Open Access Article
Open Access ArticleRecent developments in electrochemical sensors for detecting hydrazine with different modified electrodes
Somayeh Tajika,
Hadi Beitollahi *b,
Sayed Zia Mohammadi
*b,
Sayed Zia Mohammadi c,
Mostafa Azimzadehd,
Kaiqiang Zhange,
Quyet Van Le
c,
Mostafa Azimzadehd,
Kaiqiang Zhange,
Quyet Van Le *f,
Yusuke Yamauchi
*f,
Yusuke Yamauchi ghi,
Ho Won Jang
ghi,
Ho Won Jang *e and
Mohammadreza Shokouhimehr
*e and
Mohammadreza Shokouhimehr *e
*e
aResearch Center for Tropical and Infectious Diseases, Kerman University of Medical Sciences, Kerman, Iran
bEnvironment Department, Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran. E-mail: h.beitollahi@yahoo.com
cDepartment of Chemistry, Payame Noor University, Tehran, Iran
dMedical Nanotechnology & Tissue Engineering Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, PO Box 89195-999, Yazd, Iran
eDepartment of Materials Science and Engineering, Research Institute of Advanced Materials, Seoul National University, Seoul 08826, Republic of Korea. E-mail: hwjang@snu.ac.kr; mrsh2@snu.ac.kr
fInstitute of Research and Development, Duy Tan University, Da Nang 550000, Vietnam. E-mail: levanquyet@dtu.edu.vn
gSchool of Chemical Engineering, Australian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Brisbane, Queensland 4072, Australia
hInternational Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), Tsukuba 3050044, Japan
iDepartment of Plant and Environmental New Resources, Kyung Hee University, 1732 Deogyeong-daero, Giheung-gu, Yongin-si, Gyeonggi-do 446-701, Republic of Korea
First published on 18th August 2020
Abstract
The detection of hydrazine (HZ) is an important application in analytical chemistry. There have been recent advancements in using electrochemical detection for HZ. Electrochemical detection for HZ offers many advantages, e.g., high sensitivity, selectivity, speed, low investment and running cost, and low laboriousness. In addition, these methods are robust, reproducible, user-friendly, and compatible with the concept of green analytical chemistry. This review is devoted to the critical comparison of electrochemical sensors and measuring protocols used for the voltammetric and amperometric detection of the most frequently used HZ in water resources with desirable recovery. Attention is focused on the working electrode and its possible modification which is crucial for further development.
1. Introduction
According to the studies conducted, hydrazine (N2H4) (HZ) is one of the simplest molecules, which consists of two amino groups. Indeed, this molecule was initially synthesized in 1875. It is a strongly poisonous colorless oily liquid with increased abrasiveness and reducibility.1–10 It is notable that as a powerful reducing agent and strongly reactive base, HZ has a widespread usage in fuel cells, insecticides, photography, emulsifiers, chemicals, herbicides, and dyes. Its fabrication, utilization, and disposal lead to severe contamination to the environment.11–13 Moreover, researchers and scientists commonly use HZ as a pharmaceutical derivative14,15 such as phenyl-aminothiourea and anti-psychotic drug 1-isoniacyl-2-isopropylhydrazine, isoniazid, anti-infective drug 5-nitrofuran methyl-hydrazine derivative,16 anti-tumor drug methyl phenyl-hydrazine,17 etc.18–21 Another research claimed that it is possible to use HZ for big boiler water due to the respective function for removing oxygen.22 Indeed, as HZ has high enthalpy of combustion, it is frequently applied as fuel for propulsion systems and electro-chemical fuel cells.23 In addition, HZ can be readily dissolved in water and adsorbed by dermal and oral tissues so that it causes multiple poisonous complications for the blood, liver, and kidney.5 Notably, the US Environmental Protection Agency defined HZ as one of the potent carcinogens, which would limit the threshold in the drinking water to below 10 ppb (0.3 μmol L−1).6–8 Hence, procuring effective pathways for determining the HZ concentration at low concentrations and wider range is highly necessary in industry and healthcare objectives.9,10 Here, general techniques provided for detecting HZ in the trace amounts are presented: chromatography,24 capillary electrophoresis,25 chemiluminescence,26 titrimetry,27 spectrophotometry,28 and surface enhanced Raman spectroscopy.29 The above methods are limited because they are laborious, need complicated equipment and instrumentation, and are expensive. Nonetheless, it is expected that electro-chemical methods have some hopes because of the advantages of the increased sensitivity, simplified operations, affordability, shorter time for analyses, and capabilities for simultaneous detection of multiple elements.30–33 Thus, the present review aimed at highlighting new advancements and understanding the modified electrodes exploited for HZ electro-chemical sensing.2. Electrochemical sensing modified electrodes
As demonstrated by the studies, the electro-analytical methods are incredibly considered for the analyte sensing because of the respective instrumental simplification, affordability, and portability.34–37 There is enough knowledge that traditional electrodes cause a number of severe concerns as a result of their slow surface kinetics that would seriously affect the electrodes selectivity and sensitivity. In general, the analytes on the traditional electrodes show a wide peak and usually any peak does not appear at lower concentrations. For reasons related to the broadness of the peak on the unmodified electrodes, resolving the analyte peaks would be a hard task because of reduction potential or very close oxidation. However, traditional electrodes suffer from limitations to address such a kind of analyte that would make them less interesting for availability in the markets. In fact, moderate electrode reaction of the analytes on the bare traditional electrode surface would need higher potentials for proceeding the reaction at high rates, which highly amount to their formal redox potentials. It should be mentioned that the kinetically hindered electrode reactions need an appropriate electro-catalyst that may make fast the electro-chemical reaction and decline the respective redox potential.38 Studies revealed that the chemically modified electrodes (CME) have a conductive substrate modified with mono layers, electro-active thin films or thick coatings. Actually, the modified electrodes have been built for a specific utilization that would not be feasible with a bare conductive electrode. In addition, modifying the conductive substrate can lead to the augmented electron transfer kinetics. Such modifications include irreversible absorption, covalent bonding, self-assembled layers, electro-polymerization, and so forth. It is notable that surface modifications have a catalytic role. Moreover, sensitivity of the measurements in the electro-analytical utilizations would be determined by very little alterations in the surface features.39–42 Usually, the modified surfaces lead to the cases below:(1) Transferring the physico-chemical features of the modifier to the electrode.
(2) Greater electro-catalytic activities because of application of substances with larger surface areas that would allow more acceptable sensitivity.
(3) The selectivity towards the analyte because of the immobilized functional groups and dopants.
(4) Rapid diffusion kinetics in a number of substances.
(5) Extracting and accumulating an analyte at the electrode.
According to some studies, function of the modified electrodes is basically dependent on the modifier features, which is utilized in the modification procedure.43–45 In addition, adjustment of the electro-chemical sensor, which use the conductive mediators like nanomaterials, ionic liquid (IL), and conductive polymers can cause assessing the biological specimens and medicines at the nanomolar level.46–48
3. CME possessing nanomaterials for detection of HZ
As working electrode is the most important part of an electrochemical sensor many modifications enabled selective and sensitive determination of HZ. Common working electrodes from diverse forms of carbon based electrodes such as carbon paste electrodes (CPE), glassy carbon electrodes (GCE), pencil graphite electrodes (PGE) and screen printed electrodes (SPE) to metallic solid electrodes like gold electrode, and so on are proven to be effective in detection of HZ. Over the last years, the development of electrochemical sensors based on nanomaterial-modified electrodes, has become one of the most active research areas.49–51 A diversity of nanomaterials with well-controlled physicochemical features, surface charge, shape and dimension are produced by significant advances in synthetic methodologies.52,53 There are many types of nanomaterials employed for the modification of electrodes for electrochemical sensing of HZ including: carbon based nanomaterials carbon nanotubes (CNTs), graphene (GR), metal and metal oxide nanoparticles (NPs) and their composites, and so on. Owing to the high reactive surface area, high conductivity, large surface-to-volume ratio, and electrocatalytic properties, nanomaterials based electrochemical sensors exhibit dramatically increased sensitivity in electrochemical analysis of HZ. This section has been focused on the electrochemical detection of HZ based on different sensing platforms (nanomaterials modified CPEs, nanomaterials modified GCEs, nanomaterials modified GPEs, nanomaterials modified SPEs, and nanomaterials modified gold electrodes).3.1. Modifying CPE with nanomaterials
It is widely accepted that CPE is an electrode procured by filling a binder and a conductive carbon powder mix into an electrode tube or coating them on the electrode surface via smoothly mixing. In addition, preparing different CMEs based on the conditions of the object, which should be experimented, is a simple operation. Moreover, it is easy to remove, update, and re-apply the surface of the electrode.54 Here, it is notable that amongst the working electrodes in the electro-chemical systems, CPE has a widespread utilization due to multiple benefits like simple modification, reproducible surface, lower background current, very high potential window, lower ohmic resistance, and affordability.55Asadi et al. proposed a newly developed high-performance and affordable sensor in the alkaline medium on the basis of the cobalt-based zeolitic imidazolate framework (ZIF-67). In fact, synthesis of the ZIF-67 nanocrystals with a rhombododecahedral shape and big surface area has been done at the room temperature and described through multiple methods. For the next stage, ZIF-67 modified CPE (ZIF-67/CPE) has been procured. In addition, Ag NPs have been generated on the porous support (ZIF-67/CPE) by reducing the Ag ions in the constant potential (Ag/ZIF-67/CPE). Actually, electro-chemical functions of the designed sensor have been examined by cyclic voltammetry (CV), amperometry (AMP), and electrochemical impedance spectroscopy (EIS). AMP outputs have been found that have acceptable electrocatalytic activities of the sensor to measure HZ with 2 linear dynamic range (LDR) between 4 and 326 μM and 326 and 4700 μM and limit of detection (LOD) (S/N = 3) equal to 1.45 μM. In addition, researchers proved its successful utilization to detect HZ in different water samples with reasonable recovery (Fig. 1).56
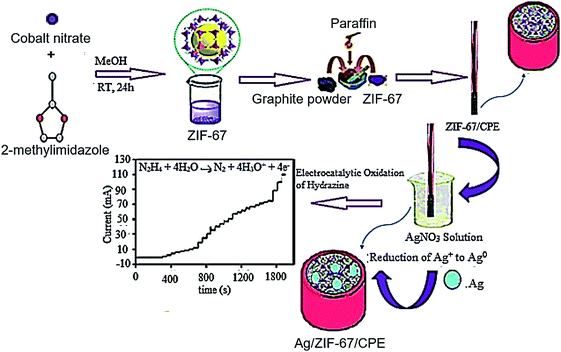 | ||
| Fig. 1 Diagram of Ag/ZIF-67/CPE production for electro-catalytic oxidation of HZ. Reused with permission from ref. 56. Copyright (2019) Springer. | ||
One of the studies addressed the synthesis of β-nickel hydroxide nano-platelets and employed them as a modifier on a CPE to detect HZ. In fact, synthesis would be on the basis of a 1-phase hydro-thermal technique by means of L-arginine, which operates as one of the agents for adjusting the pH-value and shaping nano-platelets. It should be noted that the modified electrode composition has been optimized by making changes in the numbers of the nano-platelets. Thus, most acceptable outputs have been obtained by a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 weight ratio for nano-platelets, graphite, and mineral oil (the binder). Moreover, CV has been used to study electro-chemical features of the modified CPE. It has been found that HZ diffusion coefficient is 1.56 × 10−5 cm2 s−1. Furthermore, an AMP procedure has been implemented with the merits below. (a) A working applied potential equal to 500 mV (versus Ag/AgCl reference electrode), (b) a LDR between 1 and 1300 μM, (c) 0.28 μM LOD, (d) a relative standard deviation (RSD) equal to 1.3% (for n = 3 at a level of 5 μM), and (e) a sensitivity of 1.33 μA μM−1 cm−2. At the end, the introduced sensor has been substantially utilized for quantifying HZ in the spiked tap water samples so that the recoveries have been 97 ± 2.3% (n = 3).57
20 weight ratio for nano-platelets, graphite, and mineral oil (the binder). Moreover, CV has been used to study electro-chemical features of the modified CPE. It has been found that HZ diffusion coefficient is 1.56 × 10−5 cm2 s−1. Furthermore, an AMP procedure has been implemented with the merits below. (a) A working applied potential equal to 500 mV (versus Ag/AgCl reference electrode), (b) a LDR between 1 and 1300 μM, (c) 0.28 μM LOD, (d) a relative standard deviation (RSD) equal to 1.3% (for n = 3 at a level of 5 μM), and (e) a sensitivity of 1.33 μA μM−1 cm−2. At the end, the introduced sensor has been substantially utilized for quantifying HZ in the spiked tap water samples so that the recoveries have been 97 ± 2.3% (n = 3).57
Benvidi et al. design a magnetic bar CPE (MBCPE) modified with Fe3O4 magnetic NPs (Fe3O4 NPs) and 2-(3,4-dihydroxyphenyl)benzothiazole (DPB) for electrochemical detection of HZ. Initially, the researchers self-assembled DPB on the Fe3O4 NPs. Afterwards, the resultant Fe3O4 NPs/DPB composite has been adsorbed on the developed MBCPE. Then, they applied MBCPE for attracting the magnetic NPs to the electrode surface. It should be mentioned that as the newly designed electrode has high conductivity and larger efficient surface area, it would have a very big current response for electro-catalytic oxidation of HZ. Actually, the voltammetric procedures have been applied for studying HZ electro-chemical behaviors on MBCPE/Fe3O4 NPs/DPB in the phosphate buffer solution (PBS, pH = 7.0). Finally, the presence of DPB reduced HZ oxidation potential and increased the catalytic current. Based on the results, dependence of the electro-catalytic current on the HZ concentration showed two linear ranges between 0.1 and 0.4 μM as well as 0.7 and 12.0 μM with a LOD equal to 18.0 nM. Moreover, concurrent detection of HZ and phenol has been examined by MBCPE/Fe3O4 NPs/DPB electrode. In addition, the voltammetric tests exhibited a linear range between 100 and 470 μM and LOD equal to 24.3 μM for phenol. Furthermore, the introduced electrode has been utilized for detecting phenol and HZ in the water samples (Fig. 2).58
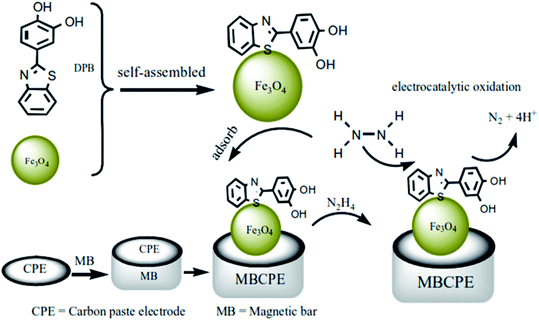 | ||
| Fig. 2 Diagram of the electro-chemical detection of the HZ based on the MBCPE/Fe3O4 NPs/DPB electrode. Reused with permission from ref. 58. Copyright (2016) Elsevier. | ||
Teymoori et al. investigated the direct electrochemical oxidation of HZ in the presence of bisphenol A (BPA) at a CuO NPs/IL/CPE. The combination of the good conductive 1-hexyl-3-methylimidazolium hexafluorophosphate and CuO NPs resulted in an electrode with attractive properties for the determination of HZ in the presence of BPA. The LDR were obtained in the ranges of 0.05–150 and 0.2–175 μM with the LOD 0.03 and 0.1 μM for HZ and BPA, respectively. High stability, sensitivity, selectivity and reproducibility, fast response, the ease of preparation, and surface renewal made the sensor well suitable for the determination of HZ in the presence of BPA, which are important pollutants in the environment. Finally, this new sensor was used for the determination of HZ and BPA in some water samples such as river water and wastewater.59
In their study, Mazloum Ardakani et al. investigated homogeneous electro-catalytic oxidation of HZ via indigocarmine (IND) as a mediator at the surface of the TiO2 NPs modified CPE. Moreover, CV has been utilized for studying the electro-chemical behaviors of IND at various scan rates. Results showed linear voltammetric response of the modified electrode versus HZ concentration in a range between 3.0 × 10−8 and 7.0 × 10−6 M with differential pulse voltammetry (DPV) technique. Based on the results, LOD has been equal to 27.3 nM. Thus, for evaluating the recommended technique utility to the real samples, the modified CPE has been utilized for detecting HZ in the water samples.60
Esfandiari Baghbamidi et al. dealt with the synthesis and utilization of a ferrocene-derivative compound, 2,7-bis(ferrocenyl ethynyl)fluoren-9-one (2,7-BFE) in order to fabricate the modified graphene oxide (GO) nanosheets paste electrode. They also examined HZ electro oxidation at the modified electrode surface by means of the electro-chemical procedures. Based on the optimal conditions, a linear increase has been found between the square wave voltammetric (SWV) peak current of HZ and HZ concentrations in a range between 2.2 × 10−7 and 3.0 × 10−4 M and LOD has been equal to 9.8 × 10−8 M for HZ. At the end, researchers utilized the modified electrode has for detecting HZ in a number of water samples.61
Beitollahi et al. modified a CPE with 2-(4-oxo-3-phenyl-3,4-dihydroquinazolinyl)-N′-phenyl-hydrazine-carbothioamide, IL (n-hexyl-3-methylimidazolium hexafluorophosphate), and magnetic core–shell Fe3O4@SiO2/MWCNT nanocomposite. They examined the HZ electro-oxidation at the modified electrode surface via electro-chemical procedures. Their modified electrode showed a remarkable improvement in the voltammetric sensitivity towards HZ in comparison with the bare electrode. In addition, SWV exhibited the LDR between 7.0 × 10−8 and 5.0 × 10−4 M and LOD of 40.0 nM for HZ. In addition, Beitollahi et al. specified diffusion coefficients and kinetic variables like the electron transfer coefficient and heterogeneous rate constant for oxidizing HZ. It has been shown that the procured modified electrode has an excellent resolution between the HZ and phenol voltammetric peaks, making it appropriate to determine HZ in the presence of phenol in the real samples.62
Another research conducted by Mahmoudi Moghaddam et al. introduced an electro-chemical sensor for selectively and sensitively detecting HZ in the presence of phenol via the bulk modification of CPE with TiO2 NPs and Mn(III) salen. It should be mentioned that the increased peak separation, reasonable sensitivity and stability enable the proposed technique to analyze HZ separately and concurrently alongside with phenol. In addition, the researchers determined SWV, LDR between 3 × 10−8 and 4.0 × 10−4 M with LOD = 10.0 nM for HZ. At the end, the suggested technique has been utilized for detecting HZ and phenol in a number of real samples (Fig. 3).63
Mazloum Ardakani et al. addressed the utilization of a newly designed CPE modified by N,N′-(2,3-dihydroxy-benzylidene)-1,4-phenylenediamine (DHBPD) and TiO2 NPs to detect HZ. They dealt with the mediated oxidation of HZ at the modified electrode. According to the researchers' findings, based on the optimal conditions of pH = 8.0 in CV, they observed strong decline of the over-potential for oxidizing HZ at the modified electrode. Moreover, DPV showed a LDR between 1.0 × 10−8 and 4.0 × 10−6 M and LOD (3σ) of 9.15 nM for HZ. Ultimately, researchers utilized the recommended technique for detecting HZ in the water samples with the standard addition procedure.64
Mazloum Ardakani et al. used a CPE modified by quinizarine (QZ) and TiO2 NPs. According to the research, the modified electrode has very good property of the electro-catalytic oxidization of HZ. In addition, a linear increase has been found between the DPV peak currents of HZ and the respective concentrations in a range between 0.5 μM and 1900.0 μM and LOD has been equal to 77 nM. Therefore, it could be concluded that their technique can be employed to detect HZ in the water samples with a standard addition procedure.65
For example, Amiripour et al. fabricated a simplified, effective and affordable electro-chemical sensor on the basis of the bi-metallic Au–Cu NPs supported on the P nano-zeolite modified CPE (Au–Cu/NPZ/CPE). They also examined the sensor effectiveness to detect HZ in the trace level. It should be mentioned that CV, chronoamperometry, and AMP procedures in 0.1 M PBS have been used to evaluate electro-chemical features of HZ at Au–Cu/NPZ/CPE surface. Outputs showed that the newly designed sensor enjoys the increased electro-catalytic activities at a partially lower potentials in comparison with the remaining modified electrodes like Au/NPZ/CPE, Au–Cu/CPE, Cu/NPZ/CPE, and so on. In addition, this sensor enjoys desirable analytical features to detect HZ like low LOD (0.04 μM), fast response time (3 s), wider linear range (0.01 to 150 mM), and higher sensitivity (99.53 μA mM−1), which have been associated with the synergic effects of Au–Cu bi-metallic, porous structure, and larger surface areas of NPZ. Moreover, the Au–Cu/NPZ/CPE sensor abilities have been substantially experimented in real samples with reasonable accuracy.66
In another study, Rostami et al. manufactured a new electro-chemical sensor through a CPE modified with CuO doped in the ZSM-5 NPs (CuO/ZSM-5 NPs/CPE) (Fig. 4). However, in order to sensitively detect HZ and hydroxylamine (HY), AMP and DPV procedures have been employed. In addition, the AMP measurements showed linear correlation of the current response and HY concentration within the range between 25 μM and 0.9 mM as well as 0.9 and 4.5 mM. Moreover, researchers found linear association in the range between 20 μM and 0.9 mM as well as 0.9 and 7.0 mM for HZ. Furthermore, low LOD of 3.2 μM and 3.6 μM (S/N = 3) respectively have been observed for HZ and HY. At the end, it has been found that this sensor would have reasonable stability, generalizability, anti-interference capabilities, and simplified operations.67
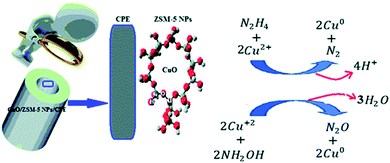 | ||
| Fig. 4 Simultaneous detection system of HA and HZ at CuO/ZSM-5 NPs/CPE. Reused with permission from ref. 67. Copyright (2017) Royal Chemical Society. | ||
3.2. Modifying GCE with nanomaterials
According to the studies, the glass-like carbon that is frequently referred to as the glassy carbon or vitreous carbon has been considered as a non-graphitizing or non-graphitizable, carbon that would combine the glassy and ceramic features with the graphite properties. In fact, higher temperature resistance, hardness (7 Mohs), lower density, lower electrical resistance, lower friction, lower thermal resistance, very high resistance to the chemical attacks, and impermeability to the gas and liquid are the most significant features. Actually, the glassy carbon has become a useful material with the extensive implementation because of the respective physicochemical properties.68–70Maleki et al. also prepared Ag@C core–shell nano-sphere by means of hydro-thermal procedure. In fact, they proposed a novel electro-chemical sensor to analyze the pollutant HZ on the basis of the immobilization of poly(alizarin yellow R) and Ag@C core–shell on the GCE (PAYR/Ag@C/GCE). Moreover, Maleki et al. applied CV and impedimetry for confirming the construction of the sensor. In addition, AMP and CV have been employed for examining the sensor electro-catalytic oxidation features. As demonstrated by their findings, PAYR/Ag@C exhibits specific electro-catalytic features to detect HZ. Furthermore, linear range (1 μM to 1320 μM), LOD = 250 nM, and sensitivity of 0.0211 μA μM−1 have been determined for the oxidation peak. It has been concluded that the new sensor would have several merits like higher sensitivity, HZ oxidation at lower potential, lower LOD, and utilization for the real samples.71
Ahmad et al. also synthesized the thin nickel oxide (NiO) nano-sheets by means of the wet chemistry procedure and modified their surface with the gold (Au) NPs using the reduction procedure for obtaining a novel hybrid (Au NPs–NiO nano-sheets) nanomaterial. Actually, the synthesized NiO nano-sheets and hybrid (Au NPs–NiO nano-sheets) nanomaterials have been additionally applied for modifying GCE in order to make the electro-chemical-based HZ sensors. Ahmad et al. used the constructed sensors for detection of HZ through CV. The produced sensing features of the hybrid nanomaterial-based sensor have been relatively more reasonable than the NiO nano-sheets based sensors alone. Additionally, the hybrid nanomaterial-based sensors have been described comprehensively, showing very good sensitivity equal to 31.75 μA nM−1 cm−2. It should be noted that low LOD of HZ sensor has been as low as nearly 0.05 nM that is remarkably more reasonable than the remaining metal oxide-based HZ sensors.72
Furthermore, Yang and Li decorated the GO nano sheets with AuNPs and multi-walled CNTs (MWCNTs) with the cetyltrimethyl ammonium bromide (CTAB) as a molecular linker. It is notable that the produced binder-free electrode has been shown as AuNP/MWCNT/CTAB/GO/GCE. Yang and Lee utilized CV, EIS, DPV, and AMP for investigating the electro-chemical behavior of AuNP/MWCNT/CTAB/GO/GCE. Results demonstrated very good catalytic ability of the modified electrode toward HZ oxidation and hydrogen peroxide (H2O2) reduction. Based on the findings, HZ oxidation current and H2O2 reduction current respectively have been linear with their concentrations in a range between 1.0 and 1000 μM as well as 10 and 5000 μM. Finally, LOD for H2O2 and HZ respectively have been 1.78 μM and 0.38 μM.73
Via a creative method, Zhang et al. fabricated the Au NPs-embedded N-doped porous carbon anchored on the reduced graphene oxide (Au NPs@NPC–RGO) nano-sheet using a confinement preparation procedure in the frame structure of ZIF-67. The above ternary hybrid materials exhibited very acceptable sensitivity to detect hazardous HZ in the liquid or gas phases. The researchers made an electro-chemical sensor on the basis of Au NPs@NPC–RGO modified GCE (Au NsPs@NPC–RGO/GCE) to detect HZ in the liquid phase, which would result in a linear detection ranging between 0.05 and 1.00 μM and LOD = 9.6 nM. Then, they assembled a functional electro-chemical gas sensor based on Au NPs@NPC–RGO to detect HZ gas under air environment. The above gas sensor showed LOD = 1.8 ppm with quick response and recovery. Finally, merits provided by the electro chemical sensors based on AuNPs@NPC–RGO is attributable to the main design component of the sensing materials with compositional and structural benefits causing Au NPs, NPC, and RGO synergy (Fig. 5).74
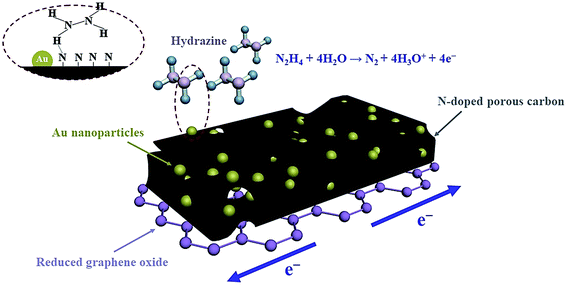 | ||
| Fig. 5 Diagram of the sensing system of Au NPs@NPC–RGO. Reused with permission from ref. 74. Copyright (2019) Elsevier. | ||
Ismail et al. designed a simplified synthesis of mesoporous Au/ZnO nanocomposite via the photo-chemical reduction strategy. It has been shown that Au NPs with the sizes in a range between 4 and 10 nm experienced homogenous distribution over the mesoporous ZnO surface. Therefore, researchers used diverse electro-chemical procedures like CV, EIS, and AMP (i–t response) and evaluated the synthesized mesoporous Au/ZnO nanocomposite as the effective electro-chemical sensor for detecting HZ. According to the findings, mesoporous Au/ZnO modified GCE would yield greater HZ oxidation peak at a comparatively less potential of nearly 0.45 V versus Ag/AgCl (Ipa = 2.6 μA, 150% greater than pure mesoporous ZnO). In addition, the relationship between the current versus HZ concentration has been linear (R2 = 0.9973) in a concentration range between 0.2 and 14.2 μM with a diffusion-controlled kinetics procedure. Analyses also indicated that LOD and sensitivity of the sensor respectively are 0.242 μM and 0.873 μA μM−1 cm−2. However, a main finding in the synthesized mesoporous Au/ZnO nanocomposite of Ismail et al. relates to the considerable significant to detect HZ in the presence of diverse interferences. Additionally, the mesoporous Au/ZnO sensor displayed lengthy stability, higher selectivity and sensitivity, generalizability, and quick kinetics detection in 10 s.75
Moreover, Rahman et al. applied the wet-chemical (coprecipitation) method for preparing the cadmium oxide (CdO) NPs decorated with the MWCNTs at low temperatures. Results showed a thin layer of CdO/CNT deposited on a GCE with the coating binder for obtaining a chemical sensor that has been consequently utilized for detecting m-tolyl HZ hydrochloride (m-THyd) in the buffer medium via an electro-chemical procedure for environmental safety. The current calibration curve versus m-THyd concentration has been linear (r2 = 0.9903) in a LDR between 0.01 nM and 0.1 mM. In addition, researchers computed sensitivity (25.7911 μA μM−1 cm−2) of the chemical sensor using GCE surface area (0.0316 cm2) and the calibration curve slope. Furthermore, LOD = 4.0 ± 0.2 pM has been determined using the signal to the noise ratio at 3. Such findings suggested that their CdO/CNT nanocomposite would be a hopeful electro-chemical sensor to detect dangerous toxins in order to largely cleanse the environment.76
In their study, Huang et al. dealt with anchoring CeO2-encapsulated Au NPs to the RGO (RGO/Au@CeO2) by an interfacial autoredox reaction in a solution consisting of tetra-chloroauric acid and Ce(III) on a solid support. Then, the produced material has been inserted on a GCE and has been utilized as one of the electro-chemical HZ sensors at the trace levels. According to the findings, electro-catalytic activities of the modified GCE toward the HZ oxidation has been remarkably augmented in comparison with just RGO/CeO2 or CeO2 encapsulated AuNPs or AuNPs loaded on the CeO2 modified with RGO. Such an increase could be attributable to RGO very good conductivity and larger surface area, and influential interactions between reversible Ce4+/Ce3+ and Au6+/Au0 redox mechanisms. It has been found that this sensor, which had been well acted at a peak voltage of 0.35 V (versus the saturated calomel electrode), exhibits a broad linear range (between 10 nM and 3 mM), low LOD (3.0 nM), and reasonable stability and selectivity. Finally, the sensor experienced a successful application to monitor HZ in the spiked environmental water specimens and to in vitro tracking of HZ in the cells based on its potent cytotoxicity (Fig. 6).77
