C | (3) |
Weber and Morris plot of qt versus t1/2 is shown in ESI Fig. 3† for β-galactosidase adsorption on A-LMS_5 at offered protein concentration of 30 mg g−1. The plot qt–t1/2 consisted of two linear sections indicating that two steps contribute to the adsorption process. The first step is the bulk and film diffusion, while second step is pore diffusion. The calculated parameters of the intra-particle diffusion model are summarized in ESI Table 2.† Since the value of kid, related to the pore diffusion, is very low, it can be concluded that very slow diffusion of enzymes from binding cites of A-LMS_5 into the inner-pores occurs which could be attributed to limitation of the available vacant binding sites for diffusion into pores and/or pore blockage. This conclusion is in accordance with two times higher value of intercept C2, for bulk and film diffusion, than C1 for pore diffusion, meaning that in the second stage of adsorption process, the density of the attached enzyme molecules on A-LMS_5 surface is higher than in stage one. Thus first stage of β-galactosidase adsorption onto A-LMS_5 may be governed by the initial intra-particle transport of enzyme molecules controlled by bulk and film diffusion process and later part is controlled by pore diffusion. Similar dual nature of adsorption process with initial linear dependence and then plateau could be found in the literature.30,33
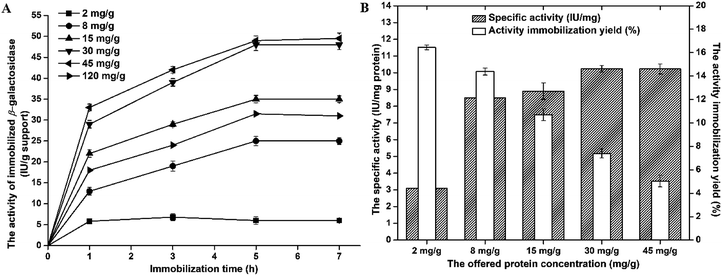
The activity of immobilized β-galactosidase on A-LMS_5 displayed the similar trend as protein loading, meaning that with increase of the offered protein concentration up to 30 mg per g support, the activity also increases. At the offered protein concentration of 45 mg g−1, the immobilized β-galactosidase on A-LMS_5 exhibited the same activity of 45 IU per g support as at the offered protein concentration of 30 mg g−1. The further increase of offered protein concentration resulted in decrease of immobilized enzyme activity for approximately 40%. Although, the maximum protein loading was achieved after 3 h at all offered protein concentrations, the maximum activity was reached only after 5 h. Additionally, if we analyse the activity immobilization yield and the specific activity of immobilized β-galactosidase on A-LMS_5 (Fig. 3B), it can be seen that specific activity of 10.22 IU per mg of attached proteins was obtained when immobilization is performed at offered protein concentrations 30 mg g−1 and 45 mg g−1, indicating that almost all attached enzyme molecules on A-LMS_5 occupy active conformation. Comparison of obtained results with the results reported for β-galactosidase immobilized on commercial porous amino support (Purolite Lifetech ECR8409), it can be observed that, although β-galactosidase immobilized on Purolite Lifetech ECR8409 expressed 3 times higher activity, the specific activity was 10 times lower than in case of immobilization on A-LMS-5.34 The highest activity immobilization yield (16%) was obtained at lowest offered protein concentration (2 mg g−1) while further increase of offered protein concentration led to decrease in activity yield to 5%. In summary, with respect to presented results for protein loading, the activity, the specific activity and the activity immobilization yield, it was shown that β-galactosidase immobilization on A-LMS_5 exhibited the best results when the immobilization was carried out at pH 4.5 and the offered protein concentration of 30 mg per g support for 5 h.
