Open Access Article
Open Access ArticleInduction of ADCC by a folic acid–mAb conjugate prepared by tryptophan-selective reaction toward folate-receptor-positive cancer cells†
Hiroshi Tagawa‡
a,
Katsuya Maruyama‡b,
Koichi Sasaki c,
Natsuki Konoueb,
Akihiro Kishimuraacd,
Motomu Kanai
c,
Natsuki Konoueb,
Akihiro Kishimuraacd,
Motomu Kanai b,
Takeshi Mori
b,
Takeshi Mori *ac,
Kounosuke Oisaki
*ac,
Kounosuke Oisaki *b and
Yoshiki Katayama
*b and
Yoshiki Katayama *acdef
*acdef
aGraduate School of Systems Life Sciences, Kyushu University, Fukuoka, Japan
bGraduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan
cDepartment of Applied Chemistry, Faculty of Engineering, Kyushu University, Fukuoka, Japan
dInternational Research Center for Molecular Systems, Kyushu University, Fukuoka, Japan
eCenter for Advanced Medical Innovation, Kyushu University, Fukuoka, Japan
fDepartment of Biomedical Engineering, Chung Yuan Christian University, Taoyuan, Taiwan
First published on 29th April 2020
Abstract
We developed conjugates between monoclonal antibody (mAb) and folic acid (FA) by using a tryptophan (Trp)-selective reaction, which yields relatively homogenous products compared to conventional methods. The obtained mAb–FA conjugates showed significant cellular cytotoxicity toward folate receptor-expressing cancer cells, demonstrating that the conjugates retained the Fc region's original function.
Introduction
Monoclonal antibodies (mAbs) have been used as cancer treatments because of their highly specific binding to antigens via the Fab region. The Fc region of a mAb also plays a crucial role by expressing effector functions as well as increasing blood retention time.1 There are three proteins that recognize the Fc region of IgG to induce these functions (Fig. 1). Fcγ receptors (FcγRs) and complement component 1q (C1q) recognize the Fc region to exert effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).2,3 Neonatal Fc receptor (FcRn) recognizes the Fc region to extend the blood half-life of IgG.4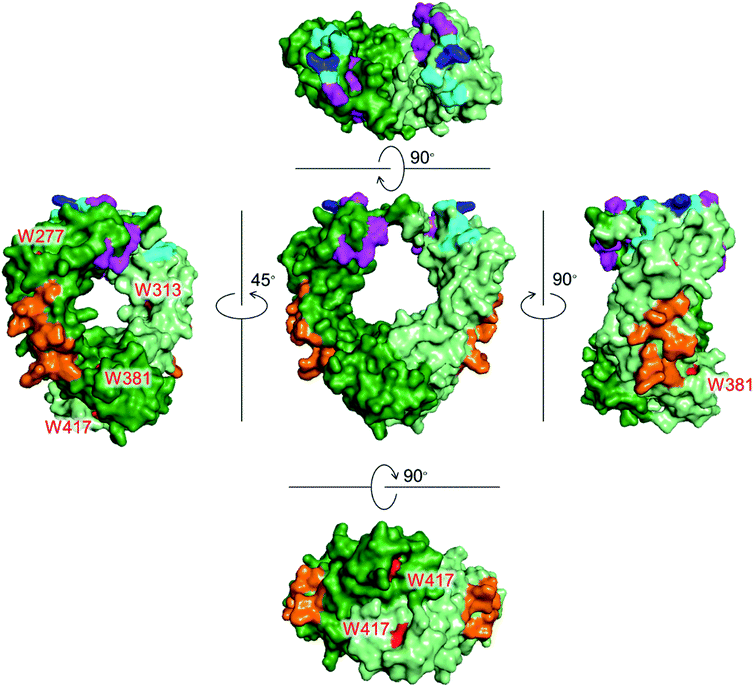 | ||
| Fig. 1 Crystal structures of the Fc region of IgG1 with Trp residues shown in red. Each chain is shown in green and light green colors. Recognition residues of the three proteins are shown in different colors: magenta (FcγRIIIa, CD16a), cyan (C1q), blue (overlapped residues of FcγRIIIa and C1q), and orange (FcRn). PDB ID: 1E4K. | ||
Conjugation of mAbs to small molecules, such as drugs, fluorophores, and ligands, is widely performed to expand the functions of mAb-based therapeutics. For the effector functions to be retained in the mAb conjugates, it is crucial that the critical residues in the Fc regions are preserved. For the mAb-conjugation of small molecules, lysine and cysteine residues have been used.5–8 Since lysine residues are abundant on the surface of the Fc region, lysine-specific modification may disturb the recognition of effector proteins. Cysteine residues exist that at the hinge region are typically used for conjugation. However, cleavage of these disulfide bonds potentially lowers the structural stability of mAbs and abrogates their effector functions.9,10 To overcome these problems, recombinant gene modification has been utilized to introduce natural and non-natural amino acids as a modifiable residue.5,11 However, such recombinant methods require screening process to determine a site of the gene modification to satisfy efficient protein expression with a native conformation and a high yield in the chemical conjugation reaction.
Tryptophan (Trp) is the least abundant (∼1%),12 and the least surface-exposed proteinogenic amino acid, and each Trp residue has a variety of solvent accessibilities. As shown in Fig. 1, Trp residues in the Fc region do not overlap with the recognition residues of the three proteins. Thus, the controlled conjugation via Trp is not expected to disturb the recognition areas of the three effector proteins. We previously developed a tryptophan (Trp)-selective conjugation of proteins using a stable organoradical, 9-azabicylo[3.3.1]nonane N-oxyl (ABNO).13,14
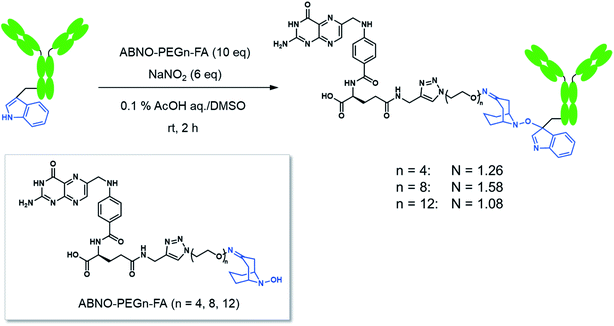
Here we conducted a Trp-selective conjugation of a mAb to folic acid (FA), which is a well-known ligand of cancer cells (Fig. 2A). The FA–mAb conjugates showed ADCC against folate receptor-α (FR-α) positive cancer cells without conventional binding via the Fab region, suggesting minimized impairment of the Fc functions by Trp-conjugation (Fig. 2B).
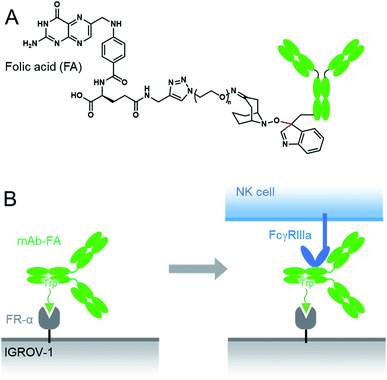 | ||
| Fig. 2 The structures of the mAb–FA conjugates via Trp reside (A) and schematic illustration of ADCC induction by the mAb–FA conjugates which bind to the FR-α on a cancer cell via FA (B). | ||
Results and discussion
FA has been widely used as a targeting ligand for drug delivery and imaging of cancer cells because some kinds of cancer cells overexpress FR-α, which has a high affinity toward FA.15,16 Pioneering works for the conjugation of mAbs with FA were reported by two groups.17,18 One of the groups applied the conjugates to induce ADCC and succeeded in enhancing cytotoxic activity of natural killer (NK) cells toward FR-α-expressing cancer cells.17 In these previous reports, FA was directly conjugated on the lysine residues of mAbs, which may impair the Fc functions. Moreover, the direct conjugation of FA produced mixtures of conjugates reacted via α- and γ-carboxyl groups of FA, although FA conjugated via the α-carboxyl group was reported to be irrelevant for binding to FR-α.19 Hence, heterogeneous structures of the conjugates prepared by the direct conjugation would vary the efficacy of ADCC of each conjugate molecule. In order to prepare homogeneous conjugates, here we utilized reagents of ABNO-PEGn-FA14 for Trp-selective conjugation of a mAb (Fig. 3). These reagents enable γ-carboxyl group-selective conjugation of FA on Trp residues of a mAb via a flexible PEG linker. Even if the Trp residues in the Fc region are conjugated by the reagent, the mAb–FA conjugate is expected to induce ADCC via recognition by FcγRIIIa20,21 because there is no Trp residue in the FcγRIIIa recognition motif of the Fc region (Fig. 1).mAb–FA conjugates were prepared based on our previous report in which we established the modification procedure of a protein with ABNO-PEGn-FA reagents (Fig. 3).14 Here we used a mAb, namely ofatumumab (anti-CD20 IgG1), which can induce ADCC toward CD20-positive cancer cells. mAb and ABNO-PEGn-FA reagents reacted with a 10-fold molar excess of the reagents. The modification ratio of FA (N = FA/mAb) was found to be 1–2 in all the conjugates in spite of the excess molar ratio of ABNO-PEGn-FA to mAb in the reaction. This result suggests that the structures of the conjugates were relatively homogeneous, and some highly exposed Trp residues in the mAb were modified with ABNO-PEGn-FA. According to solvent accessible surface area of four Trp residues estimated from reported crystal structures including Fig. 1, Trp313 and Tpr417 of the Fc region are significantly exposed comparing with other Trp residues (Fig. S8 and Table S1†). Thus, these residues are possible modification sites of ABNO-PEGn-FA.
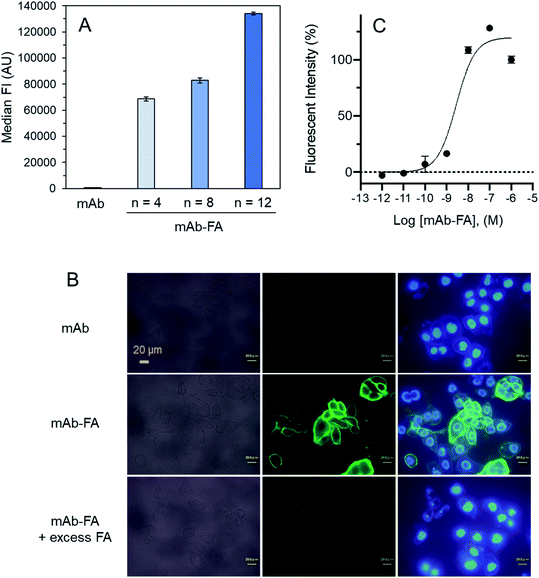
We examined effect of linker lengths of the mAb–FA conjugates on their binding to FR-α on cancer cells. We tested three types of mAb–FA conjugates with different linker lengths with IGROV-1 cells (FR-α+, CD20−). The conjugates bound to the cells were detected using a FITC-labeled anti-IgG-Fc antibody and analyzed by flow cytometry (Fig. 4A). The result shows that the fluorescence intensity increased with the length of the PEG linker, and the conjugate with a linker of n = 12 showed the highest binding amount. This may indicate presence of steric repulsion between the conjugate and FR-α which was weakened with increasing linker length. We previously reported crosslinking molecules between FR-α and IgG.20,22 In these studies, the PEG linker of n = 6 was enough to avoid the steric repulsion. One of the possible reasons in the difference of the optimum linker lengths between the present and previous works would be the difference in the modification positions in the Fc region. Hereafter, we used the n = 12 conjugate. Fig. 4B shows the fluorescence microscopy image of IGROV-1 cells treated with the conjugate. Fluorescence resulting from the bound conjugate was clearly observed on the cell surface, whereas the original anti-CD20 mAb did not. Excess FA diminished the binding of the conjugate. These results demonstrate that the mAb–FA specifically binds to the FR-α on the cell surface and the PEG linker length critically affects this binding. Fig. 4C shows the fluorescence intensity of cells, depending on the concentration of the conjugate. A dissociation constant (Kd) calculated from the curve was 4.0 nM, which agrees with the reported values for FA and FR-α (0.1–1 nM).16
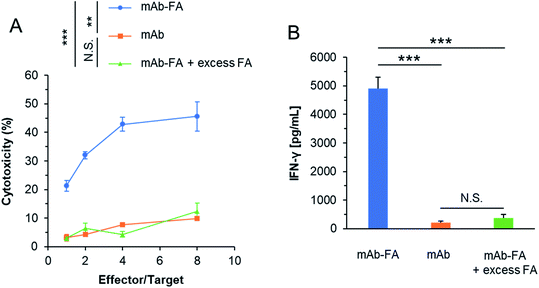
We evaluated whether the mAb–FA conjugate bound to IGROV-1 cells can activate NK cells and induce ADCC. The conjugates and NK cells (KHYG-1/CD16a-158V) were added to IGROV-1 cells and incubated for 16 hours. Afterward, the cytotoxicity was determined by LDH assay. As shown in Fig. 5A, the conjugate showed significant cytotoxicity with increasing effector/target ratio, while the mAb or mAb–FA with excess FA did not induce cytotoxicity at any effector/target ratios. We directly confirmed the activation of the NK cells upon mixing with IGROV-1 cells by secretion of interferon-γ (IFN-γ). As shown in Fig. 5B, significant amount of IFN-γ was secreted from NK cells in the presence of the mAb–FA conjugate. Such significant secretion of IFN-γ was not observed in the presence of the mAb or mAb–FA with excess FA. These results demonstrate that the mAb–FA conjugate which bind to cancer cells via FA/FR-α recognition induces ADCC by FcγR-mediated activation of NK cells as illustrated in Fig. 2B. Hence, the Trp-selective conjugation did not compromise the recognition of the Fc region of the conjugate by FcγR on NK cells. In the pioneering work of mAb–FA conjugate by Li et al., FA was conjugated on to lysine residues of mAb without a spacer.17 In this work, increment of cytotoxicity of the conjugate from an original mAb was not so significant comparing with the present study. This smaller increment in the previous report may be due to lack of the spacer and modification on the recognition residues of the Fc region by FcγR.
Conclusions
Here we applied a Trp-selective reaction for the conjugation of mAb to FA. The prepared anti-CD20–FA conjugate bound to FR-α on the target cells and induced ADCC via NK cells, showing that non-Fab binding to the target cells could exert intracellular signaling in NK cells to induce ADCC. The retention of the mAb's ADCC function would result from the fact that Trp residues are not included in the FcγR recognition residues in the Fc region. The Trp-selective conjugation is a promising method to add extra functions to mAbs with preserving the functions of the Fc region. Preparation of bispecific mAbs will be one of the attractive targets to apply the current method.Conflicts of interest
There are no conflicts to declare.References
- S. W. de Taeye, T. Rispens and G. Vidarsson, The Ligands for Human IgG and Their Effector Functions, Antibodies, 2019, 8, 30 CrossRef CAS PubMed.
- W. Wang, A. K. Erbe, J. A. Hank, Z. S. Morris and P. M. Sondel, NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy, Front. Immunol., 2015, 6, 368 Search PubMed.
- E. E. Idusogie, L. G. Presta, H. Gazzano-Santoro, K. Totpal, P. Y. Wong, M. Ultsch, Y. G. Meng and M. G. Mulkerrin, Mapping of the C1q Binding Site on Rituxan, a Chimeric Antibody with a Human IgG1 Fc, J. Immunol., 2000, 164, 4178–4184 CrossRef CAS PubMed.
- T. T. Kuo, K. Baker, M. Yoshida, S. W. Qiao, V. G. Aveson, W. I. Lencer and R. S. Blumberg, Neonatal Fc receptor: from immunity to therapeutics, J. Clin. Immunol., 2010, 30, 777–789 CrossRef CAS PubMed.
- S. C. Alley, N. M. Okeley and P. D. Senter, Antibody-drug conjugates: targeted drug delivery for cancer, Curr. Opin. Chem. Biol., 2010, 14, 529–537 CrossRef CAS PubMed.
- A. Beck, L. Goetxh, C. Dumontet and N. Corvaia, Strategies and challenges for the next generation of antibody-drug conjugates, Nat. Rev. Drug Discov., 2017, 16, 315–337 CrossRef CAS PubMed.
- Q.-Y. Hu, F. Brti and R. Adamo, Towards the next generation of biomedicines by site-selective conjugation, Chem. Soc. Rev., 2016, 45, 1691–1719 RSC.
- O. Koniev and A. Wagner, Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation, Chem. Soc. Rev., 2015, 44, 5495–5551 RSC.
- S. Panowski, S. Bhakta, H. Raab, P. Polakis and J. R. Junutula, Site-specific antibody drug conjugates for cancer therapy, mAbs, 2014, 6, 34–45 CrossRef PubMed.
- P. Sondermann, R. Huber, V. Oosthuizen and U. Jacob, The 3.2Å Crystal Structure of the Human Igg1 Fc Fragment-Fc-Gamma-Riii Complex, Nature, 2000, 406, 267–273 CrossRef CAS PubMed.
- K. Tsuchikama and Z. An, Antibody-drug conjugates: recent advances in conjugation and linker chemistries, Protein Cell, 2018, 9, 33–46 CrossRef CAS PubMed.
- D. Gilis, S. Massar, N. J. Cerf and M. Rooman, Optimality of the genetic code with respect to protein stability and amino-acid frequencies, Genome Biol., 2001, 2, 1–12 CrossRef PubMed.
- Y. Seki, T. Ishiyama, D. Sasaki, J. Abe, Y. Sohma, K. Oisaki and M. Kanai, Transition Metal-Free Tryptophan-Selective Bioconjugation of Proteins, J. Am. Chem. Soc., 2016, 138, 10798–10801 CrossRef CAS PubMed.
- K. Maruyama, K. J. Malawska, N. Konoue, K. Oisaki and M. Kanai, Synthesis of Tryptophan–Folate Conjugates, Synlett, 2020 DOI:10.1055/s-0039-1691735.
- I. G. Campbell, T. A. Jones, W. D. Foulkes and J. Trowsdale, Folate-binding Protein Is a Marker for Ovarian Cancer, Cancer Res., 1991, 51, 5329–5338 CAS.
- C. Chen, J. Ke, X. Edward Zhou, W. Yi, J. S. Brunzelle, J. Li, E. L. Yong, H. E. Xu and K. Melcher, Structural basis for molecular recognition of folic acid by folate receptors, Nature, 2013, 500, 486–489 CrossRef CAS PubMed.
- H. Li, Y. Lu, L. Piao, J. Wu, X. Yang, S. V. Kondadasula, W. E. Carson and R. J. Lee, Folate-immunoglobulin G as an anticancer therapeutic antibody, Bioconjugate Chem., 2010, 21, 961–968 CrossRef CAS PubMed.
- D. M. Kranz, T. A. Patrickt, K. E. Briglet, M. J. Spinella and E. J. Roy, Specifically Target Folate-Receptor-Positive Tumor Cells for Lysis, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 9057–9061 CrossRef CAS PubMed.
- S. Wang and P. S. Low, Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells, J. Controlled Release, 1998, 53, 39–48 CrossRef CAS PubMed.
- K. Sasaki, M. Harada, Y. Miyashita., H. Tagawa, A. Kishimura, T. Mori and Y. Katayama, Fc-binding antibody-recruiting molecules exploit endogenous antibodies for anti-tumor immune responses, Chem. Sci., 2020, 11, 3208–3214 RSC.
- T. Ji, J. Lang, B. Ning, F. Qi, H. Wang, Y. Zhang, R. Zhao, X. Yang, L. Zhang, W. Li, X. Shi, Z. Qin, Y. Zhao and G. Nie, Enhanced Natural Killer Cell Immunotherapy by Rationally Assembling Fc Fragments of Antibodies onto Tumor Membranes, Adv. Mater., 2019, 31, 1–8 Search PubMed.
- K. Sasaki, Y. Miyashita, D. Asai, D. Funamoto, K. Sato, Y. Yamaguchi, Y. Mishima, T. Iino, S. Takaishi, J. Nagano, A. Kishimura, T. Mori and Y. Katayama, A peptide inhibitor of antibody-dependent cell-mediated cytotoxicity against EGFR/folate receptor-α double positive cells, MedChemComm, 2018, 9, 783–788 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03291c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |