Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Individual and synergistic protective properties of Salvia officinalis decoction extract and sulfasalazine against ethanol-induced gastric and small bowel injuries
Saber Jedidiabc,
Foued Alouib,
Kais Rtibi a,
Houcem Sammarib,
Houcine Selmib,
Ahmed Rejebd,
Lamjed Toumib and
Hichem Sebai
a,
Houcem Sammarib,
Houcine Selmib,
Ahmed Rejebd,
Lamjed Toumib and
Hichem Sebai *a
*a
aUnité de Physiologie Fonctionnelle et Valorisation des Bio-Ressources, Université de Jendouba, Institut Superieur de Biotechnologie de Beja, Avenue Habib Bourguiba, B.P. 382, 9000 Beja, Tunisia. E-mail: sebaihichem@yahoo.fr; Fax: +216 78 459 098; Tel: +216 97 249 486
bLaboratoire des Ressources Sylvo-Pastorales, Université de Jendouba, Institut Sylvo-Pastoral de Tabarka, B.P. 345, 8110 Tabarka, Tunisia
cUniversite de Carthage, Faculté des Sciences de Bizerte, 7021 Jarzouna, Tunisia
dLaboratoire d'Anatomie Pathologique, Université de Manouba, Ecole Nationale de Médecine Vétérinaire de Sidi Thabet, 2020 Sidi Thabet, Tunisia
First published on 30th September 2020
Abstract
The present study was carried out to determine the phytochemical composition of Salvia officinalis flowers decoction extract (SOFDE) as well as its individual and/or synergistic actions with sulfasalazine against ethanol (EtOH)-induced peptic ulcer in Wistar rats. In this respect, rats were divided into six groups of eight animals each: control, EtOH, EtOH + sulfasalazine (SULF, 100 mg kg−1, b.w., p.o.), mixture: MIX (SOFDE, 50 mg kg−1 b.w., p.o. + SULF, 50 mg kg−1, b.w., p.o.) and EtOH + two doses of SOFDE (100 and 200 mg kg−1 b.w., p.o.). In vitro, the phytochemical and the antioxidant properties were determined using colorimetric analysis. HPLC-PDA/ESI-MS assay was used to identify the distinctive qualitative profile of phenolic compounds. Our results firstly indicated that SOFDE is rich in total tannins, flavonols, anthocyanins and a moderate concentration of total carotenoids. Chromatographic techniques allowed the identification of 13 phenolic compounds and the major ones are quinic acid, protocatechuic acid, gallic acid and salviolinic acid. SOFDE also exhibited an important in vitro antioxidant activity using the β-carotene bleaching method. In vivo, SOFDE and the mixture provide significant protection against ethanol-induced gastric and duodenal macroscopic and histological alterations. Also, SOFDE alone or in combination with SULF, showed a significant protection against the secretory profile disturbances, lipid peroxidation, antioxidant enzyme activities and non-enzymatic antioxidant level depletion induced by alcohol administration. Importantly, we showed that EtOH acute intoxication increased gastric and intestinal calcium, free iron, magnesium and hydrogen peroxide (H2O2) levels, while SOFDE/MIX treatment protected against all these intracellular mediators' deregulation. We also showed that alcohol treatment significantly increased the C-reactive protein (CRP) and alkaline phosphatase (ALP) activities in plasma. The SOFDE and MIX treatment significantly protected against alcohol-induced inflammation. More importantly, we showed in the present work that the mixture exerted a more important effect than SOFDE and SULF each alone indicating a possible synergism between these two molecules. In conclusion, our data suggests that SOFDE and SULF exerted a potential synergistic protective effect against all the macroscopic, histological and biochemical disturbances induced by EtOH intoxication. This protection might be related in part to its antioxidant and anti-inflammatory properties as well as by negatively regulating Fenton reaction components such as H2O2 and free iron.
Introduction
Peptic ulcer is caused by the loss of balance between aggressive and defensive factors of the gastric and duodenal mucosa.1 Also, it can be caused by Helicobacter pylori,2 the use of non steroidal anti-inflammatory drugs,3 smoking,4 the imbalance between the secretion of hydrochloric acid as well as the production of calcium bicarbonate to buffer the pH in the gastrointestinal microbiota.5Alcohol consumption is a well-known risk factor for tissue injury and gastroduodenal ulcer. It also affects other organs such as the heart, kidneys, brain, liver and pancreas.6,7
Moreover, previous studies have shown an inhibitory effect on the synthesis of prostaglandins leading to lesions of the gastric mucosa. This disease may also be related to neutrophil activation leading to an excessive production of reactive oxygen species (ROS).8 Under physiological conditions, ROS are produced in small quantities during cellular respiration and metabolism, which are important for several physiological processes.9,10 However, the intracellular imbalance between their genesis and degradation contributes to oxidative stress. This situation has been accompanied by significant damage of lipids, proteins and nucleic acids leading to cell death.11,12
The mixture of bioactive compounds from plant and synthetic drugs is an alternative for the protection and/or treatment of various pathologies and to substitute commercial drugs known for their unpredictable side effects.13 However, in the gastrointestinal system, prolonged use of drugs (anticholinergic drugs, histamine H2-receptor antagonists, antacids) and especially with relatively high doses can exhibit toxic side effects leading to severe constipation, diarrhea, hypersensitivity reactions such as rashes, fever, central nervous system aberrations14 and colorectal cancer.15 In women, misoprostol can cause malformations of the embryo.16 Importantly, we recently used a mixture between sage and loperamide as a strategy to fight against castor oil-induced diarrhea.17
Salvia officinalis (Lamiaceae family) is known as a medicinal and aromatic plant due to its richness of natural active substances.18 Due to its antioxidant19 and anti-inflammatory20 properties, sage extracts exhibit many beneficial health effects such as phytoestrogenic,21 neurprotective,22 anti-microbial23 and anticancer24 activities. More importantly, this plant has been widely used in the treatment of most gastrointestinal diseases like diarrhea and dyspepsia.17,25
Salvia officinalis is an inexhaustible reservoir of chemical compounds such as alkaloids, carbohydrate, fatty acids, glycosidic derivatives (flavonoid glycosides, saponins), phenolic compounds (coumarins, flavonoids, tannins), polyacetylenes, steroids, terpenes (monoterpenes, diterpenes, triterpenoids).26,27 However, Mansourabadi et al.28 reported that flavonoids extracted from Salvia officinalis presented anti-inflammatory properties in carrageenan model of mouse and induced analgesic effect in a dose-dependent manner. In addition, several others molecules such as manool, carnosol and ursolic acid have been previously showed for their anti-inflammatory properties.29,30
Hence, the present study aimed to investigate the individual and synergistic protective properties of Salvia officinalis flowers decoction extract and sulfasalazine against ethanol-induced peptic ulcer in rat.
Experimental
Reagents and chemicals
2-Thio-barbituric acid (TBA), epinephrine, bovine catalase, trichloroacetic acid (TCA), butylated hydroxyl toluene (BHT) were from Sigma chemicals Co. (Sigma-Aldrich GmbH, Steinheim, Germany). Ethanol (EtOH), sulfasalazine, sodium chloride (NaCl 0.9%) were purchased from central pharmacy of Tunisia. All the other chemicals used were of analytical grade.Plant material and decoction extract
The sage flowers were cultivated in the region of Ain Draham (NW-Tunisia) during April 2018 and identified by Dr Imen Bel Hadj Ali, Associate professor in the Higher Institute of Biotechnology of Béja-Tunisia. The voucher specimens (No. SO.321) have been deposited with the Herbarium of the Higher Institute of Biotechnology of Béja. The plant material was dried in the open air and powdered in an electric blender. The decoction was made with distilled water (1/5; w/v) at 100 °C during five minutes under magnetic agitation. The homogenate was filtered by Whatman filter papers and was evaporated at 40 °C in a ventilated oven.Phytochemical properties and antioxidant capacity
The quantification of flavonols was determined as previously described by Rigane et al.34 Briefly, 1 mL AlCl3 (20%) was added to 1 ml of the extract and 3 mL of sodium acetate (50 mg mL−1). After incubation for 2 hours and 30 minutes, the absorbance was read at 440 nm. Results were expressed as mg of rutin equivalents per 100 gram of dry matter (mg RE/100 g DM).
Total carotenoids in SOFDE was evaluated by the procedure described by Marina et al.35 Firstly, 1 mL of the extract was mixed with 1 mL of distilled water and 2 mL of extraction solvent (hexane/acetone/ethanol; 50/25/25%; v/v/v) and the mixture was vortexed for 1 min and centrifuged at 6000g at 5 °C for 10 min. The upper layer of hexane containing the pigments was recovered and expelled to a 25 mL volumetric flask. The remaining layer was subjected to a second extraction (same procedure as the extract) and the hexane layers were combined and adjusted to 25 mL. The total carotenoid content was evaluated by adding 1 mL of hexane extract by measuring the absorbance at 450 nm and was expressed in β-carotene using the absorbance coefficient of 2500 according to the following formula:
| Total carotenoids (μg mL−1) = A450 × volume (mL) × 1000/2500 × weight of the sample (g) |
Total anthocyanin compounds were evaluated according to the differentiation and using two buffers: KCl at pH 1.0 (0.025 M) and CH3COONa (0.025 M) pH 4.5 (0.4 M). 400 μL of the extract were mixed with 3.6 mL of the buffer (1), followed by 400 μL of the other, in buffer (2). The reaction mixture was incubated during 30 minutes in the dark and the absorbances are then read at 510 and 700 nm. The concentration of anthocyanin pigment in the extract is expressed as mg cyanidine equivalent glucosyl-3/g dry matter (mg ECy/g DM).36
| AA% = 100 × [(AE(0min) − AE(120min))/(A0(0min) − A0(120min))] |
AE(0min) et AE(120 min): absorbance of the sample analyzed at t = 0 and t = 120 min, respectively.
Animals were divided into six groups of eight animals each. Group 1 and 2 served as controls and received distilled water (10 mL kg−1, b.w., p.o.) for 15 days. Groups 3 and 4 were pre-treated with various doses of the SOFDE (100, 200 mg kg−1, b.w., p.o.), group 5 received sulfasalazine (100 mg kg−1, b.w., p.o.), while group 6 was pre-treated with the mixture (MIX: SOFDE, 50 mg kg−1 b.w., p.o. + sulfasalazine, 50 mg kg−1, b.w., p.o.). However, preliminary experiment indicated that the selected doses present the lowest that gives significant protective effects. Rats were fasted for 18 h before the last administration of SOFDE, MIX or reference molecules. After 60 min, each animal, except group 1, was received EtOH (4 g kg−1, b.w.) by oral administration. Two hours later, rats were anaesthetized by intraperitoneal administration of sodium pentobarbital (40 mg kg−1, b.w.) and sacrificed by decapitation,38 blood was collected and plasma processed for electrolytes (free iron, calcium and magnesium), alkaline phosphatase (ALP) and C-reactive protein (CRP) determinations.
| PSA (%) = 100 × (A517 (control) × A517 (sample)/A517 (control)). |
The activity of catalase was recorded by measuring the initial rate of H2O2 disappearance at 240 nm.44 The reaction mixture contained 33 mM H2O2 in 50 mM phosphate buffer (pH 7) and the CAT activity was calculated using the extinction coefficient of 40 mM−1 cm−1 for H2O2.
The activity of glutathione peroxidase was quantified following the procedure of Flohé and Gunzler.45 Briefly, 1 mL of reaction mixture containing 0.2 mL of gastric or intestinal mucosa supernatant, 0.2 mL of phosphate buffer 0.1 M pH 7.4, 0.2 mL of GSH (4 mM) and 0.4 mL of H2O2 (5 mM) was incubated at 37 °C for 1 min and the reaction was stopped by the addition of 0.5 mL TCA (5%, w/v). After centrifugation at 1500g for 5 min, aliquot (0.2 mL) from supernatant was combined with 0.5 mL of phosphate buffer 0.1 M pH 7.4 and 0.5 mL DTNB (10 mM) and absorbance was read at 412 nm. The GPx activity was expressed as nM of GSH consumed/min/mg protein.
The level of GSH was performed by colorimetric method using the method of Sedlak and Lindsay.47 In fact, 5 mL of supernatant was mixed with 4 mL of cold distilled water and 1 mL of TCA (50%). The tubes were vortexed for 10 minutes and centrifuged at 1200 g for 15 minutes. 2 mL supernatant was mixed with 4 mL of 0.4 M Tris buffer (pH 8.9). 0.1 mL of DTNB (0.01 M) were added to the reaction medium. The absorbance was recorded rapidly at 412 nm against the blank containing only the buffer.
Results
Phytochemical composition of Salvia officinalis flowers decoction extract (SOFDE) and in vitro antioxidant capacity
| Parameters | Contents |
|---|---|
| a Data are expressed as mean ± SEM (n = 3); SEM: standard error of the mean; DM: dry matter; TAE: tannic acid equivalent; CG: cyanidine glucosyl-3; RE: rutin equivalent. | |
| Iron (μmol L−1) | 3.29 ± 0.08 |
| Magnesium (mmol L−1) | 4.25 ± 0.02 |
| Extraction yield (%) | 17.22 ± 0.13 |
| Calcium (mmol L−1) | 6.64 ± 0.04 |
| Total tannins (mg TAE/g DM) | 60.41 ± 3.87 |
| Flavonols (mg RE/g DM) | 1.99 ± 0.02 |
| Total carotenoids (μg/100 mL) | 1.67 ± 0.09 |
| Total anthocyanins content (mg CG/g DM) | 3.79 ± 0.29 |
| β-Carotene bleaching inhibition IC50 (μg mL−1) | 56.77 ± 2.34 |
| Butylated hydroxytoluene IC50 (μg mL−1) | 20.83 ± 0.71 |
The HPLCPDAESI-MS/MSLC-HRESIMS analysis of SOFDE allowed to the identification of 13 phenolic compounds. Six phenolic acids which include quinic acid, protocatechuic acid, gallic acid, 1,3-di-O-caffeoyquinic acid, p-coumaric acid and salviolinic acid (Table 2). In addition, the chromatographic elution profile of flavonoids (Fig. 1) showed seven flavonoids compounds such as naringin, quercetin, kampherol, apegenin-7-o-glucoside, trans cinnamic, luteolin-7-O-glucoside and cirsilineol.
| Peak no. | Identificationa | Formula | [M]− H m/zb | Retention time (min) | Concentration (ppm) |
|---|---|---|---|---|---|
| a The compounds are suggested according to the dictionary of natural products and the characteristic fragmentation pattern.b The formulae were deduced from the quasi molecular ion peak [M + H]+. | |||||
| 1 | Quinic acid | C7H12O6 | 191.00 | 2.142 | 181.883 |
| 2 | Gallic acid | C7H6O5 | 169.00 | 3.986 | 158.708 |
| 9 | 1,3-Di-O-caffeoylquinic acid | ýC25H24O12 | 515.00 | 16.761 | 6.433 |
| 3 | Protocatchuic acid | C7H6O4 | 153.00 | 6.825 | 155.249 |
| 11 | p-Coumaric acid | C9H8O3 | 163.00 | 20.642 | 279.886 |
| 16 | Luteolin-7-O-glucoside | C21H20O11 | 447.00 | 59.969 | 48.608 |
| 20 | Naringin | C27H32O14 | 579.00 | 25.560 | 16.987 |
| 21 | Apegenin-7-O-glucoside | C21H20O10 | 431.00 | 67.712 | 14.217 |
| 23 | Salviolinic acid | C36H30O16 | 717.00 | 28.004 | 268.318 |
| 24 | Trans cinnamic | C9H802 | 147.00 | 31.617 | 733.142 |
| 25 | Quercetin | C21H20O11 | 301.00 | 31.740 | 40.272 |
| 26 | Kampherol | C15H10O6 | 285.00 | 31.840 | 1.708 |
| 31 | Cirsilineol | C18H16O7 | 343.00 | 38.998 | 5.271 |
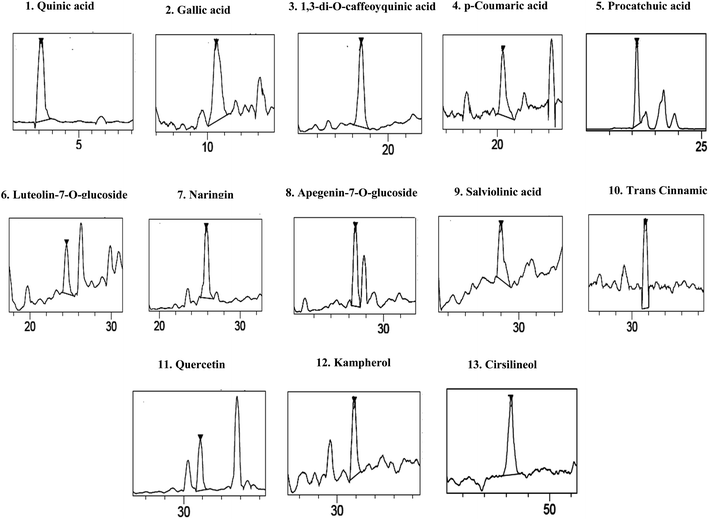 | ||
| Fig. 1 Chromatographic profile and characterization of phenolic compounds of Salvia officinalis L. flowers decoction extract (SOFDE) (assignments of peaks are given in Table 2). | ||
| Groups | Weight of stomach content (g) | Protection (%) | Weight of intestinal content (g) | Protection (%) |
|---|---|---|---|---|
| a *: p < 0.05 compared to control group and #: p < 0.05 compared to EtOH group. | ||||
| Control | 0.51 ± 0.18 | 0.34 ± 0.37 | ||
| EtOH | 5.39 ± 1.36* | 0 | 2.13 ± 0.90* | 0 |
| EtOH + SOFDE-100 | 4.57 ± 1.22# | 15.21 | 0.98 ± 0.72# | 53.99 |
| EtOH + SOFDE-200 | 4.08 ± 0.87# | 24.30 | 0.75 ± 0.30# | 64.79 |
| EtOH + SULF | 3.85 ± 1.43# | 41.56 | 0.56 ± 0.12# | 73.71 |
| EtOH + MIX | 2.99 ± 1.03# | 59.37 | 0.39 ± 0.09# | 81.69 |
| Groups | Mucus weight (g) | Protection (%) | Ulcer index (mm2) | Protection (%) | Small bowel injury index (mm2) | Protection (%) |
|---|---|---|---|---|---|---|
| a *: p < 0.05 compared to control group and #: p < 0.05 compared to EtOH group. | ||||||
| Control | 4.53 ± 0.57 | 0 | ||||
| EtOH | 1.74 ± 0.56* | 0 | 73.60 ± 4.05* | 0 | 20.82 ± 3.09* | |
| EtOH + SOLAE-100 | 2.62 ± 0.98# | 50.57 | 51.48 ± 3.83# | 30.05 | 13.34 ± 4.01# | 35.93 |
| EtOH + SOLAE-200 | 2.95 ± 0.46# | 69.54 | 39.80 ± 1.50# | 45.92 | 10.26 ± 1.37# | 50.72 |
| EtOH + SULF | 2.82 ± 0.26# | 62.07 | 29.75 ± 0.26# | 59.58 | 7.41 ± 0.36# | 64.41 |
| EtOH + MIX | 3.02 ± 0.19# | 73.56 | 10.13 ± 2.36# | 86.24 | 6.94 ± 0.43# | 66.67 |
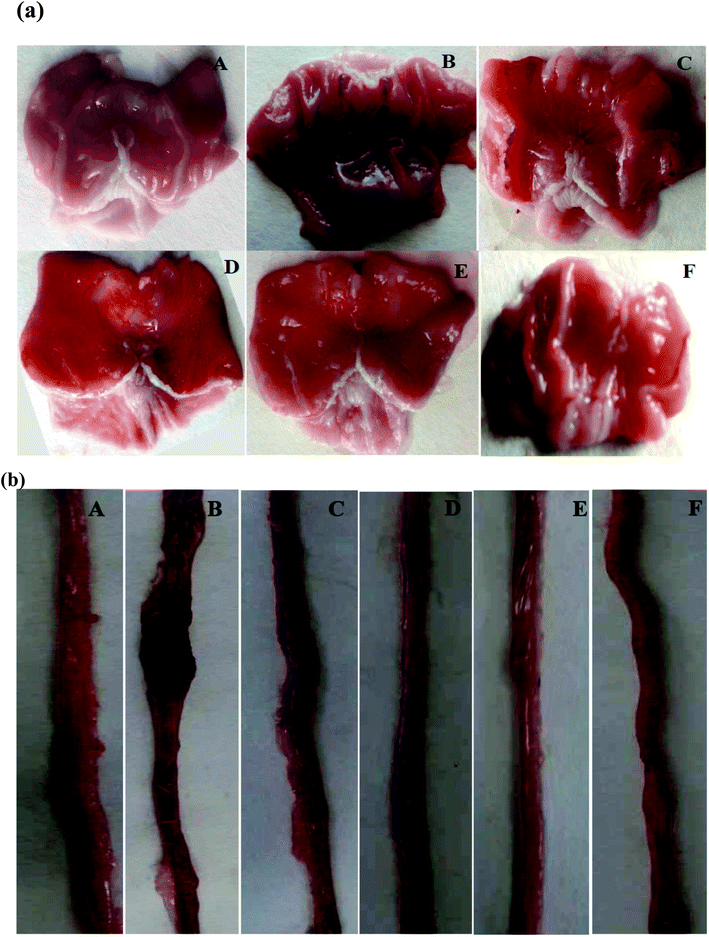
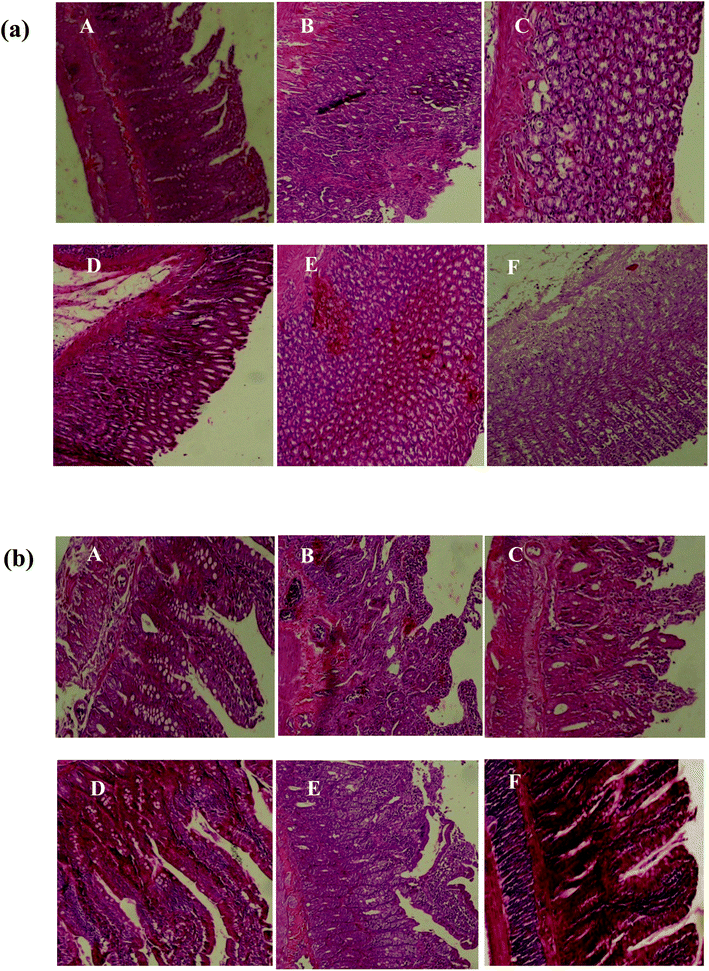
Pretreatment with SOFDE showed a dose-dependent protection of the gastric and intestinal mucosa as revealed by the reduction of lesions, mucosal and submucosal edema as well as leucocytes infiltration. Importantly, we showed that the group received the mixture registered the most important protection. A similar protective effect had also observed in sulfasalazine pretreated rats.
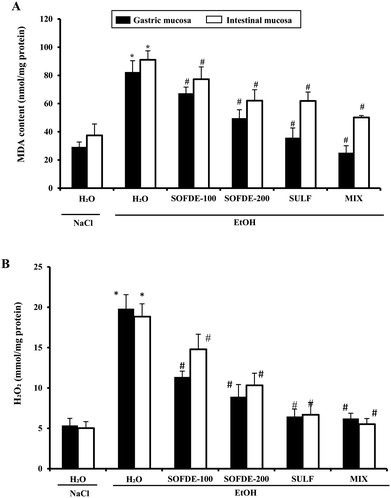
We also showed the effect of EtOH and SOFDE on intracellular mediator such as hydrogen peroxide level in gastric and intestinal mucosa (Fig. 4). In addition, alcohol group had a significant increase in hydrogen peroxide level in gastric and intestinal tissues when compared to negative control group. SOFDE and sulfasalazine treatment significantly and dose-dependently reduced the EtOH-induced this intracellular mediator deregulation. More importantly, our result found that MDA and H2O2 levels were reversed by MIX pre-treatment more significantly than sulfasalazine and SOFDE each alone.
| Groups | Free iron | Calcium | Magnesium | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gastric mucosa (μmol per mg per protein) | Intestinal mucosa (μmol per mg per protein) | Plasma (μmol L−1) | Gastric mucosa (mmol per mg per protein) | Intestinal mucosa (mmol per mg per protein) | Plasma (mmol L−1) | Gastric mucosa (μmol per mg per protein) | Intestinal mucosa (μmol per mg per protein) | Plasma (μmol L−1) | |
| a *: p < 0.05 compared to control group and #: p < 0.05 compared to EtOH group. | |||||||||
| Control | 22.02 ± 1.92 | 7.37 ± 1.05 | 1.28 ± 0.06 | 0.66 ± 0.08 | 0.73 ± 0.08 | 0.501 ± 0.04 | 194.32 ± 20.78 | 209.27 ± 19.69 | 24.30 ± 2.60 |
| EtOH | 63.09 ± 4.07* | 20.18 ± 1.78* | 2.26 ± 0.21* | 2.57 ± 0.32* | 3.11 ± 0.85* | 0.99 ± 0.08* | 364.63 ± 17.87* | 334.46 ± 17.12* | 98.66 ± 4.20* |
| EtOH + SOFDE-100 | 50.01 ± 3.90# | 17.37 ± 1.40# | 2.02 ± 0.13# | 2.48 ± 0.78# | 2.70 ± 0.58# | 0.86 ± 0,07# | 297.09 ± 19.74# | 289.61 ± 18.21# | 84.57 ± 4.09# |
| EtOH + SOFDE-200 | 44.22 ± 1.41# | 15.62 ± 1.33# | 1.69 ± 0.09# | 1.87 ± 0.54# | 2.26 ± 0.30# | 0.71 ± 0.05# | 266.26 ± 19.01# | 263.46 ± 20.21# | 63.70 ± 3.44# |
| EtOH + SULF | 34.13 ± 3.87# | 12.99 ± 2.56# | 1.42 ± 0.15# | 2.08 ± 0.79# | 2.08 ± 0.78# | 0.77 ± 0.06# | 231.69 ± 22.61# | 244.77 ± 21.22# | 53.22 ± 5.33# |
| EtOH + MIX | 30.45 ± 3.39# | 8.42 ± 1.69# | 1.31 ± 0.08# | 1.44 ± 0.41# | 1.31 ± 0.35# | 0.55 ± 0.07# | 200.86 ± 21.10# | 216.74 ± 17.12# | 49.51 ± 3.67# |
| Groups | C-Reactive protein (CRP) (μg dL−1) | Alkaline phosphatase (ALP) plasma (U L−1) at 37 °C |
|---|---|---|
| a *: p < 0.05 compared to control group and #: p < 0.05 compared to EtOH group. | ||
| Control | 0.34 ± 0.01 | 68.29 ± 3.72 |
| EtOH | 1.48 ± 0.37* | 190.44 ± 5.04* |
| EtOH + SLAE-100 | 0.92 ± 0.21# | 161.33 ± 1.67# |
| EtOH + SLAE-200 | 0.79 ± 0.12# | 105.42 ± 4.30# |
| EtOH + SULF | 0.69 ± 0.08# | 83.19 ± 5.86# |
| EtOH + MIX | 0.46 ± 0.01# | 71.27 ± 8.84# |
Discussion
In the present work, we investigated the phytochemical properties as well as the individual or synergistic protective actions of Salvia officinalis flowers decoction extract and sulfasalazine on EtOH-induced peptic ulcer.The phytochemical study firstly showed that SOFDE presents an important antioxidant activity assessed by to the bleaching inhibition potency of β-carotene (IC50 = 56.77 ± 2.34 μg mL−1). A similar result was obtained by Martins et al.49 who observed a good β-carotene bleaching inhibition (IC50 = 50.87 ± 3.73 μg mL−1). The antioxidant activity of SOFDE could be, in part, attributed to its high phenolic compounds levels. In this context, our data also suggest that SOFDE presents a high concentration of flavonols, total tannins and a moderate concentration of total anthocyanins and carotenoids. These levels fully corroborated those of Akhondzadeh et al.22 The antioxidant capacity is mainly attributed to the hight level of phenolic acids and flavonoids in this fraction such as quinic, protocatchuic, 1,3-di-O-caffeoyquinic, p-coumaric and salviolinic acids, and naringin, quercetin, kampherol, apigenin-7-o-glucoside, luteolin-7-o-glucoside and cirsilineol. It has been previously found that the majority of these bioactive molecules has been identified in aqueous leaf extracts and has been shown for a high antioxidant capacity against DPPH radical.32
In vivo, we firstly revealed that acute alcohol administration distorted the gastric and duodenal mucosa and submucosa, which are accompanied by surface coating, epithelial cells alterations, as well as edema and leukocyte infiltration. Indeed, prostaglandin deficiency within the digestive mucosa is defined as the major pathogenic mechanism of the ethanol-induced digestive system diseases. The deficit in endogenous prostaglandins plays an essential role in the pathogenic process by making the mucosa more vulnerable to the aggression, without direct implication in the digestive lesions.50 Our data are in line with previous report using EtOH as ulceration inducer.51 Ethanol causes injures in the vascular endothelial cells of the gastric and intestine mucosa and induces microcirculatory disturbance and hypoxia, related to massive production of free radicals.52
Importantly, our data showed a protective effect of subaccute treatment with SOFDE (15 days) against gastric and intestinal fluid accumulation, weight of mucus and lesions induced by EtOH administration. Our extract also contributed in the reduction of macroscopic and histopathological observed damages. The therapeutic effect of MIX is more pronounced than SULF (100 mg kg−1, b.w., p.o.) used as reference molecule as well as the high dose of SOFDE (200 mg kg−1, b.w., p.o.). This beneficial effect can be explained by the fact that this drug has a single course of action53 while sage decoction extract acts through several different mechanisms.54 When those two mechanisms are combined in the gastrointestinal tract, synergism will occur through several biochemical targets and pathways. However, for phenolic compounds many mechanisms might be involved such as intracellular mediators by chelation of metal ions (Fe2+, Cu2+), membrane stabilization,55 inhibition of pepsinogen production56 and increased mucus production, characterized by a film formed by the polymerization of glycoproteins which makes it possible to trap the bicarbonates and delay the penetration of endoluminous H+ ions. This situation establish a pH gradient ranging from less than 3 at the level of the luminal face of this layer, to more than 7 on the mucosal surface.57 However, the mixture of natural active substances and drugs has been used in previous work to treat cancer diseases58 as well as its antibacterial activity.59
In the present study, we also showed a high concentration of total tannins (60.41 ± 3.87 mg TAE/g DM). These molecules could prevent ulcer development throughout vasoconstricting effects or to their proteins precipitation in the ulceration site, producing an impermeable coating over the lining that restrains gut secretions and defends the underlying mucosa from lesions.60 We also found a high level of flavonls (kaempferol and quercetin), that present anti-ulcer and gastroprotective properties.61
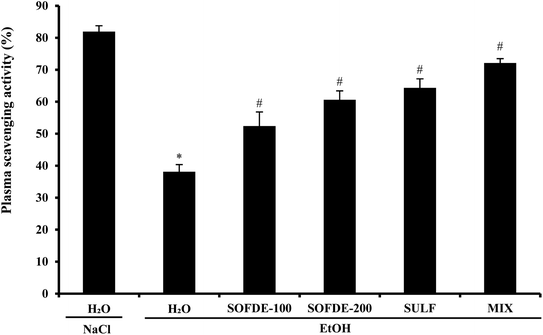
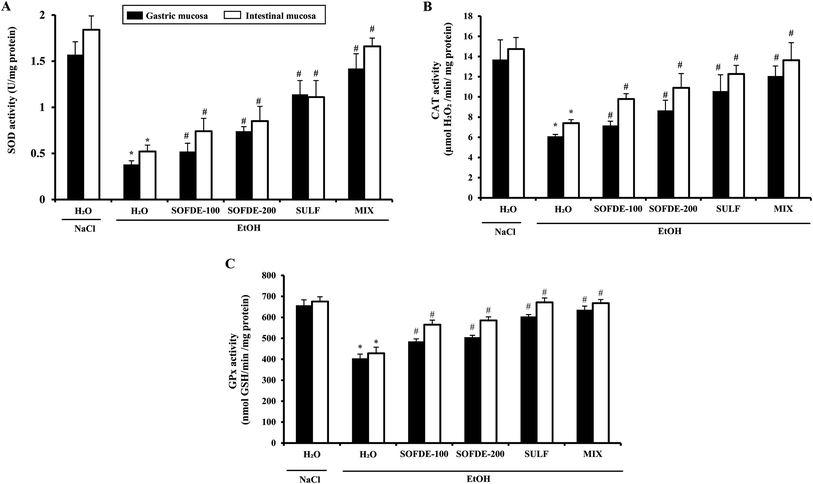
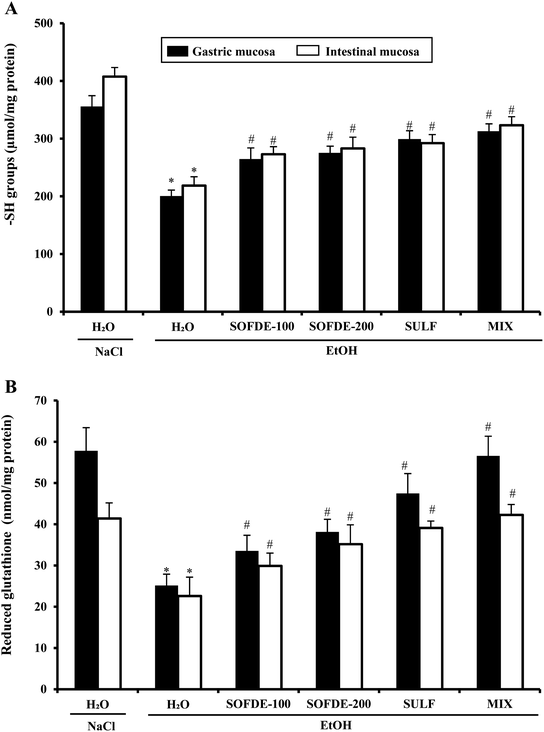
We also showed that EtOH intoxication induced a depletion of plasma scavenging activity (PSA), as well as an increase of hydrogen peroxide and MDA level, which is defined as an indicator of the ROS generation in tissues and plasma. In addition, we observed a decrease of thiol group and GSH levels, as well as depletion antioxidant enzyme activities such SOD, CAT and GPx. However, its well known that acute administration of EtOH leads oxidative imbalance through several pathways such as the generation of reactive oxygen species.62 Indeed, SOD converts the reactive superoxide radical to H2O2, which was decreased in the gastric and intestine mucosa. When this intracellular mediator was not scavenged by CAT, it could leads to lipid peroxidation after generation of hydroxyl radical.63 More importantly, we showed that SOFDE and sulfasalazine administration each alone or in combination (MIX) abolished acute EtOH-induced oxidative stress in the gastric and the duodenal mucosa. These finding are similar with previous study which has reported that the sage decoction extract contains a good amount of total polyphenols, flavonoïds, condensed tannins and a high level of rosmarinic and salviolinic acids and apegenin-7-O-glucoside.49 These molecules are the primal source of the antioxidant ability of this plant and are including in the scavenging of free radicals as hydroxyl radical (OH˙) which is the major cause of lipid peroxidation.64 Furthermore, those sulfhydryl groups are in part involved in gastric cytoprotection65 as well as in the maintain of mucosal barrier integrity and free radicals scavenging.
Importantly, we showed an increase of intracellular mediators such as calcium, free iron and magnesium in plasma, gastric and duodenal mucosa in response to oxidative stress induced by ethanol administration. These data are in line with several previous studies.66,67 However, we can now suggest that SOFDE exerts a beneficial effect by chelating free iron and scavenging H2O2 and regulation of the calcium and magnesium homeostasis. Our results also supposes that pretreatment with SOFDE protects against overcharge of cells of the gastric and intestinal mucosa by free iron and H2O2 induced by ethanol sub-acute administration. Moreover, these later are involved in the generation of hydroxyl radical (OH˙),68 which plays the major role in oxidative damage by affecting the molecular structures.69 In this respect, Jan et al.70 conclude that living organisms create a complex endogenous and exogenous antioxidant defense system to restrict the production of this damaging radical.
Finally, we have shown in the present work that EtOH intoxication induced inflammation as assessed by a significant increase in plasma CRP and ALP when compared to control group (P < 0.05). On the other hand, the pre-treatment with SOFDE, sulfasalazine and MIX significantly decreased the studied biomarkers. These data are in line with those of Mosli et al.71 Indeed, several studies have shown that EtOH is associated with an inflammatory state via the expression of pro-inflammatory cytokines.51 However, oxidative stress is well known to be related to inflammatory gastric and bowel disease.72 Moreover, quinic, salviolinic, procatchuic and p-coumaric acids which are identified with abandoned amount in SOFDE possess an important anti-inflammatory activity.29,73
Conclusion
In conclusion, our data clearly demonstrated strong synergic protective effects between Salvia officinalis decoction extract and sulfasalazine against ethanol-induced gastric and small bowel injuries. This gastro-duodenal protection might be due in part to its antioxidant and anti-inflammatory properties as well as its opposite effects on intracellular mediators such as hydrogen peroxide, free iron and calcium. Therefore, the mixture between bioactive compounds from plant and standards drugs is considered as an alternative to protect against gastro-intestinal disorders and to avoid unpredictable side effects of commercial drugs.Ethical consideration
All procedures on animals in this study were approved with the National Institute of Health recommendations for the use and care of animals.Abbreviations
| CAT | CAT |
| EtOH | Ethanol |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| H2O2 | Hydrogen peroxide |
| MDA | Malondialdehyde |
| MIX | Mixture |
| ROS | Reactive oxygen species |
| –SH | Sulfhydryl groups |
| SOD | Superoxide dismutase |
| SOFDE | Salvia officinalis flowers decoction extract |
| SULF | Sulfasalazine |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support of the Tunisian Ministry of Higher Education and Scientific Research and the Institution of Agricultural Research and Higher Education are appreciatively acknowledged.References
- S. X. M. Dong, C. C. Y. Chang and K. J. Rowe, A collection of the etiological theories, characteristics, and observations/phenomena of peptic ulcers in existing data, Data Brief, 2018, 19, 1058–1067 CrossRef.
- H. Suzuki, E. Iwasaki and T. Hibi, Helicobacter pylori and gastric cancer, Gastric Cancer, 2009, 12, 79–87 CrossRef.
- H. E. Vonkeman, R. M. Klok, M. J. Postma, J. R. Brouwers and M. A. van de Laar, Direct medical costs of serious gastrointestinal ulcers among users of NSAIDs, Drugs Aging, 2007, 24, 681–690 CrossRef.
- P. Maity, K. Biswas, S. Roy, R. K. Banerjee and U. Bandyopadhyay, Nov Smoking and the pathogenesis of gastroduodenal ulcer recent mechanistic update, Mol. Cell. Biochem., 2003, 253, 329–338 CrossRef CAS.
- L. Ceglia, S. S. Harris, H. M. Rasmussen and B. Dawson-Hughes, Activation of the calcium sensing receptor stimulates gastrin and gastric acid secretion in healthy participants, Osteoporosis Int., 2009, 20, 71–78 CrossRef CAS.
- L. Bujanda, The effects of alcohol consumption upon the gastrointestinal tract, Am. J. Gastroenterol., 2000, 95, 3374–3382 CrossRef CAS.
- J. Sun, J. Fu, Y. Zhong, L. Li, C. Chen, X. Wang, L. Wang, Y. Hou, H. Wang, R. Zhao, X. Zhang, M. Yamamoto, Y. Xu and J. Pi, NRF2 mitigates acute alcohol-induced hepatic and pancreatic injury in mice, Food Chem. Toxicol., 2018, 121, 495–503 CrossRef CAS.
- M. Hamaguchi, T. Watanabe, K. Higuchi, K. Tominaga, Y. Fujiwara and T. Arakawa, Mechanisms and roles of neutrophil infiltration in stress-induced gastric injury in rats, Dig. Dis. Sci., 2001, 46, 2708–2715 CrossRef CAS.
- N. M. Grüning, M. Rinnerthaler, K. Bluemlein, M. Mülleder, M. M. Wamelink, H. Lehrach, C. Jakobs, M. Breitenbach and M. Ralser, Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells, Cell Metab., 2011, 14, 415–427 CrossRef.
- B. Halliwell, Biochemistry of oxidative stress, Biochem. Soc. Trans., 2007, 35, 1147–1150 CrossRef CAS.
- B. C. Almroth, J. Sturve, A. Berglund and L. Förlin, Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers, Aquat. Toxicol., 2005, 73, 171–180 CrossRef CAS.
- T. N. Litvin, M. Kamenetsky, A. Zarifyan, J. Buck and L. R. Levin, Kinetic properties of "soluble" adenylyl cyclase. Synergism between calcium and bicarbonate, J. Biol. Chem., 2003, 278, 15922–15926 CrossRef CAS.
- N. Tashiro, S. Budhathoki, K. Ohnaka, K. Toyomura, S. Kono, T. Ueki, M. Tanaka, Y. Kakeji, Y. Maehara, T. Okamura, K. Ikejiri, K. Futami, T. Maekawa, Y. Yasunami, K. Takenaka, H. Ichimiya and R. Terasaka, Constipation and colorectal cancer risk: the Fukuoka Colorectal Cancer Study, Asian Pac. J. Cancer Prev., 2011, 12, 2025–2030 Search PubMed.
- P. Barbero, R. Liascovich, R. Valdez and A. Moresco, Misoprostol teratogenicity: a prospective study in Argentina, Arch. Argent. Pediatr., 2011, 109, 226–231 Search PubMed.
- B. U. Lindberg, U. Broomé and B. Persson, Proximal colorectal dysplasia or cancer in ulcerative colitis. The impact of primary sclerosing cholangitis and sulfasalazine: results from a 20-year surveillance study, Dis. Colon Rectum, 2001, 44, 77–85 CrossRef CAS.
- H. Pachajoa and C. Isaza, First case of Moebius-Poland syndrome in child prenatally exposed to misoprostol, Neurol Barc Spain, 2011, 26, 502–503 CAS.
- S. Jedidi, K. Rtibi, S. Selmi, F. Aloui, H. Selmi, D. Wannes, H. Sammari, N. Dhawefi, C. Abbes and H. Sebai, Phytochemical/antioxidant properties and individual/synergistic actions of Salvia officinalis L. aqueous extract and loperamide on gastrointestinal altering motor function, J. Med. Food, 2019, 22, 235–1245 CrossRef.
- J. B. Walker, K. J. Sytsma, J. Treutlein and M. Wink, Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae, Am. J. Bot., 2004, 91, 1115–1125 CrossRef.
- N. M. Rasmy and A. A. Hassan, Foda MI and El-Moghazy MM Assessment of the antioxidant activity of sage (Salvia officinalis L.) extracts on the shelf life of mayonnaise, World J. Dairy Food Sci., 2012, 7, 28–40 Search PubMed.
- M. Fawzi, Z. Kamel and S. Farhan, Anti-inflammatory effect of sage (Salvia officinalis) extracts abstract on oral health, Iraqi Dent. J., 2017, 39, 1–6 CrossRef.
- M. Monsefi, M. Abedian, Z. Azarbahram and M. J. Ashraf, Salvia officinalis L. induces alveolar bud growing in adult female rat mammary glands, Avicenna J. Phytomed., 2015, 5, 560–567 CAS.
- S. Akhondzadeh, M. Noroozian, M. Mohammadi, S. Ohadinia, A. H. Jamshidi and M. Khani, Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial, J. Clin. Pharm. Ther., 2003, 28, 53–59 CrossRef CAS.
- D. Stanojevic, L. Comic, O. Stefanovic and S. Solujic-Sukdolak, In vitro synergistic antibacterial activity of Salvia officinalis and some preservatives, Arch. Biol. Sci., 2010, 62, 175–183 CrossRef.
- A. Jedinák, M. Mucková, D. Kost'álová, T. Maliar and I. Masterova, Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis, Z. Naturforsch., C: J. Biosci., 2006, 61, 777–782 Search PubMed.
- J. T. Coon and E. Ernst, Systematic review: herbal medicinal products for non-ulcer dyspepsia, Aliment. Pharmacol. Ther., 2002, 16, 1689–1699 CrossRef.
- M. Wang, H. Kikuzaki, N. Zhu, S. Sang, N. Nakatani and C. T. Ho, Isolation and structural elucidation of two new glycosides from sage (Salvia officinalis L.), J. Agric. Food Chem., 2000, 48, 235e238 Search PubMed.
- P. Badiee, A. R. Nasirzadeh and M. Motaffaf, Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species, J. Pharm. Technol. Drug Res., 2012, 1, 7 CrossRef.
- A. M. Mansourabadi, H. M. Sadeghi, N. Razavi and E. Rezvani, Anti-inflammatory and analgesic properties of salvigenin, Salvia officinalis flavonoid extracted, Adv. Herb. Med., 2015, 1, 31e41 Search PubMed.
- D. Baricevic, S. Sosa, R. Della Loggia, A. Tubaro, B. Simonovska, A. Krasna and A. Zupancic, Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid, J. Ethnopharmacol., 2001, 75, 125–132 CrossRef CAS.
- H. Nicolella, J. Senedese, R. Furtado, R. Veneziani and D. Tavares, Evaluation of antiinflammatory potential of diterpene manool in macrophages by quantification of nitric oxide, J. Int. Soc. Antioxid. Nutr. Health, 2015, 1, 124e128 Search PubMed.
- AOAC, Official methods of analysis, ed. Association of Official Analytical Chemists, Washington DC, 1990 Search PubMed.
- S. Jedidi, H. Selmi, F. Aloui, K. Rtibi, M. Jridi, C. Abbes and H. Sebai, Comparative studies of phytochemical screening, HPLC-PDA-ESIMS/MS-LC/HR-ESI-MS analysis, antioxidant capacity and in vitro fermentation of officinal sage (Salvia officinalis L.) cultivated in different biotopes of Northwestern Tunisia, Chem. Biodiversity, 2019, 16, e1900394 Search PubMed.
- T. S. Kujala, J. M. Loponen, K. D. Klika and K. Pihlaja, Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds, J. Agric. Food Chem., 2000, 48, 5338–5342 CrossRef CAS.
- G. Rigane, R. Ben Salem, S. Sayadi and M. Bouaziz, Phenolic composition, isolation and structure of a new deoxyloganic acid derivative from Dhokar and Gemri-Dhokar olive cultivars, J. Food Sci., 2011, 76, 965–973 CrossRef.
- I. H. Marina, O. Velumuttu and E. K. Pekka, Carotenoids in finish foods: vegetables, fruits and berries, J. Agric. Food Chem., 1989, 37, 655–659 CrossRef.
- M. M. Giusti and R. E. Wrolstad, Anthocyanins: characterization and measurement with UV-visible spectroscopy Current protocols in food analytical chemistry banner, John Wiley & Sons, New York, 2001, pp. 19–32 Search PubMed.
- T. Kulisica, A. Radonic, V. Katalinic and M. Milos, Use of different methods for testing antioxidant activity of oregano essential oil, Food Chem., 2004, 85, 633–640 CrossRef.
- K. Rtibi, I. Hammami, S. Selmi, D. Grami, H. Sebai, M. Amri and L. Marzouki, Phytochemical properties and pharmacological effects of Quercus ilex L. aqueous extract on gastrointestinal physiological parameters in vitro and in vivo, Biomed. Pharmacother., 2017, 94, 787–793 CrossRef.
- G. D. Dicarlo, N. Mascolo, A. A. Izzo, F. Capasso and G. Autore, Effects of quercetin on gastrointestinal tract in rats and mice, Phytother. Res., 1994, 8, 42–45 CrossRef CAS.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Use of a free radical method to evaluate antioxidant activity, LWT--Food Sci. Technol., 1995, 28, 25–30 CrossRef CAS.
- H. H. Draper and M. Hadley, Malondialdehyde determination as index of lipid peroxidation, Methods Enzymol., 1990, 186, 421–431 CAS.
- B. Dingeon, J. P. Ferry and A. Roullet, Automatic assay of blood sugar by Trinder's method, Ann. Biol. Clin., 1975, 33, 3–13 CAS.
- P. Kakkar, B. Das and P. N. Viswanathan, Modified spectrophotometric assay of SOD, Indian J. Biochem. Biophys., 1984, 2, 130–132 Search PubMed.
- H. Aebi, Catalase in vitro, Methods Enzymol., 1984, 105, 121–126 CAS.
- L. Flohé and W. A. Gunzler, Assays of glutathione peroxidase, Methods Enzymol., 1984, 105, 14–121 Search PubMed.
- G. L. Ellman, Tissue sulfhydryl groups, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef CAS.
- J. Sedlak and R. H. Lindsay, Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman's reagent, Anal. Biochem., 1968, 25, 192–205 CrossRef CAS.
- E. F. Hartree, Determination of protein: a modification of the Lowry method that gives a linear photometric response, Anal. Biochem., 1972, 48, 422–427 CrossRef CAS.
- N. Martins, L. Barros, C. Santos-Buelga, M. Henriques, S. Silva and I. C. Ferreira, Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L, Food Chem., 2015, 1, 378–385 CrossRef.
- K. Amandeep, S. Robin, S. Ramica and K. Sunil, Peptic ulcer: a review on etiology and pathogenesis, Int. Res. J. Pharm., 2012, 3, 34–38 Search PubMed.
- S. Selmi, K. Rtibi, D. Grami, H. Sebai and L. Marzouki, Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat, Lipids Health Dis., 2017, 16, 152 CrossRef.
- H. Suzuki, T. Nishizawa, H. T. Sugawa, S. Mogami and T. Hibi, Roles of oxidative stress in stomach disorders, J. Clin. Biochem. Nutr., 2015, 50, 35–39 CrossRef.
- J. Dale, N. Alcorn, H. Capell and R. Madhok, Combination therapy for rheumatoid arthritis: methotrexate and sulfasalazine together or with other DMARDs, Nat. Clin. Pract. Rheumatol., 2007, 3, 450–458 CrossRef CAS.
- D. Procházková, I. Boušová and N. Wilhelmová, Antioxidant and prooxidant properties of flavonoids, Fitoter, 2011, 82, 513–523 CrossRef.
- E. Czinner, K. Hagymasi, A. Blazovics, A. Kery, E. Szoke and E. Lemberkovics, The in vitro effect of Helichysi flos on microsomal lipid peroxidation, J. Ethnopharmacol., 2001, 77, 31–35 CrossRef CAS.
- J. Alanko, A. Riutta, P. Holm, I. Mucha, H. Vapatalo and T. Metsa-Ketela, Modulation of arachidonic acid metabolism by phenols: relation to their structure and antioxidant/prooxidant properties, Free Radicals Biol. Med., 1999, 26, 193–201 CrossRef CAS.
- H. F. Gufo Kamguia, C. Fokunang, B. Ngameni, B. Njinkio Nono and E. Tembe-Fokunang, Effet cytoprotecteur de l’extrait aqueux des racines de Dorstenia psilurus sur l’ulcère gastrique chez les rats males de la souche Wistar, Health Sci. Dis., 2011, 12(4), 8–18 Search PubMed.
- D. H. Zhou, X. Wang, M. Yang, X. Shi, W. Huang and Q. Feng, Combination of low concentration of (−)epigallocatechingallate (EGCG) and curcumin strongly suppresses the growth of non small cell lung cancer in vitro and in vivo through causing cell cycle arrest, Int. J. Mol. Sci., 2013, 14, 12023–12036 CrossRef.
- M. Bhardwaj, B. R. Singh, D. K. Sinha, V. Kumar, O. R. PrasannaVadhana, S. Varan Singh, K. R. Nirupama, P. Shree and B. S. Archana Saraf, Potential of herbal drug and antibiotic combination therapy: a new approach to treat multidrug resistant bacteria, Pharmac. Anal. Acta, 2016, 7, 1–4 Search PubMed.
- N. Z. deJesus, H. de Souza Falcão, I. F. Gomes, T. J. de Almeida Leite, G. R. de Morais Lima, J. M. Barbosa-Filho, J. F. Tavares, M. S. da Silva, P. F. de Athayde-Filho and L. M. Batista, Tannins, peptic ulcers and related mechanisms, Int. J. Mol. Sci., 2012, 13, 3203–3228 CrossRef CAS.
- P. Hodek, P. Trefil and M. Stiborova, Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450, Chem.-Biol. Interact., 2002, 139, 1–21 CrossRef CAS.
- R. V. Pérez-Gallardo, L. S. Briones, A. L. Díaz-Pérez, S. Gutiérrez, J. S. Rodríguez-Zavala and J. Campos-García, Reactive oxygen species production induced by ethanol in Saccharomyces cerevisiae increases because of a dysfunctional mitochondrial iron-sulfur cluster assembly system, FEMS Yeast Res., 2013, 13, 804–819 CrossRef.
- H. Yin, L. Xu and N. A. Porter, Free radical lipid peroxidation: mechanisms and analysis, Chem. Rev., 2011, 111, 5944–5972 CrossRef CAS.
- S. M. Kadhim, M. T. Mohammed, O. M. Ahmed and A. M. N. Jassim, Study of some Salvia officinalis L. (sage) components and effect of their aqueous extract on antioxidant, Int. J. Chem. Sci., 2016, 14, 711–719 CAS.
- G. Natale, G. Lazzeri, V. Lubrano, R. Colucci, C. Vassalle, M. Fornai, C. Blandizzi and M. delTacca, Mechanisms of gastroprotection by lansoprazole pretreatment against experimentally induced injury in rats: role of mucosal oxidative damage and sulfhydryl compounds, Toxicol. Appl. Pharmacol., 2004, 195, 62–72 CrossRef CAS.
- T. Günther, J. Vormann and J. A. McGuigan, Buffering and activity coefficient of intracellular free magnesium concentration in human erythrocytes, Biochem. Mol. Biol. Int., 1995, 37, 871–875 Search PubMed.
- R. D. Rainbow, D. Macmillan and J. G. McCarron, The sarcoplasmic reticulum Ca2+ store arrangement in vascular smooth muscle, Cell Calcium, 2009, 46, 313–322 CrossRef CAS.
- E. G. Bagryanskaya, O. A. Krumkacheva, M. V. Fedin and S. R. A. Marque, Development and application of spin traps, spin probes, and spin labels, Methods Enzymol., 2015, 563, 365–396 CAS.
- J. K. Willcox, S. L. Ash and G. L. Catignani, Antioxidants and prevention of chronic disease, Crit. Rev. Food Sci. Nutr., 2004, 44, 275–295 CrossRef CAS.
- A. T. Jan, M. Azam, K. Siddiqui, A. Ali, I. Choi and Q. M. R. Haq, Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants, Int. J. Mol. Sci., 2015, 5, 29592–29630 CrossRef.
- M. H. Mosli, G. Zou, S. K. Garg, S. G. Feagan, J. K. MacDonald, N. Chande, W. J. Sandborn and B. G. Feagan, C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis, Am. J. Gastroenterol., 2015, 110, 802–819 CrossRef.
- I. M. Balmus, A. Ciobica, A. Trifan and C. Stanciu, The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: clinical aspects and animal models, Saudi J. Gastroenterol., 2016, 22, 3–17 CrossRef.
- C. F. Lima, P. B. Andrade, R. M. Seabra, M. Fernandes-Ferreira and C. Pereira-Wilson, The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats, J. Ethnopharmacol., 2005, 97, 383–389 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |