Open Access Article
Open Access ArticleChemically anchored two-dimensional-SiOx/zero-dimensional-MoO2 nanocomposites for high-capacity lithium storage materials†
Soohwan Kim,
Hyundong Yoo and
Hansu Kim *
*
Department of Energy Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul, 04763, Republic of Korea. E-mail: khansu@hanyang.ac.kr
First published on 4th June 2020
Abstract
Silicon oxides are promising alternatives for graphite anodes in lithium-ion batteries. SiOx nanosheets exhibit favorable anodic performances, including outstanding capacity retention and dimensional stability, due to their unique two-dimensional (2D) microstructures, but suffer from low specific capacity and poor initial coulombic efficiency. Here we demonstrate that chemically anchoring of molybdenum dioxide (MoO2) nanoparticles on the surface of 2D-SiOx nanosheets via a Mo–O–Si bond boosts both the reversible capacity and initial coloumbic efficiency without sacrificing the useful properties of 2D-SiOx nanosheets. The enhancements can be attributed to the introduction of a zero-dimensional MoO2 nano-object, which offers abnormal storage sites for lithium. The proposed nano-architecturing shows how we can maximize the advantages of 2D nanomaterials for energy storage applications.
Introduction
Lithium-ion batteries (LIBs) are used in diverse application such as portable electronic devices, electric vehicles, and energy storage systems1–3 due to their relatively high energy and power densities.4,5 Growing demands for batteries in various applications are driving efforts to advance battery performance.6,7 However, state-of-the-art LIB technology based on graphite anodes appears to have reached its limit,8,9 and numerous materials have been suggested for alternative anode materials.10–13 Silicon-based materials offer a high theoretical capacity (3580 mA h g−1, Li3.75Si) and relatively low operating potential (∼0.4 V vs. Li/Li+),14–16 but several constraints must be addressed before such materials can be used in practical applications. For example, volume changes of approximately 300% during lithiation and delithiation can undermine cycling performance.16–18 To overcome this drawback, various Si-based anode materials, such as SiOx-based composites, Si-carbon nanocomposites, and Si-transition metal alloys, have been proposed to minimize the mechanical strain induced by severe volume changes.19–23Recently, we developed two-dimensional (2D) SiOx nanosheets for use as anode materials in LIBs through a scalable and cost-effective sol–gel process.24 The 2D-SiOx nanosheets moderated volume expansion, enhanced cycling performance, and suppressed volume changes by taking advantage of the unique nano-architecture of 2D nanosheets. First, the absolute volume changes in thin-layered SiOx (thickness of approximately 8 nm) are much smaller than those of other Si-based anode materials. Second, randomly stacked 2D-SiOx nanosheet electrodes incorporate abundant free spaces that can accommodate volume changes in SiOx.24
Despite such advantages, several technical issues remained to be resolved, including a low reversible capacity less than 600 mA h g−1 and a poor initial coulombic efficiency of approximately 30%. To address these problems, we designed nanocomposites of 2D-SiOx nanosheets with zero-dimensional (0D) molybdenum dioxide (MoO2) nanoparticles because devising complex with 0D nanomaterials would be an alternative for complementing imperfection of 2D nanomaterials.25,26 We anticipated that such nanosheets would not require sacrificing the advantages of 2D-SiOx nanosheets because unique electrochemical properties of nanostructured MoO2. Nanostructured MoO2 has a lithium storage capacity far beyond its theoretical capacity based on conversion reactions with lithium due to its abnormal lithium storage reaction mechanism, as well as has a highly stable cycling performance over prolonged cycles.27–29 We placed 0D-MoO2 nanoparticles on the surface of 2D-SiOx nanosheets using simple carbothermic reduction of MoO3 with urea. In the proposed 2D-SiOx/0D-MoO2 nanocomposites, 0D-MoO2 nanoparticles bonded tightly to 2D-SiOx nanosheets via a Mo–O–Si bond, providing highly stable capacity retention upon repeated charge–discharge cycles. The resulting synergistic effect through creating chemical bonds with 0D-MoO2 nanoparticles without sacrificing the unique properties of SiOx-based anodes, would be another successful example that show how 2D nanomaterial overcomes its drawbacks with maintaining its unique properties for electrochemical energy storage applications.
Experimental
Material preparation
2D-SiOx nanosheets and MoO3 were prepared by our previously reported synthetic route.24,28 2D-SiOx nanosheets were prepared by interfacial sol–gel reaction of trichlorosilane (Tokyo Chemical Industry, > 98%) at the interface between water and air. Trichlorosilane (1.5 mL) was centered in the middle of a glass cylinder (diameter: 14 cm) filled with deionized water (200 mL) at room temperature. After 10 min, shredded-sheet-like hydrogen silsesquioxane (HSQ, HSiO1.5) was obtained at the surface of the deionized water. The particles were filtered and washed several times with deionized water to remove the residual acid. Filtered powders were collected and dried in a convection oven at 80 °C until the moisture was completely evaporated. The obtained HSQ was heat-treated at 1000 °C for 1 h under a 4% H2/Ar atmosphere at a heating rate of 20 °C min−1 and a flow rate of 0.5 L min−1 to generate 2D-SiOx nanosheets. MoO3 was prepared from the precipitation of an ammonium molybdate tetrahydrate (Sigma-Aldrich, 81.0–83.0% MoO3 basis) aqueous solution (0.05 M, 100 mL) using nitric acid solution (3 mL) (Sigma-Aldrich, 70%). The solution was stirred at room temperature for 3 h and its precipitates were filtered and washed several times with deionized water to remove the residual acid. Filtered MoO3 powders were collected and dried in a convection oven at 80 °C. 2D-SiOx/0D-MoO2 nanocomposites were synthesized by simple mixing of starting materials and carbothermic reduction of the mixture with urea. First, the obtained 2D-SiOx nanosheets were sonicated for 20 min in ethanol (30 mL) (Daejung Chemicals & Metals, 99.9%) and the obtained MoO3 was stirred for 1 h in ethanol (15 mL) to obtain well-dispersed particles. Then, dispersed 2D-SiOx nanosheets and MoO3 particles were mixed together and stirred for 1 h at room temperature. After 1 h, the mixture was stirred and heated at 70 °C to evaporate the ethanol, and the obtained powders were then mixed with urea (Sigma-Aldrich, > 98%) (MoO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea = 2
urea = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by weight) for carbothermic reduction of MoO3. Finally, 2D-SiOx/0D-MoO2 nanocomposites were obtained after heat treatment of the urea-mixed powders at 500 °C for 2 h under an N2 atmosphere at a heating rate of 10 °C min−1.
1, by weight) for carbothermic reduction of MoO3. Finally, 2D-SiOx/0D-MoO2 nanocomposites were obtained after heat treatment of the urea-mixed powders at 500 °C for 2 h under an N2 atmosphere at a heating rate of 10 °C min−1.
Structural characterization
The surface morphology and microstructures of the particles were characterized by field emission scanning electron microscope (FEI Verios G4 UC) and high-resolution transmission electron microscope (JEOL JEM-2100F). The microstructures of the samples were further identified by an X-ray diffractometer (Rigaku SmartLab) equipped with a 3D pixel semiconductor detector using Cu-Kα radiation. A Raman spectrometer (Horiba Jobin-Yvon LabRam ARAMIS) with an Ar-ion laser at a wavelength of 514 nm was employed to identify the obtained 2D/0D nanocomposites. The chemical bonds of the samples were further investigated using an FTIR spectrophotometer (Thermo Scientific Nicolet 6700).Electrochemical measurements
Electrochemical tests were performed using CR2032 coin-type cells with a lithium metal counter electrode. The working electrodes were prepared by coating slurries on 10 μm-thick copper foil with a mass loading of 1.0 mg cm−2. The slurries, containing 2D-SiOx nanosheets and 2D-SiOx/0D-MoO2 nanocomposites as the active materials were made by mixing the active material (70 wt%), Super-P as a conductive agent (10 wt%), and poly(acrylic acid) (PAA) as a binder (20 wt%) in deionized water. The slurry-coated electrodes were dried at 120 °C for 2 h in a vacuum oven. The coin-type cells were assembled in an Ar-filled glove box using a polyethylene membrane (Celgard 2400 membrane) as a separator and 1 M LiPF6 dissolved in a mixed solvent of ethylene carbonate and ethyl methyl carbonate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) with additives of fluoroethylene carbonate (2 wt%) (Panax Etec Co., Ltd.) as an electrolyte. The galvanostatic charge/discharge measurement was carried out using a battery-testing system (BaSyTec Cell Test System) at current densities of 200 mA g−1 at a voltage range of 0.005 to 3.0 V versus Li/Li+. Charging (lithium insertion) was performed in a constant current–constant voltage mode and discharging was performed with in a constant-current mode. The structural changes of the discharged and charged 2D-SiOx/0D-MoO2 nanocomposites electrodes were analysed by ex situ XRD analysis during cycling.
7 v/v) with additives of fluoroethylene carbonate (2 wt%) (Panax Etec Co., Ltd.) as an electrolyte. The galvanostatic charge/discharge measurement was carried out using a battery-testing system (BaSyTec Cell Test System) at current densities of 200 mA g−1 at a voltage range of 0.005 to 3.0 V versus Li/Li+. Charging (lithium insertion) was performed in a constant current–constant voltage mode and discharging was performed with in a constant-current mode. The structural changes of the discharged and charged 2D-SiOx/0D-MoO2 nanocomposites electrodes were analysed by ex situ XRD analysis during cycling.
Results and discussion
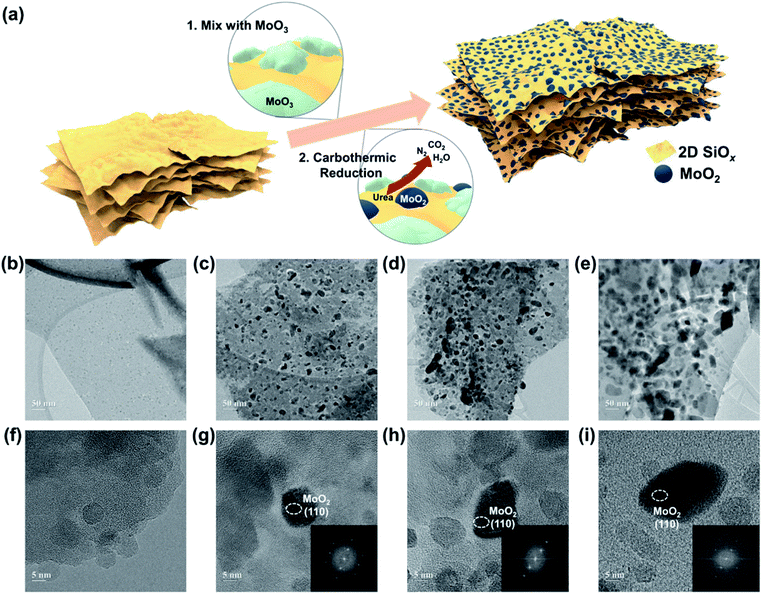
Fig. 1a illustrates a synthetic route for the proposed 2D-SiOx/0D-MoO2 nanocomposites, in which 0D-MoO2 nanoparticles fabricated by carbothermic reduction of MoO3 particles are chemically anchored to the surface of 2D-SiOx nanosheets. Urea performed a dual role of reducing agent and dispersant for reduction of MoO3 to MoO2.30 Fig. 1b–e shows transmission electron microscopy (TEM) images of the 2D-SiOx nanosheets and 2D-SiOx/0D-MoO2 nanocomposites with different amounts of MoO2 (20, 30, and 40 wt%). As previously reported,24 bare 2D-SiOx nanosheets have a 2D sheet-like morphology (width: ∼20 μm, thickness: ∼8 nm) with a smooth surface (Fig. 1b). Due to very small sizes of 0D-MoO2 nanoparticles, it was hard to observe them using a scanning electron microscopy (SEM) images (Fig. S1†), but TEM images of 0D-MoO2 nanoparticles reveal that MoO2 nanoparticles with the diameter of about 10 nm were formed on the 2D-SiOx nanosheets (Fig. 1c–e). The size and amounts of the nanoparticles increased as MoO2 was added. High-resolution TEM images with corresponding fast Fourier transformation (FFT) patterns confirmed the formation of 0D-MoO2 nanoparticles from the d-spacing values for the (110) plane of MoO2, with sizes ranging between 5 nm and 20 nm (Fig. 1f–i).X-ray diffraction (XRD) patterns confirmed the formation of MoO2 on the surface of the 2D-SiOx nanosheets (Fig. 2a). All XRD patterns showed a broad Bragg peak at 2θ = 21.8°, which is a general characteristic of amorphous Si suboxides.31–34 Other Bragg peaks appeared in the XRD patterns of 2D-SiOx/0D-MoO2 nanocomposites at 2θ = 26.0°, 37.0°, 53.5°, 60.2°, 66.6°, and 78.7°, which corresponded to MoO2.35,36 The strongest peak in the XRD patterns of 2D-SiOx/0D-MoO2 nanocomposites at 2θ = 26.0° corresponded to the (110) plane of MoO2, which matched the aforementioned FFT pattern analysis on the 2D-SiOx/0D-MoO2 nanocomposites. Fig. 2b shows Raman spectra of the 2D-SiOx nanosheets and 2D-SiOx/0D-MoO2 nanocomposites, with the spectra for MoO2 particles included as a reference. 2D-SiOx/0D-MoO2 nanocomposites showed typical bands for MoO2,37 while Raman spectra for the 2D-SiOx nanosheets only showed a single Raman band near 480 cm−1, corresponding to amorphous Si,38 which was commonly observed as non-stoichiometric SiOx. No band for amorphous Si was observed for the 2D-SiOx/0D-MoO2 nanocomposites because the intensity of the Raman bands for MoO2 were much stronger than that of amorphous Si and they overlapped the MoO2 bands.
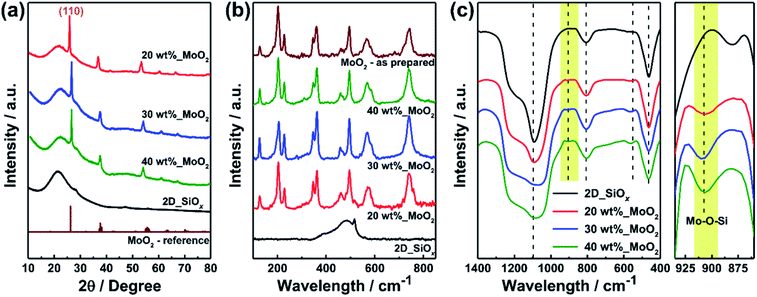 | ||
| Fig. 2 (a) XRD patterns, (b) Raman spectra, and (c) FTIR spectra for the 2D-SiOx/0D-MoO2 nanocomposites with the different amounts of MoO2. | ||
To identify specific structures of the 2D-SiOx/0D-MoO2 nanocomposites, it was necessary to determine whether chemical bonding occurred in 2D-SiOx nanosheets and 0D-MoO2 nanoparticles. Fig. 2c shows Fourier transform infrared (FTIR) spectra of the 2D-SiOx nanosheets and 2D-SiOx/0D-MoO2 nanocomposites. Peaks at 1100, 806, and 470 cm−1 in all samples were assigned to asymmetric vibration of Si–O–Si, symmetric stretching vibration of Si–O–Si, and bending vibration of Si–O–Si, respectively,39–41 from the 2D-SiOx nanosheets. Mo–O–Si bond and symmetric stretching of Mo–O–Mo near 908 and 560 cm−1 were also observed in all 2D-SiOx/0D-MoO2 nanocomposite samples.42,43 The appearance of a Mo–O–Si bond in the FTIR spectra implies that the 0D-MoO2 nanoparticles were tightly anchored with the 2D-SiOx nanosheets through chemical bonding, not just placed on the surface of 2D-SiOx nanosheets.
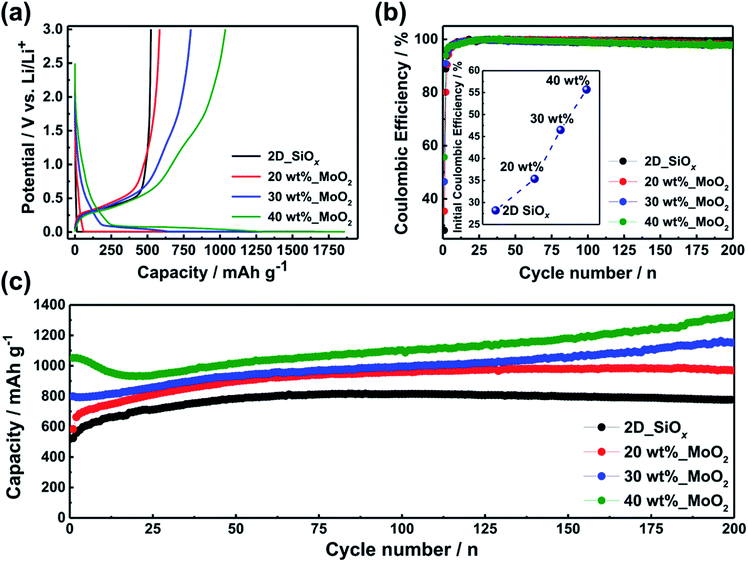
Voltage profiles of the 2D-SiOx nanosheets and 2D-SiOx/0D-MoO2 nanocomposites electrodes with different amounts of MoO2 (20, 30, and 40 wt%) for the first cycle are shown in Fig. 3a. The 2D-SiOx nanosheets electrode delivered an initial reversible capacity of 524.0 mA h g−1, while the 2D-SiOx/0D-MoO2 nanocomposites electrodes exhibited higher initial reversible capacities of 584.0 mA h g−1 (20 wt%), 799.2 mA h g−1 (30 wt%), and 1051.6 mA h g−1 (40 wt%). The initial reversible capacities of the 2D-SiOx/0D-MoO2 nanocomposites electrodes increased as the amount of MoO2 increased, eventually doubling that of bare 2D-SiOx nanosheets electrode. The 2D-SiOx/0D-MoO2 nanocomposites electrodes also showed enhanced initial coulombic efficiencies of 35.4% (20 wt%), 46.5% (30 wt%), and 55.7% (40 wt%) compared with the 2D-SiOx nanosheets electrode (28.2%) (Fig. 3b). The improved performances can be attributed to the exceptional lithium storage behavior of nanostructured MoO2,29 which could compensate for the low initial capacity and poor initial coulombic efficiency of the 2D-SiOx nanosheet electrode.24 Fig. 3c shows the long-term cycling performance of the 2D-SiOx nanosheet electrode and 2D-SiOx/0D-MoO2 nanocomposite electrodes at a constant current density of 200 mA h g−1 for 200 cycles. All samples exhibited highly stable cycling performances over 200 cycles with stable coulombic efficiencies (Fig. 3b and c). A gradual increase in reversible capacities of all the samples was evident during cycling due to the gradual activation of amorphous SiOx matrix toward lithium-ion insertion and extraction.23,24,32,44 A further increase in the reversible capacities of the 2D-SiOx/0D-MoO2 nanocomposites electrodes was also evident. The increase in the capacity of the 2D-SiOx/0D-MoO2 nanocomposites electrodes can be attributed to the activation of MoO2 nanoparticles associated with the unique microstructural evolution of nanostructured MoO2.45,46 These increasing trends were also observed in charge–discharge curves for selected cycles (Fig. S2†). This cycling performance was due to the favorable microstructures and dimensional stability of the 2D-SiOx nanosheets as well as the outstanding lithium storage properties of 0D-MoO2 nanoparticles.24,28,45–47
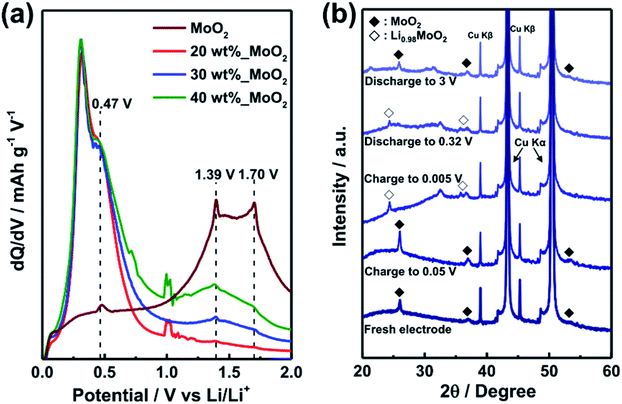
Differential capacity plots (DCPs) of the 2D-SiOx/0D-MoO2 nanocomposites electrode upon first discharge within a voltage range of 0.0 to 2.0 V (vs. Li/Li+) revealed the aforementioned reaction pathways of the 2D-SiOx/0D-MoO2 nanocomposite electrode (Fig. 4a). Among four different DCPs peaks, the sharp peak of the 2D-SiOx/0D-MoO2 nanocomposite electrodes at 0.32 V corresponded to the delithiation of a-LixSi.16,48,49 Three other peaks at 0.47 V, 1.39 V, and 1.70 V reflected the delithiation of LixMoO2,50,51 which also originated from the delithiation reaction of the MoO2 electrode (the brown line in Fig. 4a). The electrochemical reaction process of the 0D-MoO2 nanoparticles anchored on the 2D-SiOx nanosheets during delithiation helped compensate for the low initial capacity and poor initial coulombic efficiency of the 2D-SiOx nanosheet electrode.24 To investigate the improved lithium storage mechanism of the 2D-SiOx/0D-MoO2 nanocomposite electrode, we explored how the crystalline structures of the 2D-SiOx/0D-MoO2 nanocomposites changed during the electrochemical reaction. Fig. 4b shows ex situ XRD patterns of the 2D-SiOx/0D-MoO2 nanocomposite electrodes for the first cycle. We found that the Bragg peaks for MoO2 shifted to a lower angle upon lithium insertion due to the phase transition of MoO2 to Li0.98MoO2. We found no Bragg peak corresponding to the metallic Mo or Li2O phases, which are typical reaction products formed by conversion reaction of MoO2 with lithium ions. This implies that the 2D-SiOx/0D-MoO2 nanocomposites did not follow conventional conversion reactions of MoO2,52 but reacted instead with lithium ions through another lithium storage mechanism. It is not surprising that nanostructured MoO2 did not follow conventional conversion reactions. Shon et al. reported that ordered mesoporous MoO2 exhibited an extraordinarily high capacity from abnormal lithium storage sites.29 According to Shon et al., upon lithiation, lithium ions firstly inserted into the lattice of MoO2 lead to the formation of a lithium-intercalated MoO2 crystalline phase up to Li0.98MoO2. Next, more lithium ions were stored at the interface between the LixMoO2 domains as a metallic state, resulting in the formation of amorphous metallic lithium clusters.29 This reaction process created no other crystalline phases, except for those of MoO2 and LixMoO2. This reaction pathway matched the ex situ XRD patterns (Fig. 4b). Reverse phase transition was observed upon subsequent discharge,53,54 revealing that the 2D-SiOx/0D-MoO2 nanocomposites underwent highly reversible phase transition during lithiation and delithiation, explaining the stable cycling performance of the 2D-SiOx/0D-MoO2 nanocomposite electrode. The presented exceptional reaction pathways of 0D-MoO2 particles played an important role in improving the lithium storage properties of the 2D-SiOx nanosheets without degrading the superior physicochemical properties of 2D-SiOx nanosheets.
Conclusions
We demonstrated a rational design for chemically anchored 2D-SiOx/0D-MoO2 nanocomposites using a Mo–O–Si bond through a carbothermic reduction route with urea as a reducing agent. Introduction of 0D-MoO2 nanoparticles helped compensate for the shortcomings of 2D-SiOx nanosheets in terms of reversible capacity and initial coulombic efficiency without sacrificing cycling performance. Such improvements can be achieved through a synergistic effect of the unique properties of the 2D-SiOx nanosheets (favorable microstructures and dimensional stability) and the 0D-MoO2 nanoparticles (abnormal lithium storage sites and highly reversible phase transition). The results showed that our material approach to enhance lithium storage properties by creating bonds with the 0D-MoO2 nanoparticles at the surface of 2D-SiOx nanosheets can maximize the unique physicochemical properties of 2D nanomaterial-based nanocomposites for next-generation electrochemical energy storage application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Samsung Research Funding Center for Future Technology (SRFC-MA1401-52).Notes and references
- B. Scrosati, Nature, 2011, 473, 448–449 CrossRef CAS.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- E. C. Evarts, Nature, 2015, 526, S93–S95 CrossRef CAS PubMed.
- N. Nitta, F. X. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- J. Lu, Z. H. Chen, Z. F. Ma, F. Pan, L. A. Curtiss and K. Amine, Nat. Nanotechnol., 2016, 11, 1031–1038 CrossRef CAS PubMed.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243–3262 RSC.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- F. Y. Cheng, J. Liang, Z. L. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- G. Jeong, Y. U. Kim, H. Kim, Y. J. Kim and H. J. Sohn, Energy Environ. Sci., 2011, 4, 1986–2002 RSC.
- C. M. Park, J. H. Kim, H. Kim and H. J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC.
- S. M. Hwang, Y. G. Lim, J. G. Kim, Y. U. Heo, J. H. Lim, Y. Yamauchi, M. S. Park, Y. J. Kim, S. X. Dou and J. H. Kim, Nano Energy, 2014, 10, 53–62 CrossRef CAS.
- M. Pramanik, M. Imura, J. J. Lin, J. Kim, J. H. Kim and Y. Yamauchi, Chem. Commun., 2015, 51, 13806–13809 RSC.
- D. Bresser, S. Passerini and B. Scrosati, Energy Environ. Sci., 2016, 9, 3348–3367 RSC.
- T. D. Hatchard and J. R. Dahn, J. Electrochem. Soc., 2004, 151, A838–A842 CrossRef CAS.
- U. Kasavajjula, C. S. Wang and A. J. Appleby, J. Power Sources, 2007, 163, 1003–1039 CrossRef CAS.
- M. N. Obrovac and L. J. Krause, J. Electrochem. Soc., 2007, 154, A103–A108 CrossRef CAS.
- R. A. Huggins and W. D. Nix, Ionics, 2000, 6, 57–63 CrossRef CAS.
- L. Y. Beaulieu, K. W. Eberman, R. L. Turner, L. J. Krause and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, A137–A140 CrossRef CAS.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353–358 CrossRef CAS PubMed.
- W. J. Zhang, J. Power Sources, 2011, 196, 13–24 CrossRef CAS.
- H. Wu and Y. Cui, Nano Today, 2012, 7, 414–429 CrossRef CAS.
- R. Yi, F. Dai, M. L. Gordin, S. R. Chen and D. H. Wang, Adv. Energy Mater., 2013, 3, 295–300 CrossRef CAS.
- M. S. Park, E. Park, J. Lee, G. Jeong, K. J. Kim, J. H. Kim, Y. J. Kim and H. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 9608–9613 CrossRef CAS PubMed.
- H. Yoo, E. Park, J. Bae, J. Lee, D. J. Chung, Y. N. Jo, M. S. Park, J. H. Kim, S. X. Dou, Y. J. Kim and H. Kim, Sci. Rep., 2018, 8, 6904 CrossRef PubMed.
- M. Zheng, D. Qiu, B. Zhao, L. Ma, X. Wang, Z. Lin, L. Pan, Y. Zheng and Y. Shi, RSC Adv., 2013, 3, 699–703 RSC.
- Z. Jian, B. Zhao, P. Liu, F. Li, M. Zheng, M. Chen, Y. Shi and H. Zhou, Chem. Commun., 2014, 50, 1215–1217 RSC.
- J. F. Ni, Y. Zhao, L. Li and L. Q. Mai, Nano Energy, 2015, 11, 129–135 CrossRef CAS.
- A. Kim, E. Park, H. Lee and H. Kim, J. Alloys Compd., 2016, 681, 301–306 CrossRef CAS.
- J. K. Shon, H. S. Lee, G. O. Park, J. Yoon, E. Park, G. S. Park, S. S. Kong, M. Jin, J. M. Choi, H. Chang, S. Doo, J. M. Kim, W. S. Yoon, C. Pak, H. Kim and G. D. Stucky, Nat. Commun., 2016, 7, 11049 CrossRef CAS PubMed.
- A. M. Chu, M. L. Qin, R. Ud-din, B. R. Jia, H. F. Lu and X. H. Qu, J. Alloys Compd., 2012, 530, 144–151 CrossRef CAS.
- H. Takezawa, K. Iwamoto, S. Ito and H. Yoshizawa, J. Power Sources, 2013, 244, 149–157 CrossRef CAS.
- E. Park, H. Yoo, J. Lee, M. S. Park, Y. J. Kim and H. Kim, ACS Nano, 2015, 9, 7690–7696 CrossRef CAS PubMed.
- E. Park, M. S. Park, J. Lee, K. J. Kim, G. Jeong, J. H. Kim, Y. J. Kim and H. Kim, ChemSusChem, 2015, 8, 688–694 CrossRef CAS PubMed.
- J. Bae, D. S. Kim, H. Yoo, E. Park, Y. G. Lim, M. S. Park, Y. J. Kim and H. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 4541–4547 CrossRef CAS PubMed.
- L. Zheng, Y. Xu, D. Jin and Y. Xie, J. Mater. Chem., 2010, 20, 7135–7143 RSC.
- X. L. Liu, D. Wu, W. X. Ji and W. H. Hou, J. Mater. Chem. A, 2015, 3, 968–972 RSC.
- P. A. Spevack and N. S. Mcintyre, J. Phys. Chem., 1992, 96, 9029–9035 CrossRef CAS.
- N. Tomozeiu, Appl. Surf. Sci., 2006, 253, 376–380 CrossRef CAS.
- H. Guo, R. Mao, X. J. Yang and J. Chen, Electrochim. Acta, 2012, 74, 271–274 CrossRef CAS.
- M. R. S. Kebria, M. Jahanshahi and A. Rahimpour, Desalination, 2015, 367, 255–264 CrossRef CAS.
- H. Sanaeishoar, M. Sabbaghan and F. Mohave, Microporous Mesoporous Mater., 2015, 217, 219–224 CrossRef CAS.
- K. Eda, J. Solid State Chem., 1991, 95, 64–73 CrossRef CAS.
- M. M. Mohamed and G. M. S. Elshafei, Spectrochim. Acta, Part A, 1995, 51, 1525–1531 CrossRef.
- B. C. Yu, Y. Hwa, J. H. Kim and H. J. Sohn, Electrochim. Acta, 2014, 117, 426–430 CrossRef CAS.
- E. Zhou, C. G. Wang, M. H. Shao, X. L. Deng and X. J. Xu, Ceram. Int., 2017, 43, 760–765 CrossRef CAS.
- M. Fenech, S. Lim, J. Cheung and N. Sharma, Phys. Chem. Chem. Phys., 2019, 21, 25779–25787 RSC.
- B. K. Guo, X. P. Fang, B. Li, Y. F. Shi, C. Y. Ouyang, Y. S. Hu, Z. X. Wang, G. D. Stucky and L. Q. Chen, Chem. Mater., 2012, 24, 457–463 CrossRef CAS.
- B. C. Yu, Y. Hwa, C. M. Park and H. J. Sohn, J. Mater. Chem. A, 2013, 1, 4820–4825 RSC.
- J. Woo and S. H. Baek, RSC Adv., 2017, 7, 4501–4509 RSC.
- K. Palanisamy, Y. Kim, H. Kim, J. M. Kim and W. S. Yoon, J. Power Sources, 2015, 275, 351–361 CrossRef CAS.
- J. B. Boland, A. Harvey, R. Y. Tian, D. Hanlon, V. Vega-Mayoral, B. Szydlowska, A. Griffin, T. Stimpel-Lindner, S. Jaskaniec, V. Nicolosi, G. Duesberg and J. N. Coleman, Nanoscale Adv., 2019, 1, 1560–1570 RSC.
- Q. Xie and D. L. Peng, in Advanced Battery Materials, ed. C. Sun, Scrivener Publishing, Massachusetts, 1st edn, 2019, ch. 3, pp. 185–189 Search PubMed.
- J. R. Dahn and W. R. Mckinnon, Solid State Ionics, 1987, 23, 1–7 CrossRef CAS.
- K. Ben-Kamel, N. Amdouni, H. Groult, A. Mauger, K. Zaghib and C. M. Julien, J. Power Sources, 2012, 202, 314–321 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FESEM images, and galvanostatic charge–discharge curves. See DOI: 10.1039/d0ra02462g |
| This journal is © The Royal Society of Chemistry 2020 |