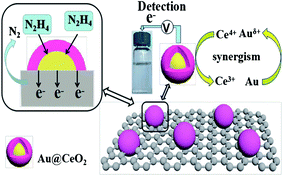 | ||
| Fig. 6 Diagram of the electro-chemical response of RGO/Au@CeO to oxidize HZ. Reused with permission from ref. 77. Copyright (2019) Springer. | ||
Nguyen et al. revealed successful fabrication of a newly designed nanostructure on the basis of the hierarchical urchin-like FeCo oxide supported carbon spheres (FeCo oxide/CSs) using a 2-phase hydro-thermal procedure accompanied by a convenient annealing phase at 300 °C under air. However, findings demonstrated that this urchin-like FeCo oxide/CSs structure exhibit better catalytic activities toward the HZ oxidation to Fe oxide/CSs, FeCo hydroxide/CSs, CSs, and Co oxide/CSs materials. Thus, results showed that FeCo oxide/CSs have a wide linear detection range between 0.1 and 516.6 μM, low LOD of 0.1 μM, and extended stability. Moreover, this material displayed reasonable selectivity toward detecting HZ in the presence of diverse interferences like ascorbic acid, uric acid, dopamine, urea, SO42−, Na+, Cl−, and K+. Of course, very good sensing function of FeCo oxide/CSs has been considered to the specific hierarchical urchin structure with the increased smoothness and density of the nanosized FeCo oxide nano-needles that would produce massive electro-active locations and greater charge transfer capability. Based on the outputs, FeCo oxide/CSs are probably a very good option for sensitively detect the HZ (Fig. 7).78
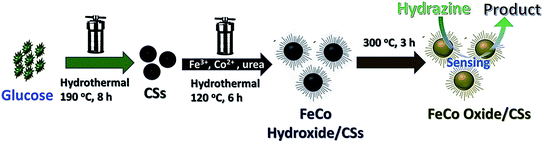 | ||
| Fig. 7 Schematic synthesis of the FeCo oxide/CSs hybrid. Reused with permission from ref. 78. Copyright (2019) Elsevier. | ||
Researchers prepared a leaf-like copper oxide (CuO) anchored onto worm-like ordered mesoporous carbon (OMC) composite to make an electro-chemical sensing platform for HZ. It is notable that, owing to the synergetic catalytic effects and certain structural characteristics, the produced nano-sized CuO integrated into OMC had reasonable electro-catalytic function to oxidize HZ. In addition, the content ratio of CuO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OMC has been optimized for improving the electro-catalytic functions. Finally, it has been revealed that CuO/OMC hybrids is able to be acted as one of the sensitive and efficient sensing platforms to detect HZ. They also have wider linear range, higher sensitivity, lower LOD, and reasonable stability.79
OMC has been optimized for improving the electro-catalytic functions. Finally, it has been revealed that CuO/OMC hybrids is able to be acted as one of the sensitive and efficient sensing platforms to detect HZ. They also have wider linear range, higher sensitivity, lower LOD, and reasonable stability.79
In another study, Duan et al. fabricated a nonenzymatic electro-chemical sensor-NiCo2S4/GCE for sensitively and selectively detecting HZ, which has been developed on the basis of the porous nanostructure and synthesized by a simple hydrothermal procedure. However, the electro-chemical measurements showed that porous NiCo2S4 sphere-based sensor has a very good voltammetric response toward oxidizing HZ with a broad linear range between 1.7 μM and 7.8 mM, sensitivity of 179.1 μA mM−1 cm−2, and low LOD equal to 0.6 μM (S/N = 3). In addition, HZ in the samples of the tap water samples has been detected by the standard addition technique. Moreover, reasonable outputs with RSD = 2.1–3.0% and recovery of 95.20–103.6% have been achieved for 5 parallel measurements. Hence, NiCo/GCE can be hopefully regarded as a newly designed option for HZ electro-chemical detection.80
In their study, Faisal et al. designed an electro-chemical procedure in order to rapidly and effectively detect HZ by means of the polythiophene (PTh)/ZnO nanocomposite modified GCE. Then, a modified sol–gel process through the F127 structure directing mediator accompanied by a chemical oxidative polymerization procedure has been used to synthesize PTh/ZnO nanocomposite. Of course, CV measurements by the PTh/ZnO modified GCEs demonstrated considerable sensing responses towards HZ in comparison with either pure ZnO or bare GCE with the diffusion-controlled electrode kinetics. In fact, efficient AMP (i–t) response has been obtained for the current modified electrode, which would yield a very fast response time of >5 seconds with a correlation coefficient (R2 = 0.9983) and a remarkable sensitivity of 1.22 μA μM−1 cm−2 in a wide linear range of HZ concentration between 0.5 and 48 μM. In addition, LOD computed from the electro-chemical measurements has been estimated as 0.207 μM (S/N = 3) that is completely less than multiple studies performed previously. The important thing is that PTh/ZnO modified electrodes have reasonable operational stability and generalizability that cause HZ sensitive detection in the presence of numerous conventional interfering molecules. Finally, outputs reported in this study show real advances toward more successful fabrication of the effective HZ chemical sensors (Fig. 8).81
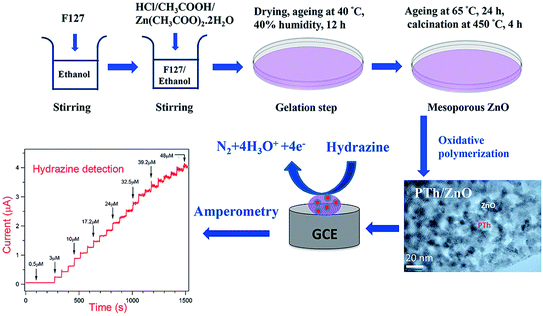 | ||
| Fig. 8 Diagram of the sol–gel synthesis of the PTh/ZnO nanocomposite with the HZ AMP detection on the modified GCE. Reused with permission from ref. 81. Copyright (2019) Elsevier. | ||
Ghasemi et al. reported the electrooxidation of HZ based on bimetallic Pt–Pd NPs formed on electrochemically reduced graphene oxide (ERGO)/GCE. Results showed that Pt–Pd/ERGO/GCE increase anodic peak current for HZ oxidation and it also has remarkable effect to facilitate kinetic of electron transfer for oxidation of HZ through decreasing the oxidation overpotential. The AMP nanosensor, best operated at a working potential of −0.71 V (vs. Ag|AgCl|KCl), has a linear response in the 0.007–5.5 mM HZ concentration range and a 1.7 μM detection limit. It has good selectivity over other species. Also, satisfactory values for detection of HZ in water sample were observed which confirm practical application of the sensor toward HZ detection.82
Based on the studies conducted so far, bimetallics catalyst has been remarkably considered because of the enhanced catalytic activities and more reasonable stability in comparison to the mono-metallica. For example, Zhang and Zheng dealt with the preparation of various Au@Ni bimetallic structures decorated GO via setting Ni and Au mass ratio. The researchers built their sensor based on the Au@Ni bi-metallic heterodimer (Au@Ni-BHD/RGO) or core–shell (Au@Ni-BCS/RGO) structure to oxidize HZ. Then, they compared electro-catalytic activities and confirmed the results using the chronoamperometry, CV, and AMP. Of course, Randles–Sevick equation has been utilized to calculate the electro-catalytic active surface area. In addition, Zhang and Zheng examined electro-catalytic functions of the Au@Ni-BHD/RGO modified electrode; the results showed a low oxidation potential of 0.07 V with 2 wide linear ranges between 0.2 μM and 1 mM as well as 1 mM and 9 mM for sensing HZ with low LOD = 0.06 μM. Correspondingly, the Au@Ni-BCS/RGO modified electrode exhibited 2 wide linear ranges between 3 μM and 1 mM as well as 1 mM and 20 mM, and low LOD = 0.9 μM for HZ. Finally, the two modified electrodes would provide higher selectivity, stability, and reasonable repeatability (Fig. 9).83
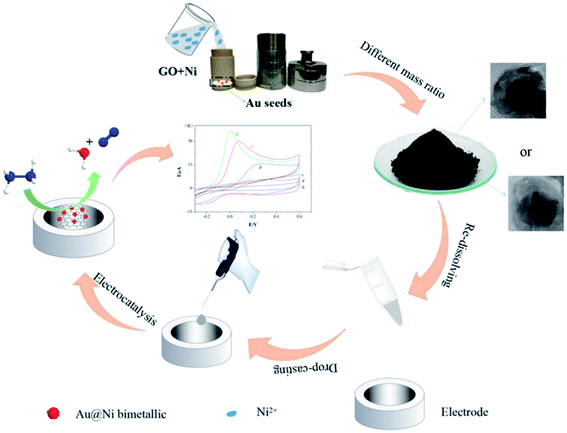 | ||
| Fig. 9 Diagram of the synthesis procedure of the modified GCE and Au@Ni bimetallic catalyst. Reused with permission from ref. 83. Copyright (2019) Elsevier. | ||
In their study, Shahid et al. procured the RGO–cobalt oxide nano-cube@gold (RGO–Co3O4@Au) nanocomposite via a one-pot hydro-thermal synthesis. According to them, RGO–Co3O4@Au nanocomposite displayed acceptable electro-catalytic activities toward HZ oxidation in the PBS (pH = 7.2). Of course, HZ has been detected by AMP procedure and the current response has been linear in a range between 10 and 620 μM. Moreover, LOD has been equal to 0.443 μM. Researchers used interferents like NO3−, Na+, SO42−, Cl−, Ag+, K+, 4-nitrophenol, ethanol, ascorbic acid and glucose to study the nanocomposite selectivity for HZ sensing. Then, they analyzed sensing diverse concentrations of HZ in the real water samples so that reasonable recoveries have been observed.84
Gao et al. synthesized the Sb2S3/perylene diimide (PDI) derivatives composite micro-spheres containing minor dendrites using solvothermal reaction. Based on their results, the composite materials displayed a very good sensitivity equal to 29.8 μA mM−1 cm2 with a low LOD = 50 pM. Moreover, more reasonable selectivity and stability have been shown in detecting HZ. According to the researchers, the proposed method efficiently regulates the composition and morphology of the Sb2S3 submicron materials and provides hopeful notions to fabricate metal sulfide–PDI amino acid derivative materials that are appropriate for catalysis, photovoltaic utilizations, and sensing (Fig. 10).85
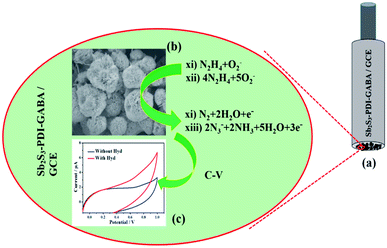 | ||
| Fig. 10 (a) The GCE coated with Sb2S3–PDI–GABA. (b) The introduced detection system of HZ, in which HZ has been oxidized to N3− by releasing the electrons over Sb2S3–PDI–GABA/GCE. (c) The observed C–V response by Sb2S3–PDI–GABA/GCE. Reused with permission from ref. 85. Copyright (2019) Elsevier. | ||
Wang et al. synthesized their newly designed electro-catalyst on the basis of the amine-functionalized Ti-based metal–organic framework (NH2-MIL-125(Ti)) embedded with Cu3P nanocrystals (signified by Cu3P@NH2-MIL-125(Ti)) and applied it for electro-catalytic detection and oxidation of HZ in the aqueous solution. Results revealed LOD of nM (S/N = 3) in a wider linear range between 5 μM and 7.5 mM for Cu3P@NH2-MIL-125(Ti)-based electro-chemical sensor. Additionally, their new sensor had higher selectivity towards HZ detection, when specific conventional interferents have been added to it, and reasonable utility in the real samples. Thus, it could be concluded that Cu3P@NH2-MIL-125(Ti) nanocomposite has hopeful options to detect HZ and provides potent utilizations in electro-analytical chemistry.86
In another investigation, Annalakshmi et al. introduced a facile, inexpensive, and fast electro-chemical sensor to detect HZ by means of trimetallic NiFeCo nano-spheres as one of the very reasonable electrocatalysts. Researchers dealt with the synthesis of the electrode modifier NiFeCo nano-spheres by one-pot simplified hydrothermal procedure. Certainly, the NiFeCo manufactured electrode showed a reasonable electro-chemical sensing function toward HZ, which is caused by its lower impedance behaviors, nano-spheres-like architecture, and synergic effects amongst the metallic NPs. Thus, Annalakshmi et al. concluded that their sensor enjoys valuable analytical function for HZ sensing with regard to LOD = 6.4 nM, wider dynamic ranges between 0.020 and 3080 μM, and fast response time (2 s). Additionally, this sensor is capable of selectively detecting HZ and can readily differentiate HZ from interfering samples. It should be noted that utility of this newly designed HZ sensor has been substantially assessed in the real water samples like lake, river, sewage water, and tap. Results showed reasonable recoveries with acceptable accuracy.87
Another study addressed the synthesis of the newly developed cobalt(II) octa-benzimidazolephthalocyanine (CoOBImPc) from cobalt(II) octa-carboxylicacid phthalocyanine (CoOCAPc) that can undergo electro-polymerization (Nemakal et al.). Moreover, different, spectroscopic and electro-chemical procedures have been used to characterize CoOBImPc, which displayed the improved electrical conductivity of 100 times in comparison with CoOCAPc. Therefore, for enhancing conductivity, the electron transfers kinetics and surface area of the Pc complex, CoOBImPc has been mixed with the RGO prior to electro-polymerization. Thus, impedance spectroscopy and super-capacitance examinations have been used to describe the electro-polymerized thin film on the surface of the electrode. Then, the modified electrodes have been utilized to detect the environmental poisonous pollutant HZ by CV in a concentration range between 100 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM with LOD equal to 40 and 33 nM, and sensitivity values of 56.8616 μA μM−1 and 40.8730 cm−2 for GCE/r-GO/poly(CoOBImPc) and GCE/poly(CoOBImPc) electrodes. According to the research design, AMP sensing of HZ has been done at the concentration range between 100 and 900 nM with LOD value equal to 40 and 33 nM and sensitivity values of 0.7432 μA nM−1 and 0.3696 cm−2 for GCE/r-GO/poly(CoOBImPc) and GCE/poly(CoOBImPc) electrodes. It has been found that the newly fabricated sensor has selective and sensitive response toward HZ even in the presence of diverse co-existing interfering samples so that the GCE/r-GO/poly(CoOBImPc) modified electrode could be substantially utilized to analyze water.88
000 nM with LOD equal to 40 and 33 nM, and sensitivity values of 56.8616 μA μM−1 and 40.8730 cm−2 for GCE/r-GO/poly(CoOBImPc) and GCE/poly(CoOBImPc) electrodes. According to the research design, AMP sensing of HZ has been done at the concentration range between 100 and 900 nM with LOD value equal to 40 and 33 nM and sensitivity values of 0.7432 μA nM−1 and 0.3696 cm−2 for GCE/r-GO/poly(CoOBImPc) and GCE/poly(CoOBImPc) electrodes. It has been found that the newly fabricated sensor has selective and sensitive response toward HZ even in the presence of diverse co-existing interfering samples so that the GCE/r-GO/poly(CoOBImPc) modified electrode could be substantially utilized to analyze water.88
In another study, Ramanathan et al. developed a low-cost modified electrode using bimetallic NPs (Co@SnO2) and honey reduced graphene oxide (HRGO) for detecting trace amounts of HZ. The HRGO and Co@SnO2 NPs were prepared using a natural reducing agent, honey. The electrocatalytic ability of the modified GCE to detect HZ was investigated. The modified electrode exhibited a wide LDR of (0–50 μL) to detect HZ and had a low LOD of 10 μL. This modified electrode had superior sensing performance compared to previously reported electrodes and also exhibited good repeatability, reproducibility, stability and ease of operation (Fig. 11).89
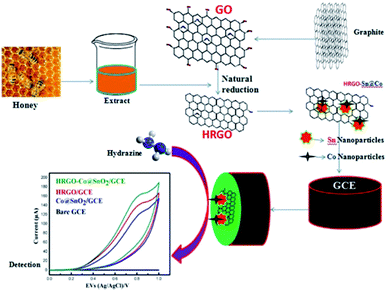 | ||
| Fig. 11 Schematic representation of the HRGO–Co@SnO2/GCE-modified surface and to detect HZ. Reused with permission from ref. 89. Copyright (2019) Springer. | ||
Sakthinathan et al. developed a RGO/platinum(II) tetraphenylporphyrin nanocomposite (RGO/Pt-TPP)-modified GCE for the selective detection of HZ. The RGO/Pt-TPP nanocomposite was successfully prepared via noncovalent π–π stacking interaction. The electrochemical detection of HZ was performed via CV and AMP. The RGO/Pt-TPP nanocomposite exhibited good electrocatalytic activity towards detection of HZ with low overpotential and high oxidation peak current. The fabricated sensor exhibited a wide LDR from 13 nM to 232 μM and a LOD of 5 nM. In addition, the fabricated sensor selectively detected HZ even in the presence of 500-fold excess of common interfering ions. The fabricated electrode exhibited good sensitivity, stability, repeatability and reproducibility. In addition, the practical applicability of the sensor was evaluated in various water samples with acceptable recoveries.90
In their study, Zheng et al. fabricated a CM–MWCNT–GCE modified electrode by electrodepositing curcumin (CM) at the surface of MWCNT modified GCE. The CM–MWCNT–GCE shows a well-defined two-electron and two-proton redox couple with the formal potential of 0.14 V (vs. saturated calomel electrode (SCE)) that results from the electrochemical oxidation product of CM, a CM derivative in quinone form. It also shows good electrocatalytic activity towards the oxidation of HZ at a reduced overpotential as well as an increased peak current compared with those at a CM modified GCE, a MWCNT modified GCE or an activated GCE. The calibration curve for HZ determination is linear in the range of 2–44 μM in pH 8.0 phosphate buffer by AMP. The LOD and the sensitivity are 1.4 μM and 22.9 nA μM−1, respectively. The modified electrode is simple in preparation, and is of character of fast response, high sensitivity and good reproducibility for HZ determination.91
Fang et al. synthesized ZnO nanoflowers by a simple process (ammonia-evaporation-induced synthetic method) and were applied to the HZ electrochemical sensor. The prepared material was immobilized onto the surface of a GCE via MWCNTs to obtain ZnO/MWCNTs/GCE. The potential utility of the constructed electrodes was demonstrated by applying them to the analytical determination of HZ concentration. An optimized LOD of 0.18 μM was obtained and with a fast response time (within 3 s). Additionally, the ZnO/MWCNTs/GCE exhibited a wide LDR from 0.6 to 250 μM and higher sensitivity for HZ than did the ZnO modified electrode without immobilization of MWCNTs.92
In another research conducted by Gowthaman et al. synthesized a novel organic–inorganic nile-blue–CeO2 (CeO2/NB) nanohybrid by environmentally benign ultrasonic irradiation method for the selective determination of the HZ in environmental water samples. For the fabrication of environmental pollutant electrochemical sensor, the prepared CeO2/NB nanohybrid was drop-casted on the electrode surface and utilized for the determination of HZ. The nanohybrid modified electrode exhibits higher electrocatalytic activity by showing enhanced oxidation current and less positive potential shift towards HZ oxidation than the bare and individual CeO2 and NB modified electrodes. The fabricated sensor with excellent reproducibility, repeatability, long-term storage stability and cyclic stability exhibited the sensational sensitivity (484.86 μA mM−1 cm−2) and specificity in the presence of 50-fold possible interfering agents with the lowest LOD of 57 nM against HZ. Utilization of the present sensor in environmental samples with excellent recovery proves it practicability in the determination of HZ in real-time application (Fig. 12).93
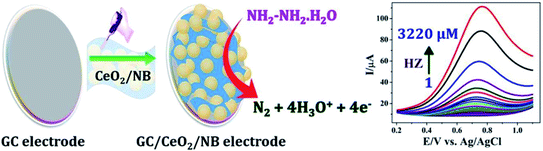 | ||
| Fig. 12 Illustration on the mechanism of electrochemical oxidation of HZ at CeO2/NB modified electrode. Reused with permission from ref. 93. Copyright (2020) Elsevier. | ||
Zhang et al. prepared a porous hybrid material from polydopamine-modified MWCNTs and RGO (P–MWCNTs/RGO). It was employed as a supporting material for an electrochemical HZ sensor. Gold NPs with a size of about 13 nm were placed on the material (Au@porous P-MWCNT/RGO). It was placed on a GCE, and CV, chronoamperometry and AMP curves were used to characterize the catalytic activity of the sensor. The kinetic parameters of the modified GCE were calculated which proved that it has a high catalytic efficiency in promoting the electron transfer kinetics of HZ. The AMP signal (obtained at a typical working potential of 0.35 V vs. SCE) has two LDR, one from 1 μM to 3 mM and one from 3 to 55 mM, with sensitivities of 524 and 98 A mM−1 cm−2, respectively. The LOD is 0.31 μM (Fig. 13).94
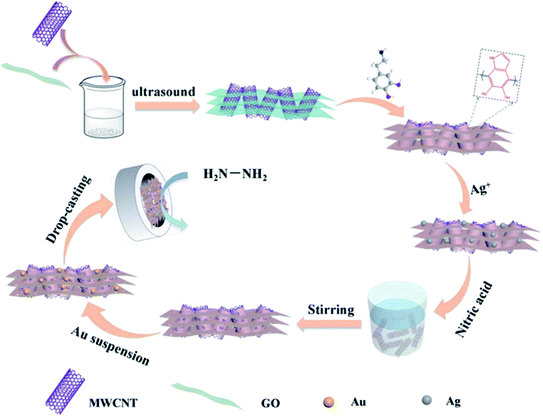 | ||
| Fig. 13 Schematic for the preparation of Au@porous polydopamine-modified MWCNT/RGO/GCE. Reused with permission from ref. 94. Copyright (2020) Elsevier. | ||
3.3. Modifying PGE with nanomaterials
According to the studies, amongst different carbon-based electrodes, researchers specially considered the PGEs because of their sp2 hybridized carbon that reveals acceptable absorption, conductivity, smaller background current, higher sensitivity, easy procurement, and surface modification features.95,96 In comparison with the remaining electrodes like the GCE, renewing the surface would contribute importantly to the consequent analyses due to the electro-chemical reactions of the molecule and can result in changes in the electrode surface characteristics.97 At the end, it is notable that consequent renewal of PGE surface for all trials can result in the sensitive and selective electro-chemical examinations of HZ.Teoman et al. procured a gold (Au) NP-modified PGE via an electro-deposition process for fast and sensitive flow injection (FI) AMP detection of HZ. Based on the CVs, Au NP-modified pre-treated PGE exhibited very good electro-catalytic activities towards oxidizing HZ because the strongly irreversible and widely seen oxidation peak at +600 mV at the pre-treated PGE switched to −167 mV at the Au NP pre-treated PGE. Moreover, they observed considerable augment in the oxidation peak current. Therefore, researchers fabricated FI AMP HZ sensor on the basis of the respective electro-catalytic oxidation at the Au NP-modified pre-treated graphite pencil electrode. In addition, Au NP-modified pre-treated PGE exhibited a linear calibration curve FI AMP current and HZ concentration in a concentration range between 0.01 and 100 μM with LOD of 0.002 μM. Finally, it is possible to apply FI AMP sensor to detect HZ in the water samples with reasonable accuracy.98
Heydari et al. addressed electrochemical deposition of the copper (Cu) nanostructures (CuNS) on a film of the MWCNTs modified PGE (MWCNTs/PGE) using CV for fabricating a CuNS–MWCNTs composite sensor (CuNS–MWCNT/PGE) to detect HZ. Of course, CuNS–MWCNTs composite has been described using CV and EIS. Based on the initial examinations, the introduced sensor has a synergistic electro-catalytic activity to oxidize HZ in the phosphate buffer. It has been found that there is a linear association between catalytic currents of SWV and HZ concentration in a range between 0.1 and 800 μM with LOD = 70 nM. In addition, the researchers observed a linear association between the AMP oxidation current and HZ concentration in a concentration range between 50 and 800 μM with LOD = 4.3 μM. It is notable that it is possible to employ this electrode to detect HZ in the real samples. Outputs showed hopeful future for using it. In addition, the empirical outputs revealed reasonable generalizability and lengthy response of the sensor to HZ, which were independent of interferences (Fig. 14).99
 | ||
| Fig. 14 Diagram of the electrode modification. Reused with permission from ref. 99. Copyright (2016) Springer. | ||
The study conducted by Aziz and Kawde dealt with the preparation of a newly designed gold NP-modified PGE (AuNP-PGE) only via immersion of a bare PGE in the AuNP solution and heating it for fifteen minutes. The AuNP-PGEs had very good electro-catalytic activity in terms of HZ oxidation and acceptable generalizability. Of course, SWV's outputs as a function of HZ concentration indicated that quantification limit of the AuNP-PGE detector is 100 nM HZ that is much lower than the amount achieved by AMP (10 μM). In addition, LOD for HZ sensing at AuNP-PGEs by means of the SWV and AMP have been equal to 42 nM and 3.07 μM. Ultimately, the modified electrode has been utilized for detecting HZ concentration in the drinking water, and reasonable outputs have been observed.100
3.4. Modifying SPE with nanomaterials
As demonstrated by the research, SPEs have had widespread utilizations in the electro-analysis because of easy utilization in several analytical chemistry operations. Moreover, the SPEs are disposable tools. Such a benefit would avoid laborious polishing (or electro-chemical treatments) step for reusing the working electrode, which would be necessary for a majority of the solid electrodes for overcoming the passivation and or pollution on their surface. Additionally, the SPEs possibly cause electro-chemical determination, which causes using just a little content of the sample in micro-liter range. It should be mentioned that SPEs would be made on the basis of the printing technology and micro-electronic tools that show, indicating wide variety of the SPEs with suitable duplicability for electro-chemical detection and affordability based on the materials.101,102Mejri et al. made a sensor based on a screen-printed carbon electrode (SPCE) loaded with the curcumin (CM)-stabilized silver NP-coated RGO magnetic spinel (FeCo2O4) nano-sheets. It should be noted that the electrode electro-catalytic activities would enable the sensitive concurrent quantification of p-nitrophenol and HZ. In general, their working potential is at −0.75 V for p-nitrophenol and at +0.15 V for HZ (both versus the pseudo Ag/AgCl). In addition, LOD is equal to 23 nM and 18 nM (at S/N = 3). Furthermore, reasonable selectivity, generality, duplicability, and storage stability have been provided. Finally, this sensor has been applied for analyzing the spiked samples of the industrial waste water and river water (Fig. 15).103
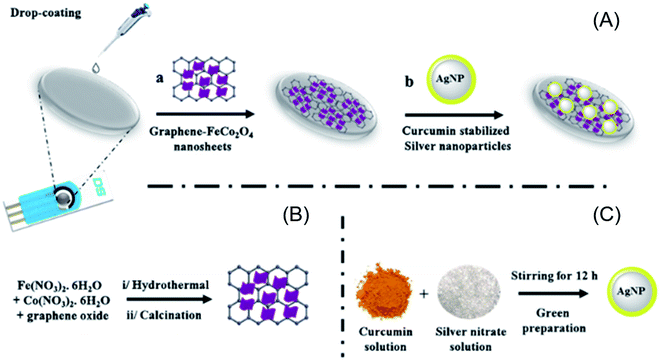 | ||
| Fig. 15 Chart of the stepwise process for fabrication of the curcumin silver NPs decorated RGO magnetic spinel FeCo2O4 nano-sheets platform (A) and procurement processes of the graphene–magnetic spinel nano-sheets (RGO–FeCo2O4) (B), and curcumin–silver NPs (C). Reused with permission from ref. 103. Copyright (2019) Springer. | ||
Ramaraj et al. synthesized poly(diallyl-dimethylammonium chloride) (PDDA)@copper(II)hexacyanoferrate nanocubes using a simple wet chemical method. CV has been employed to study the electro-catalytic behaviors of PDDA@copper(II)hexa-cyanoferrate nanocubes modified SPCE toward the electro-chemical oxidation of HZ. According to the CV findings, the greater electro-catalytic activities and the decreased oxidation potentials toward HZ in comparison to the bare SPCE has been exhibited by PDDA@copper(II)hexacyanoferrate nano-cubes modified SPCE. Then, based on the optimum conditions, AMP (i–t) technique has been applied to detect HZ. Under this condition, PDDA@copper(II)hexacyanoferrate nanocubes modified SPCE are capable of detecting HZ in the linear concentration range between 0.03 and 533.6 μM with a LOD = 10 nM. Results showed that PDDA@copper(II)hexacyanoferrate nanocubes modified SPCE has strong selectivity in the presence of the potentially active interfering compounds like higher concentration of ascorbic acid. Additionally, the introduced HZ sensor has reasonable utility with very good lengthy stability toward HZ detection.104
According to the research design, the gold NPs (AuNPs) modified cerium oxide (CeO2 or ceria) NPs (AuNP–CeO2) have been procured with the immediate deposition of AuNPs over the CeO2 surface by Sun et al. Results showed that the procured NPs enjoy very good electro-catalytic activities toward the HZ oxidation. In fact, AuNP–CeO2 has highly greater catalytic activities compared to CeO2. The calibration curve displayed linear response from 0.01 to 10 mM for detection of HZ.105
For the first time, Karrupiah et al. introduced a strongly sensitive AMP HZ sensor by means of the Au-NPs decorated actuated graphite (AG) modified SPCE. It should be mentioned that preparing AG and decorating Au-NPs on the surface of AG include simplified electro-chemical procedures. Based on the results, AG/Au-NPs composite modified electrode exhibited the enhanced catalytic responses and the decreased over-potential for HZ in comparison with the Au-NPs decorated on the actuated SPCE and or graphite SPCE. Notably, the sensor response to detect HZ ranges 2 s, which suggests very good electro-catalytic capability of the composite electrode. In fact, the least LOD of 0.57 ± 0.03 nM can be obtained by means of the AG/Au-NPs electrode. However, according to the optimal conditions, the manufactured AG/Au-NPs composite would exhibit a broader linear response to detect HZ up to 936 μM. Moreover, this sensor exhibited reasonable sensitivity and very good operational stability. Finally, it has been confirmed that the designed sensor has better selectivity even in the presence of 1000 and 200 folds of the traditional metal ions and biologically co-active interfering samples.106
3.5. Modifying gold electrodes with nanomaterials
Gold is the most widely used metallic solid electrode for electrochemical and electroanalytical purposes. The preference for this metal is attributed to its high purity, its ease of machining, and the “inertness” in the presence of almost all reagents. Gold electrodes have gained increasing preference due to two important applications: for stripping analysis and for studies involving surface modifications by self-assembling.107In their study, Rani et al. prepared a porous Zn-MOF@RGO (zinc-metal–organic framework and reduced graphene oxide) via solvothermal technique and utilized for the AMP determination of HZ. Zn-MOF@RGO solution was used to modify gold electrode for the superior electrocatalytic oxidation of HZ in real water sample. Also, the incorporation of Zn-MOF into RGO leads to the significant improvement in the performance which can be ascribed to the large surface area, excellent adsorption affinity, chemical stability and high electrical conductivity. The HZ sensor using Zn-MOF@RGO as electrocatalyst displayed a low LOD (8.7 × 10−3 μM), high sensitivity (5.4 × 10−2 μA μM−1 cm−2) and a fast response time (<2 s). Moreover, the sensor possessed excellent anti-interference property and reproducibility (Fig. 16).108
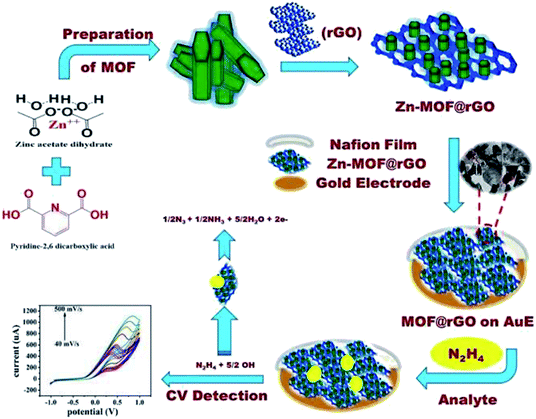 | ||
| Fig. 16 Schematic illustration of probable reaction mechanism of HZ over ZnMOF@RGO/Au electrode surface. Reused with permission from ref. 108. Copyright (2019) Elsevier. | ||
Emran et al. made electrodes by electrodepositing Pd on lanthanide (Nd, Gd, Nd/Gd)-doped titania nanotubes (TNTs; prepared using a hydrothermal method) on Au substrates were applied to the detection of HZ in alkaline media. The combination of conductive TNTs and lanthanides produced materials exhibiting remarkable electrocatalytic activity during the oxidation of HZ in a PBS at a pH of 10. A Nd–Gd-TNTs/Pd-modified Au electrode demonstrated highly reproducible behavior, a linear response from 10−5 to 10−2 M HZ, a good sensitivity value of 288.3 μA mM−1 and a high level of stability during electrochemical experiments, with a LOD of 0.15 μM. The proposed voltammetry method was also used to analyze HZ in irrigation water samples from Al Madinah Al Munawwarah City in conjunction with the standard addition technique (Fig. 17).109
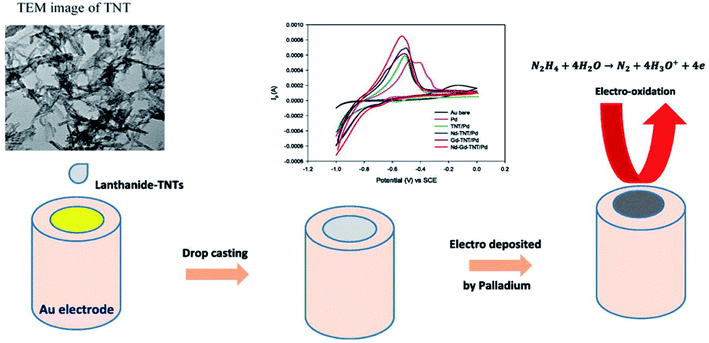 | ||
| Fig. 17 The electrochemical sensing procedure at lanthanide–TNTs/Pd. Reused with permission from ref. 109. Copyright (2020) Elsevier. | ||
Pei et al. developed a highly sensitive electrochemical sensing platform for HZ based on a highly surface-roughened nanoporous gold electrode decorated with Pt NPs (Pt/HNPG). The catalytic activity of Pt/HNPG towards electrooxidation of HZ was examined. It is found that both Pt NPs loadings and solution pH have strong influence on the electrocatalytic activity. At the potential of −0.1 V (vs. SCE), the fabricated composite electrode showed excellent sensing performance for HZ with a wide LDR of 5 μM to 6.105 mM, a LOD 1.03 μM and a high sensitivity of 3449.68 μA mM−1 cm−2. Moreover, the sensor exhibited good selectivity, repeatability and stability. It was also successfully applied for the highly sensitive determination of HZ in real water samples with satisfactory results.110
4. Analytical performances of electrochemical sensors for determination of HZ
The analytical performances of electrochemical methods depend on many factors. A variety of electrochemical sensors used for detection of HZ are presented in this review. Comparison of electrochemical method, used modifier LOD, and LDR are presented in Tables 1–5 for comparison. As shown in tables, LODs at the level of pM, nM and μM were achieved. Although, a comparison between various electrochemical sensors has been provided in this review (Tables 1–5) however this will not be true or justifiable to prove any sensor better than others on the basis of LOD, wide LDR, and modifier used for analysis. There are few more factors that should be considered for a true comparison apart from those shown in the tables. These are ease of modification procedure, response time for sensing of HZ, total cost of modification, simplicity of sample pretreatment before analysis.| Electrochemical method | Modifier | LDR | LOD | Ref. |
|---|---|---|---|---|
| AMP | ZIF-67 nanocrystals | 4–4700 μM | 1.45 μM | 56 |
| AMP | β-Nickel hydroxide nano-platelets | 1–1300 μM | 0.28 μM | 57 |
| DPV | Fe3O4 NPs/DPB | 0.1–12 μM | 18 nM | 58 |
| DPV | CuO NPs/IL | 0.05–150 μM | 0.03 μM | 59 |
| DPV | TiO2 NPs | 0.03–7 μM | 27.3 nM | 60 |
| SWV | (2,7-BFE)/GO | 0.22–300 μM | 0.098 μM | 61 |
| SWV | Fe3O4@SiO2/MWCNT/IL | 0.07–500 μM | 40.0 nM | 62 |
| SWV | TiO2 NPs/Mn(III) salen | 0.03–400 μM | 10.0 nM | 63 |
| DPV | DHBPD/TiO2 NPs | 0.01–400 μM | 9.15 nM | 64 |
| DPV | QZ/TiO2 NPs | 0.5–1900 μM | 77 nM | 65 |
| AMP | Au–Cu NPs/NPZ | 0.01–150 mM | 0.04 μM | 66 |
| AMP/DPV | CuO/ZSM-5 NPs | 20–7000 μM | 3.2 μM | 67 |
| Electrochemical method | Modifier | LDR | LOD | Ref. |
|---|---|---|---|---|
| AMP | PAYR/Ag@C | 1–1320 μM | 250 nM | 71 |
| CV | Au NPs–NiO nano-sheets | 0.0001–0.110 μM | 0.05 nM | 72 |
| AMP/DPV | AuNP/MWCNT/CTAB/GO | 1.0–1000 μM | 0.38 μM | 73 |
| AMP | AuNPs@NPC–RGO | 0.05–1.00 μM | 9.6 nM | 74 |
| AMP | Au/ZnO nanocomposite | 0.2–14.2 μM | 0.242 μM | 75 |
| AMP | CdO/CNT nanocomposite | 0.01 nM to 0.1 mM | 4.0 pM | 76 |
| AMP | RGO/Au@CeO2 | 10 nM to 3 mM | 3.0 nM | 77 |
| AMP | FeCo oxide/CSs | 0.1–516.6 μM | 0.1 μM | 78 |
| AMP | CuO/OMC hybrids | 1–2.11 × 104 μM | 0.887 μM | 79 |
| AMP | Nano NiCo2S4 | 1.7–7800 μM | 0.6 μM | 80 |
| AMP | PTh/ZnO nano composite | 0.5–48 μM | 0.207 μM | 81 |
| AMP | Pt–Pd/ERGO | 0.007–5.5 mM | 1.7 μM | 82 |
| AMP | Au@Ni-BHD/RGO | 0.2–9000 μM | 0.06 μM | 83 |
| AMP | RGO–Co3O4@Au | 10–620 μM | 0.443 μM | 84 |
| AMP | Sb2S3/PDI–GABA | 10–1300 μM | 0.05 nM | 85 |
| AMP | Cu3P@NH2-MIL-125(Ti) | 5–7500 μM | 0.079 μM | 86 |
| AMP | NiFeCo nano-spheres | 0.020–3080 μM | 6.4 nM | 87 |
| AMP | r-GO/poly(CoOBImPc) | 0.1–0.9 μM | 0.033 μM | 88 |
| CV | HRGO–Co@SnO2 | 0–50 μL | 10 μL | 89 |
| AMP | RGO/Pt-TPP | 0.013–232 μM | 5 nM | 90 |
| AMP | CM–MWCNT | 2–44 μM | 1.4 μM | 91 |
| AMP | ZnO/MWCNTs | 0.6–250 μM | 0.18 μM | 92 |
| DPV | CeO2/NB | 1–3220 μM | 57 nM | 93 |
| AMP | Au@porous P–MWCNT/RGO | 1–55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM 000 μM |
0.31 μM | 94 |
5. Conclusions
In this review article, we summarized the most significant achievements made in the field of electro-chemical sensor for quantitative detection of HZ. The nanomaterials like GR, CNT, metal and metal oxide NPs and their composites have been considered encouraging substances to construct the electrochemical sensors via amplifying signals and lowering over-potential oxidizing HZ. In general, these designed electrochemical sensors provide a sensitive and selective method for the quantification of HZ thus help in reducing environmental pollution and improving human health. Despite of great achievements in this field, there is a long way to go as these electrochemical sensors should be used more frequently for real sample analysis and would perform in situ measurements. Then these should move towards commercialization so these could be commercially available for field use.This review will be helpful to open new ideas in the field of fabrication of novel surface modified electrodes for more precise, sensitive, and selective detection of HZ by electrochemical techniques, in development of suitable electrodes for in situ studies, in fabrication of a portable device for real life applications, and in fabrication an ideal sensor for application in industrial scale.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Future Material Discovery Program (no. 2016M3D1A1027666), and Basic Science Research Program (no. 017R1A2B3009135) through the National Research Foundation of Korea, and the Neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran (Project No. 98000289).References
- W. B. Rui and N. X. Y. Qin, J. Hazard. Mater., 2011, 197, 320 CrossRef PubMed.
- D. Y. Zhou, Y. Y. Wang and J. Jia, Chem. Commun., 2015, 51, 10656 RSC.
- Z. X. Li, W. Y. Zhang and C. X. A. Liu, Sens. Actuators, B, 2017, 241, 665 CrossRef CAS.
- J. L. Fan, W. Sun and M. M. Hu, Chem. Commun., 2012, 48, 8117 RSC.
- W. D. Wang, Y. Hu and Q. Li, Inorg. Chim. Acta, 2018, 477, 206 CrossRef CAS.
- H. Beitollahi, M. A. Khalilzadeh, S. Tajik, M. Safaei, K. Zhang, H. W. Jang and M. Shokouhimehr, ACS Omega, 2020, 5, 2049 CrossRef CAS PubMed.
- M. H. Lee, B. Yoon and J. S. Kim, Chem. Sci., 2013, 4, 4121 RSC.
- S. Goswami, K. Aich and S. Das, RSC Adv., 2014, 4, 14210 RSC.
- C. Liu, K. Y. Liu and M. G. Tian, Spectrochim. Acta, Part A, 2019, 212, 42 CrossRef CAS PubMed.
- Z. Chen, X. X. Zhong and W. B. Qu, Tetrahedron Lett., 2017, 58, 2596 CrossRef CAS.
- A. Sutton, A. Burrell, D. Dixon, E. Garner, J. Gordon, T. Nakagawa, K. Ott, J. Robinson and M. Vasiliu, Science, 2011, 331, 1426 CrossRef CAS PubMed.
- A. Serov, M. Padilla, A. J. Roy, P. Atanassov, T. Sakamoto, K. Asazawa and H. Tanaka, Angew. Chem., Int. Ed., 2014, 53, 10336 CrossRef CAS PubMed.
- S. Koçak and B. Aslısen, Sens. Actuators, B, 2014, 196, 610 CrossRef.
- S. Kumari, C. B. Mishra, D. Idrees, A. Prakash, R. Yadav, M. I. Hassan and M. Tiwari, Mol. Diversity, 2017, 21, 163 CrossRef CAS PubMed.
- G. Le Goff and J. Ouazzani, Bioorg. Med. Chem., 2014, 22, 6529 CrossRef CAS PubMed.
- M. S. Tolba, M. Ahmed, A. M. K. El-Dean, R. Hassanien and M. Farouk, J. Heterocycl. Chem., 2018, 55, 408 CrossRef CAS.
- V. Sekar, S. K. Anandasadagopan and S. Ganapasam, Biofactors, 2016, 42, 623 CrossRef CAS PubMed.
- S. Tajik, Z. Dourandish, K. Zhang, H. Beitollahi, Q. V. Le, H. W. Jang and M. Shokouhimehr, RSC Adv., 2020, 10, 15406 RSC.
- D. Kovacs, J. Woelfling, N. Szabo, M. Szecsi, R. Minorics, I. Zupko and E. Frank, Eur. J. Med. Chem., 2015, 98, 13 CrossRef CAS PubMed.
- U. K. Das, Y. Ben-David and Y. Diskin-Posner, Angew. Chem., Int. Ed., 2018, 57, 2179 CrossRef CAS PubMed.
- B. Cheng, Y. Lin, M. Kuang, S. Fang, Q. Gu, J. Xu and L. Wang, Chem. Biol. Drug Des., 2015, 86, 1121 CrossRef CAS PubMed.
- C. D. C. Conceicao, R. C. Faria, O. Fatibello and A. A. Tanaka, Anal. Lett., 2008, 41, 1010 CrossRef CAS.
- S. C. Jean, K. Asazawa, T. K. Sakamoto, K. J. Yamada, H. Tanaka and P. Strasser, J. Am. Chem. Soc., 2011, 133, 5425 CrossRef PubMed.
- L. Cui, K. Jiang, D. Q. Liu and K. L. Facchine, J. Chromatogr. A, 2016, 1462, 73 CrossRef CAS PubMed.
- T. You, L. Niu, J. Y. Gui, S. Dong and E. Wang, J. Pharm. Biomed. Anal., 1999, 19, 231 CrossRef CAS PubMed.
- L. Cui, C. Ji, Z. Peng, L. Zhong, C. Zhou, L. Yan, S. Qu, S. Zhang, C. Huang, X. Qian and Y. Xu, Anal. Chem., 2014, 86, 4611 CrossRef CAS PubMed.
- H. E. Malone, Anal. Chem., 1961, 33, 575 CrossRef CAS.
- C. Liu, K. Liu, M. Tian and W. Lin, Spectrochim. Acta, Part A, 2019, 212, 42 CrossRef CAS PubMed.
- X. Gu and J. P. Camden, Anal. Chem., 2015, 87, 6460 CrossRef CAS PubMed.
- S. Tajik, H. Beitollahi, Z. Dourandish, K. Zhang, Q. V. Le, T. P. Nguyen, S. Y. Kim and M. Shokouhimehr, Sensors, 2020, 20, 3675 CrossRef PubMed.
- S. Ayaz and Y. Dilgin, Electrochim. Acta, 2017, 258, 1086 CrossRef CAS.
- M. M. Rahman, M. M. Alam and K. A. Alamry, J. Ind. Eng. Chem., 2019, 77, 309 CrossRef CAS.
- R. Ahmad, T. Bedük, S. M. Majhi and K. N. Salama, Sens. Actuators, B, 2019, 286, 139 CrossRef CAS.
- S. Tajik, H. Beitollahi, F. Garkani Nejad, K. Zhang, Q. V. Le, H. W. Jang, S. Y. Kim and M. Shokouhimehr, Sensors, 2020, 20, 3364 CrossRef PubMed.
- W. Dang, Y. Sun, H. Jiao, L. Xu and M. Lin, J. Electroanal. Chem., 2020, 856, 113592 CrossRef CAS.
- H. Beitollahi, S. Tajik, Z. Dourandish, K. Zhang, Q. V. Le, H. W. Jang, S. Y. Kim and M. Shokouhimehr, Sensors, 2020, 20, 3256 CrossRef PubMed.
- O. Sheydaei, H. Khajehsharifi and H. R. Rajabi, Sens. Actuators, B, 2020, 309, 127559 CrossRef CAS.
- B. S. Sherigara, W. Kutner and F. D'Souza, Electroanalysis, 2003, 15, 753 CrossRef CAS.
- N. Baig, M. Sajid and T. A. Saleh, TrAC, Trends Anal. Chem., 2019, 111, 47 CrossRef CAS.
- J. Liang, C. Wang, P. Zhao, Y. Wang, L. Ma, G. Zhu, Y. Hu, Z. Lu, Z. Xu, Y. Ma, T. Chen, Z. Tie, J. Liu and Z. Jin, ACS Appl. Mater. Interfaces, 2018, 10, 6084 CrossRef CAS PubMed.
- Z. Ye, C. Qin, G. Ma, X. Peng, T. Li, D. Li and Z. Jin, ACS Appl. Mater. Interfaces, 2018, 10, 39809 CrossRef CAS PubMed.
- W. Zhang, Y. Hu, L. Ma, G. Zhu, Y. Wang, X. Xue, R. Chen, S. Yang and Z. Jin, Adv. Sci., 2018, 5, 1700275 CrossRef PubMed.
- S. Tajik, H. Beitollahi, F. Garkani Nejad, M. Safaei, K. Zhang, Q. V. Le, R. S. Varma, H. W. Jang and M. Shokouhimehr, RSC Adv., 2020, 10, 21561 RSC.
- N. S. Anuar, W. J. Basirun, M. Shalauddin and S. Akhter, RSC Adv., 2020, 10, 17336 RSC.
- S. Tajik, M. A. Taher and H. Beitollahi, Electroanalysis, 2014, 26, 796 CrossRef CAS.
- H. S. Jang, D. Kim, C. Lee, B. Yan, X. Qin and Y. Piao, Inorg. Chem. Commun., 2019, 105, 174 CrossRef CAS.
- S. Mohammadi, M. A. Taher and H. Beitollahi, Microchim. Acta, 2020, 187, 1 CrossRef PubMed.
- L. Oularbi, M. Turmine, F. E. Salih and M. El Rhazi, J. Environ. Chem. Eng., 2020, 8, 103774 CrossRef CAS.
- F. Amiripour, S. N. Azizi and S. Ghasemi, Biosens. Bioelectron., 2018, 107, 111 CrossRef CAS PubMed.
- M. R. Aflatoonian, S. Tajik, B. Mohtat, B. Aflatoonian, I. Sheikh Shoaie, H. Beitollahi, K. Zhang, H. W. Jang and M. Shokouhimehr, RSC Adv., 2020, 10, 13021 RSC.
- T. Zhong, Q. Guo, Z. Yin, X. Zhu, R. Liu, A. Liu and S. Huang, RSC Adv., 2019, 9, 2152 RSC.
- M. Govindasamy, S. Manavalan, S. M. Chen, U. Rajaji, T. W. Chen, F. M. Al-Hemaid, M. Ajmal Ali and M. S. Elshikh, J. Electrochem. Soc., 2018, 165, B370 CrossRef CAS.
- D. Mohapatra, N. S. K. Gowthaman, M. S. Sayed and J. J. Shim, Sens. Actuators, B, 2020, 304, 127325 CrossRef.
- M. M. Charithra and J. G. Manjunatha, Mater. Sci. Energy Technol., 2019, 2, 365 Search PubMed.
- H. Beitollahi, H. Karimi-Maleh and H. Khabazzadeh, Anal. Chem., 2008, 80, 9848 CrossRef CAS PubMed.
- F. Asadi, S. N. Azizi and S. Ghasemi, J. Mater. Sci.: Mater. Electron., 2019, 30, 5410 CrossRef CAS.
- A. Avanes, M. Hasanzadeh-Karamjavan and G. Shokri-Jarcheloo, Microchim. Acta, 2019, 186, 441 CrossRef PubMed.
- A. Benvidi, S. Jahanbani, B. F. Mirjalili and R. Zare, Chin. J. Catal., 2016, 37, 549 CrossRef CAS.
- N. Teymoori, J. B. Raoof, M. A. Khalilzadeh and R. Ojani, J. Iran. Chem. Soc., 2018, 15, 2271 CrossRef CAS.
- M. Mazloum-Ardakani, H. Rajabi and H. Beitollahi, Chin. Chem. Lett., 2012, 23, 213 CrossRef CAS.
- S. Esfandiari-Baghbamidi, H. Beitollahi and S. Tajik, Anal. Bioanal. Electrochem., 2014, 6, 634 Search PubMed.
- H. Beitollahi, S. Tajik and S. Jahani, Electroanalysis, 2016, 28, 1093 CrossRef CAS.
- H. Mahmoudi-Moghaddam, H. Beitollahi, S. Tajik, I. Sheikhshoaie and P. Biparva, Environ. Monit. Assess., 2015, 187, 407 CrossRef PubMed.
- M. Mazloum-Ardakani, H. Rajabi, B. B. F. Mirjalili, H. Beitollahi and A. Akbari, J. Solid State Electrochem., 2010, 14, 2285 CrossRef CAS.
- M. Mazloum-Ardakani, Z. Taleat, H. Beitollahi and H. Naeimi, Nanoscale, 2011, 3, 1683 RSC.
- F. Amiripour, S. N. Azizi and S. Ghasemi, Biosens. Bioelectron., 2018, 107, 111 CrossRef CAS PubMed.
- S. Rostami, S. N. Azizi and S. Ghasemi, New J. Chem., 2017, 41, 13712 RSC.
- K. Movlaee, H. Beitollahi, M. R. Ganjali and P. Norouzi, Microchim. Acta, 2017, 184, 3281 CrossRef CAS.
- B. S. Jilani, P. Malathesh, C. D. Mruthyunjayachari and K. V. Reddy, Mater. Chem. Phys., 2020, 239, 121920 CrossRef CAS.
- T. S. K. Naik and B. K. Swamy, J. Electroanal. Chem., 2018, 826, 23 CrossRef.
- A. Maleki, R. Rezaee, H. Daraei, B. Shahmoradi and N. Amini, J. Alloys Compd., 2018, 763, 997 CrossRef CAS.
- R. Ahmad, T. Bedük, S. M. Majhi and K. N. Salama, Sens. Actuators, B, 2019, 286, 139 CrossRef CAS.
- Y. J. Yang and W. Li, Fullerenes, Nanotubes, Carbon Nanostruct., 2018, 26, 837 CrossRef CAS.
- Y. Zhang, Y. Zhang, D. Zhang, S. Li, C. Jiang and Y. Su, Sens. Actuators, B, 2019, 285, 607 CrossRef CAS.
- A. A. Ismail, F. A. Harraz, M. Faisal, A. M. El-Toni, A. Al-Hajry and M. S. Al-Assiri, Mater. Des., 2016, 109, 530 CrossRef CAS.
- M. M. Rahman, M. M. Alam and K. A. Alamry, J. Ind. Eng. Chem., 2019, 77, 309 CrossRef CAS.
- H. Huang, T. Li, Y. Sun, L. Yu, C. Wang, R. Shen, L. Ye, D. Wang and Y. Li, Microchim. Acta, 2019, 186, 46 CrossRef PubMed.
- D. M. Nguyen, L. G. Bach and Q. B. Bui, J. Pharm. Biomed. Anal., 2019, 172, 243 CrossRef CAS PubMed.
- L. Wang, T. Meng, H. Jia, Y. T. Gong, H. Wang and Y. Zhang, J. Colloid Interface Sci., 2019, 549, 98 CrossRef CAS PubMed.
- C. Duan, Y. Dong, Q. Sheng and J. Zheng, Talanta, 2019, 198, 23 CrossRef CAS PubMed.
- M. Faisal, F. A. Harraz, A. E. Al-Salami, S. A. Al-Sayari, A. Al-Hajry and M. S. Al-Assiri, Mater. Chem. Phys., 2018, 214, 126 CrossRef CAS.
- S. Ghasemi, S. R. Hosseini, F. Hasanpoor and S. Nabipour, Microchim. Acta, 2019, 186, 601 CrossRef PubMed.
- X. Zhang and J. Zheng, Appl. Surf. Sci., 2019, 493, 1159 CrossRef CAS.
- M. M. Shahid, P. Rameshkumar, W. J. Basirunc, U. Wijayantha, W. S. Chiu, P. S. Khiew and N. M. Huang, Electrochim. Acta, 2018, 259, 606 CrossRef CAS.
- Y. Gao, S. Zhang, W. Hou, H. Guo, Q. Li, D. Dong, S. Wu, S. Zhao and H. Zhang, Appl. Surf. Sci., 2019, 491, 267 CrossRef CAS.
- M. Wang, L. Yang, B. Hu, Y. Liu, Y. Song, L. He, Z. Zhang and S. Fang, Appl. Surf. Sci., 2018, 445, 123 CrossRef CAS.
- M. Annalakshmi, P. Balasubramanian, S. M. Chen and T. W. Chen, Sens. Actuators, B, 2019, 296, 126620 CrossRef CAS.
- M. Nemakal, S. Aralekallu, I. Mohammed, S. Swamy and L. K. Sannegowda, J. Electroanal. Chem., 2019, 839, 238 CrossRef CAS.
- S. Ramanathan, E. Elanthamilan, A. Obadiah, A. Durairaj, P. SanthoshKumar, J. Princy Merlin, S. Ramasundaram and S. Vasanthkumar, J. Electron. Mater., 2019, 48, 542 CrossRef CAS.
- S. Sakthinathan, S. Kubendhiran, S.-M. Chen, M. Govindasamy, F. M. A. Al-Hemaid, M. Ajmal Ali, P. Tamizhdurai and S. Sivasanker, Appl. Organomet. Chem., 2017, 31, e3703 CrossRef.
- L. Zheng and J.-f. Song, Sens. Actuators, B, 2009, 135, 650 CrossRef CAS.
- B. Fang, C. Zhang, W. Zhang and G. Wang, Electrochim. Acta, 2009, 55, 178 CrossRef CAS.
- N. S. K. Gowthaman, H. N. Lim and S. Shankar, Ultrason. Sonochem., 2020, 61, 104828 CrossRef CAS PubMed.
- X. Zhang and J. Zheng, Microchim. Acta, 2020, 187, 89 CrossRef CAS PubMed.
- Y. Yardım, Rev. Anal. Chem., 2011, 30, 37 Search PubMed.
- R. L. McCreery, Chem. Rev., 2008, 108, 2646 CrossRef CAS PubMed.
- A. Ozcan and Y. Sahin, Biosens. Bioelectron., 2010, 25, 2497 CrossRef.
- I. Teoman, S. Karakaya and Y. Dilgin, Anal. Lett., 2019, 52, 2041 CrossRef CAS.
- H. Heydari, M. B. Gholivand and A. Abdolmaleki, Mater. Sci. Eng., C, 2016, 66, 16 CrossRef CAS PubMed.
- M. A. Aziz and A. N. Kawde, Talanta, 2013, 115, 214 CrossRef PubMed.
- H. M. Mohamed, TrAC, Trends Anal. Chem., 2016, 82, 1 CrossRef CAS.
- M. Trojanowicz, TrAC, Trends Anal. Chem., 2016, 84, 22 CrossRef CAS.
- A. Mejri, A. Mars, H. Elfil and A. H. Hamzaoui, Microchim. Acta, 2019, 186, 561 CrossRef PubMed.
- S. Ramaraj, R. Sakthivel, S. M. Chen, S. Palanisamy, V. Velusamy, T. W. Chen, S. K. Ramaraj and K. Pandian, Int. J. Electrochem. Sci., 2017, 12, 5567 CrossRef CAS.
- H. Sun, S. Zhao and F. Qu, Measurement, 2012, 45, 1111 CrossRef.
- C. Karuppiah, S. Palanisamy, S. M. Chen, S. K. Ramaraj and P. Periakaruppan, Electrochim. Acta, 2014, 139, 157 CrossRef CAS.
- D. T. Sawyer and J. L. Roberts, Electrochemistry for Chemists, John Willey & Sons, New York, 1974 Search PubMed.
- S. Rani, S. Kapoor, B. Sharma, S. Kumar, R. Malhotra and N. Dilbaghi, J. Alloys Compd., 2020, 816, 152509 CrossRef CAS.
- K. M. Emran, S. M. Ali and H. E. Alanazi, J. Electroanal. Chem., 2020, 856, 113661 CrossRef CAS.
- Y. Pei, M. Hu, Y. Xia, W. Huang, Z. Li and S. Chen, Sens. Actuators, B, 2020, 304, 127416 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |