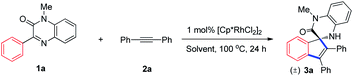Open Access Article
Open Access ArticleRh(III)-catalyzed spiroannulation of 3-arylquinoxalin-2(1H)-ones with alkynes: practical access to spiroquinoxalinones†
Yuanfei Zhang ab,
Ting Huanga,
Xinghua Lia,
Min Zhang
ab,
Ting Huanga,
Xinghua Lia,
Min Zhang b,
Ying Songa,
Kelin Huang*c and
Weiping Su
b,
Ying Songa,
Kelin Huang*c and
Weiping Su *b
*b
aGuangxi Key Laboratory of Natural Polymer Chemistry and Physics, Nanning Normal University, Nanning 530001, China
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: wpsu@fjirsm.ac.cn
cChina Academy of Science and Technology Development Guangxi Branch, Nanning 530022, China. E-mail: huanghn@sina.com
First published on 10th June 2020
Abstract
The Rh(III)-catalyzed synthesis of spiroquinoxalinone derivatives from 3-arylquinoxalin-2(1H)-ones and alkynes via a C–H functionalization/[3 + 2] annulation sequence has been developed. This method, featuring low catalyst loading, was amenable to Gram scale synthesis and tolerated a variety of functional groups and substitution patterns on the aryl rings, providing the target products in good to excellent yields.
Introduction
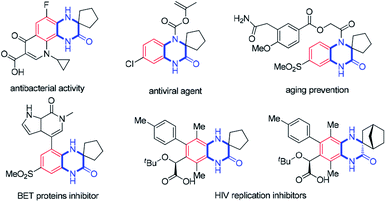
Quinoxalinone derivatives are privileged structural motifs found to have a broad spectrum of biological activities and among them spiro-1,2′-quinoxalin-3′-ones are of particular importance (Fig. 1).1,2 A handful of first-in-class entities with spiroquinoxalinone scaffolds have been employed as potential antibacterial and antiviral reagents.2a–c They are also drug candidates for preventing aging2d and inhibiting BET proteins to treat cancers.2e Conventionally, two approaches for the synthesis of spiroquinoxalinones are available: one is the nucleophilic aromatic substitution reaction of ortho-fluoro substituted nitrobenzenes with cyclic amino acids followed by reductive cyclization amide bond formation;2 the other is the use of aryl 1,2-diamines as substrates via Bargellini reaction.3 However, these procedures are either of poor atom and step economy or lack of regioselectivity, and therefore the development of methods providing practical access to complex spiroquinoxalinone frameworks that are otherwise difficult to be prepared by the established routes would be highly desirable.On the other hand, transition metal catalyzed functionalization of C–H bond has been proved to be a practical tool for the construction and modification of valuable molecules with high efficiency and regioselectivity.4 In this regard, the development of tandem C–H functionalization/[3 + 2] annulation reactions are of intense interest to the synthetic communities especially for the synthesis of N-containing spirocycles, and considerable progresses have been made.5 Such [3 + 2] annulation processes mainly stem from the pioneer works of Takai, Zhao, Cramer and others who respectively employed Re(I),6a,b Ru(II)6c and Rh(I)6d,e as the catalyst and ketimines as the directing groups to effect the cascade.6f–i In 2013, Nishimura and co-workers successfully extended the [3 + 2] annulation prototype to access spirocyclic sultams via Ir(I)-catalysis (Scheme 1a).7a Since then, a number of transition metal catalyzed C–H metalation followed by nucleophilic insertion cyclization of cyclic N-sulfonyl ketimines with alkynes or activated olefins for the construction of spirocyclic sultam cores have been successively reported.7b–f In addition, cyclic ketimines activated by electron-withdrawing groups are available to the [3 + 2] spirocyclization reactions as well (Scheme 1b).8 Duo to the weak nucleophilicity of cyclometalation intermediates generated from C–H activation, activated cyclic ketimines are commonly used to facilitate the intramolecular nucleophilic insertion, and spirocycles bearing cyclic amides on the other rings are generally formed. Taking into account that spirocycles tethered by cyclic amines are also of significant importance,2 it would be tremendously valuable yet challenging to uncover transition metal catalyzed [3 + 2] spiroannulation reactions in which imines are able to participate with concomitant formation of amine rings.9 Herein, we describe a new approach to the synthesis of spiro-1,2′-quinoxalin-3′-ones via the Rh(III) catalyzed C–H activation/[3 + 2] annulation reactions utilizing imine to trap the cyclorhodation species via a nucleophilic insertion. With only 1 mol% [Cp*RhCl2]2 as the catalyst, we have accomplished the spiroannulation reactions, which was amenable on Gram-scale, of 3-phenylquinoxalinones with alkynes. This protocol is compatible with a broad variety of functional groups and furnishes the desired products in good to excellent yields.
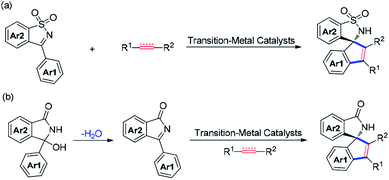 | ||
| Scheme 1 Synthesis of spirocycles from activated cyclic imines.7,8 (a) Cyclic N-sulfonylketimine. (b) N-Acyl ketimines. | ||
Results and discussion
Reaction development
Having the assumption of achieving spiroannulation reactions of cyclic imines in mind, the reaction of 1-methyl-3-phenylquinoxalin-2(1H)-one (1a)10 and 1,2-diphenylethyne (2a) catalyzed by Rh(III) was selected to verify our hypothesis. Initially, we investigated the effect of solvents on the reaction outcomes using 1 mol% [Cp*RhCl2]2 in combination of 4 mol% AgSbF6 as the catalytic system, and gratifyingly obtained the target product with 62% isolated yield when conducted in acetonitrile (Table 1, entry 3). Other solvents, such as DCE, dioxane etc., were inferior (Table 1, entries 1 and 2, see ESI† for more details). Intrigued by those primary results, PivOH was added to the reaction system as additive considering that PivOH might accelerate the C–H activation process and therefore increase the overall yield.2d,11 As expected, the introduction of one equivalent of PivOH resulted in the improvement of the yield to 86% (Table 1, entry 4). Other proton sources, such as phenol, MsOH and TFA are inferior (see ESI† for more details). The amount of AgSbF6 impacted the transformation dramatically, which did not take place without the addition of AgSbF6 and 6 mol% turned out to be the best furnishing the product in 97% yield (Table 1, entries 5–7). Lowering the catalyst loading to 0.5 mol% and 0.25 mol% decreased the yield even with elevated reaction temperature (Table 1, entries 8–11). Control experiment showed that [Cp*RhCl2]2 was indispensable for the reaction (Table 1, entry 12).| Entry | AgSbF6 (mol%) | Solvent | Additive | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.22 mmol), [Cp*RhCl2]2 (1 mol%), AgSbF6 (4 mol%), additive (1 equiv.), 2 mL of solvent, 100 °C, 24 h.b Isolated yields.c 0.5 mmol scale, 0.5 mol% of [Cp*RhCl2]2 was used.d 0.5 mmol scale, 0.25 mol% of [Cp*RhCl2]2 was used.e The reaction ran at 120 °C for 24 h.f In the absence of [Cp*RhCl2]2. | ||||
| 1 | 4 | DCE | — | <10 |
| 2 | 4 | Dioxane | — | <10 |
| 3 | 4 | CH3CN | — | 62 |
| 4c | 4 | CH3CN | PivOH | 86 |
| 5 | 5 | CH3CN | PivOH | 91 |
| 6 | 6 | CH3CN | PivOH | 97 |
| 7 | 0 | CH3CN | PivOH | 0 |
| 8c | 3 | CH3CN | PivOH | 76 |
| 9d | 1.5 | CH3CN | PivOH | 60 |
| 10c,e | 3 | CH3CN | PivOH | 78 |
| 11d,e | 1.5 | CH3CN | PivOH | 65 |
| 12f | 6 | CH3CN | PivOH | 0 |
Substrate scope
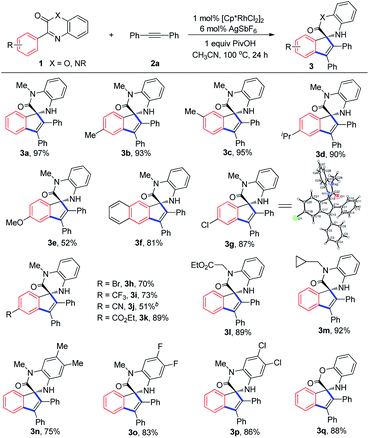
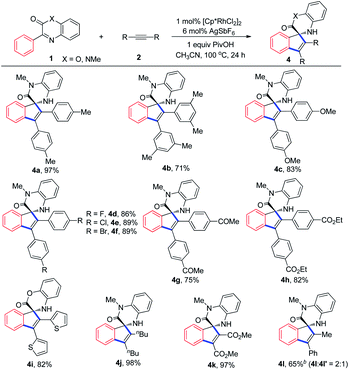
With the optimized reaction conditions established, 3-phenylquinoxalinones with different substituents or substitution patterns on the aryl rings were used to evaluate the substrate scope of the reaction. As depicted in Table 2, methyl, isopropyl and methoxyl-substituted quinoxalinones as well as 3-(naphthalen-2-yl)quinoxalinone were all suitable substrates for the transformation and the desired products were generally obtained with satisfying yields (3b–3f). The reaction preferred to occur at the less hindered site since 3c and 3f were exclusively formed. The reaction was also compatible with chloro, bromo and electron-withdrawing substituents, such as trifluoromethyl, nitrile and ester groups, and delivered the corresponding products in good yields (3g–3k). The structure of 3g was unambiguously determined by X-ray diffraction analysis.12 3-Phenylquinoxalinones bearing ester and cyclopropyl groups on the amide moiety could be easily transformed into the target products with yields of 89% and 92% respectively (3l and 3m). Methyl, fluoro and chloro substituents on the quinoxalinone rings did not affect the reaction much (3n–3p). Conversion of 3-phenyl-2H-benzo[b][1,4]oxazin-2-one to the spirocyclization product was achieved as well (3q). The reaction conditions are generally compatible with electron-donating and electron-withdrawing substituents on both aryl rings, and electron-donating groups tend to lower the reaction yields. Furthermore, because of the coordination of nitrile group to the active Rh centre impeded the catalytic cycle, only 30% yield of 3j was obtained when 1 mol% of [Cp*RhCl2]2 was used.Subsequently, we investigated the substrate scope with respect to the internal alkynes (Table 3). Symmetrical alkynes having both electron-donating and electron-withdrawing groups on the aryl rings reacted smoothly with 1a, giving yields ranging from 71% to 97% (4a–4h). The chloro and bromo substitutes within 4e and 4f provide the possibility for further derivations. 1,2-Di(thiophen-2-yl)ethyne was found to be able to participate in the reaction even if Rh(III) catalyst has been reported by several research groups to activate the α-position of thiophenes (4i).13 Similarly, other kind of symmetrical alkynes were appropriate reaction partners too (4j–4k). Under the optimized conditions, prop-1-yn-1-ylbenzene, an unsymmetrical alkyne, afforded two separable isomers in excellent yield with moderate selectivity (4l). Although both symmetrical and unsymmetrical internal alkynes are suitable substrates, terminal alkynes, such as phenylacetylene and ethyl propiolate, failed to deliver the desired products.
Synthetic utilities
To demonstrate the synthetic utility of the transformation, the reactions of 1a and 1h with 2a were then conducted on Gram scale using the standard reaction conditions and satisfying yields, namely 95% and 68%, were acquired (Scheme 2a). Moreover, subsequent conversions of the spirocyclic products were viable. For example, 3h could undergo Suzuki-coupling with phenylboronic acid yielding the biaryl product (4m) with high efficiency (Scheme 2b). The reduction of amide group in 3h with DIBAL-H at 0 °C was realized bringing about spirotetrahydroquinoxaline derivative 4n in 87% yield.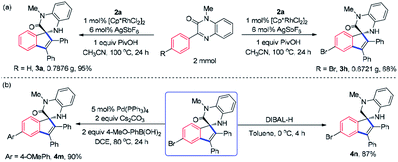 | ||
| Scheme 2 Gram scale synthesis and product derivations. (a) Gram scale synthesis of 3a and 3h. (b) Derivations of 3h. | ||
Mechanistic studies
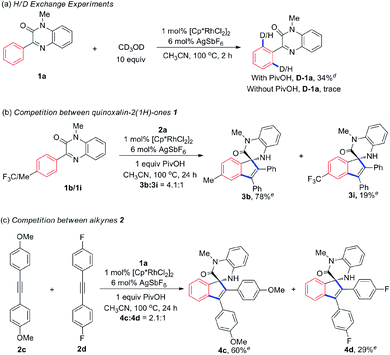
To gain insights into the reaction mechanism, an H/D exchange experiment was firstly carried out. Interestingly, we observed 34% of H/D exchange upon the addition of PivOH, which is in sharp contrast to the result when PivOH was absent (Scheme 3a). This phenomenon indicates that PivOH might help to accelerate the chelation-directed C–H activation process.2d,11 Intermolecular competition experiment between 1b and 1i disclosed that the electron-rich quinoxalinones were more reactive, suggesting a PivOH-assisted electrophilic substitution mechanism for C–H cyclorhodation (Scheme 3b).14 Additional competition experiment with regard to alkynes revealed that the reaction favored to convert electron-rich alkynes to spirocyclic product (Scheme 3c), which can be explained by a kinetic coordination of alkyne to metal center and is consistent with the previous reports on Rh(III)-catalyzed annulation reactions.7e,14a,15Proposed reaction mechanism
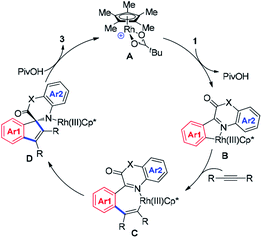
Based on the observations and considerations above, a plausible mechanism was proposed and depicted in Scheme 4. Cationic rhodium A coordinated with quinoxalinone 1 followed by the electrophilic substitution of rhodium to the aryl ring to generate rhodacycle B with the assistance of PivO−. Subsequently, a seven-membered metallacycle complex C came into being via the insertion of alkyne, and intramolecular addition of nucleophilic Rh–C bond to imine within C simultaneously occurred affording intermediate D. On protonation by PivOH, the spirocyclic products 3 were formed with the concomitant releasing of the Rh(III) catalyst. PivOH acted as proton shuttle in the catalytic cycle thus benefited the overall yield.11bConclusions
In conclusion, we have devised a practical access to spiroquinoxalinone derivatives from cyclic imines and alkynes via a tandem Rh(III)-catalyzed C–H functionalization/[3 + 2] annulation sequence. By employing only 1 mol% of Rh(III) catalyst, spiroquinoxalinones bearing a broad range of functional groups and substitution types could be efficiently synthesized on Gram scale with good to excellent yields. Key mechanistic findings illustrated the reason why PivOH was of beneficial effect on the reaction yield. Further studies on the annulation reaction of unactivated cyclic imines affording complex molecules of important biological applications are in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Science Foundation of China (Grant No. 21602221), the Start-up Grant from Nanning Normal University (Grant No. 602021239229) and the “BAGUI Scholar” program of Guangxi province is greatly appreciated.Notes and references
- (a) D.-S. Su, M. K. Markowitz, R. M. DiPardo, K. L. Murphy, C. M. Harrell, S. S. O'Malley, R. W. Ransom, R. S. L. Chang, S. Ha, F. J. Hess, D. J. Pettibone, G. S. Mason, S. Boyce, R. M. Freidinger and M. G. Bock, Discovery of a Potent, Non-peptide Bradykinin B1 Receptor Antagonist, J. Am. Chem. Soc., 2003, 125, 7516 CrossRef CAS PubMed; (b) J. Zhou, M. Ba, B. Wang, H. Zhou, J. Bie, D. Fu, Y. Cao, B. Xu and Y. Guo, Synthesis and Biological Evaluation of Novel Quinoxalinone-based HIV-1 Reverse Transcriptase Inhibitors, MedChemComm, 2014, 5, 441 RSC; (c) P. E. Mahaney, M. B. Webb, F. Ye, J. P. Sabatucci, R. J. Steffan, C. C. Chadwick, D. C. Harnish and E. J. Trybulski, Synthesis and Activity of a New Class of Pathway-selective Estrogen Receptor Ligands: Hydroxybenzoyl-3,4-dihydroquinoxalin-2(1H)-ones, Bioorg. Med. Chem., 2006, 14, 3455 CrossRef CAS PubMed; (d) D. V. Smil, S. Manku, Y. A. Chantigny, S. Leit, A. Wahhab, T. P. Yan, M. Fournel, C. Maroun, Z. Li, A.-M. Lemieux, A. Nicolescu, J. Rahil, S. Lefebvre, A. Panetta, J. M. Besterman and R. Deziél, Novel HDAC6 Isoform Selective Chiral Small Molecule Histone Deacetylase Inhibitors, Bioorg. Med. Chem. Lett., 2009, 19, 688 CrossRef CAS PubMed.
- (a) A. M. A. Qaisi, Y. M. A. Al-Hiari, J. A. Y. Zahra and M. M. M. El-Abadelah, Preparation of Quinolones and Pyridoquinoxalinones as Antibacterials, EP Pat. 1721898A1, 2006; (b) M. Christoph, R. Guenther, K. J. Peter, R. Manfred, P. Arno and B. Martin, Combination of Quinoxalines and Nucleosides for Treating Viral Infection and Preparation of the Quinoxalines, EP Pat. 657166A1, 1995; (c) T. Kenji, T. Yoshiyuki, I. Tsutomu, K. Takashi, A. Toshiyuki, S. Shuichi, T. Yoshinori and I. Masafumi, Preparation of heterocycle-fused phenylacetic acid derivatives as HIV replication inhibitors, PCT Int. Appl. WO 2014119636A1, 2014; (d) A. Zavoronkovs, A. Aliper, P. Mamoshina, A. Artemov and I. Ozerov, Withaferin Compositions for Prevention of Aging, US Pat. 20180125865A1, 2018; (e) A. P. Combs, T. P. Maduskuie Jr and N. Falahatpisheh, Preparation of 1H-pyrrolo[2,3-c]pyridin-7(6H)-ones and Pyrazolo[3,4-c]pyridin-7(6H)-ones as Inhibitors of BET Proteins, PCT Int. Appl. WO 2015164480A1, 2015.
- T. A. Alanine, S. Stokes and J. S. Scott, Practical Synthesis of 3,3-Substituted Dihydroquinoxalin-2-ones From Aryl 1,2-diamines Using the Bargellini Reaction, Tetrahedron Lett., 2006, 57, 4386 CrossRef.
- For selected reviews: (a) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Recent Advances in Directed C–H Functionalizations Using Monodentate Nitrogen-based Directing Groups, Org. Chem. Front., 2014, 1, 843 RSC; (b) F. W. Patureau, J. Wencel-Delord and F. Glorius, Cp*Rh-Catalyzed C–H Activations: Versatile Dehydrogenative Cross-Couplings of C sp2 C–H Positions with Olefins, Alkynes, and Arenes, Aldrichimica Acta, 2012, 45, 31 CAS; (c) G. Song and X. Li, Substrate Activation Strategies in Rhodium(III)-Catalyzed Selective Functionalization of Arenes, Acc. Chem. Res., 2015, 48, 1007 CrossRef CAS PubMed; (d) L. Ackermann, Carboxylate-Assisted Transition-Metal-Catalyzed C–H Bond Functionalizations: Mechanism and Scope, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (e) J. C. Lewis, R. G. Bergman and A. J. Ellman, Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C–H Bond Activation, Acc. Chem. Res., 2008, 41, 1013 CrossRef CAS PubMed; (f) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Q. Yu, Palladium-Catalyzed Transformations of Alkyl C–H Bonds, Chem. Rev., 2017, 117, 8754 CrossRef CAS PubMed; (g) T. Satoh and M. Miura, Oxidative Coupling of Aromatic Substrates with Alkynes and Alkenes under Rhodium Catalysis, Chem.–Eur. J., 2010, 16, 11212 CrossRef CAS PubMed.
- (a) M. Gulías and J. L. Mascareñas, Metal-Catalyzed Annulations Through Activation and Cleavage of C–H Bonds, Angew. Chem., Int. Ed., 2016, 55, 11000 CrossRef PubMed; (b) Y. Yang, K. Li, Y. Cheng, D. Wan, M. Li and J. You, Rhodium-catalyzed Annulation of Arenes with Alkynes Through Weak Chelation-assisted C–H Activation, Chem. Commun., 2016, 52, 2872 RSC.
- (a) Y. Kuninobu, A. Kawata and K. Takai, Rhenium-Catalyzed Formation of Indene Frameworks via C–H Bond Activation: [3 + 2] Annulation of Aromatic Aldimines and Acetylenes, J. Am. Chem. Soc., 2005, 127, 13498 CrossRef CAS PubMed; (b) Y. Kuninobu, Y. Tokunaga, A. Kawata and K. Takai, Insertion of Polar and Nonpolar Unsaturated Molecules into Carbon–Rhenium Bonds Generated by C–H Bond Activation: Synthesis of Phthalimidine and Indene Derivatives, J. Am. Chem. Soc., 2006, 128, 202 CrossRef CAS PubMed; (c) J. Zhang, A. Ugrinov and P. Zhao, Ruthenium(II)/N-Heterocyclic Carbene Catalyzed [3 + 2] Carbocyclization with Aromatic N-H Ketimines and Internal Alkynes, Angew. Chem., Int. Ed., 2013, 52, 6681 CrossRef CAS PubMed; (d) D. N. Tran and N. Cramer, Enantioselective Rhodium(I)-Catalyzed [3 + 2] Annulations of Aromatic Ketimines Induced by Directed C–H Activations, Angew. Chem., Int. Ed., 2011, 50, 11098 CrossRef CAS PubMed; (e) D. N. Tran and N. Cramer, Rhodium-Catalyzed Dynamic Kinetic Asymmetric Transformations of Racemic Allenes by the [3 + 2] Annulation of Aryl Ketimines, Angew. Chem., Int. Ed., 2013, 52, 10630 CrossRef CAS PubMedFor other examples, see: (f) P. Zhao, F. Wang, K. Han and X. Li, Ruthenium- and Sulfonamide-Catalyzed Cyclization Between N-Sulfonyl Imines and Alkynes, Org. Lett., 2012, 14, 5506 CrossRef CAS PubMed; (g) W. P. Liu, D. Zell, M. John and L. Ackermann, Manganese-Catalyzed Synthesis of cis-β-Amino Acid Esters through Organometallic C–H Activation of Ketimines, Angew. Chem., Int. Ed., 2015, 54, 4092 CrossRef CAS PubMed; (h) X. Cong, G. Zhan, Z. Mo, M. Nishiura and Z. Hou, Diastereodivergent [3 + 2] Annulation of Aromatic Aldimines with Alkenes via C–H Activation by Half-Sandwich Rare-Earth Catalysts, J. Am. Chem. Soc., 2020, 142, 5531 CrossRef CAS PubMed; (i) T. Fukutani, N. Umeda, K. Hirano, T. Satoh and M. Miura, Rhodium-catalyzed Oxidative Coupling of Aromatic Imines with Internal Alkynes via Regioselective C–H Bond Cleavage, Chem. Commun., 2009, 5141 RSC.
- For selected examples on [3 + 2] annulation of N-sulfonylketimines, see: (a) T. Nishimura, Y. Ebe and T. Hayashi, Iridium-Catalyzed [3 + 2] Annulation of Cyclic N-Sulfonyl Ketimines with 1,3-Dienes via C–H Activation, J. Am. Chem. Soc., 2013, 135, 2092 CrossRef CAS PubMed; (b) T. Nishimura, M. Nagamoto, Y. Ebe and T. Hayashi, Enantioselective [3 + 2] Annulation via C–H Activation Between Cyclic N-acyl Ketimines and 1,3-Dienes Catalyzed by Iridium/Chiral Diene Complexes, Chem. Sci., 2013, 4, 4499 RSC; (c) B. Liu, P. Hu, Y. Zhang, Y. Li, D. Bai and X. Li, Rh(III)-Catalyzed Diastereodivergent Spiroannulation of Cyclic Imines with Activated Alkenes, Org. Lett., 2017, 19, 5402 CrossRef CAS PubMed; (d) S. T. Mei, H. W. Liang, B. Teng, N. J. Wang, L. Shuai, Y. Yuan, Y. C. Chen and Y. Wei, Spirocyclic Sultam and Heterobiaryl Synthesis through Rh-Catalyzed Cross-Dehydrogenative Coupling of N-Sulfonyl Ketimines and Thiophenes or Furans, Org. Lett., 2016, 18, 1088 CrossRef CAS PubMed; (e) L. Dong, C. H. Qu, J. R. Huang, W. Zhang, Q. R. Zhang and J. G. Deng, Rhodium-Catalyzed Spirocyclic Sultam Synthesis by [3 + 2] Annulation with Cyclic N-Sulfonyl Ketimines and Alkynes, Chem.–Eur. J., 2013, 19, 16537 CrossRef CAS PubMed; (f) H. Liu, J. Li, M. Xiong, J. Jiang and J. Wang, Cp*CoIII-Catalyzed C–H Alkenylation/Annulation to Afford Spiro Indenyl Benzosultam, J. Org. Chem., 2016, 81, 6093 CrossRef CAS PubMed.
- (a) M. Nagamoto and T. Nishimura, Catalytic [3 + 2] Annulation of Ketimines with Alkynes via C–H Activation by a Cationic Iridium(cod) Complex, Chem. Commun., 2014, 50, 6274 RSC; (b) J.-R. Huang, L. Qin, Y.-Q. Zhu, Q. Song and L. Dong, Multi-site Cyclization via Initial C–H Activation Using Rhodium(III) Catalyst: Rapid Assembly of Frameworks Containing Indoles and Indolins, Chem. Commun., 2015, 51, 2844 RSC; (c) S. Sharma, Y. Oh, N. K. Mishra, U. De, H. Jo, R. Sachan, H. S. Kim, Y. H. Jung and I. S. Kim, Rhodium-Catalyzed [3 + 2] Annulation of Cyclic N-Acyl Ketimines with Activated Olefins: Anticancer Activity of Spiroisoindolinones, J. Org. Chem., 2017, 82, 3359 CrossRef CAS PubMed.
- C. Zhu, J. Luan, J. Fang, Z. X. hao, X. Wu, Y. Li and Y. Luo, A Rhodium-Catalyzed [3 + 2] Annulation of General Aromatic Aldimines/Ketimines and N-Substituted Maleimides, Org. Lett., 2018, 20, 5960 CrossRef CAS PubMed.
- A. Carrër, J.-D. Brion, S. Messaoudi and M. Alami, Palladium(II)-Catalyzed Oxidative Arylation of Quinoxalin-2(1H)-ones with Arylboronic Acids, Org. Lett., 2013, 15, 5606 CrossRef PubMed.
- (a) D. L. Davies, S. A. Macgregor and C. L. McMullin, Computational Studies of Carboxylate-Assisted C–H Activation and Functionalization at Group 8–10 Transition Metal Centers, Chem. Rev., 2017, 117, 8649 CrossRef CAS PubMed; (b) M. Lafrance and K. Fagnou, Palladium-Catalyzed Benzene Arylation: Incorporation of Catalytic Pivalic Acid as a Proton Shuttle and a Key Element in Catalyst Design, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS PubMed; (c) D. Zhao, W. Wang, S. Lian, F. Yang, J. Lan and J. You, Phosphine-Free, Palladium-Catalyzed Arylation of Heterocycles through C–H Bond Activation with Pivalic Acid as a Cocatalyst, Chem.–Eur. J., 2009, 15, 1337 CrossRef CAS PubMed; (d) J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, [RhIIICp*]-Catalyzed Dehydrogenative Aryl–Aryl Bond Formation, Angew. Chem., Int. Ed., 2012, 51, 2247 CrossRef CAS PubMed.
- Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre. Deposition number CCDC 1993099 (3g). Detailed information can be found in the ESI.†.
- (a) Y. Zhang, H. Zhao, M. Zhang and W. Su, Carboxylic Acids as Traceless Directing Groups for the Rhodium(III)-Catalyzed Decarboxylative C–H Arylation of Thiophenes, Angew. Chem., Int. Ed., 2015, 54, 3817 CrossRef CAS PubMed; (b) V. P. Reddy, R. Qiu, T. Iwasaki and N. Kambe, Rhodium-Catalyzed Intermolecular Oxidative Cross-Coupling of (Hetero)Arenes with Chalcogenophenes, Org. Lett., 2013, 15, 1290 CrossRef CAS PubMed; (c) J. Wencel-Delord, C. Nimphius, H. Wang and F. Glorius, Rhodium(III) and Hexabromobenzene-A Catalyst System for the Cross-Dehydrogenative Coupling of Simple Arenes and Heterocycles with Arenes Bearing Directing Groups, Angew. Chem., Int. Ed., 2012, 51, 13001 CrossRef CAS PubMed; (d) J. Dong, Z. Long, F. Song, N. Wu, Q. Guo, J. Lan and J. You, Rhodium or Ruthenium-Catalyzed Oxidative C–H/C–H Cross-Coupling: Direct Access to Extended π-Conjugated Systems, Angew. Chem., Int. Ed., 2013, 52, 580 CrossRef CAS PubMed.
- (a) H. Wang, M. Moselage, M. J. González and L. Ackermann, Selective Synthesis of Indoles by Cobalt(III)-Catalyzed C–H/N–O Functionalization with Nitrones, ACS Catal., 2016, 6, 2705 CrossRef CAS; (b) W. Ma, R. Mei, G. Tenti and L. Ackermann, Ruthenium(II)-Catalyzed Oxidative C–H Alkenylations of Sulfonic Acids, Sulfonyl Chlorides and Sulfonamides, Chem.–Eur. J., 2014, 20, 15248 CrossRef CAS PubMed.
- (a) D. R. Stuart, P. Alsabeh, M. Kuhn and K. Fagnou, Rhodium(III)-Catalyzed Arene and Alkene C–H Bond Functionalization Leading to Indoles and Pyrroles, J. Am. Chem. Soc., 2010, 132, 18326 CrossRef CAS PubMed; (b) T. K. Hyster and T. Rovis, Rhodium-Catalyzed Oxidative Cycloaddition of Benzamides and Alkynes via C–H/N–H Activation, J. Am. Chem. Soc., 2010, 132, 10565 CrossRef CAS PubMed; (c) Z. Zhou, G. Liu, Y. Chen and X. Lu, Rhodium (III)-Catalyzed Redox-Neutral C–H Annulation of Arylnitrones and Alkynes for the Synthesis of Indole Derivatives, Adv. Synth. Catal., 2015, 357, 2944 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectral data and copies of NMR. CCDC 1993099. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03348k |
| This journal is © The Royal Society of Chemistry 2020 |