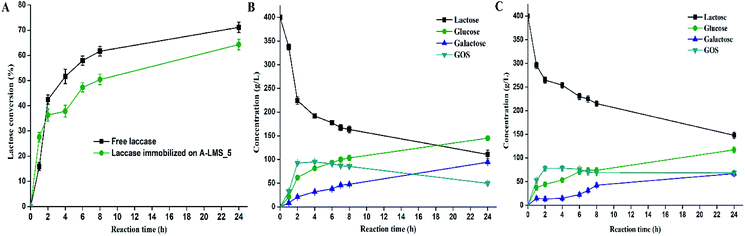
In present study, GOS synthesis was performed at previously determined optimal conditions: at initial lactose concentration of 400 g L−1, pH 4.5 and 50 °C.13 In order to estimate immobilized β-galactosidase on A-LMS_5 affinity towards transgalactosylation and possible introduction of mass transfer limitations by immobilization, the GOS synthesis was carried out with the amounts of free β-galactosidase that provide equal initial hydrolytic activity (9.73 IU mL−1) of free β-galactosidase and immobilized on A-LMS_5. The kinetics of lactose conversion using the soluble and immobilized biocatalysts are presented in Fig. 4A. The concentration of reaction species (glucose, galactose, lactose and total GOS) of lactose bioconversion as a function of reaction time is presented at Fig. 4B for free enzyme and Fig. 4C for immobilized enzyme.
The maximum amount of total GOS of 78.2 g L−1 for the immobilized enzyme was achieved at lactose conversion of 36.5% after 4 h. For the same time, the free β-galactosidase produced the maximum amount of GOS of 94.95 g L−1 at 52% lactose conversion. Also, it can be seen that hydrolysis and transgalactosylation occur simultaneously during first two hours with the lactose conversion rate of 112 g L−1 h−1 and 132 g L−1 h−1 by free and immobilized enzyme, respectively.
Additionally, it can be noted that transgalactosylation dominates the lactose bioconversion, since the A-LMS-5 immobilized preparation have produced 53 g L−1 of glucose and 78.2 g L−1 of total GOS meaning that GOS production was 1.5-times higher than glucose production. After four hours, the concentration of GOS exhibited slight decrease suggesting that hydrolysis was slowly becoming predominant.
Since the same hydrolytic activities of both free and immobilized enzyme were employed in the reaction, it can be concluded that immobilization did not change enzyme specificity towards the reaction of transgalactosylation thus the 1.5-times higher GOS productivity (g L−1 h−1) was achieved by free enzyme.
The perspective for utilization of β-galactosidase immobilized on A-LMS_5 in GOS synthesis lies in the fact that the high GOS productivity of 19.5 g L−1 h−1 was achieved. In addition, the developed immobilized preparation provides the means for facilitated GOS downstream processing and multiple reuse of the same batch of the biocatalyst, including the application in different bioreactor configurations.
Immobilization of laccase on amino-modified microspheres with 5 wt% of alginate
After it has been proven that A-LMS_5 has good prospects as support for immobilization of β-galactosidase from A. oryzae, in further experiments, the possible use of A-LMS_5 as a carrier for immobilization was also investigated for another enzyme, laccase form M. thermophila. Generally, laccases (EC 1.10.3.2, p-diphenol: dioxygen oxidoreductase) from a family of multicopper containing oxidoreductases, also known as blue copper oxidases, couple the four-electron reduction of dioxygen to water with the oxidation of variety of organic substrates, including anilines, phenols, aromatic thiols, some inorganic compounds, by a one-electron transfer mechanism. Typical fungal laccase is protein of approximately (60–70) kDa with acidic isoelectric point (pI), ranging from pH 3.0 to 7.0, usually around pH 4.0.37,38 Laccases, from various different sources, have vast possible industrial applications and biotechnological potential, including the use of enzyme as a bioleaching agent in the paper industry; degradation of PAHs or cleavage of aromatic rings; decolorization of dyes in the textile industry, cosmetics, bioremediation of waste waters from food industry; as biosensors for detection of phenolic compounds and many others.39 So far, laccase have been successfully immobilized on different carriers, including glass beads, nanoparticles, molecular sieves, silica gel, microspheres, carbon nano-tubes, carbon paste, chitosan, graphite powder, membrane (magnetic carbon paste–chitosan/silica), nanofibers, glutaraldehyde and nanoflowers.1,18,40–45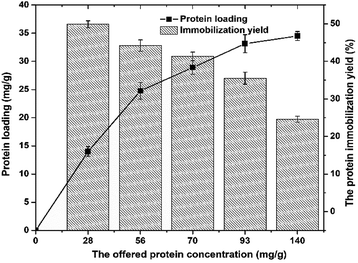 | ||
| Fig. 5 The effect of the offered protein concentration on protein loading (■) and the protein immobilization yield (bars). | ||
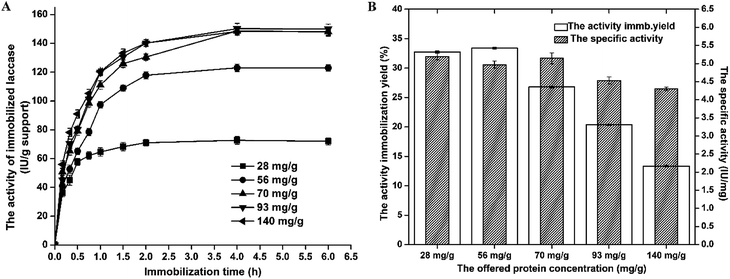
For the initial laccase concentrations of 28–140 mg g−1, the protein loading increases up from 14 mg per g support to 34.5 mg per g support, respectively. The highest concentration of immobilized laccase was 34.5 mg per g of carrier obtained with 140 mg g−1 initial enzyme concentration. From the data obtained, it can be noted that increase of the offered protein concentration from 93 mg g−1 to 140 mg g−1 did not lead to significant increase in protein loading.
So, it can be concluded that maximum binding capacity of A-LMS-5 for laccase is around 35 mg g−1, which is approximately 6 times higher than in case of β-galactosidase. Regarding the protein immobilization yield, the opposite trend can be seen, since continuous decrease of efficiency occurred with the increase of offered protein concentration (Fig. 5). The highest obtained value was 50% for 20 mg g−1 offered protein concentration, and for the highest offered protein concentration, the lowest immobilization yield of 24.6% was determined. Similar results can be found in the literature, for laccase immobilized on microspheres, but for the different origin of laccase and different composition of microspheres, mainly polymeric, as well as some natural carriers.41,46,47
In some cases of natural carriers, like nanostructured bacterial cellulose, enzyme loading were lower than obtained in the case of A-LMS_5 carrier.47 The higher enzyme loading have been reported for only several similar carriers by size and shape, such as porous polyvinyl alcohol beads containing halloysite nanotubes (PVA/HNTs) where enzyme loadings reached 237.02 mg g−1,41 but this material is not beneficial from environmental point of view as our support which contains natural component (kraft lignin) with unexplored utilization capacities. Therefore, the results obtained suggest that A-LMS_5 has good prospects for application in laccase immobilization.
As previously reported,48 dimensions of the laccase from M. thermophila are 6.74 nm × 12.84 nm × 16.36 nm, and in comparison with dimensions of β-galactosidase, it can be assumed that diffusion of laccase molecule into the pores can occur at least to some extent as in case of β-galactosidase, and significantly higher protein loading obtained with laccase is good confirmation of this assumption. Since obtained high enzyme loadings (35 mg g−1) do not always result in adequate increase in the activity of immobilized enzyme, due to the facts that its specific activity depends on its microenvironment and possibility of steric hindrance,46 in following experiments, the activity of laccase immobilized on A-LMS_5 at different offered protein concentrations was investigated (Fig. 6).
The highest activity yield (33.4%) was obtained at offered protein concentration of 56 mg g−1 while further increase of offered protein concentration led to decrease in activity yield down to 13.4%. As a conclusion, with respect to obtained results for all examined parameters that describe efficiency of applied immobilization method, it was shown that laccase immobilization on A-LMS_5 exhibited the best results when the immobilization was carried out at pH 5.0 and the offered protein concentration of 70 mg per g support for 4 h.
