Open Access Article
Open Access ArticleThe Baeyer–Villiger rearrangement with metal triflates: new developments toward mechanism†
Piotr Latos ,
Agnieszka Siewniak
,
Agnieszka Siewniak ,
Magdalena Sitko and
Anna Chrobok
,
Magdalena Sitko and
Anna Chrobok *
*
Department of Chemical Organic Technology and Petrochemistry, Silesian University of Technology, Krzywoustego 4, 44-100 Gliwice, Poland. E-mail: Anna.Chrobok@polsl.pl
First published on 4th June 2020
Abstract
Based on MS analysis, the mechanism of the Baeyer–Villiger oxidation of cyclic ketones with hydrogen peroxide using metal triflates (Ga(OTf)3 and Er(OTf)3) as catalysts was proposed. In the case of cyclohexanone as a substrate, dimeric, trimeric and tetrameric peroxide structures were detected.
The Baeyer–Villiger oxidation (BV) of cyclic ketones is a convenient method of lactone preparation. Lactones are specialty chemicals which are used, among others, in the pharmaceutical, flavour and fragrances, and agrochemical industries.1 Typical oxidants in the BV reaction are highly reactive organic peracids. However, due to their numerous disadvantages, such as sensitivity to shock and temperature, generation of corrosive waste, and hazardous storage and handling, attention is turned to more environmentally friendly hydrogen peroxide.2 H2O2 is safer than peracids, in particular in concentrations lower than 70%. The use of H2O2 in oxidation reaction results in the formation of water as a by-product.2 Nevertheless, hydrogen peroxide is kinetically inert and it is necessary to activate it.1–3 One of the types of catalysts used for hydrogen peroxide activation are Lewis acids,4 which are characterized by high activity and selectivity, however, they suffer from poor stability in an aqueous environment. Tin-containing zeolites are one of the most promising Lewis acidic catalysts for the BV reaction. Their advantages include both their high activity and stability during recycling.5
Another interesting proposition to answer the issue concerning hydrolytic stability of Lewis acidic catalysts for BV oxidation is the use of metal trifluoromethanesulfonates (triflates). Some of them exhibit both relatively high hydrolytic stability and Lewis acidity.6,7 Berkessel et al. demonstrated that rare earth triflates, especially Sc(OTf)3, were extremely active in BV oxidation as catalysts using hydrogen peroxide as an oxidant. However, these studies were presented only for the oxidation of very reactive cyclobutanones.8
In our earlier work, silica-bound gallium(III) triflate was used for the oxidation of 2-adamantanone yielded unexpected 95% of lactone with 99% selectivity.9 Encouraged by this result a number of metal triflates, with tin(II) triflate as the most active, were proved to be active catalysts in Baeyer–Villiger oxidation of 2-adamantanone, giving full conversion of ketone after short reaction time (20 minutes using 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 molar ratio of Sn(OTf)2
2.0 molar ratio of Sn(OTf)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ketone
ketone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 wt% H2O2).10
30 wt% H2O2).10
Although the BV oxidation was discovered in 1899, attempts to establish the mechanism took the next fifty years.1 A generally accepted mechanism of the BV oxidation of carbonyl compounds with peracid assumes that in the first stage an attack of peracidic nucleophilic oxygen on the carbonyl carbon of the ketone occurs leading to a formation of tetraedric intermediate product, called Criegee adduct or intermediate. The second stage involves an 1,2-anionotropic rearrangement. This mechanism was confirmed by von E. Doering by a labeling experiment with benzophenone-O18.11 In case of using peracids as oxidants the presence of catalyst is optional.2 When using hydrogen peroxide as the oxidant the addition of catalyst is required. Depending on the catalyst used various BV oxidation pathways are proposed. Catalysts can activate the ketone or oxidant, or both of them, and usually one of the activation methods dominates (Fig. 1).2
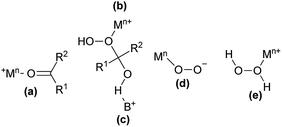 | ||
| Fig. 1 Types of catalytic activation in the BV oxidation with hydrogen peroxide: (a) electrophilic activation of a ketone by the Lewis acid; (b) electrophilic activation of the Criegee adduct by the Lewis acid; (c) nucleophilic activation of the Criegee adduct by the Brønsted acid; (d) nucleophilic activation of hydrogen peroxide by the Lewis acid; (e) electrophilic activation of hydrogen peroxide by the Lewis acid.2 M – Lewis acid, B – Brønsted acid. | ||
Metal triflates are generally considered as Lewis acids, however some of them can partially hydrolyze to triflic acid under the influence of water. Hence, they may participate in the activation of the ketone, the Criegee intermediate or hydrogen peroxide, both by Lewis or Brønsted sites.
Herein, we decided to take a deeper look at the BV oxidation mechanism with hydrogen peroxide using selected metal triflates. The studies shed light for the role of metal triflates in the non-classical approach of lactones formation concerning the high-energy compounds such as peroxides as intermediate compounds.
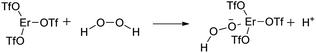
In the preliminary studies, two model metal triflates which significantly vary in hydrolytic stability: gallium(III) triflate and erbium(III) triflate were used to compare their stability in the presence of water. Test based on the reaction with retinyl acetate was conducted (Fig. 2). In this test, the presence of Brønsted acid in the test sample is confirmed by the formation of retinyl cation (blue color) from retinyl acetate (yellow) and detected by UV-Vis spectroscopy.12 Brønsted acid (triflic acid) in the studied samples of metal triflate may be formed by slow hydrolysis in the presence of traces of water. Therefore, triflic acid was used as a benchmark for these studies.
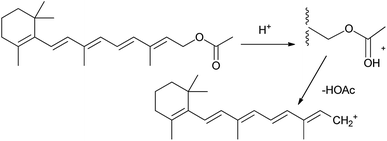 | ||
| Fig. 2 Retinol carbocation formation.12 | ||
In order to limit the water content in UV-Vis tests, anhydrous nitrobenzene was used as a solvent. Results indicates (Fig. 3) that triflate with rare earth metal Er(OTf)3 was characterized by high hydrolytic stability and the presence of retinol carbocation was not observed while Ga(OTf)3 underwent partial hydrolysis as only part of the retinol acetate was converted to carbocation during tests. Due to the high reactivity of triflic acid, its amount used for the analysis was three times lower than that of metal triflates, which were used in the same molar amount.
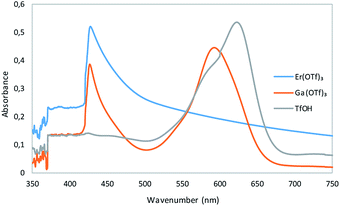 | ||
| Fig. 3 UV-Vis spectroscopy of reaction mixtures consisting of metal triflates or triflic acid with retinyl acetate. | ||
In the case of triflic acid, all retinol acetate was transformed into retinol carbocation. The obtained results confirmed that Er(OTf)3 is hydrolytically stable Lewis acid comparing to Ga(OTf)3 which can undergo hydrolysis.
Next, the BV oxidation reactions of cyclic ketones of varied reactivity were carried out in the presence of two molar excess of 60 wt% aq. H2O2 and Er(OTf)3 or Ga(OTf)3. The following cyclic ketones were selected: cyclobutanone, 2-adamantanone, norcamphor, 2-methylcyclohexanone and cyclohexanone. Secondary groups in cyclic ketones are more prone to migrate than primary alkyl groups in BV oxidation. Therefore, norcamphor, 2-adamantanone and 2-methylcyclohexanone are more reactive then cyclohexanone which is moreover non-strained and hardly reactive in BV oxidation. Very reactive, strained cyclobutanone is readily oxidized with H2O2.
Results shown in Table 1 confirmed that metal triflates are highly active catalyst in BV oxidation of such cyclic ketones as 2-methylcyclohexanone, cyclobutanone and 2-adamantanone.
| Ketone | Metal triflate | Ketone conversion (%) | Selectivity (%) |
|---|---|---|---|
| a Ketone, 0.67 mmol; 60 wt% aq. H2O2, 2.01 mmol; metal triflate, 10 mol%; toluene, 5 ml; 70 °C, conversion and selectivity were determined using GC. | |||
| 2-Methylcyclohexanone | Ga(OTf)3 | 100 | 99 |
| 2-Methylcyclohexanone | Er(OTf)3 | 97 | 94 |
| Cyclobutanone | Ga(OTf)3 | 100 | 100 |
| Cyclobutanone | Er(OTf)3 | 98 | 100 |
| 2-Adamantanone | Ga(OTf)3 | 97 | 99 |
| 2-Adamantanone | Er(OTf)3 | 94 | 80 |
| Norcamphor | Ga(OTf)3 | 98 | 99 |
| Norcamphor | Er(OTf)3 | 96 | 84 |
| Cyclohexanone | Ga(OTf)3 | 99 | 6 |
| Cyclohexanone | Er(OTf)3 | 82 | 10 |
However, our attempts to apply the developed reaction conditions for the synthesis of ε-caprolactone showed that the selectivity towards the lactone formation dropped significantly. In the reaction products the mixture of dimeric, trimeric or polymeric peroxides was detected. The quantitative analysis of this complex post reaction mixture was not possible, concerning the low thermal stability of peroxide species. The presence of dimeric peroxides has been already reported in the literature. The formation of dimeric peroxide was postulated by Baeyer and Villiger in their early works.1,13 However, numerous studies have shown that this peroxide under the influence of Lewis or Brønsted acids could not be converted into the appropriate lactone with high yields and therefore was considered as a “dead end”.1,14,15 The unique studies conducted by Berkessel group showed that oxidation of cyclohexanone with hydrogen peroxide catalyzed by Brønsted acid, such as p-toluenesulfonic acid yielded ε-caprolactone only when carried out in the presence of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as a solvent16,17 (Fig. 4). Authors postulated that perfuorinated solvent facilitates the conversion of dimeric peroxide to lactone by creating strong hydrogen bonds to anions.
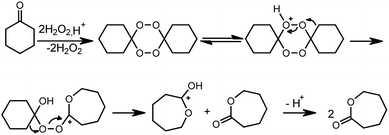 | ||
| Fig. 4 Nonclassical mechanism of the BV oxidation with 50 wt% aq. H2O2 in fluorinated alcohols.17 | ||
Few examples of the formation of trimeric peroxides are known in the literature.2 The synthesis of trimeric peroxides from 1,1′-dihydroperoxydicycloalkyl peroxides and ketones in the presences of 70% perchloric acids was described by Sanderson and Zeiler.18 Vennerstrom et al. observed the formation of trimeric peroxides – hexaoxonane derivatives – during ozonolysis of tetrahydro-4H-pyran-4-one.19 Hong and co-workers described the synthesis of hexaoxonane derivatives in the cyclocondensation of ketones with gem-dihydroperoxide catalysed by acid.20 The oxidation of acetone (linear ketone) with 30% hydrogen peroxide leading to formation of tetrameric acetone peroxide was also presented.21 However, to the best of our knowledge, the path of formation of trimeric peroxides and higher peroxides has not yet been presented. That encouraged us to gain an insight into the mechanism of the Baeyer–Villiger oxidation with hydrogen peroxide using metal triflates as catalysts and demonstrate if the triflate groups may play the similar role as a fluorinated solvent.
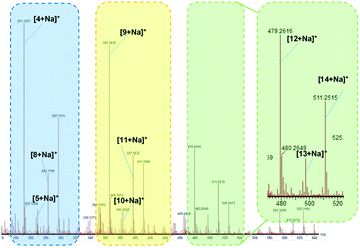
For gallium(III) triflate the conversion of cyclohexanone after 3 hours reached 99% while the selectivity towards ε-caprolactone was only 6% (Table 1). In order to clarify this phenomenon, we conducted high resolution MS analysis which showed that various peroxygen substances were present in the reaction mixture. Based on the outcome, we proposed a reaction mechanism in the presence of Ga(OTf)3 (Fig. 5). In the first stage, nucleophilic addition of hydrogen peroxide to the ketone occurs, resulting in the formation of perhydrate 2. The perhydrate reacts with the cyclohexanone molecule to give dihydroxyperoxide 3. Then several paths are possible in which compound 3 undergoes further transformations. Spirobisperoxide 4 is formed in path a, which can be transformed according to the mechanism proposed by Berkessel.17 The transformations of 10 to 11 and of 13 to 14 proceed in exactly the same way as the transformation of 5 to 8 presented in Fig. 5, but for the sake of readability of the scheme only the final product is presented. The assumption that metal triflates can form hydrogen bonds like HFIP was used. However, gallium triflate was used in a catalytic amount, not as a solvent, so this is not the main path in which compound 3 is converted. In path b, compound 3 reacts with perhydrate to give trimeric peroxide 9 which then rearranges to 11. All intermediates, as well as transient states, were visible and identified on the MS spectrum (Fig. 6, see also in ESI, Fig. S1†).
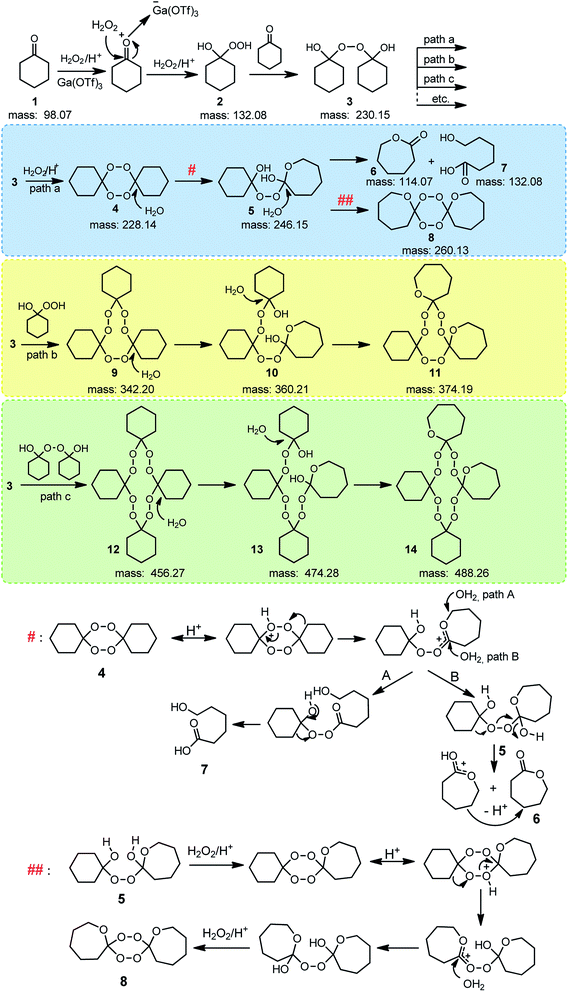 | ||
| Fig. 5 Proposition of the BV oxidation mechanism of cyclohexanone with 60 wt% aq. H2O2, in the presence of Ga(OTf)3. # and ## according to.17 | ||
A completely different mechanism was observed when reactions were conducted in the presence of erbium(III) triflate which shows high hydrolytic stability. In this case, triflic acid is not formed. Thus, the hydroxyl group of cyclohexanol of intermediate 3 was not protonated and cyclized to form the six membered cyclic peroxide 4. Instead of them the linear peroxides were presented in the reaction mixture. The proposed mechanism was not described yet in the literature (Fig. 7 and MS spectrum see in ESI, Fig. S2†).
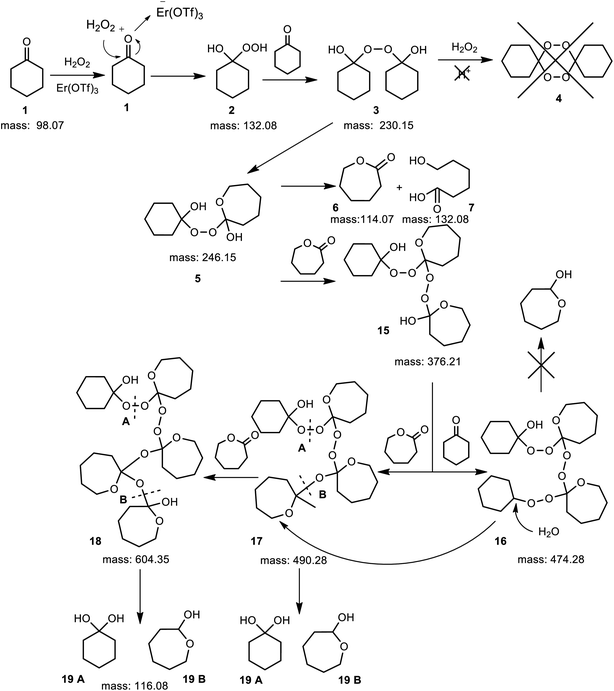 | ||
| Fig. 7 Proposition of the BV oxidation mechanism of cyclohexanone with 60 wt% aq. H2O2, in the presence of Er(OTf)3. | ||
The experiments conducted in the same reaction conditions with 2-methylcyclohexanone, cyclobutanone and 2-adamantone showed that they can be converted to lactone in high yields (Table 1). In the presence of gallium(III) triflate the conversion of these ketones was higher than 97% and selectivity towards lactone reached 99%, while for erbium(III) triflate the conversion and selectivity were higher than 94% and 80%, respectively. MS analysis confirmed that the reaction proceeded in a similar way in the presence of both Ga(OTf)3 and Er(OTf)3 (see ESI, Fig. S3–S8†).
In both cases the formation of spirobisperoxide and lactone was observed according to the mechanism shown in Fig. 4 described in the literature.17 For the formation of a cyclic peroxide a labile proton is required. In case of Er(OTf)3 which is hydrolytically stable this proton could be created through the interaction between erbium(III) triflate and hydrogen peroxide (Fig. 8). Lower selectivity may also indicate a lower strength of Brønsted acid generated in situ in the reaction mixture.
In summary, based on the MS analysis the new mechanisms were evaluated concerning the formation of dimeric and trimeric peroxides when cyclohexanone was used as a substrate. On the other hand, when other ketones were used as the substrates the formation of spirobisperoxide was observed and the reactions proceeded with high conversion and selectivity. These studies develop the knowledge of the reactivity of cyclic ketones with hydrogen peroxide in the presence of metal triflates.
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- M. Renz and B. Meunier, J. Org. Chem., 1999, 737 CAS.
- G. J. Brink, I. W. C. E. Arends and R. A. Sheldon, Chem. Rev., 2004, 104, 4105 CrossRef CAS PubMed.
- C. Jiménez-Sanchidrián and J. R. Ruiz, Tetrahedron, 2008, 64, 2011 CrossRef.
- R. A. Michelin, P. Sgarbossa, A. Scarso and G. Strukul, Coord. Chem. Rev., 2010, 254, 646 CrossRef CAS.
- A. Corma, L. T. Nemeth, M. Renz and S. Valencia, Nature, 2001, 412, 423 CrossRef CAS PubMed.
- S. Kobayashi and K. Manabe, Pure Appl. Chem., 2000, 72, 1373 CAS.
- S. Kobayashi, M. Sugiura, H. Kitagawa and W. W. L. Lam, Chem. Rev., 2002, 102, 2227 CrossRef CAS PubMed.
- A. Berkessel, E. Ashkenazi and M. R. M. Andreae, Appl. Catal., A, 2003, 254, 27 CrossRef CAS.
- M. Markiton, A. Ciemięga, K. Maresz, A. Szelwicka, J. Mrowiec-Białoń and A. Chrobok, New J. Chem., 2018, 42, 13602 RSC.
- M. Markiton, A. Szelwicka, S. Boncel, S. Jurczyk and A. Chrobok, Appl. Catal., A, 2018, 556, 81 CrossRef CAS.
- W. v. E. Doering and E. J. Dorfman, J. Am. Chem. Soc., 1953, 75, 5595 CrossRef CAS.
- P. E. Blatz and D. L. J. Pipert, J. Am. Chem. Soc., 1968, 90, 1296 CrossRef CAS.
- A. Baeyer and V. Villiger, Ber. Dtsch. Chem. Ges., 1900, 33, 858 CrossRef CAS.
- L. Ruzicka and M. Stoll, Helv. Chim. Acta, 1928, 11, 1159 CrossRef CAS.
- W. Dilthey, M. Inckel and H. Stephan, J. Prakt. Chem., 1940, 154, 219 CrossRef CAS.
- A. Berkessel and M. R. M. Andreae, Tetrahedron Lett., 2001, 42, 2293 CrossRef CAS.
- A. Berkessel, M. R. M. Andreae, H. Schmickler and J. Lex, Angew. Chem., Int. Ed., 2002, 41, 4481 CrossRef CAS PubMed.
- J. R. Sanderson and A. G. Zeiler, Synthesis, 1975, 6, 388 CrossRef.
- Y. Dong and J. L. Vennerstrom, J. Org. Chem., 1998, 63, 8582 CrossRef CAS.
- A. B. Rode, K. Chung, Y. W. Kim and I. S. Hong, Energy Fuels, 2010, 24, 1636 CrossRef CAS.
- H. Jiang, G. Chu, H. Gong and Q. Qiao, J. Chem. Res., 1980, 2, 34 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: MS spectra, experimental procedures. See DOI: 10.1039/d0ra03335a |
| This journal is © The Royal Society of Chemistry 2020 |