Lindane is a member of organochlorine (OC) pesticide class, and it has been used in plant protection and pest control, in medicinal applications and for the reduction of crop loss during the storage. It belongs to a group of chemicals known as persistent organic pollutants, or POPs, due to their durability in the environment, toxicity and tendency to bioaccumulation in the soil and water.49 Therefore, in recent years, the possible use of immobilized laccase was studied for the bioremediation of water and soil contaminated with lindane.14
For evaluation of lindane degradation by free and immobilized laccase on A-LMS_5, the gas chromatography coupled with mass spectroscopy (GC-MS) was used as described in the ESI.† The degradation of lindane was monitored with the amounts of free laccase and laccase immobilized on A-LMS_5 of equal initial activities (3.5 IU), in order to obtain adequate estimation of laccase specificity towards lindane degradation. The obtained results are presented on Fig. 7, and compared with results obtained in our previously published study where lindane degradation was performed with laccase immobilized on amino functionalized silica nanoparticles (AFNS).14
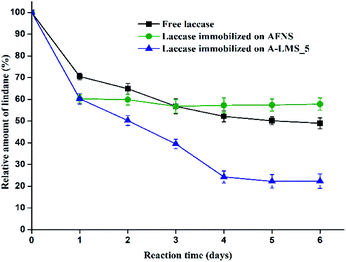 | ||
| Fig. 7 The relative amount of lindane according to the initial amount (controls) after treatment with free laccase, laccase immobilized on AFNS and laccase immobilized on A-LMS_5. | ||
In the preliminary experiments the investigation of potential adsorption of lindane by A-LMS_5 was performed, since their very good adsorption characteristics were reported in the literature.8,9 The samples prepared with only A-LMS_5 and lindane of the same initial concentration used for all other experiments, were monitored for lindane presence by analyzing the hexane extracts of these samples on GC-MS. The obtained results confirmed that A-LMS_5 adsorbed 66% of the initial lindane concentration during first 24 h. Moreover, during further incubation (lasting 6 days in total), the obtained results showed that after the first day there was no significant change in the amount of adsorbed lindane on A-LMS_5 microspheres. So, it should be emphasized that the data presented on Fig. 7 relate to the initial lindane concentration of remained 34% in the solution, as the starting concentration of lindane degradation by laccase immobilized on A-LMS_5, shown on the graph as 100%. In that way, the data presented on Fig. 7 are all relating only to the lindane degradation by enzyme, free or immobilized.
After the first day of the reaction, lindane was degraded to 70.51% of initial concentration by free laccase, 61.65% by laccase immobilized on AFNS and to 60.29% by laccase immobilized on A-LMS_5. After the second day, the degradation continued to 64.89%, 59.85% and 50.26%, respectively. In the sample with immobilized laccase on A-LMS_5, the concentration of lindane continued to decrease during time, and after the fourth day was 24.26% and in the next two days remained almost the same, with minimum of remained lindane concentration of 22.4%. For AFNS immobilized laccase, however, the degradation level had no significant change after the first day.
The obtained results confirm that immobilized laccase on A-LMS_5 has prolonged activity with lindane as a substrate and degrades lindane more efficiently than free laccase or laccase immobilized on AFNS. The high activity of the immobilized laccase can be explained by the fact, that formed electrostatic interactions engaged in the adsorption of enzyme to the carrier orientate enzyme in a way that enabling readily access of substrate.
Therefore, A-LMS_5 has good prospects as a support for laccase immobilization and potential application in degradation of pesticide lindane.
Analysis of interactions formed between enzymes and A-LMS_5
In order to study the type of interactions formed between enzymes (β-galactosidase and laccase) and A-LMS_5 microspheres, and to evaluate versatility of A-LMS microspheres as support for enzyme immobilization, the newly developed immobilized preparations were treated separately with Triton X-100 and 1 M CaCl2. As already stated, it can be presumed that both enzymes at pH above their pI will form electrostatic interactions with positively charged amino groups of carrier since they will be overly negatively charged. Notwithstanding the electrostatic interaction, the hydrophobic regions of enzyme molecules could form the hydrophobic interactions with carrier, also hydrogen or van-der-Waal forces could be formed.22The treatment of immobilized preparation with 1 M solution of CaCl2 will provide the insight on the amount of enzyme attached via electrostatic interactions because they will be removed. On the other hand, with treatment with 1% solution of Triton X-100, hydrophobically attached enzyme molecules will be removed, so their contribution to the activity of immobilized enzyme can be estimated.
After 60 min of incubation in desorption solution of Triton X-100, laccase immobilized on A-LMS_5 retained 45% of initial oxidative activity (activity before the treatment), while immobilized preparation treated with 1 M CaCl2 resulted in decrease of 45% in laccase initial activity (ESI Fig. 4A†). So, it can be concluded that laccase attachment on A-LMS_5 microspheres is governed almost equally with both hydrophobic and electrostatic interactions. In case of β-galactosidase the result of treatment with Triton X-100 and 1 M CaCl2 showed that almost all enzyme molecules (90%) are predominantly attached on A-LMS surface via electrostatic interactions (ESI Fig. 4B†).
In previously reported studies, it has been proven that high density of carboxylic groups is distributed over the entire surface of the β-galactosidase from A. oryzae.32 So, it can be assumed that enzyme molecules are dominantly adsorbed on a positively charged surface of A-LMS_5 via electrostatic attractions of negatively charged carboxyl groups exposed in the region opposite to active site. The established interactions orients enzyme in such way that allows easy access of the substrate to the active site which resulted in high specific activity obtained during immobilization of this enzyme on A-LMS_5. The overall low binding capacity and protein immobilization yield obtained for β-galactosidase immobilized on A-LMS_5 microspheres could be explained by the fact that although the scaffold of A-LMS_5 is predominately hydrophobic,50 these microspheres are synthetized from kraft lignin which possess high number of phenolic –OH groups, and at pH 4.5, these groups are negatively charged. Between these groups and negatively charged carboxylic groups of enzyme, localized repulsion interactions could be formed, resulting in overall low binding capacity of A-LMS_5 in case of β-galactosidase. On the other hand, in case of laccase from M. thermophila, 7 times higher protein loading then in case of β-galactosidase indicates that scaffold and structure of A-LMS_5 have positive effect on adsorption process. Generally, laccase have an affinity to organic supports, which is significantly higher comparing to the inorganic carriers.51
Therefore, it is evident that formed hydrophobic and electrostatic interactions between laccase and A-LMS_5 directed adsorption towards region opposite of enzyme active site resulting in undisturbed access of both applied substrates which is proven by obtained high activities.
In order to confirm assumptions regarding attachment of β-galactosidase and laccase onto A-LMS_5, the samples of A-LMS_5, laccase, β-galactosidase and the newly developed immobilized preparations were characterized using FT-IR spectroscopy (Fig. 8). At A-LMS_5 FT-IR spectra, it could be seen the board absorption band at 3243 cm−1 which is assigned to phenolic O–H stretching vibrations overlapped with N–H stretching vibrations (primary and secondary amines).8 The bands at 2842 cm−1 and 2934 cm−1 are assigned to symmetric and asymmetric C–H stretching vibrations of methylene group, while the bands appearing at 1601–1025 cm−1 are ascribed to C–N stretching vibrations.8 All these absorption bands at A-LMS FT-IR spectra indicate successful synthesis of A-LMS_5 microspheres by copolymerization between kraft lignin, PEI and epoxychloropropane. It should be noted that an enzyme FT-IR spectrum could contain nine amide bands, with vibrational contributions from both protein backbone and amino acid side chains. Of particular interest are the absorptions associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of peptide backbone denoted as Amide I (1600–1700 cm−1), whereas those associated with N–H bending denoted as Amide II (1550–1450 cm−1).52 At the spectra of laccase and β-galactosidase, the Amide I and Amide II bands appear at 1630 and 1516 cm−1 (Fig. 8).
O stretching of peptide backbone denoted as Amide I (1600–1700 cm−1), whereas those associated with N–H bending denoted as Amide II (1550–1450 cm−1).52 At the spectra of laccase and β-galactosidase, the Amide I and Amide II bands appear at 1630 and 1516 cm−1 (Fig. 8).
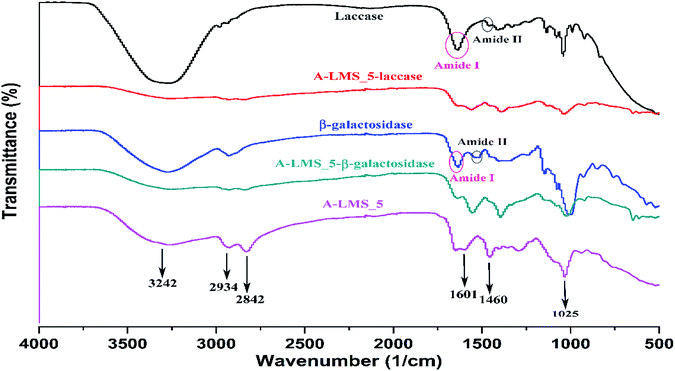 | ||
| Fig. 8 FT-IR spectra of A-LMS_5, laccase, β-galactosidase, laccase immobilized on A-LMS_5 and β-galactosidase immobilized on A-LMS_5. | ||
By comparing the A-LMS_5 spectra with spectrums of developed immobilized preparations, it could be noted that after immobilization the shift in the maximum of the peaks toward lower wavelengths as well as changes in the intensity of the peaks happened. For example, the A-LMS spectra showed a slightly more pronounced peak at 1590 cm−1 in relation to the peak at 1601 cm−1, while at spectrums of immobilized preparation it was the other way around. Moreover, the Amide I and Amide II bands appear at A-LMS_5-laccase and A-LMS_5-β-galactosidase spectrums. All these stated facts prove that both enzymes have been successfully attached onto the surface of A-LMS_5 microspheres.
Conclusion
In recent years, novel support materials for enzyme immobilization have been developed, based on natural materials and bio-waste, with diverse applications in environmental, pharmaceutical, food and chemical industry sectors, due to their lower cost, non-toxicity and biocompatibility. One of these materials, in this study, kraft-lignin, in the form of porous amino-modified microspheres (A-LMS) was investigated as a potential support for immobilization of β-galactosidase from A. oryzae and Novozym® 51003 laccase, and obtained preparations were evaluated in synthesis of galacto-oligosaccharide (GOS) and degradation of pesticide lindane, respectively. The effect of emulsifier concentration on morphological and physical characteristics of A-LMS was considered regarding the efficiency of immobilization process, and it has been shown that microspheres produced using 5 wt% of emulsifier (A-LMS_5) have pore shape, size and distribution for enzyme attachment by far better than microspheres produced using 10 wt% of emulsifier (A-LMS_10). Moreover, β-galactosidase immobilized on A-LMS_5 demonstrated good specificity towards GOS synthesis from lactose, while immobilized laccase preparation exhibited good activity in reaction of pesticide lindane degradation. From all the presented results, it can be concluded that A-LMS, has good perspective in various large-scale industrial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract No. 451-03-68/2020-14/200135 and Contact No. 451-03-68/2020-14/200287). The authors are also grateful to Directorate of Measures and Precious Metals for the technical support. The contribution of COST Action LignoCOST (CA17128), supported by COST (European Cooperation in Science and Technology), in promoting interaction, exchange of knowledge and collaborations in the field of lignin valorisation is gratefully acknowledged.Notes and references
- K. Adinarayana, J. P. Francisco, B. Antonio and A. Miguel, Recent Pat. Biotechnol., 2008, 2, 10–24 CrossRef PubMed.
- R. C. Minussi, G. M. Pastore and N. Durán, Trends Food Sci. Technol., 2002, 13, 205–216 CrossRef CAS.
- R. A. Sheldon, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- J.-C. Janson, in Book Microspheres and Microcapsules in Biotechnology: Design, Preparation and Application, ed. G. Ma and Z.-G. Si, CRC press, 1st edn, 2013, vol. 4, pp. 123–152, DOI:10.1201/b14540-5.
- J. Zdarta, A. S. Meyer, T. Jesionowski and M. Pinelo, Catalysts, 2018, 8, 92 CrossRef.
- F. S. Chakar and A. J. Ragauskas, Ind. Crops Prod., 2004, 20, 131–141 CrossRef CAS.
- A. Popovic, J. Rusmirovic, Z. Velickovic, Ž. Radovanović, M. Ristic, V. Pavlovic and A. Marinkovic, Int. J. Biol. Macromol., 2020, 156, 1160–1173 CrossRef CAS PubMed.
- A. Popović, J. Rusmirović, S. Lević, A. Božić, T. Kovačević, T. Stevanović and A. Marinković, in 31st Int. Congr. Process Ind., 2018, vol. 31, pp. 235–239 Search PubMed.
- J. Zdarta, L. Klapiszewski, A. Jedrzak, M. Nowicki, D. Moszynski and T. Jesionowski, Catalysts, 2017, 7, 14 CrossRef.
- J. Zdarta, Ł. Klapiszewski, M. Wysokowski, M. Norman, A. Kołodziejczak-Radzimska, D. Moszyński, H. Ehrlich, H. Maciejewski, A. L. Stelling and T. Jesionowski, Mar. Drugs, 2015, 13, 2424–2446 CrossRef CAS PubMed.
- C. Zhang, L. Gong, Q. Mao, P. Han, X. Lu and J. Qu, RSC Adv., 2018, 8, 14414–14421 RSC.
- K. Banjanac, M. Carević, M. Ćorović, A. Milivojević, N. Prlainović, A. Marinković and D. Bezbradica, RSC Adv., 2016, 6, 97216–97225 RSC.
- J. Bebić, K. Banjanac, M. Ćorović, A. Milivojević, M. Simović, A. Marinković and D. Bezbradica, Chin. J. Chem. Eng., 2020 DOI:10.1016/j.cjche.2019.12.025.
- M. Carević, M. Ćorović, M. Mihailović, K. Banjanac, A. Milisavljević, D. Veličković and D. Bezbradica, Int. Dairy J., 2016, 54, 50–57 CrossRef.
- F. Sheikhi, M. Roayaei Ardakani, N. Enayatizamir and S. Rodriguez-Couto, Indian J. Microbiol., 2012, 52, 701–707 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- A. Dyal, K. Loos, M. Noto, S. W. Chang, C. Spagnoli, K. V. Shafi, A. Ulman, M. Cowman and R. A. Gross, J. Am. Chem. Soc., 2003, 125, 1684–1685 CrossRef CAS PubMed.
- S. Gilani, G. Najafpour, A. Moghadamnia and A. Kamaruddin, Int. J. Eng., Trans. A, 2016, 29, 13 Search PubMed.
- A. P. Tavares, C. G. Silva, G. Dražić, A. M. Silva, J. M. Loureiro and J. L. Faria, J. Colloid Interface Sci., 2015, 454, 52–60 CrossRef CAS.
- R.-L. Tseng, F.-C. Wu and R.-S. Juang, J. Taiwan Inst. Chem. Eng., 2010, 41, 661–669 CrossRef CAS.
- D.-H. Zhang, L.-X. Yuwen and L.-J. Peng, J. Chem., 2013, 2013, 946248 Search PubMed.
- N. R. Mohamad, N. H. C. Marzuki, N. A. Buang, F. Huyop and R. A. Wahab, Biotechnol. Biotechnol. Equip., 2015, 29, 205–220 CrossRef CAS PubMed.
- A. Wang, H. Wang, S. Zhu, C. Zhou, Z. Du and S. Shen, Bioprocess Biosyst. Eng., 2008, 31, 509–517 CrossRef CAS PubMed.
- K. A. Joshi, M. Prouza, M. Kum, J. Wang, J. Tang, R. Haddon, W. Chen and A. Mulchandani, Anal. Chem., 2006, 78, 331–336 CrossRef CAS PubMed.
- W. Tischer and F. Wedekind, in Biocatalysis-from discovery to application, Springer, 1999, pp. 95–126 Search PubMed.
- G. Bayramoglu, Y. Tunali and M. Y. Arica, Catal. Commun., 2007, 8, 1094–1101 CrossRef CAS.
- T. M. Costa, S. M. J. Silva and M. L. Negreiros-Fransozo, Braz. Arch. Biol. Technol., 2006, 49, 117–123 CrossRef.
- C. Guerrero, C. Vera, N. Serna and A. Illanes, Bioresour. Technol., 2017, 232, 53–63 CrossRef CAS PubMed.
- C. Chakrapani, C. Babu, K. Vani and K. S. Rao, J. Chem., 2010, 7, S419–S427 CAS.
- H. Heydarzadeh Darzi, S. Gilani, M. Farrokhi, S. Nouri and G. Karimi, Int. J. Eng., Trans. B, 2019, 32, 207–216 Search PubMed.
- M. M. Maksimainen, A. Lampio, M. Mertanen, O. Turunen and J. Rouvinen, Int. J. Biol. Macromol., 2013, 60, 109–115 CrossRef CAS PubMed.
- N. Abdel-Ghani, E. Rawash and G. El-Chaghaby, Global J. Environ. Sci. Manage., 2016, 2, 11–18 CAS.
- M. Simović, A. Milivojević, M. Ćorović, K. Banjanac and D. Bezbradica, Int. J. Food Sci. Technol., 2019, 54, 3074–3082 CrossRef.
- D. P. Torres, M. d. P. F. Gonçalves, J. A. Teixeira and L. R. Rodrigues, Compr. Rev. Food Sci. Food Saf., 2010, 9, 438–454 CrossRef CAS.
- H. Eskandarloo and A. Abbaspourrad, Food Chem., 2018, 251, 115–124 CrossRef CAS.
- P. Baldrian, FEMS Microbiol. Rev., 2006, 30, 215–242 CrossRef CAS PubMed.
- H. Claus, Micron, 2004, 35, 93–96 CrossRef CAS PubMed.
- J. Su, J. Fu, Q. Wang, C. Silva and A. Cavaco-Paulo, Crit. Rev. Biotechnol., 2018, 38, 294–307 CrossRef CAS PubMed.
- D. Brady and J. Jordaan, Biotechnol. Lett., 2009, 31, 1639 CrossRef CAS PubMed.
- N. Durán, M. A. Rosa, A. D'Annibale and L. Gianfreda, Enzyme Microb. Technol., 2002, 31, 907–931 CrossRef.
- M. Fernández-Fernández, M. Á. Sanromán and D. Moldes, Biotechnol. Adv., 2013, 31, 1808–1825 CrossRef PubMed.
- M. Hartmann and X. Kostrov, Chem. Soc. Rev., 2013, 42, 6277–6289 RSC.
- L. Zhu, L. Gong, Y. Zhang, R. Wang, J. Ge, Z. Liu and R. N. Zare, Chem.–Asian J., 2013, 8, 2358–2360 CrossRef CAS PubMed.
- T.-T. Xia, C.-Z. Liu, J.-H. Hu and C. Guo, Chem. Eng. J., 2016, 295, 201–206 CrossRef CAS.
- L. Chen, M. Zou and F. F. Hong, Front. Microbiol., 2015, 6, 1245 Search PubMed.
- C. Pezzella, M. E. Russo, A. Marzocchella, P. Salatino and G. Sannia, BioMed Res. Int., 2014, 2014, 1–11 CrossRef PubMed.
- H. A. Ernst, L. J. Jørgensen, C. Bukh, K. Piontek, D. A. Plattner, L. H. Østergaard, S. Larsen and M. J. Bjerrum, PLoS One, 2018, 13, 11 Search PubMed.
- K. Bańka, G. Buszewicz, P. Listos and R. Mądro, Bull. Vet. Inst. Pulawy, 2010, 54, 655–659 Search PubMed.
- D. Ekeberg, K. S. Gretland, J. Gustafsson, S. M. Bråten and G. E. Fredheim, Anal. Chim. Acta, 2006, 565, 121–128 CrossRef CAS.
- T. Jesionowski, J. Zdarta and B. Krajewska, Adsorption, 2014, 20, 801–821 CrossRef CAS.
- C. Morhardt, B. Ketterer, S. Heißler and M. Franzreb, J. Mol. Catal. B: Enzym., 2014, 107, 55–63 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03439h |
| This journal is © The Royal Society of Chemistry 2020 |